Abstract
Three-finger toxins (TFTs) comprise one of largest families of snake venom toxins. While they are principal to and the most toxic components of the venoms of the Elapidae snake family, their presence has also been detected in the venoms of snakes from other families. The first TFT, α-bungarotoxin, was discovered almost 50 years ago and has since been used widely as a specific marker of the α7 and muscle-type nicotinic acetylcholine receptors. To date, the number of TFT amino acid sequences deposited in the UniProt Knowledgebase free-access database is more than 700, and new members are being added constantly. Although structural variations among the TFTs are not numerous, several new structures have been discovered recently; these include the disulfide-bound dimers of TFTs and toxins with nonstandard pairing of disulfide bonds. New types of biological activities have also been demonstrated for the well-known TFTs, and research on this topic has become a hot topic of TFT studies. The classic TFTs α-bungarotoxin and α-cobratoxin, for example, have now been shown to inhibit ionotropic receptors of γ-aminobutyric acid, and some muscarinic toxins have been shown to interact with adrenoceptors. New, unexpected activities have been demonstrated for some TFTs as well, such as toxin interaction with interleukin or insulin receptors and even TFT-activated motility of sperm. This minireview provides a summarization of the data that has emerged in the last decade on the TFTs and their activities.
Keywords: Three-finger toxins, Snake, Venom, Structure, Biological activity
Core tip: The three-finger toxins (TFTs) of snake venoms are principal to and the most toxic components of elapid venoms. Over 700 TFT amino acid sequences are listed in the UniProt Knowledgebase currently, with new members added constantly. The past decade has also seen multitudinous new discoveries, including structural variations in TFTs (i.e. disulfide-bound dimers), new types of biological activities for the well-known TFTs (e.g., α-bungarotoxin’s inhibition of ionotropic receptors of γ-aminobutyric acid), and other new, unexpected activities for the TFTs (i.e. interaction with interleukin or insulin receptors and activation of sperm motility). This minireview provides an up-to-date overview of these data.
INTRODUCTION
Three-finger toxins (TFTs) form an abundant family of nonenzymatic proteins found in snake venoms[1]. The TFTs were so-named according to their characteristic spatial structure, in which three loops (fingers) protrude from the central core, stabilized by four conserved disulfide bonds. The TFTs contain from 57 to 82 amino acid residues, with some toxin types having an extra fifth disulfide bond, located in either their central loop II or N-terminal loop I. The position of the bond affects the toxin’s biological activity.
The TFTs manifest a wide array of biological activities, ranging from selective interaction with certain receptor types to nonselective cell lysis[2]. Typically, the TFTs represent the main components of elapid venoms[3]. Thus, in the venom of the desert coral snake Micrurus tschudii, 95% of toxins are represented by TFTs[4]. Their presence has also been detected in the venoms of snakes from other families. For example, TFTs were found in different snake genera from colubrid family[5,6]. The TFTs also represent one of the largest families of snake toxins, having more than 700 TFT amino acid sequences deposited in the UniProt Knowledgebase free-access database. Intriguingly, nontoxic structural counterparts of the TFTs have been found in animal organisms, namely the lymphocyte antigen 6 (Ly6) proteins. Based on their commonality of three-finger folding, the TFTs and Ly6 proteins are combined into one Ly6/neurotoxin family[7].
The first TFT discovered, α-bungarotoxin (α-Bgt), was published almost 50 years ago[8]. Since then, α-Bgt has become a widely used specific marker of the α7 and muscle-type nicotinic acetylcholine receptors (nAChRs). In addition, a tremendous number of other TFTs have been discovered, and new members of this family possessing original structures and biological activities are emerging constantly. Moreover, new activities have been recognized for the well-known TFTs, and the discovery of new activities for both the new and the well-known TFTs may be regarded as a recent trend in toxinology.
This minireview briefly summarizes the data obtained for TFTs during the last decade (Table 1). The toxins with new structural features appearing in the recent literature are considered herein; those that have garnered the most interest is the covalently-bound TFT dimers. In addition, the recently discovered uncommon biological activities of some TFTs are discussed; these include the so-called mambalgins that exert a potent analgesic effect upon central and peripheral injection and represent the most remarkable discovery of late.
Table 1.
Novel three-finger toxin biological effects and their potential applications
| Toxin | Effect/Target | Potential application | |
| Impact on signal transduction | Mambalgins | Inhibitors of ASICs | Analgesics |
| Micrurotoxins | Modulators of GABAA receptor | Biochemical instruments for the GABAA receptor study | |
| α-Neurotoxins | Inhibitors of GABAA receptor | ||
| Muscarinic toxin MTα | Antagonist of α2B-adrenoceptor | Treatment of blood pressure disorders | |
| Toxin CM-3 | Interaction with α1A-adrenoceptor | ||
| Toxin AdTx1 (ρ-Da1a) | Specific and selective inhibitor for the α1A-adrenoceptor | ||
| Toxin ρ-Da1b | Antagonist of α2A-adrenoceptors | ||
| Toxin Tx7335 | Potassium channel activator | Biochemical instrument for the study of potassium channels | |
| Calliotoxin (δ-elapitoxin-Cb1a) | Activator of voltage-gated sodium channel | Biochemical instrument for the study of sodium channels | |
| Impact on blood coagulation | Toxin KT-6.9 | Inhibitor of platelet aggregation | Treatment of blood coagulation disorders |
| Ringhalexin | Inhibitor of FX activation | ||
| Exactin | Inhibitor of FX activation | ||
| Insulinotropic activity | Cardiotoxin-I | Induction of insulin secretion from β-cells | Treatment of type 2 diabetes |
| Impact on sperm motility | Actiflagelin | Activator of sperm motility in vitro | Infertility treatment |
ASIC: Acid sensing ion channel; GABAA: Type A receptor of gamma-amino butyric acid; FX: Factor X.
RECENTLTY-DISCOVERED TFTS WITH NEW STRUCTURAL FEATURES
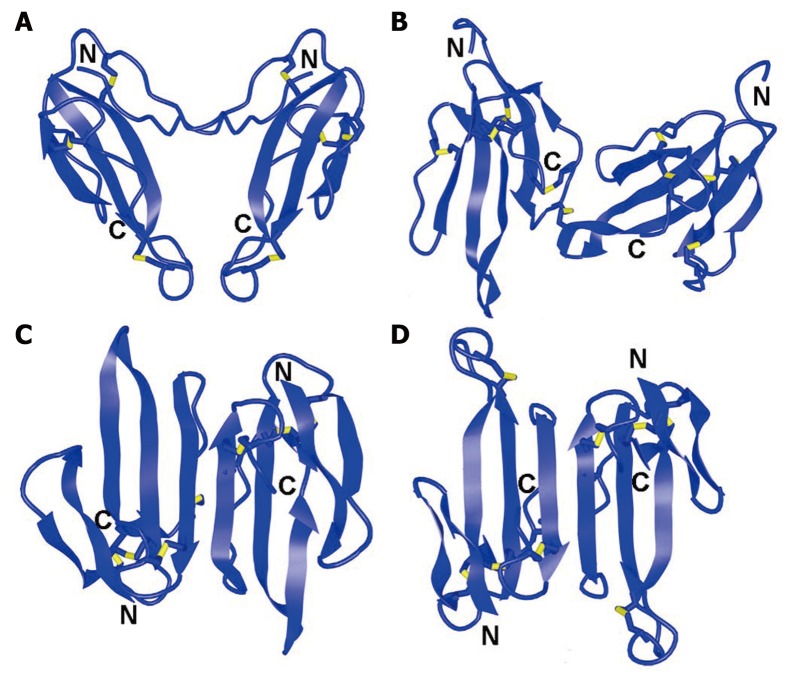
Ten years ago, the first data revealing covalently-bound TFT dimers were published[9]. Disulfide-bound dimers of TFTs, including heterodimers formed by α-cobratoxin (α-CTX; a long-chain α-neurotoxin) with different cytotoxins and the homodimer of α-CTX were isolated from the Naja kaouthia cobra venom. Determination of the homodimer crystal structure allowed identification of the intermolecular disulfides formed by Cys3 in the protomer one and Cys20 in the protomer two, and other way round (Figure 1A). All other disulfides in protomers had the same pairing as in natural α-CTX[10].
Figure 1.
Spatial structures of dimeric three-finger toxins. A: Homodimer of α-cobratoxin, Protein Data Bank Identification code (PDB ID): 4AEA; B: Irditoxin, PDB ID: 2H7Z; C: Haditoxin, PDB ID: 3HH7; D: κ-bungarotoxin, PDB ID: 1KBA. Disulfide bonds are shown in yellow. N and C indicate N- and C-terminus, respectively.
The dimerization itself strongly affected the biological activity of the original toxins, with the cytotoxic activity of cytotoxins within dimers being completely abolished. However, the dimers were found to retain most of the α-CTX capacity to interact with Torpedo and α7 nAChRs as well as with the Lymnea stagnalis acetylcholine-binding protein. Moreover, in contrast to the α-CTX monomer, the α-CTX dimer acquired the capacity to interact with α3β2 nAChR, similar to that seen with κ-bungarotoxin (κ-Bgt), a dimer with no disulfides between its monomers (Figure 1D). Collectively, these data show that dimerization of three-fingered neurotoxins is essential for binding to heteromeric α3β2 nAChRs.
In 2009, from venom of the brown cat snake Boiga irregularis a new heterodimeric TFT, irditoxin, was isolated[11]. Irditoxin spatial structure determined by X-ray analysis displayed two subunits possessing a three-finger fold, characteristic for nonconventional toxins (Figure 1B). The subunits in the irditoxin dimer are connected by an interchain disulfide bond, formed by extra cysteine residues present in each subunit. In contrast to the dimeric toxins discussed above, irditoxin does not inhibit mouse neuronal α3β2 and α7 nAChRs. However, irditoxin is a bird- and reptile-specific postsynaptic neurotoxin which inhibits the chick muscle nAChR three orders of magnitude more efficiently than the mouse receptor. In vivo, it was lethal to birds and lizards and was nontoxic toward mice[11].
Covalently-bound dimers are undoubtedly the most interesting TFT posttranslational modification recently found. A new TFT forming a noncovalent dimer was discovered recently as well; this neurotoxin, haditoxin, was isolated from the venom of king cobra Ophiophagus hannah[12]. The high-resolution X-ray analysis revealed that haditoxin is a homodimer (Figure 1C), with a structure very similar to that of κ-Bgt (Figure 1D). However, in contrast to κ-Bgt, the amino acid sequences of the monomeric subunits of haditoxin correspond to those of α-neurotoxins of the short-chain type. It should be noted that κ-Bgt targets the neuronal α3β2 and α4β2 nAChRs and α-neurotoxins of the short-chain type block the muscle-type nAChRs only, while haditoxin demonstrated new pharmacological features, being antagonist toward muscle (αβγδ) and neuronal (α7, α3β2, and α4β2) nAChRs, and having the highest affinity (IC50 180 nmol/L) for α7 nAChRs[12].
The above data indicate that TFT dimerization may underlie the capacity for interaction with neuronal nAChRs.
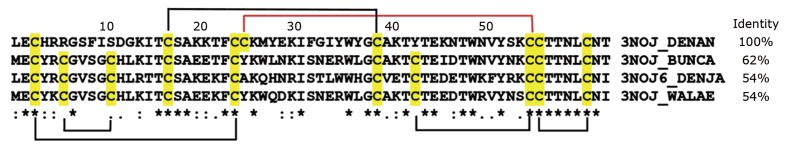
The disulfide bonds play an essential role in maintaining the spatial structure of TFTs, and their pairing pattern is largely conserved. However, an unusual disulfide bond scaffold was found in a TFT isolated from the venom of eastern green mamba Dendroaspis angusticeps[13]. This toxin, named Tx7335, has the highest amino acid sequence similarity to the TFTs of nonconventional type, but differs from them in the number and positions of cysteines (Figure 2). Similar to α-neurotoxins of the short-chain type, Tx7335 has only eight cysteines, while nonconventional toxins have ten cysteines. Furthermore, Tx7335 contains a tyrosine residue at position 43, which is occupied by a cysteine in all TFTs, while a cysteine is found at position 25. In most other TFTs this position is occupied by a tyrosine (Figure 2). According to structure modeling, Cys55 may make a disulfide bond with Cys25; due to the spatial proximity of these residues no major distortion in the three-finger structure occurs. This novel arrangement of disulfides may explain the unique functional effects observed for Tx7335. Indeed, it has been shown to activate the bacterial pH-gated potassium channel KcsA by a dose-dependent increase in both mean open time and open probability. Moreover, Tx7335 binds at the KcsA extracellular domain at a site probably different from that of channel inhibitors[11].
Figure 2.
Alignment of amino acid sequence of toxin Tx7335 with those of nonconventional toxins. Cysteine residues are marked in yellow. Black lines indicate the locations of the typical disulfide bond in nonconventional toxins; red line indicates the unusual disulfide bond 25-55 in Tx7335. 3NOJ_DENAN: Toxin Tx7335 from Dendroaspis angusticeps (Eastern green mamba); 3NOJ_BUNCA: Bucandin from Bungarus candidus (Malayan krait); 3NOJ6_DENJA: Toxin S6C6 from Dendroaspis jamesoni kaimosae (Eastern Jameson's mamba); 3NOJ_WALAE: Actiflagelin from Walterinnesia aegyptia (desert black snake).
Several new TFTs retaining the classical arrangement of disulfide bonds but possessing novel structural features have been identified recently. So, from the venom of black mamba Dendroaspis polylepis polylepis, a non-typical long-chain TFT, α-elapitoxin-Dpp2d (α-EPTX-Dpp2d), was isolated[14]. α-EPTX-Dpp2d contains an amidated C-terminal arginine, a posttranslational modification that had not been observed before in snake TFTs. Biological activity studies showed that, at a 1 μmol/L concentration, the α-EPTX-Dpp2d potently inhibited neuronal α7 (IC50 58 nmol/L) and muscle-type nAChRs (IC50 114 nmol/L) however showed no effect on α3β2 and α3β4 receptors. Therefore, this amidation was deemed to have no significant effect on the toxin’s selectivity, as the activity profile is fairly similar to that of the classic long-chain TFTs with a free carboxyl termini[14].
It was shown that the earlier characterized TFT Oh9-1[15] from Ophiophagus hannah venom may represent a new group of competitive nAChR antagonists, known as the Ω-neurotoxins[16]. Electrophysiology experiments on Xenopus oocytes showed that Oh9-1 inhibited rat muscle-type α1β1εδ (adult, IC50 3.1 µmol/L) andα1β1γδ (fetal, IC50 5.6 µmol/L) and rat neuronal α3β2 nAChRs (IC50 50.2 µmol/L), but manifested low or no affinity for other human and rat neuronal subtypes. Interestingly, Oh9-1 potentiated the human glycine receptor (homopentamer composed of α1 subunits), with activity increase by about 2-fold. Alanine-scan mutagenesis showed a novel mode of interaction with the ACh binding pocket of nAChRs via a set of functional amino acid residues that are different from those in the classical α-neurotoxins. Herewith, the central loop of Oh9-1 interacts with α1β1εδ nAChR by both sides of the β-strand, while only one side of the β-strand interacts with the α3β2 receptor[16].
The taxon-specific dimeric TFT irditoxin, isolated from a rear-fanged snake, was discussed above. Another taxon-specific TFT, fulgimotoxin, was isolated from the venom of the rear-fanged green vine snake Oxybelis fulgidus[17]. This toxin is a monomer and contains five disulfides, typical for the nonconventional TFTs. It is highly neurotoxic to lizards; however, mice are unaffected. Similar to other colubrid TFTs, fulgimotoxin has an extended N-terminal amino acid sequence and a pyroglutamic acid at the N-terminus.
The longest TFT, the nonconventional toxin BMLCL, consisting of 82 amino acid residues and five disulfide bridges, was identified in Bungarus multicinctus venom[18]. Earlier studies of biological activity revealed no interaction with the muscarinic acetylcholine receptors (mAChRs) M1 and M2 nor with the muscle-type nAChR[19]. However, recent studies showed that BMLCL interacted efficiently with both α7 (IC50 43 nmol/L) and muscle-type nAChR (IC50 31 nmol/L)[19]. Thus, the longest TFT functions as an antagonist of nAChRs.
It should be noted that so far, no TFTs have been found in the venoms of snakes from the Viperidae family; however, transcripts encoding these toxins were identified in venom gland transcriptomes of several Viperidae species. To address the question of biological activity of Viperidae TFTs, two toxins were obtained by heterologous expression in Escherichia coli. Based on the nucleotide sequences of cDNA encoding TFTs in the venom glands of vipers Azemiops feae and Vipera nikolskii, the corresponding genes optimized for bacterial expression were synthesized[20]. Expressed A. feae TFT (TFT-AF) and V. nikolskii TFT (VN-TFT), both of the nonconventional type, were refolded under the conditions elaborated on earlier for cobra TFTs. The biological activity of the toxins obtained was studied by electrophysiological techniques, calcium imaging, and radioligand analysis. Both toxins inhibited neuronal α3-containing and muscle-type nAChRs in the micromolar concentration range, but they were each very weak antagonists of neuronal α7 nAChRs. Thus, viper TFTs can function as antagonists of nAChRs of neuronal and muscle-type[20].
RECENTLY DISCOVERED TFTS WITH NEW BIOLOGICAL ACTIVITIES
Novel TFTs affecting signal transduction
The most fascinating biological activity of TFTs discovered during the last decade is their capacity to interact with acid-sensing ion channels (ASICs). ASICs are proton activated and Na+-selective ion channels, widely distributed throughout the peripheral and central nervous systems (CNS) in vertebrates. ASICs take part in an array of physiological processes, from synaptic plasticity and neurodegeneration to pain sensation. Therefore, the finding of new regulatory modes for these proteins opens up a new avenue of research for pain management, as well as for addiction or fear.
The new mambalgins class of TFTs has been characterized as potent, rapid and reversible inhibitors of ASICs, based on studies with the protein from African black mamba (Dendroaspis polylepis) venom[21]. The mambalgins are composed of 57 amino acids and eight cysteine residues, and have about 50% amino acid sequence identity to other snake TFTs. While mambalgins were found to be nontoxic in mice, they were found to exert a potent analgesic effect, as strong as that of morphine but causing much less tolerance than morphine and no respiratory distress. Pharmacological studies showed that mambalgins produce their analgesic effect through the blockade of heteromeric channels containing ASIC1a and ASIC2a subunits in CNS and of channels including ASIC1b subunit in nociceptors. Mambalgins were also shown to inhibit heteromeric channels including ASIC1a and ASIC1b subunits, homomeric rodent and human ASIC1a channels and homomeric rodent ASIC1b channels, the IC50s being in the range from 11 nmol/L to 252 nmol/L[22,23].
The structure of an ASIC1a–mambalgin-1 complex was determined by cryoelectron microscopy at a resolution of 5.4 Å[24]. The data obtained showed that mambalgin-1 binds precisely to the thumb domain of ASIC1a but not to the acid-sensing pocket, as suggested earlier[25]. However, mambalgin-1 binding induced conformational changes in the thumb domain of the channel, which may disturb the sensing of an acidity in ASIC1a[24]. The structural data obtained might provide a structural basis for further development of ASIC modulators.
No less significant than the discovery of mambalgins was the finding of TFTs that interact with ionotropic GABA receptors (GABAA). Almost simultaneously, three research groups found that snake TFTs were able to bind GABAA receptors[26-28]. Thus, two TFTs, called micrurotoxin 1 (MmTX1) and 2 (MmTX2), were isolated from Costa Rican coral snake (Micrurus mipartitus) venom and sequenced[26]. It was shown that at subnanomolar concentrations MmTX1 and MmTX2 increased receptor affinity for the agonist by binding to allosteric site, and thus potentiated opening and macroscopic desensitization of the receptor. The authors suggested that at the molecular level, the α+/β− subunit interface might be involved in toxin action. When injected into mouse brain, both toxins evoked seizures against the background of reduced basal activity[26]. The discovery of toxins enhancing GABAA receptor sensitivity to agonist established a new class of ligands for this receptor family.
In 2006, it was shown that α-Bgt, a classical blocker of α7 and muscle-type nAChRs, binds to and blocks GABAA receptors containing the interface of β3/β3 subunit[29]. No effects were observed for α-Bgt on heterooligomeric GABAA receptors which contain α-, β- and γ-subunits or α-, β- and δ-subunits. However, recently, two research groups independently showed that α-Bgt and some other TFTs could bind to recombinant and native GABAA receptors[27,28]. Both electrophysiology experiments and fluorescent measurements with α-Bgt coupled to Alexa-Fluor 555 revealed the highest toxin affinity to α2β2γ2 receptor subtype[27]. GABA reduced fluorescent labeling by α-Bgt, suggesting that the α-Bgt binding site overlaps the GABA binding site at the interface of β/α subunits[27].
Binding at the β/α subunit interface was demonstrated for the long-chain α-neurotoxin α-CTX[28], and this toxin interacted more efficiently with the GABAA receptor than α-Bgt. Electrophysiology experiments showed mixed competitive and noncompetitive α-CTX action, with highest affinity of this toxin being to the α1β3γ2 receptor (IC50 236 nmol/L). Other receptor subtypes were inhibited less potently, as follows: α1β2γ2 ≈ α2β2γ2 > α5β2γ2 > α2β3γ2 and α1β3δ. Among the several TFTs studied, the long α-neurotoxins Ls III (Laticauda semifasciata) and neurotoxin I (Naja oxiana) as well as the nonconventional toxin WTX (Naja kaouthia) interacted with the GABAA receptor. These data demonstrate that GABAA receptors are a target for diverse TFTs, including the very well-studied α-Bgt and α-CTX.
Among the vast variety of TFTs there is a class of toxins that interact with mAChRs, which are G-protein coupled receptors (GPCRs)[30]. For many years, the mAChRs were thought to be the only GPCRs affected by TFTs; however, over the last decade, several TFTs capable of interacting with other GPCRs, namely adrenoreceptors of different types, were reported.
It was shown that muscarinic toxin α (MTα) was a more potent antagonist for the α2B-adrenoceptor than for mAChR[31]. MTα inhibited the α2B-adrenoceptor, but did not affect the α2A-, α2C-, α1A- or α1B-adrenoceptors. In ligand binding experiments, MTα superseded the radioligand efficiently (IC50 3.2 nmol/L) and decreased the maximum binding without any influence on the radioligand affinity, demonstrating a noncompetitive inhibition mode[31]. The study of another MT, MTβ, showed nonselective low affinity interaction with the five muscarinic receptor subtypes[32]. Study of the toxin CM-3 (having undefined biological function to date) and MTβ demonstrated high efficacy for α-adrenoceptors and particularly a subnanomolar affinity for the receptor of α1A-subtype[32]. Both toxins were isolated more than 20 years ago from the venom of the African mamba Dendroaspis polylepis[33,34]. No or very weak affinity of these toxins were found for muscarinic receptors in the work of Blanchet et al[32].
Targeted searches for toxins interacting with α-adrenoceptors have yielded novel information in the last decade. The interactions of fractions obtained from green mamba (Dendroaspis angusticeps) venom with α1-adrenoceptors were tested in binding experiments using 3H-prazosin as a radioligand[35]. A new TFT inhibitor, AdTx1 (renamed later as ρ-Da1a[36]), comprising 65 amino acid residues with four disulfide bridges, was found. ρ-Da1a showed subnanomolar affinity with Ki of 0.35 nmol/L and demonstrated high specificity for the human adrenoceptor of α1A-subtype. Interestingly, the biological activity profile of ρ-Da1a appeared very similar to those of MTβ and CM-3; however, these latter two toxins interacted more potently than ρ-Da1a with α1B- and α1D-adrenoceptor subtypes[32]. ρ-Da1a was, thus, characterized as a specific and selective peptide inhibitor for the α1A-adrenoceptor, acting as a potent relaxant of smooth muscle[35].
Using a similar targeted screening approach, but with application of 3H-rauwolscine as a radioligand, the effects of venom fractions obtained from green mamba on α2-adrenoceptors from rat brain synaptosomes were studied[37]. A novel TFT, ρ-Da1b, comprising 66 amino acid residues with four disulfide bridges was isolated. It inhibited binding of 3H-rauwolscine to the three α2-adrenoceptor subtypes by 80% with affinity in the range of 14-73 nmol/L and with Hill coefficient of about unity. Furthermore, calcium imaging experiments on human α2A-adrenoceptors expressed in mammalian cells showed that ρ-Da1b was an antagonist of this adrenoceptor type[37].
The structural scaffold of aminergic TFTs that are known to interact with various α-adrenergic, muscarinic and dopaminergic receptors was used to generate experimental toxins with new functions. Specifically, the ancestral protein resurrection methodology was applied to identify the functional substitutions that might happen during evolution, and then utilize them for molecular design[38]. Six variants of ancestral toxin (AncTx, 1-6) were generated, and their biological activity was studied. AncTx1 was found to be the toxin possessing to date the highest selectivity to α1A-adrenoceptor. AncTx5 was the strongest inhibitor for α2-adrenoceptor of the three subtypes. The toxin ρ-Da1a affinities for the α1- and α2C-adrenoceptor subtypes were modulated most strongly by amino acids at positions 28, 38 and 43 in the evolutionary pathway[38]. Thus, this molecular engineering study represents the first successful attempt to engineer more potent aminergic TFTs.
Among the snake venoms, the mamba ones are unique in their variety of toxins affecting signal transductions[39]. A multitude of toxins capable of disturbing the different stages of cholinergic and adrenergic (see above paragraphs) transmission have been isolated from these venoms. Several toxins affecting voltage-gated ion channels have been isolated as well. The very recently discovered TFT Tx7335, in eastern green mamba Dendroaspis angusticeps venom, interacts with the KcsA potassium channel[13]. The unusual structure of this toxin was discussed above. Interestingly, Tx7335 is a channel activator but not an inhibitor, as evidenced by its ability to increase in a dose-dependent mode both mean open times and open probabilities of KcsA incorporated in artificial bilayers; yet, the Tx7335 binding site on KcsA is distinct from that of the canonical pore-blocker toxins. The authors of this study suggested that the toxin allosterically reduced inactivation of KcsA that results in increase of potassium flow through the channel[13].
Blue coral snake Calliophis bivirgatus belong to the Elapidae family of snakes the neurotoxic venoms of which typically produce the flaccid paralysis. However it was shown that the C. bivirgatus venom uniquely produced spastic paralysis[40]. The toxin producing this paralysis was isolated and called calliotoxin (protein name: δ-elapitoxin-Cb1a). Although calliotoxin is a TFT, it has low amino acid sequence similarity to the other known toxins. It comprises 57 amino acid residues with four disulfide bridges in the classical scaffold. Biological activity studies using HEK293 cells heterologously expressing NaV1.4 showed that the voltage-dependence of channel activation was shifted to more hyperpolarized potentials by calliotoxin. It inhibited inactivation and produced significant ramp currents. These data conformed with profound effects of calliotoxin on contractile force in preparation of isolated skeletal muscle. Thus, calliotoxin represents a functionally novel class of TFTs and is the first activator of voltage-gated sodium channel purified from snake venoms[40].
Novel TFTs affecting blood coagulation
TFTs affecting blood coagulation are not so numerous as those affecting signal transduction. Nevertheless, a new member of the TFT family that is capable of influencing different stages of blood coagulation appeared recently. TFTs inhibiting both primary and secondary hemostasis have been reported.
Primary hemostasis involves platelets, which immediately form a plug at the site of injury. A novel TFT which inhibits the human platelet aggregation process in a dose-dependent manner was purified from cobra Naja kaouthia venom and named KT-6.9[41]. KT-6.9 was shown to inhibit platelet aggregation induced by adenosine diphosphate (ADP), thrombin and arachidonic acid but not by collagen and ristocetin. It was 25-times more active than the antiplatelet drug clopidogrel. Based on the data showing significant inhibition (70%) of the platelet aggregation induced by ADP, the authors suggested toxin binding to ADP receptors located on the platelet surface[41].
As for secondary hemostasis, two TFTs capable of inhibiting the extrinsic tenase complex (ETC) were purified from the venom of African ringhals cobra Hemachatus haemachatus[42,43]. ETC activates conversion of factor X (FX) to factor Xa (FXa) and represents an important target for the development of novel anticoagulants. A novel TFT anticoagulant, ringhalexin (the ringhals extrinsic tenase complex inhibitor) was shown to inhibit FX activation with an IC50 of 123.8 nmol/L[42]. As an inhibitor of mixed type, on chick biventer cervicis muscle preparations ringhalexin manifested an irreversible weak neurotoxicity. The amino acid sequence of ringhalexin is 94% identical to that of NTL2, an uncharacterized neurotoxin-like protein from Naja atra. X-ray crystallography of ringhalexin revealed a typical three-finger structure stabilized by four conserved disulfide bridges[42].
Another novel anticoagulant TFT from Hemachatus haemachatus venom, called exactin, can specifically and potently inhibit the activation of FX by ETC (IC50 116.49 nmol/L), similar to ringhalexin[43]. It is also a mixed-type inhibitor of ETC and weakly inhibits FX activation by intrinsic tenase complex (IC50 4.05 µmol/L) and prothrombin activation by prothrombinase complex (IC50 17.66 µmol/L). In contrast to other TFT anticoagulants that are structurally similar to snake cytotoxins, exactin manifests structural similarity to postsynaptic neurotoxins. It also has 82% identity to the weak toxin CM1b from H. haemachatus venom and 58% identity to a number of Ophiophagus hannah neurotoxins, including the Ω-neurotoxin Oh9-1 discussed above.
Novel TFTs with unexpected biological activities
The last decade has also seen the discovery of several new TFTs possessing quite unusual biological activities.
TFTs, being structurally well defined, thermally stable and resistant to proteolysis, are very good subjects for directed evolution. When a randomization scheme was applied to α-neurotoxin amino acid residues in the loops involved in binding with nAChRs, followed by the cDNA display screening method, new modulators of the interleukin-6 receptor (IL-6R) were obtained[44]. The proteins obtained possessed nanomolar affinity and high specificity for IL-6R. The IL-6-dependent cell proliferation assay revealed both antagonists and agonists in the protein pool. Application of the size minimization procedure resulted in proteins with the molecular mass of about one-third of the original toxin; no significant loss of activities was observed. Moreover, the loops important for function were identified[44]. In another work by the same group, directed evolution was applied to produce a trypsin inhibitor based on the TFT scaffold[45]. The DNA sequences converged after seven rounds of selection. The recombinant proteins obtained were good inhibitors s of trypsin (Ki of 33-450 nmol/L). Three groups of proteins had Ki values close to those of soybean trypsin inhibitor and bovine pancreatic trypsin inhibitor. Two proteins inhibited chymotrypsin and kallikrein as well. The authors suggested that the technique developed may be widely applied for the targeted generation of different regulatory molecules based on the TFT motif[45].
Studies of a TFT cardiotoxin showed a quite unexpected effect on insulin secretion. The fractions of cobra Naja kaouthia venom obtained by combination of ultrafiltration and reversed-phase high-performance liquid chromatography were screened for insulinotropic activity using the rat INS-1E β-cell line[46]. Only one fraction of the total 22 obtained induced secretion of insulin from the INS-1E cells with no influence on cell integrity and viability. Liquid chromatography-tandem mass spectrometry analysis revealed that this fraction represented the cardiotoxin-I (CTX-I) isolated earlier from Naja kaouthia venom. Analysis of the isolated CTX-I toxin in INS-1E cells showed that its insulin stimulation ability persisted even in the absence of glucose. In contrast to typical cobra cardiotoxin, CTX-I did not induce direct hemolysis of human erythrocytes and showed no potent vasoconstriction capability. Based upon this toxin, a truncated analogue [Lys(52)CTX-I(41-60)] was obtained by structure-guided modification[47]. This analogue showed insulinotropic activity similar to CTX-I and appeared to exert its action through Kv channels[47]. As such, it may serve as a basis for the design of new therapeutic agents for the treatment of type 2 diabetes (Table 1).
A new paradoxical TFT, nakoroxin, was isolated from the cobra Naja kaouthia venom[48]. Nakoroxin belongs to the group of orphan TFTs (group “XX”), the biological activities of which are practically unknown. Nakoroxin was not cytotoxic to rat pheochromocytoma PC12 cells nor to human lung carcinoma HT1080 cells. It did not inhibit the binding of α-Bgt to α7 or muscle-type nAChRs, but potentiated the binding of α-Bgt to the acetylcholine-binding protein from Lymnaea stagnalis. The reason for this unusual property of nakoroxin is not clear.
Another quite interesting TFT, actiflagelin, was isolated from cobra Walterinnesia aegyptia venom by combination of reverse-phase and ion-exchange chromatography[49]. Actiflagelin activated in vitro motility of sperm from OF1 male mice. The amino acid sequence established by Edman sequencing combined with tandem mass spectrometry analyses showed that the protein comprised 63 amino acid residues with five disulfide bonds, the pattern of which corresponded to that of nonconventional toxins. Actiflagelin had a noticeable homology to bucandin, a nonconventional toxin from Bungarus candidus venom[49]. The authors suggested that the protein found may have therapeutic potential for cases of infertility when the problem is related to the sperm motility.
CONCLUSION
TFTs were among the first toxins isolated from snake venoms. At present, they form one of the largest toxin families and their number is increasing constantly. Several TFTs are used as sophisticated pharmacological tools to study the function and structure of their molecular targets. The new TFTs that have emerged recently possess both novel structural and functional characteristics, expanding the possibilities for their future applications. The TFTs discovered during the last decade have good prospects to be transformed into novel drugs. For example, mambalgins are perfect candidates for the design of powerful analgesics, and on the basis of CTX-I possessing insulinotropic activity, new therapeutics for the treatment of diabetes may be created. The data presented in this minireview (Table 1) show that TFTs continue to be important and promising for both basic science and medicine.
Footnotes
Conflict-of-interest statement: Utkin YN declares no conflict of interest related to this publication.
Manuscript source: Invited manuscript
Peer-review started: September 3, 2018
First decision: October 26, 2018
Article in press: December 5, 2018
Specialty type: Biochemistry and molecular biology
Country of origin: Russia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sabatier JM S- Editor: Ma RY L- Editor: A E- Editor: Wu YXJ
References
- 1.Utkin Y, Sunagar K, Jackson TNW, Reeks T, Fry BG. 2015. Three-Finger Toxins (3FTxs). Fry BG, editor. Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery. New York: Oxford University Press; pp. 215–227. [Google Scholar]
- 2.Kini RM, Doley R. Structure, function and evolution of three-finger toxins: mini proteins with multiple targets. Toxicon. 2010;56:855–867. doi: 10.1016/j.toxicon.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Utkin YN. Three-finger toxins, a deadly weapon of elapid venom--milestones of discovery. Toxicon. 2013;62:50–55. doi: 10.1016/j.toxicon.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Sanz L, Pla D, Pérez A, Rodríguez Y, Zavaleta A, Salas M, Lomonte B, Calvete JJ. Venomic Analysis of the Poorly Studied Desert Coral Snake, Micrurus tschudii tschudii, Supports the 3FTx/PLA₂ Dichotomy across Micrurus Venoms. Toxins (Basel) 2016;8 doi: 10.3390/toxins8060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dashevsky D, Debono J, Rokyta D, Nouwens A, Josh P, Fry BG. Three-Finger Toxin Diversification in the Venoms of Cat-Eye Snakes (Colubridae: Boiga) J Mol Evol. 2018 doi: 10.1007/s00239-018-9864-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Modahl CM, Mrinalini, Frietze S, Mackessy SP. Adaptive evolution of distinct prey-specific toxin genes in rear-fanged snake venom. Proc Biol Sci. 2018:285. doi: 10.1098/rspb.2018.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsetlin VI. Three-finger snake neurotoxins and Ly6 proteins targeting nicotinic acetylcholine receptors: pharmacological tools and endogenous modulators. Trends Pharmacol Sci. 2015;36:109–123. doi: 10.1016/j.tips.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Chang CC, Lee CY. isolation of neurotoxins from the venom of Bungarus multicinctus and their modes of neuromuscular blocking action. Arch Int Pharmacodyn Ther. 1963;144:241–257. [PubMed] [Google Scholar]
- 9.Osipov AV, Kasheverov IE, Makarova YV, Starkov VG, Vorontsova OV, Ziganshin RKh, Andreeva TV, Serebryakova MV, Benoit A, Hogg RC, Bertrand D, Tsetlin VI, Utkin YN. Naturally occurring disulfide-bound dimers of three-fingered toxins: a paradigm for biological activity diversification. J Biol Chem. 2008;283:14571–14580. doi: 10.1074/jbc.M802085200. [DOI] [PubMed] [Google Scholar]
- 10.Osipov AV, Rucktooa P, Kasheverov IE, Filkin SY, Starkov VG, Andreeva TV, Sixma TK, Bertrand D, Utkin YN, Tsetlin VI. Dimeric α-cobratoxin X-ray structure: localization of intermolecular disulfides and possible mode of binding to nicotinic acetylcholine receptors. J Biol Chem. 2012;287:6725–6734. doi: 10.1074/jbc.M111.322313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pawlak J, Mackessy SP, Sixberry NM, Stura EA, Le Du MH, Ménez R, Foo CS, Ménez A, Nirthanan S, Kini RM. Irditoxin, a novel covalently linked heterodimeric three-finger toxin with high taxon-specific neurotoxicity. FASEB J. 2009;23:534–545. doi: 10.1096/fj.08-113555. [DOI] [PubMed] [Google Scholar]
- 12.Roy A, Zhou X, Chong MZ, D'hoedt D, Foo CS, Rajagopalan N, Nirthanan S, Bertrand D, Sivaraman J, Kini RM. Structural and functional characterization of a novel homodimeric three-finger neurotoxin from the venom of Ophiophagus hannah (king cobra) J Biol Chem. 2010;285:8302–8315. doi: 10.1074/jbc.M109.074161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivera-Torres IO, Jin TB, Cadene M, Chait BT, Poget SF. Discovery and characterisation of a novel toxin from Dendroaspis angusticeps, named Tx7335, that activates the potassium channel KcsA. Sci Rep. 2016;6:23904. doi: 10.1038/srep23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang CI, Reeks T, Vetter I, Vergara I, Kovtun O, Lewis RJ, Alewood PF, Durek T. Isolation and structural and pharmacological characterization of α-elapitoxin-Dpp2d, an amidated three finger toxin from black mamba venom. Biochemistry. 2014;53:3758–3766. doi: 10.1021/bi5004475. [DOI] [PubMed] [Google Scholar]
- 15.Chang LS, Liou JC, Lin SR, Huang HB. Purification and characterization of a neurotoxin from the venom of Ophiophagus hannah (king cobra) Biochem Biophys Res Commun. 2002;294:574–578. doi: 10.1016/S0006-291X(02)00518-1. [DOI] [PubMed] [Google Scholar]
- 16.Hassan-Puttaswamy V, Adams DJ, Kini RM. A Distinct Functional Site in Ω-Neurotoxins: Novel Antagonists of Nicotinic Acetylcholine Receptors from Snake Venom. ACS Chem Biol. 2015;10:2805–2815. doi: 10.1021/acschembio.5b00492. [DOI] [PubMed] [Google Scholar]
- 17.Heyborne WH, Mackessy SP. Identification and characterization of a taxon-specific three-finger toxin from the venom of the Green Vinesnake (Oxybelis fulgidus; family Colubridae) Biochimie. 2013;95:1923–1932. doi: 10.1016/j.biochi.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Chung C, Wu BN, Yang CC, Chang LS. Muscarinic toxin-like proteins from Taiwan banded krait (Bungarus multicinctus) venom: purification, characterization and gene organization. Biol Chem. 2002;383:1397–1406. doi: 10.1515/BC.2002.158. [DOI] [PubMed] [Google Scholar]
- 19.Utkin YN, Kasheverov IE, Kudryavtsev DS, Andreeva TV, Starkov VG, Ziganshin RH, Kuznetsov DV, Anh HN, Thao NT, Khoa NC, et al. Nonconventional three-finger toxin BMLCL from krait Bungarus multicinctus venom with high affinity interacts with nicotinic acetylcholine receptors. Dokl Biochem Biophys. 2015;464:294–297. doi: 10.1134/S1607672915050099. [DOI] [PubMed] [Google Scholar]
- 20.Makarova YV, Kryukova EV, Shelukhina IV, Lebedev DS, Andreeva TV, Ryazantsev DY, Balandin SV, Ovchinnikova TV, Tsetlin VI, Utkin YN. The First Recombinant Viper Three-Finger Toxins: Inhibition of Muscle and Neuronal Nicotinic Acetylcholine Receptors. Dokl Biochem Biophys. 2018;479:127–130. doi: 10.1134/S1607672918020205. [DOI] [PubMed] [Google Scholar]
- 21.Diochot S, Baron A, Salinas M, Douguet D, Scarzello S, Dabert-Gay AS, Debayle D, Friend V, Alloui A, Lazdunski M, et al. Black mamba venom peptides target acid-sensing ion channels to abolish pain. Nature. 2012;490:552–555. doi: 10.1038/nature11494. [DOI] [PubMed] [Google Scholar]
- 22.Baron A, Diochot S, Salinas M, Deval E, Noël J, Lingueglia E. Venom toxins in the exploration of molecular, physiological and pathophysiological functions of acid-sensing ion channels. Toxicon. 2013;75:187–204. doi: 10.1016/j.toxicon.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Brzezicki MA, Zakowicz PT. Mambalgins, the Venom-origin Peptides as a Potentially Novel Group of Analgesics: Mini Review. CNS Neurol Disord Drug Targets. 2018;17:87–97. doi: 10.2174/1871527317666171221110419. [DOI] [PubMed] [Google Scholar]
- 24.Sun D, Yu Y, Xue X, Pan M, Wen M, Li S, Qu Q, Li X, Zhang L, Li X, et al. Cryo-EM structure of the ASIC1a-mambalgin-1 complex reveals that the peptide toxin mambalgin-1 inhibits acid-sensing ion channels through an unusual allosteric effect. Cell Discov. 2018;4:27. doi: 10.1038/s41421-018-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salinas M, Besson T, Delettre Q, Diochot S, Boulakirba S, Douguet D, Lingueglia E. Binding site and inhibitory mechanism of the mambalgin-2 pain-relieving peptide on acid-sensing ion channel 1a. J Biol Chem. 2014;289:13363–13373. doi: 10.1074/jbc.M114.561076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosso JP, Schwarz JR, Diaz-Bustamante M, Céard B, Gutiérrez JM, Kneussel M, Pongs O, Bosmans F, Bougis PE. MmTX1 and MmTX2 from coral snake venom potently modulate GABAA receptor activity. Proc Natl Acad Sci USA. 2015;112:E891–E900. doi: 10.1073/pnas.1415488112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hannan S, Mortensen M, Smart TG. Snake neurotoxin α-bungarotoxin is an antagonist at native GABA(A) receptors. Neuropharmacology. 2015;93:28–40. doi: 10.1016/j.neuropharm.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudryavtsev DS, Shelukhina IV, Son LV, Ojomoko LO, Kryukova EV, Lyukmanova EN, Zhmak MN, Dolgikh DA, Ivanov IA, Kasheverov IE, et al. Neurotoxins from snake venoms and α-conotoxin ImI inhibit functionally active ionotropic γ-aminobutyric acid (GABA) receptors. J Biol Chem. 2015;290:22747–22758. doi: 10.1074/jbc.M115.648824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCann CM, Bracamontes J, Steinbach JH, Sanes JR. The cholinergic antagonist alpha-bungarotoxin also binds and blocks a subset of GABA receptors. Proc Natl Acad Sci USA. 2006;103:5149–5154. doi: 10.1073/pnas.0600847103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Servent D, Blanchet G, Mourier G, Marquer C, Marcon E, Fruchart-Gaillard C. Muscarinic toxins. Toxicon. 2011;58:455–463. doi: 10.1016/j.toxicon.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Koivula K, Rondinelli S, Näsman J. The three-finger toxin MTalpha is a selective alpha(2B)-adrenoceptor antagonist. Toxicon. 2010;56:440–447. doi: 10.1016/j.toxicon.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Blanchet G, Upert G, Mourier G, Gilquin B, Gilles N, Servent D. New α-adrenergic property for synthetic MTβ and CM-3 three-finger fold toxins from black mamba. Toxicon. 2013;75:160–167. doi: 10.1016/j.toxicon.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 33.Joubert FJ. The amino acid sequence of protein CM-3 from Dendroaspis polylepis polylepis (black mamba) venom. Int J Biochem. 1985;17:695–699. doi: 10.1016/0020-711x(85)90367-2. [DOI] [PubMed] [Google Scholar]
- 34.Jolkkonen M, Van Giersbergen PL, Hellman U, Wernstedt C, Oras A, Satyapan N, Adem A, Karlsson E. Muscarinic toxins from the black mamba Dendroaspis polylepis. Eur J Biochem. 1995;234:579–585. doi: 10.1111/j.1432-1033.1995.579_b.x. [DOI] [PubMed] [Google Scholar]
- 35.Quinton L, Girard E, Maiga A, Rekik M, Lluel P, Masuyer G, Larregola M, Marquer C, Ciolek J, Magnin T, et al. Isolation and pharmacological characterization of AdTx1, a natural peptide displaying specific insurmountable antagonism of the alpha1A-adrenoceptor. Br J Pharmacol. 2010;159:316–325. doi: 10.1111/j.1476-5381.2009.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maïga A, Mourier G, Quinton L, Rouget C, Gales C, Denis C, Lluel P, Sénard JM, Palea S, Servent D, et al. G protein-coupled receptors, an unexploited animal toxin targets: Exploration of green mamba venom for novel drug candidates active against adrenoceptors. Toxicon. 2012;59:487–496. doi: 10.1016/j.toxicon.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Rouget C, Quinton L, Maïga A, Gales C, Masuyer G, Malosse C, Chamot-Rooke J, Thai R, Mourier G, De Pauw E, et al. Identification of a novel snake peptide toxin displaying high affinity and antagonist behaviour for the α2-adrenoceptors. Br J Pharmacol. 2010;161:1361–1374. doi: 10.1111/j.1476-5381.2010.00966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanchet G, Alili D, Protte A, Upert G, Gilles N, Tepshi L, Stura EA, Mourier G, Servent D. Ancestral protein resurrection and engineering opportunities of the mamba aminergic toxins. Sci Rep. 2017;7:2701. doi: 10.1038/s41598-017-02953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rowan EG, Harvey AL. Snake toxins from mamba venoms: unique tools for the physiologist. Acta Chim Slov. 2011;58:689–692. [PubMed] [Google Scholar]
- 40.Yang DC, Deuis JR, Dashevsky D, Dobson J, Jackson TN, Brust A, Xie B, Koludarov I, Debono J, Hendrikx I, et al. The Snake with the Scorpion's Sting: Novel Three-Finger Toxin Sodium Channel Activators from the Venom of the Long-Glanded Blue Coral Snake (Calliophis bivirgatus) Toxins (Basel) 2016;8 doi: 10.3390/toxins8100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chanda C, Sarkar A, Sistla S, Chakrabarty D. Anti-platelet activity of a three-finger toxin (3FTx) from Indian monocled cobra (Naja kaouthia) venom. Biochem Biophys Res Commun. 2013;441:550–554. doi: 10.1016/j.bbrc.2013.10.125. [DOI] [PubMed] [Google Scholar]
- 42.Barnwal B, Jobichen C, Girish VM, Foo CS, Sivaraman J, Kini RM. Ringhalexin from Hemachatus haemachatus: A novel inhibitor of extrinsic tenase complex. Sci Rep. 2016;6:25935. doi: 10.1038/srep25935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Girish VM, Kini RM. Exactin: A specific inhibitor of Factor X activation by extrinsic tenase complex from the venom of Hemachatus haemachatus. Sci Rep. 2016;6:32036. doi: 10.1038/srep32036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naimuddin M, Kobayashi S, Tsutsui C, Machida M, Nemoto N, Sakai T, Kubo T. Directed evolution of a three-finger neurotoxin by using cDNA display yields antagonists as well as agonists of interleukin-6 receptor signaling. Mol Brain. 2011;4:2. doi: 10.1186/1756-6606-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cai W, Naimuddin M, Inagaki H, Kameyama K, Ishida N, Kubo T. Directed evolution of three-finger toxin to produce serine protease inhibitors. J Recept Signal Transduct Res. 2014;34:154–161. doi: 10.3109/10799893.2013.865747. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen TT, Folch B, Létourneau M, Vaudry D, Truong NH, Doucet N, Chatenet D, Fournier A. Cardiotoxin-I: an unexpectedly potent insulinotropic agent. Chembiochem. 2012;13:1805–1812. doi: 10.1002/cbic.201200081. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen TT, Folch B, Létourneau M, Truong NH, Doucet N, Fournier A, Chatenet D. Design of a truncated cardiotoxin-I analogue with potent insulinotropic activity. J Med Chem. 2014;57:2623–2633. doi: 10.1021/jm401904q. [DOI] [PubMed] [Google Scholar]
- 48.Osipov AV, Meshcheryakova AV, Starkov VG, Ziganshin RK, Oustitch TL, Peters LE, Tsetlin VI, Utkin YN. New paradoxical three-finger toxin from the cobra Naja kaouthia venom: Isolation and characterization. Dokl Biochem Biophys. 2017;475:264–266. doi: 10.1134/S1607672917040068. [DOI] [PubMed] [Google Scholar]
- 49.Abd El-Aziz TM, Al Khoury S, Jaquillard L, Triquigneaux M, Martinez G, Bourgoin-Voillard S, Sève M, Arnoult C, Beroud R, De Waard M. Actiflagelin, a new sperm activator isolated from Walterinnesia aegyptia venom using phenotypic screening. J Venom Anim Toxins Incl Trop Dis. 2018;24:2. doi: 10.1186/s40409-018-0140-4. [DOI] [PMC free article] [PubMed] [Google Scholar]