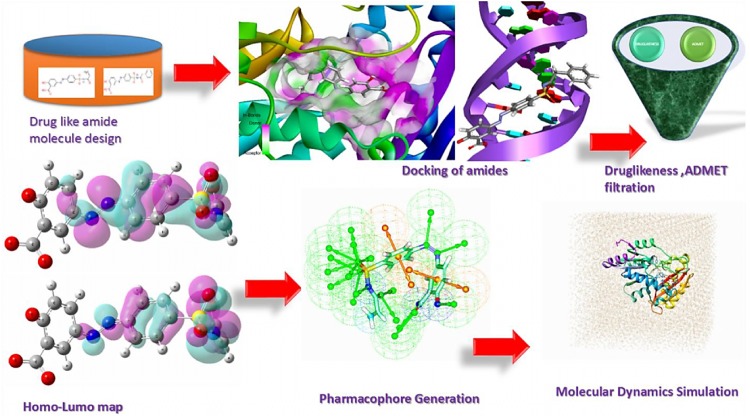
Abstract
Abstract
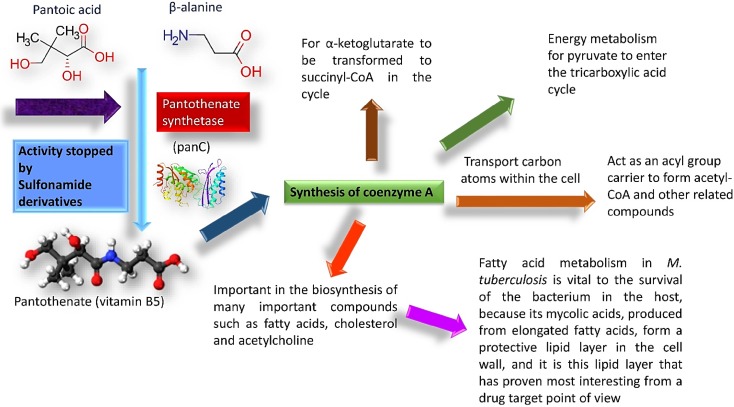
Pantothenate is a crucial enzyme for the synthesis of coenzyme A and acyl carrier protein in Mycobacterium tuberculosis and Staphylococcus aureus. It is indispensable for the growth and survival of these bacteria. Amides analogs are designed and have been used as inhibitors of pantothenate synthetase. Molecular docking approach has been used to design and predict the drug activity of molecule to the specific disease. In this work, more than hundred amides have been screened by Discovery Studio molecular docking programme to search best suitable molecule for the treatment of Mycobacterium tuberculosis. Pharmacophore generation has been done to recognize the binding modes of inhibitors in the receptor active site. To observe the stability and flexibility of inhibitors molecular dynamics (MD) simulation has been done; Lipinski’s rule of five protocols is followed to screen drug likeness and ADMET (absorption, distribution, metabolism, excretion and toxicity) filtration is also used to value toxicity. DFT computation of optimized geometry and derivation of MOs has been used to correlate the drug likeness. The small difference in energy between HOMO and LUMO may help to activate the drug in the protein environment quickly. 2-Hydroxy-5-[(E)-2-{4-[(prop-2-enamido)sulfonyl]phenyl}diazen-1-yl]benzoic acid (M1) shows best theoretical efficiency against Mycobacterium tuberculosis (MTB) pantothenate synthetase and so does 2-hydroxy-5-[(E)-2-{4-[(2-phenylacetamido)sulfonyl]phenyl}diazen-1-yl]benzoic acid (M2) against Staphylococcus aureus pantothenate synthetase. These compounds also bind to Adenine–Thymine region of tuberculosis DNA.
Graphical abstract
Keywords: Pantothenate synthetase inhibitors, Heterocyclic amide compounds, Structure based drug design, Molecular docking, ADMET, MD simulation
Introduction
Virtually one-third of the human population of the world is suffering from Mycobacterium tuberculosis (MTB) infection (Onyango 2011). Despite the existence of approved drug against TB, it continues to claim approximately 1.5 million lives every year due to drug-resistant TB problem (multidrug resistant tuberculosis, MDR-TB and extensively drug resistant tuberculosis, XDR-TB). So, there is an urgent need to develop new anti-TB drugs (Onyango 2011). In the search for MTB vaccine, growth and virulence of pantothenate synthetase (panC) auxotrophs has been severely collaborated, sustaining the theory of the necessity of this enzyme and its prettiness as an antimicrobial target (White et al. 2007).
Pantothenate synthetase (PS) plays critical roles in many cellular processes like, fatty acid metabolism. It catalyzes the adenosine triphosphate (ATP)-dependent condensation of pantoate and β-alanine to form pantothenate (vitamin B5), and key precursor for the synthesis of coenzyme A (CoA) and acyl carrier protein (ACP) (von Delft et al. 2001). Upon action of fatty acid synthases on Acetyl-CoA and NADPH fatty acids are generated which combine with glycerol followed by phosphorylation could form phospholipid (Leonardi and Jackowski 2007). The bulk of the lipid bilayers those make up cell wall and surround the organelles within the cells have been synthesized from phospholipids (Berg et al. 2002; Chaffey et al. 2003). Lipid-rich cell wall of MTB is an essential element of intracellular survival and pathogenicity, and also thought to contribute to the difficulty of effectively delivering antimicrobial agents into the cell. The significance of this lipid-rich cell wall is underscored by the large number of genes (approximately 250) encoding enzymes in fatty acid metabolism present in the MTB genome, making this pathway a promising target for new antibacterial drug discovery (Cole et al. 1998). Indeed, several anti-tubercular agents are known to inhibit cell wall biosynthesis.
PanC is absent in mammals. It searches pantothenate from their diet using pantothenate permease, of which, there is no homolog in MTB (Table 1) (Grassl 1992; Vallari and Rock 1985). Literature search shows that MTB mutant defective in the de novo biosynthesis of pantothenate is highly attenuated in both immune compromised and immune competent mice. It points out that functionality of pantothenate in biosynthetic pathway is crucial (Fig. 1) for virulence of MTB (Wang and Eisenberg 2003). Different industries invest over 50 billion dollars on research and development each year to identify potential new drug targets. Pantothenate Synthetase may explore an opportunity to design the drug resistant TB drugs (Overington et al. 2006).
Table 1.
Percent identity matrix (PAM) for searching similarities between different species containing Pantothenate synthetase
| Seq. no. | PDB chain | Species | Similarities in percentage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3N8H_A|PDBID|CHAIN|SEQUENCE | Francisellatularensis | 100 | 36.4 | 39.46 | 38.52 | 37.93 | 35.57 | 38.67 | 34.24 | 34.4 | 37.26 | 38.58 | 36.29 |
| 2 | 4EFK_A|PDBID|CHAIN|SEQUENCE | Mycobacterium tuberculosis | 36.4 | 100 | 43.93 | 43.84 | 41.79 | 43.96 | 45.71 | 39.21 | 44.81 | 41.08 | 40.36 | 36.43 |
| 3 | 3Q10_A|PDBID|CHAIN|SEQUENCE | Yersinia pestis | 39.46 | 43.93 | 100 | 71.73 | 72.82 | 44.96 | 50.18 | 40.57 | 45.42 | 44.91 | 44.24 | 38.87 |
| 4 | 1IHO_A|PDBID|CHAIN|SEQUENCE | Escherichia coli | 38.52 | 43.84 | 71.73 | 100 | 87.99 | 44.73 | 52.16 | 40.71 | 46.89 | 45.55 | 46.76 | 40.5 |
| 5 | 3MUE_A|PDBID|CHAIN|SEQUENCE | Salmonella enterica | 37.93 | 41.79 | 72.82 | 87.99 | 100 | 41.01 | 49.47 | 39.86 | 45.42 | 44.56 | 43.88 | 40.28 |
| 6 | 3UK2_A|PDBID|CHAIN|SEQUENCE | Burkholderiathailandensis | 35.57 | 43.96 | 44.96 | 44.73 | 41.01 | 100 | 54.48 | 40.73 | 44.78 | 45.88 | 44.53 | 37.32 |
| 7 | 5KWV_A|PDBID|CHAIN|SEQUENCE | Neisseria gonorrhoeae | 38.67 | 45.71 | 50.18 | 52.16 | 49.47 | 54.48 | 100 | 37.91 | 46.1 | 43.86 | 46.72 | 38.49 |
| 8 | 3AG6_A|PDBID|CHAIN|SEQUENCE | Staphylococcus aureus | 34.24 | 39.21 | 40.57 | 40.71 | 39.86 | 40.73 | 37.91 | 100 | 41.82 | 43.11 | 45.71 | 42.91 |
| 9 | 1UFV_A|PDBID|CHAIN|SEQUENCE | Thermusthermophilus | 34.4 | 44.81 | 45.42 | 46.89 | 45.42 | 44.78 | 46.1 | 41.82 | 100 | 50.91 | 51.09 | 40.15 |
| 10 | 3INN_A|PDBID|CHAIN|SEQUENCE | Brucellamelitensis | 37.26 | 41.08 | 44.91 | 45.55 | 44.56 | 45.88 | 43.86 | 43.11 | 50.91 | 100 | 50.36 | 41.05 |
| 11 | 2EJC_A|PDBID|CHAIN|SEQUENCE | Thermotoga maritima | 38.58 | 40.36 | 44.24 | 46.76 | 43.88 | 44.53 | 46.72 | 45.71 | 51.09 | 50.36 | 100 | 52.33 |
| 12 | 3MXT_A|PDBID|CHAIN|SEQUENCE | Campylobacter jejuni | 36.29 | 36.43 | 38.87 | 40.5 | 40.28 | 37.32 | 38.49 | 42.91 | 40.15 | 41.05 | 52.33 | 100 |
Fig. 1.
Activity of pantothenate synthetase
Amides are known to play a crucial role in supramolecular anion sensing technology (McMurry et al. 2017). They may also be used as antibacterial agents against Gram positive and Gram negative bacteria in the future design of drugs (Stefańska et al. 2015). Amide derivatives (Table 2) show numerous types of biological features as anthelmintic, antihistaminic, antifungal, and antibacterial including Staphylococcus aureus and Mycobacterium tuberculosis (Yildiz et al. 2008; Jagessar and Rampersaud 2007; The Sulfa Derivatives in the Treatment of Tuberculosis 1944).
Table 2.
Selected amides (IUPAC names are given) showing docking score (CDOCKER energy) against Mycobacterial and Staphylococcus p.s. They have been passed the Lipinski’s rule and ADMET filter
| IUPAC name of amides | MTB | SA | Lipinski’s rule | ADMET | |
|---|---|---|---|---|---|
| A | Sulfamethoxazole | a.u. | a.u. | ||
| 1 | (2S)-2-amino-4-({4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}carbamoyl)butanoic acid | − 44.63 | − 46.94 | + | + |
| 2 | (2S)-2-amino-N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}-3-phenylpropanamide | − 46.53 | − 55.40 | + | + |
| 3 | 2-Amino-N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}benzamide | − 45.32 | − 47.30 | + | + |
| 4 | 2-Hydroxy-N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}benzamide | − 56.43 | − 53.08 | + | + |
| 5 | 4-({4-[(5-Methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}amino)benzene-1-sulfonamide | − 48.41 | − 48.83 | + | + |
| 6 | 4-({4-[(5-Methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}amino)benzene-1-sulfonamide | − 50.02 | − 50.64 | + | + |
| 7 | 4-Methyl-N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}benzene-1-sulfonamide | − 40.11 | − 47.32 | + | + |
| 8 | 5-({4-[(5-Methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}amino)naphthalene-1-sulfonamide | − 35.012 | − 52.06 | + | + |
| 9 | 6-({4-[(5-Methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}amino)pyridine-3-carboxamide | − 50.57 | − 48.74 | + | + |
| 10 | N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}acetamide | − 37.12 | − 36.75 | + | + |
| 11 | N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}benzamide | − 45.62 | − 46.02 | + | + |
| 12 | N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}hexanamide | − 41.38 | − 49.40 | + | + |
| 13 | N-(5-methyl-1,2-oxazol-3-yl)-4-(N-methylhydrazido)benzene-1-sulfonamide | ND | − 55.37 | + | + |
| 14 | 4-(N-butylbenzenesulfonamido)-N-(5-methyl-1,2-oxazol-3-yl)benzene-1-sulfonamide | − 38.70 | − 38.31 | + | + |
| 15 | N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}prop-2-enamide | − 38.21 | − 35.20 | + | + |
| 16 | N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}pyrazine-2-carboxamide | − 45.32 | − 46.67 | + | + |
| 17 | N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}pyridine-3-carboxamide | ND | − 47.12 | + | + |
| 18 | (2R)-4-carbamoyl-2-({4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}amino)butanoic acid | − 53.47 | − 57.71 | + | + |
| 19 | (2R,3S)-N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}-3-[(4E,7E)-nona-4,7-dienoyl]oxirane-2-carboxamide | − 44.86 | − 62.80 | + | + |
| 20 | (2S)-2,6-diamino-N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}hexanamide | − 50.59 | − 52.40 | + | + |
| 21 | N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}-1,3-benzothiazole-2-sulfonamide | − 40.30 | − 49.45 | + | + |
| 22 | N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}-1,3-thiazole-2-sulfonamide | − 41.62 | − 45.85 | + | + |
| 23 | N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}-2,3-dihydro-1H-indene-5-sulfonamide | 41.73 | − 47.61 | + | + |
| 24 | N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}-2-phenoxyacetamide | − 42.48 | − 49.78 | + | + |
| 25 | 3-Amino-N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}benzamide | − 48.74 | − 49.06 | + | + |
| 26 | N-(4-ethoxyphenyl)-N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}acetamide | − 44.95 | − 50.17 | + | + |
| 27 | N-methyl-N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}acetamide | − 38.90 | − 39.89 | + | + |
| 28 | N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}thiophene-2-sulfonamide | − 38.80 | − 42.56 | + | + |
| 29 | N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}-1,2,3,4-tetrahydroisoquinoline-7-sulfonamide | − 45.4053 | − 49.9116 | + | + |
| 30 | 1-Methyl-N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}-3-oxo-1,3-dihydro-2,1-benzothiazole-5-sulfonamide | − 42.03 | − 56.77 | + | + |
| 31 | N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}-2-propylpentanamide | − 42.87 | − 48.82 | + | + |
| 32 | N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}-3-oxo-N-[(3S)-2-oxooxolan-3-yl]octanamide | ND | − 63.91 | + | + |
| 33 | N-[2-({4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}amino)ethyl]isoquinoline-5-sulfonamide | ND | − 37.33 | + | + |
| 34 | N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}-2-oxopropanamide | − 42.18 | − 40.96 | + | + |
| 35 | 4-(4-Chlorophenyl)-N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}piperazine-1-carboximidamide | ND | ND | + | + |
| 36 | 4-Amino-N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}-1H-imidazole-5-carboxamide | − 43.80 | − 41.97 | + | + |
| 37 | 5-Hydroxy-N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}naphthalene-1-sulfonamide | − 39.17 | − 51.45 | + | + |
| 38 | 4-Benzenesulfonamido-N-(5-methyl-1,2-oxazol-3-yl)benzene-1-sulfonamide | − 43.03 | − 46.33 | + | + |
| 39 | 4-Hydroxy-3-methoxy-N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}benzamide | − 48.55 | − 70.46 | + | + |
| 40 | N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}-N-phenyl-1H-pyrazole-5-carboxamide | − 48.013 | − 50.82 | + | + |
| 41 | N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}-N-phenylformamide | − 38.78 | − 44.14 | + | + |
| 42 | N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}piperidine-1-sulfonamide | − 41.12 | − 49.65 | + | + |
| 43 | 4-[(1,2-Benzoxazol-3-yl)methanesulfonamido]-N-(5-methyl-1,2-oxazol-3-yl)benzene-1-sulfonamide | − 41.82 | − 49.84 | + | + |
| 44 | N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}-1-benzothiophene-2-sulfonamide | − 43.82 | − 9.82 | + | + |
| 45 | 4-(N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}acetamido)benzoic acid | − 34.11 | − 50.71 | + | + |
| 46 | (2R)-2-[(N-hydroxyformamido)methyl]-4-methyl-N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}pentanamide | ND | − 59.30 | + | + |
| 47 | 1,3-Dimethyl-N-{4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl}-2-oxo-2,3-dihydro-1H-1,3-benzodiazole-5-carboxamide | − 46.39 | ND | + | + |
| B | Sulfadiazine | ||||
| 48 | (2S)-4-carbamoyl-2-({4-[(pyrimidin-2-yl)sulfamoyl]phenyl}amino)butanoic acid | − 48.54 | − 65.49 | + | − |
| 49 | (2S)-2-amino-3-phenyl-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}propanamide | − 46.06 | − 52.08 | + | + |
| 50 | 2-Amino-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}benzamide | − 44.51 | − 43.50 | + | + |
| 51 | 2-Hydroxy-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}benzamide | − 46.65 | − 44.73 | + | − |
| 52 | 2-Phenyl-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}acetamide | − 43.36 | − 46.87 | + | + |
| 53 | 4-Amino-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}benzene-1-sulfonamide | − 41.56 | − 48.64 | + | + |
| 54 | 4-Methyl-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}benzene-1-sulfonamide | − 47.37 | − 49.44 | + | + |
| 55 | 6-({4-[(Pyrimidin-2-yl)sulfamoyl]phenyl}amino)pyridine-3-carboxamide | − 35.78 | − 51.18 | + | + |
| 56 | N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}acetamide | − 35.78 | − 37.34 | + | + |
| 57 | N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}benzamide | − 46.84 | − 45.60 | + | + |
| 58 | N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}hexanamide | − 46.99 | − 47.66 | + | + |
| 59 | N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}formamide | − 46.02 | − 37.80 | + | + |
| 60 | 4-(N-butylbenzenesulfonamido)-N-(pyrimidin-2-yl)benzene-1-sulfonamide | − 37.22 | − 50.71 | + | + |
| 61 | N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}prop-2-enamide | − 38.178 | − 38.06 | + | + |
| 62 | N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}pyrazine-2-carboxamide | − 43.31 | − 45.02 | + | + |
| 63 | N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}pyridine-3-carboxamide | − 45.56 | − 44.12 | + | + |
| 64 | (2R,3S)-3-[(4E,7E)-nona-4,7-dienoyl]-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}oxirane-2-carboxamide | ND | − 61.01 | + | − |
| 65 | (2S)-2,6-diamino-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}hexanamide | − 51.11 | − 54.59 | + | − |
| 66 | N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}-1,3-benzothiazole-2-sulfonamide | − 48.19 | − 47.25 | + | + |
| 67 | N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}-1,3-thiazole-2-sulfonamide | − 40.52 | − 43.90 | + | + |
| 68 | 2-Phenoxy-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}acetamide | − 49.30 | − 48.70 | + | + |
| 69 | 3-Amino-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}benzamide | − 47.69 | − 49.00 | + | + |
| 70 | (2R)-4-carbamoyl-2-({4-[(pyrimidin-2-yl)sulfamoyl]phenyl}amino)butanoic acid | − 53.28 | − 60.18 | + | − |
| 71 | N-(4-ethoxyphenyl)-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}acetamide | − 41.89 | − 52.01 | + | + |
| 72 | N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}acetamide | − 35.78 | − 37.34 | + | + |
| 73 | N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}thiophene-2-sulfonamide | − 42.40 | − 43.95 | + | + |
| 74 | N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}-1,2,3,4-tetrahydroisoquinoline-7-sulfonamide | − 44.78 | − 52.40 | + | + |
| 75 | 1-Methyl-3-oxo-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}-1,3-dihydro-2,1-benzothiazole-5-sulfonamide | − 40.17 | − 43.95 | + | − |
| 76 | 2-Propyl-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}pentanamide | − 45.87 | − 52.11 | + | − |
| 77 | 3-Oxo-N-[(3S)-2-oxooxolan-3-yl]-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}octanamide | − 41.03 | − 56.66 | + | − |
| 78 | 2-Oxo-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}propanamide | ND | − 41.98 | + | − |
| 79 | 4-(4-Chlorophenyl)-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}piperazine-1-carboximidamide | − 42.016 | − 49.23 | + | + |
| 80 | 4-Amino-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}-1H-imidazole-5-carboxamide | − 38.34 | − 47.26 | + | + |
| 81 | 5-Hydroxy-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}naphthalene-1-sulfonamide | − 43.3 | − 46.86 | + | − |
| 82 | 4-Benzenesulfonamido-N-(pyrimidin-2-yl)benzene-1-sulfonamide | − 44.59 | − 45.62 | + | + |
| 83 | N-ethyl-4-hydroxy-3-methoxy-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}benzamide | − 40.13 | − 49.39 | + | − |
| 84 | N-phenyl-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}-1H-pyrazole-5-carboxamide | − 48.63 | − 43.00 | + | + |
| 85 | N-phenyl-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}formamide | ND | − 51.9081 | + | + |
| 86 | N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}piperidine-1-sulfonamide | − 39.31 | − 47.16 | + | + |
| 87 | 4-[(1,2-Benzoxazol-3-yl)methanesulfonamido]-N-(pyrimidin-2-yl)benzene-1-sulfonamide | − 45.79 | − 48.93 | + | + |
| 88 | N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}-1-benzothiophene-2-sulfonamide | − 41.86 | − 49.57 | + | + |
| 89 | 4-(N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}acetamido)benzoic acid | − 48.16 | − 50.66 | + | + |
| 90 | (2R)-2-[(N-hydroxyformamido)methyl]-4-methyl-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}pentanamide | ND | − 51.64 | + | + |
| 91 | 1,3-Dimethyl-2-oxo-N-{4-[(pyrimidin-2-yl)sulfamoyl]phenyl}-2,3-dihydro-1H-1,3-benzodiazole-5-carboxamide | − 19.93 | − 46.00 | + | + |
| C | Sulfadoxine | ||||
| 92 | (2S)-4-carbamoyl-2-({4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}amino)butanoic acid | ND | ND | + | − |
| 93 | (2S)-2-amino-N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}-3-phenylpropanamide | − 40.08 | ND | + | − |
| 94 | 2-Amino-N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}benzamide | − 41.83 | − 49.59 | + | − |
| 95 | N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}-2-hydroxybenzamide | ND | − 71.28 | + | − |
| 96 | N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}-2-phenylacetamide | − 44.46 | − 48.99 | + | − |
| 97 | 4-Amino-N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}benzene-1-sulfonamide | − 44.53 | − 53.77 | + | − |
| 98 | N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}-4-methylbenzene-1-sulfonamide | − 44.03 | ND | + | − |
| 99 | 6-({4-[(5,6-Dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}amino)pyridine-3-carboxamide | − 43.82 | ND | + | − |
| 100 | N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}acetamide | − 41.15 | − 42.00 | + | − |
| 101 | N-{4-[(5,6-Dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}benzamide | − 46.17 | ND | + | − |
| 102 | N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}hexanamide | − 42.91 | − 51.01 | + | − |
| 103 | N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}formamide | − 47.49 | − 40.98 | + | − |
| 104 | N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}prop-2-enamide | ND | − 44.25 | + | − |
| 105 | N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}pyrazine-2-carboxamide | − 47.52 | ND | + | − |
| 106 | N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}pyridine-3-carboxamide | − 46.09 | − 51.93 | + | − |
| 107 | (2R)-4-carbamoyl-2-({4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}amino)butanoic acid | ND | ND | + | − |
| 108 | (2S)-2,6-diamino-N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}hexanamide | − 46.87 | ND | + | − |
| 109 | N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}-1,3-thiazole-2-sulfonamide | ND | ND | + | − |
| 110 | N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}-2-phenoxyacetamide | − 47.79 | − 56.89 | + | − |
| 111 | 3-Amino-N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}benzamide | − 52.91 | − 50.61 | + | − |
| 112 | 1-{4-[(5,6-Dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}-3-(4-ethoxyphenyl)urea | ND | ND | + | − |
| 113 | N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}-N-methylacetamide | ND | − 47.15 | + | − |
| 114 | N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}thiophene-2-sulfonamide | ND | − 50.31 | + | − |
| 115 | 4-Amino-N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}-1H-imidazole-5-carboxamide | NA | NA | − | NA |
| 116 | N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}-2-oxopropanamide | − 46.87 | − 46.82 | + | − |
| 117 | N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}-2-propylpentanamide | ND | − 53.05 | + | + |
| 118 | 4-Amino-N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}-1H-imidazole-5-carboxamide | NA | NA | − | NA |
| 119 | 4-Benzenesulfonamido-N-(5,6-dimethoxypyrimidin-4-yl)benzene-1-sulfonamide | − 53.75 | − 51.50 | − | − |
| 120 | N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}-N-phenyl-1H-pyrazole-5-carboxamide | ND | ND | − | NA |
| 121 | N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}-N-phenylformamide | − 41.87 | − 53.59 | + | + |
| 122 | N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}piperidine-1-sulfonamide | ND | ND | − | − |
| 123 | 4-(N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}acetamido)benzoic acid | ND | ND | − | NA |
| 124 | (2R)-N-{4-[(5,6-dimethoxypyrimidin-4-yl)sulfamoyl]phenyl}-2-[(N-hydroxyformamido)methyl]-4-methylpentanamide | ND | ND | − | NA |
| D | Sulfasalaine | ||||
| 125 | 5-[(E)-2-(4-{[(1S)-3-carbamoyl-1-carboxypropyl]sulfamoyl}phenyl)diazen-1-yl]-2-hydroxybenzoic acid | ND | ND | − | − |
| 126 | 5-[(E)-2-(4-{[(2S)-2-amino-3-phenylpropanamido]sulfonyl}phenyl)diazen-1-yl]-2-hydroxybenzoic acid | ND | − 83.98 | + | + |
| 127 | 5-[(E)-2-(4-{[(2-aminophenyl)formamido]sulfonyl}phenyl)diazen-1-yl]-2-hydroxybenzoic acid | − 47.53 | − 82.68 | + | − |
| 128 | 2-Hydroxy-5-[(E)-2-(4-{[(2-hydroxyphenyl)formamido]sulfonyl}phenyl)diazen-1-yl]benzoic acid | − 47.56 | − 60.56 | + | − |
| 129 | 2-Hydroxy-5-[(E)-2-{4-[(2-phenylacetamido)sulfonyl]phenyl}diazen-1-yl]benzoic acid (M2) | − 62.18 | − 85.41 | + | + |
| 130 | 2-Hydroxy-5-[(E)-2-{4-[(4-methylbenzenesulfonyl)sulfamoyl]phenyl}diazen-1-yl]benzoic acid | − 54.11 | − 68.96 | + | − |
| 131 | 5-[(E)-2-[4-(acetamidosulfonyl)phenyl]diazen-1-yl]-2-hydroxybenzoic acid | − 50.51 | − 57.42 | + | + |
| 132 | 2-Hydroxy-5-[(E)-2-[4-(phenylformamido)sulfonylphenyl]diazen-1-yl]benzoic acid | − 46.29 | − 58.41 | + | + |
| 133 | 5-[(E)-2-[4-(hexanamidosulfonyl)phenyl]diazen-1-yl]-2-hydroxybenzoic acid | − 42.93 | − 81.40 | + | + |
| 134 | 2-Hydroxy-5-[(E)-2-(4-formamidosulfonylphenyl)diazen-1-yl]benzoic acid | − 61.32 | − 74.46 | + | + |
| 135 | 2-Hydroxy-5-[(E)-2-{4-[(prop-2-enamido)sulfonyl]phenyl}diazen-1-yl]benzoic acid (M1) | − 66.41 | − 70.68 | + | + |
| 136 | 2-Hydroxy-5-[(E)-2-(4-{[(pyridin-3-yl)formamido]sulfonyl}phenyl)diazen-1-yl]benzoic acid | − 68.67 | − 66.03 | + | − |
| 137 | 5-[(E)-2-{4-[(4-aminobenzenesulfonyl)sulfamoyl]phenyl}diazen-1-yl]-2-hydroxybenzoic acid | NA | NA | − | NA |
| 138 | 2-Hydroxy-5-[(E)-2-{4-[(4-methylbenzenesulfonyl)sulfamoyl]phenyl}diazen-1-yl]benzoic acid | NA | NA | − | NA |
| 139 | 2-Hydroxy-5-[(E)-2-(4-{[(pyrazin-2-yl)formamido]sulfonyl}phenyl)diazen-1-yl]benzoic acid | NA | NA | + | NA |
| 140 | 2-Hydroxy-5-[(E)-2-{4-[(2-phenoxyacetamido)sulfonyl]phenyl}diazen-1-yl]benzoic acid | − 62.84 | − 79.97 | + | − |
| 141 | 5-[(E)-2-(4-{[(3-aminophenyl)formamido]sulfonyl}phenyl)diazen-1-yl]-2-hydroxybenzoic acid | − 56.08 | − 77.70 | + | + |
| 142 | 5-[(E)-2-(4-{[N-(4-ethoxyphenyl)acetamido]sulfonyl}phenyl)diazen-1-yl]-2-hydroxybenzoic acid | ND | − 29.85 | + | + |
| 143 | 2-Hydroxy-5-[(E)-2-{4-[(N-methylacetamido)sulfonyl]phenyl}diazen-1-yl]benzoic acid | − 50.24 | − 78.35 | + | − |
| 144 | 2-Hydroxy-5-[(E)-2-{4-[(thiophene-2-sulfonyl)sulfamoyl]phenyl}diazen-1-yl]benzoic acid | − 71.17 | − 75.69 | + | − |
| 145 | 5-[(E)-2-(4-{[(1R)-3-carbamoyl-1-carboxypropyl]sulfamoyl}phenyl)diazen-1-yl]-2-hydroxybenzoic acid | NA | NA | − | NA |
| 146 | 5-[(E)-2-(4-{[(2S)-2,6-diaminohexanamido]sulfonyl}phenyl)diazen-1-yl]-2-hydroxybenzoic acid | NA | NA | − | NA |
| 147 | 2-Hydroxy-5-[(E)-2-{4-[(1,3-thiazole-2-sulfonyl)sulfamoyl]phenyl}diazen-1-yl]benzoic acid | NA | NA | − | NA |
| 148 | 2-Hydroxy-5-[(E)-2-{4-[(2-propylpentanamido)sulfonyl]phenyl}diazen-1-yl]benzoic acid | ND | − 72.11 | + | + |
| 149 | 2-Hydroxy-5-[(E)-2-{4-[(2-oxopropanamido)sulfonyl]phenyl}diazen-1-yl]benzoic acid | − 46.87 | − 72.11 | + | − |
| 150 | 5-[(E)-2-{4-[(benzenesulfonyl)sulfamoyl]phenyl}diazen-1-yl]-2-hydroxybenzoic acid | − 53.75 | − 78.77 | + | − |
| 151 | 2-Hydroxy-5-[(E)-2-{4-[(N-phenylformamido)sulfonyl]phenyl}diazen-1-yl]benzoic acid | − 41.85 | − 58.47 | + | + |
| 152 | 5-[(E)-2-(4-{[(4-amino-1H-imidazol-5-yl)formamido]sulfonyl}phenyl)diazen-1-yl]-2-hydroxybenzoic acid | NA | NA | − | NA |
| 153 | 2-Hydroxy-5-[(E)-2-{4-[(piperidine-1-sulfonyl)sulfamoyl]phenyl}diazen-1-yl]benzoic acid | NA | NA | − | NA |
| 154 | 5-[(E)-2-(4-{[N-(4-carboxyphenyl)acetamido]sulfonyl}phenyl)diazen-1-yl]-2-hydroxybenzoic acid | NA | NA | − | NA |
| 155 | C1 (MTB) | − 40.58 | NA | + | + |
| 156 | C2 (SA) | NA | − 44.31 | + | + |
In the table ‘ND’ denotes there were no docking pose for the molecules, ‘NA’ denotes the molecules hadn’t passed the Lipinski rule. ‘+’ sign denotes that the amides have passed Lipinski’s rule and ADMET filter, ‘−’ denotes they haven’t passe Lipinski’s rule and ADMET filter
On the other hand, sulfa drugs show potent anti-microbial activity and literature has shown that they are active against Mycobacterium tuberculosis and Staphylococcus aureus (Bartzatt et al. 2010; Holloway et al. 2016). In this work, a series of amide functionalized sulfa drugs have been designed by modifying sulfa drugs amides by structure based drug design. Amides have been docked to examine their action against Mycobacterium tuberculosis (MTB) and Staphylococcus aureus (SA).
According to some scientists at Lilly Research Laboratories, Eli Lilly & Company, USA two compounds with 4-cyano-1-methyl-3-(4-phenylphenyl)pyrrole-2-carboxylic acid core structure, inhibited MTB pantothenate synthetase (Kumar et al. 2013).The MIC50 values of the two compounds were high (55 and 118 µM. This suggested the growth inhibitory properties were due to PanC-mediated inhibition[a].The IUPAC name of the compounds are 3‐{[1,1′‐biphenyl]‐4‐yl}‐4‐cyano‐5‐(ethylsulfanyl)‐1‐methyl‐1H‐pyrrole‐2‐carboxylic acid (compound 1) and 3‐{[1,1′‐biphenyl]‐4‐yl}‐4‐cyano‐5‐ethyl‐1‐methyl‐1H‐pyrrole‐2‐carboxylic acid (compound 2). In the present work it has been showed that 2-hydroxy-5-[(E)-2-{4-[(prop-2-enamido)sulfonyl]phenyl}diazen-1-yl]benzoic acid and 2-hydroxy-5-[(E)-2-{4-[(2-phenylacetamido)sulfonyl]phenyl}diazen-1-yl]benzoic acid showed better docking score than compound 1 (C1) and compound 2 (C2). Sulfonamide, sulfamoyl groups as co-crystalized structure in the crystal structure of pantothenate synthetase proves their efficiency in binding and inhibition.
Out of 154 different amides of sulfa drugs 93 derivatives show higher docking score (binding affinity) than C1 (Table 3). For SA also some compounds exhibit docking score higher than C2 (Table 4) which are used to treat bacterial infection by susceptible microorganisms (Kumar et al. 2013; Wu et al. 2003). Molecular dynamics (MD) simulations have been done to observe the effect of explicit solvent molecules on protein (Pantothenate Synthetase) ligand (amides) complex structure and stability to achieve time-averaged attributes of the bimolecular system, along with diverse thermodynamic parameters. Drug likeness has been examined following Lipinski’s rule of five filter (Lipinski et al. 2001). The molecular orbitals are used to calculate the electronic configuration, molecular reactivity and stability of the compounds by density functional theory (DFT) computational process (Rozhenko 2014). Pharmacophore map generation has also done to observe steric and electronic features of best docked amides that ensured the optimal interactions with a receptor (Pantothenate Synthetase) and to block its biological response (Wermuth et al. 1998). ADMET (absorption, distribution, metabolism, excretion and toxicity) filtration has been applied to check toxicity of the compounds (Hou and Wang 2008). Two compounds out of 154 amides, 2-hydroxy-5-[(E)-2-{4-[(prop-2-enamido)sulfonyl]phenyl} diazen-1-yl]benzoic acid (M1, Table 2) and 2-hydroxy-5-[(E)-2-{4-[(2-phenylacetamido)sulfonyl]phenyl}diazen-1-yl]benzoic acid (M2, Table 2) exhibit better theoretical drug potency than approved and have crossed ADMET and Lipinski’s rule of five filter (Table 2). These two compounds also bind to Adenine–Thymine region of tuberculosis DNA (Sirajuddin et al. 2013).
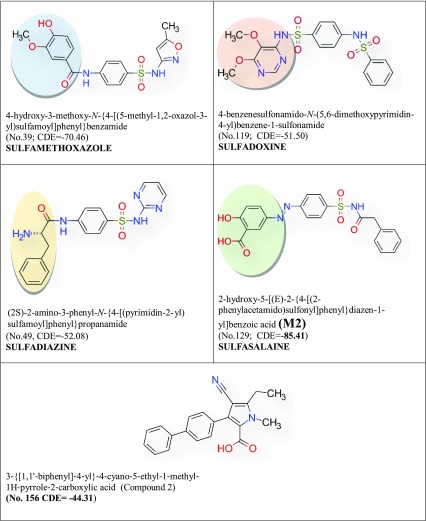
Table 3.
Selected sulfonamides of higher docking score than C2 with SA pantothenate synthetase and passed the Lipinski’s rule and ADMET filter. IUPAC names (Serial no; Table 2) and CDOCKER energy (CDE).The name of the drug group are given below. Added functional groups are in coloured circle
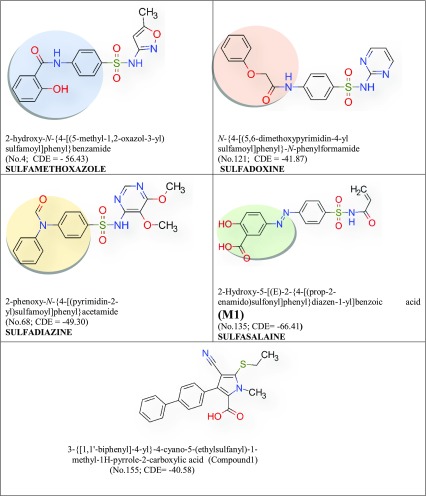
Table 4.
Selected sulfonamides of higher docking score than C1 with MTB pantothenate synthetase and passed the Lipinski’s rule and ADMET filter. IUPAC names (Serial No. Table 2) and CDOCKER energy (CDE). The name of the drug group are given below. Added functional groups are in coloured circle
Methods and materials
Sequence alignment analysis
Functional similarity between two or more sequences of amino acids has been carried out by using in silico multiple-sequence alignment (MSA) and is important for finding the functionality of proteins and evolution history of the species (Nguyen and Pan 2013).
High throughput virtual screening (HTVS)
Structure-based drug discovery is a vital process for fast and cost-effective lead discovery and proven to be more efficient than the conventional wet lab drug discovery (Lionta et al. 2014; Pradhan and Sinha 2017). High-Throughput Screening (HTS) is an approach to drug discovery that has become a standard tool for structure-based drug discovery. It is basically a process of screening of large number of drug like molecules against selected and specific drug targets. HTS are used for screening of various types of libraries, including combinatorial chemistry, genomics, protein, and peptide libraries (Szymański et al. 2012).
Drug-like amide molecule preparation
We have collected 154 small Lipinski’s filter passed amide molecule compounds from the PubChem database which inhibit pantothenate synthetase enzyme (Database Resources of the National Center for Biotechnology Information 2013). The compounds are drawn by compiling sulfa drugs with amides attached to the amino group of sulfa drugs by Accelrys Draw v4. The 3D coordinates and change of ionization are done by Discovery Studio 4 software’s ligand preparation wizard. Compounds are prepared using the “Prepare Ligand” protocol in Discovery Studio v4; default parameters towards performing the CDOCKER simulation with sdf files (The Sulfa Derivatives in the Treatment of Tuberculosis 1944).
a. Docking preparation of pantothenate synthetase
Pantothenate synthetase of MTB has been retrieved from RCSB PDB database with five bound-inhibitors. The protein data bank (pdb) file (pdb id: 4efk) showed a dimer of two chains. However, in the present study, only the monomeric unit (A-chain) has been used in the docking studies because it has two N, N-dimethylthiophene-3-sulfonamide bound inhibitors. The pantothenate synthetase of SA was retrieved from RCSB PDB with three bound-inhibitors (pdb id: 3ag6). The pdb file was a dimer of two chains, only the monomeric unit (A- chain) was used in the docking studies.
The bounded inhibitors from the pdb structure are removed before docking. Prepare Protein protocol has been executed such as inserting missing atoms in incomplete residues, modeling missing loop regions, and removing waters from protein. The default parameter values are mostly kept the invariant in the Prepare Protein protocol of Discovery Studio 4.
b. Molecular docking
Molecular docking of 154 selected pantothenate synthetase inhibitors to the receptor enzyme has been carried out in the present study by using CDOCKER with Discovery Studio v4. To perform flexible docking, for the small-molecules all torsion angles are set to be free. CDOCKER is a powerful CHARMm-based docking method that has been used to generate highly accurate docked poses. In this refinement application, the ligands were conceded to tilt around the rigid receptor (Wu et al. 2003). In the CDOCKER simulation the following parameters are used: top hits-10, random conformations-10, orientations to refine-10, and force field-CHARMm. For the assurance of potential relationships between pantothenate synthetase and amides, predicted CDOCKER energy values of the best docked conformations of small-molecule inhibitors (amides and approved drugs) are selected as preliminary binding conformations and saved for observing interactions between pantothenate synthetase and amides.
Drug likeness
Lipinski’s rule of five (RO5) describes four simple physicochemical factors ranges (MWT < 500, log P < 5, H-bond donors < 5, H bond acceptors < 10) related with 90% of orally active drugs that have accomplished phase II clinical status (Lipinski et al. 2001). These physicochemical factors are related with aqueous solubility, intestinal permeability and oral bioavailability. Because all parameters can be easily computed, the RO5 (or its variants) has become the most widely useful filter in virtual library design (Lipinski 2004).
Quantum chemistry calculation
DFT (density functional theory) calculations have been performed by Gaussian 09W (Frisch et al. 2009). Gaussian calculation setup has been done in Gaussian 09 software using Becke’s three-parameter exchange potential and Lee–Yang–Parr correlation functional (B3LYP) theory with basis set 6–31G (Becke 1993; Gill et al. 1992; Devlin et al. 1995). The surfaces (molecular orbital, density, potential) and electrostatic potential charges (EPS) have calculated the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) (Fukui et al. 1952). Chemical reactivity, intermolecular interactions and kinetic stability of molecules are characterized by the energy difference of HOMO–LUMO functions (Rauk 1994; Fleming 2011; Strom and Wilson 2013; Brownell et al. 2013). Interaction between the HOMO of the drug (amides) and the LUMO of the receptor (pantothenate synthetase) is the key factor of drug activity. Tight binding of drugs with the receptor can be achieved by increasing HOMO energy and decreasing LUMO energy in the drug molecule (El-Henawy et al. 2013).
Pharmacophore generation
A pharmacophore model is an ensemble of steric and electronic features that is necessary to ensure the optimal supramolecular interactions with a specific biological target and to trigger (or block) its biological response (Wermuth et al. 1998). The generated pharmacophore models based on receptor-ligand interactions have confirmed all substantial interactions in the compound-receptor interaction modes (Meduru et al. 2016). In a structure-based pharmacophore model, possible interaction area between the drug target (receptor) and ligands are examined (Böhm 1992).
The structure-based pharmacophore (SBP) method employed in Discovery Studio is a typical example of a macromolecule-based approach. SBP converts LUDI interaction maps within the protein-binding site into catalyst pharmacophoric features: H-bond acceptor, H-bond donor and hydrophobe. The computer program LUDI is a new method for the de novo design of enzyme inhibitors (Böhm 1992; Yang 2010). Its interaction maps comprise of a large number of catalyst features (Yang 2010).
In the present work, pharmacophore generation executed by receptor-ligand pharmacophore generation with Discovery Studio 4 for study the interactions between protein and ligand. The receptor-ligand pharmacophore generation produces few pharmacophore models from a receptor-ligand complex. The model is generated from the features that are related to the receptor-ligand docking interactions. The following ligand features types are considered: hydrogen bond acceptor, hydrophobic feature, ionizable feature and aromatic ring.
Drug–DNA interaction
Drugs (amides) can interact with different coordinates (groove) of DNA, creating different binding patterns (Chaires 1998). Each pattern has its own significance (Chen et al. 1993). Study and recognition of these patterns lead to fruitful estimate of binding modes and site selectivity which will be contributory for developments in the understanding of new drug molecules as potent and selective gene-regulatory drugs (Chaires 1998; Chen et al. 1993).
To find the binding pattern of Amides with MTBDNA (pdb id: 3pvp), docking method is used. Drug-DNA docking is done by autodock vina software on Windows platform with 8 Gb RAM and Intel I 5 processor (Trott and Olson 2010).
ADMET
Before a drug applies pharmacodynamics effect on the body via interaction with its target, it must transport through the body to reach the site of drug action. Pharmacokinetics denotes to the expedition of the drug from its point of entrance to the site of action. Generally speaking, this process can be defined by the following phases: absorption, distribution, metabolism, excretion and toxicity called ADMET (Roncaglioni et al. 2013). Competence of the drug to reach pharmacologically active concentration at the drug targets without any undesirable effect is taken care by ADMET properties which include the calculation of a series of factors (Lin et al. 2013).
In the present study, ADMET has been carried out by evaluating water solubility, human intestinal absorption, oral bioavailability, blood–brain barrier penetration, transporter, plasma protein binding, volume of distribution, CYP450, toxicity etc. by support vector machine (SVM) algorithm (Cheng et al. 2012).
Molecular dynamics simulation
Molecular dynamics simulation (MD simulation) is calculated to confirm further the interaction strength and stability of the receptor-ligand complex determined from molecular docking by Discovery Studio’s standard dynamics cascade wizard.
The same pdb file which is modified for docking and 2-hydroxy-5-[(E)-2-{4-[(prop-2-enamido) sulfonyl] phenyl} diazen-1-yl] benzoicacid are taken as protein–ligand complexes. For DNA MD simulation, same DNA is chosen. Prior to performing MD simulations, charm force field has been applied to each of the protein–ligand complexes and solvation is set to explicit periodic boundary (Brooks et al. 1983). The parameters for MD simulations are set with following conditions: both steepest descent of energy minimization and steps of conjugate gradient minimization are done in order to obtain constant and reasonable conformation of biomolecules (Petrova and Solov’ev 1997; Hestenes and Stiefel 1952). The system was heated from an initial temperature of 50 K to the target temperature of 300 K, and the equilibration steps are done. Moreover, the parameters of electrostatics are chosen as particle mesh Ewald (PME) for long-range electrostatic constrains (Darden et al. 1993). The total production time of 53 ps simulations and are performed with NVT (dynamics without temperature/pressure control) (Beard and Qian 2010). Default setting values are adopted for other parameters.
Results and discussions
Sequence alignment analysis
The sequence alignment analysis has been carried out by Clustal Omega and the results are tabulated in Table 1. These results used to observe similarities between different pantothenate synthetase (Sievers and Higgins 2014). There is no significant match between MTB pantothenate synthetase and other pantothenate synthetase. Clustal Omega reveals that Neisseria gonorrhoeae pantothenate synthetase has 45.71% similarity with MTB pantothenate synthetase.
Clustal Omega with the support of percent identity matrix (PIM) shows that SA pantothenate synthetase has 45.71% similarity with Thermotoga maritima pantothenate synthetase (Chenna et al. 2003). These sequences give an idea of biological species evolution. The sequence similarity would be a motive for drug designers to work with other species containing pantothenate synthetase.
Docking and bond analysis
The specific binding of a ligand (drug) to a drug target molecule is the key to drug action. Each ligand binds favorably to a specific site on the surface of the target molecule. Identification of the ligand-binding site for each specific protein molecule is crucially important when trying to find a suitable drug molecule for the target, and it is also important to understand the function of the protein (Soga et al. 2007). The docking analysis scores of 93 compounds with MTB and SA pantothenate synthetase have been recorded in Table 2. On comparing with CDOCKER energy of C1 and C2, useful tuberculosis drugs A (Table 1) and B (Table 2) show best score for MTB. The CDOCKER energy of M1 − 66.41 a.u. (MTB pantothenate synthetase) and energy of C1 is − 40.58 a.u. M2 shows best score for SA, and CDOCKER energy is − 70.69 a.u. and − 44.31 a.u. for C2.
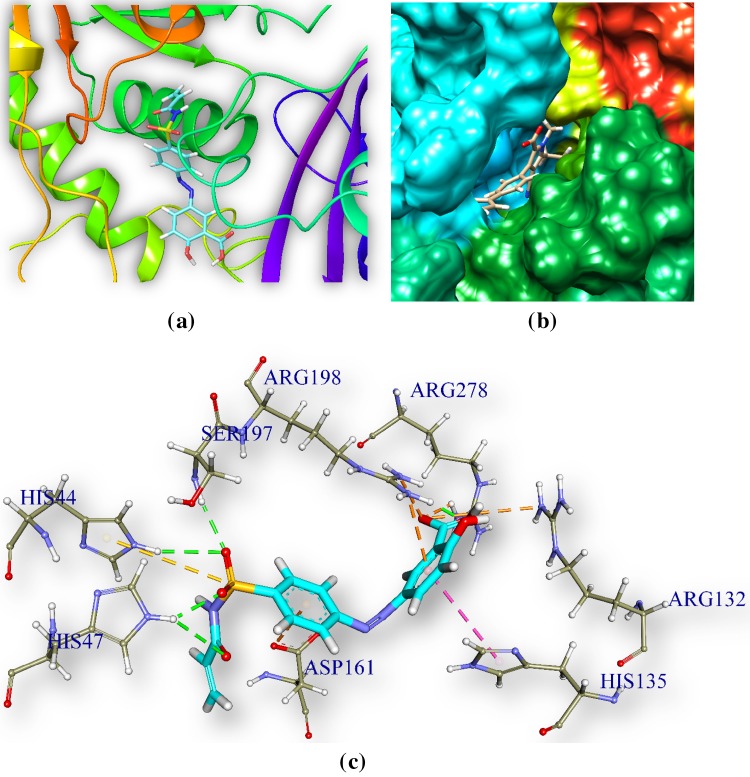
2-Hydroxy-5-[(E)-2-{4-[(prop-2-enamido)sulfonyl]phenyl}diazen-1-yl]benzoic acid (M1) and pantothenate synthetase
M1 is docked in the active site of pantothenate synthetase of MTB. It forms 9 hydrogen bonds with amino acids of the pantothenate synthetase in the ranging distance 2.78–3.34 Å (Table 5, Fig. 2). It also forms 2 electrostatic bonds with amino acids of the ps. The hydrogen bond forming amino acids are asparagine (ARG278), arginine (ARG198), serine (SER197 and SER19), tyrosine (TYR82). The CDOCKER energy is − 66.41 a.u. and CDOCKER energy is − 39.54.
Table 5.
Noncovalent bond distances between 2-hydroxy-5-[(E)-2-{4-[(prop-2-enamido) sulfonyl] phenyl} diazen-1-yl] benzoic acid, C1 and pantothenate synthetase
| Noncovalent bond distances between M1 (Lig1) (Table 1) and MTB ps | Distance (Å) | Bonding category | Noncovalent bond distances between C1 (Lig2) and MTB pantothenate synthetase | Distance (Å) | Bonding category |
|---|---|---|---|---|---|
| A:ARG198:NH2—Lig1:O26 | 2.70 | Electrostatic | A:GLY158:HN—Lig2:N21 | 2.23 | Hydrogen bond |
| A:ARG278:NH1—Lig1:O9 | 2.78 | Hydrogen bond | Lig2:H44—A:TYR82:OH | 3.043 | Hydrogen bond |
| A:ARG198:NH1—Lig1:O9 | 2.83 | Hydrogen bond | A:GLN164:HE22—Lig2 | 3.09 | Hydrogen bond |
| A:ARG278:NH1—Lig1:O8 | 2.87 | Electrostatic | A:ASP161:OD2—Lig2 | 3.53 | Electrostatic |
| A:SER197:OG—Lig1:O23 | 3.20 | Hydrogen bond | |||
| A:SER197:N—Lig1:O23 | 3.28 | Hydrogen bond | |||
| A:TYR82:OH—Lig1:O25 | 3.34 | Hydrogen bond | |||
| A:ARG198:NH2—Lig1 | 3.52 | Hydrogen bond | |||
| A:ASP161:OD2—Lig1 | 3.53 | Hydrogen bond | |||
| A:ARG132:NH2—Lig1:O8 | 3.60 | Hydrogen bond | |||
| A:ARG198:NH1—Lig1:O8 | 3.81 | Hydrogen bond |
Fig. 2.
Docked comprehensive perception of MTB pantothenate synthetase and M1 after docking. a p.s is represented by ribbon and M1 is represented by stick and coloured according to elements. b secondary structure of p.s represented by hydrophobic surface and M1 represented is by stick model, c interactions of M1 with p.s amino acids. Bonds are in dots. M1 surrounding amino acids are in three letters code, represented in blue
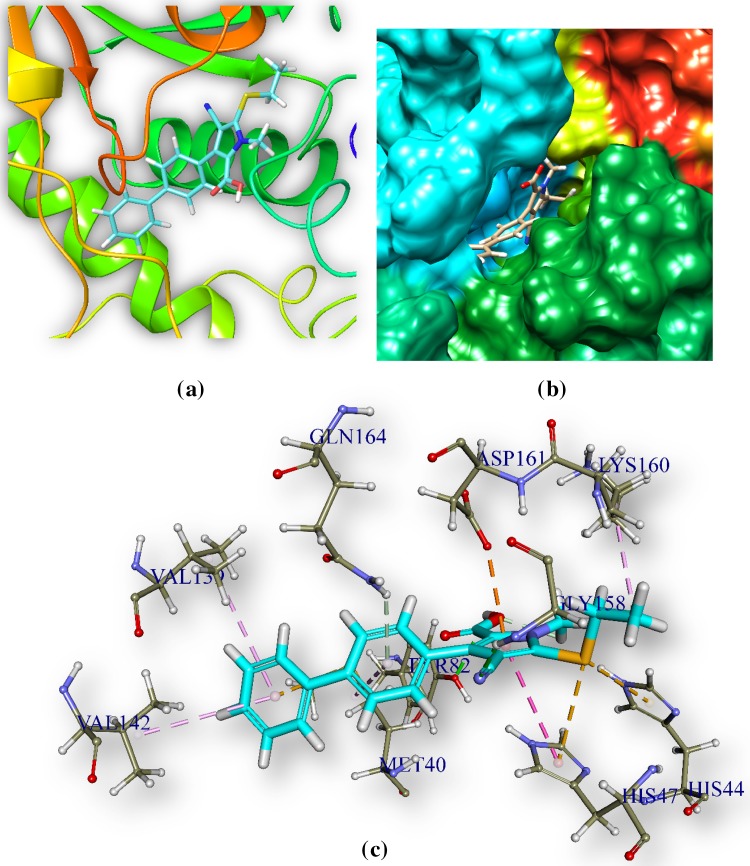
C1 and pantothenate synthetase
C1 (Table 1) is docked in the active site of pantothenate synthetase of MTB. It forms three hydrogen bonds with amino acids of the pantothenate synthetase within the ranging distance of 2.23–3.09 Å (Table 5, Fig. 3).The hydrogen bond forming amino acids are Glycine (GLY158), Tyrosine TYR82, Glycine GLN164, Aspartate ASP161. The CDOCKER energy is − 40.58 a.u. and CDOCKER energy is − 5.52 a.u (Fig. 3).
Fig. 3.
Docked comprehensive perception of pantothenate synthetase and C1 interaction after docking. Picture legends representation are same as Fig. 1
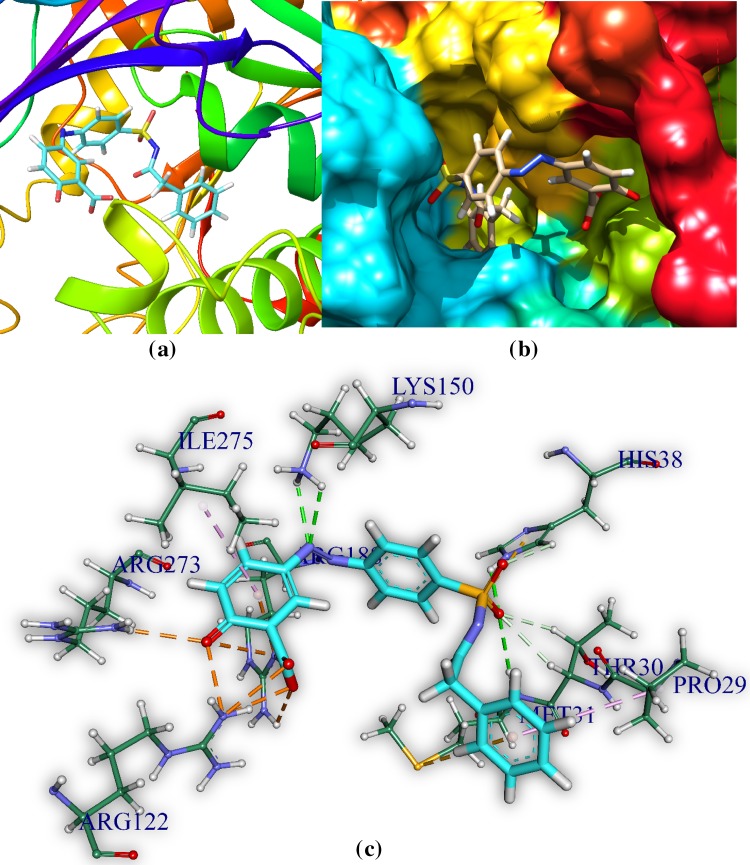
5-[(E)-2-{4-[(4-aminobenzenesulfonyl) sulfamoyl] phenyl} diazen-1-yl]-2-hydroxybenzoic acid (M2) and Staphylococcus aureus pantothenate synthetase
M2 is docked in the active site of Staphylococcus aureus pantothenate synthetase. It forms twelve hydrogen bonds ranging between 1.67 and 3.08 Å (Table 6, Fig. 4). It also forms an electrostatic interaction at a distance of 3.60 Å. The hydrogen bond forming amino acids are serine (SER186), glutamine (GLN154), lysine (LYS150), arginine (ARG188) histidine (HIS38), methionine (MET31) and threonine (THR30). The CDOCKER energy is − 85.41 a. u. followed by CDOCKER energy is 57.64 a.u. It also forms 1 electrostatic bonds with amino acids of the ps
Table 6.
Recognizable bonds between M2, C2 and Staphylococcus aureus Pantothenate synthetase
| Noncovalent bond distances bonds between M2 (LIG3) and SA pantothenate synthetase | Distance (Å) | Bonding category | Noncovalent bond distances between C2 (Lig4) and SA ps | Distance (Å) | Category |
|---|---|---|---|---|---|
| A: LYS150:HZ3 - :LIG3: O | 1.67 | Hydrogen bond; Electrostatic | A:ARG188:HH22—:LIG4:O | 1.9 | Hydrogen bond; Electrostatic |
| A: SER186: HG - :LIG3: O | 1.91 | Hydrogen bond | A:HIS38:HE2 - :LIG4:O | 2.11 | Hydrogen bond |
| A: GLN154:HE22 - :LIG3: O | 1.93 | Hydrogen bond | A:LYS150:HZ3 - :LIG4:N | 2.43 | Hydrogen bond |
| A: LYS150:HZ2 - :LIG3: O | 2.15 | Hydrogen bond; Electrostatic | A:LYS150:HZ2 - :LIG4:N | 2.63 | Hydrogen bond |
| A:ARG188:HH22 - :LIG3:N | 2.26 | Hydrogen bond | A:THR30:HA - :LIG4:O | 2.72 | Hydrogen bond |
| A: HIS38:HE2 - :LIG3: O | 2.38 | Hydrogen bond | A:ARG273:HH11 - :LIG4:O | 2.75 | Hydrogen bond; Electrostatic |
| A: MET31: HN - :LIG3: O | 2.45 | Hydrogen bond | A:MET31:HN - :LIG4:O | 2.77 | Hydrogen bond |
| A: THR30: HA - :LIG3: O | 2.51 | Hydrogen bond | A:THR30:HB - :LIG4:O | 2.78 | Hydrogen bond |
| A: SER186:HB2 - :LIG3: O | 2.68 | Hydrogen bond | A:ARG122:NH2 - :LIG4:O | 2.83 | Electrostatic |
| A: LYS150:HE1 - :LIG3: O | 2.69 | Hydrogen bond | A:HIS38:HD2 - :LIG4:O | 2.94 | Hydrogen bond |
| A: SER186:HB2 - :LIG3: O | 2.81 | Hydrogen bond | A:ARG122:NH2 - :LIG4:O | 2.95 | Electrostatic |
| A: SER186: HA - :LIG3: O | 3.08 | Hydrogen bond | A:ARG122:NH2 - :LIG4:O | 3.00 | Electrostatic |
| A:ARG188:NH2 - :LIG3 | 3.60 | Electrostatic | A:ARG188:NH2 - :LIG4 | 3.19 | Electrostatic |
| A:ARG188:HH11 - :LIG4:O | 3.20 | Hydrogen bond; Electrostatic | |||
| A:ARG188:NH2 - :LIG4:O | 3.90 | Electrostatic |
Fig. 4.
Docked comprehensive perception M2 and Staphylococcus aureus pantothenate synthetase after docking. Picture legends representation is same as Fig. 2
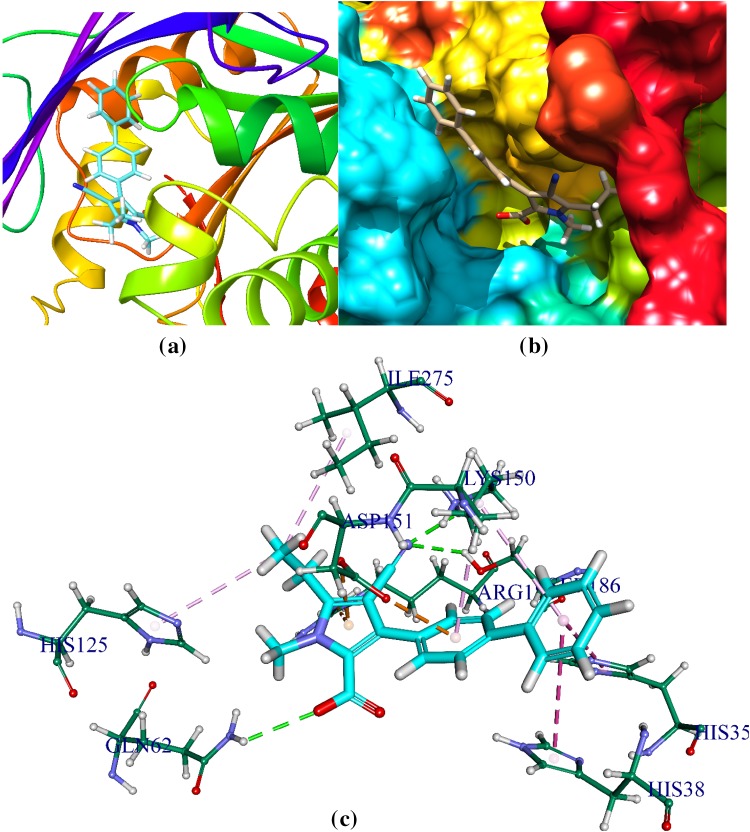
C2 and pantothenate synthetase
C2 is docked in the active site of SA pantothenate synthetase. It forms three hydrogen bonds with amino acids of the pantothenate synthetase within the ranging distance of 1.9–3.9 Å (Table 6, Fig. 5).The hydrogen bond forming amino acids are Arginine (ARG122,ARG188, ARG273), Histidine (HIS38), Lysine(LYS150), Threonine (THR30), Methionine(MET31). It also forms 1 electrostatic bonds with amino acids of the ps. The CDOCKER energy is − 44.31 a.u and CDOCKER energy is − 9.07 a.u.
Fig. 5.
Comprehensive perception of C2 and Staphylococcus aureus pantothenate synthetase interaction after docking. Picture legends representation are same as Fig. 2
ADMET property analysis
There are total 26 parameters in ADMET data which were available in literature (Cheng et al. 2012). In the present work a ‘+’ sign is marked when 23 parameters are positive (81%) and ‘++’ is assigned (Table 7) when more than 23 parameters are passed (Cheng et al. 2012). Pharmacokinetic properties and toxicities are predicted by ADMET which can predict permeability for BBB (blood–brain barrier), HIA (human intestinal absorption), P-glycoprotein substrate/inhibitor, renal organic cation transporter, etc. ADMET result shows 2-hydroxy-5-[(E)-2-{4-[(prop-2-enamido)sulfonyl]phenyl}diazen-1-yl]benzoic acid and 2-hydroxy-5-[(E)-2-{4-[(2-phenylacetamido)sulfonyl]phenyl}diazen-1-yl] benzoic acid are positive (+) in HIA, BBB permeability. It suggests that the molecules are well absorbed in human body (Table 7). Inhibition and initiation of P-glycoprotein have been reported as the causes of drug–drug interactions (Lin and Yamazaki 2003). Two best docked molecules (M1 and M2) are P-glycoprotein non-inhibitor. Data in Table 8 show the best fitted ligand in permissible limit (Lin et al. 2013). Organiccation transporters are responsible for drug absorption and disposition in the kidney, liver, and intestine (Zhang et al. 1998). ADMET result of two best docked molecules shows that they are non-inhibitor of renal organic cation transporter. The human cytochromes P450 (CYPs), particularly isoforms 1A2, 2C9, 2D6 and 3A4 are responsible for about 90% oxidative metabolic reactions. Inhibition of CYP enzymes will lead to inductive or inhibitory failure of drug metabolism (Uttamsingh et al. 2005).
Table 7.
ADMET properties of M1 and C1
| ADMET properties | M1 | C1 | ||
|---|---|---|---|---|
| Value | Probability | Value | Probability | |
| Absorption | ||||
| Blood–brain barrier | + | 0.6541 | + | 0.8722 |
| Human intestinal absorption | + | 0.8131 | + | 0.9727 |
| Caco-2 permeability | – | 0.6518 | + | 0.5769 |
| P-glycoprotein substrate | Non-substrate | 0.8458 | Non-substrate | 0.8551 |
| P-glycoprotein inhibitor | Non-inhibitor | 0.8955 | Non-inhibitor | 0.8246 |
| Non-inhibitor | 0.8994 | Inhibitor | 0.7104 | |
| Renal organic cation transporter | Non-inhibitor | 0.9100 | Non-inhibitor | 0.7486 |
| Distribution | ||||
| Subcellular localization | Mitochondria | 0.5965 | Mitochondria | 0.7434 |
| Metabolism | ||||
| CYP450 2C9 substrate | Non-substrate | 0.5802 | Non-substrate | 0.6852 |
| CYP450 2D6 substrate | Non-substrate | 0.8518 | Non-substrate | 0.8225 |
| CYP450 3A4 substrate | Non-substrate | 0.6784 | Non-substrate | 0.6153 |
| CYP450 1A2 inhibitor | Non-inhibitor | 0.8434 | Non-inhibitor | 0.5117 |
| CYP450 2C9 inhibitor | Inhibitor | 0.8248 | Inhibitor | 0.6145 |
| CYP450 2D6 inhibitor | Non-inhibitor | 0.8765 | Non-inhibitor | 0.8789 |
| CYP450 2C19 inhibitor | Non-inhibitor | 0.7830 | Inhibitor | 0.5904 |
| CYP450 3A4 inhibitor | Non-inhibitor | 0.7587 | Non-inhibitor | 0.8283 |
| CYP inhibitory promiscuity | Low CYP inhibitory promiscuity | 0.7264 | High CYP inhibitory promiscuity | 0.8456 |
| Toxicity | ||||
| Human Ether-a-go–go-related gene inhibition | Weak inhibitor | 0.9825 | Weak inhibitor | 0.9972 |
| Non-inhibitor | 0.8904 | Non-inhibitor | 0.7206 | |
| AMES toxicity | Non AMES toxic | 0.7342 | Non AMES toxic | 0.7595 |
| Carcinogens | Non-carcinogens | 0.5385 | Non-carcinogens | 0.8117 |
| Fish toxicity | High FHMT | 0.9977 | High FHMT | 0.9710 |
| Tetrahymena pyriformis toxicity | High TPT | 0.8832 | High TPT | 0.8240 |
| Honey bee toxicity | Low HBT | 0.7391 | Low HBT | 0.7051 |
| Biodegradation | Not ready biodegradable | 0.9836 | Not ready biodegradable | 0.9936 |
| Acute oral toxicity | III | 0.6643 | III | 0.4468 |
| Carcinogenicity (three-class) | Non-required | 0.5722 | Non-required | 0.5471 |
Table 8.
ADMET properties of M2 and C2
| ADMET properties | M2 | C2 | ||
|---|---|---|---|---|
| Value | Probability | Value | Probability | |
| Absorption | ||||
| Blood–brain barrier | + | 0.6917 | + | 0.8646 |
| Human intestinal absorption | + | 0.7727 | + | 0.9974 |
| Caco-2 permeability | – | 0.7290 | + | 0.6995 |
| P-glycoprotein Substrate | Non-substrate | 0.8093 | Non-substrate | 0.7334 |
| P-glycoprotein Inhibitor | Non-inhibitor | 0.9111 | Non-inhibitor | 0.8353 |
| Non-inhibitor | 0.8988 | Non-inhibitor | 0.6629 | |
| Renal organic cation transporter | Non-inhibitor | 0.8985 | Non-inhibitor | 0.7101 |
| Distribution | ||||
| Subcellular localization | Mitochondria | 0.5996 | Mitochondria | 0.7147 |
| Metabolism | ||||
| CYP450 2C9 Substrate | Non-substrate | 0.5626 | Non-substrate | 0.6468 |
| CYP450 2D6 substrate | Non-substrate | 0.8561 | Non-substrate | 0.8068 |
| CYP450 3A4 substrate | Non-substrate | 0.6266 | Non-substrate | 0.5928 |
| CYP450 1A2 inhibitor | Non-inhibitor | 0.8540 | Non-inhibitor | 0.6955 |
| CYP450 2C9 inhibitor | Inhibitor | 0.7520 | Inhibitor | 0.5256 |
| CYP450 2D6 inhibitor | Non-inhibitor | 0.8908 | Non-inhibitor | 0.9153 |
| CYP450 2C19 inhibitor | Non-inhibitor | 0.7418 | Non-inhibitor | 0.6502 |
| CYP450 3A4 inhibitor | Non-inhibitor | 0.8894 | Non-inhibitor | 0.7977 |
| CYP inhibitory promiscuity | Low CYP inhibitory promiscuity | 0.5715 | High CYP inhibitory promiscuity | 0.7357 |
| Toxicity | ||||
| Human Ether-a-go–go-related gene inhibition | Weak inhibitor | 0.9740 | Weak inhibitor | 0.9905 |
| Non-inhibitor | 0.8964 | Non-inhibitor | 0.8962 | |
| AMES toxicity | Non AMES toxic | 0.7293 | Non AMES toxic | 0.8398 |
| Carcinogens | Non-carcinogens | 0.5519 | Non-carcinogens | 0.8317 |
| Fish toxicity | High FHMT | 0.9604 | High FHMT | 0.9634 |
| Tetrahymena pyriformis toxicity | High TPT | 0.6599 | High TPT | 0.8293 |
| Honey bee toxicity | Low HBT | 0.7513 | Low HBT | 0.8206 |
| Biodegradation | Not ready biodegradable | 0.9449 | Not ready biodegradable | 0.9806 |
| Acute oral toxicity | III | 0.6801 | II | 0.6630 |
| Carcinogenicity (three-class) | Non-required | 0.5950 | Non-required | 0.6214 |
The Ames test is a widely employed method that uses bacteria to test whether a given chemical can cause cancer. More formally, it is a biological assay to assess the mutagenic potential of chemical compounds (Ames et al. 1972; Mortelmans and Zeiger 2000).
Human Ether-à-go–go-Related Gene (hERG) is a gene sensitive to drug binding (Sanguinetti and Tristani-Firouzi 2006). ADMET results show that the drugs M1, M2 are weak inhibitor and non-inhibitor of hERG (predictor I and II) (Sanguinetti and Tristani-Firouzi 2006). The aqueous solubility (logS) of a compound considerably affects its absorption and distribution properties. The predicted logS values of the studied compounds are within the acceptable limit (Vyas et al. 2013). The solubility (logS) of organic molecules in water is considered in the ADMET, because this parameter generally has important impact on many ADMET concerned properties of drugs, such as uptake, distribution, transport and eventually bioavailability (Hou et al. 2004). Understanding of ADMET properties together with their measurement and prediction gives an idea about the dose size and dose frequency, drugs solubility, metabolism of drug and its toxicity. Before synthesizing drug like molecules in laboratory, a profound knowledge of drug properties will save time and resources.
Density functional theory analysis
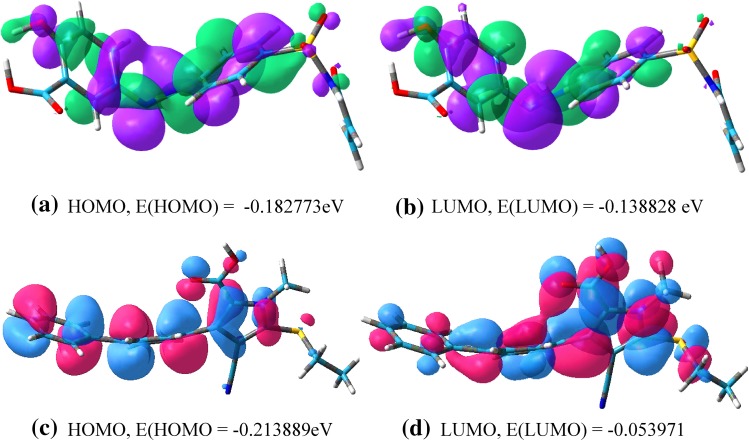
HSFs from the best binding pose have been transferred to Gauss view 5 and the difference in HOMO and LUMO energy, known as band gap, indicates the electronic excitation energy, necessary to compute the molecular reactivity and stability of the compounds (Becke 1993; Gill et al. 1992; Devlin et al. 1995; Fukui et al. 1952). For 2-hydroxy-5-[(E)-2-{4-[(prop-2-enamido)sulfonyl]phenyl} diazen-1-yl] benzoic acid, eigen values of HOMO and LUMO are − 0.182773 and − 0.138828 eV respectively and the HOMO–LUMO gap is − 0.043945. For compound 1, eigen values of HOMO and LUMO are − 0.213889 and − 0.053971 eV and the HOMO–LUMO gap is − 0.159918 eV (Figs. 5, 6; Table 6).
Fig. 6.
a, b HOMO and LUMO plots of M1. The positive electron density has been shown in green color while negative in violet. c, d HOMO and LUMO plots of C1. The positive electron density has been shown in red color while negative in blue
Pharmacophore generation
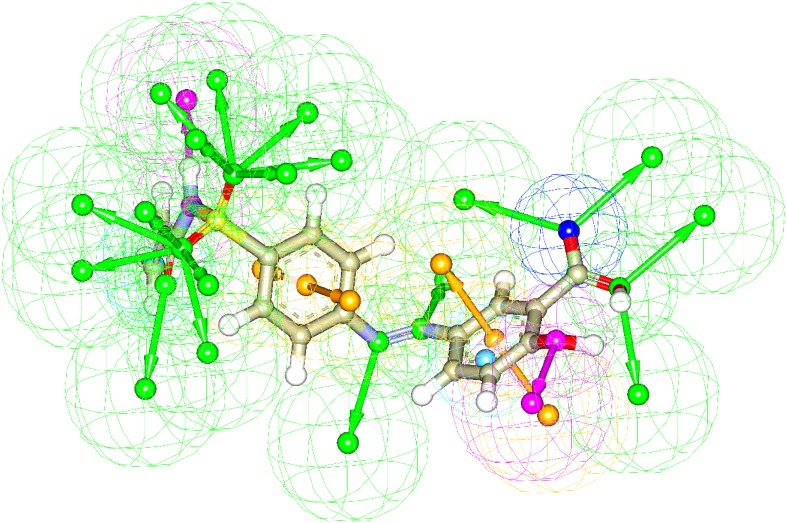
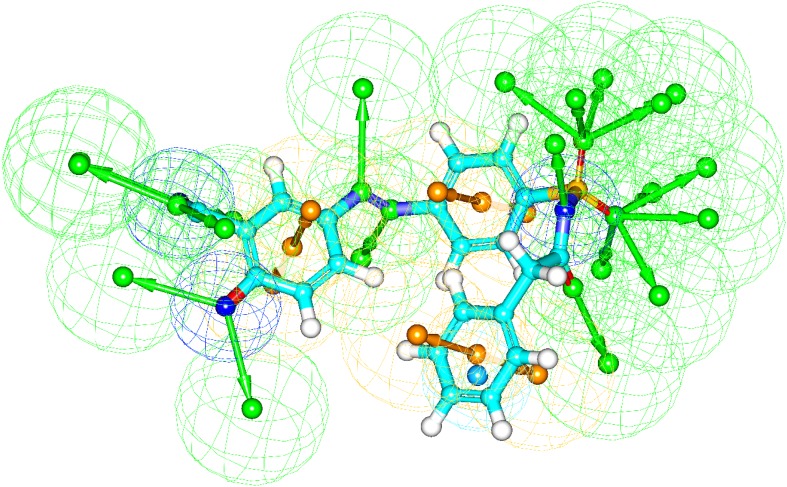
The generated pharmacophore models based on receptor-ligand interactions by docking have confirmed all major interactions in the drug-receptor interaction modes. The number of features, feature set and selectivity score from pharmacophore generation are observed from the two best docked molecules. Pharmacophore generation of 2-Hydroxy-5-[(E)-2-{4-[(prop-2-enamido) sulfonyl]phenyl} diazen-1-yl]benzoic acid (M1) and 2-hydroxy-5-[(E)-2-{4-[(2-phenylacetamido)sulfonyl] phenyl}diazen-1-yl]benzoic acid (M2) are shown in Figs. 7 and 8, respectively.
Fig. 7.
The result of pharmacophore features of M1 based on receptor-ligand pharmacophore generation. The hydrogen bond acceptor, hydrogen bond donor, positive ionizablefeature, aromatic ring and negative ionizable features are shown as green, orange and blue respectively
Fig. 8.
The result of pharmacophore features of M2 based on receptor-ligand pharmacophore generation. The hydrogen bond acceptor, hydrogen bond donor, positive ionizablefeature, aromatic ring and negative ionizable features are shown as green, orange, and blue respectively
Drug–DNA interaction
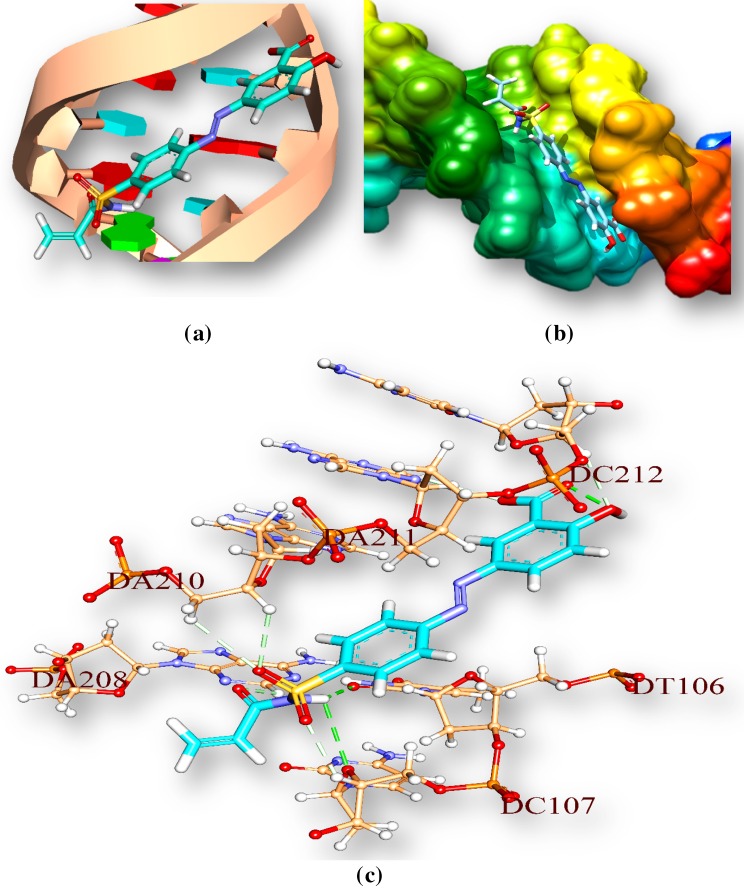
The drug (amides)–DNA interactions have been computationally examined by docking techniques used to study interactions between DNA and small ligand molecules those are potentially pharmaceutical interest. Autodock vina software is used for docking (Trott and Olson 2010). Drug-DNA interaction gives an idea about binding pattern of drug with DNA. Amides compounds (drug) docked with M. tuberculosis’s DNA is used to understand the drug-DNA interaction. We have observed that amide compounds interact with AT-rich regions (represented by red and turquoise coloured rings respectively) of DNA in the minor groove by forming hydrogen bonding and hydrophobic interactions (Fig. 9). The amino group of guanine (represented by green ring) prevents 2-hydroxy-5-[(E)-2-{4-[(prop-2-enamido) sulfonyl] phenyl} diazen-1-yl] benzoic acid from binding to the G·C base pairs by steric hindrance (Table 9), and thus conferring AT-selectivity on the drug molecule. Minor groove binding molecules are usually constructed of a series of heterocyclic or aromatic hydrocarbon rings that possess rotational freedom. This allows these molecules to fit into the minor groove, with displacement of water; these drugs can form hydrogen bonds to bases (Sangeetha Gowda et al. 2014).
Fig. 9.
M1 interaction with Tuberculosis’s DNA in minor groove. a Drug is represented by stick and double helical DNA structure is represented by ladder and rings, b double helical structure of DNA represented by M1 represented by stick model and are coloured according to elements, c interactions of ligand with DNA base pairs (A, T, G, C); the interaction types. Hydrogen bonds are in green. Ligand surrounding base pairs are in three letters code represented in black
Table 9.
Noncovalent bond distances between M1 and DNA (A and B chain)
| Bonds | Distance (Å) | Category | Type |
|---|---|---|---|
| M1:H2 - :M1:O | 2.30036 | Hydrogen bond | Conventional hydrogen bond |
| M1:H9 - A:DT106:O2 | 2.36896 | Hydrogen bond | Conventional hydrogen bond |
| M1:H9 - A:DC107:O4′ | 3.0371 | Hydrogen bond | Conventional hydrogen bond |
| A:DC107:H4′ - :M1:O | 2.57249 | Hydrogen bond | Carbon hydrogen bond |
| B:DA208:H2 - :M1:O | 2.50191 | Hydrogen bond | Carbon hydrogen bond |
| B:DA210:H5′2 - :M1:O | 2.91783 | Hydrogen bond | Carbon hydrogen bond |
| B:DA210:H4′ - :M1:O | 2.95761 | Hydrogen bond | Carbon hydrogen bond |
| B:DA211:H2 - :M1:O | 2.69911 | Hydrogen bond | Carbon hydrogen bond |
| B:DC212:H4′ - :M1:O | 2.54721 | Hydrogen bond | Carbon hydrogen bond |
Molecular dynamics simulation
The MD simulation study of best docked molecules with Mtb and S. aureus pantothenate synthetase and Mtb DNA are carried out for 53 ps by 50 thousand steps. MD production run and the trajectory of the various energy profiles are created and analyzed.
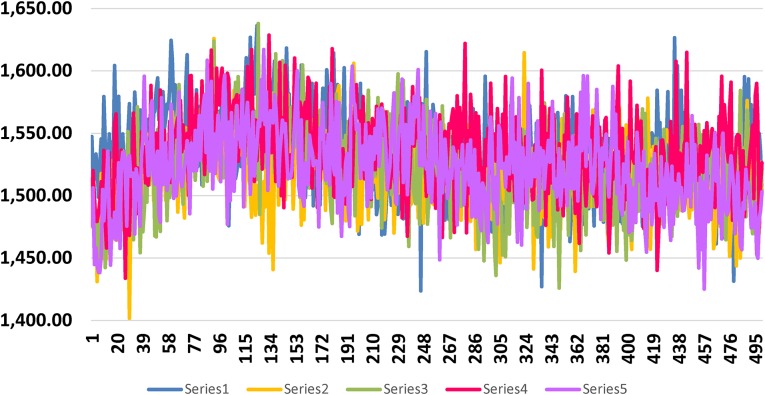
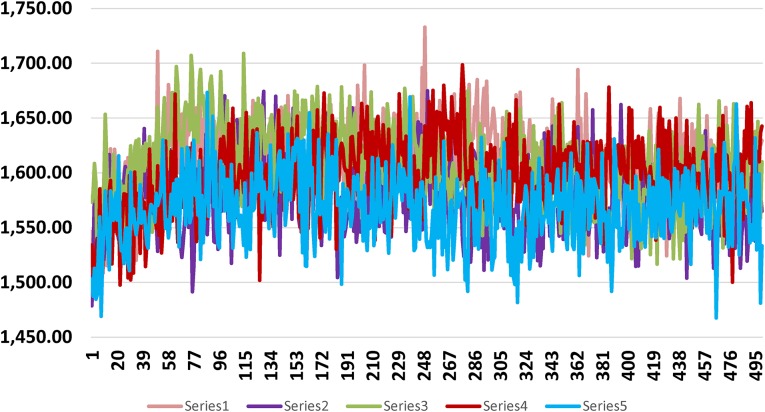
Potential energy of M1 after MD simulation was − 14,633 kcal/mol and C1 − 14,607.2 kcal/mol and bond energy was 1526.3 and 1503.13 kcal/mol respectively. Same for S. aureus pantothenate M2 − 16,245.9 kcal/mol bond energy was 1642.68 and 1533.34 kcal/mol respectively.
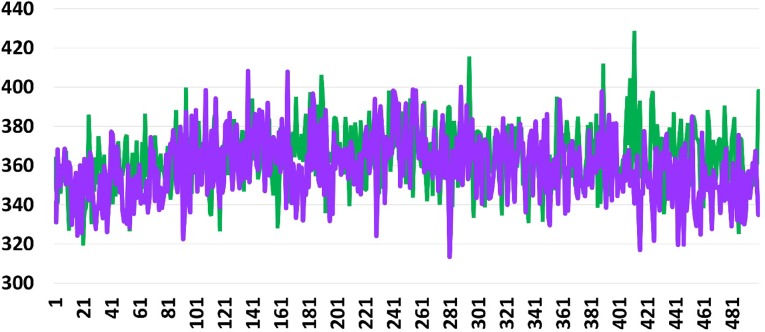
For Mtb DNA and 2-hydroxy-5-[(E)-2-{4-[(prop-2-enamido) sulfonyl]phenyl} diazen-1-yl] benzoic acid potential energy was − 2394.2 kcal/mol and bond energy was 398.096 kcal/mol. For C1 potential energy was − 2357.67 kcal/mol bond energy 334.856 kcal/mol. Bond energy graphs are shown in Figs. 10, 11 and 12.
Fig. 10.
Shows bond energy graph of M1, other four molecules of Table 3 and C1 with pantothenate synthetase of Mycobacterium tuberculosis. Bond energy and number of trajectory frames are plotted along X- and Y-axis respectively. Best docked molecule is in blue and C1 is in pink. (Series numbers are maintained as in Table 3)
Fig. 11.
Shows bond energy graph of M2 with pantothenate synthetase of S. aurues. X axis and Y-axis represent the bond energy and number of trajectory frames respectively. Best docked molecule is in pink and C2 is in blue. (Series numbers are maintained as in Table 4)
Fig. 12.
Shows bond energy graph of 2-hydroxy-5-[(E)-2-{4-[(prop-2-enamido) sulfonyl] phenyl} diazen-1-yl] benzoic acid and C1 with DNA of Mycobacterium tuberculosis. X-axis and Y-axis represent bond energy and number of trajectory frames respectively. Best docked molecule is in green and C1 is in violet
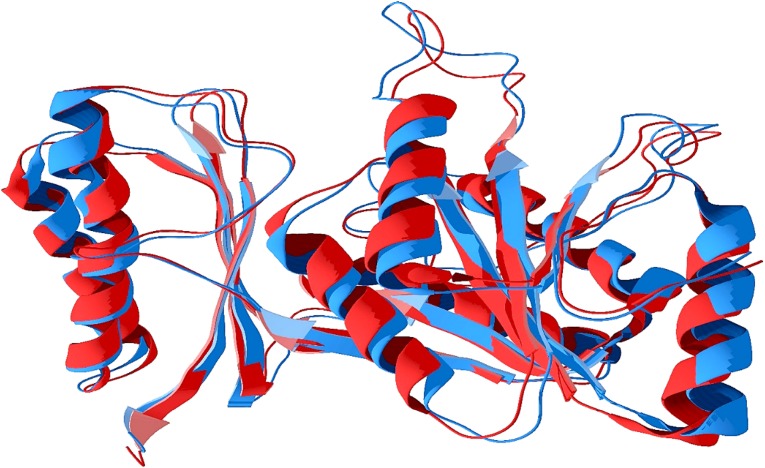
As the graphs revealed that in all situations for the best docked molecules, the bond strength are increased from initial position, ended from the starting point of bond strength and it was higher till then end compared to corresponding drugs. So it can be clearly predicted that molecules formed stable conformation with Mtb and S. aureus pantothenate synthetase and DNA. Super imposed structures of first conformation (trajectory frame) and last conformation of Mtb pantothenate synthetase show the deviation of end point of the dynamics from the initial point of dynamics (Fig. 13).
Fig. 13.
Shows results of molecular dynamics, last conformation (structure) superimposed (red) with first conformation (blue) of pantothenate synthetase of Mycobacterium tuberculosis
Conclusion
Amide functionalized sulfa drugs show potent anti-microbial activity and also active against MTB and S. aureus in vivo. The best docked compounds have better docking score than approved drugs and also show better ADMET efficiency.
Out of 154 compounds, 2-hydroxy-5-[(E)-2-{4-[(prop-2-enamido)sulfonyl]phenyl}diazen-1-yl] benzoic acid and 2-hydroxy-5-[(E)-2-{4-[(2-phenylacetamido)sulfonyl]phenyl}diazen-1-yl] benzoic acid exhibit significantly higher docking score than approved drugs, C1 and C2. Molecular orbital, pharmacophore, drug likeness and ADMET predicted the idea about electrostatic pharmacological and nontoxic properties. Molecular dynamics simulation enriches the knowledge of stability of drug like molecule. We hope, will open new avenues to amide drug research. This computational prediction about a better tuberculosis drug will encourage and assist experimentalists to a great extent to design and synthesize potential new drugs for removal of this epidemic disease, spreading globally with a rapid span.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sayantan Pradhan, Email: sayan23us@gmail.com.
Chittaranjan Sinha, Email: crsjuchem@gmail.com.
References
- Ames BN, Gurney EG, Miller JA, Bartsch H. Carcinogens as frameshift mutagens: metabolites and derivatives of 2-acetylaminofluorene and other aromatic amine carcinogens. Proc Natl Acad Sci USA. 1972;69(11):3128–3132. doi: 10.1073/pnas.69.11.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzatt R, Cirillo SL, Cirillo JD. Sulfonamide agents for treatment of Staphylococcus MRSA and MSSA infections of the central nervous system. Cent Nerv Syst Agents Med Chem. 2010;10(1):84–90. doi: 10.2174/187152410790780109. [DOI] [PubMed] [Google Scholar]
- Beard DA, Qian H. Chemical biophysics: quantitative analysis of cellular systems. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- Becke AD. Density-functional thermochemistry. The role of exact exchange. J Chem Phys. 1993;98(7):5648–5652. [Google Scholar]
- Berg JM, Tymoczko JL, Stryer L. Biochemistry. 5. New York: W. H. Freeman; 2002. [Google Scholar]
- Böhm H-J. The computer program LUDI: a new method for the de novo design of enzyme inhibitors. J Comput Aided Mol Des. 1992;6(1):61–78. doi: 10.1007/BF00124387. [DOI] [PubMed] [Google Scholar]
- Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M. CHARMM: a program for macromolecular energy, minimization, and dynamics calculations. J Comput Chem. 1983;4(2):187–217. [Google Scholar]
- Brownell LV, Robins KA, Jeong Y, Lee Y, Lee D-C. Highly systematic and efficient HOMO–LUMO energy gap control of thiophene-pyrazine-acenes. J Phys Chem C. 2013;117(48):25236–25247. [Google Scholar]
- Chaffey N, Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular biology of the cell, 4th edn. Ann Bot. 2003;91(3):401. [Google Scholar]
- Chaires JB. Drug–DNA interactions. Curr Opin Struct Biol. 1998;8(3):314–320. doi: 10.1016/s0959-440x(98)80064-x. [DOI] [PubMed] [Google Scholar]
- Chen AY, Yu C, Gatto B, Liu LF. DNA minor groove-binding ligands: a different class of mammalian DNA topoisomerase I inhibitors. Proc Natl Acad Sci USA. 1993;90(17):8131–8135. doi: 10.1073/pnas.90.17.8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F, Li W, Zhou Y, Shen J, Wu Z, Liu G, Lee PW, Tang Y. admetSAR: a comprehensive source and free tool for assessment of chemical ADMET properties. J Chem Inf Model. 2012;52(11):3099–3105. doi: 10.1021/ci300367a. [DOI] [PubMed] [Google Scholar]
- Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the clustal series of programs. Nucl Acid Res. 2003;31(13):3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, 3rd, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393(6685):537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- Darden T, York D, Pedersen L. Particle mesh Ewald: an N·log(N) method for Ewald sums in large systems. J Chem Phys. 1993;98(12):10089–10092. [Google Scholar]
- Database Resources of the National Center for Biotechnology Information Nucl Acids Res. 2013;41(Database issue):D8–D20. doi: 10.1093/nar/gks1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin FJ, Finley JW, Stephens PJ, Frisch MJ. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields: a comparison of local, nonlocal, and hybrid density functionals. J Phys Chem. 1995;99(46):16883–16902. [Google Scholar]
- El-Henawy AA, Khowdiary MM, Badawi AB, Soliman HM. In vivo anti-leukemia, quantum chemical calculations and ADMET investigations of some quaternary and isothiouronium surfactants. Pharmaceuticals. 2013;6(5):634–649. doi: 10.3390/ph6050634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I. Molecular orbitals and organic chemical reactions. Reference. Amsterdam: Wiley; 2011. [Google Scholar]
- Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Petersson G. Gaussian 09. Wallingford: Gaussian. Inc; 2009. [Google Scholar]
- Fukui K, Yonezawa T, Shingu H. A molecular orbital theory of reactivity in aromatic hydrocarbons. J Chem Phys. 1952;20(4):722–725. [Google Scholar]
- Gill PMW, Johnson BG, Pople JA, Frisch MJ. The performance of the Becke–Lee–Yang–Parr (B–LYP) density functional theory with various basis sets. Chem Phys Lett. 1992;197(4):499–505. [Google Scholar]
- Grassl SM. Human placental brush-border membrane Na(+)-pantothenate cotransport. J Biol Chem. 1992;267(32):22902–22906. [PubMed] [Google Scholar]
- Hestenes MR, Stiefel E (1952) Methods of conjugate gradients for solving linear systems, vol 49
- Holloway KA, Rosella L, Henry D. The impact of WHO essential medicines policies on inappropriate use of antibiotics. PLoS One. 2016;11(3):e0152020. doi: 10.1371/journal.pone.0152020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T, Wang J. Structure – ADME relationship: still a long way to go? Exp Opin Drug Metab Toxicol. 2008;4(6):759–770. doi: 10.1517/17425255.4.6.759. [DOI] [PubMed] [Google Scholar]
- Hou TJ, Xia K, Zhang W, Xu XJ. ADME evaluation in drug discovery. 4. Prediction of aqueous solubility based on atom contribution approach. J Chem Inf Comput Sci. 2004;44(1):266–275. doi: 10.1021/ci034184n. [DOI] [PubMed] [Google Scholar]
- Jagessar RC, Rampersaud D. Amides as antimicrobial agents. Life Sci J. 2007;4(4):46–49. [Google Scholar]
- Kumar A, Casey A, Odingo J, Kesicki EA, Abrahams G, Vieth M, Masquelin T, Mizrahi V, Hipskind PA, Sherman DR, Parish T. A high-throughput screen against pantothenate synthetase (PanC) identifies 3-biphenyl-4-cyanopyrrole-2-carboxylic acids as a new class of inhibitor with activity against Mycobacterium tuberculosis. PLoS One. 2013;8(11):e72786. doi: 10.1371/journal.pone.0072786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi R, Jackowski S (2007) Biosynthesis of pantothenic acid and coenzyme A. EcoSal Plus 2(2) [DOI] [PMC free article] [PubMed]
- Lin JH, Yamazaki M. Role of P-glycoprotein in pharmacokinetics: clinical implications. Clin Pharmacokinet. 2003;42(1):59–98. doi: 10.2165/00003088-200342010-00003. [DOI] [PubMed] [Google Scholar]
- Lin K, Tibbitts J, Shen BQ. Pharmacokinetics and ADME characterizations of antibody-drug conjugates. Methods Mol Biol (Clifton NJ) 2013;1045:117–131. doi: 10.1007/978-1-62703-541-5_7. [DOI] [PubMed] [Google Scholar]
- Lionta E, Spyrou G, Vassilatis DK, Cournia Z. Structure-based virtual screening for drug discovery: principles, applications and recent advances. Curr Top Med Chem. 2014;14(16):1923–1938. doi: 10.2174/1568026614666140929124445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;1(4):337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1–3):3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- McMurry JE, Ballantine DS, Hoeger CA, Peterson VE. Fundamentals of general, organic and biological chemistry. London: Pearson Education, Limited; 2017. [Google Scholar]
- Meduru H, Wang Y-T, Tsai JJP, Chen Y-C. Finding a potential dipeptidyl peptidase-4 (DPP-4) inhibitor for type-2 diabetes treatment based on molecular docking, pharmacophore generation, and molecular dynamics simulation. Int J Mol Sci. 2016;17(6):920. doi: 10.3390/ijms17060920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortelmans K, Zeiger E. The Ames salmonella/microsome mutagenicity assay. Mutat Res. 2000;455(1–2):29–60. doi: 10.1016/s0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]
- Nguyen KD, Pan Y. A knowledge-based multiple-sequence alignment algorithm. IEEE/ACM Trans Comput Biol Bioinf. 2013;10(4):884–896. doi: 10.1109/TCBB.2013.102. [DOI] [PubMed] [Google Scholar]
- Onyango R. State of the globe: tracking tuberculosis is the test of time. J Glob Infect Dis. 2011;3(1):1–3. doi: 10.4103/0974-777X.77287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5(12):993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- Petrova SS, Solov’ev AD. The origin of the method of steepest descent. Hist Math. 1997;24(4):361–375. [Google Scholar]
- Pradhan S, Sinha C. Combating prostate cancer by sulfonamide compounds: Theoretical prediction. J Indian Chem Soc. 2017;94(10):1113–1122. [Google Scholar]
- Rauk A. Orbital interaction theory of organic chemistry. Amsterdam: Wiley; 1994. [Google Scholar]
- Roncaglioni A, Toropov AA, Toropova AP, Benfenati E. In silico methods to predict drug toxicity. Curr Opin Pharmacol. 2013;13(5):802–806. doi: 10.1016/j.coph.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Rozhenko AB. Density functional theory calculations of enzyme-inhibitor interactions in medicinal chemistry and drug design. In: Gorb L, Kuz’min V, Muratov E, editors. Application of computational techniques in pharmacy and medicine. Dordrecht: Springer; 2014. pp. 207–240. [Google Scholar]
- Sangeetha Gowda KR, Mathew BB, Sudhamani CN, Naik HSB. Mechanism of DNA binding and cleavage. Biomed Biotechnol. 2014;2(1):1–9. [Google Scholar]
- Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440(7083):463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- Sievers F, Higgins DG. Clustal omega, accurate alignment of very large numbers of sequences. Method Mol Biol (Clifton NJ) 2014;1079:105–116. doi: 10.1007/978-1-62703-646-7_6. [DOI] [PubMed] [Google Scholar]
- Sirajuddin M, Ali S, Badshah A. Drug–DNA interactions and their study by UV-Visible, fluorescence spectroscopies and cyclic voltametry. J Photochem Photobiol B. 2013;124:1–19. doi: 10.1016/j.jphotobiol.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Soga S, Shirai H, Kobori M, Hirayama N. Use of amino acid composition to predict ligand-binding sites. J Chem Inf Model. 2007;47(2):400–406. doi: 10.1021/ci6002202. [DOI] [PubMed] [Google Scholar]
- Stefańska J, Antoszczak M, Stępień K, Bartoszcze M, Mirski T, Huczyński A. Tertiary amides of Salinomycin: a new group of antibacterial agents against Bacillus anthracis and methicillin-resistant Staphylococcus epidermidis. Bioorg Med Chem Lett. 2015;25(10):2082–2088. doi: 10.1016/j.bmcl.2015.03.085. [DOI] [PubMed] [Google Scholar]
- Strom ET, Wilson AK (2013) Pioneers of quantum chemistry. Am Chem Soc vol 1122
- Szymański P, Markowicz M, Mikiciuk-Olasik E. Adaptation of high-throughput screening in drug discovery—toxicological screening tests. Int J Mol Sci. 2012;13(1):427–452. doi: 10.3390/ijms13010427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The sulfa derivatives in the treatment of tuberculosis Can Med Assoc J. 1944;51(5):467. [PMC free article] [PubMed] [Google Scholar]
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttamsingh V, Lu C, Miwa G, Gan LS. Relative contributions of the five major human cytochromes P450, 1A2, 2C9, 2C19, 2D6, and 3A4, to the hepatic metabolism of the proteasome inhibitor bortezomib. Drug Metab Dispos Biol Fate Chem. 2005;33(11):1723–1728. doi: 10.1124/dmd.105.005710. [DOI] [PubMed] [Google Scholar]
- Vallari DS, Rock CO. Isolation and characterization of Escherichia coli pantothenate permease (panF) mutants. J Bacteriol. 1985;164(1):136–142. doi: 10.1128/jb.164.1.136-142.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Delft F, Lewendon A, Dhanaraj V, Blundell TL, Abell C, Smith AG. The crystal structure of E. coli pantothenate synthetase confirms it as a member of the cytidylyltransferase superfamily. Structure. 2001;9(5):439–450. doi: 10.1016/s0969-2126(01)00604-9. [DOI] [PubMed] [Google Scholar]
- Vyas VK, Ghate M, Goel A. Pharmacophore modeling, virtual screening, docking and in silico ADMET analysis of protein kinase B (PKB beta) inhibitors. J Mol Graph Model. 2013;42:17–25. doi: 10.1016/j.jmgm.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Wang S, Eisenberg D. Crystal structures of a pantothenate synthetase from M. tuberculosis and its complexes with substrates and a reaction intermediate. Protein Sci A Publ Protein Soc. 2003;12(5):1097–1108. doi: 10.1110/ps.0241803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wermuth CG, Ganellin CR, Lindberg P, Mitscher LA. Glossary of terms used in medicinal chemistry (IUPAC recommendations 1998) Pure Appl Chem. 1998;70:1129. [Google Scholar]
- White EL, Southworth K, Ross L, Cooley S, Gill RB, Sosa MI, Manouvakhova A, Rasmussen L, Goulding C, Eisenberg D, Fletcher TM., 3rd A novel inhibitor of Mycobacterium tuberculosis pantothenate synthetase. J Biomol Screen. 2007;12(1):100–105. doi: 10.1177/1087057106296484. [DOI] [PubMed] [Google Scholar]
- Wu G, Robertson DH, Brooks CL, 3rd, Vieth M. Detailed analysis of grid-based molecular docking: a case study of CDOCKER-A CHARMm-based MD docking algorithm. J Comput Chem. 2003;24(13):1549–1562. doi: 10.1002/jcc.10306. [DOI] [PubMed] [Google Scholar]
- Yang SY. Pharmacophore modeling and applications in drug discovery: challenges and recent advances. Drug Discov Today. 2010;15(11–12):444–450. doi: 10.1016/j.drudis.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Yildiz I, Ertan T, Bolelli K, Temiz-Arpaci O, Yalcin I, Aki E. QSAR and pharmacophore analysis on amides against drug-resistant S. aureus. SAR QSAR Environ Res. 2008;19(1–2):101–113. doi: 10.1080/10629360701844159. [DOI] [PubMed] [Google Scholar]
- Zhang L, Brett CM, Giacomini KM. Role of organic cation transporters in drug absorption and elimination. Annu Rev Pharmacol Toxicol. 1998;38:431–460. doi: 10.1146/annurev.pharmtox.38.1.431. [DOI] [PubMed] [Google Scholar]