Figure 7.
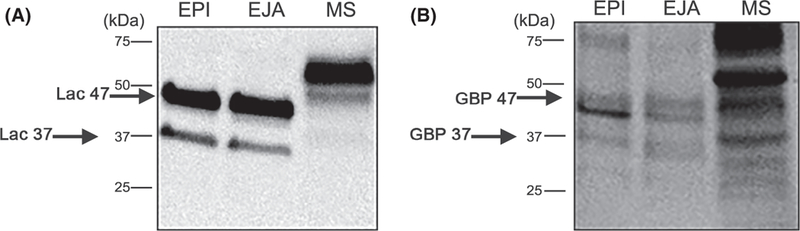
Lactadherin from porcine and mouse spermatozoa binds suLeX. (A) Proteins from porcine sperm epididymal (EPI) and ejaculated (EJA) plasma membranes (20 µg per well), and mouse epididymal spermatozoa (MS) were separated by SDS-PAGE and subjected to western blot analysis using anti-mouse lactadherin antibody. Two bands in porcine spermatozoa were detected with the lactadherin antibody migrating as 37 and 47 kDa (Lac 37 and Lac 47 labeled by black arrows). (B) After the western blot, the same membrane was stripped and incubated with biotinylated suLeX. Glycan-binding proteins (GBP) matching the migration of lactadherin (37 and 47 kDa) were detected (black arrows) from porcine spermatozoa and an abundant 60 kDa band that matched the migration of mouse sperm lactadherin was also detected.
