Abstract
Social impairment is a core feature of schizophrenia that presents a major barrier toward recovery. Some of the psychotic symptoms are partly ameliorated by medication but the route to recovery is hampered by social impairments. Since existing social skills interventions tend to suffer from lack of availability, high-burden and low adherence, there is a dire need for an effective, alternative strategy. The present study examined the feasibility and acceptability of Multimodal Adaptive Social Intervention in Virtual Reality (MASI-VR) for improving social functioning and clinical outcomes in schizophrenia. Out of eighteen patients with schizophrenia who enrolled, seventeen participants completed the pre-treatment assessment and 10 sessions of MASI-VR, but one patient did not complete the post-treatment assessments. Therefore, the complete training plus pre- and post-treatment assessment data are available from sixteen participants. Clinical ratings of symptom severity were obtained at pre- and post-training. Retention rates were very high and training was rated as extremely satisfactory for the majority of participants. Participants exhibited a significant reduction in overall clinical symptoms, especially negative symptoms following 10 sessions of MASI-VR. These preliminary results support the feasibility and acceptability of a novel virtual reality social skills training program for individuals with schizophrenia.
Keywords: social skills training, virtual reality, schizophrenia, social cognition, psychosocial intervention, computerized training, negative symptoms, social attention
1. Introduction
Social impairment is a core feature of schizophrenia that presents a major barrier toward recovery. This impairment is present from the premorbid stage, persists over the course of the illness, and predicts outcome (Green et al., 2008). Pharmacotherapy has been largely inadequate for ameliorating social impairments in schizophrenia. Validated behavioral interventions, such as Social Skills Training (SST) (Kreyenbuhl et al., 2010; Swartz et al., 2007) target a broad range of social domains by practicing pragmatic living skills, often in groups (Bellack, 2005; Heinssen et al. 2000; Turner et al, 2017). Many of these psychosocial interventions yield only modest effect sizes for social outcome (Kopelowicz et al., 2006; Pfammatter et al., 2006) with perhaps the exception of the SST, which seem to be more effective than other social interventions (Granholm and Harvey, 2018). Interventions targeting social cognition, which involve training of specific cognitive and emotional processes thought to underlie social interactions such as emotion recognition and theory-of-mind, have proven more effective (Kopelowicz et al., 2006: Kurtz and Mueser, 2008; Granholm et al., 2005; Lindenmayer et al., 2013; Penn et al., 2007). However, low generalizability, modest treatment effects and low compliance remain challenges to implementation (Kurtz and Mueser, 2008). Importantly, most individuals with severe mental illness do not have access to social interventions, and even with access, low retention rates present challenges in implementation and efficacy of training programs (Kreyenbuhl et al., 2010; Kurtz and Mueser, 2008).
Despite these practical shortcomings, there is a broad consensus on effective strategies for improving social functioning. One candidate treatment target is social attention, the fast orientation and allocation of attentional resources towards socially relevant or significant stimuli (e.g., people, faces, eyes) (Nummenmaa and Calder, 2009). Social attention is impaired in individuals with schizophrenia (Minassian et al., 2005; Russell et al., 2008; Loughland et al., 2002a, 2002b) and associated with poor social outcome (Russell et al., 2008). Another successful learning strategy involves structured didactic procedures with concrete goals, role-playing, and rehearsal (Bellack, 2004). For efficient learning it is also important to provide targeted feedback and rapid reinforcement, which allow for dynamic adjustments of task difficulty based on performance. Social learning occurs when the patient’s goals and capacity are well calibrated so that errors are minimized, and targeted behaviors are reinforced (Swartz et al., 2010). Since all forms of learning involve mental simulation, imitation, and repetition in a wide variety of situations (Park et al., 2008; Matthews et al., 2012; Mazza et al., 2010), role-playing exercises in multiple contexts can help generalization. Lastly, optimal arousal and attention are indispensable for engaging the individual and facilitating learning (e.g., Corrigan et al., 1990; Nakamura and Csikszentmihalyi, 2002).
Such optimal learning parameters are difficult to accommodate within currently existing treatment protocols. Conventional social interventions are also limited by the time and effort required of patients and therapists, low adherence, lack of personalization, low generalizability and small treatment effects. Some of these hurdles can be addressed by incorporating available technologies such as Virtual Reality (VR) (Welch et al., 2009; Bekele et al., 2013a,b) into social interventions. The advantages of VR include flexibility, controllability, and an extensive repertoire of stimuli, while remaining low-burden, cost efficient, and safe (Strickland, 1997). VR further provides simulation and adaptive rehearsal essential to practicing new skills across multiple contexts. This capacity also promotes generalization of skills to real world applications (Strickland, 1997; Mitchell et al., 2007). While VR cannot replace interpersonal interactions, the controllable complexity of VR allows the user to navigate realistic social interactions across multiple scenarios with minimal distraction, confusion or distress (Moore et al., 2000; Tartaro et al., 2007; Standen et al., 2005).
VR interventions have already been used successfully in a range of settings aiming to understand mechanisms of psychosis (Veling et al., 2016; Freeman et al., 2010) and to assess and improve symptoms and functional outcomes in schizophrenia (for a review, see Rus-Calafell et al., 2018). Such studies demonstrate both the tolerability of VR in those with schizophrenia and other psychotic disorders (e.g., Pot-Kolder et al., 2016; Rus-Calafell et al., 2018; Rus-Calafell, Gutiérrez-Maldonado, & Ribas-Sabaté, 2014; Valmaggia, Freeman, & Green, 2007) as well as demonstrating the potential utility of VR as both a stand-alone and supplemental treatment tool for various medical and psychological interventions (Freeman et al., 2017; Kurtz et al., 2007; Rus-Calafell et al., 2014; Valmaggia, 2017; Freeman et al., 2008). For instance, VR has been shown to enhance medication adherence (Kurtz et al., 2007) and help individuals cope with auditory hallucinations (Craig et al., 2017; Du Sert et al, 2018). Furthermore, a randomized clinical trial of VR for vocational training found significant improvement in cognition, work outcome, and work-related self-efficacy after just ten, 30-minute VR sessions across five weeks (Tsang and Man, 2013). In addition, recent studies also suggests that VR may improve social function in schizophrenia. For example, Rus-Calafell et al. (2014) found that 16, one-hour sessions of VR SST improved negative symptoms, social anxiety, avoidance and social functioning in a group of 12 individuals with schizophrenia and schizoaffective disorder. Furthermore, most of these gains were maintained at a four-month follow-up (Rus-Calafell et al., 2014). Interestingly, Park et al. (2011) found in a larger, randomized controlled trial of SST with VR role-playing that individuals with schizophrenia in the VR training group exhibited greater improvement in conversational skills and assertiveness than a group that received SST with traditional role-playing. These findings add to a larger literature demonstrating VR’s efficacy in the treatment of neurological disorders (Burge et al., 2009; Yip et al., 2013), cognitive impairments (McGeorge et al., 2001), phobias (Mühlberger et al., 2001), body image disturbances (Riva, 2002), and autism spectrum disorder (Kandalaft et al., 2013; Ke et al., 2013; Parsons et al., 2004), which emphasizes the clear positive transfer from virtual to real life (Riva, 2002; Zhang et al., 2003; Tsang and Man, 2013; Lam et al., 2004).
To summarize, both immersive and non-immersive VR programs can incorporate empirically derived strategies for optimal learning via personalized exercises and rapid feedback in low-demand settings. The major aim of this initial feasibility and acceptability study was to design and implement a potentially effective, high-compliance VR social skills training game for individuals with schizophrenia by capitalizing on technological innovations in adaptive, non-immersive VR technology. If successful, this approach could result in an alternative or an adjunctive intervention to behavioral treatments administered by therapists, which are effective but unfortunately unavailable or unaffordable to a majority of help-seeking individuals in the U.S. Based on the past VR research and findings from social neuroscience, we hypothesized that social simulation exercises in a low-stress, low-demand setting (i.e. playing an easy social video game on a non-immersive VR platform) would improve social functioning in individuals with schizophrenia. Our bottom-up approach emphasizes learning by simulation and repeated exposure to similar but not identical situations that require a degree of social problem solving. The novelty of this non-immersive, VR-based social training ‘game’ for use in populations with schizophrenia requires a demonstration of feasibility and acceptability before the efficacy can be established. In other words, we need to know whether the participants are able and willing to visit the laboratory bi-weekly over the course of at least five weeks (feasibility), and whether the game is sufficiently engaging and motivating but not burdensome (acceptability).
Thus, the present study is an initial report of the feasibility and acceptability of a 10-session VR social skills training ‘game’ (Multimodal Adaptive Social Intervention in Virtual Reality, MASI-VR) that targeted social attention in individuals with schizophrenia. This ‘game’ did not involve any therapy, but instead, we focused on training one’s ability to make ‘small talk’ in different environments that are familiar with most people (e.g. shop, bus stop and cafeteria); participants were asked to interact with various characters to obtain personal information from them.
2. Methods
2.1. Participants.
Individuals from the community who met the DSM-5 criteria for schizophrenia and were taking antipsychotic medication (SZ) were recruited from outpatient day facilities in Nashville. All participants were recruited via advertisements placed in these facilities. Diagnosis was confirmed with the Structured Clinical Interview for DSM-5 (SCID-5RV; First et al., 2015) conducted by MA level clinical psychologists. Those who conducted clinical interviews for symptoms and assessments were not involved in the 5-week social training game. In order to further ensure blindness, symptoms scores and assessment scores were not examined until all participants had completed the training and post assessment. Moreover, those involved in the training were blind to the symptoms scores and baseline assessment scores of the participants. Out of 19 patients assessed for eligibility, 18 met the inclusion criteria and consented to enroll in the study. Exclusion criteria included history of head injury or seizures, drug abuse, neurological diseases (e.g., stroke, tumors), or estimated IQ <70. Intellectual functioning was measured to ensure participants understood and freely consented to the study, as required by the Vanderbilt University Review Board policies. Screening for cognitive ability also allowed us to ensure the participants met the basic reading and comprehension required to understand all procedures, tasks and measures that they are asked to undertake. IQ was measured using both North American Adult Reading Test- Revised (NART-R; Blair and Spreen, 1989) and the WASI (Wechsler, 1999). No participant met the exclusion criteria for IQ.
Demographic and clinical information are summarized in Table 1. The study was explained to potential participants by laboratory research staff and research assistants rigorously trained to explain the study procedure as scripted in the research protocol approved by the Institutional Review Board of Vanderbilt University. All participants provided written informed consent after full explanation of study procedures. Participants were paid after each visit.
Table 1.
Participant demographic information
| SZ-PRE (N=18) |
SZ-Completed (N=16) |
|
|---|---|---|
| Characteristic | Mean (SD) | Mean (SD) |
| M/F | 10/8 | 9/7 |
| Age (years) | 48.78 (7.42) | 48.63 (6.98) |
| Education ears) | 13.11 (1.94) | 13.19 (1.97) |
| Medication (mg/day)a | 285.41 (254.84) | 260.44 (242.48) |
| NART-R | 102.02 (8.71) | 102.69 (8.81) |
| WASI | 98.27 (15.31) | 98.75 (16.21) |
| Handednessb | 68.06 (40.95) | 66.56 (42.73) |
NART-R: National Adult Reading Test, Revised (Blair & Spreen, 1989); WASI: Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999)
All patients were medicated. Antipsychotic dosage was converted to chlorpromazine (CPZ) equivalent (Andreasen et al., 2010).
Edinburgh Handedness Inventory (Oldfield, 1971). Scores range from -100 (completely left-handed) to +100 (completely right-handed).
2.2. Study procedure.
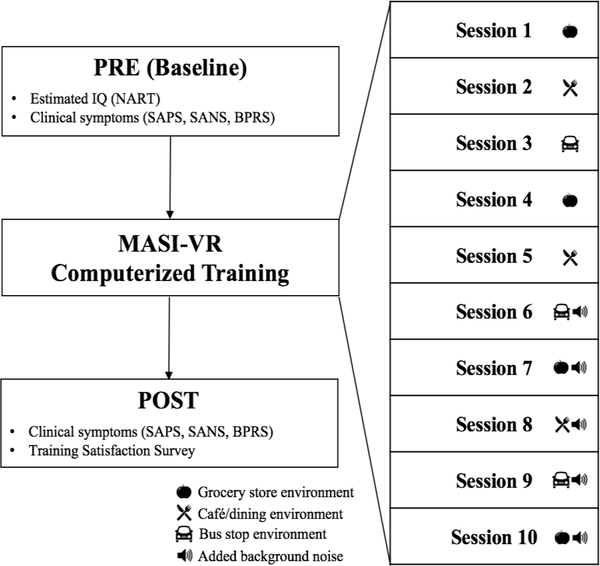
All participants completed a pre-training (PRE) baseline visit, which included assessments of intellectual functioning, social engagement and clinical symptoms (See Figure 1 for a diagram of study visits). Following the baseline visit, participants were asked to return and complete ten sessions of the MASI-VR training program, scheduled approximately twice per week (number of days to complete all ten sessions, M=38.8, SD =14.9). Participants also completed a post-training (POST) assessment, scheduled within two weeks of the final training session. At the post-training visit, symptoms were reassessed and participants completed a training satisfaction survey.
Figure 1.
Study procedure
2.3. MASI-VR
The goal of MASI-VR (for further detail, see Bekele et al., 2016; Bekele et al., 2014) is to enhance social skills in an engaging and low-burden environment. The low-burden environment was achieved by administering relatively easy tasks in a university campus (rather than hospital) setting, without time pressure. MASI-VR was administered on a desktop computer in the form of a video game, making it engaging for participants. Because this was a non-immersive VR game, potential side-effects of immersive VR such as dizziness or nausea could be minimized. The social parameters of MASI-VR could be repeatedly explored and systematically altered to avoid habituation. Social interaction difficulty could also be varied to enhance skill training. In the real, physical world, micro-level, non-verbal social skills such as eye contact, facial expression, social distance and gesture are continuously modified according to the demands of social situations (Spence, 2003). Such micro-level skills are then integrated with appropriate macro-level strategies, such as starting conversations, greeting people, and requesting help or information. In the MASI-VR, we promoted the use of both micro and macro-level social skills by requiring participants to engage avatars by directly looking at them and to, pay attention to the avatar’s facial expressions and body language across three different social contexts (bus stop, shop and cafeteria). MASI-VR games did not involve open ended interactions. Instead, the participant interacted with the avatar by using a keyboard to select from text-based menus that provided structured statements from which a subject may choose to initiate or continue an interaction. Avatars conveyed facial expressions and natural speech via prerecorded sound files. The avatar’s speech was pre-recorded by native English speakers to ensure that the prosody and intonation were similar to what the participants would encounter in a real-life social interaction. Additionally, a pre-recorded game-narrator provided verbal instructions for general game navigation and training feedback following conversational selections. Notably, there were no negative consequences to making one choice versus another in the game (no loss of points), making it a low-stakes, training program.
The content of the MASI-VR was created to exercise an important aspect of the SST curriculum that focuses on starting a conversation with an unfamiliar person in order to make requests or ask for information. Every session of the MASI-VR required the participant to complete 12 social “missions”. At the beginning of each session, the participant selected a mission from four available options on the computer screen. A mission (e.g., “Find out the avatar’s favorite TV show”) began with the participant approaching, selecting and interacting with a new person (an avatar) in a naturalistic environment (a grocery store, café, or bus stop) through variable sequences of conversations. Once the mission was selected, the participant was free to explore the virtual space at his/her pace to select an avatar to engage. This avatar’s face was occluded behind a translucent green mask until the participant fixated her/his eyes on the avatar’s face, fully engaging attention on that avatar. Fixating one’s gaze on the avatar removed the green mask to reveal the face of the avatar. Then, the participants could choose from a list of four conversational options presented on the screen to tackle the mission. If participant chose an incorrect response, an audio feedback informed the participant why the response was not ideal for that particular situation, and participants were asked to select again (e.g., “Very good try. This question might get you some of the information you are trying to learn, but remember to offer information about yourself. Try again.”). Real time feedback was provided to the participant after each response was made, allowing for strategy updates, and in-vivo learning opportunities. See Figure 2 for illustrations of the game.
Figure 2. Sample scenes from MASI-VR.
All environments display the number of completed missions (in green), failed missions (in red), and mission target (red text below mission count) on the upper left side of the screen. (A) Participants explore the virtual environment (shown, cafeteria). (B) Participants must select avatars to approach and interact with (shown, bus stop) (C) Once an avatar is selected, participants must fixate on the avatar prior to initiating a conversation (shown, grocery store). (D) The participant receives feedback after selecting a conversation topic and exchanging with the avatar (shown, grocery store).
Social missions varied in difficulty, determined by the number of conversational inquiries and responses required for mission completion. For an easy scenario, a participant could interact with the avatar with minimal effort (i.e., one appropriate social inquiry). A medium level of difficulty required the participant to link two appropriate social ‘bids’ together to attain a practical social goal, (e.g., joining a table occupied by strangers at a busy cafeteria). For high difficulty level interactions, the social goal could be attained only after the participant linked several appropriate social bids together. Missions were considered unsuccessful if the participant chose more than two ‘incorrect’ conversational responses during an interaction. Missions were ordered by difficulty to scaffold training. Thus, participants completed four easy, four medium and finally, four hard missions sequentially at each training session. As a result, error allowance remained the same while the number of opportunities to make errors increased with the difficulty level.
After five training sessions, naturalistic environmental background noise was added to each of the VR environments to increase mission difficulty. For example, instead of interacting with an avatar at a bus stop during an off-peak time, now the bus stop was made noisier and busier to reflect the rush hour. This scaffolded approach to mission difficulty, both across and within missions and training sessions, minimized the potential risk of participant disengagement due to perceived or real difficulty. There was no time limit to complete a mission. Therefore, every participant completed all 12 missions per session but the rate at which they worked through these missions varied. During these interactions, the key to learning was maintaining optimal attentional engagement levels. Game performance indices included the number of missions correctly completed, error rates (i.e., number of incorrect questions posed to the avatar), duration of time to completion, and social engagement latency (time it took to select and engage with an avatar).
2.4. Assessments.
Intellectual ability was assessed with the National Adult Reading Test, Revised (NART-R; Blair and Spreen, 1989) a widely-used measure to assess premorbid intelligence in schizophrenia research (O’Carroll et al., 1992; Morrison et al., 2000) and WASI (Wechsler, 1999). Severity of general psychiatric symptoms in SZ was assessed with the Brief Psychiatric Rating Scale (BPRS, Overall and Gorham, 1962). Severity of positive and negative psychotic symptoms was measured with the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984) and Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1983), respectively. Social engagement and function over the past three months were assessed by the Social Functioning Scale (SFS; Birchwood et al., 1990). Advanced clinical graduate students or a MA-level research assistant with established interrater reliability conducted all clinical symptom interviews. Interviewers were not involved in the 10-session training of the specific participants to avoid potential rater bias.
3. Results
3.1. Participant retention.
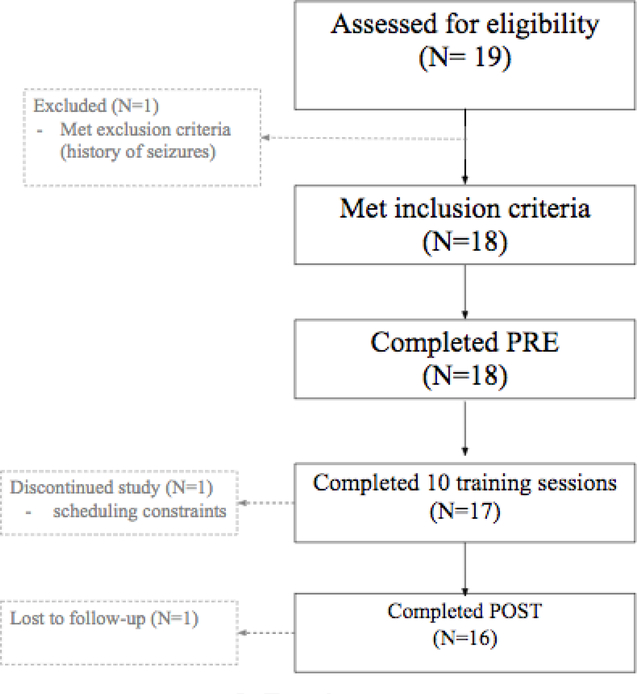
Figure 3 summarizes the enrollment process for the study. Amongst the 19 SZ participants screened for eligibility, 18 met inclusion criteria for the study and completed the initial baseline assessment (PRE). One person who did not meet the inclusion criteria has a history of seizures. Out of the 18 participants who began to train, one participant dropped out after the fourth session due to increasing work demands. Seventeen patients completed all 10 sessions of VR training. One participant completed 10 sessions of training but did not return for the post-treatment assessment. Therefore, although 17 people completed the required training, the missing post-treatment assessment and satisfaction survey data from one participant reduced the final completion rate to 16/18. To summarize, 16 patients successfully completed the full study protocol, including the initial PRE assessment, 10 sessions of VR social skills training, and post-intervention assessments, yielding an overall retention rate of 89%.
Figure 3.
Flow chart of participant recruitment and retention
3.2. MASI-VR training acceptability.
See Table 2 for survey results. Survey feedback indicated high levels of overall satisfaction with the MASI-VR training program (94% of participants reported some degree of satisfaction, and 81% endorsed Extreme Satisfaction). No participants reported dissatisfaction with the training. The majority of participants (81%) also found the training to be helpful, of acceptable length, and would participate again or recommend it to others. Participants endorsed a range of difficulty levels for training, with exactly half indicating that the game was neither too easy nor too difficult. See Supplement 1 for additional participant feedback and comments regarding training.
Table 2.
Post-training survey results
| Acceptability: | ||||
| Overall Training Satisfaction | Extremely Satisfied | A Little Satisfied | Neither Satisfied nor Dissatisfied | Unsatisfied (a little or extremely) |
| 81.2% | 12.5% | 6.2% | 0% | |
| Training Length Acceptable? | Yes | OK | Unsure | No |
| (total program period) | 81.2% | 12.5% | 6.2% | 0% |
| (training session length) | 87.5% | 12.5% | 0% | 0% |
| Compensation Acceptable? | Yes | OK | No | |
| 93.7% | 6.2% | 0% | ||
| Would you recommend the training to someone else? | Yes | Maybe | No | |
| 81.2% | 12.5% | 6.2% | ||
| Feasibility: | ||||
| Training Difficulty | Easy | Neither | Difficult | |
| 43.7% | 50% | 6.2% | ||
| Ease of Attending Study | Easy | Neither | Difficult | |
| 93.7% | 6.2% | 0% | ||
3.3. Outcome measures.
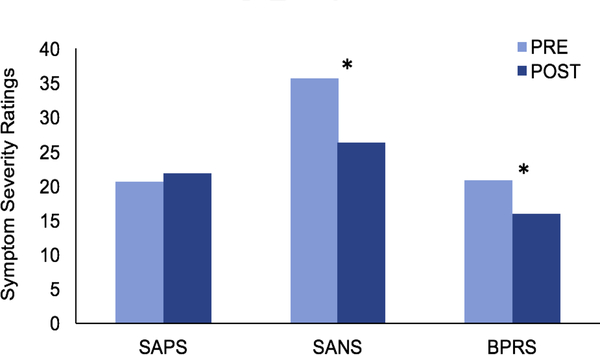
Overall psychiatric symptom severity as measured by the BPRS significantly improved from PRE (M= 21.0, SD= 8.65) to POST (M= 16.06, SD = 7.54) training, F(1,15) = 8.83, p = 0.01, η2 =0.23 (See Figure 4). Negative symptom severity assessed by SANS also significantly decreased from PRE (M= 36.44, SD= 13.06) to POST training (M= 26.37, SD= 10.79), F(1,15) = 8.64, p = 0.01, η2 =0.22. These results indicate medium-to-large effect sizes for both overall psychiatric and negative symptoms improvement. No improvement of positive symptoms was observed from PRE-to-POST training. No significant changes overall or across subscales of the SFS were observed from PRE-to-POST training.
Figure 4. Symptom change from PRE-to-POST training.
SAPS: Schedule for the Assessment of Positive Symptoms; SANS: Schedule for the Assessment of Negative Symptoms; BPRS: Brief Psychiatric Rating Scale. *Significant change from PRE-to- POST, p < 0.05.
4. Discussion
The high rate of participant retention and positive feedback in response to the training length, satisfaction, and helpfulness indicate that a VR-based game intervention (MASI-VR) is both feasible and acceptable for individuals with schizophrenia. Importantly, the majority of participants who completed all 10 sessions of training rated MASI-VR to be extremely satisfactory and helpful. We note that the acceptability and feasibility of MASI-VR was assessed with self-report questionnaires. This positive feedback, alongside the high retention rates throughout the study, suggest that individuals with chronic schizophrenia are able to participate and benefit from computerized interventions such as MASI-VR despite relatively low exposure to this type of game system. The retention rate was better than many current psychosocial group interventions for individuals with schizophrenia (Granholm et al., 2005; Granholm et al., 2014). Additionally, non-immersive systems like MASI-VR can be easily adapted for use at home, and therefore, it could be used as an adjunct social intervention, together with more intensive treatments requiring skilled therapists and in-person attendance (e.g., psychosocial skills groups). In the absence or unavailability of established therapies due to cost, systems such as the MASI-VR could fill in the gap.
The reduction in symptoms, and particularly negative symptoms, following MASI-VR training is very promising. Pharmacological treatments have a limited impact on negative symptoms (Fusar-Polli et al, 2015). Current psychosocial interventions demonstrate only moderate or highly heterogeneous efficacy (Lutgens et al., 2017, Remington et al., 2016), with the exception of the SST, which seems to reduce both negative symptoms and improve social functioning (Granholm and Harvey, 2018; Turner et al, 2017). It is important to note that the content of the MASI-VR was designed to exercise an important aspect of the SST curriculum. As the SFS inquiries about an individual’s engagement over the prior three months, no change was expected during the duration of the intervention. However, follow-up testing may reveal more significant improvements.
The low cost and low burden nature of this intervention, given demonstrated improvement in reduction of negative symptoms, make MASI-VR a potential candidate to tackle this current gap in treatment. Importantly, although we were primarily focused on improving social skills, reducing negative symptoms may be a crucial gateway towards translation of these skills. For example, without reducing anhedonia or apathy, the participant may never have an opportunity to practice the skills acquired. Thus, we consider the significant reduction of negative symptoms an initial proof of concept for MASI-VR.
There are caveats. First, this study did not include a control condition, precluding our ability to differentiate specific from nonspecific treatment effects. It is possible that coming to the laboratory bi-weekly for five weeks and interacting with the experimenters contributed to improvements in symptoms. A randomized clinical trial in the future is needed to determine whether the MASI-VR training successfully targets social impairments. Secondly, this preliminary study did not directly examine targeted mechanisms of social attention. Future work will utilize eye-tracking data to investigate potential changes in social attention, the primary mechanistic target of MASI-VR. Moreover, it is unknown skills learned in the MASI-VR game translate to social functioning in the real world. Finally, our sample size limits the generalizability of our findings but our initial findings of high acceptability and improvements in negative symptoms warrant further investigation.
In sum, high levels of acceptability, low dropout rate, and predominantly positive feedback support the feasibility of a VR social skills training game for individuals with schizophrenia. These participants were not only able to complete the 10 sessions of training, they also reported enjoyment and satisfaction. The preliminary finding of reduced negative symptoms following 10-session-training highlights the potential of MASI-VR as an effective and accessible intervention for social impairments in schizophrenia.
Supplementary Material
Highlights.
This novel VR social skills training is feasible for patients with schizophrenia
This protocol was acceptable to patients with schizophrenia
Clinical symptoms, especially negative symptoms improved after 10 sessions
Participants reported high levels of training satisfaction and real-world utility
Acknowledgments
This work was supported by NARSAD, MH106748 and Gertrude Conaway Vanderbilt Endowment. We would like to thank Justin Hong, Jacqueline Roig, Andrea Prada, and Eunsol Chon for help with data collection. We also would like to acknowledge Andy Tomarken and Steve Hollon for their support. We are especially grateful to our participants for their patience and dedication.
Footnotes
Declaration of Conflict of Interest: No conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC, 1984. Scale for the assessment of positive symptoms (SAPS). University of Iowa, Iowa City [Google Scholar]
- Andreasen NC, 1983. Scale for the assessment of negative symptoms (SANS). University of Iowa, Iowa City. [Google Scholar]
- Bekele E, Lahiri U, Swanson AR, Crittendon JA, Warren ZE, Sarkar N, 2013. A step towards developing adaptive robot-mediated intervention architecture (ARIA) for children with autism. IEEE Trans Neural Syst Rehabil Eng 21 (2), 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekele E, Zheng Z, Swanson A, Crittendon J, Warren Z, Sarkar N, 2013. Understanding how adolescents with autism respond to facial expressions in virtual reality environments. IEEE Trans Vis Comput Graph 19 (4), 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekele E, Bian D, Zheng Z, Peterman JS, Park S, Sarkar N, 2014. Responses during Facial Emotional Expression Recognition Tasks Using Virtual Reality and Static IAPS Pictures for Adults with Schizophrenia. VAMR Part II. LNCS 8526: 225–235 [Google Scholar]
- Bekele E, Bian D, Peterman JS,, Park S, Sarkar N, 2016. Design of a Virtual Reality System for Affect Analysis in Facial Expressions (VR-SAAFE); application to schizophrenia. IEEE Trans Neural Syst Rehabil Eng pp 99). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellack AS, Mueser KT, Gingerich S, Agresta J, 2004. Social Skills Training for Schizophrenia: A Step-by-Step Guide. Guilford Publications, New York. [Google Scholar]
- Bellack AS, Dickinson D, Morris SE, Tenhula WN, 2005. The development of a computer-assisted cognitive remediation program for patients with schizophrenia. Isr J Psychiatry Relat Sci 42 (1), 5–14. [PubMed] [Google Scholar]
- Birchwood M, Smith JO, Cochrane R, Wetton S, Copestake SONJ, 1990. The social functioning scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br J Psychiatry 157 (6), 853–859. [DOI] [PubMed] [Google Scholar]
- Blair JR, Spreen O, 1989. Predicting premorbid IQ: a revision of the National Adult Reading Test. Clin Neuropsychol 3 (2), 129–136.. [Google Scholar]
- Burge J, Lane T, Link H, Qiu S, Clark VP, 2009. Discrete dynamic Bayesian network analysis of fMRI data. Human Brain Mapp 30 (1), 122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig TK, Rus-Calafell M, Ward T, Leff JP, Huckvale M, Howarth E, et al. , 2018. AVATAR therapy for auditory verbal hallucinations in people with psychosis: a single-blind, randomised controlled trial. Lancet Psychiatry 5 (1), 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan PW, Liberman RP, Engel JD, 1990. From noncompliance to collaboration in the treatment of schizophrenia. Hosp Community Psychiatry 41 (11), 1203–1211. [DOI] [PubMed] [Google Scholar]
- Du Sert OP, Potvin S, Lipp O, Dellazizzo L, Laurelli M, Breton R, et al. , 2018. Virtual reality therapy for refractory auditory verbal hallucinations in schizophrenia: A pilot clinical trial. Schizophr Res pii: S0920–9964 (18), 30108–7. [DOI] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, Spitzer RL, 2015. Structured Clinical Interview for DSM-5 Research Version. American Psychiatric Association; Arlington, VA. [Google Scholar]
- Freeman D, 2008. Studying and treating schizophrenia using virtual reality: A new paradigm. Schizophr Bull 34 (4), 605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D, Pugh K, Vorontsova N, Antley A, Slater M, 2010. Testing the continuum of delusional beliefs: An experimental study using virtual reality. J Abnorm Psychol 119 (1), 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D, Reeve S, Robinson A, Ehlers A, Clark D, Spanlang B, et al. , 2017. Virtual reality in the assessment, understanding, and treatment of mental health disorders. Psychol Med 47 (14), 2393–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, et al. , 2015. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr. Bull 41 (4), 892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Harvey PD, 2018. Social Skills Training for Negative Symptoms of Schizophrenia. Schizophr Bull 44 (3), 472–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, McQuaid JR, McClure FS, Auslander LA, Perivoliotis D, Pedrelli P, et al. , 2005. A randomized, controlled trial of cognitive behavioral social skills training for middle-aged and older outpatients with chronic schizophrenia. Am J Psychiatry 162 (3), 520–529. [DOI] [PubMed] [Google Scholar]
- Granholm E, Holden J, Link PC, McQuaid JR, 2014. Randomized clinical trial of cognitive behavioral social skills training for schizophrenia: Improvement in functioning and experiential negative symptoms. J Consult Clin Psychol 82 (6), 1173–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Penn DL, Bentall R, Carpenter WT, Gaebel W, Gur RC, et al. , 2008. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr Bull 34 (6), 1211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinssen RK, Liberman RP, Kopelowicz A, 2000. Psychosocial skills training for schizophrenia: lessons from the laboratory. Schizophr Bull, 26 (1), 21–46. [DOI] [PubMed] [Google Scholar]
- Kandalaft MR, Didehbani N, Krawczyk DC, Allen TT, Chapman SB, 2013. Virtual reality social cognition training for young adults with high-functioning autism. J Autism Dev Disord 43 (1), 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke F, Im T, 2013. Virtual-reality-based social interaction training for children with high-functioning autism. J Educ Res 106 (6), 441–461. [Google Scholar]
- Kopelowicz A, Liberman RP, Zarate R, 2006. Recent advances in social skills training for schizophrenia. Schizophr Bull 32, S12–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman AF, Kreyenbuhl J, Buchanan RW, Dickerson FB, Dixon LB, Goldberg R, et al. , 2004. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2003. Schizophr Bull 30 (2), 193–217. [DOI] [PubMed] [Google Scholar]
- Kurtz MM, Baker E, Pearlson GD, Astur RS, 2006. A virtual reality apartment as a measure of medication management skills in patients with schizophrenia: a pilot study. Schizophr Bull 33 (5), 1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz MM, Mueser KT 2008. A meta-analysis of controlled research on social skills training for schizophrenia. J Consult Clin Psychol 76 (3), 491–504. [DOI] [PubMed] [Google Scholar]
- Lam YS, Tam SF, Man DWK, Weiss PL, 2004. Evaluation of a computer-assisted 2D interactive virtual reality system in training street survival skills of people with stroke. Proc ICDVRAT V pp. 27–32. [Google Scholar]
- Lindenmayer JP, McGurk SR, Khan A, Kaushik S, Thanju A, Hoffman L, et al. , 2012. Improving social cognition in schizophrenia: a pilot intervention combining computerized social cognition training with cognitive remediation Schizophr Bull 39 (3), 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughland CM, Williams LM, Gordon E, 2002a. Visual scanpaths to positive and negative facial emotions in an outpatient schizophrenia sample. Schizophr Res 55 (1), 159–170. [DOI] [PubMed] [Google Scholar]
- Loughland CM, Williams LM, Gordon E, 2002b. Schizophrenia and affective disorder show different visual scanning behavior for faces: a trait versus state-based distinction? Biol Psychiatry 52 (4), 338–348. [DOI] [PubMed] [Google Scholar]
- Lutgens D, Gariepy G, Malla A, 2017. Psychological and psychosocial interventions for negative symptoms in psychosis: Systematic review and meta-analysis. Br J Psychiatry 210 (5) 324–332. [DOI] [PubMed] [Google Scholar]
- Matthews N, Gold BJ, Sekuler R, Park S, 2011. Gesture imitation in schizophrenia. Schizophr Bull 39 (1), 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M, Lucci G, Pacitti F, Pino MC, Mariano M, Casacchia M, et al. , 2010. Could schizophrenic subjects improve their social cognition abilities only with observation and imitation of social situations? Neuropsychol Rehabil 20 (5), 675–703. [DOI] [PubMed] [Google Scholar]
- McGeorge P, Phillips LH, Crawford JR, Garden SE, Sala SD, Milne AB, et al. , 2001. Using virtual environments in the assessment of executive dysfunction. Presence: Teleoperators Virtual Environ 10 (4), 375–383. [Google Scholar]
- Minassian A, Granholm E, Verney S, Perry W, 2005. Visual scanning deficits in schizophrenia and their relationship to executive functioning impairment. Schizophr Res 74 (1), 69–79. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Parsons S, Leonard A, 2007. Using virtual environments for teaching social understanding to 6 adolescents with autistic spectrum disorders. J Autism Dev Disord 37 (3), 589–600. [DOI] [PubMed] [Google Scholar]
- Moore D, McGrath P, Thorpe J, 2000. Computer-aided learning for people with autism-a framework for research and development. IJIER 37 (3), 218–228. [Google Scholar]
- Morrison G, Sharkey V, Allardyce J, Kelly RC, McCreadie RG, 2000. Nithsdale schizophrenia surveys 21: a longitudinal study of National Adult Reading Test stability. Psychol Med 30 (3), 717–720. [DOI] [PubMed] [Google Scholar]
- Mühlberger A, Herrmann MJ, Wiedemann G, Ellgring H, Pauli P, 2001. Repeated exposure of flight phobics to flights in virtual reality. Behav Res Ther 39 (9), 1033–1050. [DOI] [PubMed] [Google Scholar]
- Nakamura J, Csikszentmihalyi M, 2002. The concept of flow, in: Snyder CR, Lopez SJ (Eds.), Handbook of Positive Psychology. Oxford University Press, New York, pp. 89–105. [Google Scholar]
- Nummenmaa L, Calder AJ, 2009. Neural mechanisms of social attention. Trends Cogn Sci 13 (3), 135–143. [DOI] [PubMed] [Google Scholar]
- O’Carroll R, Walker M, Dunan J, Murray C, Blackwood D, Ebmeier KP, et al. , 1992. Selecting controls for schizophrenia research studies: The use of the national adult reading test (NART) is a measure of pre-morbid ability. Schizophr Res 8 (2), 137–141. [DOI] [PubMed] [Google Scholar]
- Oldfield RC, 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9 (1), 97–113. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR, 1962. The brief psychiatric rating scale. Psychological Reports 10 (3), 799–812. [Google Scholar]
- Park S, Matthews NL, Gibson C, 2008. Imitation, simulation, and schizophrenia. Schizophr Bull 34 (4), 698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KM, Ku J, Choi SH, Jang HJ, Park JY, Kim, et al. , 2011. A virtual reality application in role-plays of social skills training for schizophrenia: a randomized, controlled trial. Psychiatry Res 189 (2), 166–172. [DOI] [PubMed] [Google Scholar]
- Parsons S, Mitchell P, Leonard A, 2004. The use and understanding of virtual environments by adolescents with autistic spectrum disorders. J Autism Dev Disord 34 (4), 449–466. [DOI] [PubMed] [Google Scholar]
- Penn DL, Roberts DL, Combs D, Sterne A, 2007. Best practices: the development of the social cognition and interaction training program for schizophrenia spectrum disorders. Psychiatr Serv, 58 (4), 449–451 [DOI] [PubMed] [Google Scholar]
- Pfammatter M, Junghan UM, Brenner HD, 2006. Efficacy of psychological therapy in schizophrenia: conclusions from meta-analyses. Schizophr Bull, 32, S64–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pot-Kolder R, Veling W, Geraets C, van der Gaag M, 2016. Effect of virtual reality exposure therapy on social participation in people with a psychotic disorder (VRETp): study protocol for a randomized controlled trial. Trials 17 (25). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington G, Foussias G, Fervaha G, Agid O, Takeuchi H, Lee J, et al. , 2016. Treating negative symptoms in schizophrenia: an update. Curr Treat Options Psychiatry 3 (2), 133–150.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva G, 2002. Virtual reality for health care: the status of research. Cyberpsychol Behav 5 (3), 219–225. [DOI] [PubMed] [Google Scholar]
- Rus-Calafell M, Garety P, Sason E, Craig TJK, Valmaggia LR, 2018. Virtual reality in the assessment and treatment of psychosis: a systematic review of its utility, acceptability and effectiveness. Psychol Med 48 (3), 362–391. [DOI] [PubMed] [Google Scholar]
- Rus-Calafell M, Gutiérrez-Maldonado J, Ribas-Sabaté J, 2014. A virtual reality-integrated program for improving social skills in patients with schizophrenia: a pilot study. J Behav Ther Exp Psychiatry 45 (1), 81–89. [DOI] [PubMed] [Google Scholar]
- Russell TA, Green MJ, Simpson I, Coltheart M, 2008. Remediation of facial emotion perception in schizophrenia: concomitant changes in visual attention. Schizophr Res 103 (1), 248–256. 10.1016/j.schres.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Spence SH, 2003. Social skills training with children and young people: Theory, evidence and practice. Child Adolesc Ment Health 8 (2), 84–96. [DOI] [PubMed] [Google Scholar]
- Standen PJ, Brown DJ, 2005. Virtual reality in the rehabilitation of people with intellectual disabilities. Cyberpsychol Behav 8 (3), 272–282. [DOI] [PubMed] [Google Scholar]
- Strickland D, 1997. Virtual reality for the treatment of autism. Stud Health Technol Inform 44, 81–86. [PubMed] [Google Scholar]
- Swartz RJ, Costa AL, Beyer BK, Reagan R, Kallick B, 2010. Thinking-Based Learning: Promoting Quality Student Achievement in the 21st Century. Teachers College Press, New York. [Google Scholar]
- Swartz MS, Perkins DO, Stroup TS, Davis SM, Capuano G, Rosenheck RA, et al. , 2007. Effects of antipsychotic medications on psychosocial functioning in patients with chronic schizophrenia: findings from the NIMH CATIE study. Am J Psychiatry 164 (3), 428–436. [DOI] [PubMed] [Google Scholar]
- Tartaro A, Cassell J, 2007. Using virtual peer technology as an intervention for children with autism, in: Lazar J, (Ed.) Towards Universal Usability: Designing Computer Interfaces for Diverse User Populations. John Wiley & Sons, Ltd., New York, pp. 231–262. [Google Scholar]
- Tsang MM, Man DW, 2013. A virtual reality-based vocational training system (VRVTS) for people with schizophrenia in vocational rehabilitation. Schizophr Res, 144 (1–3), 51–62. [DOI] [PubMed] [Google Scholar]
- Turner DT, McGlanaghy E, Cuijpers P, van der Gaag M, Karyotaki E, MacBeth A, 2018. A Meta-analysis of social skills training and related interventions for psychosis. Schizophr Bull 44 (3), 475–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valmaggia LR, Freeman D, Green C, Garety P, Swapp D, Antley A, et al. , 2007. Virtual reality and paranoid ideations in people with an ‘at-risk mental state’for psychosis. Br J Psychiatry Suppl 191 (51), s63–s68. [DOI] [PubMed] [Google Scholar]
- Valmaggia L, 2017. The use of virtual reality in psychosis research and treatment. World Psychiatry 16 (3), 246–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veling W, Pot-Kolder R, Counotte J, van Os J, van der Gaag M, 2016. Environmental social stress, paranoia and psychosis liability: a virtual reality study. Schizophr Bull 42 (6), 1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D 1999. Wechsler Abbreviated Scale of Intelligence®(WASI®). The Psychological Corporation, New York, NY. [Google Scholar]
- Welch KC, Lahiri U, Liu C, Weller R, Sarkar N, Warren Z, 2009. An affect-sensitive social interaction paradigm utilizing virtual reality environments for autism intervention. LNCS 5612, 703–712. [Google Scholar]
- Yip BC, Man DW, 2013. Virtual reality-based prospective memory training program for people with acquired brain injury. NeuroRehabilitation 32 (1), 103–115. [DOI] [PubMed] [Google Scholar]
- Zhang L, Abreu BC, Seale GS, Masel B, Christiansen CH, Ottenbacher KJ, 2003. A virtual reality environment for evaluation of a daily living skill in brain injury rehabilitation: reliability and validity. Arch Phys Med Rehabil 84 (8), 1118–1124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.