Abstract
The gut microbiome is increasingly recognized for its role in human health and disease. Initial evidence indicates that gut microbial dysbiosis is associated with several pancreatic diseases. Although it is not known if these associations are causative, gut dysbiosis is hypothesized to mediate chronic pro-inflammatory changes in the pancreas. Further mechanistic and epidemiological studies of the microbiome are needed. Ultimately, targeted modulation of the microbiota could have therapeutic value.
Introduction:
The human gastrointestinal tract has a rich microbial community consisting of more than 1014 microorganisms and over 5,000,000 genes.1, 2 Firmicutes and Bacteroidetes are the most prevalent bacteria constituting 80–90% of the gut microbiota3. Gut microbiota play a major role in human physiology through effects on metabolism, modulation of the mucosal immune system, vitamin production, facilitation of digestion and modulation of intestinal architecture. The gut microbiome influences the immune system through its effect on systemic metabolism effects as well as its antigenic effects. Normal gut bacteria play a crucial role in maintaining gut mucosal integrity. The interplay between the gut microbiota, immune system and intestinal barrier limits the growth of pathogenic flora4 and disruption of this homeostasis leads to microbial imbalance known as ‘dysbiosis’.5 The gut microbiome is known to have major effects on systemic metabolism6,7 but there are thousands of metabolites in the plasma and in the gut, many of which remain uncharacterized.7 A large portion of the variance in fecal metabolites can be explained by the composition of the fecal microbiome.7, 8 Dysbiosis is associated with the pathogenesis of several gastrointestinal diseases (inflammatory bowel disease, irritable bowel syndrome)9, as well as other diseases such as obesity, the metabolic syndrome, diabetes10–12 and pancreatic diseases including pancreatic cancer.13,14
The normal pancreas is not in direct contact with the gut microbiota and was previously not considered to have a microbiome of its own. However, gut flora can migrate into the pancreas and may influence the pancreatic microenvironment even in subjects with an otherwise normal pancreas.15 Gut microbiota alterations are found in pancreatic disease and may play a role in the pathogenesis of several pancreatic diseases including acute pancreatitis, chronic pancreatitis and pancreatic cancer.14 However, there is still only limited evidence supporting a causal relationship between gut dysbiosis and pancreatic diseases. Many common factors influence the composition of the human microbiome including diet16, 17, commonly used medications (e.g. proton pump inhibitors18, metformin19), and genetics20–22 necessitating carefully controlled studies to evaluate relationships between the microbiome and disease. For this reason, many of the insights to date have come from animal models. In this review, we will discuss relevant human and animal studies that have provided insights into the role of the gut microbiome in the pathogenesis of pancreatic diseases.
Acute pancreatitis
Acute pancreatitis is among the most common gastrointestinal disorders requiring hospitalization with an annual incidence of 13–45 cases per 100,000 persons in United States.23,24 Regardless of the etiology, acute pancreatitis is the result of premature intra-acinar activation of trypsinogen and other proteolytic enzymes resulting in pancreatic acinar injury, upregulation of pro-inflammatory mediators, release of cytokines, systemic inflammatory response and microcirculatory injury.25 In the setting of acute pancreatitis, microcirculatory injury and hypovolemia can lead to can lead to gut mucosal ischemia and reperfusion injury resulting in loss of gut barrier integrity and translocation of gut flora causing local and systemic infections.26 In a meta-analysis of 18 studies, Wu et al. found that 59% of patients with acute pancreatitis have gut barrier dysfunction.27 In one study, circulating bacterial DNA representative of gut microbiota was found in 68.8% patients with acute pancreatitis.28 Circulating bacteria likely contribute to the mortality from acute pancreatitis by causing infection within necrotic areas of the pancreas. Mortality in patients with infected pancreatic necrosis and organ failure is approximately double that found in patients with sterile pancreatic necrosis and organ failure.29 The microbial composition of infected pancreatic necrosis was previously dominated by gram-negative flora of GI origin, such as Enterobacteriaceae. However, in the recent years, the widespread use of prophylactic antibiotics have shifted the dominant flora to Staphylococcus, Enterococcus and Candida.30 Patients at higher risk of infected pancreatic necrosis are those who have been previously treated with antibiotics. While patients with infected pancreatic necrosis have higher mortality, the microbial spectrum of infected necrosis does not appear to influence mortality.31
Necrotizing pancreatitis is known to impair gastrointestinal motility and in animal models results in small intestinal bacterial overgrowth (SIBO). In these models, duodenal bacterial overgrowth is associated with bacterial translocation and pancreatic infection.32 In a rat model of necrotizing pancreatitis, bacterial translocation and subsequent infected pancreatic necrosis was found to be more frequent from the small bowel than the colon.33
Experimental necrotizing pancreatitis induces pro-inflammatory cytokines and reduces antimicrobial peptide expression (alpha-defensins and lysozyme) in the distal ileum.34 These antimicrobial peptides help maintain gut microbiota homeostasis and barrier function.35 Antimicrobial peptides are also secreted by pancreatic acinar cells. Knockout of the Ca2+ channel Orai1 in pancreatic acinar cells (Orai1−/−) in mice reduced secretion of cathelicidin-related peptide (CRAMP), the major antimicrobial secreted by the pancreas, and resulted in increased small bowel and colonic bacterial colonization, increased intestinal permeability and bacterial translocation with resultant systemic infection and mortality. Mortality was reduced with antibiotics, short chain free fatty acids, and synthetic CRAMP.36
Interestingly, in pancreatic acinar cells, sensing of bacterial antigens by pathogen recognition receptors such as Toll like receptors (TLR), and the nucleotide binding oligomerization domain (NOD)-like receptors (NLRs) are thought to contribute to the pathogenesis of pancreatitis. Pancreatitis in mice can develop by chronic low-dose cerulein stimulation with NOD1 agonist stimulation, effects that are prevented in NOD1 knockout mice.37 NOD1 can bind to peptidoglycan peptides in pancreatic acinar cells.38
Patients with acute pancreatitis are more likely than healthy volunteers to have gut dysbiosis (higher Enterobacteriaceae and Enterococcus populations, lower Bifidobacteria).39 These pre-clinical and human studies that implicate a role for intestinal dysbiosis in the pathogenesis and severity of acute pancreatitis raise the question of whether modulating the gut microbiome might be beneficial. Altering the ‘acute pancreatitis-associated microbiota’ with ‘physiological gut microbiota’ using broad-spectrum antibiotics and antimicrobial peptides might be expected to improve outcome. Benign physiological gut microbiota such as Lactobacillus alone and Bifidobacterium are thought to help maintain gut barrier function and limit the growth of pathogenic flora. The potential value of using probiotics for patients with severe acute pancreatitis has been evaluated in randomized controlled trials and summarized in a Cochrane review and a meta-analysis.40, 41 Overall, probiotics have been shown to have no significant beneficial nor adverse effects in patients with severe acute pancreatitis. One caveat is that these trials had significant heterogeneity with respect to patient characteristics and probiotic regimens. One of these trials, conducted in the Netherlands, the ‘Probiotics in Pancreatitis Trial’ (PROPATRIA),42 randomized 296 patients with predicted severe acute pancreatitis to a multispecies probiotic mixture containing two different Bifidobacterium species, three different Lactobacillus species and one Lactococcus species versus placebo. The infectious complications were similar among the two groups, but the probiotic group had higher mortality (16% vs 6%) and incidence of bowel ischemia (6% vs 0%) compared to the placebo group. The high load of probiotic mixture used in this study was suspected to be a cause of the increased mortality in the treated group.43 These studies highlight the challenges of trying to beneficially manipulate the gut microbiome in the setting of acute pancreatitis.
Chronic pancreatitis
Several studies have evaluated the gut microbiome in patients with chronic pancreatitis.44 Evidence for gut microbial dysbiosis is suggested by the frequent observation of small intestinal bacterial overgrowth (SIBO) in patients with chronic pancreatitis. SIBO is thought to be more likely to arise in patients with chronic pancreatitis as a result of reduced pancreatic synthesis of anti-microbial peptides, impaired motility, abnormal chyme formation in the small intestinal lumen and from reduced alkalization due to reduced bicarbonate rich pancreatic secretory capacity.45, 46 SIBO can exacerbate pancreatic exocrine insufficiency (PEI) suggesting that treatment of SIBO would help patients with PEI.47 In a meta-analyses by Capurso et al., the mean prevalence of SIBO in patients with chronic pancreatitis was reported to be 36% (95%CI 17–60%),48 although significant heterogeneity between studies was noted. In a subsequent study, Jandhayala et al. examined 16 patients with chronic pancreatitis, 14 with chronic pancreatitis with diabetes and 10 healthy controls.49 Phyla abundances were different in the three patient groups, with reductions in the abundance of Bacteroidetes and increases in the ratio of Firmicutes to Bacteroidetes ratio among patients with chronic pancreatitis. There was a significant reduction in Faecalibacterium prausnitzii and Ruminococcus bromii between the control, chronic pancreatitis without diabetes and chronic pancreatitis with diabetes groups. Faecalibacterium prausnitzii is known to contribute to intestinal barrier homeostasis and integrity.50, 51 Plasma endotoxin was detected in many of these patients and correlated negatively with glycemic status indicating that gut microbial dysbiosis was associated with the metabolic alterations of chronic pancreatitis.49 Additional studies are needed to evaluate the role of gut dysbiosis in the setting of chronic pancreatitis.
Pancreatic cancer
The incidence of pancreatic cancer has been rising and is expected to be the second leading cause of death in United States by 2030.52 The reasons for this increase in incidence are not fully understood but could in part be related to an increased incidence of known risk factors for pancreatic cancer including obesity, diabetes and the metabolic syndrome. Obesity and diabetes are known to be associated with changes in the gut microbiome,10–12 and some of their metabolic consequences could be mediated in part through effects on the gut microbiome. Chronic pancreatitis is also a risk factor for developing pancreatic cancer, with the risk increasing with the duration of disease.53–55 Since gut dysbiosis has been postulated to contribute to chronic pancreatic inflammation, it may also contribute to the pathogenesis of pancreatic cancer in the setting of chronic pancreatitis.
Gut dysbiosis has been hypothesized to promote the development of many types of cancer through systemic mechanisms, particularly metabolic changes that can influence precancerous cells and immune cells.56 For example, short chain fatty acids produced in the gut with the help of the gut microbiome have metabolic effects on immune cells.57 In the colon, adherent bacteria such as Bacteroides fragilis are thought to promote adenomas and cancers as they produce enterotoxins that have tumorigenic effects including effects on signaling, cell adhesion molecules and cytokine alterations.58, 59 Consistent with their role in modulating the immune response, gut bacterial profiles are recognized as influencing the immune response directed towards tumors.15, 60 Several studies have shown that gut microbiome profiles influence the immune therapy response to checkpoint inhibitor therapy.61–63
Several studies have evaluated the influence of the microbiome on the pathogenesis of pancreatic cancer (summarized in Table 1). For example, several studies have found differences in the oral microbial flora between patients with pancreatic cancer compared to controls. Fan et al.64 and Michaud et al.65 found higher levels of Porphyromonas gingivalis in patients with pancreatic cancer than controls; the highest concentration of Porphyromonas gingivalis was associated with a twofold increase in pancreatic cancer risk, although a smaller study did not find this association.66 Porphyromonas gingivalis is an important contributor to periodontal disease and has been postulated to cause systemic inflammation.67 Other oral microbial differences found between patient groups with pancreatic cancer, chronic pancreatitis and healthy controls were described by Farrell et al.68 Since these were case/control studies, it is not yet clear if these microbial changes contribute to disease pathogenesis or are merely a consequence of the disease. Recent studies have also identified bacteria within the tumor microenvironment of human pancreatic cancers 15 and within other tumors.69 The bacteria identified in human pancreatic cancers are representative of the major genus of gut bacteria such as Proteobacteria.69,15 Recent studies in genetically engineered mouse models of pancreatic neoplasia have shed additional light on the role of gut bacteria in pancreatic tumorigenesis. Using the Ptf1aCre; LSL-KrasG12D (KC) and Ptf1aCre, LSL-KrasG12D, Trp53R172H (KPC) mouse models of pancreatic neoplasia, Pushalkar et al. found oral antibiotics protected against neoplastic progression. They also found repopulating germ free KC mice with feces from pancreatic cancer bearing KPC mice or with Bifidobacterium pseudolongum accelerated disease progression. B. pseudolongum could also be detected within the pancreata of treated mice. Changes in the gut microbiome resulted in changes in immune cells within the tumor microenvironment. Microbial ablation led to a reduction in immunosuppressive CD206+ M2-like tumor-associated macrophages (TAMs) with a concomitant increase in tumor-protective M1-like TAMs, and an increase in the CD8/CD4 ratio within the tumor microenvironment, changes that reversed with repopulation of the microbiome. Similarly, another recent study using wild type C57BL/6J, Rag1 knock-out (lacks mature T and B cells), KPC and Ptenfl/fl mice demonstrated that gut microbial depletion with antibiotics resulted in an increase in Th1 (IFNγ+CD4+CD3+) and Tc1(IFNγ+CD8+CD3+) cells in the tumor microenvironment and a reduction in pancreatic tumor burden.70
Table 1:
Studies examining microbiota alterations in patients with pancreatic cancer
| Study author, year of publication | Number of cases with pancreatic cancer, number of controls | Sample type and microbial characterization | Microbial alterations (increases) | Microbial alterations (decreases) | Limitations |
|---|---|---|---|---|---|
| Pushalkar et al, 201815 | 32, 31 | Fecal, 16S rRNA gene sequencing |
Proteobacteria, Actinobacteria, Fusobacteria, Verrucomicrobia |
sample size | |
| Pushalkar, 201815 | 12, not reported | pancreatic cancer, 16S rRNAgene sequencing |
Proteobacteria, Sphingobacteria |
sample size | |
| Fan et al, 201864 | 361, 371 | Salivary, 16S rRNA gene sequencing |
Porphyromonas Aggregatibacter |
Leptotrichia, Fusobacteria |
|
| Geller et al, 201769 | 113, 20 | Pancreatic tumors, 16S rRNAgene sequencing | Proteobacteria | ||
| Torres et al, 201566 | 8, 22 | Salivary, 16S rRNA gene sequencing |
Leptotrichia Bacteroides |
Porphyromonas | sample size |
| Michaud et al, 201365 | 405, 410 | Plasma antibody levels to oral bacteria |
Porphyromonas gingivalis |
Streptococcus mitis |
|
| Farrell et al, 201268 | 38, 38 | Salivary, 16S rRNA gene sequencing |
Neisseria elongate, Streptococcus mitis |
sample size |
Experimental models have also found that intratumoral bacteria alter the metabolism of nucleoside chemotherapeutics such as gemcitabine.69,71 Bacteria are also detected in pancreatic cyst fluids including cysts associated with IPMN as well as non-neoplastic cysts, but the significance of these bacteria is not clear.72 Despite these intriguing studies, the evidence that bacteria within the pancreas contributes to human pancreatic cancer pathogenesis is still limited.
Conclusions:
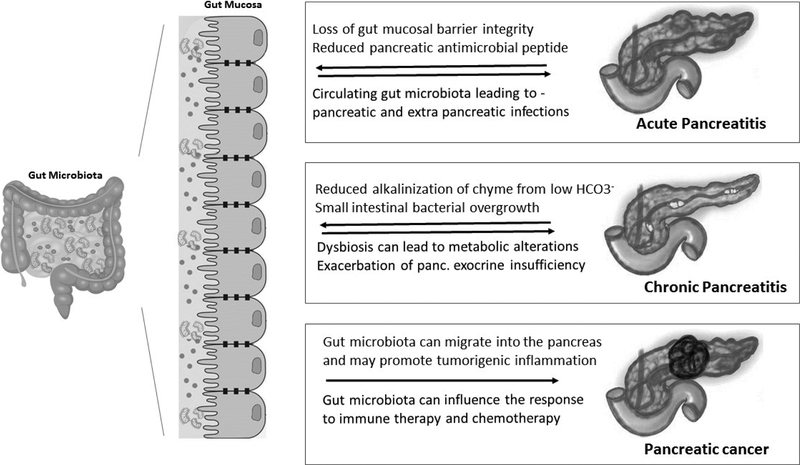
Gut microbial dysbiosis is thought to contribute to the pathogenesis of pancreatic diseases (Figure 1). Much of the evidence for this comes from animal models and these models will continue to be valuable for testing hypotheses. There is still much to understand about the causes and consequences of gut microbial dysbiosis as well as the contributions played by alterations in the abundance of different species. There are many large-scale efforts underway to characterize the human microbiome, the dietary, genetic, pathologic and pharmacological variables that influence it and its metabolic effects. Unraveling these associations is a necessary step towards better understanding the role of the microbiome in human pancreatic disease. Evaluating the gut microbiome of patients at risk of pancreatic disease in prospective studies is necessary to better understand the role of gut dysbiosis in human pancreatic disease pathogenesis.
Figure 1:
Overview of interactions between gut microbiota and pancreatic disease
Acknowledgments
Grant Support: This work was supported by NIH grants (U01CA210170, R01CA176828 and CA62924).
Abbreviations:
- SIBO
Small intestinal bacterial overgrowth
- DNA
Deoxyribonucleic acid
- CRAMP
Cathelicidin-related anti-microbial peptide
- TLR
Toll like receptors
- NLR
Nucleotide binding oligomerization domain (NOD)-like receptors
- PEI
Pancreatic exocrine insufficiency
- LPS
Lipopolysaccharide
- IFN γ
Interferon gamma
- TAM
Tumor-associated macrophages
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts statement
Vikesh Singh: Consultant for Abbvie, Ariel Precision Medicine, and Akcea Therapeutics
Rupjyoti Talukdar: Advisory Board member for Abbott India and Dr. Reddy’s Laboratories.
None of the other authors have disclosures
REFERENCES
- 1.Forbes JD, Van Domselaar G, Bernstein CN. The Gut Microbiota in Immune-Mediated Inflammatory Diseases. Front Microbiol. 2016;7:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–33. [DOI] [PubMed] [Google Scholar]
- 3.Lucas Lopez R, Grande Burgos MJ, Galvez A, et al. The human gastrointestinal tract and oral microbiota in inflammatory bowel disease: a state of the science review. APMIS. 2017;125(1):3–10. [DOI] [PubMed] [Google Scholar]
- 4.Pagliari D, Piccirillo CA, Larbi A, et al. The Interactions between Innate Immunity and Microbiota in Gastrointestinal Diseases. J Immunol Res. 2015;2015:898297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jandhyala SM, Talukdar R, Subramanyam C, et al. Role of the normal gut microbiota. World journal of gastroenterology. 2015;21(29):8787–803. Epub 2015/08/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. The New England journal of medicine. 2013;368(17):1575–84. Epub 2013/04/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujisaka S, Avila-Pacheco J, Soto M, et al. Diet, Genetics, and the Gut Microbiome Drive Dynamic Changes in Plasma Metabolites. Cell reports. 2018;22(11):3072–86. Epub 2018/03/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zierer J, Jackson MA, Kastenmuller G, et al. The fecal metabolome as a functional readout of the gut microbiome. Nature genetics. 2018. Epub 2018/05/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sartor RB, Wu GD. Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic Approaches. Gastroenterology. 2017;152(2):327–39.e4. Epub 2016/10/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenblum S, Turnbaugh PJ, Borenstein E. Metagenomic systems biology of the human gut microbiome reveals topological shifts associated with obesity and inflammatory bowel disease. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(2):594–9. Epub 2011/12/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4. Epub 2008/12/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science (New York, NY). 2013;341(6150):1241214 Epub 2013/09/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Li J. Pathogenic Microorganisms and Pancreatic Cancer. Gastrointest Tumors. 2015;2(1):41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Signoretti M, Roggiolani R, Stornello C, et al. Gut microbiota and pancreatic diseases. Minerva Gastroenterol Dietol. 2017;63(4):399–410. [DOI] [PubMed] [Google Scholar]
- 15.Pushalkar S, Hundeyin M, Daley D, et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018;8(4):403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. Epub 2013/12/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science (New York, NY). 2011;334(6052):105–8. Epub 2011/09/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llorente C, Jepsen P, Inamine T, et al. Gastric acid suppression promotes alcoholic liver disease by inducing overgrowth of intestinal Enterococcus. Nature communications. 2017;8(1):837 Epub 2017/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–6. Epub 2015/12/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolde R, Franzosa EA, Rahnavard G, et al. Host genetic variation and its microbiome interactions within the Human Microbiome Project. Genome medicine. 2018;10(1):6 Epub 2018/01/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li D, Achkar JP, Haritunians T, et al. A Pleiotropic Missense Variant in SLC39A8 Is Associated With Crohn’s Disease and Human Gut Microbiome Composition. Gastroenterology. 2016;151(4):724–32. Epub 2016/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imhann F, Vich Vila A, Bonder MJ, et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2018;67(1):108–19. Epub 2016/11/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1252–61. Epub 2013/04/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peery AF, Crockett SD, Barritt AS, et al. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology. 2015;149(7):1731–41 e3. Epub 2015/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144(6):1180–93. [DOI] [PubMed] [Google Scholar]
- 26.Andersson R, Wang XD. Gut barrier dysfunction in experimental acute pancreatitis. Ann Acad Med Singapore. 1999;28(1):141–6. [PubMed] [Google Scholar]
- 27.Wu LM, Sankaran SJ, Plank LD, et al. Meta-analysis of gut barrier dysfunction in patients with acute pancreatitis. Br J Surg. 2014;101(13):1644–56. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Wang C, Tang C, et al. Bacteremia in patients with acute pancreatitis as revealed by 16S ribosomal RNA gene-based techniques*. Crit Care Med. 2013;41(8):1938–50. [DOI] [PubMed] [Google Scholar]
- 29.Werge M, Novovic S, Schmidt PN, et al. Infection increases mortality in necrotizing pancreatitis: A systematic review and meta-analysis. Pancreatology. 2016;16(5):698–707. Epub 2016/07/28. [DOI] [PubMed] [Google Scholar]
- 30.Sahar N, Kozarek RA, Kanji ZS, et al. The microbiology of infected pancreatic necrosis in the era of minimally invasive therapy. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2018. Epub 2018/04/21. [DOI] [PubMed] [Google Scholar]
- 31.Caldas C, Hahn SA, da Costa LT, et al. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma [published erratum appears in Nat Genet 1994 Dec;8(4):410]. Nature genetics. 1994;8(1):27–32. [DOI] [PubMed] [Google Scholar]
- 32.Van Felius ID, Akkermans LM, Bosscha K, et al. Interdigestive small bowel motility and duodenal bacterial overgrowth in experimental acute pancreatitis. Neurogastroenterol Motil. 2003;15(3):267–76. [DOI] [PubMed] [Google Scholar]
- 33.Fritz S, Hackert T, Hartwig W, et al. Bacterial translocation and infected pancreatic necrosis in acute necrotizing pancreatitis derives from small bowel rather than from colon. Am J Surg. 2010;200(1):111–7. [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Huang C, Wang J, et al. Dysbiosis of intestinal microbiota and decrease in paneth cell antimicrobial peptide level during acute necrotizing pancreatitis in rats. PLoS One. 2017;12(4):e0176583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong W Shaping the gut microbiome from the pancreas. Sci Signal. 2017;10(472). [DOI] [PubMed] [Google Scholar]
- 36.Ahuja M, Schwartz DM, Tandon M, et al. Orai1-Mediated Antimicrobial Secretion from Pancreatic Acini Shapes the Gut Microbiome and Regulates Gut Innate Immunity. Cell Metab. 2017;25(3):635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe T, Sadakane Y, Yagama N, et al. Nucleotide-binding oligomerization domain 1 acts in concert with the cholecystokinin receptor agonist, cerulein, to induce IL-33-dependent chronic pancreatitis. Mucosal immunology. 2016;9(5):1234–49. Epub 2016/01/28. [DOI] [PubMed] [Google Scholar]
- 38.Tsuji Y, Watanabe T, Kudo M, et al. Sensing of commensal organisms by the intracellular sensor NOD1 mediates experimental pancreatitis. Immunity. 2012;37(2):326–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan C, Ling Z, Huang Y, et al. Dysbiosis of Intestinal Microbiota Associated With Inflammation Involved in the Progression of Acute Pancreatitis. Pancreas. 2015;44(6):868–75. [DOI] [PubMed] [Google Scholar]
- 40.Gou S, Yang Z, Liu T, et al. Use of probiotics in the treatment of severe acute pancreatitis: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2014;18(2):R57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poropat G, Giljaca V, Hauser G, et al. Enteral nutrition formulations for acute pancreatitis. Cochrane Database Syst Rev. 2015(3):CD010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Besselink MG, Timmerman HM, Buskens E, et al. Probiotic prophylaxis in patients with predicted severe acute pancreatitis (PROPATRIA): design and rationale of a double-blind, placebo-controlled randomised multicenter trial [ISRCTN38327949]. BMC Surg. 2004;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morrow LE, Gogineni V, Malesker MA. Synbiotics and probiotics in the critically ill after the PROPATRIA trial. Curr Opin Clin Nutr Metab Care. 2012;15(2):147–50. [DOI] [PubMed] [Google Scholar]
- 44.Memba R, Duggan SN, Ni Chonchubhair HM, et al. The potential role of gut microbiota in pancreatic disease: A systematic review. Pancreatology. 2017;17(6):867–74. [DOI] [PubMed] [Google Scholar]
- 45.Bures J, Cyrany J, Kohoutova D, et al. Small intestinal bacterial overgrowth syndrome. World journal of gastroenterology : WJG. 2010;16(24):2978–90. Epub 2010/06/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dutta SK, Russell RM, Iber FL. Impaired acid neutralization in the duodenum in pancreatic insufficiency. Dig Dis Sci. 1979;24(10):775–80. [DOI] [PubMed] [Google Scholar]
- 47.Ni Chonchubhair HM, Bashir Y, Dobson M, et al. The prevalence of small intestinal bacterial overgrowth in non-surgical patients with chronic pancreatitis and pancreatic exocrine insufficiency (PEI). Pancreatology. 2018. [DOI] [PubMed] [Google Scholar]
- 48.Capurso G, Signoretti M, Archibugi L, et al. Systematic review and meta-analysis: Small intestinal bacterial overgrowth in chronic pancreatitis. United European Gastroenterol J. 2016;4(5):697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jandhyala SM, Madhulika A, Deepika G, et al. Altered intestinal microbiota in patients with chronic pancreatitis: implications in diabetes and metabolic abnormalities. Sci Rep. 2017;7:43640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wrzosek L, Miquel S, Noordine ML, et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC biology. 2013;11:61 Epub 2013/05/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossi O, van Berkel LA, Chain F, et al. Faecalibacterium prausnitzii A2–165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses. Scientific reports. 2016;6:18507 Epub 2016/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer research. 2014;74(11):2913–21. Epub 2014/05/21. [DOI] [PubMed] [Google Scholar]
- 53.Kirkegard J, Mortensen FV, Cronin-Fenton D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. The American journal of gastroenterology. 2017;112(9):1366–72. Epub 2017/08/02. [DOI] [PubMed] [Google Scholar]
- 54.Lowenfels AB, Maisonneuve P, DiMagno EP, Elitsur Y, Gates LK Jr., Perrault J, Whitcomb DC Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89(6):442–6. [DOI] [PubMed] [Google Scholar]
- 55.Duell EJ, Lucenteforte E, Olson SH, et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23(11):2964–70. Epub 2012/07/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garrett WS. Cancer and the microbiota. Science. 2015;348(6230):80–6. Epub 2015/04/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–6. Epub 2009/10/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dejea CM, Fathi P, Craig JM, et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science (New York, NY). 2018;359(6375):592–7. Epub 2018/02/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dejea CM, Wick EC, Hechenbleikner EM, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(51):18321–6. Epub 2014/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967–70. Epub 2013/11/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sears CL, Pardoll DM. The intestinal microbiome influences checkpoint blockade. Nature medicine. 2018;24(3):254–5. Epub 2018/03/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science (New York, NY). 2018;359(6371):97–103. Epub 2017/11/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science (New York, NY). 2018;359(6371):91–7. Epub 2017/11/04. [DOI] [PubMed] [Google Scholar]
- 64.Fan X, Alekseyenko AV, Wu J, et al. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut. 2018;67(1):120–7. Epub 2016/11/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Michaud DS, Izard J, Wilhelm-Benartzi CS, et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut. 2013;62(12):1764–70. Epub 2012/09/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Torres PJ, Fletcher EM, Gibbons SM, et al. Characterization of the salivary microbiome in patients with pancreatic cancer. PeerJ. 2015;3:e1373 Epub 2015/11/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang JS, Tsai CR, Chen LT, et al. Investigating the Association Between Periodontal Disease and Risk of Pancreatic Cancer. Pancreas. 2016;45(1):134–41. Epub 2015/10/17. [DOI] [PubMed] [Google Scholar]
- 68.Farrell JJ, Zhang L, Zhou H, et al. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut. 2012;61(4):582–8. Epub 2011/10/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geller LT, Barzily-Rokni M, Danino T, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science (New York, NY). 2017;357(6356):1156–60. Epub 2017/09/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sethi V, Kurtom S, Tarique M, et al. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology. 2018. Epub 2018/04/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lehouritis P, Cummins J, Stanton M, et al. Local bacteria affect the efficacy of chemotherapeutic drugs. Scientific reports. 2015;5:14554 Epub 2015/09/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li S, Fuhler GM, Bn N, et al. Pancreatic cyst fluid harbors a unique microbiome. Microbiome. 2017;5(1):147 Epub 2017/11/11. [DOI] [PMC free article] [PubMed] [Google Scholar]