Abstract
Sulfonamide derivatives have been used in pharmaceutics for decades. Here we report a new approach to release sulfonamides efficiently using a bioorthogonal reaction of sulfonyl sydnonimines and dibenzoazacyclooctyne (DIBAC). The second-order rate constant of the cycloaddition reaction can be up to 0.62 M−1 s−1, and the reactants are highly stable under physiological conditions. Most significantly, we also discovered the mutual orthogonality between the sydnonimine–DIBAC and benzonorbornadiene–tetrazine cycloaddition pairs, which can be used for selective and simultaneous liberation of sulfonamide and primary amine drugs.
Sulfonamides constitute an important family of drugs that have been extensively applied in human and veterinary medicine due to their general stability, bioavailability, and ease of preparation.1 The compounds of this class (Fig. 1) have been clinically in use for decades, and some of which showed potent antitumor activity.2 Over decades, various strategies for controlled release of drugs under biological conditions have been developed,3 such as multi-function polymeric nanoformulations4 and photoresponsive biomaterials for targeted drug delivery.5 However, in the case of sulfonamides, few examples had been reported, such as the use of an epichlorohydrin-crosslinked semi-interpenetrating polymer network to control the release of the sulfonamide drug sulpiride.6 In biological systems, the physiological triggers, such as pH value,7 are subtle, more general and reliable triggers that involve rapid bond formation and selective bond cleavage8 to release drug molecules are highly desirable.
Fig. 1.
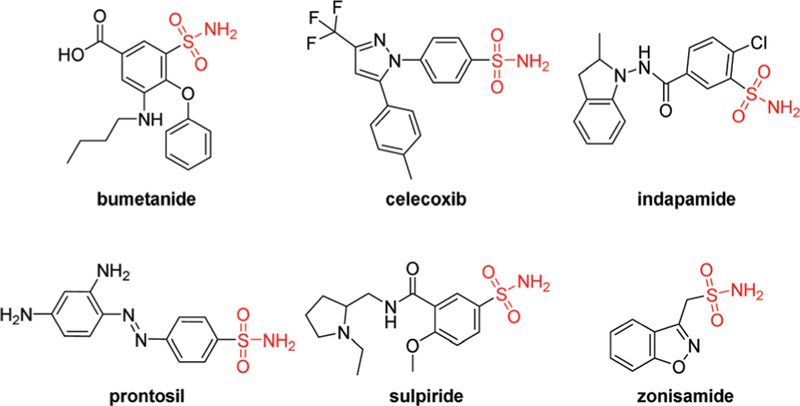
Sulfonamide drugs in clinical use.
In recent years, with bioorthogonal chemistry as a powerful tool,9 click-release reactions have been developed and applied to drug delivery, enzyme activation, and so on.10 In 2013, Robillard and coworkers reported the inverse-electrondemand Diels–Alder (IEDDA) reaction of carbamate-modified trans-cyclooctene (TCO) with tetrazine that allows release of a primary amine drug doxorubicin attached to the TCO after the cycloaddition step under ambient conditions.10a Royzen et al. applied this drug release strategy into treating soft tissue sarcoma in vivo.10g Very recently, Weissleder and coworkers discovered that the TCO–tetrazine-ligation-based drug release is sensitive to pH value, and can only achieve partial release due to an intramolecular side reaction.11 They further designed and synthesized the tetrazine-acids for rapid cleavage reactions that are less sensitive to pH value. In addition, the Gamble group reported the strain-promoted 1,3-dipolar cycloaddition of TCO and azide, the product of which undergoes degradation, hydrolysis, and elimination to release doxorubicin.10d
Sydnone and its derivatives are a relatively new class of bioorthogonal reagents, introduced into bioorthogonal reactions by Taran,12,13 and some of them have been used in click-release reactions.14 Chin first described the reaction of N-phenyl sydnone with strained alkyne bicyclo-[6.1.0]-nonyne (BCN) in protein labeling.15 The rate constant in MeOH/H2O (55 : 45) was measured to be 0.054 M−1 s−1 at 21 °C. Previously we predicted that dibenzoazacyclooctyne (DIBAC) had better reactivity than BCN with N-phenyl sydnone by DFT calculations. Our prediction was confirmed by the Murphy group, and the measured rate constant is 0.90 M−1 s−1 (Fig. 2a).16 Inspired by the fact that sydnone cycloaddition is followed by the extrusion of CO2, which has a very low barrier,16 we envision that the sulfonyl sydnonimine cycloaddition will lead to the simultaneous generation of sulfonyl isocyanate, which is extremely unstable in aqueous media and quantitatively hydrolyzed to form CO2 and sulfonamide.17 Therefore, a new strategy for the controlled release of sulfonamides is proposed: sulfonyl-modified sydnonimines (N6-sulfonyl-SIN 4, with aliphatic and aromatic substituents) undergo 1,3-dipolar cycloadditions with DIBAC 5, resulting in rapid release of sulfonamide 8 via sulfonyl isocyanate 7 (Fig. 2b).
Fig. 2.
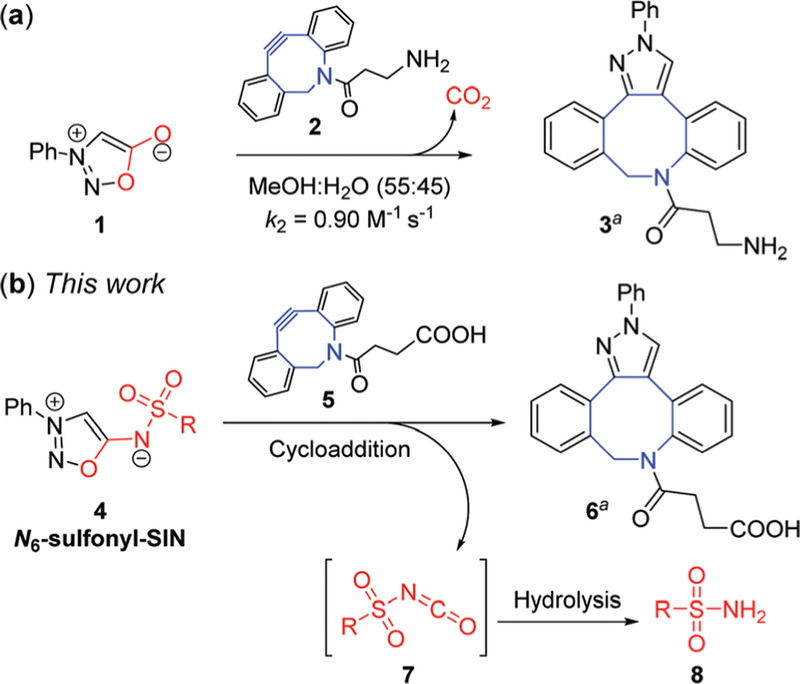
(a) The (3+2) cycloaddition reaction of sydnone; (b) release of sulfonamides through the (3+2) reaction of N6-sulfonyl sydnonimines. aOnly one regioisomer is depicted.
Before launching into experiments, we carried out DFT calculations at the M06-2X level of theory18 on the (3+2) cycloaddition of N6-methanesulfonyl-SIN 4a and DIBAC. The computed activation free energy for this reaction is 21.5 kcal mol−1 (TS_4a-DIBAC, Fig. 3), corresponding to a predicted second-order rate constant of 0.12 M−1 s−1, which is reasonably fast for a bioorthogonal reaction. Subsequently, we synthesized sydnonimine hydrochloride 9.19 After optimization, we found that lowering the reaction temperature to −10 to 0 °C, the desired products N6-sulfonyl-SIN 4a–c can be obtained in good yields from the reaction of 9 and sulfonyl chlorides 10 in dichloromethane with triethylamine (Fig. 4).20
Fig. 3.
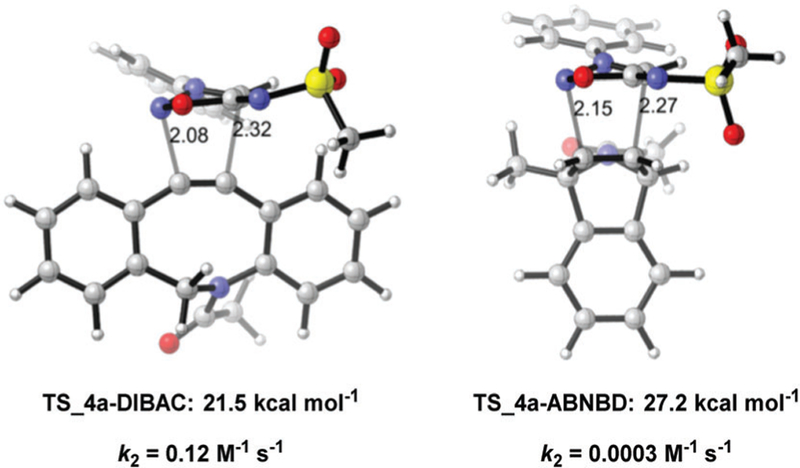
Transition-state structures for the (3+2) cycloadditions of 4a with DIBAC and ABNBD (activation free energies and predicted rate constants are shown below each structure).
Fig. 4.
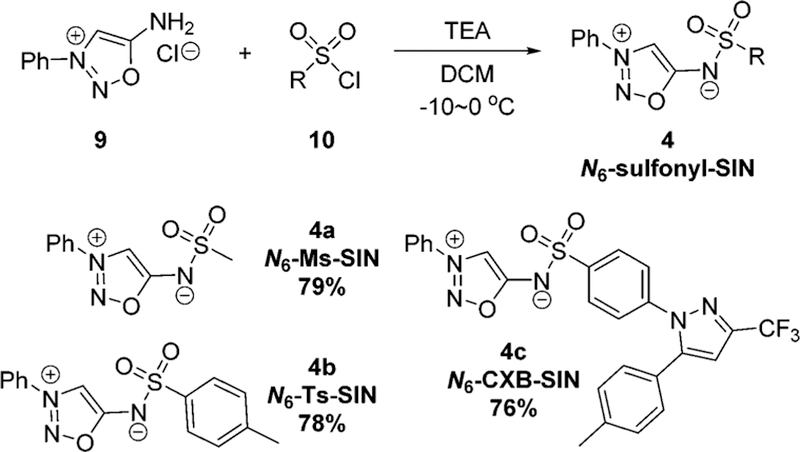
Synthesis of N6-sulfonyl sydnonimines.
To test the feasibility of the click-release strategy, we monitored the cycloaddition reaction of 4a (c0 = 2 mM) with DIBAC-COOH 5 (c0 = 2.2 mM) in 9 : 1 DMSO-d6/D2O at 22 °C with 1H NMR spectroscopy (Fig. 5a). The process of the reaction is characterized by the disappearance of the methyl proton signal of 4a at 2.97 ppm (▪, Fig. 5a). After 2 hours, we observed 40% conversion of 4a into the cycloaddition product 6 (★, Fig. 5a) and methanesulfonamide (8a) (*, δ= 2.91 ppm, Fig. 5a). After 6 hours, 65% of the starting material was consumed. Nearly complete transformation took place within 24 hours, and no side product or intermediate signals were observed during the whole process. This result confirmed our prediction of the effciency of the cycloaddition reaction and the release of sulfonamide. We also monitored the reaction of pro-drug 4c (N6-CXB-SIN) with 5 in 9 : 1 DMSO-d6/D2O using the same method (see ESI†). The release of drug 8c (celecoxib, CXB) is very effcient.
Fig. 5.
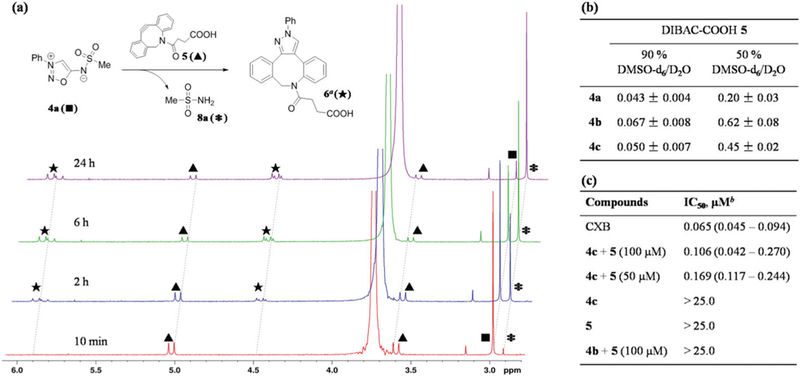
(a) 1H NMR analysis of the reaction of N6-Ms-SIN 4a (2 mM) and DIBAC-COOH 5 (2.2 mM) in DMSO-d6/D2O (9 : 1 v/v). δ = 3.82 ppm is H2O. aOnly one regioisomer is depicted. (b) Measured rate constants (k2, in M−1 s−1) for reactions between 4a–c and 5 at 22 °C. (c) IC50 values against human COX-2. b 95% confidence interval (n = 2) is given in parentheses.
We further studied the kinetics of the reactions of N6-sulfonyl-SIN 4a–c with 5. As shown in Fig. 5b, the measured second-order rate constants (k2) of N6-methanesulfonyl-SIN 4a was 0.043 M−1 s−1 in 9 : 1 DMSO-d6/D2O at 22 °C, and N6-sulfonyl-SIN 4b–c have better reactivities than 4a (0.067 M−1 s−1 and 0.050 M−1 s−1 for 4b and 4c, respectively). To our delight, the second-order rate constants are increased by 5–9 fold in 1 : 1 DMSO-d6/D2O (Fig. 5b).21 Moreover, the stability of pro-drug N6-CXB-SIN 4c is tested in phosphate-buffered saline (PBS) and fetal bovine serum (FBS) at 37 °C. No degeneration was observed within a week in PBS/DMSO (1 : 1 v/v), while less than 20% of 4c decomposed after a week in FBS/PBS (1 : 1 v/v). The high stability of the pro-drug and efficiency of the click-release reaction make 4c very promising for in vivo applications.
Celecoxib is a non-steroidal anti-inflammatory drug (NSAID), a specific COX-2 inhibitor for pain and inflammation without inhibiting COX-1,22 and also shows potent antitumor activity.23 Since the bioorthogonal release of CXB has proved to be effcient, we further test the inhibition effect of this method against human COX-2 (Fig. 5c). When pro-drug 4c was treated with DIBAC 5 (100 mM) in the system, it shows excellent performance with IC50 of 0.106 mM, which is very close to that of the direct use of CXB (IC50 of 0.065 μM). Decreasing the concentration of DIBAC to 50 μM, the measured IC50 slightly dropped to 0.169 μM. The pro-drug 4c and DIBAC 5 are both nontoxic at the testing concentration with IC50 > 25.0 μM. Control experiments indicate that the combination of 4b and 5 showed no toxicity even at maximal testing concentration, demonstrating that the cycloadduct 6 is nontoxic. These results confirmed the biostability and high cycloaddition-release effciency of 4c, which can be potentially applied to targeted drug release.
Recently, the Franzini group reported that 7-aza-benzonorbornadiene (ABNBD) derivatives react with tetrazine to liberate a primary amine drug doxorubicin.10i Tetrazines react rapidly with ABNBD but are inert to DIBAC.24 Our calculations predicted that sydnonimines have selectivity opposite to that of tetrazines, and react with DIBAC but not with ABNBD (Fig. 3). The computed activation barrier for the reaction between sydnonimine 4a and ABNBD is 27.2 kcal mol−1, corresponding to a rate constant of 3 × 10−4 M−1 s−1. Based on these facts, we designed click-release pairs that can be used for orthogonal release of two different drugs simultaneously, where sydnonimine–DIBAC and tetrazine–ABNBD cycloaddition pairs are delivery vehicles for celecoxib 8c and doxorubicin 13, respectively (Fig. 6a). 1H NMR spectroscopy confirmed that there is no reaction between sydnonimine 4c and ABNBD 11 within 25 hours (Fig. 6b). We successfully released celecoxib (after 4.5 h) or doxorubicin (after 11 h) by introducing DIBAC 5 or 3,6-di(2-pyridyl)-1,2,4,5-tetrazine (DPTz, 12) correspondingly to the system where both 4c and 11 were present (Fig. 6b). When both 5 and 12 were added, the selective and simultaneous liberation of two drugs 8c and 13 can be complete after 11 hours (Fig. 6b). While a few examples of multi-component bio-labeling using mutually orthogonal cycloaddition pairs were reported,24a,25 this established the first example of using mutually orthogonal click-release pairs to liberate multiple bioactive molecules.
Fig. 6.
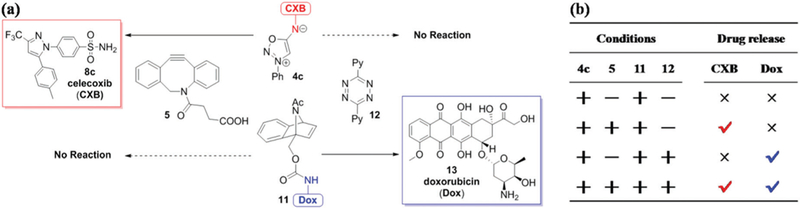
Orthogonal drug release of celecoxib and doxorubicin. (a) Mutually orthogonal click-release pairs. (b) 1H NMR analysis of drug release in DMSO-d6/D2O (9 : 1 v/v). Attendance reagent (+), or no reagent (−); release drug (√), or no drug (×).
In summary, we describe a bioorthogonal reaction of sulfonyl sydnonimines with DIBAC for effcient release of sulfonamides. The sulfonyl sydnonimine as pro-drug exhibits excellent bio-stability with low toxicity under physiological conditions, which is essential in targeted drug release. With the aid of DFT calculations, we also discovered the first mutually orthogonal click-release pairs—sydnonimine–DIBAC and tetrazine–ABNBD, which realized the liberation of sulfonamide and primary amine drugs selectively and simultaneously in one system. This method is promising for applications in dual-drug delivery and targeted therapy.
Supplementary Material
Acknowledgments
We thank Prof. Jennifer Prescher at UCI for helpful comments. This work was financially supported by the Natural Science Foundation of China (21803030), the Fundamental Research Funds for the Central Universities, the National Thousand Young Talents Program, the Jiangsu Specially-Appointed Professor Plan, and the NSF of Jiangsu Province (BK20170631) in China. K. N. H. is grateful to the National Institute of General Medical Sciences, National Institutes of Health (R01 GM109078) for support.
Footnotes
Conflicts of interest
There are no conflicts to declare.
Electronic supplementary information (ESI) available: Experimental and computational details. See DOI: 10.1039/c8cc08533a
Notes and references
- 1.(a) Supuran CT, Nat. Rev. Drug Discovery, 2008, 7, 168; [DOI] [PubMed] [Google Scholar]; (b) Supuran CT, Molecules, 2017, 22, 1642. [Google Scholar]
- 2.(a) Monti SM, Supuran CT and Simone GD, Expert Opin. Ther. Pat, 2013, 23, 737; [DOI] [PubMed] [Google Scholar]; (b) Krall N, Pretto F, Decurtins W, Bernardes GJ, Supuran CT and Neri D, Angew. Chem., Int. Ed, 2014, 53, 4231. [DOI] [PubMed] [Google Scholar]
- 3.Bernkop-Schnurch A and Jalil A, J. Controlled Release, 2018, 271, 55. [DOI] [PubMed] [Google Scholar]
- 4.(a) Ding C and Li Z, Mater. Sci. Eng., C, 2017, 76, 1440; [DOI] [PubMed] [Google Scholar]; (b) Mai BT, Fernandes S, Balakrishnan PB and Pellegrino T, Acc. Chem. Res, 2018, 51, 999. [DOI] [PubMed] [Google Scholar]
- 5.(a) Yue X, Zhang Q and Dai Z, Adv. Drug Delivery Rev, 2017, 115, 155; [DOI] [PubMed] [Google Scholar]; (b) Ruskowitz ER and DeForest CA, Nat. Rev. Mater, 2018, 3, 17087. [Google Scholar]
- 6.Hoosain FG, Choonara YE, Kumar P, Tomar LK, Tyagi C, du Toit LC and Pillay V, AAPS PharmSciTech, 2017, 18, 654. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Deng X, Ding J, Zhou W, Zheng X and Tang G, Int. J. Pharm, 2018, 535, 253. [DOI] [PubMed] [Google Scholar]
- 8.Li J and Chen PR, Nat. Chem. Biol, 2016, 12, 129. [DOI] [PubMed] [Google Scholar]
- 9.(a) Prescher JA and Bertozzi CR, Nat. Chem. Biol, 2005, 1, 13; [DOI] [PubMed] [Google Scholar]; (b) Jewett JC and Bertozzi CR, Chem. Soc. Rev, 2010, 39, 1272; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Devaraj NK and Weissleder R, Acc. Chem. Res, 2011, 44, 816; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Lang K and Chin JW, ACS Chem. Biol, 2014, 9, 16; [DOI] [PubMed] [Google Scholar]; (e) Patterson DM, Nazarova LA and Prescher JA, ACS Chem. Biol, 2014, 9, 592; [DOI] [PubMed] [Google Scholar]; (f) Lang K and Chin JW, Chem. Rev, 2014, 114, 4764; [DOI] [PubMed] [Google Scholar]; (g) Liu F, Liang Y and Houk KN, Acc. Chem. Res, 2017, 50, 2297; [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Row RD and Prescher JA, Acc. Chem. Res, 2018, 51, 1073; [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Wu H and Devaraj NK, Acc. Chem. Res, 2018, 51, 1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Versteegen RM, Rossin R, ten Hoeve W, Janssen HM and Robillard S, Angew. Chem., Int. Ed, 2013, 52, 14112; [DOI] [PubMed] [Google Scholar]; (b) Li J, Jia S and Chen PR, Nat. Chem. Biol, 2014, 10, 1003; [DOI] [PubMed] [Google Scholar]; (c) Wu H, Cisneros BT, Cole CM and Devaraj NK, J. Am. Chem. Soc, 2014, 136, 17942; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Matikonda SS, Orsi DL, Staudacher V, Jenkins IA, Fiedler F, Chen J and Gamble AB, Chem. Sci, 2015, 6, 1212; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Wu H, Alexander SC, Jin S and Devaraj NK, J. Am. Chem. Soc, 2016, 138, 11429; [DOI] [PubMed] [Google Scholar]; (f) Fan X, Ge Y, Lin F, Yang Y, Zhang G, Ngai WS, Lin Z, Zheng S, Wang J, Zhao J, Li J and Chen PR, Angew. Chem., Int. Ed, 2016, 55, 14046; [DOI] [PubMed] [Google Scholar]; (g) Mejia Oneto JM, Khan I, Seebald L and Royzen M, ACS Cent. Sci, 2016, 2, 476; [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Rossin R, van Duijnhoven SM, ten Hoeve W, Janssen HM, Kleijn LH, Hoeben FJ, Versteegen RM and Robillard MS, Bioconjugate Chem, 2016, 27, 1697; [DOI] [PubMed] [Google Scholar]; (i) Xu M, Tu J and Franzini RM, Chem. Commun, 2017, 53, 6271; [DOI] [PubMed] [Google Scholar]; (j) Rossin R, Versteegen RM, Wu J, Khasanov A, Wessels HJ, Steenbergen EJ, ten Hoeve W, Janssen HM, van Onzen A, Hudson PJ and Robillard MS, Nat. Commun, 2018, 9, 1484; [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Tu J, Xu M, Parvez S, Peterson RT and Franzini RM, J. Am. Chem. Soc, 2018, 140, 8410. [DOI] [PubMed] [Google Scholar]
- 11.Carlson JCT, Mikula H and Weissleder R, J. Am. Chem. Soc,2018, 140, 3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolodych S, Rasolofonjatovo E, Chaumontet M, Nevers MC, Creminon C and Taran F, Angew. Chem., Int. Ed, 2013, 52, 12056. [DOI] [PubMed] [Google Scholar]
- 13.Decuypere E, Plougastel L, Audisio D and Taran F, Chem. Com-mun, 2017, 53, 11515. [DOI] [PubMed] [Google Scholar]
- 14.(a) Bernard S, Audisio D, Riomet M, Bregant S, Sallustrau A, Plougastel L, Decuypere E, Gabillet S, Kumar RA, Elyian J, Trinh N, Koniev O, Wagner A, Kolodych S and Taran F, Angew. Chem., Int. Ed, 2017, 56, 15612; [DOI] [PubMed] [Google Scholar]; (b) Riomet M, Decuypere E, Porte K, Bernard S, Plougastel L, Kolodych S, Audisio D and Taran F, Chem. – Eur. J, 2018, 24, 8535. [DOI] [PubMed] [Google Scholar]
- 15.Wallace S and Chin JW, Chem. Sci, 2014, 5, 1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narayanam MK, Liang Y, Houk KN and Murphy JM, Chem. Sci, 2016, 7, 1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Field L and Settlage PH, J. Am. Chem. Soc, 1954, 76, 1222. [Google Scholar]
- 18.(a) Zhao Y and Truhlar DG, Theor. Chem. Acc, 2008, 120, 215; [Google Scholar]; (b) Zhao Y and Truhlar DG, Acc. Chem. Res, 2008, 41, 157; [DOI] [PubMed] [Google Scholar]; (c) de la Concepci´on JG, A´valos M, Cintas P and Jim´enez JL, Chem. – Eur. J, 2018, 24, 7507;29534312 [Google Scholar]; (d) Tao H, Liu F, Zeng R, Shao Z, Zou L, Cao Y, Murphy JM, Houk KN and Liang Y, Chem. Commun, 2018, 54, 5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beal EN and Tumbull K, Synth. Commun, 1992, 22, 673. [Google Scholar]
- 20.Daeniker HU and Druey J, Helv. Chim. Acta, 1962, 45, 2462. [Google Scholar]
- 21.Introducing an ester group at the para position of the N-phenyl group of sydnonimine can enhance the reaction rate by about 3 fold. For details, see ESI†.
- 22.Penning TD, Talley JJ, Bertenshaw SR, Carter JS,Collins PW, Docter S, Graneto MJ, Lee LF, Malecha JW, Miyashiro JM, Rogers RS, Rogier DJ, Yu SS, Anderson GD,Burton EG, Cogburn JN, Gregory SA, Koboldt CM, Perkins WE, Seibert K, Veenhuizen AW, Zhang YY and Isakson PC, J. Med. Chem, 1997, 40, 1347. [DOI] [PubMed] [Google Scholar]
- 23.Lu XY, Wang ZC, Ren SZ, Shen FQ, Man RJ and Zhu HL,Bioorg. Med. Chem. Lett, 2016, 26, 3491. [DOI] [PubMed] [Google Scholar]
- 24.(a) Karver MR, Weissleder R and Hilderbrand SA, Angew. Chem., Int. Ed, 2012, 51, 920; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liang Y, Mackey JL, Lopez SA, Liu F and Houk KN, J. Am. Chem. Soc, 2012, 134, 17904. [DOI] [PubMed] [Google Scholar]
- 25.(a) Willems LI, Li N, Florea BI, Ruben M, van der Marel GA and Overkleeft HS, Angew. Chem., Int. Ed, 2012, 51, 4431; [DOI] [PubMed] [Google Scholar]; (b) Patterson DM, Nazarova LA, Xie B, Kamber DN and Prescher JA, J. Am. Chem. Soc, 2012, 134, 18638; [DOI] [PubMed] [Google Scholar]; (c) Cole C, Yang J, ˇSeˇckut˙e J and Devaraj NK, ChemBioChem, 2013, 14, 205; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Patterson DM, Jones KA and Prescher JA, Mol. BioSyst, 2014, 10, 1693; [DOI] [PubMed] [Google Scholar]; (e) Sachdeva A, Wang K, Elliott T and Chin JW, J. Am. Chem. Soc, 2014, 136, 7785; [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Nikic I, Plass T, Schraidt O, Szymanski J, Briggs JAG, Schultz C and Lemke EA, Angew. Chem., Int. Ed, 2014, 53, 2245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


