Abstract
BACKGROUND & AIMS:
Biliary atresia (BA) results from a neonatal inflammatory and fibrosing obstruction of bile ducts of unknown etiology. Although the innate immune system has been linked to virus-induced mechanism of disease, the role of the inflammasome-mediated epithelial injury remains largely undefined. Here, we hypothesized that disruption of the inflammasome suppresses the neonatal proinflammatory response and prevents experimental BA.
METHODS:
We determined the expression of key inflammasome-related genes in livers from infants at diagnosis of BA and in extrahepatic bile ducts (EHBDs) of neonatal mice after infection with rotavirus (RRV) immediately after birth. Then, we determined the impact of the wholesale inactivation of the genes encoding IL-1R1 (Il1r1−/−), NLRP3 (Nlrp3−/−) or Caspase-1 (Casp1−/−) on epithelial injury and bile duct obstruction.
RESULTS:
IL1R1, NLRP3 and CASP1 mRNA increased significantly in human livers at the time of diagnosis, and in extrahepatic bile ducts of RRV-infected mice. In Il1r1−/− mice, the epithelial injury of EHBDs induced by RRV was suppressed, with an inability of dendritic cells (DCs) to activate natural killer (NK) cells. A similar protection was observed in Nlrp3−/− mice, with decreased injury and inflammation of livers and EHBDs. Long-term survival was also improved. In contrast, the inactivation of the Casp1 gene had no impact on tissue injury, and all mice died. Tissue analyses in Il1r1−/− and Nlrp3−/− mice showed decreased population of DC and NK cells and suppressed the expression of type-1 cytokines and chemokines.
CONCLUSIONS:
Inflammasome genes are overexpressed at diagnosis of BA in humans and in the BA mouse model. In the experimental model, the targeted loss of IL1R1 or NLRP3, but not of Capase-1, protected neonatal mice against RRV-induced bile duct obstruction.
Keywords: Biliary atresia, NLRP3, IL-1 signaling, epithelial injury
Introduction
Biliary atresia (BA) results from a rapidly progressing inflammation and obstruction of extrahepatic bile ducts (EHBDs) in early infancy and is the most common indication for pediatric liver transplantation1–3. The etiology of BA includes environmental triggers in the genetically susceptible host4, 5, followed by an over-activation of the neonatal immune response in the liver and EHBD6, 7. We and others previously reported several cellular (dendritic cells [DCs], natural killer [NK] cells and CD8+ T cells) and molecular (IL-8, IL-15, IFN-γ, TNFα) effectors of bile duct epithelial injury8–16. Despite this progress, very little is known about how molecular sensors and related circuits regulate the hepatobiliary injury and duct obstruction in BA.
The activation of the innate immune system in liver diseases is functionally related to the activation of NLRP3 (NOD-, LRR- and pyrin domain-containing 3) and its related molecules of the inflammasome17–20. Upon recognizing danger signals from pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), NLRP3 is activated, followed by the recruitment of the adapter apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC), cleavage of procaspase-1 into activated caspase-1, and the maturation and secretion of IL-1β and IL-18, inducing pyroptosis and inflammatory tissue injuries21. In this paper, we investigated the role of the inflammasome in biliary atresia. First, we quantified the expression of inflammasome-related genes in diseased tissues based on the role of this molecular cascade as an effector of tissue injury. Human livers and the EHBD of experimental BA had increased mRNA expression for IL1R1, NLRP3 and CASP1. Then, to test the hypothesis that disruption of the inflammasome suppresses the neonatal proinflammatory response, we injected rhesus rotavirus type A (RRV) into mice lacking IL-1R1, NLRP3 or Caspase-1. Among these molecules, the loss of NLRP3 most predominantly suppressed the phenotype of experimental BA, with decreased epithelial injury, bile duct obstruction, and increased survival.
Materials and Methods
Human Tissue, Microarray and Immunohistochemical Analyses
We obtained mRNA expression data from the genome-wide expression datasets of human liver samples of BA, intrahepatic cholestasis and healthy controls reported by us previously and submitted to the Gene Expression Omnibus (data accessible at NCBI GEO database; accession GSE46995)22. The datasets were extracted as Affymetrix CEL files and analyzed with the GeneSpring software (Agilent Technologies, Santa Clara, CA, USA) for analyses. Immunohistochemistry was performed in de-identified paraffin-embedded liver sections from our biorepository (obtained at the time of diagnosis of BA and age-matched infants with intrahepatic cholestasis); normal control sample consisted of a biobanked biopsy sample from a liver donor. Immunohistochemistry was performed on paraffinembedded tissue sections from human subjects as described previously (13), using rabbit polyclonal antibodies that recognized human NLRP3 (HPA012878, 1:200), CASP1 (HPA003056, 1:500) and IL1R1 (HPA005823, 1:500), all from SIGMA life science, MO USA, after blocking with goat serum overnight at 4°C. Biotinylated anti-rabbit antibody (Vector Laboratories) was used as secondary antibody followed by detection using avidin/biotin (VECTASTAIN ABC reagent PK-4001; Vector Laboratories) and the 3,3’diaminobenzidine (DAB) substrate (Vector kit, SK-4100; Vector Laboratories).
Experimental model of BA
Wild type (WT) BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA) and IL1R1-deficient (Il1r1−/−) mice were a kind gift from Holly Rosenzweig (Department of Opthalmology, Oregon Health & Science University, Portland, OR). To generate mice lacking functional NLRP3 (Nlrp3−/−) mice or Caspase-1 (Casp1−/−), we backcrossed individual knockout mice with WT BALB/c female mice for 12 generations to obtain a breeding pair of heterozygous mice to generate homozogous male and female mice. Then, newborn Il1r1−/−, Nlrp3−/−, Caspase-1−/− and WT BALB/c mice were injected intraperitoneally with 1.5 × 105 fluorescent-forming units (ffu) of RRV in 20 μl, or equal volume of normal saline (NS) as control, within 24 hours of birth to induce experimental BA as described previously9, with main pathobiological processes triggered by RRV that include: 1) epithelial injury and obstruction of EHBD, 2) inflammation and expansion of portal tracts with secondary hepatocyte injury, 3) persistent increase in serum bilirubin and aminotransferases, and 4) mortality in >90% of mice. Of note, livers have fibrosis but the mice die before the development of cirrhosis. Although interrelated, these processes may be differentially impacted by individual mechanisms of tissue injury8–16. At the time of serum and tissue harvest, we sacrificed all mice in each litter and analyzed tissues and cells individually (when needing to correlate with clinical phenotype) or polled (when tissue or number of cells were not enough for the assays) to avoid any potential bias that could be incurred from a purposeful selection of individual mice. The Institutional Animal Care and Use Committee (IACUC) of the Cincinnati Children’s Research Foundation approved all animal experiments and protocols.
Histopathology and serum hepatic biochemistry
Livers, EHBDs, and other organs were harvested from neonatal mice using a dissecting microscope. Paraffin-embedded sections of livers, EHBDs, and kidneys were examined after staining with hematoxylin and eosin. Total bilirubin (Total Bilirubin Reagent Set; Pointe Scientific, Inc. Canton, MI) and alanine transaminase (ALT; DiscretPak ALT Reagent Kit; Catachem, Inc. Oxford, CT) were quantified in serum samples according to the manufacturer’s instructions and as described previously9.
Flow Cytometric Analysis
Mononuclear cells were isolated from livers of neonatal mice as described previously and subjected to antibody-staining for flow cytometric analysis using fluorescence-activated cell sorting (FACS) Canto II dual-laser flow cytometer (BD Biosciences); data were analyzed using the FlowJo software (Tree Star Inc., Ashland, OR). Fluorochrome-conjugated mouse antibodies were detailed in CTAT tables of supplementary materials.
mRNA expression by real time PCR
RNA was isolated from livers and EHBD of RRV- or saline-injected mice using the RNeasy Mini Kit according to the manufacturer’s protocol (QIAGEN), as described previously. Gene expression was quantified by real time PCR using specific primers and a Stratagene Mx3005P thermocycler (Agilent Technologies, Inc., Santa Clara, CA) using specific primers (detailed in CTAT table 1.4 sequence based reagents).
Isolation and co-culture of hepatic NK and DC cells
Hepatic NK cells were purified using magnetic-activated cell sorting (MACS) CD49b (DX5) Microbeads and MidiMACS Separator (Miltenyi Biotec) as described previously10.
Hepatic DCs were purified from minced liver tissue incubated with collagenase IV (SigmaAldrich) for 30 minutes at 37°C in RPMI and centrifuged in 33% percoll. Single cell suspension of DCs was obtained by MACS according to the manufacturer’s protocol (Miltenyi Biotec). In brief, cells were incubated with anti-mPDCA1 micro beads and passed over 2 consecutive LS columns to enrich pDC populations. For isolation of Cd11c+, hepatic mononuclear cells were incubated with pan DC micro beads (Miltenyi Biotec) on 2 consecutive magnetic bead columns. The purity of individual DC populations was around 90% as determined by flow cytometric analysis. Co-culture of purified DCs with NK cells was performed using 1:1 ratio (1×105 cells) in a U bottom 96-well plate at 37°C for 48 hours. After the co-culture assay, cells were pooled and the activation markers of NK cells were measured by Nkg2d and Cd107a staining on Cd49+ gated cells by flow cytometric analysis.
Statistical analysis
Statistical analysis was performed using the GraphPad PRISM Version 6.03 Software (GraphPad Software, San Diego, CA). Values are expressed as mean ± standard deviation (SD) and statistical significance is determined by unpaired 2-tailed t test with significance set at P<0.05. Survival curves were created by Kaplan-Meier analysis. All experiments included WT as a control group to be compared with gene knockout mice; to control for the RRV challenge, saline was injected to separate groups for each knockout mouse strain. The numbers of mice for each group are described in the figure legends and in the text (where appropriate).
For further details regarding the materials used, please refer to the CTAT table and supplementary information.
Results
Increased expression of inflammasome-related genes
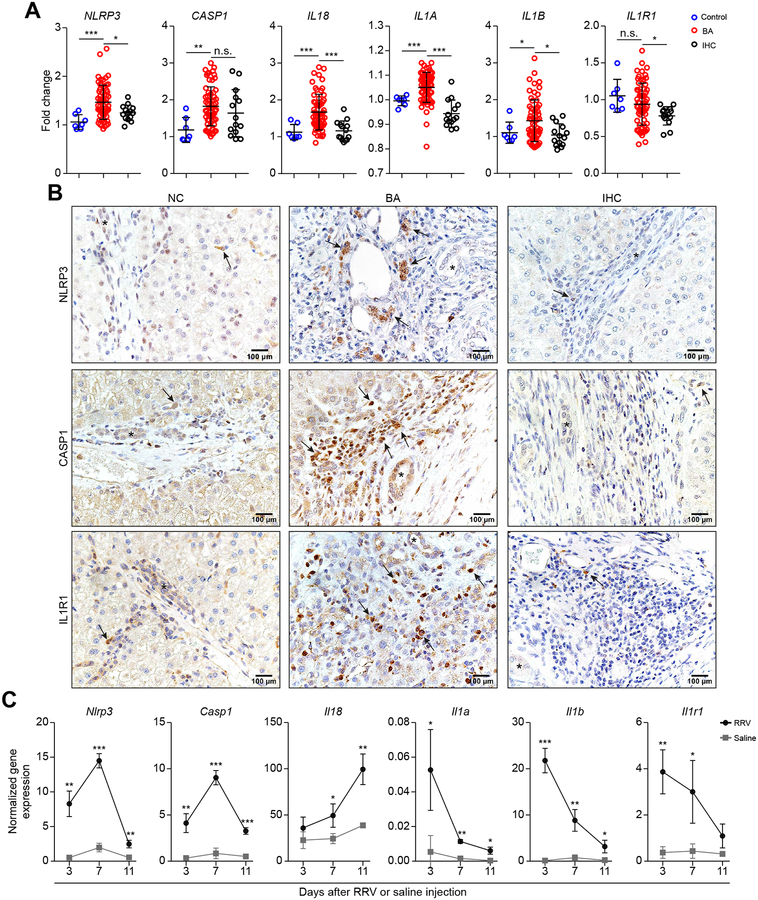
In search for preliminary evidence that elements of the inflammasome may be related to pathogenesis of BA, we first quantified the mRNA expression of inflammasome-related genes in livers from infants with BA at the time of diagnosis and from a control group without liver disease (NC) and a disease control of intrahepatic cholestasis (IHC). The expression of NLRP3, IL18, IL1A, and IL1B increased in BA above NC and IHC, with variable patterns for CASP1 and IL1R1 (Figure 1A). At the protein level, immunohistochemical staining showed increased numbers of portal mononuclear cells expressing NLRP3, CASP1 and IL1R1 (Figure 1B); we focused on these proteins because they were the subject of the mechanistic studies of this paper. To investigate whether these increases relate to pathogenesis of tissue injury at the mechanistic level, we first quantified the expression of these genes in the neonatal mouse model of BA. For these experiments, gene expression was quantified in EHBDs, the main initial site of tissue injury (tissue that is not available for analysis in humans). In EHBDs, Nlrp3, Casp1, Il18, Il1a, Il1b, and Il1r1 increased at most time points of epithelial injury (3 days), lumen obstruction (7 days), and atresia (11 days) (Figure 1C). The combined human and murine data implied that RRV infection activated the inflammasome, and raised the possibility that this circuit may be relevant to bile duct injury.
Fig. 1. Expression of inflammasome in human livers and murine EHBDs.
(A) mRNA expression in human livers of BA at diagnosis (BA; N=64, <6 months of age) compared to children without liver disease (control; N=7, 22–42 months of age) and intrahepatic cholestasis (IHC; N=14, < 6 months of age). (B) Immunohistochemistry staining of NLRP3, CASP1 and IL1R1 proteins in portal tracts of human liver sections (arrows denote positive signals). (C) mRNA expression in murine EHBDs at 3, 7, and 11 days; N=3 per group/time point. Expression levels were normalized to Hprt. *P < 0.05, **P < 0.01, ***P < 0.001.
Loss of IL-1R1 suppresses bile duct injury and improves cholestasis
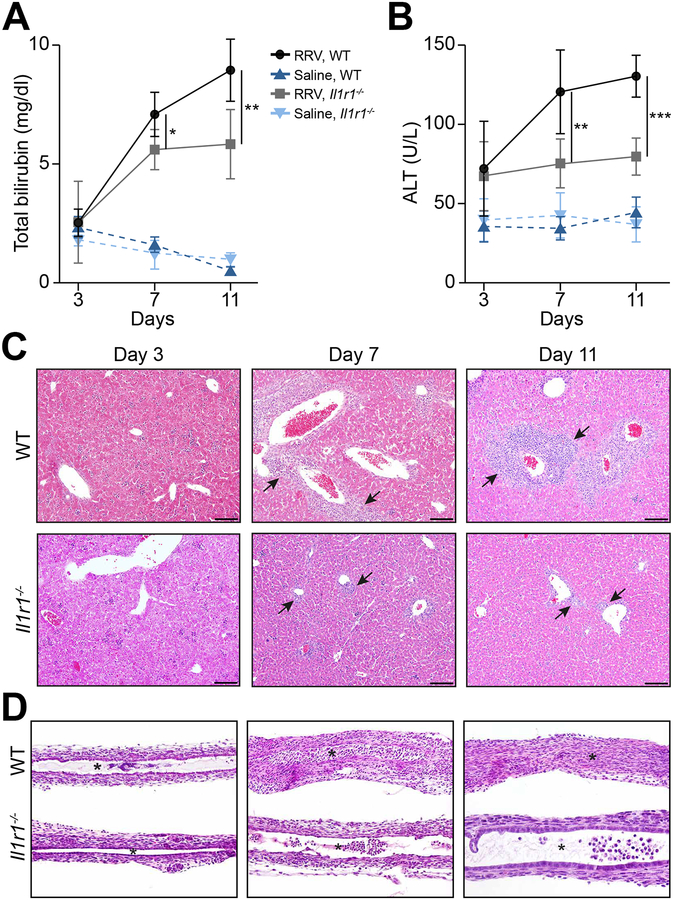
To directly explore how these genes relate to bile duct injury, we first investigated IL-1α/β→IL-1R1 signaling based on their simultaneous increase in mRNA expression during the epithelial and obstructive phases of bile duct injury. We injected RRV or saline into Il1r1−/− and WT newborn mice in the first 24 hours of life. In WT mice, RRV resulted in jaundice within 7 days, similar to findings reported previously23. In Il1r1−/− mice, RRV also induced jaundice, but a comparison of serum total bilirubin showed lower levels than WT mice, which was associated with lower serum ALT (Figure 2A and B). Consistent with a decrease in hepatobiliary injury, the histology of Il1r1−/− livers showed less portal inflammation (Figure 2C). In EHBDs, the loss of IL-1R1 delayed the epithelial injury to 7 days (compared to 3 days in WT mice), which recovered by day 11 days and was not accompanied by lumen obstruction (N=13 for Il1r1−/− mice; N=15 for WT mice; Figure 2D). The pattern of delayed epithelial injury in Il1r1−/− mice pointed to a potential role for IL-1R1 signaling in the early activation of the neonatal immune response to RRV.
Fig. 2. Loss of IL-1R1 improves cholestasis and tissue injury.
(A and B) Serum total bilirubin and ALT levels in WT and Il1r1−/− mice. N=3–7 per group/time point; *P <0.05, **P <0.01, ***P < 0.001. Representative sections stained with H&E showing improvement in portal inflammation (liver, panel C) and restoration of duct epithelium and patent lumen (EHBD, panel D) in Il1r1−/− mice after RRV (compared to WT controls). N=13 for Il1r1−/− mice; N=15 for WT mice. Arrows denote portal tracts; *Denotes duct lumen; scale bar: 100μm; magnification=100× for EHBD.
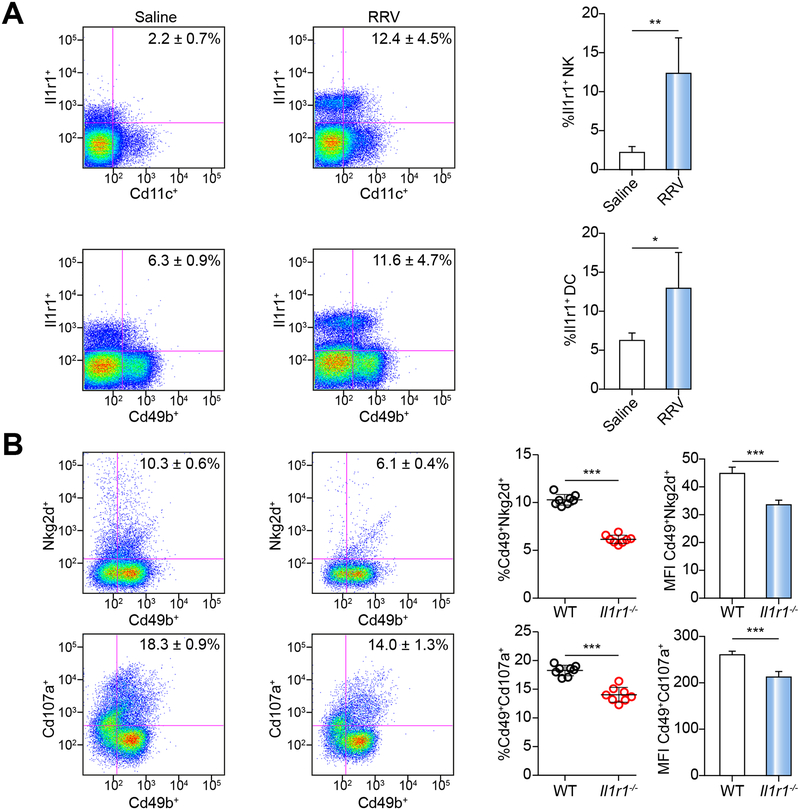
The lack of IL-1R1 suppresses the activation of NK cells by DCs
To investigate the potential mechanisms by which the lack of IL-1R1 protects the duct epithelium, we quantified the protein expression in DC and NK cells by FACS. We focused on these cells because of a previous report that the activation of DCs by RRV is an important mechanism of NK cell-dependent injury of RRV-infected cholangiocytes10, 12, 24. In WT mice, the expression of IL-1R1 increased in hepatic DC and NK cells 7 days after RRV (Figure 3A). To test if the increased IL-1R1 is important for NK cell activation, we harvested DCs from livers of Il1r1−/− and WT mice 3 days after RRV infection and co-cultured them with hepatic NK cells from non-infected WT mice. After 48 hours of co-culture, we assessed NK cell activation by FACS. The co-culture of RRV-primed DCs with RRV-naïve NK cells induced the overexpression of Nkg2d and Cd107a in NK cells from WT mice, which was not observed in NK cells from Il1r1−/− mice (Figure 3B). Taken together, these results indicate that the expression of IL-1R1 by DCs is an important mechanism used to activate NK cells and injure the biliary epithelium after RRV. These findings raised the possibility that other inflammasome proteins upstream of IL-1α/β may have similar properties in driving epithelial injury of bile ducts in experimental BA.
Fig. 3. IL-1R1 expression and function in DC and NK cells.
(A) Flow cytometric analysis of IL-1R1 expressing dendritic cells (Cd11c+) and NK cells (Cd49b+) at 7 days after saline or RRV injection; N=4 livers per group/time point. *P < 0.05, **P < 0.01. (B) Expression levels of Nkg2d and Cd107a on naïve NK cells. N=8 livers per group. MFI, mean fluorescent intensity. ***P < 0.001.
Genetic depletion of Nlrp3 suppresses tissue injury and improves survival
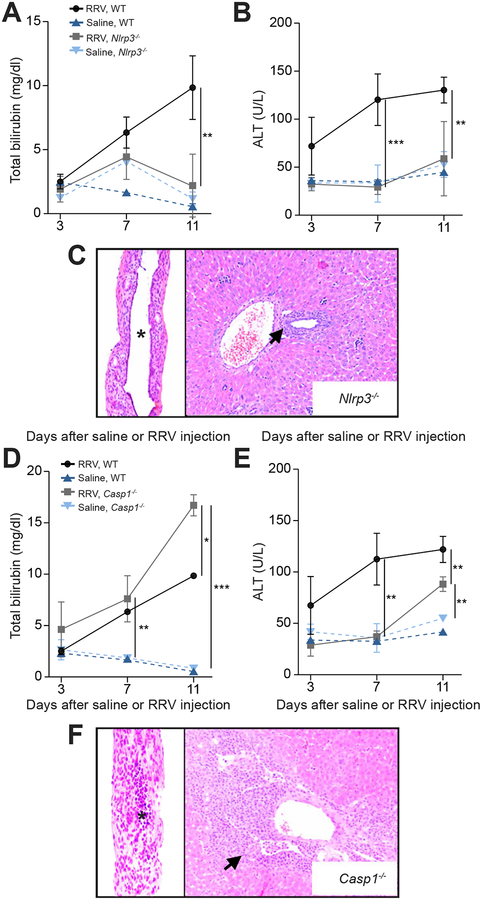
Based on the early position of NLRP3 in the inflammasome signaling and on the mRNA increase in human livers and EHBDs of the experimental model, we applied the same RRV-induced experimental challenge to Nlrp3–/– mice. Serum levels of total bilirubin increased at 7 days in Nlrp3−/− mice, but decreased to baseline levels at day 11 after RRV challenge, with serum ALT levels remaining at baseline at both time points (Figure 4A and B). Consistent with this biochemical improvement, the lobular and portal inflammation of Nlrp3−/− mice were mild and the EHBDs had intact epithelium and no evidence of lumen obstruction (Figure 4C) (N=17 for Nlrp3−/− mice; N=14 for Casp1−/− mice). To quantify the degree of biliary epithelial injury in Nlrp3−/− and Il1r1−/− mice compared to WT mice, we calculated the epithelial surface area of EHBD after RRV in all these knockout mice compared to WT. Morphometric quantification showed the highest epithelial surface area in EHBDs of Nlrp3−/− mice, which was above the surface area of EHBDs from Il1r1−/− and WT mice (P<0.05; Supplementary Figure 1).
Fig. 4. Genetic depletion of NLRP3 suppresses cholestasis, portal inflammation and bile duct obstruction.
Serum total bilirubin (A and D) and ALT levels (B and E) in Nlrp3−/−, Casp1−/− and WT mice; N=3–7 per group/time point. *P < 0.05, **P < 0.01, ***P < 0.001. (C and F) Representative panels of H&E staining of liver and EHBD 11 days after RRV injection in Nlrp3−/− (N=17), with intact epithelium and patent lumen (C), while Casp1−/− mice (N=14) have obstructed lumen and expanded portal inflammation (F). Arrows denote portal tracts. * denotes duct lumen; magnification × 200.
Next, we applied the same RRV challenge to Casp1−/− mice to investigate whether Caspase-1 mediates the molecular signal downstream of NLRP3. In Casp1−/− mice, the serum bilirubin levels increased at 7 and 11 days despite a less prominent increase in ALT (Figure 4D and E), with most mice dying after 11 days. Histological investigation at 11 days after RRV infection revealed severe portal inflammation in the liver and a diffuse epithelial injury of EHBDs, with evidence of lumen obstruction (Figure 4F), similar to the pattern seen in WT mice infected with RRV.
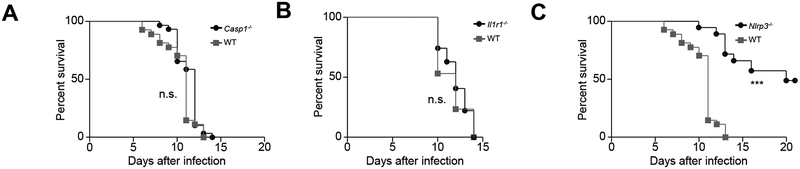
The early death of Casp1−/− mice prompted us to perform survival analyses in all three knockout mouse lines. After RRV infection, 100% of Casp1−/− mice died by 14 days (Figure 5A), with all mice showing obstructed/atretic bile ducts. In Il1r1−/− mice, despite the improved liver histology and patent EHBDs (discussed above), the loss of IL-1R1 resulted in 100% death by 14 days after RRV (Figure 5B), suggesting non-hepatic causes for the generalized mortality. In histological surveys of several tissues in search for the potential causes of death in Il1r1−/− mice, the only non-hepatic tissue with abnormalities was the kidney, which had a diffuse glomerular and tubular injury that was also present but less extensive in RRV-infected WT mice (Supplementary Figure 2A). In addition, assays to quantify RRV from several tissues revealed a persistence of RRV in the liver, EHBDs and spleens beyond 11 days of Il1r1−/− mice (Supplementary Figure 2B). Among all mouse strains, the best survival (48.6%) was of Nlrp3−/− mice, which was in keeping with the improved liver biochemistry and tissue histology discussed above (Figure 5C).
Fig. 5. Survival analysis of Casp1−/−, Il1r1−/−, and Nlrp3−/− mice.
Survival curves after RRV infection show increased mortality in WT, Casp1−/− and Il1r1−/− mice, while 48.6% of Nlrp3−/− mice survive beyond 15 days of life. N=29 for Casp1−/−, N=27 for of Il1r1−/−, N=34 for Nlrp3−/− mice. For WT control mice, N=27 in A and C, and N=17 in B. ***P < 0.001.
To investigate whether an aberrant pattern of RRV infection in the liver may relate to the severity of tissue injury and persistent elevation of liver biochemistry, we performed immunohistochemistry in paraffin-embedded sections 7 days after RRV infection. RRV was easily detected in cells of portal tracts of WT and Casp1−/− mice (consistent with the more severe phenotype), and either absent or occasionally present in Il1r1−/− and Nlrp3−/− mice (with less affected tissue histology) (Supplementary Figure 3). An examination of the hepatic parenchyma in several liver sections identified only occasional or no immunostaining of RRV in hepatocytes regardless of the mouse genotype or severity the experimental disease (Supplementary Figure 3). Altogether, the low survival of Il1r1−/− mice may be due to the RRV spread in non-hepatic tissues. In contrast, the findings of increased expression of RRV in portal tracts of WT and Casp1−/− mice may relate to their worse tissue injury and poor outcome, with minimal or no evidence of infected hepatocytes.
Loss of NLRP3 or IL-1R1 lowers the population and activation DC and NK cells
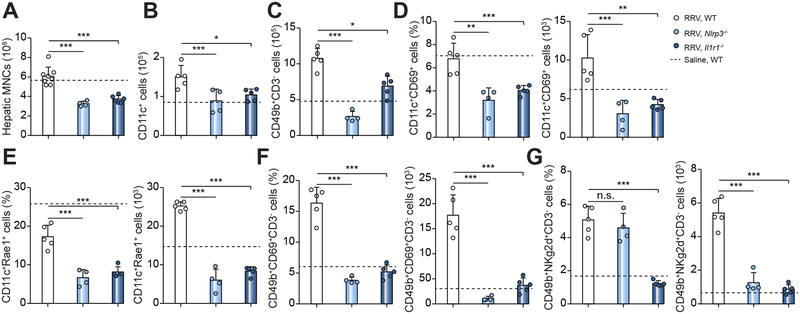
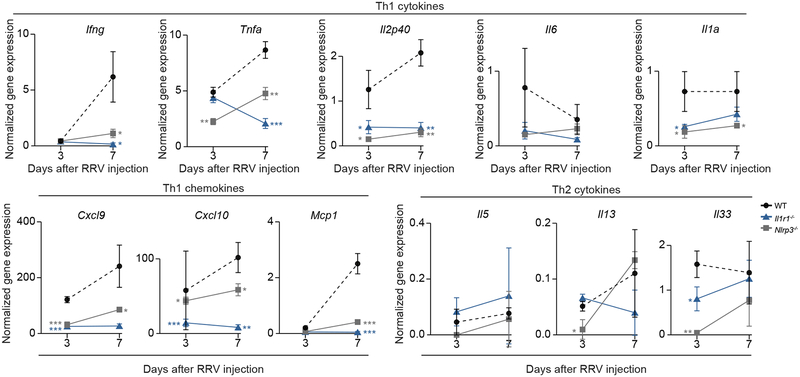
To explore the potential cellular mechanisms responsible for the improved tissue phenotype in Nlrp3−/− and Il1r1−/− mice, we evaluated the infiltration and activation of DCs and NK cells in vivo. We focused on these immune cells based on our earlier findings that IL-1R1 plays a regulatory role in the DC-dependent activation of NK cells in culture (above). We first quantified the number of total hepatic mononuclear cells, DCs and NK cells by FACS, and found that they were lower in Nlrp3−/− and Il1r1−/− mice than in WT mice 3 days after RRV challenge (Figure 6A–C). Then we checked the activation status of hepatic DCs and NK cells. Compared to RRV-challenged WT mice, the populations of Cd69+ or Rae1+ DCs and Cd69+ or Nkg2d+ NK cells were lower after RRV infection of Nlrp3−/− and Il1r1−/− mice than WT controls (Figure 6D–G). The quantification of cytokine expression at the mRNA level showed a suppression of the type 1 cytokines Ifng, Tnfa, IL12p40, and IL1a, and the chemokines Cxcl9, Cxcl10 and Mcp1, with no consistent increase in mRNA expression for IL6 or type2 cytokines IL5, IL13 or IL33 (Figure 7). Taken together, the results indicate that the disruption of the inflammasome signaling induced by the loss of NLRP3 or IL1R1 suppressed the infiltration and activation of DCs and NK cells within the liver and protected the duct epithelium against RRV-induced cholangiocyte injury.
Fig. 6. Population and activation of DCs and NK cells in neonatal livers after RRV infection.
Flow cytometric analysis of immune cells from livers at 3 days showing the lower number of hepatic total mononuclear cells (A), dendritic cells (CD11c+) (B), and NK cells (CD49b+) (C). The activation of DC and NK is blunted with lower percentage of DCs positive for CD69+ (D) or RAE1+(E), and NK cells positive for CD69+ (F) or NKg2d+ (G). N=4–8 livers per group. *P < 0.05, **P < 0.01, ***P < 0.001. The dash line represents values of WT mice injected with normal saline.
Fig. 7. Hepatic mRNA expression of proinflammatory genes after RRV infection.
mRNA expression of type-1 cytokines and chemokines as well as type-2 cytokines at 3 and 7 days after RRV injection in Nlrp3−/−, Il1r1−/− and WT mice. Data were normalized to Hprt; N=3 per group/time point. *P < 0.05, **P <0.01, ***P < 0.001.
Discussion
We found that the expression of inflammasome-related genes increased in livers of infants with BA at the time of diagnosis and in bile ducts excised from neonatal mice subjected to a model of experimental BA. Directly examining their regulatory role in pathogenesis of tissue injury, we found that the targeted inactivation of the Nlrp3 and Il1r1 genes decreased epithelial injury and prevented the obstruction of EHBDs, while the loss of functional Caspase-1 did not protect against the development of the full phenotype of experimental BA. In an in vitro co-culture system, DCs lacking IL-1R1 signaling failed to activate NK cells upon exposure to RRV. Consistent with this finding, the loss of NLRP3 or and IL-1R1 decreased the hepatic infiltration and activation of DC and NK cells and suppressed expression of type 1 cytokines and chemokines after RRV infection. Collectively, the data are in keeping with a regulatory role of specific components of the inflammasome in the pathogenic mechanisms of bile duct injury in experimental BA.
The epithelial injury in experimental BA has been mechanistically linked to innate immunity12, 25–27. Here, we extend the innate signals to NLRP3 and IL-1R1, individual elements of a molecular complex responsive to danger signals from infection, stress or sterile damage. NLRP3 has already been linked to liver injury in hepatitis C20, alcoholic liver disease and nonalcoholic fatty liver disease and fibrosis17, 28, as well as some autoimmune diseases29, metabolic syndrome, and cancer30, 31. IL-1R1 is linked to the inflammasome by its binding to IL-1β, a proinflammatory cytokine whose activation is downstream of NLRP332. Upon binding, IL-1R1 signaling is an important mediator of the innate immunity and inflammation in diseases of the liver17, 18 and other systems33. Together, IL-1β and IL-18 have been shown to jointly drive pathology of diseases, but do so at different stages of the tissue injury34. In this regard, the early increase in mRNA expression for IL1b within 3 days of RRV, compared to the increase in IL18 at days 7 and 11, suggested that IL-1β may be one of the initiators of immune-mediated injury in experimental BA.
In keeping with a key role for IL-1R1, impairment in its signals rendered DCs unable to express the activating properties to NK cells, decreased the hepatic infiltration by DCs and NK cells, and substantially decreased the epithelial injury induced by RRV. This finding is consistent with the previous reports that depletion of DCs or NK cells prevents cholangiocyte injury and maintains the continuity of bile duct epithelium in experimental BA12. Despite the prevention of tissue injury, the loss of IL-1R1 did not improve mortality. In regard to the cause of death in the WT mice infected with RRV, the severity of liver injury and obstructive cholestasis in infected mice are major pathological contributors, but the poor nutrition and dehydration secondary to maternal neglect of the sick pup are other important factors. These factors were also present in Casp1−/− mice, but in Il1r1−/− mice the combination of an extensive glomerular and tubular kidney injury and the persistence of RRV in EHBD, livers, and spleen beyond 11 days point to potential influences of ongoing virus insult and extrahepatic spread in the decreased survival of mice lacking IL1-R1. Thus, the cause of death in experimental biliary atresia may vary depending on the molecule being targeted and its role in specific biological circuit. In contrast, the overall improvement in Nlrp3 mutant mice probably results from a suppressed inflammatory response that allows for epithelial repair of extrahepatic bile ducts and less liver inflammation. Based on these data, any potential inflammasome-based therapies would need to target Nlrp3 to suppress inflammation and facilitate hepatobiliary repair.
With the protective phenotype induced by the loss of NLRP3 and IL-1R1, it was surprising that the inactivation of the Casp1 gene had no impact on the epithelial injury, duct obstruction and the full expression of the BA phenotype in neonatal mice. The intermediate position of Caspase-1 downstream from NLRP3 and upstream of IL-1β suggest that the proinflammatory properties of NLRP3 may use non-canonical signaling pathways to produce epithelial injury without a molecular dependence on the activating cleavage of pro-IL-1β via caspase-1. Although we have not formally examined how Caspase-1 directly induces or protects epithelial injury, the ongoing injury and obstruction of EHBDs in Casp1−/− mice may suggest that Caspase-1 is not at all involved in pathogenesis of biliary injury or may directly or indirectly suppress inflammation and minimize epithelial injury in experimental biliary atresia. Such a paradigm is consistent with the previous report that Caspase-1 is actually hepatoprotective by reducing liver injury induced by inflammatory insults35. In the future, based on the recognized role of the inflammasome in modulating the effects of the intestinal microbiota in hepatic and biliary physiology, we will perform comprehensive analyses of the intestinal microbiome in WT and knockout mice, followed by mechanistic experiments to more precisely identify potential bacterial elements that may influence the activation of DC and NK cells and injury of EHBDs in experimental biliary atresia.
In summary, we found that the disruption of the inflammasome by the loss of NLRP3 or IL-1R1 signaling suppressed the activation of DCs and their ability to activate NK cells. As a consequence, neonatal mice became resistant to the epithelial injury-inducing properties of RRV, maintained the patency of EHBDs, and prevented the experimental phenotype of BA. The inferred roles of NLRP3 and IL-1R1 in regulating the early cellular activation advances our understanding of the early events that commit the neonatal innate immune system towards the infected biliary epithelium, and sets in motion the adaptive immune response that populates the livers of neonatal mice and infants with BA. The findings also raise the possibility that the modulation of inflammasome signaling may represent potential novel therapeutic approaches to block progression of the disease.
Supplementary Material
Acknowledgements
We thank Bryan Donnelly for the assays to titer the RRV load in hepatic, biliary and non-hepatic tissues.
Financial support statement: Supported by the NIH grants DK-64008 and DK-83781 to JAB and by the Integrative Morphology and the Gene Analysis Cores of the Digestive Health Center (DK-78392; JAB). Dr. Yang Li received the STAT (Scientific Training Award for young Talents) Award from Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. The funding sources had no involvement in study design, collection/analysis/interpretation of data, or preparation of the article for publication.
Footnotes
Conflict of interest:
The authors have declared that no conflict of interest exists.
References
- [1].Shneider BL, Moore J, Kerkar N, Magee JC, Ye W, Karpen SJ, et al. Initial assessment of the infant with neonatal cholestasis-Is this biliary atresia? PLoS One 2017;12:e0176275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Feldman AG, Mack CL. Biliary Atresia: Clinical Lessons Learned. J Pediatr Gastroenterol Nutr 2015;61:167–175. [DOI] [PubMed] [Google Scholar]
- [3].Sundaram SS, Mack CL, Feldman AG, Sokol RJ. Biliary Atresia: Indications and Timing of Liver Transplantation and Optimization of Pretransplant Care. Liver Transplant 2017;23:96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brindley SM, Lanham AM, Karrer FM, Tucker RM, Fontenot AP, Mack CL. Cytomegalovirus-specific T-cell reactivity in biliary atresia at the time of diagnosis is associated with deficits in regulatory T cells. Hepatology 2012;55:1130–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xu Y, Yu JK, Zhang RZ, Yin YX, Ye JW, Tan LM, et al. The Perinatal Infection of Cytomegalovirus Is an Important Etiology for Biliary Atresia in China. Clin Pediatr 2012;51:109–113. [DOI] [PubMed] [Google Scholar]
- [6].Asai A, Miethke A, Bezerra JA. Pathogenesis of biliary atresia: defining biology to understand clinical phenotypes. Nat Rev Gastroenterol Hepatol 2015;12:342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mack CL, Feldman AG, Sokol RJ. Clues to the etiology of bile duct injury in biliary atresia. Semin Liver Dis 2012;32:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bezerra JA, Tiao G, Ryckman FC, Alonso M, Sabla GE, Shneider B, et al. Genetic induction of proinflammatory immunity in children with biliary atresia. Lancet 2002;360:1653–1659. [DOI] [PubMed] [Google Scholar]
- [9].Shivakumar P, Campbell KM, Sabla GE, Miethke A, Tiao G, McNeal MM, et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest 2004;114:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shivakumar P, Sabla GE, Whitington P, Chougnet CA, Bezerra JA. Neonatal NK cells target the mouse duct epithelium via Nkg2d and drive tissue-specific injury in experimental biliary atresia. J Clin Invest 2009;119:2281–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shivakumar P, Sabla G, Mohanty S, McNeal M, Ward R, Stringer K, et al. Effector role of neonatal hepatic CD8(+) lymphocytes in epithelial injury and autoimmunity in experimental biliary atresia. Gastroenterology 2007;133:268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Saxena V, Shivakumar P, Sabla G, Mourya R, Chougnet C, Bezerra JA. Dendritic cells regulate natural killer cell activation and epithelial injury in experimental biliary atresia. Sci Transl Med 2011;3:102–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shivakumar P, Mizuochi T, Mourya R, Gutta S, Yang L, Luo Z, et al. Preferential TNFalpha signaling via TNFR2 regulates epithelial injury and duct obstruction in experimental biliary atresia. JCI Insight 2017;2:e88747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Okamura A, Harada K, Nio M, Nakanuma Y. Participation of natural killer cells in the pathogenesis of bile duct lesions in biliary atresia. J Clin Pathol 2013;66:99–108. [DOI] [PubMed] [Google Scholar]
- [15].Liu YJ, Li K, Yang L, Tang ST, Wang XX, Cao GQ, et al. Dendritic Cells Regulate TregTh17 Axis in Obstructive Phase of Bile Duct Injury in Murine Biliary Atresia. PLoS One 2015;10:e0136214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lages CS, Simmons J, Chougnet CA, Miethke AG. Regulatory T cells control the CD8 adaptive immune response at the time of ductal obstruction in experimental biliary atresia. Hepatology 2012;56:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tilg H, Moschen AR, Szabo G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2016;64:955–965. [DOI] [PubMed] [Google Scholar]
- [18].Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 2014;59:898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Inoue Y, Shirasuna K, Kimura H, Usui F, Kawashima A, Karasawa T, et al. NLRP3 regulates neutrophil functions and contributes to hepatic ischemia-reperfusion injury independently of inflammasomes. J Immunol 2014;192:4342–4351. [DOI] [PubMed] [Google Scholar]
- [20].Negash AA, Ramos HJ, Crochet N, Lau DTY, Doehle B, Papic N, et al. IL-1 beta Production through the NLRP3 Inflammasome by Hepatic Macrophages Links Hepatitis C Virus Infection with Liver Inflammation and Disease. Plos Pathog 2013;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jo EK, Kim JK, Shin DM, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 2016;13:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bessho K, Mourya R, Shivakumar P, Walters S, Magee JC, Rao M, et al. Gene expression signature for biliary atresia and a role for interleukin-8 in pathogenesis of experimental disease. Hepatology 2014;60:211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Oetzmann von Sochaczewski C, Pintelon I, Brouns I, Dreier A, Klemann C, Timmermans JP, et al. Rotavirus particles in the extrahepatic bile duct in experimental biliary atresia. J Pediatr Surg 2014;49:520–524. [DOI] [PubMed] [Google Scholar]
- [24].Squires JE, Shivakumar P, Mourya R, Bessho K, Walters S, Bezerra JA. Natural killer cells promote long-term hepatobiliary inflammation in a low-dose rotavirus model of experimental biliary atresia. PLoS One 2015;10:e0127191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zagory JA, Nguyen MV, Dietz W, Mavila N, Haldeman A, Grishin A, et al. Toll-like receptor 3 mediates PROMININ-1 expressing cell expansion in biliary atresia via Transforming Growth Factor-Beta. J Pediatr Surg 2016;51:917–922. [DOI] [PubMed] [Google Scholar]
- [26].Clemente MG, Patton JT, Anders RA, Yolken RH, Schwarz KB. Rotavirus Infects Human Biliary Epithelial Cells and Stimulates Secretion of Cytokines IL-6 and IL-8 via MAPK Pathway. Biomed Res Int 2015;2015:697238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Venter J, Francis H, Meng F, DeMorrow S, Kennedy L, Standeford H, et al. Development and functional characterization of extrahepatic cholangiocyte lines from normal rats. Dig Liver Dis 2015;47:964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kong SH, Yeon JE, Jet JH, Lee YS, Yoo YJ, Suh SJ, et al. Nucleotide binding and oligomerization domain like receptors (NLR) inflammasomes in patients with nonalcoholic fatty liver disease (NAFLD). Hepatology 2016;64:792a–792a. [Google Scholar]
- [29].Lalor SJ, Dungan LS, Sutton CE, Basdeo SA, Fletcher JM, Mills KH. Caspase-1-processed cytokines IL-1beta and IL-18 promote IL-17 production by gammadelta and CD4 T cells that mediate autoimmunity. J Immunol 2011;186:5738–5748. [DOI] [PubMed] [Google Scholar]
- [30].Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol 2015;12:387–400. [DOI] [PubMed] [Google Scholar]
- [31].Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 2015;21:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Elliott EI, Sutterwala FS. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev 2015;265:35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 2009;15:1170–1178. [DOI] [PubMed] [Google Scholar]
- [34].Brydges SD, Broderick L, McGeough MD, Pena CA, Mueller JL, Hoffman HM. Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. J Clin Invest 2013;123:4695–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Menzel CL, Sun Q, Loughran PA, Pape HC, Billiar TR, Scott MJ. Caspase-1 is hepatoprotective during trauma and hemorrhagic shock by reducing liver injury and inflammation. Mol Med 2011;17:1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.