Abstract
In the present review we summarize observations to date supporting the concept that neuroactive steroids are synthesized in the peripheral nervous system, regulate the physiology of peripheral nerves and exert notable neuroprotective actions. Indeed, neuroactive steroids have been recently proposed as therapies for different types of peripheral neuropathy, like for instance those occurring during aging, chemotherapy, physical injury and diabetes. Moreover, pharmacological tools able to increase the synthesis of neuroactive steroids might represent new interesting therapeutic strategy to be applied in case of peripheral neuropathy.
Keywords: Progesterone, Testosterone, Metabolism, Peripheral neuropathy, Steroidogenesis, Neuroprotection
1. Introduction
Neuroactive steroids are molecules acting in the nervous system including steroids produced by the nervous system (i.e., neurosteroids) and hormonal steroids coming from classical steroidogenic tissues (i.e., gonads and adrenal glands) [1]. Several reviews have extensively considered and discussed this topic in the central nervous system (CNS), because the first observations were obtained in the brain [2–7]. However, more recent results have indicated that the peripheral nervous system (PNS) also synthesizes and metabolizes neuroactive steroids and is a target for these molecules. Indeed, neuroactive steroids exert key physiological roles in the PNS acting on the glial [8–16] and neuronal compartments [17–19]. On this basis, new therapeutic strategies based on neuroactive steroids have been recently proposed for peripheral neuropathy [10,20]. Here, we review the state of the art on the synthesis, actions and therapeutic implications of neuroactive steroids in the PNS.
2. Synthesis of neuroactive steroids
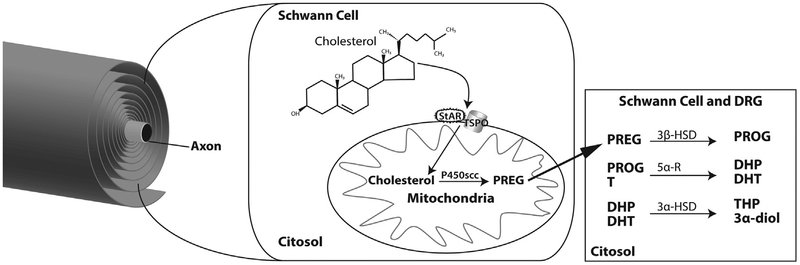
The first step of steroidogenesis is the transport of cholesterol from intracellular stores to the inner mitochondrial membrane, where cytochrome P450 side chain cleavage (P450scc), the enzyme that converts cholesterol to pregnenolone (PREG), is located (Fig. 1). This transport is facilitated by translocator protein-18 kDa (TSPO) and steroidogenic acute regulatory protein (StAR). The machinery of this first step of steroidogenesis (i.e., P450scc, TSPO and StAR) is present in Schwann cells [21,22]. Moreover, Schwann cells as well as neurons in dorsal root ganglia (DRG) are capable of converting PREG further to neuroactive steroids (Fig. 1). Indeed, Schwann cells and DRG neurons express steroidogenic enzymes such as (i) 3β-hydroxysteroid dehydrogenase, which converts PREG into progesterone (PROG) [18,19,23–27]; (ii) 5α-reductase (5α-R) type 1, which converts PROG and testosterone (T) into dihydroprogesterone (DHP) and dihydrotestosterone (DHT) respectively and (iii) 3α-hydroxysteroid dehydrogenase, which converts DHP and DHT into tetrahydroprogesterone (THP) and 5α-androstane-3α, 17β-diol (3α-diol) respectively [1,24,28–31].
Fig. 1.
Synthesis and metabolism of neuroactive steroids in the PNS. Further details are provided in the text. DRG, dorsal root ganglia; StAR, steroidogenic acute regulatory protein; TSPO, translocator protein-18 kDa; PREG, pregnenolone; PROG, progesterone; T, testosterone; DHP, dihydroprogesterone; DHT, dihydrotestosterone; THP, tetrahydroprogesterone; 3α-diol, 5α-androstane-3α, 17β-diol; P450scc, cytochrome P450 side chain cleavage; 5α-R, 5α-reductase; 3β-HSD, 3β-hydroxysteroid dehydrogenase.
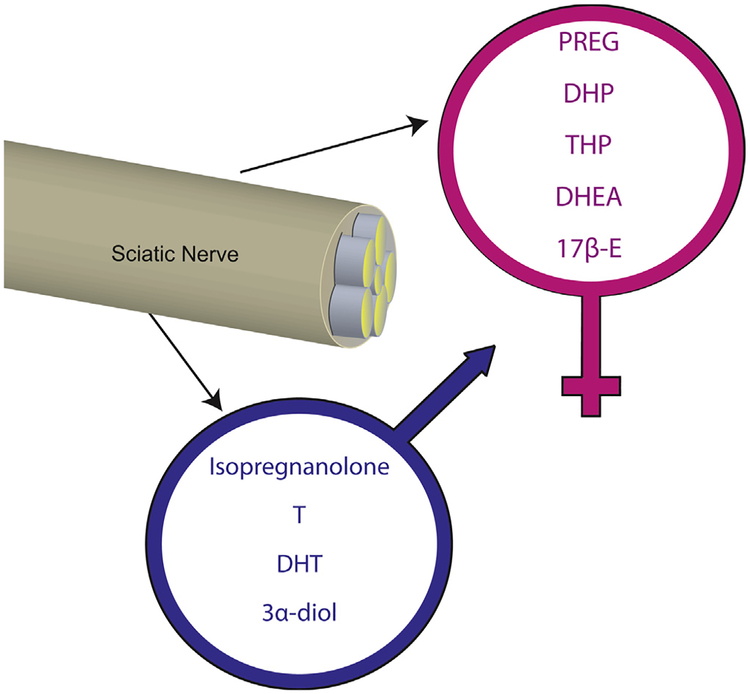
Further evidence of the steroidogenic activity of the PNS is provided by the analysis of neuroactive steroid levels by liquid chromatography tandem mass spectrometry. Indeed, PREG, PROG and its derivatives (i.e., DHP, THP and isopregnanolone), dehydroepiandrosterone (DHEA), T and its derivatives (i.e., DHT and 3α-diol) and 17β-estradiol (17β-E) are measurable in the sciatic nerve of rats [32–35]. Interestingly, the levels of neuroactive steroids are different in males and females (Fig. 2), with females having higher PREG, DHP, THP, DHEA and 17β-E levels, and males having higher levels of isopregnanolone, T, DHT and 3α-diol [36–39].
Fig. 2.
Neuroactive steroid levels in rat sciatic nerve show sexual dimorphism. Further details are provided in the text. PREG, pregnenolone; DHP, dihydroprogesterone; THP, tetrahydroprogesterone; DHEA, dehydroepiandrosterone; 17β-E, 17β-estradiol; T, testosterone; DHT, dihydrotestosterone; 3α-diol, 5α-androstane-3α, 17β-diol.
Thus, PNS express steroidogenic capability as well as the presence of consistent in situ amounts of neuroactive steroids.
3. The PNS as a physiological target of neuroactive steroids
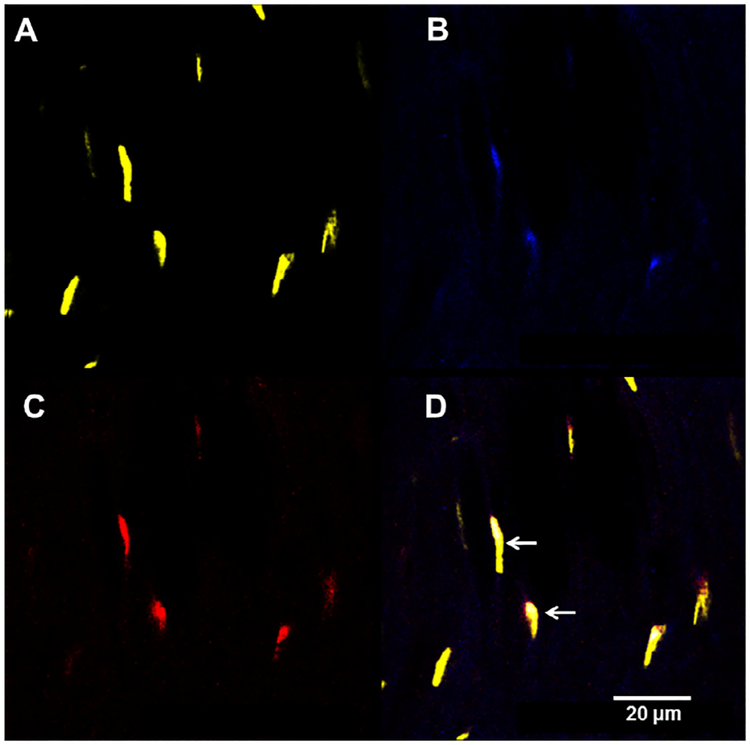
PNS is not only able to synthesize and metabolize neuroactive steroids but it is also a target for their effects. Neuroactive steroids may exert their effects by classical steroid receptors as well as non-classical steroid receptors. Indeed, classical intracellular steroid receptors, such as PROG (PR), androgen (AR), estrogen, glucocorticoid and mineralocorticoid receptors, which bind PROG, DHP, T, DHT, DHEA, estrogens and corticosteroids, have been detected in the glial (i.e., Schwann cells) and neuronal (i.e., DRG) compartments of the PNS [40–47]. Moreover, non-classical steroid receptors, such as progesterone receptor membrane component 1 (PGRMC1), GABA-A, GABA-B, NMDA, AMPA and kainate subunits, as well as sigma 1 receptor are also expressed by the different cellular components of the PNS [42,48–52]. Therefore, neuroactive steroids may regulate PNS physiology through different signaling pathways. Among the physiological effects of neuroactive steroids in the PNS, the regulation of the myelination program has been investigated extensively. For example, an important myelin protein, such as glycoprotein zero (P0) is a target of the action of PROG and its derivatives (i.e., DHP and THP) as well as of T derivatives (i.e., DHT and 3α-diol) [11,16,53,54]. Another myelin protein, the peripheral myelin protein 22 (PMP22) is under the control of THP and 3α-diol [11,16,53,54]. These physiological effects are mediated by activation of classical or non-classical steroid receptors. Observations to date indicate that the expression of P0 is under the control of classical steroid receptors, such as PR and AR, while that of PMP22, is under the control of a non-classical steroid receptor, such as GABA-A receptor [11]. A classical steroid genomic effect on P0 is supported by the presence of putative progesterone responsive elements on the P0 gene [53]. In further support of a classic genomic mechanism, steroid receptor coactivator (SRC)-1, a member of the p160 family of nuclear receptor coactivators [55], is involved in the control of P0 expression [56]. In further support of PR functioning with nuclear receptor coactivators, cells of the sciatic nerve of female rats co-express PR and SRC-2, another member of the p160 family (Fig. 3).
Fig. 3.
Sciatic nerve cells coexpress progesterone receptor (PR) and steroid receptor coactivator-2 (SRC-2) in female rats. Sciatic nerve from ovariectomized rats treated with estradiol benzoate (10 μg, sc) were immunostained for (A) nucleic acids (DAPI), (B) PR, (C) SRC-2 and (D) merged. White arrows in D point to nuclei of cells that coexpress PR and SRC-2.
P0 and PMP22 play an important role for the maintenance of the multilamellar structure of PNS myelin [57]. Therefore, consistent with the effects exerted on the proteins of peripheral myelin, PROG stimulates the synthesis of myelin membranes accelerating the time of initiation and enhancing the rate of myelin synthesis in Schwann cells co-cultured with DRG neurons [19,58]. Moreover, neuroactive steroids, such as PROG or its metabolites, DHP and THP, stimulate the gene expression of transcription factors with key role in Schwann cells physiology and their myelinating program, such as Krox-20, Krox-24, Egr-3, FosB, and Sox-10 [9,13,59].
PROG also exerts effects on the neuronal compartment. Indeed, in co-cultures of Schwann cells and DRG neurons this neuroactive steroid stimulates the expression of a small Ras-like GTP-binding protein (Rap 1b) and of phosphoribosyl diphosphate synthase-associated protein, that are two neuronal molecules involved in the myelination process [18,19]. Moreover, PROG also affects axonal outgrowth in DRG neurons. For instance, this neuroactive steroid is able to induce morphological changes, especially in the neuronal growth cones, associated with a rapid reorganization of actin filaments [42]. In agreement, the blockade of PR with the antagonist mifepristone, during development results in axonal impairment in the sciatic nerve of male rats [17].
4. Levels of neuroactive steroids are affected in peripheral neuropathy
Peripheral neuropathy is one of the most common disorders with a prevalence of about 2.4% that rises with aging to 8% in the general population [60]. Different types of peripheral neuropathy have been described. They may be inherited (e.g., Charcot–Marie–Tooth disease including demyelinating and axonal variants) or acquired, such as those occurring during aging process, after physical injury, in diabetes mellitus, vitamin deficiencies, alcoholism, kidney failure, cancer, in infections and autoimmune disorders (e.g., AIDS, hepatitis, Guillain–Barré syndrome, Lyme disease, rheumatoid arthritis, leprosy, sarcoidosis, syphilis, systemic lupus erythematosus, etc.), after exposure to toxic compounds and during drug treatment (e.g., chemotherapeutic, antiretroviral, anti-tuberculosis medications, antimicrobial drugs, lithium, etc.).
Data so far obtained indicate that the levels of several neuroactive steroids are affected in peripheral neuropathy. For instance, in an experimental model of crush injury, the levels of PREG, DHP and THP present in the distal portion of injured sciatic nerve were lowered [61]. Changes in the levels of neuroactive steroids have also been reported in an experimental model of Charcot–Marie–Tooth type 1 (CMT1A) [33] and in experimental diabetic neuropathy [35,36]. Interestingly, in these experimental models the levels of neuroactive steroids were changed in a sex-dimorphic manner by the pathology. Indeed, as demonstrated in the sciatic nerve of male and female PMP22 transgenic rats (i.e., an experimental model of CMT1A), the levels of 3α-diol were strongly decreased in males and those of isopregnanolone were strongly decreased in females [33]. In the sciatic nerve of streptozotocin (STZ)-treated animals (i.e., an experimental model of diabetes inducing peripheral neuropathy), the levels of PREG, T, DHT and 3α-diol were significantly decreased in males but not in females, while those of PROG, THP and isopregnanolone were decreased only in females [36].
Taken together these results, indicating that neurodegeneration in PNS changes the levels of neuroactive steroids, suggest that these molecules may represent promising neuroprotective agents. Further support of this idea is provided by the relationship between hormonal environment and peripheral neuropathy. Indeed, ovariectomy, but not orchidectomy, significantly counteract STZ-induced alterations on different parameters of the peripheral nerves, such as nerve conduction velocity (NCV), Na+, K+-ATPase activity, and expression of P0 and PMP22 [37]. These effects of ovariectomy were associated with an increase in the levels of DHEA, T and DHT in the sciatic nerve of diabetic rats [37]. Thus, as also demonstrated in non-pathological animals, the PNS adapts its local levels of neuroactive steroids in response to castration with sex specificity and depending on the duration of the peripheral modifications [34].
A therapy based on neuroactive steroids could be extremely important because the therapeutic agents available so far for peripheral neuropathies are very limited. Indeed, as discussed below, neuroactive steroids act as protective agents in different experimental models of peripheral neuropathy.
5. Neuroactive steroids as protective agents in the PNS
5.1. Aging
Decrease in the synthesis of P0 and PMP22 and morphological changes in peripheral nerves have been reported during aging [15,20]. Treatment with PROG or its derivatives counteract these alterations [15,20,62,63]. These effects of PROG and its derivatives seem to be a peculiarity of this class of neuroactive steroids because neither T nor its derivatives were able to influence the morphological parameters analyzed in these experiments [15,20].
5.2. Physical injury
As previously mentioned, neuroactive steroids such as PROG and DHP, increase gene expression of P0 after nerve transection [64]. Moreover, PREG and PROG counteract the decrease in the amount of myelin membranes induced by a cryolesion in the sciatic nerve of mice [25]. Furthermore, in guided regeneration of the rabbit facial nerve, PROG treatment increases the number of Schwann cell nuclei, of nonmyelinated and myelinated nerve fibers (also with an increase in their diameters), as well as of the g-ratio of myelinated nerve fibers [65]. Finally, PROG or DHP treatments counteract alterations in myelin proteins and Na+,K+-ATPase pump, stimulate reelin gene expression and also counteract nociception impairment in a crush injury model [61].
Interesting results have been also obtained with other neuroactive steroids. Indeed, T and DHT, accelerate regeneration and functional recovery of injured nerves [66–70]. After rat sciatic nerve transection, DHEA reduces the extent of denervation atrophy and induces an earlier onset of axonal regeneration [71]. This neuroactive steroid and also 17β-estradiol promote a faster return to normal values of sciatic function index and increase the number of myelinated fibers and fiber diameters after nerve crush injury in rats [72] and mice [73].
5.3. Chemotherapy-induced peripheral neurotoxicity
DHP or P treatments counteract the effects of docetaxel (i.e., a semisynthetic taxane widely employed as antineoplastic agent for the treatment of breast, ovarian, and non-small cell lung cancer). Thus, neuroactive steroid treatment prevents NCV and thermal threshold changes, degeneration of skin nerves in the footpad as well as changes in gene expression of P0, PMP22, myelin and lymphocyte-associated protein and myelin basic protein [74].
5.4. Diabetic peripheral neuropathy
In STZ-treated rats, treatment with PROG or its derivatives improves alterations in NCV, P0 and PMP22, Na+,K+-ATPase activity, thermal threshold and skin innervation density [75] and counteracts the increase in the number of fibers with myelin infoldings [76]. Similar neuroprotective effects are also exerted by treatment with T or its derivatives [77] as well as by DHEA [38]. Interestingly, DHEA exerts sex-depending neuroprotective actions, with more potent effects in female animals [38]. Moreover, DHEA prevents not only neuronal but also vascular dysfunction in this experimental model [78].
Recently, it has been reported that altered levels of neuroactive steroids and morphological changes in peripheral nerves are associated not only with changes in myelin proteins but also in the lipid components. Indeed, we demonstrated that diabetes in peripheral myelin alters phospholipids, fatty acids and cholesterol content in a pattern that can modify membrane fluidity [79,80]. Interestingly, neuroactive steroids, such as DHP or 3α-diol are able to counteract these effects. In particular, these neuroactive steroids reduce myelin structural alterations, decrease the accumulation of myelin saturated fatty acids and promote desaturation [81]. Therefore these results suggest that the myelin lipid compartment can also be considered a target for the action of neuroactive steroids.
5.5. Neuropathic pain
Neuropathic pain, an important consequence of peripheral nerve damage, is also a target for the action of neuroactive steroids [82,83]. Indeed, as reported in different experimental models, T-type calcium channels, GABA-A channels, P2X3 receptors, voltage-gated sodium channels and bradykinin signaling, which exert a role in neuropathic pain, are also affected by different kinds of neuroactive steroids [84–89]. In particular, metabolites of PROG (i.e., DHP and THP) suppress neuropathic symptoms (allodynia/hyperalgesia) evoked by antineoplastic drugs such as vincristine [90] or oxaliplatin [91]. Moreover, metabolites of T have been recently demonstrated as potential agents for the treatment of diabetic neuropathic pain [92]. Indeed, DHT counteracts the effect of diabetes on mechanical nociceptive threshold, pre- and post-synaptic components, glutamate release, astrocyte immunoreactivity and expression of interleukin-1β, while its metabolite, 3α-diol, was effective on tactile allodynia threshold, glutamate release, astrocyte immunoreactivity and the expression of substance P, toll-like receptor 4, tumor necrosis factor-α, transforming growth factor β−1, interleukin-1β and TSPO [92].
6. The induction of the synthesis of neuroactive steroids as a therapeutic tool
Because a therapeutic strategy that uses exogenous neuroactive steroids could evoke endocrine side effects, an alternative strategy could be the use of pharmacological agents that increase the synthesis of endogenous neuroactive steroids directly in the peripheral nervous system. As reported in the CNS, activation of TSPO or liver X receptor (LXR) may be considered the basis for therapeutic strategy in the neurodegenerative and psychiatric field. Indeed, TSPO ligands, like for instance XBD 173 or etifoxine, increase neurosteroidogenesis and exert anxiolytic effects without causing the classical side effects (i.e., sedation or tolerance) of benzodiazepines [7]. Beneficial effects by midazolam on behavior deficits have been also reported in an experimental model of post-traumatic stress disorder [93]. Moreover, protective effects have been also reported in experimental model of multiple sclerosis [94] or Alzheimer’s disease [95]. Similarly, activation of LXR exerts protective effects in global [96] or focal cerebral ischemia [97] as well as in neurode-generative diseases, such multiple sclerosis, Alzheimer and Parkinson diseases [98]. On this basis, similar therapeutic strategies have been also applied in the PNS. For instance, treatment of STZ-induced diabetic neuropathy in rats with the TSPO ligand, Ro5–4864, increased the levels of PREG, PROG and DHT, and counteracted the impairment of NCV and thermal threshold, restored skin innervation density and P0 gene expression, and improved Na+,K+-ATPase activity [99]. This TSPO ligand was also able to exert a beneficial effect on morphological parameters of the sciatic nerve of aged male rats by increasing the total number of myelinated fibers and decreasing the percentage of fibers with myelin decompaction [100]. Moreover, another TSPO ligand, SSR180575, has been reported to increase the survival of facial nerve motoneurons after axotomy and the regeneration of peripheral nerves [101]. Furthermore, a TSPO ligand used for the treatment of anxiety disorders, etifoxine, enhances peripheral nerve regeneration and functional recovery, increases axonal growth, causes a marked reduction in the number of macrophages and improves recovery of locomotion, motor coordination and sensory functions in experimental models of peripheral nerve lesion [102,103]. As reported in an experimental model, this ligand is also able to exert a beneficial effect on neurophatic pain evoked by an antitumoral agent, such as vincristine sulphate [104].
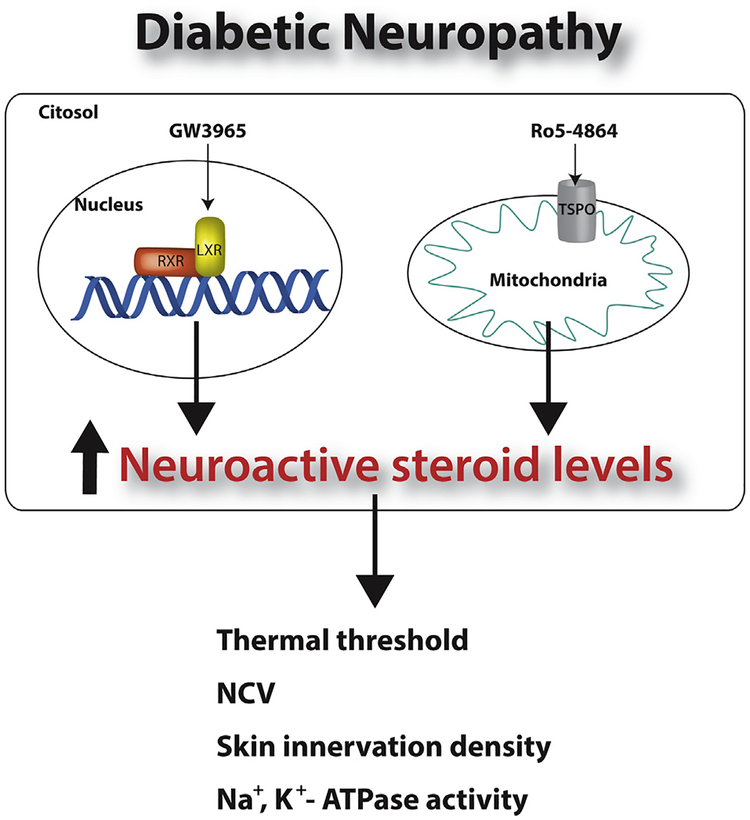
Treatment with a synthetic ligand of LXR, such as GW3965, increases the levels of PREG, PROG, DHP and 3α-diol and of molecules and enzymes involved in their synthesis, such as StAR, P450scc and 5α-R in the sciatic levels of STZ-treated animals [105]. These changes are associated with neuroprotective effects on thermal nociceptive activity, NCV and Na+,K+-ATPase activity [105]. Interestingly, LXR knock-out mice have an altered pheno-type of the myelin sheaths surrounding axons (i.e., thinner myelin sheaths), with no change in the diameter or number of axons [106], suggesting that the myelin compartment is also a target for this pharmacological tool.
On the other hand, even if these two pharmacological tools may be considered extremely promising it is also important to recall that they may also induce side-effects. For instance, it has been recently proposed that TSPO may play a role in schizophrenia susceptibility and antipsychotic-induced weight gain [107]. Moreover, an association of TSPO activation with the advancing of breast cancer has been also reported [108]. LXR activation increases triglycerides biosynthesis, an undesirable side effect for a candidate therapeutic drug. Indeed, it has been reported that in db/db mice, a model of type 2 diabetes, the synthetic LXR agonist T0901317 induced severe hepatic lipogenesis and increased plasma triglycerides [109]. In addition, studies with GW3965 and its analog SB742881 in hamster and monkey showed, unexpectedly, that these compounds increased LDL-cholesterol in the species expressing CETP. In addition Hong and colleagues demonstrated that LXR activation in monkeys induces hepatic expression of the E3 ubiquitin ligase IDOL a negative regulator of the LDL receptor thus raising plasma LDL levels [110]. This negative effect together with the hypertriglyceridemic properties are detrimental issues associated with drug discovery targeting LXR [111]. However, new ligands avoiding these side effects may represent a promising strategy for the development of novel interventions targeting LXR. In this context, it is also important to highlight that it is possible to maintain the LXR beneficial properties and avoid hepatic steatosis by changing the administration protocol of GW3965, instead of daily administration we dosed STZ-animals once a week for 4 weeks [105]. Moreover, at least in diabetic animals, activation of LXR [105] seems to be particularly interesting, because at variance to that of TSPO [99], did not induce significant changes of neuroactive steroid levels in plasma.
7. Concluding remarks
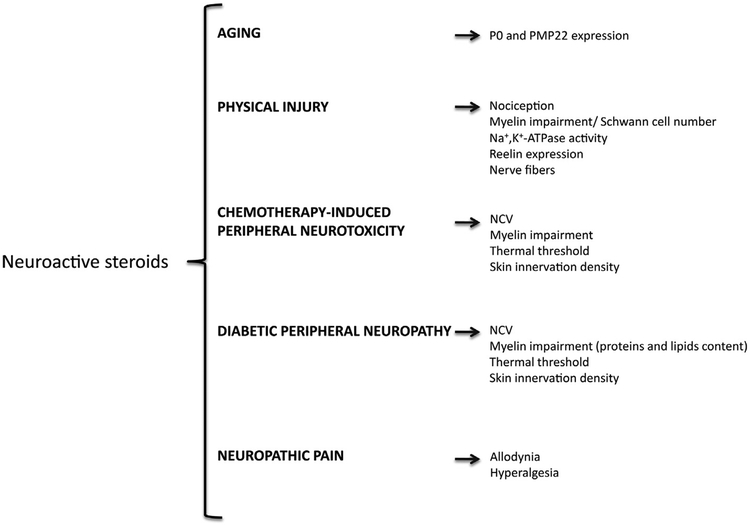
The concept that the CNS is able to produce neurosteroids and is a target for neuroactive steroids is well established and discussed in several reviews [1,5,112–115]. Here we have recapitulated this concept in the PNS, highlighting the potential efficacy of a therapeutic strategy based on administration of neuroactive steroids (Fig. 4) or pharmacological strategy that induce the synthesis of endogenous neuroactive steroids (Fig. 5) in different forms of peripheral neuropathies. Indeed, these therapeutic strategies are extremely intriguing given the many situations in which there are no effective treatments that can prevent, arrest or reverse peripheral nerve damage.
Fig. 4.
Protective effects of neuroactive steroids in healthy aging and different pathological conditions. Details are provided in the text. P0, glycoprotein zero; PMP22, peripheral myelin protein 22; NCV, nerve conduction velocity.
Fig. 5.
Pharmacological tools, able to increase neuroactive steroid levels, are able to exert protective effects in sciatic nerve of diabetic animals. LXR, liver X receptor; RXR, retinoic X receptor; TSPO, translocator protein-18 kDa; NCV, nerve conduction velocity.
Acknowledgments
The financial support of Fondazione Cariplo (Rif. 2012–0547) to R.C.M.; Ministerio de Economía y Competividad, Spain (BFU2011–30217-C03–01) to L.M.G-C; Armenise Harvard Foundation Career Development grant to N.M.; National Institutes of Health R01 DK61935 to M.J.T. are gratefully acknowledged. We thank Rebecca Muwanse and Sarah Finkelstein for their technical assistance and contribution to Fig. 3.
Abbreviations:
- 3α-diol
5α-androstane-3α, 17β-diol
- 5α-R
5α-reductase
- 17β-E
17β-estradiol
- AR
androgen receptor
- DHEA
dehydroepiandrosterone
- DHP
dihydroprogesterone
- DHT
dihydrotestosterone
- DRG
dorsal root ganglia
- P0
glycoprotein zero
- LXR
liver X receptor
- NCV
nerve conduction velocity
- P450scc
P450 side chain cleavage
- PMP22
peripheral myelin protein 22
- PNS
peripheral nervous system
- PREG
pregnenolone
- PROG
progesterone
- PR
progesterone receptor
- PGRMC1
progesterone receptor membrane component 1
- StAR
steroidogenic acute regulatory protein
- SRC-1
steroid receptor coactivator-1
- T
testosterone
- THP
tetrahydroprogesterone
- STZ
streptozotocin
- TSPO
translocator protein-18 kDa
References
- [1].Melcangi RC, Garcia-Segura LM, Mensah-Nyagan AG. Neuroactive steroids: state of the art and new perspectives. Cell Mol Life Sci 2008;65:777–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schumacher M, Sitruk-Ware R, De Nicola AF. Progesterone and progestins: neuroprotection and myelin repair. Curr Opin Pharmacol 2008;8:740–6. [DOI] [PubMed] [Google Scholar]
- [3].Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci 2005;6:565–75. [DOI] [PubMed] [Google Scholar]
- [4].Melcangi RC, Panzica GC. Neuroactive steroids and the nervous system: further observations on an incomplete tricky puzzle. J Neuroendocrinol 2013;25:957–63. [DOI] [PubMed] [Google Scholar]
- [5].Melcangi RC, Panzica G, Garcia-Segura LM. Neuroactive steroids: focus on human brain. Neuroscience 2011;191:1–5. [DOI] [PubMed] [Google Scholar]
- [6].Melcangi RC, Panzica GC. Allopregnanolone: state of the art. Prog Neurobiol 2014;113:1–5. [DOI] [PubMed] [Google Scholar]
- [7].Schule C, Nothdurfter C, Rupprecht R. The role of allopregnanolone in depression and anxiety. Prog Neurobiol 2014;113:79–87. [DOI] [PubMed] [Google Scholar]
- [8].Fex Svenningsen A, Kanje M. Estrogen and progesterone stimulate Schwann cell proliferation in a sex- and age-dependent manner. J Neurosci Res 1999;57:124–30. [DOI] [PubMed] [Google Scholar]
- [9].Mercier G, Turque N, Schumacher M. Early activation of transcription factor expression in Schwann cells by progesterone. Brain Res Mol Brain Res 2001;97:137–48. [DOI] [PubMed] [Google Scholar]
- [10].Melcangi RC, Giatti S, Pesaresi M, Calabrese D, Mitro N, Caruso D, Garcia-Segura LM. Role of neuroactive steroids in the peripheral nervous system. Front Endocrinol (Lausanne) 2011;2:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Melcangi RC, Cavarretta IT, Ballabio M, Leonelli E, Schenone A, Azcoitia I, Miguel Garcia-Segura L, Magnaghi V. Peripheral nerves: a target for the action of neuroactive steroids. Brain Res Brain Res Rev 2005;48:328–38. [DOI] [PubMed] [Google Scholar]
- [12].Lubischer JL, Bebinger DM. Regulation of terminal Schwann cell number at the adult neuromuscular junction. J Neurosci 1999;19:RC46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Guennoun R, Benmessahel Y, Delespierre B, Gouezou M, Rajkowski KM, Baulieu EE, Schumacher M. Progesterone stimulates Krox-20 gene expression in Schwann cells. Brain Res Mol Brain Res 2001;90:75–82. [DOI] [PubMed] [Google Scholar]
- [14].Desarnaud F, Do Thi AN, Brown AM, Lemke G, Suter U, Baulieu EE, Schumacher M. Progesterone stimulates the activity of the promoters of peripheral myelin protein-22 and protein zero genes in Schwann cells. J Neurochem 1998;71:1765–8. [DOI] [PubMed] [Google Scholar]
- [15].Azcoitia I, Leonelli E, Magnaghi V, Veiga S, Garcia-Segura LM, Melcangi RC. Progesterone and its derivatives dihydroprogesterone and tetrahydroprogesterone reduce myelin fiber morphological abnormalities and myelin fiber loss in the sciatic nerve of aged rats. Neurobiol Aging 2003;24:853–60. [DOI] [PubMed] [Google Scholar]
- [16].Melcangi RC, Magnaghi V, Cavarretta I, Zucchi I, Bovolin P, D’Urso D, et al. Progesterone derivatives are able to influence peripheral myelin protein 22 and P0 gene expression: possible mechanisms of action. J Neurosci Res 1999;56:349–57. [DOI] [PubMed] [Google Scholar]
- [17].Melcangi RC, Leonelli E, Magnaghi V, Gherardi G, Nobbio L, Schenone A. Mifepristone (RU 38486) influences expression of glycoprotein Po and morphological parameters at the level of rat sciatic nerve: in vivo observations. Exp Neurol 2003;184(2):930–8. [DOI] [PubMed] [Google Scholar]
- [18].Rodriguez-Waitkus PM, Lafollette AJ, Ng BK, Zhu TS, Conrad HE, Glaser M. Steroid hormone signaling between Schwann cells and neurons regulates the rate of myelin synthesis. Ann N Y Acad Sci 2003;1007:340–8. [DOI] [PubMed] [Google Scholar]
- [19].Chan JR, Rodriguez-Waitkus PM, Ng BK, Liang P, Glaser M. Progesterone synthesized by Schwann cells during myelin formation regulates neuronal gene expression. Mol Biol Cell 2000;11:2283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Melcangi RC, Azcoitia I, Ballabio M, Cavarretta I, Gonzalez LC, Leonelli E, et al. Neuroactive steroids influence peripheral myelination: a promising opportunity for preventing or treating age-dependent dysfunctions of peripheral nerves. Prog Neurobiol 2003;71:57–66. [DOI] [PubMed] [Google Scholar]
- [21].Benmessahel Y, Troadec JD, Cadepond F, Guennoun R, Hales DB, Schumacher M, et al. Downregulation of steroidogenic acute regulatory protein (StAR) gene expression by cyclic AMP in cultured Schwann cells. Glia 2004;45:213–28. [DOI] [PubMed] [Google Scholar]
- [22].Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, et al. Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 2006;27:402–9. [DOI] [PubMed] [Google Scholar]
- [23].Schumacher M, Guennoun R, Mercier G, Desarnaud F, Lacor P, Benavides J, et al. Progesterone synthesis and myelin formation in peripheral nerves. Brain Res Brain Res Rev 2001;37:343–59. [DOI] [PubMed] [Google Scholar]
- [24].Schaeffer V, Meyer L, Patte-Mensah C, Mensah-Nyagan AG. Progress in dorsal root ganglion neurosteroidogenic activity: basic evidence and pathophysiological correlation. Prog Neurobiol 2010;92:33–41. [DOI] [PubMed] [Google Scholar]
- [25].Koenig HL, Schumacher M, Ferzaz B, Thi AN, Ressouches A, Guennoun R, et al. Progesterone synthesis and myelin formation by Schwann cells. Science 1995;268:1500–3. [DOI] [PubMed] [Google Scholar]
- [26].Guennoun R, Schumacher M, Robert F, Delespierre B, Gouezou M, Eychenne B, et al. Neurosteroids: expression of functional 3beta-hydroxysteroid dehydrogenase by rat sensory neurons and Schwann cells. Eur J Neurosci 1997;9:2236–47. [DOI] [PubMed] [Google Scholar]
- [27].Coirini H, Gouezou M, Delespierre B, Liere P, Pianos A, Eychenne B, et al. Characterization and regulation of the 3beta-hydroxysteroid dehydrogenase isomerase enzyme in the rat sciatic nerve. J Neurochem 2003;84:119–26. [DOI] [PubMed] [Google Scholar]
- [28].Melcangi RC, Celotti F, Ballabio M, Poletti A, Martini L. Testosterone metabolism in peripheral nerves: presence of the 5 alpha-reductase-3 alpha-hydroxysteroid-dehydrogenase enzymatic system in the sciatic nerve of adult and aged rats. J Steroid Biochem 1990;35:145–8. [DOI] [PubMed] [Google Scholar]
- [29].Yokoi H, Tsuruo Y, Ishimura K. Steroid 5alpha-reductase type 1 immunolocalized in the rat peripheral nervous system and paraganglia. Histochem J 1998;30:731–9. [DOI] [PubMed] [Google Scholar]
- [30].Melcangi RC, Magnaghi V, Galbiati M, Martini L. Formation and effects of neuroactive steroids in the central and peripheral nervous system. Int Rev Neurobiol 2001;46:145–76. [DOI] [PubMed] [Google Scholar]
- [31].Schumacher M, Weill-Engerer S, Liere P, Robert F, Franklin RJ, Garcia-Segura LM, et al. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog Neurobiol 2003;71:3–29. [DOI] [PubMed] [Google Scholar]
- [32].Melcangi RC, Giatti S, Calabrese D, Pesaresi M, Cermenati G, Mitro N, et al. Levels and actions of progesterone and its metabolites in the nervous system during physiological and pathological conditions. Prog Neurobiol 2014;113: 56–69. [DOI] [PubMed] [Google Scholar]
- [33].Caruso D, Scurati S, Roglio I, Nobbio L, Schenone A, Melcangi RC. Neuroactive steroid levels in a transgenic rat model of CMT1A neuropathy. J Mol Neurosci 2008;34:249–53. [DOI] [PubMed] [Google Scholar]
- [34].Caruso D, Pesaresi M, Maschi O, Giatti S, Garcia-Segura LM, Melcangi RC. Effects of short- and long-term gonadectomy on neuroactive steroid levels in the central and peripheral nervous system of male and female rats. J Neuroendocrinol 2010;22:1137–47. [DOI] [PubMed] [Google Scholar]
- [35].Caruso D, Scurati S, Maschi O, De Angelis L, Roglio I, Giatti S, et al. Evaluation of neuroactive steroid levels by liquid chromatography-tandem mass spectrometry in central and peripheral nervous system: effect of diabetes. Neurochem Int 2008;52:560–8. [DOI] [PubMed] [Google Scholar]
- [36].Pesaresi M, Maschi O, Giatti S, Garcia-Segura LM, Caruso D, Melcangi RC. Sex differences in neuroactive steroid levels in the nervous system of diabetic and non-diabetic rats. Horm Behav 2010;57:46–55. [DOI] [PubMed] [Google Scholar]
- [37].Pesaresi M, Giatti S, Cavaletti G, Abbiati F, Calabrese D, Bianchi R, et al. Sex differences in the manifestation of peripheral diabetic neuropathy in gonadectomized rats: a correlation with the levels of neuroactive steroids in the sciatic nerve. Exp Neurol 2011;228:215–21. [DOI] [PubMed] [Google Scholar]
- [38].Pesaresi M, Giatti S, Cavaletti G, Abbiati F, Calabrese D, Lombardi R, et al. Sexdimorphic effects of dehydroepiandrosterone in diabetic neuropathy. Neuroscience 2011;199:401–9. [DOI] [PubMed] [Google Scholar]
- [39].Caruso D, Pesaresi M, Abbiati F, Calabrese D, Giatti S, Garcia-Segura LM, et al. Comparison of plasma and cerebrospinal fluid levels of neuroactive steroids with their brain, spinal cord and peripheral nerve levels in male and female rats. Psychoneuroendocrinology 2013;38:2278–90. [DOI] [PubMed] [Google Scholar]
- [40].Dong F, Xie W, Strong JA, Zhang JM. Mineralocorticoid receptor blocker eplerenone reduces pain behaviors in vivo and decreases excitability in small-diameter sensory neurons from local inflamed dorsal root ganglia in vitro. Anesthesiology 2012;117:1102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ye L, Xie W, Strong JA, Zhang JM. Blocking the mineralocorticoid receptor improves effectiveness of steroid treatment for low back pain in rats. Anesthesiology 2014;121:632–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Olbrich L, Wessel L, Balakrishnan-Renuka A, Boing M, Brand-Saberi B, Theiss C. Rapid impact of progesterone on the neuronal growth cone. Endocrinology 2013;154:3784–95. [DOI] [PubMed] [Google Scholar]
- [43].Luo H, Liu J, Kang D, Cui S. Ontogeny of estrogen receptor alpha, estrogen receptor beta and androgen receptor, and their co-localization with Islet-1 in the dorsal root ganglia of sheep fetuses during gestation. Histochem Cell Biol 2008;129:525–33. [DOI] [PubMed] [Google Scholar]
- [44].Melcangi RC, Magnaghi V, Galbiati M, Martini L. Glial cells: a target for steroid hormones. Prog Brain Res 2001;132:31–40. [DOI] [PubMed] [Google Scholar]
- [45].Jung-Testas I, Schumacher M, Robel P, Baulieu EE. Demonstration of progesterone receptors in rat Schwann cells. J Steroid Biochem Mol Biol 1996;58:77–82. [DOI] [PubMed] [Google Scholar]
- [46].Groyer G, Eychenne B, Girard C, Rajkowski K, Schumacher M, Cadepond F. Expression and functional state of the corticosteroid receptors and 11 beta-hydroxysteroid dehydrogenase type 2 in Schwann cells. Endocrinology 2006;147:4339–50. [DOI] [PubMed] [Google Scholar]
- [47].Jordan CL, Price RH Jr, Handa RJ. Androgen receptor messenger RNA and protein in adult rat sciatic nerve: implications for site of androgen action. J Neurosci Res 2002;69:509–18. [DOI] [PubMed] [Google Scholar]
- [48].Verkhratsky A, Steinhauser C. Ion channels in glial cells. Brain Res Brain Res Rev 2000;32:380–412. [DOI] [PubMed] [Google Scholar]
- [49].Coggeshall RE, Carlton SM. Ultrastructural analysis of NMDA, AMPA, and kainate receptors on unmyelinated and myelinated axons in the periphery. J Comp Neurol 1998;391:78–86. [DOI] [PubMed] [Google Scholar]
- [50].Dememes D, Lleixa A, Dechesne CJ. Cellular and subcellular localization of AMPA-selective glutamate receptors in the mammalian peripheral vestibular system. Brain Res 1995;671:83–94. [DOI] [PubMed] [Google Scholar]
- [51].Palacios G, Muro A, Verdu E, Pumarola M, Vela JM. Immunohistochemical localization of the sigma1 receptor in Schwann cells of rat sciatic nerve. Brain Res 2004;1007:65–70. [DOI] [PubMed] [Google Scholar]
- [52].Wessel L, Olbrich L, Brand-Saberi B, Theiss C. New aspects of progesterone interactions with the actin cytoskeleton and neurosteroidogenesis in the cerebellum and the neuronal growth cone. J Histochem Cytochem 2014;62:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Magnaghi V, Cavarretta I, Zucchi I, Susani L, Rupprecht R, Hermann B, et al. Po gene expression is modulated by androgens in the sciatic nerve of adult male rats. Brain Res Mol Brain Res 1999;70:36–44. [DOI] [PubMed] [Google Scholar]
- [54].Magnaghi V, Ballabio M, Gonzalez LC, Leonelli E, Motta M, Melcangi RC. The synthesis of glycoprotein Po and peripheral myelin protein 22 in sciatic nerve of male rats is modulated by testosterone metabolites. Brain Res Mol Brain Res 2004;126:67–73. [DOI] [PubMed] [Google Scholar]
- [55].Tetel MJ, Acharya KD. Nuclear receptor coactivators: regulators of steroid action in brain and behaviour. J Neuroendocrinol 2013;25:1209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cavarretta IT, Martini L, Motta M, Smith CL, Melcangi RC. SRC-1 is involved in the control of the gene expression of myelin protein Po. J Mol Neurosci 2004;24:217–26. [DOI] [PubMed] [Google Scholar]
- [57].D’Urso D, Brophy PJ, Staugaitis SM, Gillespie CS, Frey AB, Stempak JG, et al. Protein zero of peripheral nerve myelin: biosynthesis, membrane insertion, and evidence for homotypic interaction. Neuron 1990;4:449–60. [DOI] [PubMed] [Google Scholar]
- [58].Chan JR, Phillips 2nd LJ, Glaser M. Glucocorticoids and progestins signal the initiation and enhance the rate of myelin formation. Proc Natl Acad Sci U S A 1998;95:10459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Magnaghi V, Ballabio M, Roglio I, Melcangi RC. Progesterone derivatives increase expression of Krox-20 and Sox-10 in rat Schwann cells. J Mol Neurosci 2007;31:149–57. [DOI] [PubMed] [Google Scholar]
- [60].England JD, Asbury AK. Peripheral neuropathy. Lancet 2004;363:2151–61. [DOI] [PubMed] [Google Scholar]
- [61].Roglio I, Bianchi R, Gotti S, Scurati S, Giatti S, Pesaresi M, et al. Neuroprotective effects of dihydroprogesterone and progesterone in an experimental model of nerve crush injury. Neuroscience 2008;155:673–85. [DOI] [PubMed] [Google Scholar]
- [62].Melcangi RC, Magnaghi V, Martini L. Aging in peripheral nerves: regulation of myelin protein genes by steroid hormones. Prog Neurobiol 2000;60:291–308. [DOI] [PubMed] [Google Scholar]
- [63].Melcangi RC, Magnaghi V, Cavarretta I, Martini L, Piva F. Age-induced decrease of glycoprotein Po and myelin basic protein gene expression in the rat sciatic nerve. Repair by steroid derivatives. Neuroscience 1998;85: 569–78. [DOI] [PubMed] [Google Scholar]
- [64].Melcangi RC, Magnaghi V, Galbiati M, Ghelarducci B, Sebastiani L, Martini L. The action of steroid hormones on peripheral myelin proteins: a possible new tool for the rebuilding of myelin? J Neurocytol 2000;29:327–39. [DOI] [PubMed] [Google Scholar]
- [65].Chavez-Delgado ME, Gomez-Pinedo U, Feria-Velasco A, Huerta-Viera M, Castaneda SC, Toral FA, et al. Ultrastructural analysis of guided nerve regeneration using progesterone- and pregnenolone-loaded chitosan prostheses. J Biomed Mater Res B Appl Biomater 2005;74:589–600. [DOI] [PubMed] [Google Scholar]
- [66].Vita G, Dattola R, Girlanda P, Oteri G, Lo Presti F, Messina C. Effects of steroid hormones on muscle reinnervation after nerve crush in rabbit. Exp Neurol 1983;80:279–87. [DOI] [PubMed] [Google Scholar]
- [67].Yu WH. Effect of testosterone on the regeneration of the hypoglossal nerve in rats. Exp Neurol 1982;77:129–41. [DOI] [PubMed] [Google Scholar]
- [68].Tanzer L, Jones KJ. Neurotherapeutic action of testosterone on hamster facial nerve regeneration: temporal window of effects. Horm Behav 2004;45: 339–44. [DOI] [PubMed] [Google Scholar]
- [69].Jones KJ, Brown TJ, Damaser M. Neuroprotective effects of gonadal steroids on regenerating peripheral motoneurons. Brain Res Brain Res Rev 2001;37: 372–82. [DOI] [PubMed] [Google Scholar]
- [70].Huppenbauer CB, Tanzer L, DonCarlos LL, Jones KJ. Gonadal steroid attenuation of developing hamster facial motoneuron loss by axotomy: equal efficacy of testosterone, dihydrotestosterone, and 17-beta estradiol. J Neurosci 2005;25:4004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ayhan S, Markal N, Siemionow K, Araneo B, Siemionow M. Effect of subepineurial dehydroepiandrosterone treatment on healing of transected nerves repaired with the epineurial sleeve technique. Microsurgery 2003;23:49–55. [DOI] [PubMed] [Google Scholar]
- [72].Gudemez E, Ozer K, Cunningham B, Siemionow K, Browne E, Siemionow M. Dehydroepiandrosterone as an enhancer of functional recovery following crush injury to rat sciatic nerve. Microsurgery 2002;22:234–41. [DOI] [PubMed] [Google Scholar]
- [73].Islamov RR, Hendricks WA, Jones RJ, Lyall GJ, Spanier NS, Murashov AK. 17Beta-estradiol stimulates regeneration of sciatic nerve in female mice. Brain Res 2002;943:283–6. [DOI] [PubMed] [Google Scholar]
- [74].Roglio I, Bianchi R, Camozzi F, Carozzi V, Cervellini I, Crippa D, et al. Docetaxel-induced peripheral neuropathy: protective effects of dihydroprogesterone and progesterone in an experimental model. J Peripher Nerv Syst 2009;14:36–44. [DOI] [PubMed] [Google Scholar]
- [75].Leonelli E, Bianchi R, Cavaletti G, Caruso D, Crippa D, Garcia-Segura LM, et al. Progesterone and its derivatives are neuroprotective agents in experimental diabetic neuropathy: a multimodal analysis. Neuroscience 2007;144: 1293–304. [DOI] [PubMed] [Google Scholar]
- [76].Veiga S, Leonelli E, Beelke M, Garcia-Segura LM, Melcangi RC. Neuroactive steroids prevent peripheral myelin alterations induced by diabetes. Neurosci Lett 2006;402:150–3. [DOI] [PubMed] [Google Scholar]
- [77].Roglio I, Bianchi R, Giatti S, Cavaletti G, Caruso D, Scurati S, et al. Testosterone derivatives are neuroprotective agents in experimental diabetic neuropathy. Cell Mol Life Sci 2007;64:1158–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Yorek MA, Coppey LJ, Gellett JS, Davidson EP, Bing X, Lund DD, et al. Effect of treatment of diabetic rats with dehydroepiandrosterone on vascular and neural function. Am J Physiol Endocrinol Metab 2002;283:E1067–75. [DOI] [PubMed] [Google Scholar]
- [79].Cermenati G, Brioschi E, Abbiati F, Melcangi RC, Caruso D, Mitro N. Liver X receptors, nervous system, and lipid metabolism. J Endocrinol Invest 2013;36:435–43. [DOI] [PubMed] [Google Scholar]
- [80].Cermenati G, Abbiati F, Cermenati S, Brioschi E, Volonterio A, Cavaletti G, et al. Diabetes-induced myelin abnormalities are associated with an altered lipid pattern: protective effects of LXR activation. J Lipid Res 2012;53: 300–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Mitro N, Cermenati G, Brioschi E, Abbiati F, Audano M, Giatti S, et al. Neuroactive steroid treatment modulates myelin lipid profile in diabetic peripheral neuropathy. J Steroid Biochem Mol Biol 2014;143:115–21. [DOI] [PubMed] [Google Scholar]
- [82].Patte-Mensah C, Meyer L, Taleb O, Mensah-Nyagan AG. Potential role of allopregnanolone for a safe and effective therapy of neuropathic pain. Prog Neurobiol 2014;113:70–8. [DOI] [PubMed] [Google Scholar]
- [83].Mensah-Nyagan AG, Meyer L, Schaeffer V, Kibaly C, Patte-Mensah C. Evidence for a key role of steroids in the modulation of pain. Psychoneuroendocrinology 2009;34(Suppl 1):S169–77. [DOI] [PubMed] [Google Scholar]
- [84].Cho T, Chaban VV. Interaction between P2X3 and oestrogen receptor (ER)alpha/ERbeta in ATP-mediated calcium signalling in mice sensory neurones. J Neuroendocrinol 2012;24:789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Rowan MP, Berg KA, Milam SB, Jeske NA, Roberts JL, Hargreaves KM, et al. 17beta-estradiol rapidly enhances bradykinin signaling in primary sensory neurons in vitro and in vivo. J Pharmacol Exp Ther 2010;335:190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Todorovic SM, Pathirathna S, Brimelow BC, Jagodic MM, Ko SH, Jiang X, et al. 5beta-reduced neuroactive steroids are novel voltage-dependent blockers of T-type Ca2+ channels in rat sensory neurons in vitro and potent peripheral analgesics in vivo. Mol Pharmacol 2004;66:1223–35. [DOI] [PubMed] [Google Scholar]
- [87].Wang Q, Cao J, Hu F, Lu R, Wang J, Ding H, et al. Effects of estradiol on voltage-gated sodium channels in mouse dorsal root ganglion neurons. Brain Res 2013;1512:1–8. [DOI] [PubMed] [Google Scholar]
- [88].Lu Y, Jiang Q, Yu L, Lu ZY, Meng SP, Su D, et al. 17beta-estradiol rapidly attenuates P2X3 receptor-mediated peripheral pain signal transduction via ERalpha and GPR30. Endocrinology 2013;154:2421–33. [DOI] [PubMed] [Google Scholar]
- [89].Ayoola C, Hwang SM, Hong SJ, Rose KE, Boyd C, Bozic N, et al. Inhibition of CaV3.2 T-type calcium channels in peripheral sensory neurons contributes to analgesic properties of epipregnanolone. Psychopharmacology (Berl) 2014;231:3503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Meyer L, Patte-Mensah C, Taleb O, Mensah-Nyagan AG. Cellular and functional evidence for a protective action of neurosteroids against vincristine chemotherapy-induced painful neuropathy. Cell Mol Life Sci 2010;67:3017–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Meyer L, Patte-Mensah C, Taleb O, Mensah-Nyagan AG. Allopregnanolone prevents and suppresses oxaliplatin-evoked painful neuropathy: multi-parametric assessment and direct evidence. Pain 2011;152:170–81. [DOI] [PubMed] [Google Scholar]
- [92].Calabrese D, Giatti S, Romano S, Porretta-Serapiglia C, Bianchi R, Milanese M, et al. Diabetic neuropathic pain: a role for testosterone metabolites. J Endocrinol 2014;221:1–13. [DOI] [PubMed] [Google Scholar]
- [93].Miao YL, Guo WZ, Shi WZ, Fang WW, Liu Y, Liu J, et al. Midazolam ameliorates the behavior deficits of a rat posttraumatic stress disorder model through dual 18 kDa translocator protein and central benzodiazepine receptor and neurosteroidogenesis. PLoS One 2014;9:e101450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Daugherty DJ, Selvaraj V, Chechneva OV, Liu XB, Pleasure DE, Deng W. A TSPO ligand is protective in a mouse model of multiple sclerosis. EMBO Mol Med 2013;5:891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Barron AM, Garcia-Segura LM, Caruso D, Jayaraman A, Lee JW, Melcangi RC, et al. Ligand for translocator protein reverses pathology in a mouse model of Alzheimer’s disease. J Neurosci 2013;33:8891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Cheng O, Ostrowski RP, Liu W, Zhang JH. Activation of liver X receptor reduces global ischemic brain injury by reduction of nuclear factor-kappaB. Neuroscience 2010;166:1101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Sironi L, Mitro N, Cimino M, Gelosa P, Guerrini U, Tremoli E, et al. Treatment with LXR agonists after focal cerebral ischemia prevents brain damage. FEBS Lett 2008;582:3396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Xu P, Li D, Tang X, Bao X, Huang J, Tang Y, et al. LXR agonists: new potential therapeutic drug for neurodegenerative diseases. Mol Neurobiol 2013;48:715–28. [DOI] [PubMed] [Google Scholar]
- [99].Giatti S, Pesaresi M, Cavaletti G, Bianchi R, Carozzi V, Lombardi R, et al. Neuroprotective effects of a ligand of translocator protein-18 kDa (Ro5–4864) in experimental diabetic neuropathy. Neuroscience 2009;164:520–9. [DOI] [PubMed] [Google Scholar]
- [100].Leonelli E, Yague JG, Ballabio M, Azcoitia I, Magnaghi V, Schumacher M, et al. Ro5–4864, a synthetic ligand of peripheral benzodiazepine receptor, reduces aging-associated myelin degeneration in the sciatic nerve of male rats. Mech Ageing Dev 2005;126:1159–63. [DOI] [PubMed] [Google Scholar]
- [101].Ferzaz B, Brault E, Bourliaud G, Robert JP, Poughon G, Claustre Y, et al. SSR180575 (7-chloro-N,N,5-trimethyl-4-oxo-3-phenyl-3,5-dihydro-4H-pyridazino[4,5-b]indole-1-acetamide), a peripheral benzodiazepine receptor ligand, promotes neuronal survival and repair. J Pharmacol Exp Ther 2002;301:1067–78. [DOI] [PubMed] [Google Scholar]
- [102].Girard C, Liu S, Cadepond F, Adams D, Lacroix C, Verleye M, et al. Etifoxine improves peripheral nerve regeneration and functional recovery. Proc Natl Acad Sci U S A 2008;105:20505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Zhou X, He B, Zhu Z, He X, Zheng C, Xu J, et al. Etifoxine provides benefits in nerve repair with acellular nerve grafts. Muscle Nerve 2014;50: 235–43. [DOI] [PubMed] [Google Scholar]
- [104].Aouad M, Charlet A, Rodeau JL, Poisbeau P. Reduction and prevention of vincristine-induced neuropathic pain symptoms by the non-benzodiazepine anxiolytic etifoxine are mediated by 3alpha-reduced neurosteroids. Pain 2009;147:54–9. [DOI] [PubMed] [Google Scholar]
- [105].Cermenati G, Giatti S, Cavaletti G, Bianchi R, Maschi O, Pesaresi M, et al. Activation of the liver X receptor increases neuroactive steroid levels and protects from diabetes-induced peripheral neuropathy. J Neurosci 2010;30:11896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Makoukji J, Shackleford G, Meffre D, Grenier J, Liere P, Lobaccaro JM, et al. Interplay between LXR and Wnt/beta-catenin signaling in the negative regulation of peripheral myelin genes by oxysterols. J Neurosci 2011;31:9620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Pouget JG, Goncalves VF, Nurmi EL, Laughlin CP, Mallya KS, McCracken JT, et al. Investigation of TSPO variants in schizophrenia and antipsychotic treatment outcomes. Pharmacogenomics 2015;16:5–22. [DOI] [PubMed] [Google Scholar]
- [108].Wu X, Gallo KA. The 18-kDa translocator protein (TSPO) disrupts mammary epithelial morphogenesis and promotes breast cancer cell migration. PLoS One 2013;8:e71258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Chisholm JW, Hong J, Mills SA, Lawn RM. The LXR ligand T0901317 induces severe lipogenesis in the db/db diabetic mouse. J Lipid Res 2003;44:2039–48. [DOI] [PubMed] [Google Scholar]
- [110].Hong C, Marshall SM, McDaniel AL, Graham M, Layne JD, Cai L, et al. The LXR-Idol axis differentially regulates plasma LDL levels in primates and mice. Cell Metab 2014;20:910–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Groot PH, Pearce NJ, Yates JW, Stocker C, Sauermelch C, Doe CP, et al. Synthetic LXR agonists increase LDL in CETP species. J Lipid Res 2005;46:2182–91. [DOI] [PubMed] [Google Scholar]
- [112].Brinton RD. Neurosteroids as regenerative agents in the brain: therapeutic implications. Nat Rev Endocrinol 2013;9:241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Zheng P Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance. Prog Neurobiol 2009;89:134–52. [DOI] [PubMed] [Google Scholar]
- [114].Schumacher M, Hussain R, Gago N, Oudinet JP, Mattern C, Ghoumari AM. Progesterone synthesis in the nervous system: implications for myelination and myelin repair. Front Neurosci 2012;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Panzica GC, Balthazart J, Frye CA, Garcia-Segura LM, Herbison AE, Mensah-Nyagan AG, et al. Milestones on steroids and the nervous system: 10 years of basic and translational research. J Neuroendocrinol 2012;24:1–15. [DOI] [PubMed] [Google Scholar]