Abstract
This randomized, double-blind, placebo-controlled Phase 2 clinical trial explored NorLeu3-A(1–7) (DSC127) safety and healing efficacy in diabetic foot ulcers. Patients with chronic, non-infected, neuropathic or neuroischemic plantar Wagner Grade 1 or 2 foot ulcers (n=172) were screened for non-healing. Subjects were randomized to receive four weeks’ once-daily topical treatment with 0.03%DSC127 (n=26), 0.01%DSC127 (n=27), or Placebo (n=24), followed by 20 weeks’ Standard of Care. DSC127 was assessed for safety (including laboratory values and Adverse Events), primary efficacy (% ulcers completely epithelialized at Week 12), and durability of effect. Baseline, demography, and safety parameters were compared between ITT groups and were comparable. Dose-response curves for DSC127 effect on % area reduction from baseline at Weeks 12 (40% Placebo; 67% 0.01%DSC127; 80% 0.03%DSC127) and 24 (23% Placebo; 53% 0.01%DSC127; 95% 0.03%DSC127) followed a log-linear pattern for both ITT and PP populations. Covariate analysis compared reduction in ulcer area, depth, and volume from baseline; reductions in the 0.03%DSC127 group were greater at Weeks 12 and 24. Placebo treated ulcers healed in a median 22 weeks vs. 8.5 weeks for 0.03%DSC127 (p=0.04). This study provides preliminary evidence that DSC127 is safe and effective in accelerating the healing of diabetic foot ulcers.
Keywords: diabetes, foot ulcers, wound healing, DSC127, NorLeu3-Angiotensin 1–7
INTRODUCTION
One of the most common complications for a person with diabetes is foot ulceration (1). Part of the syndrome that may contribute to diabetic ulcers includes ischemia and loss of protective sensation (neuropathy). Because these patients suffer from neuropathy in their extremities, resulting in prolonged pressure on weight-bearing tissues, ulcers that form are often not detected by the patient until they are large or chronically infected. Infections of diabetic foot ulcers (DFU) are one of the leading causes of hospitalization and morbidity in these patients. Delayed healing of DFU is the primary pathophysiological factor leading to amputation and death. Furthermore, the cost of DFU treatment exceeds $2 billion in the United States annually. Thus, there is a need for therapy that accelerates the closure of these ulcers (2–4).
Conventional procedures to treat DFU involve protecting the wounds with off-loading devices and dressings, and using antibiotics to prevent infection. Debridement of the wounds’ necrotic edge is also used to speed the natural healing process (5). The only pharmacological agent currently approved and indicated for treatment of lower extremity diabetic neuropathic ulcers is the human recombinant platelet-derived growth factor in a carboxymethyl cellulose gel, becaplermin (Regranex ®, Ortho-McNeil-Janssen Pharmaceutical, Inc.) (6).
Two active peptides of the renin-angiotensin system (RAS), angiotensin II (AII) and angiotensin 1–7 (A(1–7)), have been shown to accelerate the healing of dermal injuries (7–11). AII is well known to stimulate increases in blood pressure; in contrast A(1–7) does not elevate blood pressure. AII stimulates wound repair after full-thickness excision in normal rats, in oxorubicin-or steroid-treated rats (7), in diabetic mice (12), after partial-thickness thermal injuries in guinea pigs (8), and after random flap injuries in rats (11).
A(1–7) was shown to be comparable to or better than AII in the acceleration of wound healing (9, 12–14). Animal testing of an analog of A(1–7), NorLeu3-A(1–7) (active pharmaceutical ingredient of DSC127, DermaSciences, Princeton, NJ), demonstrated accelerated wound healing compared to (1–7) and becaplermin (12,13,15). DSC127 delivered topically in a mucoadherant, viscoelastic vehicle was effective in re-epithelializing full-thickness wounds in normal rats and diabetic mice. Epidermal regrowth was seen as early as four days after treatment initiation. In Db/db mice (mice that are obese and diabetic due to hyperphagia following truncation of the leptin receptor) treated daily for 18 days with topical NorLeu3-A(1–7), full healing occurred in 60% of dorsal full-thickness excisions, as compared with 0% in groups treated with becaplermin or placebo (13). Histological examination showed that NorLeu3-A(1–7) accelerated collagen deposition six-fold and increased the number of blood vessels at the wound site three-fold (12). At later time points, the collagen displayed a “basket-weave” organization consistent with a regenerative process. As a result, a development program for the indication of increasing the healing of diabetic foot ulcers was initiated. This paper reports results of the Phase 2 study.
MATERIALS AND METHODS
This double-blind Phase 2 clinical study evaluated the safety, tolerance, and preliminary dose response for effectiveness of topical DSC127 to expedite closure of plantar (on the midfoot or forefoot, including the toes but excluding the heel), non-healing, chronic (1–10 month duration) neuropathic or neuroischemic, 1–6 cm2 wound area, Wagner Grade 1 or 2 foot ulcers (16) (partial- or full-thickness but not involving bone, tendon or capsule, with no sign of infection or osteomyelitis) (17,18) in subjects with Type 1 or 2 diabetes. If more than one ulcer was present that met the inclusion criteria, the larger was studied and treated according to the protocol. Non-study ulcers were treated according to institutional best practice.
Study eligibility was determined from a set of predetermined inclusion and exclusion criteria. Subjects were included as neuropathic if the ratio of ankle-to-brachial systolic blood pressure (ABI) was at least 0.8. Ulcers were considered to be neuroischemic if ABI was ≥ 0.7, or if TcPO2 in the skin surrounding the target DFU exceeded 40 mmHg, or if the same-foot great toe systolic pressure exceeded 50 mmHg. The baseline level of neuropathy of the foot was assessed using the Semmes-Weinstein filaments. Patients were considered to have site specific neuropathy sufficient for loss of protective sensation (LOPS) if they were unable to feel a #5.07 monofilament applied to at least 5 of the following 7 sites on the study foot: plantar to toes and metatarsals 1, 3 and 5 (3 sites), plantar to midfoot medial and lateral (2 sites), plantar heel (1 site), dorsal distal first interspace (1 site) (19).
Subjects were randomly assigned to the treatment groups according to a computerized by-site randomization schedule to ensure equal distribution at the time of enrollment. The study was conducted at 12 sites under an IND from the U.S. Food and Drug Administration (FDA) with approval from all sites’ Institutional Review Boards prior to enrolling patients. Written informed consent was obtained from all subjects prior to treatment. The study is listed at http://clinicaltrials.gov under Identifier # NCT00796744. The study was performed in compliance with the Declaration of Helsinki.
Subjects were randomized in a 1:1:1 ratio to one of the following three treatment groups:
| Group 1: | Control Placebo Vehicle (2% Hydroxyethyl Cellulose (HEC) with 0.1% methyl paraben, 0.02% propyl paraben) |
| Group 2: | 0.03% DSC127 in Vehicle |
| Group 3: | 0.01% DSC127 in Vehicle |
Screening and Treatment
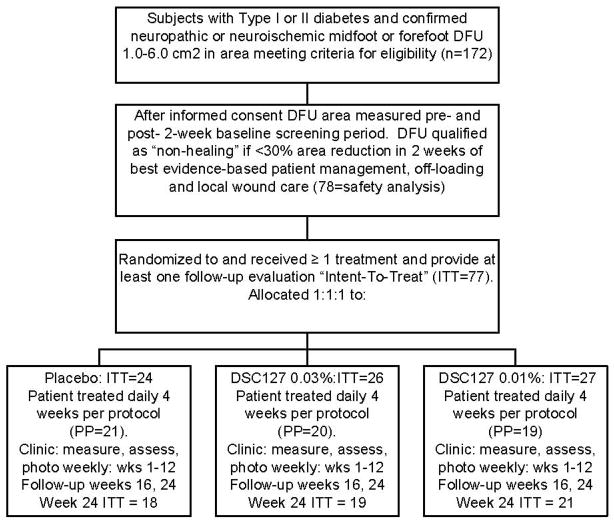
Enrolled patients experienced successive Screening, Treatment, Assessment and Durability Evaluation periods, as described in the study CONSORT Diagram (Figure 1).
Figure 1.
Consort Diagram Describing Study Subject Disposition.
Screening Period (Week −2 to Week 0): During the two-week Screening Period, the DFU was treated based on the standard of care (SOC), including debridement as determined necessary by the Investigator, dressing the wound with a transparent film, and off-loading of the affected foot with a DH Walker® removable boot (Ossur, Fotthill Ranch, CA). The wound was cleaned and dressed daily. The off-loading boot was to be worn for all routine activities. Subjects with ulcers that decreased in area by less than 30% of their baseline surface area during the Screening Period qualified as having a non-healing ulcer (20), and were enrolled into the study and randomized to treatment assignment using an electronic randomization scheme. Subjects with ulcers decreasing in area 30% or more during this period were considered to be “healing”, and therefore were not enrolled into the study (21,22).
Treatment Period (Weeks 0–4): A qualifying subject received the randomly assigned topical wound treatment applied at a thickness of about 1 mm on the first day of each of the four treatment weeks at the physician’s clinic. Thereafter, the subject self-administered the study drug daily for the remaining 6 days at home each week, for 4 weeks or until the DFU re-epithelialized, whichever occurred first. If the ulcer healed during the four weeks of treatment, treatment was stopped and the subject entered the Durability phase of the study for assessment of healing durability. Complete healing was defined as complete re-epithelialization with no drainage.
Assessment Period (Weeks 5–12): The active treatment study phase was followed by weekly observation and assessment for 8 weeks (Weeks 5–12). The ulcer was evaluated weekly; if it healed during this period, the subject then entered the Durability phase for assessment of durability. No investigational treatment was given during this Assessment Period, but all subjects received SOC dressing and off-loading.
Durability Period (Weeks 12–24): After completion of the active treatment (up to 4 weeks) and assessment period (up to study Week 12), sustained tissue integrity or absence of DFU post-healing recurrence was evaluated for all subjects at study Weeks 16 and 24 (or at 4 and 12 weeks post-healing). Off-loading and ulcer dressing requirements remained the same throughout the study.
Safety Parameters
Safety and tolerance parameters such as blood pressure changes and changes in laboratory values were evaluated at each visit. Routine standardized blood tests were performed at Screening and at Weeks 1, 3, 5, 7, 9, and 12, providing full CBC and hematology parameters during the course of the study. Primary safety outcomes were based on adverse events reportedly related to the DSC127 or placebo treatments or the off-loading modalities.
Efficacy Parameters
The primary efficacy parameter was the proportion of ulcers healed in 12 weeks, with healing defined as 100% epithelialized with no drainage. The secondary endpoints were the rate of re-epithelialization of the ulcer site, percentage of ulcers that are re-epithelialized at 3, 5, 7, 9, and 12 weeks, the time to re-epithelialization of the ulcer site, and the percent reduction from baseline surface area of the ulcer as well as depth reduction in millimeters and percent.
Assessment of the wound was performed weekly and included measurements of the wound area (for the primary efficacy endpoint). Wound dimensions (greatest length, greatest perpendicular width, and greatest depth) and ulcer tracings were taken at each visit. Tracings were subsequently evaluated for area measurement by a blinded, independent assessor using the ImageJ software program (Research Services Branch, NIH, Bethesda, MD); these results were entered into the database for analysis.
Statistical and Analytical Plans
Data management and statistical analysis were performed by McDougall Scientific Ltd. (Toronto, Canada). The results of the study were presented utilizing descriptive statistics and making comparisons between treatment groups with respect to demographic, efficacy, and safety parameters. Descriptive statistics by treatment group for continuous variables consisted of sample sizes, means, standard deviations, standard errors of least squares means, medians, and minimum and maximum values. Inferential analyses for continuous variables utilized appropriate analysis of variance (ANOVA) models. Frequencies and percentages were displayed for ordered categorical variables. Results of categorical parameters were displayed as frequencies and proportions by treatment group and were analyzed using chi-square, Mantel-Haenszel, or Fisher’s Exact Test procedures, as appropriate.
Summary statistics on demographic parameters were presented on the following parameters at baseline: age, race and ethnicity (according to FDA Guidelines), gender, height, weight, medical history, concomitant medications, vital signs, clinical laboratory parameters, and dimensions of the ulcer. Subject accounting by treatment group was displayed at each visit, and the proportion of subjects terminating prematurely for any reason, as well as due to adverse events, was compared between treatment groups. All statistical tests utilized two-sided p-values. Differences associated with p-values ≤ 0.05 were declared statistically significant, while p-values between 0.05 and 0.10 were interpreted as reflecting tendencies toward statistical significance. All analyses were conducted using SAS. Appropriate analyses of covariance or multiple regressions were performed to identify healing variables independently or jointly affecting healing.
RESULTS
Demographics
Of the 172 subjects with chronic DFU that were screened, approximately 53% did not meet the entry criteria for randomization to treatment; this included those subjects whose ulcers healed more than the 30% during this period. The safety population for this study included 78 subjects, and the relevant subject demographic and baseline characteristics of the three treatment groups are summarized in Table 1. The intent-to-treat (ITT) population comprised 77 subjects who met all criteria, received at least one treatment, and provided at least one follow-up assessment; one subject in the 0.01% DSC127 group did not receive treatment. In the ITT population, the placebo group comprised 24 subjects, the 0.01%DSC127 group 27 subjects, and the 0.03% DSC127 group 26 subjects.
Table 1.
Demographics and Baseline Characteristics (Safety Population)
| Placebo (N = 24) | 0.03% DSC127 (N = 26) | 0.01% DSC127 (N = 28) | Total (N = 78) | ||
|---|---|---|---|---|---|
| Age (yrs.) | |||||
| N | 24 | 26 | 28 | 78 | |
| Mean (SD) | 56.8 (12.77) | 56.3 (10.35) | 53.1 (12.47) | 55.3 (11.87) | |
| 95% CI | 51.4, 62.2 | 52.2, 60.5 | 48.3, 57.9 | 52.6, 58.0 | |
| Sex | |||||
| Male | 16 (66.7%) | 19 (73.1%) | 25 (89.3%) | 60 (76.9%) | |
| Female | 8 (33.3%) | 7 (26.9%) | 3 (10.7%) | 18 (23.1%) | |
| Weight (kg) | |||||
| N | 23 | 26 | 28 | 77 | |
| Mean (SD) | 113.00 (28.249) | 99.71 (23.229) | 106.65 (30.105) | 106.20 (27.549) | |
| 95% CI | 100.78, 152.22 | 90.33, 109.09 | 94.98, 118.32 | 99.95, 112.46 | |
| BMI (kg/m2) | |||||
| N | 23 | 26 | 28 | 77 | |
| Mean (SD) | 35.52 (8.387) | 33.03 (6.763) | 33.27 (7.472) | 33.86 (7.513) | |
| 95% CI | 31.89, 39.14 | 30.30, 35.77 | 30.37, 36.17 | 32.16, 35.57 | |
| Baseline Ulcer Area (cm2) | |||||
| N | 24 | 26 | 28 | 78 | |
| Mean (SD) | 1.94 (1.218) | 2.40 (1.285) | 1.91 (0.971) | 2.08 (1.166) | |
| 95% CI | 1.42, 2.45 | 1.88, 2.92 | 1.53, 2.29 | 1.82, 2.34 | |
| Race | |||||
| Hispanic/Latino | |||||
| Yes | 5 (20.8%) | 9 (34.6%) | 12 (42.9%) | 26 (33.3%) | |
| No | 19 (79.2%) | 17 (65.4%) | 16 (57.1%) | 52 (66.7%) | |
| Unknown | 0 (0.0%) | 1 (3.8%) | 0 (0.0%) | 1 (1.3%) | |
| American Indian or Alaska Native | 1 (4.2%) | 0 (0.0%) | 0 (0.0%) | 1 (1.3%) | |
| Asian | 1 (4.2%) | 0 (0.0%) | 0 (0.0%) | 1 (1.3%) | |
| White | 17 (70.8%) | 20 (76.9%) | 25 (89.3%) | 62 (79.5%) | |
| Black or African American | 4 (16.7%) | 5 (19.2%) | 3 (10.7%) | 12 (15.4%) | |
| Native Hawaiian or Other Pacific Islander | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
There were no statistically significant differences among the groups, with respect to any demographic characteristic. Of the 77 ITT subjects, 68 completed through Week 12 assessments or healed early. Of these, 58 continued study visits through Week 24 (Figure 1). Primary efficacy analysis was performed at Week 12 on the 77 subjects in the ITT and 60 subjects in the PP populations at that time point.
Safety
The proportion of subjects in each treatment group reporting adverse events was tracked continuously throughout the study and analyzed by preferred term, by body system, and by severity and relationship to study medication, as assessed by the Investigator. Adverse events were coded using MedDRA, and concomitant medications were coded using the WHO Drug Dictionary. Time points for the analysis of laboratory safety parameters were at baseline and Weeks 3, 5, 7, 9, and 12, and at the final follow-up visits (Weeks 16 and 24), where data were available to make those analyses possible. There were no significant differences among the three treatment groups, either at baseline or during the study, in any of the safety parameters at any of the time points measured.
As DSC127 contains an analog of angiotensin, blood pressure (BP) was monitored on all subjects at weekly intervals for the first 12 weeks and again at follow-up (Week 24). EKGs were performed to measure the QTc intervals at screening (for baseline), and post-first dose of treatment. There were no differences in BP readings at baseline or during the study period, and there were no differences in the QTc evaluations. In addition, subjects who were taking angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers at the time of enrollment were equally distributed among the groups and these subjects followed a similar course during the study, with no differences in adverse experiences compared to subjects not on these therapies.
Efficacy
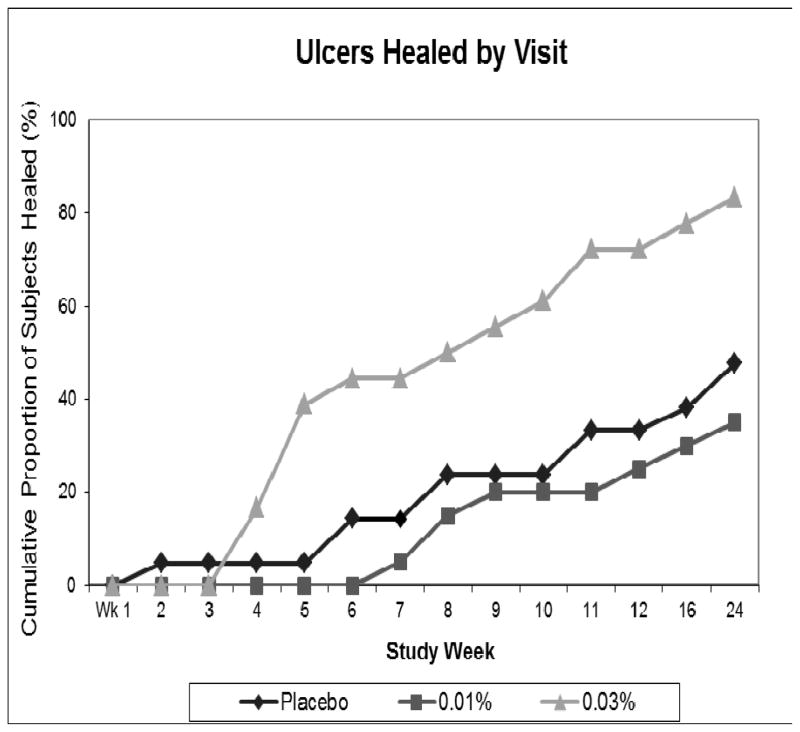
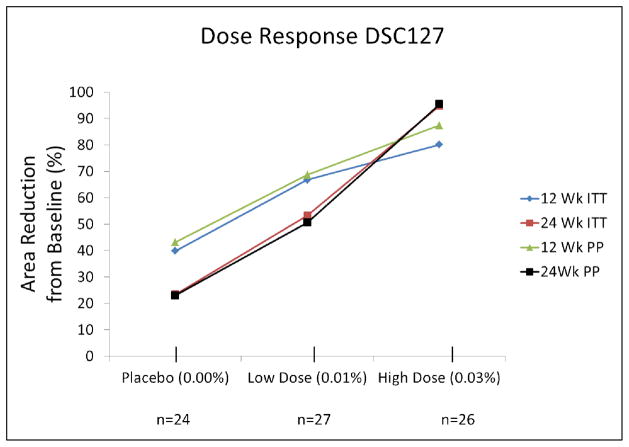
The primary efficacy endpoint was the proportion of ulcers healed by Week 12 of the study. In the ITT population, 33% in the Placebo group healed completely by Week 12, compared with 54% in the 0.03% DSC127 group (p=0.15). In the per protocol (PP) population, the proportion of Placebo-treated patients healed was 38%, compared with 65% in the 0.03% DSC127 group (p=0.09, Figure 2). After Week 3, the relative proportion of subjects healed in the 0.03% DSC127 group steadily rose above the other two groups. By Week 24, the difference in the proportion of subjects healed was statistically significant, with 46% healed in the Placebo group compared to 73% in the 0.03% DSC127 group in the ITT population (p=0.05). In the PP population, the proportions were 52% healed in the Placebo group compared with 85% in the 0.03% DSC127 group (p=0.03). A linear-log dose-response relationship was observed between DSC127 concentration and percent reduction in ulcer surface area from baseline after 12 and 24 weeks (Figure 3). By Week 24, subjects who had received the 0.03% concentration of DSC127 experienced a greater reduction in percent of baseline area than subjects who received 0.01% DSC127 or Placebo (PP p=0.019; ITT p<0.001).
Figure 2.
Cumulative proportion of ulcers that healed during the 24-week study interval for the subjects in the per protocol population that received either Placebo vehicle (n=24), 0.01% DSC127 (n=24), or 0.03% DSC127 (n=26). Subjects were followed on a weekly basis through study Week 12 (Treatment Phase) and for an additional 12 weeks (Durability Phase). Note subjects with ulcers healed before Week 12 were followed for an additional 12 weeks to assess durability of the healed ulcer. At Week 4, there was a relative increase in the proportion of ulcers healed in the 0.03% DSC127 group compared with the Placebo and 0.01% DSC127 groups.
Figure 3.
Dose-response curves for DSC127 effect on % area reduction from baseline at Weeks 12 and 24 followed a log-linear pattern similar for ITT and PP populations.
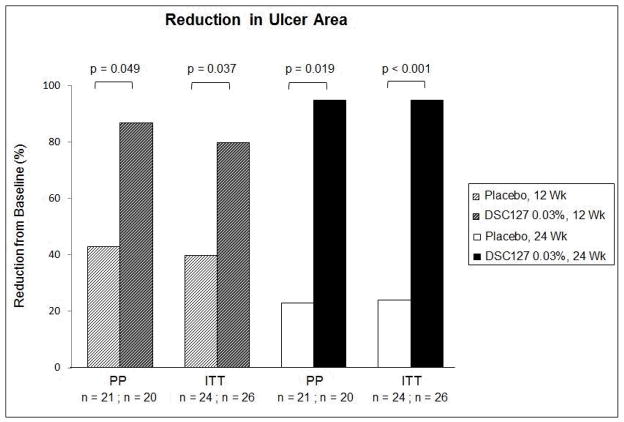
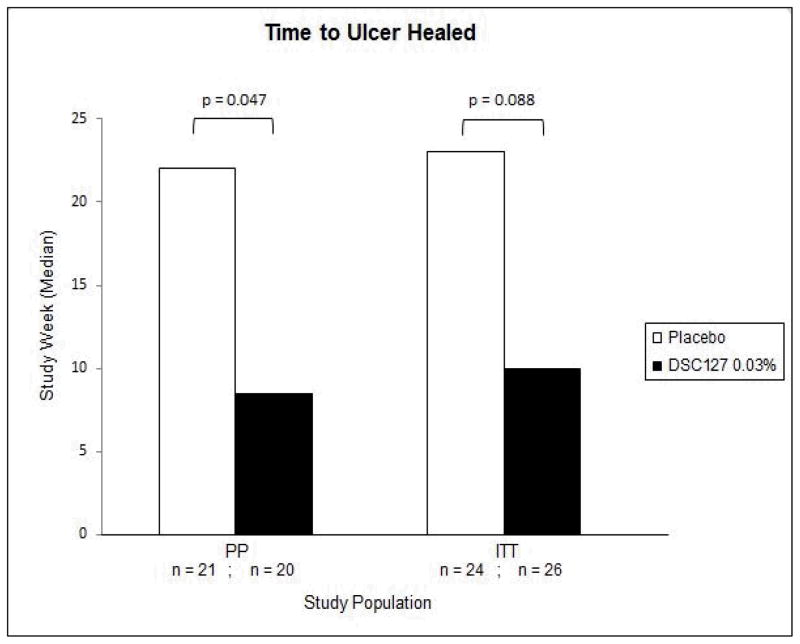
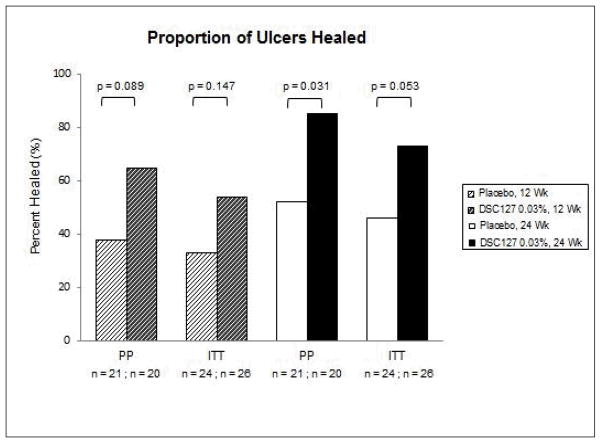
Secondary efficacy endpoints included percent area and depth reduction in the ulcer from baseline measured at 12 and 24 weeks, and the time taken to complete closure of the wound as assessed using the tracing data. Subjects treated with 0.03% DSC127 showed a statistically significant reduction in wound area compared with Placebo in both the ITT and PP populations, at both Week 12 (p=0.037, p=0.049, respectively) and Week 24 (p=0.001 for both groups; Figure 4). Depth and volume reduction paralleled these 24-week findings (p<0.05 for both ITT and PP subjects). Diabetic ulcers treated with 0.03% DSC127 healed in a median of 10 (ITT) or 8.5 (PP) weeks, compared with 23 (ITT) or 22 (PP) weeks for Placebo subjects (ITT p=0.0881; PP p=0.0471; Figure 5). The proportion of healed ulcers in both the ITT and PP populations demonstrated a greater % healed in the 0.03% DSC127 group vs. the placebo group by Week 12 (ITT p=0.147; PP p=0.089; Figure 6). At the end of the 24-week study period, the cumulative proportion of healed ulcers was significantly higher in the 0.03% DSC127 group when compared with the placebo group in the PP population (p=0.031), and the difference approached significance in the ITT population at Week 24 (p=0.053; Figure 6). There were no differences in the outcomes of secondary endpoints between the Placebo and 0.01% DSC127 groups.
Figure 4.
The 0.03% DSC127 Group had a greater reduction in ulcer area from baseline than the Placebo group at 12 and 24 weeks and in both PP and ITT populations. Some Placebo-treated ulcers enlarged between Week 12 and Week 24, while all ulcers treated with 0.03% DSC127 continued to heal. There was no difference between the 0.01% DSC127 group and the Placebo-treated control (data not shown). Analysis was conducted using a repeat measure mixed model, with treatment and visit as main effects, treatment visit interaction effect, and baseline as covariate.
Figure 5.
This figure shows the median study week that the ulcers healed for the subjects in the PP and ITT populations following treatment with either Placebo vehicle or 0.03% DSC127. There was a significant reduction in time to ulcer healing in the 0.03% DSC127 group as compared to the Placebo vehicle group for the PP population (Kaplan Meier estimated median time to heal at Week 24; p-value from the log rank test for the ITT and PP Population).
Figure 6.
Cumulative proportion of ulcers that healed during the 12-week Treatment Phase of the study for the subjects in the ITT population that received treatment with Placebo vehicle (n=24) and 0.03% DSC127 (n=26), as well as for the PP population (Placebo, n=21; 0.03% DSC127, n=20). At Week 12 there was a relative increase in the proportion of ulcers that healed in the group that received 0.03% DSC127 compared to the Placebo group for both the ITT (n=26, p=0.148) and the PP (n=20, p=0.089) populations. The cumulative proportion of ulcers that healed during the 24-week study showed a significant increase in the percent of ulcers that healed in the PP group (Placebo, 52% vs. 0.03% DSC127, 85%, p<0.05) and approached significance in the ITT population (Placebo, 45% vs. 0.03% DSC127, 73%, p=0.053). Analysis was conducted using a logistic model with treatment as a main effect.
DISCUSSION
The renin-angiotensin system is well known to play a role in dermal repair (7–9,12,13,15,23,24). Components of the RAS have been observed in skin and are modified during injury (23–28). In human skin, AT1 and AT2 receptors were found in the epidermis and in dermal vessel walls (23). Angiotensin II levels in skin were reported by Phillips to increase following wounding and may play a role in scar formation (29). Takeda hypothesized that wound healing is regulated by the balance of AT1 and AT2 receptors (30). The same expression pattern was found for angiotensinogen (the gene product from which active peptides are derived), renin, and angiotensin-converting enzyme (ACE). All RAS components were also demonstrated at the mRNA level in cultured primary keratinocytes, melanocytes (except AT2 receptors), dermal fibroblasts, and dermal microvascular endothelial cells. Recently, an upregulation of the Mas receptor, the functional receptor for A(1–7), in the skin after injury has been shown (unpublished data). Antagonism of AT1 or Mas receptors (receptors of the RAS) delays healing (13). AII was shown to accelerate dermal healing and was reported to increase survival of random skin flaps in normal rats (7,8,11). Further, A(1–7), a non-hypertensive member of the RAS and the parent to the active ingredient in DSC127, accelerated healing as well as improved survival of random flaps with nicotine-induced ischemia (9,12–14). These two active peptides of the RAS accelerate the healing of injuries, in part, through stimulation of progenitor cell proliferation and formation of granulation tissue. Further, A(1–7) may increase the rate of dermal repair by stimulating prostaglandin and release of nitric oxide.
An analog of A(1–7), NorLeu3-A(1–7) (the active ingredient of formulation DSC127) was identified and found to be more potent than A(1–7) in accelerating wound repair. Rodgers et al. demonstrated that NorLeu3-A(1–7) is effective in accelerating wound healing in a full-thickness excision wound model in diabetic mice (12,13,15). Full healing was observed in 60% of wounds of mice treated topically with NorLeu3-A(1–7); in contrast, becaplermin did not fully heal any wounds, and A(1–7) healed only 20% of the wounds. Further, administration of NorLeu3-A(1–7) reduced fibrosis and scarring in the healed wound. Based on this evidence, it was hypothesized that DSC127 (the clinical formulation of NorLeu3-A(1–7) in hydroxyethyl cellulose with parabens) may be of significant benefit in accelerating the closure of foot ulcers in diabetic subjects. Hao et al. reported an increased expression of angiotensin II as well as mRNA and protein expression of AT1 receptors in streptozotocin-induced diabetes (31). They hypothesized that the dermal RAS system was activated in these animals, and that angiotensin II receptors may mediate some of the events associated with the dermal response to streptozotocin treatment. Dermal tissue of diabetics manifests a complicated pathophysiology that renders it more susceptible to damage, including ulceration which typically becomes chronic (32).
Obtaining complete closure of a diabetic foot wound is difficult, and maintaining the integrity of the healed area is equally challenging (33). The use of off-loading devices helps the area to heal more quickly than without, and continued use of these devices after healing may help to prevent recurrence of the ulcer (34). Ulcers that are off-loaded may heal with routine care within a period of 12 weeks, if their initial healing rate increases to over 50% during 4 weeks (21). In an attempt to exclude potential study subjects who would heal with enhanced clinical care, we excluded subjects whose ulcers were on a healing trajectory, based on their capacity to heal at least 30% during the initial 2-week screening period. We included only chronic (duration 1 to 10 months) ulcers to assure that DSC127 formulations are capable of meeting the clinical challenges of these wounds.
The purpose of the current randomized, parallel-group, double-blind, placebo-controlled Phase 2 study was to evaluate the safety, tolerance, and dose response for effectiveness of topical DSC127 to expedite closure of chronic Wagner Grade 1 or 2 foot ulcers in diabetic subjects. There were no significant DSC127 effects on safety and tolerance parameters, including blood pressure changes and changes in laboratory values.
Two concentrations of DSC127 (0.01%, 0.03%) and placebo (gel vehicle without NorLeu3-A(1–7)) (n=77 total intent-to-treat [ITT] subjects) were investigated in order to show an initial dose response of DSC127 to facilitate the healing of DFU. In the Placebo group, the healing rate of 33% found in this clinical study was generally consistent with the rate reported in the literature, where 25–35% of ulcers which are off-loaded and monitored on a weekly basis were reported to heal (2,21). A similar rate of healing was seen in the 0.01% DSC127 group, at 30%. However, for the subjects who received 0.03% DSC127, 53.8% healed at 12 weeks. This increase in the proportion of subjects with healed ulcers for the ITT population is one of the largest reported in Phase 2 clinical trials. This relative increase in the proportion of subjects with healed ulcers continued through the duration of the study, achieving statistical significance by study Week 24 for both the ITT population (27% points difference between Placebo and 0.03% DSC127; p=0.05) and the PP population (33% points difference; p=0.03). Complete closure of 85% of the ulcers at Week 24 was found in the 0.03% DSC127 group of subjects who followed the protocol (PP group). The healing stimulation associated with 0.03% DSC127 persists and exceeds what one would expect from good-quality off-loading and moist wound healing.
The rate of healing of the study ulcers was improved in the treatment group, as demonstrated by both the greater reduction in the ulcer area over time and the shorter time frame in which the ulcers in the 0.03% DSC127 group reached complete healing. Wound area was significantly reduced in both the ITT and PP populations at both 12 and 24 weeks, demonstrating significant healing effect of 0.03% DSC127 over Placebo. Time to complete closure of the wounds in the ITT population was achieved in less than half the time in the treated group, when compared with Placebo (10 vs. 23 weeks, respectively). In the PP population, the time reduction was even more evident (8.5 vs. 22 weeks, respectively), and reached statistical significance (p=0.047).
In conclusion, the results of this clinical study demonstrate the preliminary safety and effectiveness of DSC127 in facilitating healing in diabetic foot ulcers. When taken together, these results warrant further investigation in pivotal clinical studies.
Acknowledgments
The authors would like to acknowledge Warren Garner, MD, Department of Surgery, Keck School of Medicine, University of Southern California; Peter Sheehan, MD, Mount Sinai School of Medicine, New York, NY; Samantha Mauro, Kainoa Peterson, Sarah Farrell, Rose Marie Cavanna-Mast, Peter Mast, Kristin Gibbons, US Biotest, San Luis Obispo, CA
The paper was supported by NIH-SBIR Grant #5R44DK076425-06 and Derma Sciences, Princeton, NJ
Footnotes
DSC127 Clinical Study Group:
David G. Armstrong, DPM, MD, PhD
University of Arizona, Tucson, Southern Arizona Limb Salvage Alliance
Peter Balingit, MD
University of California, Los Angeles – Olive View-UCLA Medical Center
Sylmar, CA
Vickie Driver, DPM
Boston University School of Medicine
Richard Galperin, DPM
Dallas, TX
Christopher Gauland, DPM
Eastern Carolina Foot & Ankle Specialists
Jason Hanft, DPM
Doctor’s Research Network, Miami, FL
Michael Molyneaux, MD
Passavant Area Hospital Wound Center
Gerit Mulder, DPM
University of California San Diego, Wound Treatment and Research Center
Alexander Reyzelman, DPM
California School of Podiatric Medicine at Samuel Merritt University, Oakland, CA
John Steinberg, DPM
Georgetown University Medical Center
Jodi Walters, DPM and Katherine Neiderer, DPM
University of Arizona, Tucson, VA Tucson
Charles Zelen, DPM FACFAS
Foot & Ankle Associates of Southwest Virginia
References
- 1.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–28. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 2.Tecilazich F, Dinh T, Veves A. Treating diabetic ulcers. Expert Opin Pharmacother. 2011;12:593–606. doi: 10.1517/14656566.2011.530658. [DOI] [PubMed] [Google Scholar]
- 3.Wrobel JS, Mayfield JA, Reiber GE. Geographic variation of lower-extremity major amputation in individuals with and without diabetes in the Medicare population. Diabetes care. 2001;24:860–4. doi: 10.2337/diacare.24.5.860. [DOI] [PubMed] [Google Scholar]
- 4.Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benhamin SM, Gregg EW, Terney EF, Rios-Burrows N, Mokdad AH, Ford ES, Imperatore G, Narayan KM. The evolving diabetes burden in the united states. Ann Intern Med. 2004;140:945–50. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong DG, Lavery LA, Nixon BP, Boulton AJM. It is not what you put on, but what you take off: techniques for debriding and offloading the diabetic foot wound. Clin Infect Dis. 2004;39:S92–9. doi: 10.1086/383269. [DOI] [PubMed] [Google Scholar]
- 6.Steed DL, Ricotta JJ, Prendergast JJ, Kaplan RJ, Webster MW, McGill JB, Schwartz SL. Promotion and Acceleration of Diabetic Ulcer Healing by Arginine-Glycine-Aspartic Acid (RGD) Peptide Matrix. RGD Study Group. Diabetes Care. 1995;18(1):39–46. doi: 10.2337/diacare.18.1.39. [DOI] [PubMed] [Google Scholar]
- 7.Rodgers K, Abiko M, Girgis W, St Amand K, Campeau J, diZerega G. Acceleration of dermal tissue repair by angiotensin II. Wound Repair Regen. 1997;5:175–83. doi: 10.1046/j.1524-475X.1997.50210.x. [DOI] [PubMed] [Google Scholar]
- 8.Rodgers KL, DeCherney AH, St Amand KM, Dougherty WR, Felix JC, Girgis WW, diZerega GS. Histologic alterations in dermal repair after thermal injury effects of topical angiotensin II. J Burn Care Rehabil. 1997;18:381–8. doi: 10.1097/00004630-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Rodgers K, Xiong S, Felix J, Roda N, Espinoza T, Maldonado S, diZerega G. Development of angiotensin (1–7) as an agent to accelerate dermal repair. Wound Repair Regen. 2001;9:238–47. doi: 10.1046/j.1524-475x.2001.00238.x. [DOI] [PubMed] [Google Scholar]
- 10.Rodgers K, Xiong S, DiZerega GS. Effect of angiotensin II and angiotensin(1–7) on hematopoietic recovery after intravenous chemotherapy. Cancer Chemother Pharmacol. 2003;51:97–106. doi: 10.1007/s00280-002-0509-4. [DOI] [PubMed] [Google Scholar]
- 11.Okuyama N, Roda N, Sherman R, Guerrero A, Dougherty WR, Nguyen T, diZerega G, Rodgers K. Angiotensin II improves random-flap viability in a rat model. Ann Plast Surg. 1999;42:313–9. doi: 10.1097/00000637-199903000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Rodgers KE, Roda N, Felix JE, Espinoza T, Maldonado S, diZerega G. Histological evaluation of the effects of angiotensin peptides on wound repair in diabetic mice. Exp Dermatol. 2003;12:784–90. doi: 10.1111/j.0906-6705.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 13.Rodgers KE, Espinoza T, Felix J, Roda N, Maldonado S, diZerega G. Acceleration of healing, reduction of fibrotic scar, and normalization of tissue architecture by an angiotensin analogue, NorLeu3-A(1–7) Plast Reconstr Surg. 2003;111:1195–206. doi: 10.1097/01.PRS.0000047403.23105.66. [DOI] [PubMed] [Google Scholar]
- 14.Baykan H, Günay GK, Özyazgan İ, Soyuer I. The Effect of Angiotensin (1–7) on Survival of Random Pattern Skin Flaps With Nicotine-Induced Ischemia in Rats. Ann Plas Surg. 2011;XX:1–6. doi: 10.1097/SAP.0b013e3182069bfd. [DOI] [PubMed] [Google Scholar]
- 15.Rodgers KE, Ellefson DD, Espinoza T, Maulhardt H, Roda N, Maldonado S, diZerega GS. Fragments of Nle-angiotensin(1–7) accelerate healing in dermal models. J Pept Res. 2005;66 (1Suppl):41–7. [Google Scholar]
- 16.Ellefson DD, diZerega GS, Espinoza T, Roda N, Maldonado S, Rodgers KE. Synergistic effects of co-administration of angiotensin 1–7 and Neupogen on hematopoietic recovery in mice. Cancer Chemother Pharmacol. 2004;53(1):15–24. doi: 10.1007/s00280-003-0710-0. [DOI] [PubMed] [Google Scholar]
- 17.Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Harkless LB, Boulton AJM. A comparison of two diabetic foot ulcer classification systems. Diabetes Care. 2001;24:84–8. doi: 10.2337/diacare.24.1.84. [DOI] [PubMed] [Google Scholar]
- 18.Jude EB, Apelqvist J, Spraul M, Martini J the Silver Dressing Group. Prospective randomized controlled study of Hydrofiber® dressing containing silver or calcium alginate dressings in non-ischaemic diabetic foot ulcers. Diabetic Medicine. 2007;24:280–8. doi: 10.1111/j.1464-5491.2007.02079.x. [DOI] [PubMed] [Google Scholar]
- 19.Rodgers LC, Driver VR, Armstrong DG. Assessment of the diabetic foot. In: Krasner DL, Rodeheaver GT, Sibbald RG, editors. Chronic Wound Care: A Clinical Source Book for Healthcare Professionals. 4. Malvern PA: HMP Communications; 2007. pp. 549–56. [Google Scholar]
- 20.Food and Drug Administration. Guidance for Industry: Chronic Cutaneous Ulcer and Burn Wounds – Developing Products for Treatment. Jun, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong DG, Lavery LA, Wu S, Boulton AJ. Evaluation of removable and irremovable cast walkers in the healing of diabetic foot wounds: a randomized controlled trial. Diabetes Care. 2005;28:551–4. doi: 10.2337/diacare.28.3.551. [DOI] [PubMed] [Google Scholar]
- 22.Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care. 2003;26:1879–82. doi: 10.2337/diacare.26.6.1879. [DOI] [PubMed] [Google Scholar]
- 23.Steckelings UM, Wollschlager T, Peters J, Henz BM, Hermes B, Artuc M. Human skin: source of and target organ for angiotensin II. Exp Dermatol. 2004;13:148–54. doi: 10.1111/j.0906-6705.2004.0139.x. [DOI] [PubMed] [Google Scholar]
- 24.Steckelings UM, Henz BM, Wiehstutz S, Unger T, Artuc M. Differential expression of angiotensin receptors in human cutaneous wound healing. Br J Dermatol. 2005;153:887–93. doi: 10.1111/j.1365-2133.2005.06806.x. [DOI] [PubMed] [Google Scholar]
- 25.Abiko M, Rodgers KE, Campeau JD, Nakamura RM, diZerega GS. Alterations of angiotensin II Receptor levels in sutured wounds in rat skin. J Invest Surg. 1996;9:447–53. doi: 10.3109/08941939609025862. [DOI] [PubMed] [Google Scholar]
- 26.Abiko M, Rodgers KE, Campeau JD, Nakamura RM, diZerega GS. Alterations of angiotensin II receptor levels in full-thickness excisional wounds in rat skin. Wound Repair Regen. 1996;4:363–7. doi: 10.1046/j.1524-475X.1996.40313.x. [DOI] [PubMed] [Google Scholar]
- 27.Kimura B, Sumners C, Phillips MI. Changes in skin angiotensin II receptors in rats during wound healing. Biochem Biophys Res Commun. 1992;187:1083–90. doi: 10.1016/0006-291x(92)91308-d. [DOI] [PubMed] [Google Scholar]
- 28.Viswanathan M, Saavedra JM. Expression of angiotensin II AT2 receptors in the rat skin during experimental wound healing. Peptides. 1992;13:783–6. doi: 10.1016/0196-9781(92)90187-8. [DOI] [PubMed] [Google Scholar]
- 29.Phillips MI, Kimura B, Gyruko R. Angiotensin receptor stimulation of transforming growth factor-β in rat skin and wound healing. In: Saavedra JM, Timmermans PBMWM, editors. Angiotensin receptors. New York: Plenum Press; 1994. pp. 377–96. [Google Scholar]
- 30.Takeda H, Katagata Y, Hozumi Y, Kondo S. Effects of Angiotensin II Receptor Signaling during Skin Wound Healing. Am J Pathol. 2004;165:1653–62. doi: 10.1016/S0002-9440(10)63422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao S, Ren M, Yang C, Lin D, Chen L, Zhu P, Cheng H, Yan L. Activation of skin renin-angiotensin system in diabetic rats. Endocr. 2011;39:242–50. doi: 10.1007/s12020-010-9428-z. [DOI] [PubMed] [Google Scholar]
- 32.Urbancic-Rovan V. Causes of diabetic foot lesions. Lancet. 2005;366:1675–6. doi: 10.1016/S0140-6736(05)67673-8. [DOI] [PubMed] [Google Scholar]
- 33.Wu SC, Jensen JL, Weber AK, Robinson DE, Armstrong DG. Use of pressure offloading devices in diabetic foot ulcers: do we practice what we preach? Diabetes Care. 2008;31(11):2118–9. doi: 10.2337/dc08-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uccioli L, Toffolo M, Volpe A, Pasqualitto P, Ferri A, Monticone G, Menzinger G. Efficacy of different shoes and insoles in reducing plantar pressures in diabetic neuropathic patients (Abstract) Diabetologia. 1997;40(1 Suppl):A-489. [Google Scholar]