Abstract
A recent study has demonstrated that porcine spermatozoa recognize with high affinity carbohydrate structures containing Lewis X motifs. Sperm adhesion to Lewis X is proposed to mediate sperm binding to the oviduct epithelium to form a reservoir. The objective of this study was to identify Lewis X-binding proteins from porcine spermatozoa as candidate receptors for oviduct glycans. To identify low-abundance proteins typically masked by proteins originating from seminal fluid, Lewis X candidate receptors were enriched from cauda epididymal boar spermatozoa. Plasma membrane preparations from cauda epididymal spermatozoa were subjected to RP-HPLC and glycan blotting assays to isolate and detect proteins that bind Lewis X. Following bottom-up LC-MS/MS analysis, among the two bands that bound sulfated Lewis X, ADAM5, which spermatozoa, was confidently identified. ADAM family members have been established as contributors to sperm entry into the oviduct. A second sulfated Lewis X-binding protein identified was the peripheral membrane protein lactadherin (also known as P47, SED1 and MFG-E8 in different species). The interaction between Lewis X and lactadherin was functionally important because competitive inhibition by soluble recombinant lactadherin reduced sperm binding to the oviduct epithelium. Furthermore, far-western blotting demonstrated that purified lactadherin could bind oviduct cells. In summary, these findings reveal that, in addition to the previously reported glycan affinity of accessory gland proteins that adhere to spermatozoa, multiple proteins intrinsic to spermatozoa have affinity for a specific oviduct glycan. Further, in addition to binding to the zona pellucida, lactadherin is now implicated in binding to oviduct glycans to promote formation of the sperm reservoir.
Keywords: cell adhesion, glycans, lactadherin, Lewis X, oviduct, SED1
INTRODUCTION
After mating or insemination, mammalian spermatozoa pass through the utero-tubal junction and bind to the oviduct epithelium forming a reservoir (Hunter, 1984; Suarez, 1987; Smith & Yanagimachi, 1991). During sperm movement in the female reproductive tract, spermatozoa undergo several maturational processes, collectively termed capacitation, that give them the ability to fertilize an oocyte (Chang, 1984). Binding to the oviduct epithelium maintains spermatozoa in an uncapacitated state characterized by inhibition of motility and hyperactivity, thereby extending sperm lifespan prior to ovulation (Rodriguez-Martinez et al., 2005). Spermatozoa remain bound to the epithelium until close to the time of ovulation when molecules present in the oviduct fluid gradually release spermatozoa which them move toward the oocyte (Mburu et al., 1996). Even if the molecule regulating sperm release are still not completely identified (Talevi & Gualteri, 2010), it is known that by retaining and slowly releasing spermatozoa, the oviduct increases the probability that oocytes will be exposed to spermatozoa that are competent for fertilization (Rodriguez-Martinez et al., 2005). Formation of the oviduct reservoir is mediated by sperm recognition of oviduct glycan structures. Distinct glycan motifs are proposed to recognize spermatozoa in different species; sialyl, galactosyl, mannosyl and fucosyl residues competitively inhibit binding of hamster, equine, porcine and bull spermatozoa to oviduct cells (DeMott et al., 1995; Lefebvre et al., 1997; Wagner et al., 2002; Sabeur & Ball, 2007). Most studies testing sperm affinity to glycans have used competitive assays which pre-incubate spermatozoa with a small selected group of glycan structures. While this is an effective experimental approach, the limited number of glycans tested usually does not include the more complex authentic oviduct glycans. Leemans et al. (2016), has demonstrated that stallion sperm binding to oviduct cells was unchanged in the presence of several distinct carbohydrate structures (Leemans et al., 2016), including galactose that has been characterized as part of a glycan/s involved in sperm-oviduct binding in the horse (Sabeur & Ball, 2007). Although there is not an obvious explanation for the different results, testing only a very limited number of glycans risks missing specific oviduct structures recognized by spermatozoa. There are tens of thousands of possible glycan structures produced by mammals.
A recent study using an array with 377 glycans, a much larger number than has been examined previously, has demonstrated that porcine spermatozoa recognize with high affinity, carbohydrate structures containing Lewis X (LeX) or biantennary 6-sialylated N-acetyllactosamine (bi-SiaLN) motifs and that recognition of these motifs mediates porcine sperm binding to the oviduct cell aggregates (Kadirvel et al., 2012; Machado et al., 2014). Lewis X is a trisaccharide formed by the monosaccharides N-acetyl glucosamine, galactose and fucose. Although receptors for this motif have not been identified in porcine spermatozoa, binding assays to fluorescein labeled glycans have shown that spermatozoa recognize LeX motifs and that receptors are found on the plasma membrane in the anterior acrosomal region, which is the region of spermatozoa that binds oviduct cells (Silva et al., 2014). Spermatozoa from mice (Kerr et al. 2004) and humans (Pang et al., 2011) bind to LeX and sialylated LeX; there-fore, it is possible that this motif contributes to sperm binding to the oviduct in multiple species.
To understand the function of sperm binding to glycans containing LeX termini, it is essential to identify LeX receptors. In this study, a multi-dimensional strategy was employed to isolate and identify LeX-binding proteins with the objective of detecting proteins that may be involved in sperm adhesion to oviduct glycans. We chose to use epididymal spermatozoa to reduce the interference of abundant seminal plasma proteins, which do not have the glycan-binding specificity to explain porcine sperm binding to oviduct cells. We predicted that this would allow detection of less abundant proteins that may have significant biological function.
MATERIALS AND METHODS
Materials
Chemicals were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA), unless stated otherwise. Carbo-free blocking solution was purchased from Vector Laboratory (Burlingame, CA, USA). SuperSignal West Pico Chemiluminescent Substrate was purchased from Thermo Scientific (Rockford, IL, USA). Precast gel (4–20%) and Precision Plus Protein Standard Kaleidoscope Pre-stained protein markers were purchased from Bio-Rad (Hercules, CA, USA). Percoll was purchased from Pharmacia (Piscataway, NJ, USA). NP-40 was purchased from Calbiochem EMD Millipore (Billerica, MA, USA). SimplyBlue SafeStain was purchased from Life Technologies (Carlsbad, CA, USA). The BCA assay kit was purchased from Thermo Scientific. Biotinylated glycans were obtained from Lectinity (Moscow, Russia). ACS grade methanol (MeOH), ACS grade acetonitrile (ACN), Optima LC-MS grade ACN, Optima LC-MS grade water and ammonium bicarbonate were purchased from Fisher Scientific (Pittsburgh, PA, USA). Deionized water (18.2 MΩ cm) was prepared with a Milli-Q Millipore system. Formic acid (≥98%) was obtained from Fluka (Buchs, Switzerland). DL-Dithiothreitol (DTT), Protease-Max surfactant and sequencing-grade modified trypsin were purchased from Promega (Madison, WI, USA). Recombinant lactadherin protein was purchased from R&D Systems (Minneapolis, MN, USA; product 2805-MF/CF).
Collection of ejaculated and epididymal spermatozoa
Cauda epididymides were collected from Calihan Pork Processors in Peoria, IL, USA. After arrival in the laboratory, the cauda epididymis was flushed retrograde with PBS (136 mM NaCl, 2.68 mM KCl, 10.4 mM Na2HPO4 and 1.76 mM KH2PO4, pH 7.4) to collect spermatozoa. Cauda epididymal spermatozoa were separated from fluid by centrifugation (800 g for 6 min at 24 °C) and washed twice with Hepes-buffered saline (HBS; 126 mM NaCl, 5 mM KCl, 18.2 mM HEPES, pH 7.4) by centrifugation (800 g for 6 min, 24 °C). SigmaFast Protease Inhibitor Cocktail was purchased from Sigma-Aldrich Co.
Pooled semen from fertile boars was provided by Prairie State Semen Inc (Champaign, IL, USA) and washed through a Percoll cushion as previously described (Kadirvel et al., 2012).
Plasma membrane isolation from epididymal porcine spermatozoa
An enriched plasma membrane fraction was collected by nitrogen cavitation followed by a sequence of high-speed centrifugation steps, as modified from published protocols (Gillis et al., 1978; Flesch et al., 1998). Washed cauda epididymal spermatozoa were resuspended in Tris-buffered sucrose solution (TBSS; 40 mM Tris Base, 250 mM sucrose and SigmaFAST Protease Inhibitor Cocktail, pH 7.4). All subsequent media used during this procedure contained this protease inhibitor cock-tail. The sperm suspension (1 × 109/mL) was subjected to nitrogen cavitation (10 min, 50 bar) in a cell disruption bomb (Parr Instrument, Moline, IL, USA). The suspension was vortexed for 1 min and centrifuged at 1000 g for 15 min at 6 °C. After centrifugation, the supernatant was collected. The pellet was washed with 3 mL of TBSS and re-centrifuged. This procedure was repeated twice. The supernatants were combined and centrifuged at 5956 g for 20 min at 6 °C. The 5956 g supernatant was collected and subsequently centrifuged at 145,250 g for 60 min at 6 °C to precipitate membranes. The pellet was washed with HBS and centrifuged again at 145,250 g for 60 min at 6 °C. After the last centrifugation, the membrane pellet was collected and solubilized in HBS with 0.1% NP-40 for 30 min in ice. The sample was centrifuged at 10,000 g for 2 min to remove any precipitate. The supernatant was collected and kept in 20 °C until further analysis. A similar procedure was used for isolation of ejaculated sperm plasma membranes.
Purification and identification of sulfated Lewis X-binding proteins from epididymal spermatozoa
After plasma membrane isolation, 2 mg of protein sample was fractionated by RP-HPLC on a GE MDLC system equipped with a Vydac C4 (214TP54) 4.6 × 250 mm reversed-phase column and operated at a flow rate of 1 mL/min. Fractions were collected over an 80 min gradient of 10% to 90% ACN in 0.1% TFA and lyophilized for further use. Each fraction was resolved by one-dimensional SDS-PAGE followed by glycan blot analysis to investigate the presence of glycan-binding proteins.
Aliquots (21–22 µL) from the collected RP-HPLC fractions were diluted in 4× LSB (final concentration: 10% glycerol, 50 mM Tris, 2% sodium dodecyl sulfate, 100 mM DTT, 0.025% Bromophenol Blue) and heated at 100 °C for 7 min. Proteins were resolved by SDS-PAGE on 8.6 × 6.7 cm 4–20% gradient gels followed by transfer onto a nitrocellulose membrane. After protein preparation and transfer, the membrane was blocked for 1 h in blocking buffer consisting of 1× Carbo-free blocking buffer diluted in Tris-Buffered Saline and Tween20 (TBST; 50 mM Tris, 150 mM NaCl, 0.1% Tween 20) containing 0.2 mM CaCl2 and 0.2 mM MgCl2; this buffer was used throughout the experiment. After the blocking step, the membrane was rinsed with TBST (2×, 10 min) and subsequently incubated with 0.5 µg/mL biotinylated suLeX that was covalently coupled to a 30 kDa poly-acrylamide chain. After glycan incubation and rinsing, bound suLeX was detected by incubation with Streptavidin Poly-HRP Conjugate (Pierce Biotechnology, Rockford, IL, USA), diluted 1 : 60,000 in blocking buffer for 30 min, and developed using ECL Western Blotting Substrate (Thermo Scientific). Glycan blot-ting was also used to determine affinity of increasing concentrations (0.5 and 1.5 lg) of recombinant mouse lactadherin protein (MFG-E8; R&D Systems) to biotinylated suLeX, LeX and N-acetyllactosamine (LN).
In-gel tryptic digestion
Excised bands were washed and destained. ProteaseMax surfactant-assisted in-gel protein digestion (Promega) was carried out according to the manufacturer’s recommended protocol. Gel slices were cut into 1 mm3 pieces and washed once with water and twice with 50% MeOH/50 mM NH4HCO3. The pieces were dehydrated with 50% ACN/50 mM NH4HCO3 and 100% ACN washes followed by drying in vacuo using a Thermo Scientific Savant SpeedVac concentrator (SVC100; Waltham, MA, USA). Proteins were reduced in a solution of 25 mM DTT in 50 mM NH4HCO3 by incubation at 56 °C for 20 min. The solution was discarded and proteins were alkylated by incubation in a solution of 55 mM iodoacetamide in 50 mM NH4HCO3 at room temperature for 20 min in the dark. The pieces were washed twice with water, dehydrated and dried in vacuo. Digestion was per-formed by rehydrating the pieces in a solution of 12 ng/lL sequencing-grade trypsin and 0.01% ProteaseMax surfactant in 50 mM NH4HCO3 for 10 min at room temperature, overlaying with a solution of 0.01% ProteaseMax surfactant in 50 mM NH4HCO3, and incubating at 37 °C for 2 h. The samples were then centrifuged briefly and the solution containing the extracted peptides was transferred to a fresh sample tube. Formic acid was added to a final concentration of 0.5% (pH ≤ 3), and the peptides were desalted using ZipTipC18 (Millipore) solid phase extraction pipette tips. Peptides were dried in vacuo and reconstituted in 20 lL 0.1% formic acid for nanoLC-MS/MS analysis.
NanoLC-MS/MS analysis
All experiments were performed on a system consisting of a Waters NanoAcquity UPLC (Milford, MA, USA) interfaced with a Thermo Scientific Q-Exactive mass spectrometer (Bremen, Germany) through a nanoelectrospray ion source. Mobile phase A consisted of 0.1% formic acid in H2O, and mobile phase B consisted of 0.1% formic acid in ACN. Four microliters of each sample was injected onto a Waters Symmetry C18 5 µm, 180 µm × 20 mm trap column at a flow rate of 5 µL/min for 5 min at 99% A/1% B. Separation was performed on a Waters BEH130 C18 1.7 µm, 75 µm × 150 mm analytical column using a linear gradient of 5–35% mobile phase B at 300 nL/min over 90 min. The Q-Exactive was operated in data-dependent mode to automatically switch between survey scan MS and MS/MS acquisition. Survey scan spectra were acquired in the orbitrap mass analyzer using a mass range of m/z 200–2000 at a resolving power of 70,000 FWHM (m/z 200) with an AGC target of 1e6. The top ten precursor ions were sequentially selected for HCD fragmentation and MS/MS analysis in the orbitrap at a resolving power of 17,500 with an AGC target of 1e5. MS/MS scans employed an isolation window of 3.0 Da and a normalized collision energy of 30. Precursors were subjected to dynamic exclusion.
Data processing
Resulting data were processed using Proteome Discover 1.4 (Thermo Fisher Scientific, San Jose, CA, USA). The Sequest HT search algorithm was used to search the MS/MS data against the UniProt Sus Scrofa complete database (September 2013) to identify proteins. The precursor tolerance was set to 5 ppm, and fragment mass tolerance was set to 0.02 Da. Carbamidomethylation of cysteine was included as a fixed modification, and oxidation of methionine and deamidation of glutamine and asparagine were included as dynamic modifications. Reported peptides were filtered to a false discovery rate of 1% using Percolator. Reported proteins were filtered to require a Sequest score >10 and at least two unique peptides used for identification. Contaminant proteins such as keratins were not included in the reported identifications.
Sperm-oviduct binding assay
Details of the sperm-oviduct binding assay are described else-where (Kadirvel et al., 2012). Briefly, 20 oviduct cell aggregates were incubated with increasing concentrations of recombinant mouse lactadherin (R&D Systems) for 30 min. Spermatozoa (final concentration 1 × 106 spermatozoa/mL) were added to aggregates and cells were incubated for 15 min. After incubation, the aggregates were washed and mounted on slides for observation using a Zeiss Axioskop and AxioCam HRc digital camera (Carl Zeiss, Thornwood, NY, USA). The circumference of the aggregates was measured by AXIOVISION V 4.5 software (Carl Zeiss). The number of sperm bound to the periphery of each aggregate was counted and normalized by the aggregate circumference. Experiments were performed in triplicate and data were analyzed by the PROC MIXED procedure in SAS (SAS Institute Inc., Cary, NC, USA). The treatment was considered a fixed factor and replicates were considered a random factor. Tukey– Kramer’s test was used for multiple comparisons between treatments.
Western blot
Protein from spermatozoa was extracted with lysis buffer containing 0.1% NP-40 diluted in HBS and 1 × protease inhibitor (SigmaFAST). Spermatozoa were incubated in lysis buffer for 1 h in a cold room (6 °C) under gentle rotation, vortexed for 1 min and subsequently submitted to two cycles of freezing and thawing in a dry ice/alcohol solution. After the last thawing, the lysate was centrifuged twice at 10,000 g for 10 min to pellet the insoluble debris and the supernatant was stored at −20 °C for further use. The protein concentration was determined by BCA assay, and protein (15 µg) was prepared and transferred as described for glycan blotting and the membrane was blocked in TBST + 5% milk. The membrane was then incubated with poly-clonal affinity-purified antibody to mouse lactadherin (MFG-E8; R&D Systems) in TBST + 2% milk. The bound antibody was visualized by incubation with chicken anti-goat IgG conjugated to horseradish peroxidase (R&D Systems) and ECL Western Blot-ting Substrate (Thermo Scientific). Mouse sperm lysates were used as positive control. After western blotting, the membrane was stripped of the antibodies by incubating in Restore Stripping Buffer (Thermo Scientific) for 10 min and the membrane was prepared for glycan blotting using biotinylated suLeX.
Oviduct proteins that bind lactadherin were detected by far-western blotting. Oviduct cell lysate (28 µg protein) was transferred to membrane, blocked with 2% BSA for 1 h, and incubated with recombinant lactadherin (2 µg/mL) for 50 min. The bound protein was visualized by incubation of the membrane with polyclonal affinity-purified antibody to mouse lactadherin as previously described for western blot.
RESULTS
Purification of sulfated Lewis X-binding proteins from epididymal spermatozoa
As a first step to purify candidate LeX receptors from spermatozoa, samples of enriched plasma membrane proteins from cauda epididymal spermatozoa were fractionated by RP-HPLC, resolving in distinct peaks. Fractions within each peak were pooled and resolved by SDS-PAGE. Proteins were transferred to nitrocellulose and the membranes were incubated with biotinylated suLeX to detect proteins with affinity for this trisaccharide. suLeX was used because it binds more spermatozoa than unsulfated LeX when used on a glycan array (Kadirvel et al., 2012) and because the other more complex LeX structures that bind spermatozoa, LeX dimers and trimers (Kadirvel et al., 2012) were not available. A comparison of the glycan structures is shown (Fig. 1). The fractions containing a suLeX-binding protein were subjected to preparative SDS-PAGE, and the gel was stained with SimplyBlue SafeStain to locate proteins that bound glycans within each fraction (Fig. 2).
Figure 1.
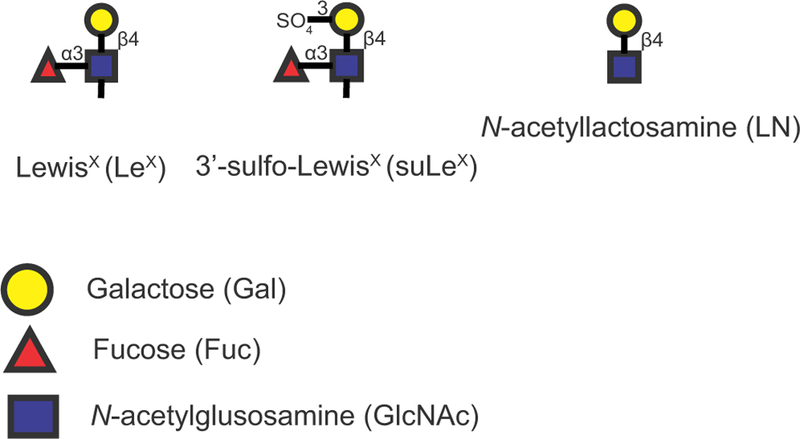
Structures of three related glycans used in these experiments. Colored shapes represent the specific monosaccharide and the specific link-age is provided. Sulfate in suLeX is on the 3 position of galactose. [Colour figure can be viewed at wileyonlinelibrary.com].
Figure 2.
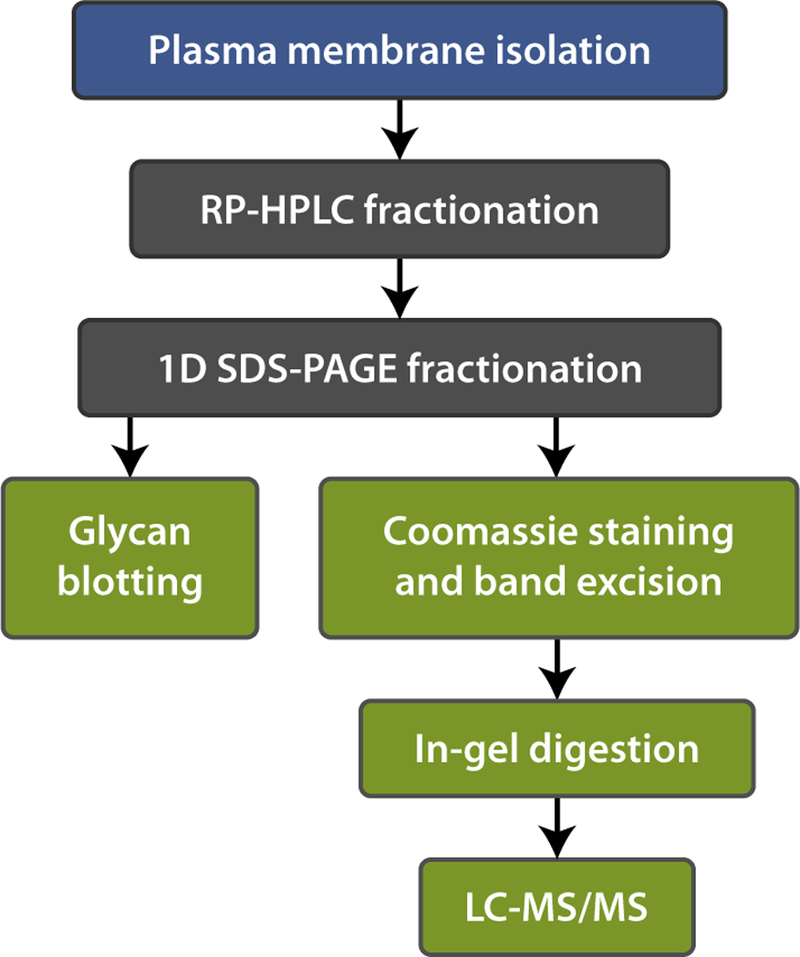
A schematic of the strategy used to identify suLeX -binding proteins in epididymal sperm lysate. Two purification steps were used: (i) RP-HPLC and (ii) 1D SDS-PAGE. The presence of suLeX-binding proteins was confirmed by glycan blotting assays. A duplicate gel was stained with Coomassie Blue and the migration of the proteins identified was matched to glycan blot results. Protein bands corresponding to bands that bound glycans were excised from the gel and analyzed by LC-MS/MS. [Colour figure can be viewed at wileyonlinelibrary.com].
Only two of the protein-containing fractions presented a clear positive signal after glycan blotting. One band (B1) of suLeX- binding proteins migrating at approximately 40 kDa was detected in fraction 1 (Fig. 3). After SDS-PAGE and staining, protein in fraction 1 contained a double band migrating at 35– kDa (Fig. 3, band B1 and B2). Based on the glycan blot result, only the higher molecular weight (MW) band (B1) bound biotinylated suLeX. The lower MW band, labeled B2, was analyzed by LC-MS/MS to further investigate a possible complex formation with B1. Another pair of protein bands migrating at 37–40 kDa was detected in fraction 2 (Band 3, B3).
Figure 3.
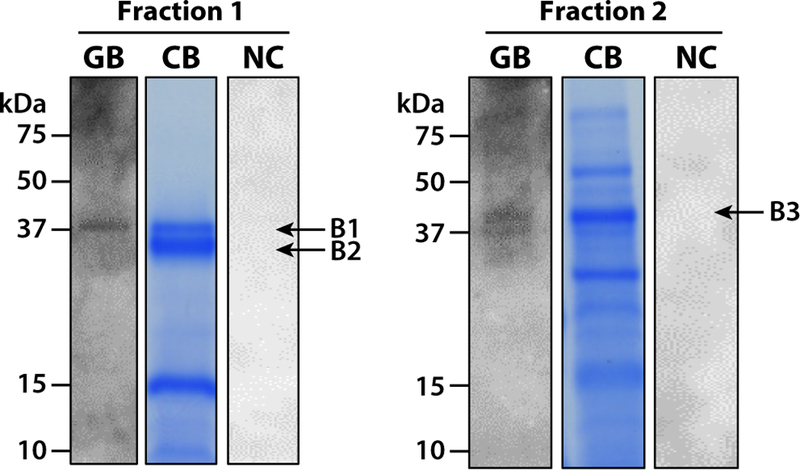
Detection of suLeX-binding proteins in two RP-HPLC fractions of enriched plasma membranes from cauda epididymal spermatozoa. Following RP-HPLC, SDS-PAGE and Coomassie Blue staining (CB) resolved proteins prior to selection for LC-MS/MS. Glycan blotting (GB) using suLeX identified suLeX-binding proteins in fractions 1 (B1) and 2 (B3). The migration of molecular weight standards is indicated on the left of each fraction. Negative control (NC) blots, where biotinylated suLeX was omitted, are shown on the right. [Colour figure can be viewed at wileyonlinelibrary.com].
Identification of suLeX-binding proteins by LC-MS/MS
Protein bands in fractions 1 and 2 detected by a Coomassie Blue stain were excised, digested in-gel with trypsin and analyzed by LC-MS/MS. Raw data were searched against the UniProt Sus Scrofa complete database for identification of peptide sequences revealed multiple proteins (Table 1).
Table 1.
LC-MS/MS identification of proteins in the RP-HPLC fractions that contained proteins that bound suLeX in glycan blotsa
| Protein name | General name | Accession | Sequest HT score | MW (kDa) | Coverage (%) | Peptides | Unique peptides | Peptide spectrum matches |
|---|---|---|---|---|---|---|---|---|
| Band B1 (bound suLeX) | ||||||||
| ADAM5 (disintegrin and metalloprotease domain-containing protein 5) |
ADAM5 | E7FM66 | 764.75 | 45.2 | 49.8 | 20 | 20 | 264 |
| Band B2 (did not bind suLeX) | ||||||||
| ADAM3a | – | A5A4F6 | 1330.39 | 82.9 | 22.9 | 19 | 19 | 774 |
| ADAM2 (Fertilin beta) | FTNB | Q866A8 | 413.46 | 81.8 | 14.4 | 11 | 11 | 244 |
| ADAM3b | – | A5HJZ3 | 352.58 | 83.5 | 22.0 | 16 | 16 | 166 |
| Band B3 (bound suLeX) | ||||||||
| Lactadherin | MFGE8 | B2CZF8 | 1773.84 | 47.8 | 60.3 | 37 | 37 | 881 |
Proteins from epididymal sperm plasma membrane were identified by LC-MS/MS. Protein bands were excised from a Coomassie-stained one-dimensional gel. Database was searched using the Sequest HT algorithm in Proteome Discoverer.
Fraction 1
LC-MS/MS results indicated that the B1 band contained a protein known as a disintegrin and metalloprotease domain-containing protein 5 (ADAM5; accession number E7FM66), which has a MW of 45.2 kDa (Uniprot Database). ADAM5 was identified with high confidence based on a Sequest HT score of 764.75 and 49.8% sequence coverage. The peptide sequences identifying ADAM5 are labeled in black in Fig. 4.
Figure 4.

Amino acid sequence of ADAM5 (accession number E7FM66) from the UniProt Sus Scrofa complete database are shown. Peptide sequences in bold type were deduced by LC-MS/MS.
The B2 band, which did not bind suLeX in the glycan blot, included ADAM3a and ADAM2 proteins, which were also confidently identified. The MWs of ADAM3a (82.9 kDa) and ADAM2 (81.8 kDa) do not match with the 35 kDa estimated MW of the gel bands (Fig. 3). However, investigation of the peptide sequences identified by LC-MS/MS (labeled in black, Fig. 5) demonstrated that only the C-terminal half of the protein sequence was present for both ADAM3a and ADAM2. This suggests that the migration as 35 kDa proteins was because of proteolysis prior to or following sperm lysis, consistent with the observation that several ADAMs are cleaved as spermatozoa pass through the epididymis (Kim et al., 2006).
Figure 5.

Amino acid sequence of ADAM3a (ac-cession number A5A4F6) and ADAM2 (acces-sion number Q866A8) from the UniProt Sus Scrofa complete database are shown. Peptide sequences in bold type were deduced by LC-MS/MS.
Fraction 2
Lactadherin (accession number B2CZF8; also known as SED1, P47 and MFG-E8 in different species, Mather, 2000) was identified in the B3 band. The detected peptides provided over 60% coverage of the entire peptide with a high Sequest score of 1773.84. The peptide sequences deduced by LC-MS/MS for lactadherin included the discoidin domain (Fig. 6).
Figure 6.

Amino acid sequences of lactadherin (accession number B2CZF8) from the UniProt Sus Scrofa complete database are shown. Pep-tide sequences in bold type were deduced by LC-MS/MS. Arrowheads indicate the putative starting and ending points of the discoidin domain.
Detection of lactadherin in spermatozoa by western blot
The presence of lactadherin on the sperm plasma membrane was investigated using an antibody against mouse lactadherin (MFG-E8; R&D Systems). The mouse antigen used to produce the antibody (residues Ala23-Cys463) showed 69% identity with the corresponding region of porcine lactadherin (data not shown). Consistent with previous reports (Ensslin & Shur, 2003), the antibody recognized a 67 kDa protein in mouse epididymal spermatozoa (MS, Fig. 7A). Boar epididymal and ejaculated spermatozoa yielded 37 and 47 kDa bands (EPI and EJA, Fig. 7A). Stripping the membrane after the western blot and further incubation with biotinylated suLeX resulted in two signals that migrated the same as lactadherin in porcine and murine spermatozoa (Fig. 7B). This confirmed that sperm lactadherin binds to suLeX. Other proteins that bound suLeX were also observed (Fig. 7B).
Figure 7.
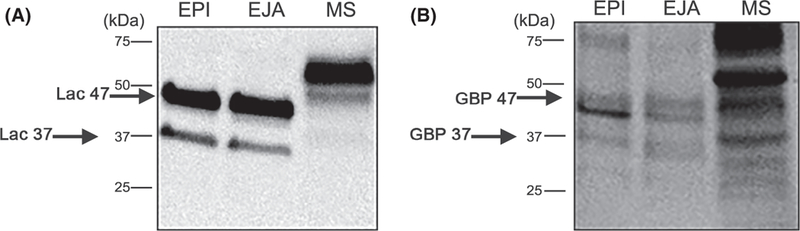
Lactadherin from porcine and mouse spermatozoa binds suLeX. (A) Proteins from porcine sperm epididymal (EPI) and ejaculated (EJA) plasma membranes (20 µg per well), and mouse epididymal spermatozoa (MS) were separated by SDS-PAGE and subjected to western blot analysis using anti-mouse lactadherin antibody. Two bands in porcine spermatozoa were detected with the lactadherin antibody migrating as 37 and 47 kDa (Lac 37 and Lac 47 labeled by black arrows). (B) After the western blot, the same membrane was stripped and incubated with biotinylated suLeX. Glycan-binding proteins (GBP) matching the migration of lactadherin (37 and 47 kDa) were detected (black arrows) from porcine spermatozoa and an abundant 60 kDa band that matched the migration of mouse sperm lactadherin was also detected.
Involvement of lactadherin on sperm binding to oviduct aggregates
To confirm that purified lactadherin binds LeX and to examine its binding specificity, recombinant Lactadherin (rLac; 0.5 and 1.5 µg) was resolved on SDS-PAGE and subsequently incubated with biotinylated suLeX, LeX and N-acetyllactosamine (LN). Glycan blot results show that rLac bound to suLeX and LeX, demonstrating that recognition of suLeX does not require the negatively charged sulfate residue (Fig. 8). In this assay, rLac also had affinity for N-acetyllactosamine, but there was no signal in the control in which no glycan was present (NC, Fig. 8). To determine the biological importance of sperm lactadherin, we tested its involvement in sperm-oviduct binding. This was accomplished by incubation of oviduct cell aggregates with rLac to occupy binding sites in oviduct cells. Pre-incubation with lactadherin reduced sperm binding to oviduct cells by 56.6% (Fig. 9A). Significant inhibition was observed at a rLac concentration as low as 2 µg/mL. We confirmed that rLac bound oviduct cells by far-western blotting. The recombinant protein detected multiple bands on a blot of oviduct epithelial cell protein across a range of molecular sizes (Fig. 9B), further validating the proposed interaction. The control in which rLac was deleted showed no non-specific bands.
Figure 8.
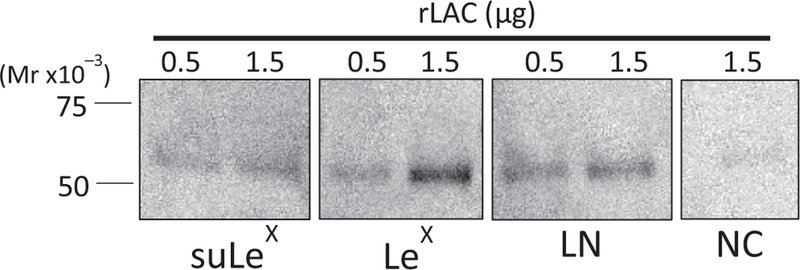
Increasing concentrations (0.5 and 1.5 µg) of rLac were resolved on SDS-PAGE and subsequently incubated with biotinylated suLeX, LeX and N-acetyllactosamine (LN). Negative control (NC), where biotinylated glycan was omitted, is shown on the right.
Figure 9.
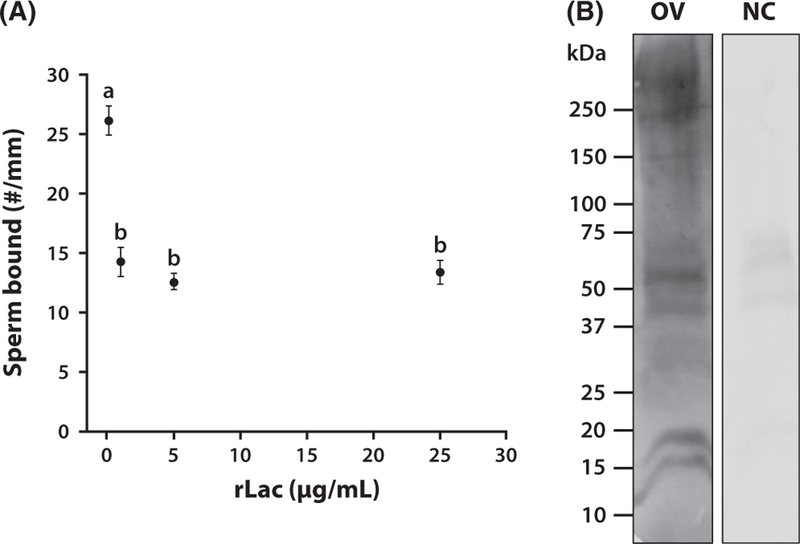
The biological function of lactadherin was assessed by inhibition of sperm binding to oviduct cells pre-incubated with increasing concentrations of recombinant lactadherin (rLac). (A) Total number of sperm bound to the periphery of each aggregate was counted and normalized by the aggregate circumference. Different superscripts indicate means that differ statistically (p < 0.01). Lactadherin competitively reduced sperm binding to oviduct cells. (B) Proteins from porcine oviduct cells (OV) were separated by SDS-PAGE and subjected to far-western blot analysis using rLac. Multiple bands in oviduct lysate were detected. A negative control (NC) where the presence of rLac was omitted is shown on the right. rLac identified many potential oviduct glycoproteins capable of an interaction.
DISCUSSION
To identify putative LeX receptors, a glycan blot was used to identify suLeX-binding proteins from RP-HPLC-fractionated lysates of epididymal spermatozoa. RP-HPLC was an efficient approach to fractionate epididymal sperm proteins with high resolution, resulting in the detection of two bands of suLeX-binding proteins. In addition, lactadherin, a protein known to be involved in cell-cell adhesion, was confirmed in independent assays to bind suLeX. Despite its implication in binding to the zona pellucida (Ensslin & Shur, 2003; Petrunkina et al., 2003), this is the first demonstration of lactadherin’s ability to bind specific glycans in any extracellular matrix.
Protein in B1, which bound suLeX in the glycan blots, was identified as ADAM5. The a disintegrin and metalloprotease (ADAMs) family is large, containing at least 33 members, 18 of which are present in the male reproductive system (Han et al., 2009). ADAM proteins have both adhesion and proteolytic function because of a multidomain structure characterized by a metalloprotease domain as well as a disintegrin domain (Wolfsberg et al., 1995b). ADAMs proteins contain a disintegrin domain that mediates cell adhesion; however, not all ADAM proteins are functional adhesion molecules (Wolfsberg et al., 1995a). There are few reports of glycan affinity of ADAMs proteins. Authors of one previous publication using asialo-α1-acid and asialo-aga-lacto- α1-acid affinity chromatography, reported that N-acetyllactosamine but not LeX had affinity for ADAM5 from epididymal boar spermatozoa (Mori et al., 2012). On the contrary, we found that porcine epididymal and ejaculated spermatozoa recognize N-acetyllactosamine but bind much less N-acetyllactosamine than suLeX (Silva et al., 2013). The preliminary results described here are consistent with a function for sperm ADAM5 in adhesion to oviduct suLeX. This hypothesis needs further validation.
A smaller protein band (B2) that did not bind to suLeX on glycan blots contained ADAM2 (also known as fertilinb), ADAM3a and ADAM3b, which are present as the 35 kDa mature form. Because suLeX bound to ADAM5 but not the other three ADAMs, it is apparent that glycan binding is specific. ADAM2, ADAM3a, ADAM3b and ADAM5 are present on the mouse sperm surface and are part of the same ADAM phylogenetic group characterized by proteins present exclusively or predominantly in the testis (Kim et al., 2006). Most ADAM proteins are also detected with a higher MW that corresponds to the precursor protein that is processed to mature form in the epididymis (Kim et al., 2006). It will be very interesting to determine if the proteins ADAM2, ADAM3a, ADAM3b and ADAM5 form a protein complex to recognize LeX. In mice, it has been speculated that ADAM5 surface localization is regulated by the ADAM1a/2 complex and that these ADAMs play a coordinated role in sperm migration to the oviduct (Kim et al., 2006).
The protein identified from B3 was lactadherin. Similar to ADAMs proteins, lactadherin also binds integrins (Raymond & Shur, 2009), but it remains unknown if this interaction is through lactadherin recognition of integrin carbohydrates. Lactadherin is the major protein of the milk fat globule membrane of the mammary gland and is also known as P47, SED1 and MFG-E8 in different species (Mather, 2000). In pigs, lactadherin was initially identified as P47, a sperm plasma membrane protein that bound to zona pellucida glycoproteins (Ensslin et al., 1998). This protein was localized in the apical ridge of epididymal and ejaculated boar spermatozoa (Petrunkina et al., 2003). The mouse homolog of P47 protein was renamed as SED1 to refer to a Secreted protein with Notch-like type II EGF repeats and C-terminal Discoidin domains (Ensslin & Shur, 2003). The EGF domain contains an arginine-glycine-aspartate sequence that is found in integrin ligands (Ruoslahti, 1996). The discoidin domain binds a multiplicity of ligands including phospholipids, carbohydrates and proteins (Kiedzierska et al., 2007).
The presence of lactadherin on the sperm plasma membrane was investigated using a commercially available polyclonal antibody against the mouse protein. The antigen used to produce the mouse antibody (residues Ala23-Cys463) showed 69% identity with the corresponding region of porcine lactadherin. As expected, mouse antibody recognized a 67 kDa protein in mouse epididymal spermatozoa and a 47 kDa protein in porcine epididymal and ejaculated spermatozoa (Fig. 7); however, we also detected a lower MW protein in pig and mouse spermatozoa in multiple western blot assays. The lower MW protein could be a proteolytic product of the full-length lactadherin protein or a different protein with cross reactivity with the antibody. At least in mouse epididymal tissue, short and long forms of lactadherin have been reported (Raymond & Shur, 2009) that would account for the smaller band. Glycan blot results show that rLac also binds to biotinylated LeX that is not sulfated, demonstrating that recognition of suLeX is not restricted to the negative sulfate residue (Fig. 8). In addition, rLac also demonstrated affinity to N-acetyllactosamine by glycan blotting. This is consistent with the observation that proteins with discoidin domains bind galactose (Kiedzierska et al., 2007), but this is the first direct evidence that lactadherin binds specific glycans.
To determine the biological importance of lactadherin, we tested its involvement in sperm-oviduct binding. The latter was accomplished by incubation of oviduct cell aggregates with rLac to occupy binding sites on oviduct cells. Pre-incubation with low concentrations of lactadherin reduced sperm binding to oviduct cells, supporting the hypothesis that lactadherin mediates sperm binding. It has been previously proposed that lactadherin facilitates sperm binding to integrins on the oviduct surface before the acrosome reaction occurs (Petrunkina et al., 2003), but this is the first time that involvement of this protein in oviduct cell binding was demonstrated directly. Multiple proteins that bind lactadherin were detected by far-western blotting, further validating the proposed interaction. Based on this result and the abundance of LeX on oviduct glycoproteins (Kadirvel et al., 2012), it is quite likely that many oviduct glycoproteins contain LeX and could interact with lactadherin.
In summary, glycan-binding proteins were isolated from boar epididymal sperm membranes using RP-HPLC and preparative SDS-PAGE. Data presented here support the hypothesis that multiple proteins coordinate binding to suLeX and potentially mediate sperm adhesion to the oviduct. This is in agreement with previous data from our group (Silva et al., 2014) that has shown that multiple glycan-binding proteins recognize suLeX and bi-SiaLN motifs and some of these glycan-binding proteins are present before sperm contact with accessory gland fluids. While multiple proteins appear to bind suLeX, in the present work we have purified and identified ADAM5 and lactadherin as suLeX-binding proteins. This is the first time that glycan-binding specificity for lactadherin has been determined and also the first time that an ADAMs family member has been implicated as a suLeX-binding protein. Because the ADAMs contain a transmembrane domain, they may induce an intracellular response through stimulation of signaling pathways, something less likely with peripheral membrane proteins such as lactadherin. The interaction of spermatozoa with oviduct glycans can affect sperm function. For example, binding to the oviduct regulates sperm intracellular Ca2+, motility and lifespan (Dobrinski et al., 1997), although it is not certain if this regulation is accomplished by binding to oviduct glycans. On the other hand, it is recognized that binding spermatozoa can influence oviduct cell gene
expression and protein secretion (Fazeli et al., 2004; Georgiou et al., 2005), which demonstrates the complex cross-talk between these two cell types. Determination of molecules capable of eliciting a cell signaling response in both spermatozoa and oviduct cells is of great importance for further understanding of how sperm adhesion to oviduct glycans can impact sperm function. The change in sperm intracellular Ca2+, sperm motility and lifespan induced by binding to the oviduct epithelium may be mediated by these candidate receptors. A molecular under standing of these complex interactions will be important to regulating fertility.
ACKNOWLEDGEMENTS
We thank Prairie State Semen Inc., Champaign, IL and Calihan Pork Producers, Peoria, IL for providing the cells and tissues used in our experiments. We thank Dr Peter Yau and Brian Imai from the Protein Sciences Facility at University of Illinois for their expertise and assistance with protein purification and analysis. This project was supported by Agriculture and Food Research Initiative Competitive Grant no. 2011–67015-20099 and 2015–67015-23228 from the USDA National Institute of Food and Agriculture. We thank the NIH shared instrument program for funding the instrument purchase (S10 RR029531) and funding support from NIH R01 NS071513 and R01 DK071801. LL acknowledges an H. I. Romnes Faculty Research Fellowship. We are also grateful to the UW School of Pharmacy Analytical Instrumentation Center for access to the Q Exactive Orbitrap mass spectrometer. NB was supported by grant #14–14-00579 of the Russian Science Foundation.
Footnotes
DISCLOSURE
The authors have nothing to disclose.
REFERENCES
- Chang MC. (1984) The meaning of sperm capacitation. A historical perspective. J Androl 5, 45–50. [DOI] [PubMed] [Google Scholar]
- DeMott RP, Lefebvre R & Suarez SS. (1995) Carbohydrates mediate the adherence of hamster sperm to oviductal epithelium. Biol Reprod 52, 1395–1403. [DOI] [PubMed] [Google Scholar]
- Dobrinski I, Smith TT, Suarez SS & Ball BA. (1997) Membrane contact with oviductal epithelium modulates the intracellular calcium concentration of equine spermatozoa in vitro. Biol Reprod 56, 861–869. [DOI] [PubMed] [Google Scholar]
- Ensslin MA & Shur BD. (2003) Identification of mouse sperm SED1, a bimotif EGF repeat and discoidin-domain protein involved in spermegg binding. Cell 114, 405–417. [DOI] [PubMed] [Google Scholar]
- Ensslin M, Vogel T, Calvete JJ, Thole HH, Schmidtke J, Matsuda T & Topfer-Petersen E. (1998) Molecular cloning and characterization of P47, a novel boar sperm-associated zona pellucida-binding protein homologous to a family of mammalian secretory proteins. Biol Reprod 58, 1057–1064. [DOI] [PubMed] [Google Scholar]
- Fazeli A, Affara NA, Hubank M & Holt WV. (2004) Sperm-induced modification of the oviductal gene expression profile after natural insemination in mice. Biol Reprod 71, 60–65. [DOI] [PubMed] [Google Scholar]
- Flesch FM, Voorhout WF, Colenbrander B, van Golde LM & Gadella BM. (1998) Use of lectins to characterize plasma membrane preparations from boar spermatozoa: a novel technique for monitoring membrane purity and quantity. Biol Reprod 59, 1530–1539. [DOI] [PubMed] [Google Scholar]
- Georgiou AS, Sostaric E, Wong CH, Snijders AP, Wright PC, Moore HD & Fazeli A. (2005) Gametes alter the oviductal secretory proteome. Mol Cell Proteomics 4, 1785–1796. [DOI] [PubMed] [Google Scholar]
- Gillis G, Peterson R, Russell L, Hook L & Freund M. (1978) Isolation and characterization of membrane vesicles from human and boar spermatozoa: methods using nitrogen cavitation and ionophore induced vesiculation. Prep Biochem 8, 363–378. [DOI] [PubMed] [Google Scholar]
- Han C, Choi E, Park I, Lee B, Jin S, Kim do H, Nishimura H & Cho C (2009) Comprehensive analysis of reproductive ADAMs: relationship of ADAM4 and ADAM6 with an ADAM complex required for fertilization in mice. Biol Reprod 80, 1001–1008. [DOI] [PubMed] [Google Scholar]
- Hunter RH. (1984) Pre-ovulatory arrest and peri-ovulatory redistribution of competent spermatozoa in the isthmus of the pig oviduct. J Reprod Fertil 72, 203–211. [DOI] [PubMed] [Google Scholar]
- Kadirvel G, Machado SA, Korneli C, Collins E, Miller P, Bess KN, Aoki K, Tiemeyer M, Bovin N & Miller DJ. (2012) Porcine sperm bind to specific 6-sialylated biantennary glycans to form the oviduct reservoir. Biol Reprod 87, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr CL, Hanna WF, Shaper JH & Wright WW. (2004) Lewis X-containing glycans are specific and potent competitive inhibitors of the binding of ZP3 to complementary sites on capacitated, acrosome-intact mouse sperm. Biol Reprod 71, 770–777. [DOI] [PubMed] [Google Scholar]
- Kiedzierska A, Smietana K, Czepczynska H & Otlewski J. (2007) Structural similarities and functional diversity of eukaryotic discoidin-like domains. Biochim Biophys Acta 1774, 1069–1078. [DOI] [PubMed] [Google Scholar]
- Kim T, Oh J, Woo JM, Choi E, Im SH, Yoo YJ, Kim DH, Nishimura H & Cho C. (2006) Expression and relationship of male reproductive ADAMs in mouse. Biol Reprod 74, 744–750. [DOI] [PubMed] [Google Scholar]
- Leemans B, Gadella BM, Stout TA, Sostaric E, De Schauwer C, Nelis H, Hoogewijs M & Van Soom A. (2016) Combined albumin and bicarbonate induces head-to-head sperm agglutination which physically prevents equine sperm-oviduct binding. Reproduction 151, 313–330. [DOI] [PubMed] [Google Scholar]
- Lefebvre R, Lo MC & Suarez SS. (1997) Bovine sperm binding to oviductal epithelium involves fucose recognition. Biol Reprod 56, 1198–1204. [DOI] [PubMed] [Google Scholar]
- Machado SA, Kadirvel G, Daigneault BW, Korneli C, Miller P, Bovin N & Miller DJ. (2014) LewisX-containing glycans on the porcine oviductal epithelium contribute to formation of the sperm reservoir. Biol Reprod 91, 140. [DOI] [PubMed] [Google Scholar]
- Mather IH. (2000) A review and proposed nomenclature for major proteins of the milk-fat globule membrane. J Dairy Sci 83, 203–247. [DOI] [PubMed] [Google Scholar]
- Mburu JN, Einarsson S, Lundeheim N & Rodriguez-Martinez H. (1996) Distribution, number and membrane integrity of spermatozoa in the pig oviduct in relation to spontaneous ovulation. Anim Reprod Sci 45, 109–121. [DOI] [PubMed] [Google Scholar]
- Mori E, Fukuda H, Imajoh-Ohmi S, Mori T & Takasaki S. (2012) Purification of N-acetyllactosamine-binding activity from the porcine sperm membrane: possible involvement of an ADAM complex in the carbohydrate-binding activity of sperm. J Reprod Dev 58, 117–125. [DOI] [PubMed] [Google Scholar]
- Pang PC, Chiu PCN, Lee C-L, Chang L-Y, Panico M, Morris HR, Haslam SM, Khoo K-H, Clark GF & Yeung WS. (2011) Human sperm binding is mediated by the sialyl-Lewis(x) oligosaccharide on the zona pellucida. Science 333, 1761–1764. [DOI] [PubMed] [Google Scholar]
- Petrunkina AM, Lakamp A, Gentzel M, Ekhlasi-Hundrieser M & Topfer-Petersen E. (2003) Fate of lactadherin P47 during post-testicular maturation and capacitation of boar spermatozoa. Reproduction 125, 377–387. [PubMed] [Google Scholar]
- Raymond AS & Shur BD. (2009) A novel role for SED1 (MFG-E8) in maintaining the integrity of the epididymal epithelium. J Cell Sci 122, 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Martinez H, Saravia F, Wallgren M, Tienthai P, Johannisson A, Vazquez JM, Martinez E, Roca J, Sanz L & Calvete JJ. (2005) Boar spermatozoa in the oviduct. Theriogenology 63, 514–535. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E (1996) RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol 12, 697–715. [DOI] [PubMed] [Google Scholar]
- Sabeur K & Ball BA. (2007) Characterization of galactose-binding proteins in equine testis and spermatozoa. Anim Reprod Sci 101, 74–84. [DOI] [PubMed] [Google Scholar]
- Silva E, Miller DJ & Bovin N. (2013) Multiple receptors for sulfated Lewis X trisaccharide and sialylated N-acetyllactosamine are present on the boar sperm plasma membrane contributing to oviduct reservoir formation. FASEB J 27, 828. [Google Scholar]
- Silva E, Kadirvel G, Jiang R, Bovin N & Miller D. (2014) Multiple proteins from ejaculated and epididymal porcine spermatozoa bind glycan motifs found in the oviduct. Andrology 2, 763–771. [DOI] [PubMed] [Google Scholar]
- Smith TT & Yanagimachi R. (1991) Attachment and release of spermatozoa from the caudal isthmus of the hamster oviduct. J Reprod Fertil 91, 567–573. [DOI] [PubMed] [Google Scholar]
- Suarez SS. (1987) Sperm transport and motility in the mouse oviduct: observations in situ. Biol Reprod 36, 203–210. [DOI] [PubMed] [Google Scholar]
- Talevi R & Gualtieri R. (2010) Molecules involved in sperm-oviduct adhesion and release. Theriogenology 73, 796–801. [DOI] [PubMed] [Google Scholar]
- Wagner A, Ekhlasi-Hundrieser M, Hettel C, Petrunkina A, Waberski D, Nimtz M & Topfer-Petersen E. (2002) Carbohydrate-based interactions of oviductal sperm reservoir formation-studies in the pig. Mol Reprod Dev 61, 249–257. [DOI] [PubMed] [Google Scholar]
- Wolfsberg TG, Primakoff P, Myles DG & White JM. (1995a) ADAM, a novel family of membrane proteins containing A Disintegrin And Metalloprotease domain: multipotential functions in cell-cell and cell-matrix interactions. J Cell Biol 131, 275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfsberg TG, Straight PD, Gerena RL, Huovila AP, Primakoff P, Myles DG & White JM. (1995b) ADAM, a widely distributed and developmentally regulated gene family encoding membrane proteins with a disintegrin and metalloprotease domain. Dev Biol 169, 378–383. [DOI] [PubMed] [Google Scholar]


