Abstract
Objective
To estimate the effects of preconceptional cardiovascular health, measured by American Heart Association (AHA) guidelines, on pregnancy outcomes in women with systemic lupus erythematosus (SLE).
Methods
The study included patients in the Hopkins Lupus Pregnancy Cohort. Body mass index (BMI), total cholesterol, and blood pressure in the most recent clinic visit prior to conception or 1st trimester were used to determine cardiovascular health (ideal, intermediate or poor health) based on AHA definitions. Outcomes included preterm birth, gestational age at birth, and small for gestational age (SGA). Multivariable linear and logistic regression models with generalized estimating equations estimated the association of each cardiovascular health factor and outcome.
Results
The analysis included 309 live births. There were 95 preterm births (31%), and of the 293 pregnancies with birth weights, 18% were SGA. Ideal BMI, total cholesterol and blood pressure were reported in 56%, 85%, and 51% of pregnancies, respectively. Intermediate BMI was associated with decreased odds of SGA (OR: 0.26; 95% CI: 0.11, 0.63), adjusted for race and prednisone use. Intermediate/poor total cholesterol was associated with increased odds of preterm birth (OR: 2.21; 95% CI: 1.06, 4.62). Intermediate/poor blood pressure was associated with decreased gestational age at birth (β: −0.96; 95% CI: −1.62, −0.29).
Conclusion
Poor/intermediate preconception cardiovascular health affects pregnancy outcomes of preterm birth and SGA infants among women with SLE. Efforts to maintain BMI, total cholesterol, and blood pressure within the recommended ideal range prior to pregnancy is important in order to improve pregnancy outcomes in women with SLE.
Keywords: birth weight, cardiovascular diseases, pregnancy, premature birth, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease which largely affects women, with disease onset typically occurring between ages 15 and 44.1 Although pregnancy outcomes in women with SLE have improved, the prevalences of preterm birth and infants born small for gestational age remain two- to six-times greater in women with SLE compared to the general population.2–4 Well established risk factors for adverse pregnancy outcomes in the general population, such as body mass index, cholesterol, physical activity, and diet, have not been investigated in SLE.
The American Heart Association (AHA)’s 2020 Impact Goals included the development of the concept of “ideal cardiovascular health,” which focuses on primary prevention and is composed of seven modifiable cardiovascular metrics: health factors (glucose, total cholesterol, and blood pressure) and health behaviors (body mass index, physical activity, diet, and cigarette smoking).5 Meeting these metrics for ideal cardiovascular health is associated with lower cardiovascular disease risk, cardiovascular mortality, and all-cause mortality among U.S. adults.6,7
Longitudinal cohort studies report that hypertension, dyslipidemia, and obesity are common co-morbidities in SLE, affecting 30-60% of patients.8–10 Maternal cardiovascular health at conception and during early pregnancy has implications for the in utero environment. Obesity at conception can lead to alterations in metabolic adjustments during gestation, affecting placental, embryonic, and fetal growth. Increased body fat is associated with increased levels of proinflammatory proteins, and obese women are more likely to enter pregnancy in a state of subclinical inflammation.11–13 In the general population, maternal obesity increases the risk of preeclampsia and delivering a large for gestational age infant.14–18
Studies have shown that hypertension is a risk factor for preterm birth in the general population.19–21 Additionally, compared to woman without hypertension, women with chronic hypertension have 5.5 times the risk of delivering a preterm, small for gestational age infant and 1.5-1.7 times the risk of delivering a term, small for gestational age infant.19,20,22 Previous research, although limited, has demonstrated that increased total cholesterol during the first trimester is associated with preterm birth in the general population, with possible modification by maternal inflammation.21,23,24
It has been theorized that maternal risk factors for cardiovascular disease may also be risk factors for fetal growth restriction and fetal programming.25 As SLE is a chronic inflammatory disease, it is important to understand the way these cardiovascular health factors affect preterm birth and fetal growth during SLE pregnancies, as they could be targeted for improved pregnancy outcomes. The objective of this analysis is to determine the proportion of pregnant women with SLE meeting the AHA’s guidelines for ideal cardiovascular health and to estimate the effects of poor and intermediate cardiovascular health on pregnancy outcomes.
Materials and Methods
Study population
The Hopkins Lupus Pregnancy Cohort has prospectively followed pregnant patients with SLE since 1987, with data available through February 2015. All patients met the American College of Rheumatology (ACR) or Systemic Lupus International Collaborating Clinics (SLICC) criteria for SLE26–28 and were enrolled following informed consent. This study is approved by the UNC IRB (Study #13-3942). Pregnant patients were seen every 4-6 weeks by a single rheumatologist. Weight, blood pressure, lupus disease activity [physician global assessment of disease activity (PGA) and SELENA SLEDAI29,30], and laboratory tests were measured at each visit. Laboratory tests included complete blood count, complement levels (C3/C4), autoantibodies, total cholesterol, and urinalysis. Pregnancy outcome data were collected from patients at the first postpartum visit or via telephone or email.
Preconceptional cardiovascular health
Given the lack in information on smoking, physical activity, diet and fasting glucose, preconceptional cardiovascular health was defined according to three available AHA’s metrics, body mass index (BMI), total cholesterol, and blood pressure, using the following criteria: BMI: (1) poor health (obese): ≥30 kg/m2; (2) intermediate health (overweight): 25-29.9 kg/m2; (3) ideal health (low/normal BMI): <25 kg/m2; total cholesterol: (1) poor health: ≥240 mg/dL; (2) intermediate health: 200–239 mg/dL or treated to goal; (3) ideal health: <200 mg/dL; blood pressure: (1) poor health: systolic ≥140 or diastolic ≥90 mm Hg; (2) intermediate health: systolic 120–139 or diastolic 80–89 mm Hg or treated to goal; (3) ideal health: <120/<80 mm Hg. Each metric was coded as a categorical variable, with “ideal health” as the referent group. Due to small sample size, poor health and intermediate health were collapsed into one exposure category for total cholesterol and blood pressure. Each metric was also analyzed as a continuous variable.
BMI, total cholesterol, and blood pressure at the most recent clinic visit in the one-year prior to conception were used to classify cardiovascular health. If a clinic visit prior to conception was unavailable, the first measurement taken during the first trimester served as a surrogate for preconception health, as minimal changes during the first trimester have been demonstrated.31,32
Pregnancy outcomes
Pregnancy outcomes of interest included gestational age at birth and birth weight for gestational age z-score. Gestational age at birth was based on the last menstrual period and categorized as preterm (<37 weeks) and term (≥37 weeks), and analyzed as a continuous variable. Birth weight for gestational age z-score was based on US population reference percentiles of birth weight, stratified by infant sex.33 Birth weight for gestational age z-score was analyzed as a continuous variable, as well as <10th percentile (small for gestational age; SGA) and >90th percentile (large for gestational age; LGA).
Covariates
Covariates of interest included race, education, age at conception, duration of SLE, and infant birth date (prior to January 1999 and January 1999-February 2015). Medication use (low dose aspirin, hydroxychloroquine, immunosuppressants, prednisone, and prednisone ≥7.5 mg/day) was defined as use ever during pregnancy. Clinical characteristics during pregnancy were defined as ever occurring during pregnancy: renal involvement (renal Lupus Activity Index >1), elevated serum creatinine (>1 mg/dl), high PGA (PGA ≥2), low C3/C4, and anti-dsDNA (ever positive). Organ system damage at conception was measured by the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI), with a score of ≥1 representing the presence of any damage.
Analysis
Differences in the prevalence of preterm birth, SGA, and LGA among live births by preconceptional cardiovascular health were analyzed descriptively by Fischer’s exact test. Unadjusted differences in mean gestational age and mean birth weight for gestational age z-score by preconceptional cardiovascular health were analyzed by ANOVA. Multivariable logistic regression models estimated odds ratios (OR) and 95% confidence intervals for the association of each maternal cardiovascular health factor and categorical pregnancy outcome of interest. Multivariable linear regression models estimated associations of each maternal cardiovascular health factor with continuous outcome measures. To account for correlation between multiple births in the same patient, generalized estimating equations (GEE) with an exchangeable correlation structure were used.34 Confounders were assessed based on combined directed acyclic graph (DAG) minimally sufficient set that was reduced based on a 10% change in beta (β) estimates for parsimony. Models with BMI as the exposure were adjusted for prednisone use during pregnancy and patient race, and blood pressure models were adjusted for renal involvement during pregnancy and patient race. For the exposure of total cholesterol, three adjusted models were estimated: 1) adjusted for patient race and prednisone use during pregnancy; 2) adjusted for patient race and hydroxychloroquine use during pregnancy; and 3) adjusted for patient race, prednisone use during pregnancy, and hydroxychloroquine use during pregnancy. All analyses were conducted with SAS 9.3 (Cary, North Carolina).
Results
During the study period, there were 515 pregnancies, of which 431 were live births. Pregnancies without any cardiovascular metrics available in the one year prior to conception or first trimester were excluded (n=122). Of the 431 births, 309 (73%) had at least one cardiovascular measure (n=291 BMI, n=275 total cholesterol, n=309 blood pressure). Of the 309 pregnancies included, 63% had a cardiovascular health measurement in the one-year prior to conception; the remaining had the measure during the first trimester. More than one singleton live birth per patient was allowed in the analysis (309 births to 261 patients).
A greater proportion of live births excluded from the analysis due to missing cardiovascular health data were to black mothers and had a pregnancy outcome date prior to 1999, compared to the selected sample. Additionally, excluded patients had shorter disease duration, lower frequency of hydroxychloroquine use during pregnancy and lower frequency of low C3/C4. There were no observed differences in live birth outcomes among included patients compared to excluded patients. Patients with a cardiovascular health measurement in the one-year prior to conception had a longer disease duration and higher frequency of hydroxychloroquine and immunosuppressant use than patients with a first trimester measurement. No differences were seen in live birth outcomes or classification of cardiovascular health among patients with preconception measures compared to patients with first trimester measures.
The majority of the 309 pregnancies included in the analysis were to white mothers, with a median age at conception of 30 years (Table 1). Hydroxychloroquine, prednisone, and immunosuppressant use during pregnancy were reported in 60%, 51%, and 15% of pregnancies, respectively. Immunosuppressant use was almost exclusively limited to azathioprine. There were 95 preterm births (31%), and of the 293 pregnancies with birth weights, 18% were SGA and 4% were LGA (Table 2).
Table 1.
Population characteristics in the Hopkins Lupus Pregnancy Cohort.
| Pregnancies | Patients | |
|---|---|---|
|
| ||
| Characteristic | n=309 | n = 261 |
|
| ||
| n (%) | n (%) | |
| Race | ||
| White | 184 (60%) | 151 (58%) |
| Black | 93 (30%) | 80 (31%) |
| Other | 32 (10%) | 30 (11%) |
| Education | ||
| HS Education (≤12 years) | 101 (33%) | 81 (31%) |
| College (13–16 years) | 141 (46%) | 120 (46%) |
| Greater than College (>16 years) | 67 (22%) | 60 (23%) |
| Pregnancy outcome date | ||
| Prior to January 1999 | 117 (38%) | |
| January 1999 – February 2015 | 192 (62%) | |
| Medication use during pregnancy* | ||
| Low dose aspirin | 162 (52%) | |
| Hydroxychloroquine | 184 (60%) | |
| Immunosuppressant | 48 (15%) | |
| Prednisone | 160 (51%) | |
| Prednisone ≥7.5 mg/day among prednisone users | 116 (73%) | |
| No medications | 22 (7%) | |
| Clinical characteristics1 | ||
| Renal involvement during pregnancy (LAI>1) | 79 (26%) | |
| Elevated serum creatinine during pregnancy (>1) | 24 (8%) | |
| High PGA during pregnancy (PGA ≥2) | 49 (16%) | |
| SDI ≥1 at conception | 114 (37%) | |
| Low C3 during pregnancy | 74 (24%) | |
| Low C4 during pregnancy | 106 (34%) | |
| Anti-dsDNA+ during pregnancy | 115 (37%) | |
| Median (IQR) | ||
| Age at conception, years | 29.9 (26.7–33.2) | |
| Disease duration, years | 5.5 (2.1–9.3) | |
| Highest PGA during pregnancy (scale: 0–3) | 1.0 (0.5–1.5) | |
| SDI at conception | 0 (0–3) | |
| Highest daily prednisone dose during pregnancy, mg | 2.5 (0–15.0) | |
| BMI, kg/m 2 | 24.3 (21.3–29.2) | |
| Total cholesterol, mg/dL | 162.0 (142.0–184.0) | |
| Systolic blood pressure, mm Hg | 116.0 (106.0–126.0) | |
| Diastolic blood pressure, mm Hg | 70.0 (64.0–80.0) | |
categories not mutually exclusive; women can be in multiple categories, therefore, percentages add up to more than 100%
Abbreviations: BMI, body mass index; C3, complement 3; C4, complement 4; HS, high school; LAI, Lupus Activity Index; PGA: Physician Global Assessment of Disease Activity
Table 2.
Birth outcomes in the Hopkins Lupus Pregnancy Cohort (n=309 pregnancies).
| Outcome | n (%) |
|---|---|
| Small for gestational age (n=293) | 53 (18%) |
| Large for gestational age (n=293) | 12 (4%) |
| Preterm birth | 95 (31%) |
| Pregnancy induced hypertension (n=252) | 15 (6%) |
| Pre-eclampsia (n=257) | 30 (12%) |
| Caesarian section (n=256) | 100 (39%) |
| Premature rupture of membranes (n=255) | 39 (15%) |
| Median (IQR) | |
| Gestational age at birth (weeks) | 38.0 (36.0–39.0) |
| Birth weight percentile (n=293) | 31.0 (12.0–53.0) |
| Birth weight z-score (n=293) | −0.51 (−1.20 – 0.06) |
| Birth weight (g) (n=293) | 2920.0 (2506.1 – 3309.0) |
BMI, total cholesterol, and blood pressure were reported to be within the ideal range in 56%, 85%, and 51% of pregnancies, respectively (Figure 1). Patients who had low/normal BMI had higher education, a lower prevalence of renal involvement, lower blood pressure, and were more frequently non-black, compared to overweight and obese women. Patients with ideal total cholesterol had higher education, higher frequency of hydroxychloroquine use, and lower BMI, compared to patients with intermediate/poor total cholesterol. Patients with ideal blood pressure had higher education, lower frequency of prednisone use, lower PGA, lower BMI, and were more frequently non-black, compared to patients with intermediate/poor blood pressure.
Figure 1.
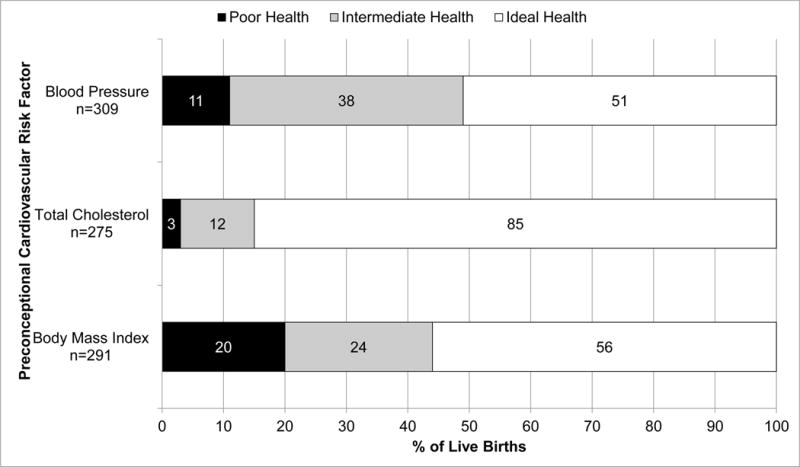
Preconceptional cardiovascular health according to American Heart Association criteria* in the Hopkins Lupus Pregnancy Cohort (n=309 pregnancies).
*Body mass index: (1) poor health (obese): ≥30 kg/m2; (2) intermediate health (overweight): 25-29.9 kg/m2; (3) ideal health (low/normal BMI): <25 kg/m2; Total cholesterol: (1) poor health: ≥240 mg/dL; (2) intermediate health: 200–239 mg/dL or treated to goal; (3) ideal health: <200 mg/dL; Blood pressure: (1) poor health: Systolic ≥140 or Diastolic ≥90 mm Hg; (2) intermediate health: Systolic 120–139 or Diastolic 80–89 mm Hg or treated to goal; (3) ideal health: <120/<80 mm Hg
In descriptive models, there was a lower frequency of preterm birth among patients who were obese (20%) compared to patients who were overweight and had low/normal BMI (39% and 31%, respectively). Frequency of SGA was lowest in patients who were overweight (8%) compared to obese and low/normal BMI (22% and 21%, respectively). The frequency of preterm birth was highest in patients with poor total cholesterol (75%) compared to patients with intermediate and ideal total cholesterol (38% and 27%, respectively). The mean gestational age at birth was lower in patients with poor blood pressure (35.8 weeks) compared to patients with intermediate and ideal blood pressure (36.4 weeks and 37.4 weeks, respectively; Table 3). In sensitivity analysis of only patients with a pre-pregnancy cardiovascular measurement were analyzed (n=195), there were no differences in the associations between cardiovascular health and pregnancy outcomes (data not shown).
Table 3.
Mean gestational age and birth weight z-scores by preconceptional cardiovascular health*, with ANOVA tests for differences in means in the Hopkins Lupus Pregnancy Cohort (n=309 pregnancies).
| Cardiovascular Health | Gestational Age | Birth Weight Z-Score |
|---|---|---|
|
| ||
| Mean (SD) | Mean (SD) | |
| Body mass index | ||
| Ideal health (low/normal BMI) | 36.7 (3.9) | −0.58 (0.92) |
| Intermediate health (overweight) | 36.8 (2.6) | −0.28 (0.82) |
| Poor health (obese) | 37.4 (3.1) | −0.51 (1.06) |
| ANOVA p-value | 0.3 | 0.09 |
| Total cholesterol | ||
| Ideal health | 37.0 (3.2) | −0.48 (0.94) |
| Intermediate health | 36.8 (2.3) | −0.48 (0.95) |
| Poor health | 34.9 (3.9) | −0.53 (0.60) |
| ANOVA p-value | 0.2 | 1.0 |
| Blood pressure | ||
| Ideal health | 37.4 (2.6) | −0.48 (0.98) |
| Intermediate health | 36.4 (3.3) | −0.50 (0.91) |
| Poor health | 35.8 (4.1) | −0.55 (0.72) |
| ANOVA p-value | 0.003 | 0.9 |
Body mass index: (1) poor health (obese): ≥30 kg/m2; (2) intermediate health (overweight): 25-29.9 kg/m2; (3) ideal health (low/normal BMI): <25 kg/m2; Total cholesterol: (1) poor health: ≥240 mg/dL; (2) intermediate health: 200–239 mg/dL or treated to goal; (3) ideal health: <200 mg/dL; Blood pressure: (1) poor health: Systolic ≥140 or Diastolic ≥90 mm Hg; (2) intermediate health: Systolic 120–139 or Diastolic 80–89 mm Hg or treated to goal; (3) ideal health: <120/<80 mm Hg
In adjusted analyses for race and prednisone use (Table 4), overweight was associated with decreased odds of SGA compared to low/normal BMI (OR: 0.26; 95% CI: 0.11, 0.63). In linear regression models, after adjusting for race and prednisone use, gestational age at birth increased with each 1 kg/m2 increase in BMI (β: 0.06; 95% CI: 0.001, 0.11), and overweight was associated with a higher birth weight-for-gestational age z-score (β: 0.32; 95% CI: 0.06, 0.59) (Table 5). Additional adjustment for low dose aspirin use during pregnancy in these models did not change the point estimates.
Table 4.
Multivariable logistic regression models for association of preconceptional cardiovascular health and pregnancy outcomes in SLE in the Hopkins Lupus Pregnancy Cohort (n=309 pregnancies).
| Cardiovascular Health | Preterm Birth | SGA | ||
|---|---|---|---|---|
|
| ||||
| OR (95% CI) | AOR (95% CI) | OR (95% CI) | AOR (95% CI) | |
| Body Mass Index* | ||||
| Ideal Health (low/normal BMI; n=163) | 1.0 | 1.0 | 1.0 | 1.0 |
| Intermediate Health (overweight; n=69) | 1.39 (0.82, 2.36) | 1.38 (0.70, 2.71)§ | 0.35 (0.15, 0.82) | 0.26 (0.11, 0.63)§ |
| Poor Health (obese; n=59) | 0.56 (0.28, 1.13) | 0.50 (0.21, 1.18)§ | 0.95 (0.44, 2.05) | 0.92 (0.42, 2.05)§ |
| Total Cholesterol† | ||||
| Ideal Health (n=235) | 1.0 | 1.0 | 1.0 | 1.0 |
| Intermediate/Poor Health (n=40) | 2.27 (1.15, 4.46) | 2.21 (1.06, 4.62)§ 1.91 (0.96, 3.79)‖ 1.93 (0.92, 4.04)¶ |
0.57 (0.21, 1.54) | 0.41 (0.14, 1.26)§ 0.58 (0.21, 1.61) 0.44 (0.14, 1.38)¶ |
| Blood Pressure‡ | ||||
| Ideal Health (n=158) | 1.0 | 1.0 | 1.0 | 1.0 |
| Intermediate/Poor Health (=151) | 1.32 (0.82, 2.12) | 1.10 (0.67, 1.79)# | 0.68 (0.39, 1.20) | 0.60 (0.33, 1.10)# |
| Continuous variables | ||||
| BMI, kg/m2 (n=291) | 0.97 (0.93, 1.01) | 0.95 (0.91, 1.00)§ | 0.99 (0.94, 1.04) | 0.98 (0.93, 1.04)§ |
| Total cholesterol, 10 mg/dL (n=275) | 1.11 (1.03, 1.19) | 1.10 (1.01, 1.19)§ 1.08 (1.01, 1.16)‖ 1.09 (1.00, 1.18)¶ |
0.91 (0.82, 1.02) | 0.90 (0.80, 1.01)§ 0.92 (0.82, 1.02)‖ 0.90 (0.80, 1.01)¶ |
| Systolic blood pressure, 10 mmHg (n=309) | 1.15 (0.99, 1.34) | 1.08 (0.92, 1.28)# | 0.88 (0.73, 1.06) | 0.85 (0.70, 1.04)# |
| Diastolic blood pressure, 10 mmHg (n=309) | 1.25 (0.99, 1.58) | 1.18 (0.93, 1.50)# | 0.79 (0.59, 1.06) | 0.75 (0.56, 1.02)# |
Body mass index: (1) poor health: ≥30 kg/m2; (2) intermediate health: 25-29.9 kg/m2; (3) ideal health: <25 kg/m2
Total cholesterol: (1) poor health: ≥240 mg/dL; (2) intermediate health: 200–239 mg/dL or treated to goal; (3) ideal health: <200 mg/Dl
Blood pressure: (1) poor health: Systolic ≥140 or Diastolic ≥90 mm Hg; (2) intermediate health: Systolic 120–139 or Diastolic 80–89 mm Hg or treated to goal; (3) ideal health: <120/<80 mm Hg
Adjusted for race (black vs. nonBblack) and prednisone use ever during pregnancy
Adjusted for race (black vs. nonBblack) and antiBmalarial use ever during pregnancy
Adjusted for race (black vs. nonBblack), prednisone use ever during pregnancy, and antiBmalarial use ever during pregnancy
Adjusted for race (black vs. nonBblack) and renal involvement during pregnancy (Renal LAI ≥1)
Table 5.
Multivariable linear regression models for association of preconceptional cardiovascular health and pregnancy outcomes in SLE in the Hopkins Lupus Pregnancy Cohort (n=309 pregnancies).
| Cardiovascular Health | Gestational Age | Birthweight for Gestational Age Z-Score | ||
|---|---|---|---|---|
| β (95% CI) | Adjusted β (95% CI) | β (95% CI) | Adjusted β (95% CI) | |
| Body Mass Index* | ||||
| Ideal Health (low/normal BMI; n=163) | Ref | Ref | Ref | Ref |
| Intermediate Health (overweight; n=69) | 0.19 (−0.65, 1.01) | 0.14 (−0.63, 0.93)§ | 0.29 (0.03, 0.56) | 0.32 (0.06, 0.59)§ |
| Poor Health (obese; n=59) | 0.75 (−0.21, 1.71) | 0.70 (−0.24, 1.65)§ | 0.08 (−0.24, 0.41) | 0.14 (−0.18, 0.45)§ |
| Total Cholesterol† | ||||
| Ideal Health (n=235) | Ref | Ref | Ref | Ref |
| Intermediate/Poor Health (n=40) | −0.53 (−1.46, 0.40) | −0.43 (−1.28, 0.41)§ −0.39 (−1.30, 0.51)‖ −0.37 (−1.22, 0.48)¶ |
0.02 (−0.29, 0.33) | 0.03 (−0.28, 0.34)§ −0.02 (−0.32, 0.29)‖ −0.02 (−0.33, 0.30)¶ |
| Blood Pressure‡ | ||||
| Ideal Health (n=158) | Ref | Ref | Ref | Ref |
| Intermediate/Poor Health (=151) | −1.14 (−1.83, −0.45) | −0.96 (−1.62, −0.29)# | 0.02 (−0.19, 0.24) | 0.09 (−0.12, 0.31)# |
| Continuous variables | ||||
| BMI, kg/m2 (n=291) | 0.05 (−0.004, 0.11) | 0.06 (0.001, 0.11)§ | 0.01 (−0.01, 0.03) | 0.02 (−0.01, 0.04)§ |
| Total cholesterol, 10 mg/dL (n=275) | −0.07 (−0.18, 0.04) | −0.06 (−0.15, 0.04)§ −0.06 (−0.16, 0.04)‖ −0.05 (−0.14, 0.04)¶ |
0.01 (−0.02, 0.03) | 0.01 (−0.02, 0.04)§ 0.004 (−0.02, 0.03)‖ 0.005 (−0.02, 0.03)¶ |
| Systolic blood pressure, 10 mmHg (n=309) | −0.46 (−0.71, −0.21) | −0.39 (−0.63, −0.15)# | 0.004 (−0.06, 0.07) | 0.03 (−0.04, 0.10)# |
| Diastolic blood pressure, 10 mmHg (n=309) | −0.60 (−0.98, −0.22) | −0.52 (−0.89, −0.14)# | 0.01 (−0.09, 0.11) | 0.04 (−0.06, 0.15)# |
Body mass index: (1) poor health: ≥30 kg/m2; (2) intermediate health: 25-29.9 kg/m2; (3) ideal health: <25 kg/m2
Total cholesterol: (1) poor health: ≥240 mg/dL; (2) intermediate health: 200–239 mg/dL or treated to goal; (3) ideal health: <200 mg/Dl
Blood pressure: (1) poor health: Systolic ≥140 or Diastolic ≥90 mm Hg; (2) intermediate health: Systolic 120–139 or Diastolic 80–89 mm Hg or treated to goal; (3) ideal health: <120/<80 mm Hg
Adjusted for race (black vs. non-black) and prednisone use ever during pregnancy
Adjusted for race (black vs. non-black) and anti-malarial use ever during pregnancy
Adjusted for race (black vs. non-black), prednisone use ever during pregnancy, and anti-malarial use ever during pregnancy
Adjusted for race (black vs. non-black) and renal involvement during pregnancy (Renal LAI ≥1)
In logistic regression models adjusted for race and anti-malarial use (Table 4), intermediate/poor total cholesterol was associated with increased odds of preterm birth (OR: 1.91; 95% CI: 0.96, 3.79). No association was seen between cholesterol and SGA. In linear regression models (Table 5), no associations were observed between total cholesterol and gestational age at birth or birth-weight-for-gestational age z-score. Additional adjustment for disease activity in these models did not impact the results.
The odds of preterm birth were only slightly increased and not significant for patients with intermediate/poor blood pressure in logistic regression models (Table 4) after adjustment for race and renal involvement (OR: 1.10; 95% CI: 0.67, 1.79), and no association was observed between blood pressure and SGA. However, in linear regression models (Table 5), intermediate/poor blood pressure was associated with decreased gestational age at birth (β: −0.96; 95% CI: −1.62, −0.29), adjusted for race and renal involvement.
Discussion
The analysis highlights the importance of SLE patients having BMI, total cholesterol, and blood pressure within the ideal range prior to pregnancy in order to improve pregnancy outcomes. In unadjusted analyses, women with ideal weight, cholesterol, or blood pressure had fewer preterm deliveries, and the mean gestational age at birth increased when preconceptional blood pressure was in the ideal range.
In the general population, an analysis of women of reproductive age (20-44 years) from the National Health and Nutrition Examination Survey estimated the prevalence of overweight and obesity in the United States 2003-2008 to be 23% and 29%, respectively.35 Our cohort had a similar distribution of pre pregnancy BMI, with 24% and 20% of women overweight and obese, respectively. Analyses of the Nationwide Inpatient Sample reported that the diagnosis of hypertension prior to pregnancy is more common among women with SLE than women without SLE3. Additionally, among women who gave birth in the United States in 2002, women with SLE had almost three times the prevalence of hypertensive disorders than the general population, with 8% of the general population having a hypertensive disorder.36 As expected, the prevalence of poor and intermediate pre pregnancy blood pressure was high in this cohort, with approximately half of patients having blood pressure ≥120/≥80 mm Hg or blood pressure treated to goal.
The effects of preconceptional cardiovascular health on preterm birth seen in this analysis were consistent with studies of the general population. In the general population, there is a U-shaped association of pre-pregnancy BMI and preterm birth, with the frequency of preterm birth highest among underweight women and obese women.16,37 It is important to note, however, that the indication for preterm birth should be considered. Several studies have demonstrated an increased risk of indicated preterm birth but decreased risk of spontaneous preterm birth in obese patients.38–40 Although data are limited, studies have reported that 70-75% of preterm births in women with SLE are medically indicated.41,42 Reasons for a medically indicated preterm delivery in SLE include maternal blood pressure, pre-eclampsia, proteinuria, decreased amniotic fluid volume, intrauterine growth restriction, HELLP syndrome, and SLE flare.43 A limitation of the present analysis is data specifying indication for preterm births were not collected; therefore, we were unable to determine if preconception cardiovascular health increased the risk of spontaneous preterm birth, indicated preterm birth, or both.
Our finding of increased risk of preterm birth in patients with intermediate and poor preconception cholesterol are supported by previous general population studies. The CARDIA study reported a U-shaped association of pre-pregnancy cholesterol and preterm birth, with the lowest and highest tertiles of pre-pregnancy cholesterol increasing the risk of preterm birth.44 This association was supported by a case-control analysis in the Pregnancy Exposures and Preeclampsia Prevention (PEPP) study, which found the risk of preterm birth in patients with high cholesterol during the first 15 weeks of pregnancy to be almost three times the risk of patients with normal cholesterol.23
The results of our linear regression models show a decrease in gestational age at birth in patients with intermediate and poor preconception blood pressure. This supports previous findings in both the general population and SLE cohorts that hypertension is associated with gestational age at birth.19–21,45
Infant size did not appear to be associated with maternal cholesterol or blood pressure, but overweight women had the fewest small for gestational age infants. In adjusted models, overweight women had an almost 40% increased risk of preterm birth compared to low/normal BMI women; however, somewhat surprisingly, overweight women had a 74% decreased risk of a SGA infant compared to women with low/normal BMI. In linear models, overweight women had a greater birthweight for gestational age z-score compared to women with low/normal BMI in linear models. While studies show that low dose aspirin may decrease the risk of preeclampsia and preterm birth among women with a high risk of these complications, additional adjustment for low dose aspirin use during pregnancy did not change our results. It is possible that our small sample size did not have sufficient power to detect an effect of aspirin use with pregnancy outcomes. Of particular interest, there was no observed difference in the frequency of LGA births by preconceptional BMI, which is in contrast to pregnancies in the general population.17,46,47 However, as power was limited by the low frequency of LGA births in the analysis, these results should be considered preliminary.
Our study suffered from some limitations. Cardiovascular health data were not available for all live births in the cohort, and it is unknown how the cardiovascular health of these patients differed. Data were also unavailable for the four remaining AHA cardiovascular metrics (glucose, physical activity, diet, and cigarette smoking); therefore, we were unable to assess the combined effects of cardiovascular risk factors. As these are important factors for pregnancy outcomes in the general population, poor health in each of these metrics may be associated with an increased risk of preterm birth among SLE patients. Additionally, the data were collected at a single academic center and therefore may not be representative of all SLE patients. Finally, the sample size of the analytic cohort limited our statistical power, particularly for discrete outcomes (preterm birth, SGA, and LGA). While the cohort is larger than other SLE pregnancy cohorts, the modest sample size does not provide sufficient power to detect small differences in outcomes. Even so, the results of analyses with continuous variable mirrored that of categorical variables, giving us confidence in our results.
The findings of our analysis have important implications for SLE patients during pregnancy. Of particular interest is the apparent inverse association of preterm birth in obese patients, but an increased risk of preterm birth in overweight patients. This suggests that efforts to normalize maternal weight prior to pregnancy may improve pregnancy outcomes, but the finding warrants further study. Additionally, having a further understanding of SLE patients who are able to maintain ideal cardiovascular health will be important in order to develop future targeted interventions. Previous studies have found that among SLE patients, pregnancy increases the risk of future major cardiovascular events and a poor pregnancy outcome increases the risk cardiovascular mortality.48 Interventions to improve the cardiovascular health of patients prior to pregnancy would improve pregnancy outcomes, as well as benefit the long-term health of SLE patients.
This analysis is the first to examine the AHA’s guidelines for cardiovascular health in patients with SLE prior to conception, as well as determine the effects of suboptimal preconceptional cardiovascular health on live birth outcomes. Our results suggest that ideal cardiovascular health, in addition to well managed lupus disease activity, is an important component to a successful pregnancy outcome. This finding underscores the importance of pre-pregnancy counseling for women with lupus to ensure that prior to pregnancy their cardiovascular health is optimized in accordance with the American Heart Association’s guidelines, in order to reduce the risk of preterm births and improve the overall cardiovascular health of SLE patients. Interventions to improve preconceptional cardiovascular health may involve weight loss, increased exercise, and appropriate blood pressure control. This analysis increases our perspective on risk factors for pregnancy complications in women with lupus beyond measures of lupus disease activity and management.
Acknowledgments
Funding: The Hopkins Lupus Cohort is funded by NIAMS AR 43727 and 69572.
Footnotes
Competing Interests The authors have no conflicts of interest to disclose.
Contributor Information
Amanda M. Eudy, Department of Epidemiology, University of North Carolina Chapel Hill Gillings School of Global Public Health, Chapel Hill, NC.
Anna Maria Siega-Riz, Department of Epidemiology, University of North Carolina Chapel Hill Gillings School of Global Public Health, Chapel Hill, NC.
Stephanie M. Engel, Department of Epidemiology, University of North Carolina Chapel Hill Gillings School of Global Public Health, Chapel Hill, NC.
Nora Franceschini, Department of Epidemiology, University of North Carolina Chapel Hill Gillings School of Global Public Health, Chapel Hill, NC.
Annie Green Howard, Department of Biostatistics, University of North Carolina Chapel Hill Gillings School of Global Public Health, Chapel Hill, NC.
Megan E. B. Clowse, Division of Rheumatology, Department of Medicine, Duke University Medical Center, Durham, NC.
Michelle Petri, Division of Rheumatology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD.
References
- 1.Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus. 2006;15:308–18. doi: 10.1191/0961203306lu2305xx. [DOI] [PubMed] [Google Scholar]
- 2.Petri M, Daly R, Pushparajah D. Healthcare costs of pregnancy in systemic lupus erythematosus: retrospective observational analysis from a US health claims database. J Med Econ. 2015;18:967–73. doi: 10.3111/13696998.2015.1066796. [DOI] [PubMed] [Google Scholar]
- 3.Clowse MEB, Jamison M, Myers E, James AH. A national study of the complications of lupus in pregnancy. AJOG. 2008;199:127 e1–6. doi: 10.1016/j.ajog.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnabe C, Faris P, Hude Q. Canadian pregnancy outcomes in rheumatoid arthritis and systemic lupus erythematosus. Int J Rheumatol. 2011 doi: 10.1155/2011/345727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction the American Heart Association’s Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 6.Yang Q, Cogswell ME, Flanders W, Hong Y, Zhang Z, Loustalot F, et al. Trends in cardiovascular health metrics and associations with all-cause and cvd mortality among us adults. JAMA. 2012;307:1273–83. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125:987–95. doi: 10.1161/CIRCULATIONAHA.111.049122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertoli AM, Alarcón GS, Calvo-Alén J, Fernández M, Vilá LM, Reveille JD. Systemic lupus erythematosus in a multiethnic US cohort: clinical features, course, and outcome in patients with late-onset disease. Arthritis Rheum. 2006;54:1580–7. doi: 10.1002/art.21765. [DOI] [PubMed] [Google Scholar]
- 9.Urowitz MB, Gladman DD. Atherosclerosis and lupus – the SLICC study. Lupus. 2007;16:925–8. doi: 10.1177/0961203307085259. [DOI] [PubMed] [Google Scholar]
- 10.Urowitz MB, Gladman D, Ibañez D, Fortin P, Sanchez-Guerrero J, Bae S, et al. Clinical manifestations and coronary artery disease risk factors at diagnosis of systemic lupus erythematosus: data from an international inception cohort. Lupus. 2007;16:731–5. doi: 10.1177/0961203307081113. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–5S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 12.King JC. Maternal obesity, metabolism, and pregnancy outcomes. Annu Rev Nutr. 2006;26:271–91. doi: 10.1146/annurev.nutr.24.012003.132249. [DOI] [PubMed] [Google Scholar]
- 13.Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr. 2001;4:611–24. doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- 14.Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103:219–24. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- 15.Wolf M, Kettyle E, Sandler L, Ecker JL, Roberts J, Thadhani R. Obesity and preeclampsia: the potential role of inflammation. Obstet Gynecol. 2001;98:757–62. doi: 10.1016/s0029-7844(01)01551-4. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharya S, Campbell D, Liston W, Bhattacharya S. Effect of body mass index on pregnancy outcomes in nulliparous women delivering singleton babies. BMC Public Health. 2007;7:168. doi: 10.1186/1471-2458-7-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. AJOG. 2004;191:964–8. doi: 10.1016/j.ajog.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 18.Lu GC, Rouse DJ, DuBard M, Cliver S, Kimberlin D, Hauth JC. The effect of the increasing prevalence of maternal obesity on perinatal morbidity. AJOG. 2001;185:845–9. doi: 10.1067/mob.2001.117351. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert WM, Young AL, Danielsen B. Pregnancy outcomes in women with chronic hypertension: a population-based study. J Reprod Med. 2007;52:1046–51. [PubMed] [Google Scholar]
- 20.Catov JM, Nohr EA, Olsen J, Ness RB. Chronic hypertension related to risk for preterm and term small for gestational age births. Obstet Gynecol. 2008;112:290–6. doi: 10.1097/AOG.0b013e31817f589b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chatzi L, Plana E, Daraki V, Karakosta P, Alegkakis D, Tsatsanis C, et al. Metabolic syndrome in early pregnancy and risk of preterm birth. Am J Epidemiol. 2009;170:829–36. doi: 10.1093/aje/kwp211. [DOI] [PubMed] [Google Scholar]
- 22.Haelterman E, Bréart G, Paris-Liado J, Dramaix M, Tchobroutsky C. Effect of uncomplicated chronic hypertension on the risk of small-for-gestational age birth. Am J Epidemiol. 1997;145:689–95. doi: 10.1093/aje/145.8.689. [DOI] [PubMed] [Google Scholar]
- 23.Catov JM, Bodnar LM, Kip KE, Hubel C, Ness RB, Harger G, et al. Early pregnancy lipid concentrations and spontaneous preterm birth. AJOG. 2007;197:610 e1–7. doi: 10.1016/j.ajog.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 24.Catov JM, Bodnar LM, Ness RB, Barron SJ, Roberts JM. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. Am J Epidemiol. 2007;166:1312–9. doi: 10.1093/aje/kwm273. [DOI] [PubMed] [Google Scholar]
- 25.Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ. 2002;325:157–60. doi: 10.1136/bmj.325.7356.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 27.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 28.Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–86. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petri M, Buyon J, Kim M. Classification and definition of major flares in SLE clinical trials. Lupus. 1999;8:685–91. doi: 10.1191/096120399680411281. [DOI] [PubMed] [Google Scholar]
- 30.Buyon JP, Petri MA, Kim MY, Kalunian KC, Grossman J, Hahn BH, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142:953–62. doi: 10.7326/0003-4819-142-12_part_1-200506210-00004. [DOI] [PubMed] [Google Scholar]
- 31.Potter JM, Nestel PJ. The hyperlipidemia of pregnancy in normal and complicated pregnancies. AJOG. 1979;133:165–70. doi: 10.1016/0002-9378(79)90469-1. [DOI] [PubMed] [Google Scholar]
- 32.Fattah C, Farah N, Barry SC, O’Connor N, Stuart B, Turner MJ. Maternal weight and body composition in the first trimester of pregnancy. Acta Obstet Gynecol Scand. 2010;89:952–5. doi: 10.3109/00016341003801706. [DOI] [PubMed] [Google Scholar]
- 33.Oken E, Kleinman K, Rich-Edwards J, Gillman M. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatrics. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 35.Tinker SC, Hamner HC, Berry RJ, Bailey LB, Pfeiffer CM. Does obesity modify the association of supplemental folic acid with folate status among nonpregnant women of childbearing age in the United States? Birth Defects Res A Clin Mol Teratol. 2012;94:749–55. doi: 10.1002/bdra.23024. [DOI] [PubMed] [Google Scholar]
- 36.Chakravarty EF, Nelson L, Krishnan E. Obstetric hospitalizations in the United States for women with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006;54:899–907. doi: 10.1002/art.21663. [DOI] [PubMed] [Google Scholar]
- 37.Haas JS, Fuentes-Afflick E, Stewart AL, Jackson RA, Dean ML, Brawarsky P, et al. Prepregnancy health status and the risk of preterm delivery. Arch Pediatr Adolesc Med. 2005;159:58–63. doi: 10.1001/archpedi.159.1.58. [DOI] [PubMed] [Google Scholar]
- 38.Nohr EA, Bech BH, Vaeth M, Rasmussen KM, Henriksen TB, Olsen J. Obesity, gestational weight gain and preterm birth: a study within the Danish National Birth Cohort. Paediatr Perinat Epidemiol. 2007;21:5–14. doi: 10.1111/j.1365-3016.2007.00762.x. [DOI] [PubMed] [Google Scholar]
- 39.Smith GC, Shah I, Pell JP, Crossley JA, Dobbie R. Maternal obesity in early pregnancy and risk of spontaneous and elective preterm deliveries: a retrospective cohort study. Obstet Gynecol Surv. 2007;62:299–300. doi: 10.2105/AJPH.2005.074294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hendler I, Goldenberg RL, Mercer BM, Iams JD, Meis PJ, Moawad AH, et al. The Preterm Prediction Study: Association between maternal body mass index and spontaneous and indicated preterm birth. AJOG. 2005;192:882–6. doi: 10.1016/j.ajog.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 41.Carvalheiras G, Vita P, Marta S, Trovão R, Farinha F, Braga J, et al. Pregnancy and systemic lupus erythematosus: review of clinical features and outcome of 51 pregnancies at a single institution. Clin Rev Allergy Immunol. 2010;38:302–6. doi: 10.1007/s12016-009-8161-y. [DOI] [PubMed] [Google Scholar]
- 42.Eudy AM, Jayasundara M, Haroun T, Neil L, James AH, Clowse ME. Reasons for cesarean and medically indicated deliveries in pregnancies in women with systemic lupus erythematosus. Lupus. 2018;27:351–6. doi: 10.1177/0961203317720525. [DOI] [PubMed] [Google Scholar]
- 43.Clark CA, Spitzer KA, Nadler JN, Laskin CA. Preterm deliveries in women with systemic lupus erythematosus. J Rheumatol. 2003;30:2127–32. [PubMed] [Google Scholar]
- 44.Catov JM, Ness RB, Wellons MF, Jacobs DR, Roberts JM, Gunderson EP. Prepregnancy lipids related to preterm birth risk: the coronary artery risk development in young adults study. J Clin Endocrinol Metab. 2010;95:3711–8. doi: 10.1210/jc.2009-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petri M, Howard D, Repke J, Goldman DW. The Hopkins lupus pregnancy center: 1987–1991 Update. Am J Reprod Immunol. 1992;28:188–91. doi: 10.1111/j.1600-0897.1992.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 46.Stuebe AM, Landon MB, Lai Y, Spong CY, Carpenter MW, Ramin SM, et al. Maternal BMI, glucose tolerance, and adverse pregnancy outcomes. AJOG. 2012;207:62 e1–7. doi: 10.1016/j.ajog.2012.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khashan AS, Kenny LC. The effects of maternal body mass index on pregnancy outcome. Eur J Epidemiol. 2009;24:697–705. doi: 10.1007/s10654-009-9375-2. [DOI] [PubMed] [Google Scholar]
- 48.Soh MC, Nelson-Piercy C, Dib F, Westgren M, McCowan L, Pasupathy D. Association between pregnancy outcomes and death from cardiovascular causes in parous women with systemic lupus erythematosus: a study using Swedish population registries. Arthritis Rheumatol. 2015;67:2376–82. doi: 10.1002/art.39218. [DOI] [PubMed] [Google Scholar]


