Abstract
Matrix-assisted laser desorption/ionization(MALDI) mass spectrometry (MS) is a rapid and sensitive analytical method that is well suited for determining molecular weights of peptides and proteins from complex samples. MALDI-MS can be used to profile the peptides and proteins from single-cell and small tissue samples without the need for extensive sample preparation. Furthermore, the recently developed MALDI imaging technique enables mapping of the spatial distribution of signaling molecules in tissue samples. Several examples of signaling molecule analysis at the single-cell and single-organ levels using MALDI-MS technology are highlighted followed by an outlook of future directions.
Keywords: Neuropeptides, Mass spectrometric imaging, MALDI-MS, Direct tissue profiling, Single-cell measurements
Introduction
The complexity and plasticity of synaptic connections within the nervous system provides the molecular basis for production of behaviors. Thus the knowledge of signaling molecules involved in synaptic function and their mechanism of action is essential for understanding how the nervous system regulates physiological processes. A growing body of evidence has shown that neuropeptides, which are one of the most diverse classes of signaling molecules, are central to many physiological processes and behaviors, such as feeding, stress, reproduction, and many others [1, 2]. There has therefore been a long-lasting interest in characterizing these molecules and determining their bioactivities in neural circuits.
Because the mammalian nervous system is extremely complex, the study of neuropeptides and their functions at the cellular and circuit levels has been performed with simple model organisms such as arthropods. For example, the crustacean stomatogastric nervous system (STNS) consists of several linked, motor-pattern-generating networks that produce coordinated motor output, which provides an excellent platform for studying neuropeptide activities [3]. Furthermore, many neuropeptide families have structurally related homologs in vertebrates, and increasing evidence suggests that many of the signaling molecules and pathways underlying complex behaviors are conserved across species. Thus the study of arthropod models may also provide useful insights for a better understanding of the nervous system in vertebrates including mammals.
In the early days, characterization of neuropeptides was achieved by extensive chromatography separation coupled with Edman degradation, which requires a large amount of sample material to start with. The development in biological mass spectrometry dramatically accelerates the pace of neuropeptide discovery. In this process, the matrix-assisted laser desorption/ionization (MALDI) type of instrument provides several unique advantages: (1) compatibility with complex sample background with high tolerance for physiological salts and impurities; (2) minimum sample amount is required (nano- to picomolar level); (3) high chemical specificity; (4) MS/MS capability enables de novo sequencing of novel peptides; and (5) fast acquisition and straightforward data interpretation. Thus MALDI-MS can be used to directly profile neuropeptide content from tissue samples of interest without tissue extraction and separation. When coupled with gas-phase fragmentation techniques, such as post source decay (PSD) and collision induced dissociation (CID), neuropeptide discovery can be achieved at the single-animal, single-organ, or even single-cell level [4, 5]. Furthermore, the recent development of MALDI imaging technique enables mapping of neuropeptide distribution in complex organs, which provides comparable chemical information as immunohistochemistry.
Tissue-based MS strategies
For neuropeptide profiling, especially single-cell analysis, careful tissue dissection and single-cell isolation are crucial to the quality of the acquired mass spectra. Micromanipulation techniques using micro glass capillary or small diameter glass micropipette are involved to isolate the cells and transfer them to the sample plate (Fig. 1) [6]. After certain sample preparation steps, a single cell or small piece of tissue is covered with a small droplet of matrix solution before MS analysis.
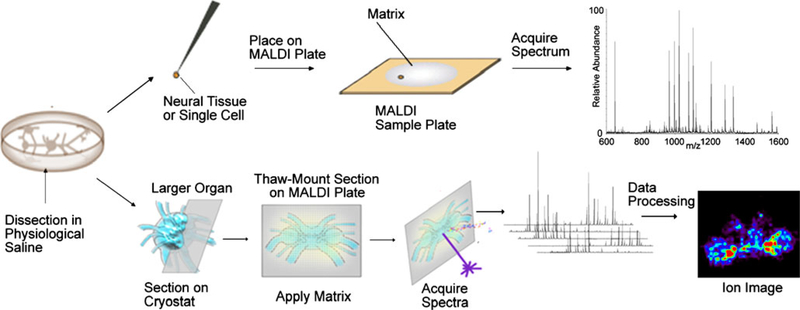
Fig. 1.
Overview of tissue-based mass spectrometric analysis strategies for neuropeptide discovery and distribution study. First, the tissue is dissected from the animal. For single-cell or direct tissue analysis, the sample can be rinsed to reduce salt content and then placed on the MALDI target. MALDI matrix is then deposited onto the surface of the tissue by the dried-droplet method before MS analysis. MALDI imaging can be used to study the distribution of neuropeptides in larger and more complex organs, such as the crab brain. The organ is sectioned via a cryostat and thaw-mounted on the MALDI plate. MALDI matrix is coated by using an airbrush or automatic matrix spotter, and then mass spectra are acquired in a raster mode using predetermined step size. After data are processed by imaging programs, ion images of specific neuropeptides can be recovered by plotting the peak intensities in the mass spectra with the x, y coordinate of each spot
Sample preparation plays a pivotal role in obtaining quality mass spectra from samples as complex as biological tissues. The physiological salt concentrations associated with neurons from marine specimens or saline solution used for dissection interfere greatly with MALDI analysis. Extracellular salts and physiological saline can be removed in a stepwise fashion by spot-to-spot cell transfers to a fresh matrix drop on the sample plate [7]. An alternative simplified preparation method involves replacing the physiological saline with the 2,5-dihydroxybenzoic acid (DHB) matrix solution during dissection, so that the salt removal occurs simultaneously with neuronal isolation. For direct tissue analysis, rinsing samples firstly in acidified methanol followed by dilute DHB (10 mg ml–1) aqueous solution was reported to efficiently remove physiological saline and extract the neuropeptides from the cells for sensitive MS detection [8].
The emerging mass spectrometric imaging (MSI) technique is another tissue-based MS analysis method, which reveals spatial distribution of analytes. As shown in Fig. 1, mass spectra are acquired according to a predefined Cartesian grid. This array of mass spectra is then processed into a cohesive image where each pixel contains the data from the corresponding spectrum. This technique can simultaneously generate numerous ion density maps with high chemical specificity. MSI provides the information on spatial distribution of target molecules which will be completely lost through pooling and homogenization required by most of the solution-based MS analysis techniques. Table 1 highlights examples of various tissue-based MALDI-MS analysis of neuropeptides in a wide range of model organisms.
Table 1.
Application of tissue-based mass spectrometric analysis for studying neuropeptides
| Organisms | Specimens | Neuropeptides | Analysis methods | Refs. |
|---|---|---|---|---|
| Mollusk | ||||
| Lymnaea stagnalis | Single identified neuron and neuroendocrine system in brain | Ovulation-inducing caudodorsal cell hormone (CDCH), egg-laying hormone and etc. | Single-cell profiling | [5, 9, 10, 42] |
| Aplysia californica | Individual neurons, and individual vesicles from exocrine atrial gland | α-bag cell peptide, small cardioactive peptides, egg-laying hormone, insulin-related peptides etc. | Single-cell profiling, salts removal for marine specimen; MALDI imaging of single neuron; quantitation of cell–cell signaling molecules | [4, 7, 11, 12, 16, 19, 39] |
| Aplysia vaccaria and Phyllaplysia taylori | Individual bag cell | Egg-laying hormone and related peptides | Single-cell profiling combined with ESI-FTICR analysis of tissue extract | [43] |
| Nematode | ||||
| Ascaris suum | Nerve tissues | FaRPs | Direct tissue profiling | [44] |
| Crustaceans | ||||
| Crayfish | ||||
| Procambarus clarkii | Brain tissue | SIFamide | Direct tissue profiling combined with LC-MS/MS analysis of extract and molecular cloning | [45] |
| Orconectes limosus | Neurosecretory cell | CHH precursors | Single-cell profiling combined with immunostaining | [46] |
| Lobster | ||||
| Homarus americanus | PO and STG | Orcokinins | Direct tissue profiling combined with PSD sequencing | [47] |
| Crabs | ||||
| Cancer borealis | PO, SG, STG, CoG, brain and gut tissue PO and brain | Neuropeptides from multiple families (RFamide, CabTRPs, orcokinins etc.) | Direct tissue profiling; In cell accumulation on MALDI-FTICR for sensitivity improvement 2D and 3D MALDI imaging | [8, 48][18, 49] |
| Cancer productus | Neuroendocrine tissues, midgut tissue | Neuropeptides from multiple families, including CabTRPs | Direct profiling | [47, 50] |
| Carcinus maenas | Multiple neural organs (SG, PO and brain) | Neuropeptides from multiple families (RFamide, CabTRPs, orcokinins etc.) | Direct profiling coupled with LC-MS/MS sequencing of novel peptides | [51] |
| Insects | ||||
| Fruit fly | ||||
| Drosophila melanogaster | Nerve tissues | Multiple neuropeptide families (FaRPs, adipokinetic hormones etc.) | Direct profiling coupled with genomic prediction | [52] |
| Cockroach | ||||
| Periplaneta americana | Identified individual neurons in terminalganglion | Allatotropin-related peptide | Single-cell profiling | [14] |
| Posterolateral cells of thoracic ganglia | FaRPs | Single-cell profiling | [13] | |
| Heteroptera | ||||
| Nezara viridula;Euschistus servus;Acrosternum hilare; Banasa dimiata | Antennal lobes and abdominal nerves | Allatotropin- and tachykinin-related peptides | MALDI direct profiling | [53] |
| Ticks | ||||
| Ixodes ricinus;Boophilus microplus | Central nervous system | Periviscerokinin peptides | Single-cell profiling | [54] |
| Mammals Rat | Individual pituitary cells | Pro-opiomelanacortin prohormone-derived peptides | Single-cell profiling | [15] |
| Brain sections | Brain peptides such as vasopressinmelanotropin, somatostatin,and neurotensin | Direct tissue and MALDI imaging with solid ionic matrix | [22] | |
| Spinal cord sections | Multiple brain peptides, such as substance P | MALDI imaging usingMALDI-FTICR | [55] | |
| SIMS for lipids and MALDI imaging for peptides | [28] |
FaRP FMRFamide-related peptide, CHH crustacean hyperglycemic hormone, SG sinus gland, PO pericardial organ, CoG commissural ganglion, STG stomatogastric ganglion, FTICR Fourier transform ion cyclotron resonance
Profiling of neuropeptides in single cells
The study of individual identified cells is extremely valuable for neuropeptide discovery, since fewer analytes are present in the cell compared to tissue extract, which simplifies the analysis. Furthermore, it is also important for the purpose of studying chemically heterogeneous nervous system where adjacent cells can use a distinct set of neuropeptides during intercellular communication. The knowledge of neuropeptide complements at the single-cell level is often critical to understand the interplay of signaling molecules involved in cell–cell communication and system-generated behaviors.
Mollusk serves as a good model system for the development of improved sample preparation techniques for single-cell analysis because their neurons are relatively large and their simpler nervous systems ease the dissection procedure and related sample handling techniques for MS detection. To date, various freshwater and marine species have been studied, including Lymnaea stagnalis, Aplysia californica, and Aplysia vaccaria, to name a few [9–12] (Table 1). With the development of sample preparation methods and the advancement of MS instrumentation, many more organisms have been studied by using singlecell analysis in recent years, such as individual insect neurons [13, 14] and mammalian cells [15]. In addition, using vesicles from the exocrine atrial gland of Aplysia californica as a model, Sweedler and coworkers reported the detection of a wide range of bioactive peptides within individual vesicles (1–2 μm)[16].
Direct tissue analysis
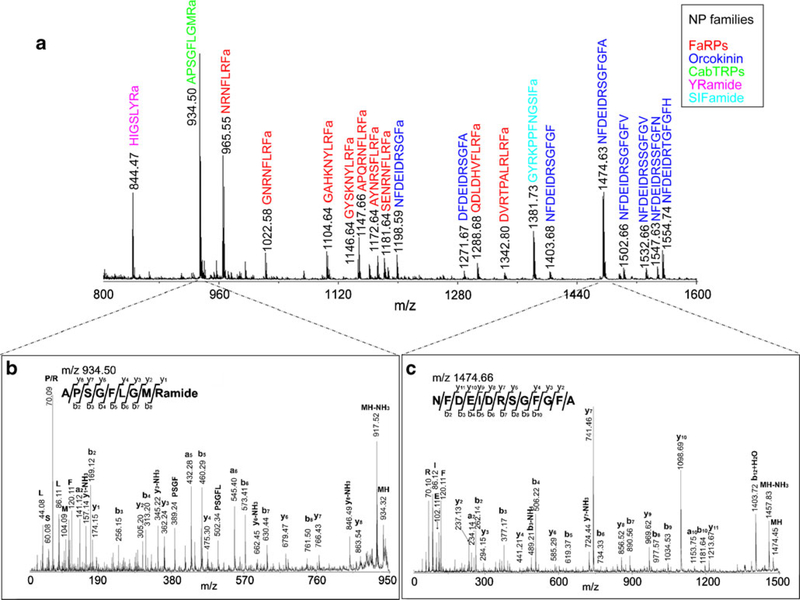
In addition to mass spectral analysis of single cells or organelles, various studies have been published on the analysis of neuronal clusters or neuroendocrine tissues. This method is widely used to obtain a snapshot of neuropeptide contents in a target organ with minimal sample handling and preparation, and is particularly useful for smaller cells that are difficult to isolate. As shown in Table 1, extensive applications have been found in the analysis of neuropeptides from decapod crustaceans, where tissues isolated from multiple neuronal organs of different crustacean species were analyzed directly using MALDI-based instrumentation. Both MALDI-TOF/TOF and MALDI Fourier transform mass spectrometers (FTMS) have been utilized, with the latter offering high mass accuracy and high resolving power for neuropeptide analysis. While MALDI-TOF/TOF generally provides better sensitivity for low-level signaling peptide analysis, MALDI-FTMS instruments offer unique ion trapping and storing capability. This feature enables the use of an in-cell accumulation technique to improve the detection sensitivity of complex tissue samples [8, 17, 18]. In combination with in situ fragmentation reactions offered by each instrument, the peptide identities can be directly determined from minute quantities of tissue samples. Figure 2 shows a representative mass spectrum of a piece of Cancer borealis brain tissue acquired on a MALDI-TOF/TOF instrument. Numerous neuropeptides from several different families are identified. The high energy CID fragmentation capability of MALDI-TOF/TOF provides abundant and informative sequence-specific fragment ions from selected peptides for sequence assignment.
Fig. 2.
Direct analysis of a piece of Cancer borealis brain tissue using MALDI-TOF/TOF. a The mass spectrum shows numerous identified neuropeptides from several different families, which are labeled with their corresponding peptide sequences. Peptides from different families are indicated with different colors. FaRPs, FMRFamide-related peptides; CabTRPs, Cancer borealis tachykinin-related peptides. In situ CID fragmentation enables peptide characterization directly from tissue sample: b APSGFLGMRa (m/z 934.5), and c NFDEIDRSGFGFA (m/z 1474.7). The presence of b- and y-type fragment ions is indicated by horizontal lines above (y ions) or below (b ions) the corresponding amino acid residues in the peptide sequence in each spectrum
MALDI imaging of neuronal tissues
MSI is a valuable technology because the location of biologically active molecules is often related to their functions. Furthermore, MSI has emerged as a powerful tool to investigate expression changes that occur as a result of normal cognitive functioning or development. Over the last decade, numerous developments in sample preparation and instrumentation have been achieved to improve the quality and spatial resolution of the MALDI imaging technique. For sample preparation, glycerol stabilization was reported to offer acceptable preservation of cell morphology and prevention of neuropeptide redistribution [19]. To achieve better MSI sensitivity, thinner tissue sections have been shown to provide better detection sensitivity [20]. Depositing a thin layer of gold on the tissue surface was shown to increase the signal intensities for the peptide detection [21]. The development of solid ionic matrix was also of great success. The ionic matrix made from conventional MALDI matrix with organic bases (e.g., aniline) was demonstrated to provide better spectral quality, more homogeneity of crystallization, and better resistance to laser irradiation [22]. Although the resolution of the MSI technique is often limited by the diameter of laser beams that are coupled with most MALDI-MS (100 μm), a novel technique was recently reported to enable imaging of features smaller than the laser beam size by complete ablation of the MALDI matrix coating the sample at each sample position and moving the sample target at a distance less than the diameter of the laser beam before repeating the process [23].
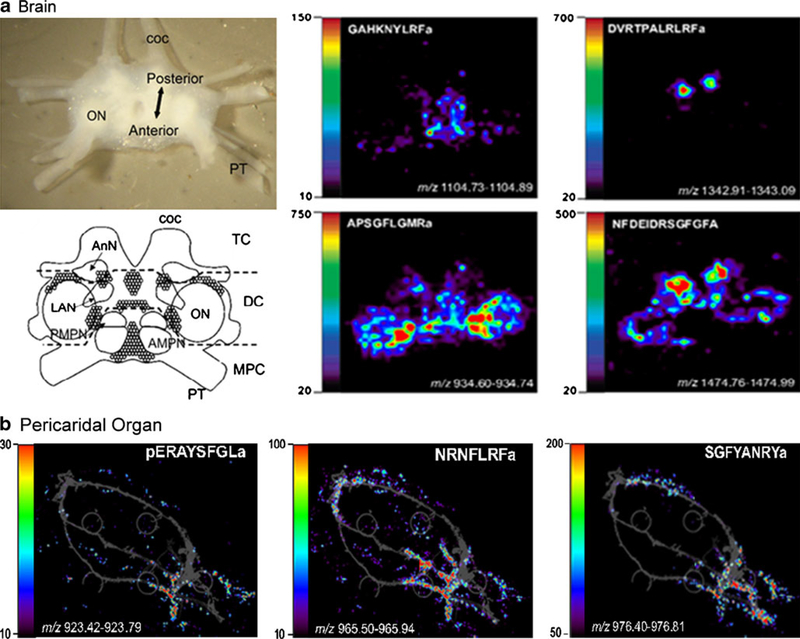
MALDI-MSI has been extensively used for studying the localization of proteins [24] or pharmaceuticals [25, 26] in tissue. More recently, the use of MSI to map the distribution of neuropeptides has gained increased attention. As an example, direct molecular imaging of nervous tissue from the freshwater snail Lymnaea stagnalis at subcellular spatial resolution was achieved by using matrix-enhanced SIMS [27]. Recently, both SIMS and MALDI imaging were applied to study the distribution of neuropeptides, lipids, and small molecules in rat spinal cord, and several neuropeptides were detected to be colocalized, such as substance P and somatostatin-14 [28]. MSI has also been used to profile the neuropeptides present in neuronal ganglia in Aplysia and to compare peptide contents of the cell bodies and the neuronal processes (neurites) in isolated Aplysia neurons [19, 29]. Additionally, MALDI imaging has been employed to study the differential distribution of multiple neuropeptide families in the brain and a neuroendocrine structure pericardial organ from the crab Cancer borealis [30]. In a recent report, Chen et al. introduced a simple three-dimensional MALDI imaging technique to establish a detailed description of neuropeptide localization in the crab brain, which showed great correlation with the morphological structures [18]. Figure 3 shows representative MALDI images of neuropeptides in crustacean neuronal tissues, including brain and pericardial organ, which shows differential distribution of neuropeptides from different families.
Fig. 3.
MALDI mass spectrometric imaging of neuropeptides in two neuronal organs from Cancer borealis. a MALDI images of three different neuropeptide families in the brain, including two RFamide isoforms on the top panels, crustacean tachykinin-related peptide (CabTRP 1a) and an orcokinin peptide from left to right on the bottom panels. MALDI-MS images of select peptides demonstrate colocalization of members of the same neuropeptide family as well as exceptions to this trend. The amino acid sequence and mass to charge ratio of each peptide is labeled in each image. b MALDI images of three different neuropeptide families in pericardial organ (PO), including A-type allatostatin, FaRP, Arg-Tyr-amide (RYamide) peptide from left to right. Peptides from different families show differential distribution patterns in PO
Traditionally the spatial localization and relative amounts of bioactive peptides are visualized with immunostaining or in situ hybridization techniques. Immunostaining uses fluorescent dye, enzyme, or radioactive compound linked to antibody to probe the target protein or peptides of interest in the tissue. It has the advantage of excellent sensitivity and high spatial resolution; however, specific antibodies are needed to identify and localize the peptides. Immunostaining also lacks specificity if multiple neuropeptides cross-react with the antibodies. For in situ hybridization, a nucleotide probe binds or hybridizes to the target gene transcript and is visualized. Thus, prior knowledge of nucleotide sequence is necessary. MSI is more flexible than immunostaining or in situ hybridization because the peptides of interest do not need to be preselected. The sample preparation of MSI is also relatively simple without the need for extensive washing and fixation. Furthermore, numerous target molecules with diverse chemical structures can be analyzed simultaneously, and their colocalization patterns can be visualized readily that yield valuable information regarding possible interactions among different neuropeptides.
Outlook
MALDI-MS has been utilized to analyze many different types and sizes of tissue samples ranging from individual organelles to large tissue sections. In combination with multiple fragmentation techniques, such as PSD and CID, this methodology can be used to discover novel neuropeptides directly from extremely small amounts of samples and localized regions. Furthermore, using MALDI imaging technology, one can resolve the spatial distribution of neuropeptides simultaneously, which may provide further insight into understanding of the functions of target molecules. We expect more exciting applications of these tissue-based mass spectral techniques to the study of a wide range of model organisms in the coming years, which will accelerate the discovery of novel neuropeptides and expand our knowledge of their biological functions. Several aspects that will impact these applications are highlighted below.
In situ chemical derivatization
With the advancement of tandem mass spectrometric techniques, one can determine the amino acid sequence of a peptide of interest via gas-phase MS/MS fragmentation directly from tissue samples [4, 5]. However this task is typically more difficult compared to the analysis of pooled tissue extract using an ESI type of instrument, partially due to the complex nature of tissue samples and low abundance of peptide(s) of interest. This problem may be alleviated by chemical derivatization, such as reductive dimethylation, esterification, to name a few, which have been developed and widely used in proteomics and peptidomics studies. These simple derivatization reactions help to improve ionization and fragmentation efficiency and thus the quality of resulting MS/MS spectra. Recently, Franck et al. reported that sulfonation of the N-terminus of tryptic peptides helped to improve on-tissue identification of proteins by enhancing fragmentation [31]. By using appropriate derivatization reagents, it is possible to achieve higher sensitivity for MALDI detection, and it is also practical to get better MS/MS spectra by reducing the formation of internal fragments and enhancing sequence-related ions.
Microscale separation
Strategies employing microscale extraction and separation are attractive to further improve the sensitivity of neuropeptide analysis in the organ and cellular domains. For example, capillary electrophoresis has been demonstrated for the analysis of single-cell and single-organ extracts with improved dynamic range and spectral quality compared to crude samples [32, 33]. Furthermore, microcolumn liquid chromatography coupled with MALDI-TOF-MS was also reported to improve the analysis of peptides in single neurons [34].
Quantitation
Obtaining quantitative information of signaling molecules especially in single cells or a localized region is crucial to understanding their functions. It is well known that the signal intensity generated on MALDI-MS is often affected by the sample background and biological matrix. However, when the experimental conditions are carefully controlled and normalization is properly performed, valuable information can be obtained regarding to the relative abundance of certain peptides present in different samples, which is often referred to as a semiquantitation technique. Jiménez et al. conducted several studies on the application of MALDI-TOF-MS for (semi)quantitative, label-free profiling of single cells and neuroendocrine tissues in the freshwater snail Lymnaea stagnalis and rat [5, 35–37]. The levels of several neuropeptides were observed to be greatly changed between different neurons or under different physiological conditions. For more accurate measurement, a technique via in-cell combination of isotopically labeled ions with MALDI-FTICR instrumentation has been developed, enabling quantitation of two separate tissue samples [38]. Recently, Rubakhin et al. adopted stable isotope labeling with d0- and d4-succinic anhydride and iTRAQ (isobaric tagging reagents for relative and absolute quantitation) for quantitative microanalysis of peptides in individual Aplysia californica neurons and small pieces of tissues. Absolute quantitation was also achieved by standard addition [39].The potential application of these methods to quantitatively measure neuropeptides directly from individual neurons or tissue samples could have great impact.
Single-cell sampling
For single-cell profiling, microscale dissection is usually the most challenging aspect, especially for smaller and tightly packed neurons. Recently, a novel massively parallel sample preparation technique has been developed to generate single-cell-sized samples for mammalian brain sections using beads embedded in Parafilm [40]. A laser capture microdissection technique was also developed and used to harvest a small number of cells from complex tissue sections [41]. These continued technique improvements coupled with development of modern MS instruments will further accelerate our pace of neuropeptide study and significantly expand our knowledge about cell-to-cell signaling.
Acknowledgement
Preparation of this manuscript was supported in part by a National Science Foundation CAREER Award (CHE-0449991), National Institutes of Health through grant 1R01DK071801. L.L. acknowledges an Alfred P. Sloan Research Fellowship and Vilas Associate Award.
Contributor Information
Ruibing Chen, Research Center of Basic Medical Sciences, Tianjin Medical University, Tianjin 300070, China.
Lingjun Li, Department of Chemistry & School of Pharmacy, University of Wisconsin-Madison, 777 Highland Avenue, Madison, WI 53705-2222, USA lli@pharmacy.wisc.edu.
References
- 1.Audsley N, Weaver RJ (2009) Neuropeptides associated with the regulation of feeding in insects. Gen Comp Endocrinol 162:93–104 [DOI] [PubMed] [Google Scholar]
- 2.Mercier AJ, Friedrich R, Boldt M (2003) Physiological functions of FMRFamide-like peptides (FLPs) in crustaceans. Microsc Res Tech 60:313–324 [DOI] [PubMed] [Google Scholar]
- 3.Marder E, Bucher D (2007) Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol 69:291–316 [DOI] [PubMed] [Google Scholar]
- 4.Li L, Garden RW, Romanova EV, Sweedler JV (1999) In situ sequencing of peptides from biological tissues and single cells using MALDI-PSD/CID analysis. Anal Chem 71:5451–5458 [DOI] [PubMed] [Google Scholar]
- 5.Jiménez CR, Li KW, Dreisewerd K, Spijker S, Kingston R, Bateman RH, Burlingame AL, Smit AB, van Minnen J, Geraerts WP (1998) Direct mass spectrometric peptide profiling and sequencing of single neurons reveals differential peptide patterns in a small neuronal network. Biochemistry 37:2070–2076 [DOI] [PubMed] [Google Scholar]
- 6.Li L, Garden RW, Sweedler JV (2000) Single-cell MALDI: a new tool for direct peptide profiling. Trends Biotechnol 18:151–160 [DOI] [PubMed] [Google Scholar]
- 7.Garden RW, Moroz LL, Moroz TP, Shippy SA, Sweedler JV (1996) Excess salt removal with matrix rinsing: direct peptide profiling of neurons from marine invertebrates using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Mass Spectrom 31:1126–1130 [DOI] [PubMed] [Google Scholar]
- 8.Kutz KK, Schmidt JJ, Li L (2004) In situ tissue analysis of neuropeptides by MALDI FTMS in-cell accumulation. Anal Chem 76:5630–5640 [DOI] [PubMed] [Google Scholar]
- 9.Li KW, Hoek RM, Smith F, Jimenez CR, van der Schors RC et al. (1994) Direct peptide profiling by mass spectrometry of single identified neurons reveals complex neuropeptide-processing pattern. J Biol Chem 269:30288–30292 [PubMed] [Google Scholar]
- 10.Li KW, Jimenez CR, Van Veelen PA, Geraerts WP (1994) Processing and targeting of a molluscan egg-laying peptide prohormone as revealed by mass spectrometric peptide fingerprinting and peptide sequencing. Endocrinology 134:1812–1819 [DOI] [PubMed] [Google Scholar]
- 11.Floyd PD, Li L, Rubakhin SS, Sweedler JV, Horn CC, Kupfermann I, Alexeeva VY, Ellis TA, Dembrow NC, Weiss KR, Vilim FS (1999) Insulin prohormone processing, distribution, and relation to metabolism in Aplysia californica. J Neurosci 19: 7732–7741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garden RW, Moroz TP, Gleeson JM, Floyd PD, Li L, Rubakhin SS, Sweedler JV (1999) Formation of N-pyroglutamyl peptides from N-Glu and N-Gln precursors in Aplysia neurons. J Neurochem 72:676–681 [DOI] [PubMed] [Google Scholar]
- 13.Neupert S, Predel R (2005) Mass spectrometric analysis of single identified neurons of an insect. Biochem Biophys Res Commun 327:640–645 [DOI] [PubMed] [Google Scholar]
- 14.Neupert S, Schattschneider S, Predel R (2009) Allatotropin-related peptide in cockroaches: identification via mass spectrometric analysis of single identified neurons. Peptides 30:489–494 [DOI] [PubMed] [Google Scholar]
- 15.Rubakhin SS, Sweedler JV (2007) Characterizing peptides in individual mammalian cells using mass spectrometry. Nat Protoc 2:1987–1997 [DOI] [PubMed] [Google Scholar]
- 16.Rubakhin SS, Garden RW, Fuller RR, Sweedler JV (2000) Measuring the peptides in individual organelles with mass spectrometry. Nat Biotechnol 18:172–175 [DOI] [PubMed] [Google Scholar]
- 17.Ma M, Chen R, Ge Y, He H, Marshall AG, Li L (2009) Combining bottom-up and top-down mass spectrometric strategies for de novo sequencing of the crustacean hyperglycemic hormone from Cancer borealis. Anal Chem 81:240–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen R, Hui L, Sturm RM, Li L (2009) Three dimensional mapping of neuropeptides and lipids in crustacean brain by mass spectral imaging. J Am Soc Mass Spectrom 20:1068–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubakhin SS, Greenough WT, Sweedler JV (2003) Spatial profiling with MALDI MS: distribution of neuropeptides within single neurons. Anal Chem 75:5374–5380 [DOI] [PubMed] [Google Scholar]
- 20.Sugiura Y, Shimma S, Setou M (2006) Thin sectioning improves the peak intensity and signal-to-noise ratio in direct tissue mass spectrometry. J Mass Spectrom Soc Jpn 54:45–48 [Google Scholar]
- 21.Altelaar AF, Klinkert I, Jalink K, de Lange RP, Adan RA, Heeren RM, Piersma SR (2006) Gold-enhanced biomolecular surface imaging of cells and tissue by SIMS and MALDI mass spectrometry. Anal Chem 78:734–742 [DOI] [PubMed] [Google Scholar]
- 22.Lemaire R, Tabet JC, Ducoroy P, Hendra JB, Salzet M, Fournier I (2006) Solid ionic matrixes for direct tissue analysis and MALDI imaging. Anal Chem 78:809–819 [DOI] [PubMed] [Google Scholar]
- 23.Jurchen JC, Rubakhin SS, Sweedler JV (2005) MALDI-MS imaging of features smaller than the size of the laser beam. J Am Soc Mass Spectrom 16:1654–1659 [DOI] [PubMed] [Google Scholar]
- 24.Groseclose MR, Andersson M, Hardesty WM, Caprioli RM (2007) Identification of proteins directly from tissue: in situ tryptic digestions coupled with imaging mass spectrometry. J Mass Spectrom 42:254–262 [DOI] [PubMed] [Google Scholar]
- 25.Hsieh Y, Chen J, Korfmacher WA (2007) Mapping pharmaceuticals in tissues using MALDI imaging mass spectrometry. J Pharmacol Toxicol Meth 55:193–200 [DOI] [PubMed] [Google Scholar]
- 26.Drexler DM, Garrett TJ, Cantone JL, Diters RW, Mitroka JG, Prieto Conaway MC, Adams SP, Yost RA, Sanders M (2007) Utility of imaging mass spectrometry (IMS) by matrix-assisted laser desorption ionization (MALDI) on an ion trap mass spectrometer in the analysis of drugs and metabolites in biological tissues. J Pharmacol Toxicol Methods 55:279–288 [DOI] [PubMed] [Google Scholar]
- 27.Altelaar AF, Van Minnen J, Jimenez CR, Heeren RM, Pierma SR (2005) Direct molecular imaging of Lymnaea stagnalis nervous tissue at subcellular spatial resolution by mass spectrometry. Anal Chem 77:735–741 [DOI] [PubMed] [Google Scholar]
- 28.Monroe EB, Annangudi SP, Hatcher NG, Gutstein HB, Rubakhin SS, Sweedler JV (2008) SIMS and MALDI MS imaging of the spinal cord. Proteomics 8:3746–3754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kruse R, Sweedler JV (2003) Spatial profiling invertebrate ganglia using MALDI MS. J Am Soc Mass Spectrom 14:752–759 [DOI] [PubMed] [Google Scholar]
- 30.DeKeyser SS, Kutz-Naber KK, Schmidt JJ, Barrett-Wilt GA, Li L (2007) Imaging mass spectrometry of neuropeptides in decapod crustacean neuronal tissues. J Proteome Res 6:1782–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franck J, El Ayed M, Wisztorski M, Salzet M, Fournier I (2009) On-tissue N-termial peptide derivatization for enhancing protein identification in MALDI mass spectrometric imaging strategies. Anal Chem 81:8305–8317 [DOI] [PubMed] [Google Scholar]
- 32.Sheeley SA, Miao H, Ewing MA, Rubakhin SS, Sweedler JV (2005) Measuring D-amino acid-containing neuropeptides with capillary electrophoresis. Analyst 130:1198–1203 [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Ma M, Chen R, Li L (2008) Enhanced neuropeptide profiling via capillary electrophoresis off-line coupled with MALDI FTMS. Anal Chem 80:6168–6177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsieh S, Dreisewerd K, van der Schors RC, Jimenez CR, Stahi-Zeng J, Hillenkamp F, Jorgenson JW, Geraerts WP, Li KW (1998) Separation and identification of peptides in single neurons by microcolumn liquid chromatography-matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and postsource decay analysis. Anal Chem 70:1847–1852 [DOI] [PubMed] [Google Scholar]
- 35.Jiménez CR, Li KW, Dreisewerd K, Mansvelder HD, Brussaard AB, Reinhold BB, Van der Schors RC, Karas M, Hillenkamp F, Burbach JP, Costello CE, Geraerts WP (1997) Pattern changes of pituitary peptides in rat after salt-loading as detected by means of direct, semiquantitative mass spectrometric profiling. Proc Natl Acad Sci U S A 94:9481–9486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiménez CR, ter Maat A, Pieneman A, Burlingame AL, Smit AB,Li KW (2004) Spatio-temporal dynamics of the egg-laying-inducing peptides during an egg-laying cycle: a semiquantitative matrix-assisted laser desorption/ionization mass spectrometry approach. J Neurochem 89:865–875 [DOI] [PubMed] [Google Scholar]
- 37.Jiménez CR, Li KW, Smit AB, Janse C (2006) Auto-inhibitory control of peptidergic molluscan neurons and reproductive senescence. Neurobiol Aging 27:763–769 [DOI] [PubMed] [Google Scholar]
- 38.DeKeyser SS, Li L (2006) Matrix-assisted laser desorption/ionization Fourier transform mass spectrometry quantitation via in cell combination. Analyst 131:281–290 [DOI] [PubMed] [Google Scholar]
- 39.Rubakhin SS, Sweedler JV (2008) Quantitative measurements of cell-cell signaling peptides with single-cell MALDI MS. Anal Chem 80:7128–7136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monroe EB, Jurchen JC, Koszczuk BA, Losh JL, Rubakhin SS, Sweedler JV (2006) Massively parallel sample preparation for the MALDI MS analyses of tissues. Anal Chem 78:6826–6832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mustafa D, Kros JM, Luider T (2008) Combining laser capture microdissection and proteomics techniques. Methods Mol Biol 428:159–178 [DOI] [PubMed] [Google Scholar]
- 42.Jiménez CR, van Veelen PA, Li KW, Wildering WC, Geraerts WP, Tjaden UR, van der Greef J (1994) Neuropeptide expression and processing as revealed by direct matrix-assisted laser desorption ionization mass spectrometry of single neurons. J Neurochem 62:404–407 [DOI] [PubMed] [Google Scholar]
- 43.Li L, Garden RW, Floyd PD, Moroz TP, Gleeson JM, Sweedler JV, Pasa-Tolic L, Smith RD (1999) Egg-laying hormone peptides in the aplysiidae family. J Exp Biol 202:2961–2973 [DOI] [PubMed] [Google Scholar]
- 44.Yew Y, Dikler S, Stretton AO (2003) De novo sequencing of novel neuropeptides directly from Ascaris suum tissue using matrix-assisted laser desorption/ionization time-of-flight/time-of-flight. Rapid Commun Mass Spectrom 17:2693–2698 [DOI] [PubMed] [Google Scholar]
- 45.Yasuda A, Yasuda-Kamatani Y, Nozaki M, Nakajima T (2004) Identification of GYRKPPFNGSIFamide (crustacean-SIFamide) in the crayfish Procambarus clarkii by topological mass spectrometry analysis. Gen Comp Endocrinol 135:391–400 [DOI] [PubMed] [Google Scholar]
- 46.Redeker V, Toullec JY, Vinh J, Rossier J, Soyez D (1998) Combination of peptide profiling by matrix-assisted laser desorption/ionization time of flight mass spectrometry and immunodetection on single glands or cells. Anal Chem 70:1805–1811 [DOI] [PubMed] [Google Scholar]
- 47.Li L, Pulver SR, Kelley WP, Thirumalai V, Sweedler JV, Marder E (2002) Orcokinin peptides in developing and adult crustacean stomatogastric nervous systems and pericardial organs. J Comp Neurol 444:227–244 [DOI] [PubMed] [Google Scholar]
- 48.Christie AE, Kutz-Naber KK, Stemmler EA, Klein A, Messinger DI, Dickinson PS (2007) Midgut epithelial endocrine cells are a rich source of the neuropeptides APSGFLGMRamide (Cancer borealis tachykinin-related peptide Ia) and GYRKPPFNGSIFamide (Gly1-SIFamide) in the crabs Cancer borealis, Cancer magister and Cancer productus. J Exp Biol 210:699–714 [DOI] [PubMed] [Google Scholar]
- 49.DeKeyser SS, Kutz-Naber KK, Schmidt JJ, Barrett-Wilt GA, Li L (2007) Imaging mass spectrometry of neuropeptides in decapod crustacean neuronal tissues. J Proteome Res 6:1782–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu Q, Kutz KK, Schmidt JJ, Hsu YW, Messinger DI, Cain SD, de la Iglesia HO, Christie AE, Li L (2005) Hormone complement of the Cancer productus sinus gland and pericardial organ: an anatomical and mass spectrometric investigation. J Comp Neurol 493:607–626 [DOI] [PubMed] [Google Scholar]
- 51.Ma M, Bors EK, Dickinson ES, Kwiatkowski MA, Sousa GL, Henry RP, Smith CM, Towle DW, Christie AE, Li L (2009) Characterization of the Carcinus maenas neuropeptidome by mass spectrometry and functional genomics. Gen Comp Endocrinol 161:320–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wegener C, Gorbashov A (2008) Molecular evolution of neuropeptides in the genus Drosophila. Genome Biol 9:R131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neupert S, Russell WK, Russell DH, Lopez JD Jr, Predel R, Nachman RJ (2009) Neuropeptides in Heteroptera: identification of allatotropin-related peptide and tachykinin-related peptides using MALDI-TOF mass spectrometry. Peptides 30:483–488 [DOI] [PubMed] [Google Scholar]
- 54.Neupert S, Predel R, Russell WK, Davies R, Pietrantonio PV, Nachman RJ (2005) Identification of tick periviscerokinin, the first neurohormone of Ixodidae: single cell analysis by means of MALDI-TOF/TOF mass spectrometry. Biochem Biophys Res Commun 338:1860–1864 [DOI] [PubMed] [Google Scholar]
- 55.Taban IM, Altelaar AF, van der Burgt YE, McDonnell LA, Heeren RM, Fuchser J, Baykut G (2007) Imaging of peptides in the rat brain using MALDI-FTICR mass spectrometry. J Am Soc Mass Spectrom 18:145–151 [DOI] [PubMed] [Google Scholar]