Abstract
Oral intake of beta-hydroxy-beta-methylbutyrate (HMB), arginine, and glutamine may ameliorate muscle loss by stimulating protein synthesis and decreasing protein degradation while simultaneously decreasing inflammation. Previous studies provide evidence for improvement in body composition with dietary supplementation of these ingredients among patients with muscle-wasting diseases. The objectives of this study were to examine the effects of this amino acid mixture on lean body mass, muscle volume, and physical function among healthy older adults.
Thirty-one community-dwelling men and women, ages 65–89, were randomized to either two oral doses of the amino acid supplement (totaling 3g HMB, 14g arginine, 14g glutamine) or placebo daily for six months. At baseline and month six, lean body mass was measured by air displacement plethysmography, dual-energy X-ray absorptiometry and four-compartment model. Muscle volume of quadriceps was quantified by magnetic resonance imaging (MRI), and participants performed a battery of tests to assess physical function.
As compared to the placebo group, the treatment group exhibited improvement in a timed stair climb (p = 0.016) as well as significant increases in lean body mass by all methods of assessment (p < 0.05). Regional analysis by DXA revealed increased arm lean mass in the supplement group only (p = 0.035). However, no change was observed in MRI-derived quadriceps volume.
Dietary supplementation with HMB, arginine, and glutamine improved total body lean mass among a small sample of healthy older adults. Further research is indicated to elucidate mechanisms of action and to determine whether supplementation may benefit frail elders.
Keywords: Beta-hydroxy-beta-methylbutyrate, Arginine, Glutamine, Elderly, Lean Body Mass
Introduction
A major health issue facing older adults worldwide is the progressive loss of muscle mass with advancing age. Even with normal healthy aging, skeletal muscle mass decreases by as much as 2% each year after age 50 y (Morley et al. 2010), and this age-related loss of muscle is correlated with declines in physical function and activities of daily living (Jones et al. 2009).
Underlying etiologies for muscle loss with age are multifactorial. Contributing factors include a decreased anabolic response of aging muscle to amino acids increased cell damage by reactive oxygen species (Roubenoff 2007; Jensen 2008) and circulating pro-inflammatory cytokines (Burton and Sumukadas 2010; Schaap et al. 2006; Meng and Yu 2010). To date, researchers have failed to identify effective means to prevent or reverse muscle loss. While resistance exercise appears to offer the most promise (Hunter, McCarthy, and Bamman 2004), exercise training is not feasible for all older adults, particularly those with limited mobility or high risk for fall-related injury. Therefore, effective dietary interventions are needed. Such dietary interventions may include oral amino acid supplementation.
Beta-hydroxy-beta-methylbutyrate (HMB) is an active metabolite of the amino acid leucine (Zanchi et al. 2010) that may improve muscle protein turnover by activating mTOR cell signaling pathways (Pimentel et al. 2011; Fitschen et al. 2013; Eley et al. 2007) and inhibiting protein degradation by proteasomes (Portal et al. 2010). HMB may further decrease muscle protein breakdown by reducing inflammation (Eley, Russell, and Tisdale 2008a; Townsend et al. 2013; Hsieh et al. 2006). The amino acid arginine may work synergistically with HMB to decrease oxidative stress (Potenza, Nacci, and Mitolo-Chieppa 2001) andinflammation, thereby attenuating muscle loss. Like HMB, arginine may also have direct anabolic effects on skeletal muscle through regulation of mTOR cell signaling pathways (Yao et al. 2008).
As the most abundant free amino acid in the body (Melis et al. 2004). glutamine also serves as an anabolic substrate. In vitro studies provide evidence that glutamine can upregulate muscle protein synthesis (Wu and Thompson 1990) (Chiu et al. 2012; Xi et al. 2011). Antioxidant and immunomodulatory properties of glutamine may also complement HMB and arginine by decreasing muscle damage from reactive oxygen species and pro-inflammatory cytokines (Roth 2008).(Xi et al. 2011).
Previous studies with HMB, arginine, and glutamine provide evidence for favorable effects on body composition of patients with cancer and acquired immune deficiency syndrome (AIDS) (May et al. 2002; Berk et al. 2008) (Clark et al. 2000). While these previous studies have led to the widespread clinical use of this amino acid mixture for muscle-wasting diseases, effects on body composition of healthy older adults are unknown. Thus, the present study aimed to determine whether this dietary supplement may affect body composition in the general population of healthy older adults.
Other studies investigating similar mixtures of amino acids among healthy older adults suggest promise. A study by Flakoll et al. reported a trend for increased fat-free mass determined by bioelectrical impedance analysis (BIA) (p = 0.08) among untrained older women who supplemented their diets with HMB, arginine, and lysine for 12 weeks (Flakoll et al. 2004). Another group of researchers supplemented the diets of older men and women with a combination of HMB, arginine, and lysine without exercise for a period of 12 months. They reported improvement in fat-free mass estimated by BIA (p = 0.002), but they saw no effects on muscle strength or physical function (Baier et al. 2009). Citing research associating circulating vitamin D [25(OH)D] with muscle strength and function (Ceglia 2009), these researchers re-analyzed the data, stratifying participants by vitamin D status (< 30 vs > 30 ng/mL). Post-hoc analysis revealed improvement in muscle strength was specific to individuals with circulating 25(OH)D > 30 ng/ml (Fuller et al. 2011).
Taken together, previous studies suggest benefit from supplementation, and they hint at a possible synergistic effect of amino acids and vitamin D. However, many previous studies were limited by estimates of lean body mass from the proxy measure of BIA, a method prone to variability depending on hydration status (Smith 1993) and prediction equations (Mattsson and Thomas 2006). Estimates of fat mass and lean mass by air displacement plethysmography (BOD POD) and dual-energy X-ray absorptiometry (DXA) are more robust measures compared to BIA (Smith 1993), and the four-compartment model of body composition is considered the gold standard for assessing fat mass and fat-free mass (Malina 2007; Minderico et al. 2008). Likewise, the method of magnetic resonance imaging (MRI) is considered a gold-standard for detecting small changes in individual muscle groups (Mattsson and Thomas 2006).
Thus, the primary purpose of this study was to determine whether six-month dietary supplementation with an amino acid mixture of HMB, arginine, and glutamine would affect lean body mass and thigh muscle volume of healthy older adults as determined by the robust methods of air displacement plethysmography, DXA, four-compartment model and MRI. A secondary objective was to examine the effects of supplementation on physical function.
Subjects and Methods
Participants
Participants were 31 ambulatory, community-dwelling men and women, ages 65y and older. Sample size calculations were based on published data showing increases in fat-free mass over eight and twelve weeks (Vukovich, Stubbs, and Bohlken 2001; Flakoll et al. 2004). Based on these studies, assuming a change in fat-free mass of 0.5 kg, standard deviation of change 0.4, a two-sided paired t-test, and a significance level of 5%, 80% power to detect a significant change in fat-free mass within each group would be achieved with eight participants per group. Enrolling fourteen participants per group would allow for 80% power to detect significant between-group differences assuming a difference in the change in fat-free mass between treatment and placebo groups of 0.45 kg (+ 0.4), two-sided individual samples t-test, and a significance level of 5%. Exclusion criteria included steroid/androgen use within the previous three months, body mass index (BMI) ≤ 18.5 or ≥ 40 kg/m2, cognitive impairment, terminal illness, or a history of uncontrolled hypertension or diabetes. Each individual provided written consent, and the protocol was approved by the Institutional Review Board for Human Use at the University of Alabama at Birmingham (UAB).
Study Design
For this double-blind, placebo-controlled trial, participants were randomized to supplement their usual diets with either the amino acid supplement (Juven® by Abbott Nutrition, Columbus, Ohio) or a calorically-similar placebo (isomaltulose, citric acid, and flavoring) for six months. Supplements and placebo were provided in individual packets in powder form. Participants were instructed to mix each packet with 240 ml of fluid and to take one packet with breakfast and another with dinner each day. Subjects were also instructed to keep their usual diets and activity patterns stable throughout the study. Two daily doses of the supplement provided a total of 3g HMB, 14g L-arginine, and 14g L-glutamine in the experimental group. A randomization schedule was generated by a statistician using PROC PLAN in SAS Version 9.1, with randomization further stratified by sex. For the purpose of blinding, supplement and placebo doses were provided in coded packets by Abbott Nutrition (Columbus, OH), and neither subjects nor investigators were aware of group assignment. Compliance to the supplement protocol was monitored using daily log forms with check boxes.
Over the 6-month study period, participants came to the testing facility once every four weeks to pick up supplement/placebo, turn in log forms, and obtain weight measurements. Weight was measured using a calibrated scale, and height was recorded from wall-mounted stadiometer. Dietary intake was monitored by 24-hour recalls administered at baseline and monthly afterwards. Recalls were administered by a single-trained staff member using a multi-pass method. Dietary intake data were analyzed using Nutrition Data System for Research software (Nutrition Coordinating Center, Minneapolis, MN, 2014). At baseline and month six, subjects provided a fasted blood sample for analysis of 25(OH)D, and they performed additional tests for body composition and physical function.
Body Composition
Following an eight-hour overnight fast, total body density, percent body fat, and fat-free mass were assessed by whole body air displacement plethysmography (BOD POD Body Composition System version 1.69; Life Measurement Instruments, Concord, CA). Participants removed all jewelry and wore form-fitting swimsuits and swim caps during testing. Body density was calculated from measurements of body mass and body volume; then, the 2-compartment model of Siri (WE 1961) was used to calculate percent body fat from density. Fat-free mass was calculated by subtracting fat mass from total body mass.
Bone-free lean mass, percent body fat, and total bone mineral content were measured by dual energy X-ray absorptiometry (DXA) (Lunar iDXA; GE Healthcare, Madison, WI; software version 12.3). Leg lean mass and arm lean mass were also quantified from DXA. Full-body scans were obtained with participants lying in a supine position, wearing light clothing.
Total body water (TBW) was determined by deuterium dilution as previously described (Schoeller et al. 1980). An initial urine sample was collected after an 8-hr overnight fast. Immediately afterwards, each subject consumed a 10-gram dose of deuterium oxide (2H20, 99.9%; Cambridge Isotope Laboratories, Inc.) from a sterile plastic container. The container was filled with 50 ml of tap water, and subjects were asked to drink the water. All participants voided three hours after consuming the isotope dose. A final urine sample was collected at four hours post-dose. Urine samples were analyzed in duplicate for 2H by isotope ratio mass spectrometry (Delta V Advantage IRMS with High Temperature Conversion Elemental Analyzer; Thermo Scientific, Inc.). TBW was calculated from the following formula: TBW = dose of tracer / (isotope enrichment of post-dose sample minus isotope in pre-dose sample).
Body mass and density from BOD POD, total bone mineral content from DXA, and TBW from deuterium dilution were used to compute percent body fat from the four-compartment model method previously described by Baumgartner et al. (Baumgartner et al. 1991). This model was derived from a population of ambulatory men and women, ages 65–94y. Fat-free mass was calculated by subtracting fat mass from total body mass.
Quadriceps Muscle Volume
Quadriceps muscle volume was determined by magnetic resonance imaging (MRI) using a 3-Tesla magnet with a whole-body coil (Philips Achieva System; Andover, MA). T1 proton-weighted, trans-axial images 1-cm thick spaced 0.5-cm apart were collected at the midpoint of the thigh. The same technician determined regions of interest (ROI) by outlining the selected muscles and signal intensity thresholds for contractile tissue (SliceOmatic Image Analysis Software, version 4.2; Tomovision, Montreal, Canada). Volumes of contractile and non-contractile tissue were determined by converting the number of pixels within the ROI to cross-sectional area (cm2) by spatial calibration. The cross-sectional area of each image was summed to calculate volume.
Physical Function
Participants performed three different tests of physical function at baseline and month six. All tests were administered following a 3-minute warmup on a stationary bike. The tests were selected to represent typical activities of daily living and included an eight-foot up-and-go test, 25-foot walk test, and timed stair climb.
Up-and-go test.
The up-and-go test was performed as previously described by Podsiadlo and Richardson (Podsiadlo and Richardson 1991). A stop watch was used to measure the time required for a participant to stand from a seated position, walk briskly to circle a cone placed eight feet from the front of the chair, and return to a seated position. Time required to complete the task was recorded to the nearest 0.01 second, and the best score of two consecutive trials was used in the statistical analysis.
Twenty-five-foot walk test.
A stop watch was used to time participants as they walked a 25-ft distance on a flat surface. To allow for acceleration and deceleration, the course included 15 ft on both sides of the 25-ft distance. Participants were asked to walk as fast as possible without running (Hunter et al. 1995). Time was recorded to the nearest 0.01 second, and the best score of two consecutive trials was used in the statistical analysis.
Timed stair climb.
Participants were asked to ascend one flight of 12 steps (seven inches in ht) as quickly and safely as possible. Subjects were instructed to take only one stair at a time and to use the handrail only as needed for stability (Skelton et al. 1995). A timing mat was used to measure time to the nearest 0.001 second, and the final score was recorded as the average of two consecutive trials.
Circulating Vitamin D
Blood was sampled from an antecubital vein after an eight-hour overnight fast, and serum was analyzed for circulating 25(OH)D. Serum measurements for one participant from the treatment group and another from the placebo group were not recorded due to inability of the phlebotomist to obtain venous blood samples from these individuals. Serum 25(OH)D was quantified using IDS ELISA plates (Immuno Diagnostic Systems; Fountain Hills, AZ) with a volume size of 25 μl in duplicate. Minimum sensitivity is 6.8 nmol/l, inter-assay coefficient of variation (CV) is 4.94%, and intra-assay CV is 4.83%.
All body composition tests, physical function assessments, and laboratory assays were conducted by the Core Laboratory of UAB’s Diabetes and Research Center, Nutrition Obesity Research Center, and Center for Clinical and Translational Science.
Statistics
Pearson chi-square tests were used to examine group differences in gender, and the nonparametric Mann-Whitney U test was used to compare age between the two groups. Independent t-tests were used to compare all other variables between groups at baseline and month six.
Changes within each group from baseline to month six were examined by paired t-tests. Changes over time were also examined by repeated-measures ANOVA. Cross-sectional studies have associated low circulating concentrations of vitamin D with low muscle mass, strength, and physical function (Ceglia 2009; Bischoff-Ferrari, Stähelin, and Walter 2011; Moreno et al. 2011). Because older adults are more prone to vitamin D insufficiency compared to younger adults (Holick 2008; Morley 2009), serum 25(OH)D was included as a covariate in all statistical models.
All calculations were two-sided with a significance level of p < 0.05 and were performed using SPSS 24.0 for Windows.
Results
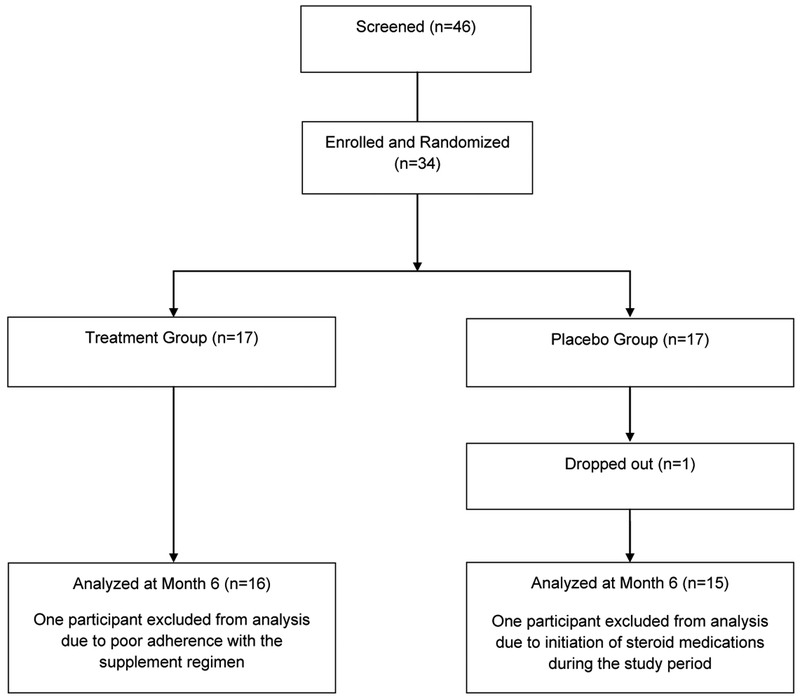
Data are reported for 31 men and women who completed the study (Figure 1). Although 40 participants were originally recruited, six of those individuals were excluded after an initial screening visit because they did not meet the inclusion criteria or because they opted not to enroll. One participant dropped out shortly after baseline testing, and another was excluded due to concomitant use of steroid medications during the study period. Data from one participant was excluded from statistical analysis for failure to meet the compliance rate. Minimal compliance with the supplement regimen was predetermined as 67% of the recommended dose of two packets per day for six months. Compliance rates for remaining participants in the treatment and placebo groups were 98.3% ± 0.04 and 97.9% ± 0.03, respectively.
Figure 1.
Participants Included and Excluded
As displayed in Table 1, demographic characteristics were similar between the two groups. No group differences were identified at baseline or month six for energy intake or macronutrient intake, and no changes in these variables were observed from baseline to month six within either group (data not shown). Independent t-tests revealed no group differences in any measure of body composition, physical function, or serum 25(OH)D at baseline or month six. No changes were observed within either group for weight, BMI, or 25(OH)D levels during the study period (Table 2). Likewise, no changes in fat mass were detected by BOD POD, DXA, or four-compartment model (data not shown).
Table 1.
Baseline Characteristics of Treatment and Placebo Groups
| Variable | Treatment Group | Placebo Group | P-value |
|---|---|---|---|
| Males/females | 7/9 | 7/8 | 0.870 |
| Caucasian/ African American/Asian American | 14/2/0 | 14/0/1 | 0.226 |
| Age (years) | 72.3 ± 6.6 | 70.6 ± 5.2 | 0.451 |
Table 2.
Body Composition and Physical Function Measures at Baseline and Month Six for Treatment (N = 16) and Placebo (N = 15) Groups
| Measure | Baseline | Month 6 | Change within groupa | Time × Treatment (unadjusted)b |
Time × Treatment Adjusted for 25(OH)Db |
|---|---|---|---|---|---|
| Body Weight (kg) • Treatment • Placebo |
85.4 ± 14.8 79.9 ± 16.3 |
86.4 ± 14.5 80.5 ± 16.1 |
p = 0.063 p = 0.246 |
p = 0.610 |
p = 0.336 |
| BMI (kg/m2) • Treatment • Placebo |
29.4 ± 4.5 27.5 ± 4.0 |
29.7 ± 4.3 27.7 ± 3.9 |
p = 0.073 p = 0.406 |
p = 0.506 |
p = 0.220 |
| BODPOD FFM (kg) • Treatment • Placebo |
52.7 ± 12.0 49.0 ± 12.6 |
54.3 ± 12.7 48.7 ± 11.7 |
p = 0.012 p = 0.622 |
p = 0.021 |
p = 0.004 |
| DXA bone-free lean mass (kg) • Treatment • Placebo |
49.2 ± 10.0 46.8 ± 11.3 |
50.3 ± 10.7 46.7 ± 10.9 |
p = 0.003 p = 0.688 |
p = 0.009 |
p = 0.005 |
| 4-Compartment Model FFM (kg) • Treatment • Placebo |
51.9 ± 11.4 48.5 ± 11.0 |
52.9 ± 11.9 48.4 ± 11.4 |
p = 0.023 p = 0.745 |
p = 0.068 |
p = 0.036 |
| DXA Arm Lean (kg) • Treatment • Placebo |
5.6 ± 1.6 5.0 ± 1.9 |
5.8 ± 1.7 5.1 ± 1.8 |
p = 0.011 p = 0.682 |
p = 0.035 |
p = 0.007 |
| DXA Leg Lean (kg) • Treatment • Placebo |
16.8 ± 3.9 15.4 ± 4.0 |
17.2 ± 4.0 15.8 ± 4.1 |
p = 0.024 p = 0.025 |
p = 0.802 |
p = 0.949 |
| Thigh muscle volume (cm2) • Treatment • Placebo |
602.2 ± 141.3 549.1 ± 134.6 |
602.3 ± 132.9 546.8 ± 132.8 |
p = 0.892 p = 0.766 |
p = 0.744 |
p = 0.598 |
| Up-and-go test (s) • Treatment • Placebo |
5.60 ± 1.79 5.33 ± 0.83 |
5.54 ± 1.61 5.15 ± 0.87 |
p = 0.881 p = 0.287 |
p = 0.508 |
p = 0.322 |
| 25-ft timed walk test (s) • Treatment • Placebo |
4.06 ± 0.92 3.84 ± 0.70 |
4.01 ± 1.08 3.94 ± 0.46 |
p = 0.677 p = 0.428 |
p = 0.394 |
p = 0.497 |
| Timed stair climb (s) • Treatment • Placebo |
5.428 ± 1.978 4.555 ± 0.768 |
4.858 ± 1.662 4.607 ± 0.796 |
p = 0.002 p = 0.777 |
p = 0.016 |
p = 0.047 |
| 25-hydroxyvitamin D (ng/ml) • Treatment Group (N = 15) • Placebo Group (N = 14) |
29.8 ± 8.6 32.5 ± 8.6 |
30.7 ± 7.8 32.1 ± 9.5 |
p = 0.529 p = 0.717 |
p = 0.475 |
Mean ± SD ; BMI = body mass index, FFM = fat-free mass
paired t-tests
RM-ANOVA
Paired t-tests revealed significant improvement in total lean body mass by all methods of body composition assessment within the treatment group but not within the control group (Table 2). In addition to results of paired t-tests, Table 2 shows results of repeated-measures ANOVA both unadjusted and adjusted for circulating vitamin D. After adjustment for 25(OH)D, RM-ANOVA confirmed significant Time x Treatment interactions for fat-free mass by BOD POD (p = 0.004), bone-free lean mass by DXA (p = 0.005), and fat-free mass from the four-compartment model (p = 0.036). Results were similar when sex was included in the models in place of vitamin D (data not shown). Regional analysis of lean mass by DXA showed improvement in arm lean mass within the treatment group but not within the control group (p = 0.011; p = 0.007 for Time x Treatment interaction). RM-ANOVA models failed to show significant Time x Treatment interactions for changes in leg lean mass, and MRI analysis revealed no effects of supplementation on muscle volume of quadriceps. There was also a significant improvement in the timed stair climb among the treatment group (p = 0.002; p = 0.047 for Time x Treatment interaction) (Table 2).
Discussion
Six months of dietary supplementation with HMB, arginine, and glutamine resulted in significant improvement in total lean body mass among healthy older adults. Results were consistent among different measures including air displacement plethysmography, DXA, and the gold-standard four-compartment model. Improvements in lean body mass were independent of vitamin D status. Regional Despite improvement in participants’ physical function as assessed by a timed stair climb, regional analyses by DXA and MRI suggested that supplementation may be most influential on muscles of the upper extremity compared to weight-bearing muscles of the lower extremity.
Improvement in Lean Body Mass and Physical Function with Supplementation
A major finding of this study was the improvement in total lean body mass of healthy older adults with oral ingestion of HMB, arginine, and glutamine. Although results of previous clinical trials indicated that this amino acid mixture may favorably affect body composition in younger cohorts with cancer (May et al. 2002; Berk et al. 2008) and AIDS (Clark et al. 2000), , no studies have investigated the effects of this particular dietary supplement in healthy older adults. Previous studies examining supplementation of HMB alone in older adults have yielded equivocal results in regards to total body lean mass. In 2001, Vukovich et al. randomized healthy men and women ages 70 and older to either three grams daily of HMB or a rice flour placebo along with concomitant resistance training. Total body fat-free mass estimated by skinfold thicknesses tended to increase in the treatment group (p=0.08), but no change was detected by DXA (Vukovich, Stubbs, and Bohlken 2001). A more recent study among healthy adults ages 65y and older examined the effects of six-month HMB supplementation vs placebo, with and without resistance training, on lean body mass, strength, and function. Among non-exercisers, the treatment group showed significant improvement in lean body mass assessed by DXA (p < 0.01), leg extensor strength (p < 0.05), and time required to complete a get-up-and go functional test (p < 0.05) compared to the placebo group. Although resistance exercise significantly improved lean mass among both HMB and placebo groups, increases in lean mass did not differ between groups (Stout et al. 2013). A separate study supplemented the diets of older adults with a mixture of HMB, arginine, and lysine without exercise for a period of 12 months. They reported improvement in fat-free mass by BIA (p = 0.002) (Baier et al.). Similarly, the older adults in our study were instructed to keep physical activity levels consistent, but they were not prescribed an exercise regimen. Taken together, the collective evidence seems to suggest the greatest benefits of supplementation in the absence of resistance training. Thus, although resistance exercise is known to be the most effective strategy for attenuating age-related loss of muscle (Hunter, McCarthy, and Bamman 2004), this nutritional intervention may be particularly beneficial for people who are unable or unwilling to exercise. Because HMB is frequently marketed as an ergogenic aid among weight lifters (Zanchi et al. 2010), we expected to see the greatest improvement in weight-bearing muscle groups of the lower extremity. However, our results seemed to indicate the opposite. Arm lean mass assessed by DXA increased with supplementation while no change in muscle volume of the quadriceps was detected by MRI. These results suggest that supplementation of HMB, arginine, and glutamine may have a more dramatic treatment effect on non-weight-bearing muscles. Nonetheless, significant improvement was demonstrated in muscle function as assessed by a timed stair climb. Perhaps we would have seen more pronounced improvement in muscle function if we had selected performance tests specific to the upper extremity.
No previous studies had assessed fat-free mass by a multicomponent model. The four-compartment model derived by Baumgartner et al. (Baumgartner et al. 1991) was developed in a population of older adults similar to our cohort. It includes density from BOD POD, bone mineral content from DXA, total body water from deuterium dilution, and body mass; thus, the four-compartment model yields a more accurate estimate of fat-free mass than any of those measures alone (Malina 2007; Minderico et al. 2008). Results of this analysis provide convincing evidence that the combination HMB, arginine, and glutamine can favorably improve the ratio of fat-free mass to fat mass among older adults, even without concurrent resistance training.
Potential Mechanisms of Bioactives in the Supplement
Declines in muscle mass and function with advancing age are multifactorial. The age-related loss of muscle mass is thought to be due in part to chronic, low-grade inflammation (Singh and Newman 2011). Thus, the antioxidant and anti-inflammatory properties of HMB (Eley, Russell, and Tisdale 2008a, 2008b), arginine (Appleton 2002; Tong and Barbul 2004), and glutamine (Alpers 2006; Kim 2011), may ameliorate muscle loss. Improvement of body composition with supplementation could also result from a collective effect of the amino acids on the ‘mammalian target of rapamycin’ mTOR cell signaling pathways. The protein kinase mTOR is involved in several key aspects of muscle protein synthesis, and muscle loss with aging is thought to be largely due to changes in the stimulation of mTOR pathways (Cuthbertson et al. 2005). HMB likely had the most direct effect on activating mTOR pathways (Pimentel et al. 2011; Fitschen et al. 2013;), but to a lesser extent, both arginine (Ban et al. 2004; Yao et al. 2008) and glutamine (Chiu et al. 2012; Xi et al. 2011) have been shown to up-regulate these pathways, too. Beyond its effects on muscle protein synthesis, HMB is also known to attenuate muscle protein breakdown by means of the ubiquitin-proteosome pathway and activation of the transcription factor nuclear factor-kappaB (Eley et al. 2007). Multiple properties of arginine, such as its vasodilatory effects (Preli, Klein, and Herrington 2002) and its action as a secretagogue for anabolic hormones (Kanaley 2008) may have impacted muscle fibers as well. While it is beyond the scope of this study, more research is needed to elucidate the mechanisms by which HMB, arginine, and glutamine act synergistically to favorably affect muscle protein turnover.
This study was limited by a modest sample size, and participants were predominantly from one ethnic group (Caucasian). All participants were generally in good health, so results may not be extrapolated to older adults with frailty or comorbidities. Similarly, average vitamin D levels were approximately 30ng/ml for both treatment and placebo groups, signifying adequate vitamin D status among most of this cohort. Although participants were instructed to maintain their typical activity patterns throughout the study, the protocol did not include an exercise intervention. Nonetheless, the study was strengthened by the double-blind, placebo controlled design with stratification by sex. It was also strengthened by very robust methods of body composition assessment including MRI and the gold-standard four-compartment model.
In conclusion, results of this study provide evidence that dietary supplementation with HMB, arginine, and glutamine may ameliorate age-related loss of muscle mass among community-dwelling older adults. The observed improvement in lean mass in the absence of exercise training suggests that supplementation may hold particular promise for older adults with limited mobility or frailty. Additional investigation among these populations is warranted.
Supplementary Material
Acknowledgements
The authors are grateful to Maryellen Williams and Cindy Zeng for laboratory analyses, Alexandra Vyazovkina for mass spectrometry analysis, and the University of Alabama at Birmingham’s Center for Clinical and Translational Science for statistical consultation.
Funding
This work was supported by the National Center for Complementary and Integrative Health (F31 AT005384–01). Core laboratory support and facilities were provided by the UAB Diabetes Research Center Human Physiology Core (P30DK079626–06). Abbott Nutrition provided Juven® and placebo in coded packets.
Footnotes
Declaration of Interest
The authors report no conflicts of interest.
Registered under ClinicalTrials.gov identifier no. NCT01057082
References
- Alpers DH. Glutamine: do the data support the cause for glutamine supplementation in humans?, Gastroenterology. 2006; 130: S106–16. [DOI] [PubMed] [Google Scholar]
- Anthony JC, TG Anthony SR Kimball, and LS Jefferson. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J Nutr. 2001;131: 856S–60S. [DOI] [PubMed] [Google Scholar]
- Appleton J Arginine: Clinical potential of a semi-essential amino. Altern Med Rev. 2002; 7: 512–22. [PubMed] [Google Scholar]
- Baier S, Johannsen D, Abumrad N, Rathmacher JA, Nissen S, and Flakoll P. Year-long changes in protein metabolism in elderly men and women supplemented with a nutrition cocktail of beta-hydroxy-beta-methylbutyrate (HMB), L-arginine, and L-lysine. JPEN J Parenter Enteral Nutr. 2009; 33: 71–82. [DOI] [PubMed] [Google Scholar]
- Ban H, Shigemitsu K, Yamatsuji T, Haisa M, Nakajo T, Takaoka M, Nobuhisa T, Gunduz M, Tanaka N, and Naomoto Y. Arginine and Leucine regulate p70 S6 kinase and 4E-BP1 in intestinal epithelial cells. Int J Mol Med. 2004; 13: 537–43. [PubMed] [Google Scholar]
- Baumgartner RN, Heymsfield SB, Lichtman S, Wang J, and Pierson RN. Body composition in elderly people: effect of criterion estimates on predictive equations. Am J Clin Nutr. 1991; 53: 1345–53. [DOI] [PubMed] [Google Scholar]
- Berk L, James J, Schwartz A, Hug E, Mahadevan A, Samuels M, and Kachnic L. A randomized, double-blind, placebo-controlled trial of a beta-hydroxyl beta-methyl butyrate, glutamine, and arginine mixture for the treatment of cancer cachexia (RTOG 0122). Support Care Cancer. 2008; 16: 1179–88. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari H, Stähelin HB, and Walter P. Vitamin D effects on bone and muscle. Int J Vitam Nutr Res. 2011; 81: 264–72. [DOI] [PubMed] [Google Scholar]
- Burton LA, and Sumukadas D. Optimal management of sarcopenia. Clin Interv Aging. 2010; 5: 217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceglia L Vitamin D and its role in skeletal muscle. Curr Opin Clin Nutr Metab Care. 2009; 12: 628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu M, Tardito S, Barilli A, Bianchi MG, Dall’Asta V, and Bussolati O. Glutamine stimulates mTORC1 independent of the cell content of essential amino acids. Amino Acids. 2012; 43: 2561–7. [DOI] [PubMed] [Google Scholar]
- Clark RH, G Feleke M Yasmin Din, T, Singh G, Khan FA, and Rathmacher JA. Nutritional treatment for acquired immunodeficiency virus-associated wasting using beta-hydroxy beta-methylbutyrate, glutamine, and arginine: a randomized, double-blind, placebo-controlled study. JPEN J Parenter Enteral Nutr. 2000; 24: 133–9. [DOI] [PubMed] [Google Scholar]
- Coman D, Yaplito-Lee J, and Boneh A. New indications and controversies in arginine therapy. Clin Nutr. 2008; 27: 489–96. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, and Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005; 19: 422–4. [DOI] [PubMed] [Google Scholar]
- Eley HL, Russell ST, Baxter JH, Mukerji P, and Tisdale MJ. Signaling pathways initiated by beta-hydroxy-beta-methylbutyrate to attenuate the depression of protein synthesis in skeletal muscle in response to cachectic stimuli. Am J Physiol Endocrinol Metab. 2007; 293: E923–31. [DOI] [PubMed] [Google Scholar]
- Eley HL, Russell ST, and Tisdale MJ. Attenuation of depression of muscle protein synthesis induced by lipopolysaccharide, tumor necrosis factor, and angiotensin II by beta-hydroxy-beta-methylbutyrate. Am J Physiol Endocrinol Metab. 2008a; 295: E1409–16. [DOI] [PubMed] [Google Scholar]
- Fitschen PJ, Wilson GJ, Wilson JM, and Wilund KR. Efficacy of β-hydroxy-β-methylbutyrate supplementation in elderly and clinical populations. Nutrition. 2013; 29: 29–36. [DOI] [PubMed] [Google Scholar]
- Flakoll P, Sharp R, Baier S, Levenhagen D, Carr C, and Nissen S. 2004. ‘Effect of beta-hydroxy-beta-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women.’, Nutrition, 20: 445–51. [DOI] [PubMed] [Google Scholar]
- Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, and Rasmussen BB. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol. 2007; 582: 813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller JC, Baier S, Flakoll P, Nissen SL, Abumrad NN, and Rathmacher JA. Vitamin D status affects strength gains in older adults supplemented with a combination of β-hydroxy-β-methylbutyrate, arginine, and lysine: a cohort study. JPEN J Parenter Enteral Nutr. 2011; 35: 757–62. [DOI] [PubMed] [Google Scholar]
- Garlick PJ. The role of leucine in the regulation of protein metabolism. J Nutr. 2005; 135: 1553S–6S. [DOI] [PubMed] [Google Scholar]
- Giovannini S, Marzetti E, Borst SE, and Leeuwenburgh C. Modulation of GH/IGF-1 axis: Potential strategies to counteract sarcopenia in older adults. Mech Ageing Dev. 2008;129: 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet C, Prod’homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, and Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 2004; 18: 1586–7. [DOI] [PubMed] [Google Scholar]
- Holick MF. The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol Aspects Med. 2008; 29: 361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh LC, Chien SL, Huang MS, Tseng HF, and Chang CK. Anti-inflammatory and anticatabolic effects of short-term beta-hydroxy-beta-methylbutyrate supplementation on chronic obstructive pulmonary disease patients in intensive care unit. Asia Pac J Clin Nutr. 2006; 15: 544–50. [PubMed] [Google Scholar]
- Hunter GR, McCarthy JP, and Bamman MM. Effects of resistance training on older adults. Sports Medicine. 2004; 34: 329–48. [DOI] [PubMed] [Google Scholar]
- Hunter GR, Treuth MS, Weinsier RL, Kekes-Szabo T, Kell SH, Roth DL, and Nicholson C. The effects of strength conditioning on older women’s ability to perform daily tasks. J Am Geriatr Soc. 1995; 43: 756–60. [DOI] [PubMed] [Google Scholar]
- Jensen GL Inflammation: roles in aging and sarcopenia. JPEN J Parenter Enteral Nutr. 2008; 32: 656–9. [DOI] [PubMed] [Google Scholar]
- Jones TE, Stephenson KW, King JG, Knight KR, Marshall TL, and Scott WB. Sarcopenia--mechanisms and treatments. J Geriatr Phys Ther. 2009; 32: 39–45. [PubMed] [Google Scholar]
- Kanaley JA. Growth hormone, arginine and exercise. Curr Opin Clin Nutr Metab Care. 2008; 11: 50–4. [DOI] [PubMed] [Google Scholar]
- Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, and Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006; 291: E381–7. [DOI] [PubMed] [Google Scholar]
- Kim H Glutamine as an immunonutrient. Yonsei Med J. 2007; 52: 892–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan PA, Brown RA, and Rennie MJ. A positive relationship between protein synthetic rate and intracellular glutamine concentration in perfused rat skeletal muscle. FEBS Lett. 1987; 215: 187–91. [DOI] [PubMed] [Google Scholar]
- MacLennan PA, Smith K, Weryk B, Watt PW, and Rennie MJ. Inhibition of protein breakdown by glutamine in perfused rat skeletal muscle. FEBS Lett. 1988; 237: 133–6. [DOI] [PubMed] [Google Scholar]
- Malina RM. Body composition in athletes: assessment and estimated fatness. Clin Sports Med. 2007; 26: 37–68. [DOI] [PubMed] [Google Scholar]
- Marcora S, Lemmey A, and Maddison P. Dietary treatment of rheumatoid cachexia with beta-hydroxy-beta-methylbutyrate, glutamine and arginine: a randomised controlled trial. Clin Nutr. 2005; 24: 442–54. [DOI] [PubMed] [Google Scholar]
- Mattsson S, and Thomas BJ. Development of methods for body composition studies. Phys Med Biol. 2006; 51: R203–28. [DOI] [PubMed] [Google Scholar]
- May PE, Barber A, D’Olimpio JT, Hourihane A, and Abumrad NN. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am J Surg. 2002; 183: 471–9. [DOI] [PubMed] [Google Scholar]
- Melis GC, ter Wengel N, Boelens PG, and van Leeuwen PA. Glutamine: recent developments in research on the clinical significance of glutamine. Curr Opin Clin Nutr Metab Care. 2004; 7: 59–70. [DOI] [PubMed] [Google Scholar]
- Meng SJ, and LJ Yu. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. 2010;11: 1509–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minderico CS, Silva AM, Keller K, Branco TL, Martins SS, Palmeira AL, Barata JT, Carnero EA, Rocha PM, Teixeira PJ, and Sardinha LB. Usefulness of different techniques for measuring body composition changes during weight loss in overweight and obese women. Br J Nutr. 2008; 99: 432–41. [DOI] [PubMed] [Google Scholar]
- Moreno LA, Valtueña J, Pérez-López F, and González-Gross M. Health effects related to low vitamin D concentrations: beyond bone metabolism. Ann Nutr Metab. 2011; 59: 22–7. [DOI] [PubMed] [Google Scholar]
- Morley JE. Vitamin D redux. J Am Med Dir Assoc. 2009;10: 591–2. [DOI] [PubMed] [Google Scholar]
- Morley JE, JM Argiles WJ Evans S Bhasin, Cella D, Deutz NE, Doehner W, Fearon KC, Ferrucci L, Hellerstein MK, Kalantar-Zadeh K, Lochs H, MacDonald N, Mulligan K, Muscaritoli M, Ponikowski P, Posthauer ME, Fanelli F Rossi, Schambelan M, Schols AM, Schuster MW, Anker SD, and Cachexia Society for Sarcopenia, and Wasting Disease. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 2010; 11: 391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel GD, Rosa JC, Lira FS, Zanchi NE, Ropelle ER, Oyama LM, Oller do Nascimento CM, de Mello MT, Tufik S, and Santos RV. β-Hydroxy-β-methylbutyrate (HMβ) supplementation stimulates skeletal muscle hypertrophy in rats via the mTOR pathway. Nutr Metab (Lond). 2011; 8: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsiadlo D, and Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991; 39: 142–8. [DOI] [PubMed] [Google Scholar]
- Portal S, Eliakim A, Nemet D, Halevy O, and Zadik Z. Effect of HMB supplementation on body composition, fitness, hormonal profile and muscle damage indices. J Pediatr Endocrinol Metab. 2010; 23: 641–50. [DOI] [PubMed] [Google Scholar]
- Potenza MA, Nacci C, and Mitolo-Chieppa D. Immunoregulatory effects of L-arginine and therapeutical implications. Curr Drug Targets Immune Endocr Metabol Disord. 2001; 1: 67–77. [DOI] [PubMed] [Google Scholar]
- Preli RB, Klein KP, and Herrington DM. Vascular effects of dietary L-arginine supplementation. Atherosclerosis. 2002; 162: 1–15. [DOI] [PubMed] [Google Scholar]
- Roth E Nonnutritive effects of glutamine. J Nutr. 2008; 138: 2025S–31S. [DOI] [PubMed] [Google Scholar]
- Roubenoff R Physical activity, inflammation, and muscle loss. Nutr Rev. 2007; 65: S208–12. [DOI] [PubMed] [Google Scholar]
- Rowlands DS, and Thomson JS. Effects of beta-hydroxy-beta-methylbutyrate supplementation during resistance training on strength, body composition, and muscle damage in trained and untrained young men: a meta-analysis. J Strength Cond Res. 2009; 23: 836–46. [DOI] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SM, Deeg DJ, and Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006; 119: 526.e9–17. [DOI] [PubMed] [Google Scholar]
- Schoeller DA, van Santen E, Peterson DW, Dietz W, Jaspan J, and Klein PD. Total body water measurement in humans with 18O and 2H labeled water. Am J Clin Nutr. 1980; 33: 2686–93. [DOI] [PubMed] [Google Scholar]
- Singh T, and Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011; 10: 319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton DA, Young A, Greig CA, and Malbut KE. Effects of resistance training on strength, power, and selected functional abilities of women aged 75 and older. J Am Geriatr Soc. 1995; 43: 1081–7. [DOI] [PubMed] [Google Scholar]
- Smith DN. Bioelectrical impedance and body composition. Lancet. 1993; 341: 569. [PubMed] [Google Scholar]
- Stout JR, Smith-Ryan AE, Fukuda DH, Kendall KL, Moon JR, Hoffman JR, Wilson JM, Oliver JS, and Mustad VA. Effect of calcium β-hydroxy-β-methylbutyrate (CaHMB) with and without resistance training in men and women 65+yrs: a randomized, double-blind pilot trial. Exp Gerontol. 2013; 48: 1303–10. [DOI] [PubMed] [Google Scholar]
- Timmerman KL, and E Volpi. Amino acid metabolism and regulatory effects in aging. Curr Opin Clin Nutr Metab Care. 2008; 11: 45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong BC, and Barbul A. Cellular and physiological effects of arginine. Mini Rev Med Chem. 2004; 4: 823–32. [DOI] [PubMed] [Google Scholar]
- Townsend JR, Fragala MS, Jajtner AR, Gonzalez AM, Wells AJ, Mangine GT, Robinson EH, McCormack WP, Beyer KS, Pruna GJ, Boone CH, Scanlon TM, Bohner JD, Stout JR, and Hoffman JR. β-Hydroxy-β-methylbutyrate (HMB)-free acid attenuates circulating TNF-α and TNFR1 expression postresistance exercise. J Appl Physiol 2013; 115: 1173–82. [DOI] [PubMed] [Google Scholar]
- Vukovich MD, Stubbs NB, and Bohlken RM. Body composition in 70-year-old adults responds to dietary beta-hydroxy-beta-methylbutyrate similarly to that of young adults. J Nutr. 2001; 131: 2049–52. [DOI] [PubMed] [Google Scholar]
- WE Siri. Body composition from fluid spaces and density: analysis of methods In: Brozek J and Henschel A (eds.). Techniques for measuring body composition (National Academy of Sciences: Washington, DC: ), 1961. [Google Scholar]
- Williams JZ, Abumrad N, and Barbul A. Effect of a specialized amino acid mixture on human collagen deposition. Ann Surg. 2002; 236: 369–74; discussion 74–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GY, and Thompson JR. The effect of glutamine on protein turnover in chick skeletal muscle in vitro. Biochem J. 1990; 265: 593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi P, Jiang Z, Zheng C, Lin Y, and Wu G. Regulation of protein metabolism by glutamine: implications for nutrition and health. Front Biosci. 2011; 16: 578–97. [DOI] [PubMed] [Google Scholar]
- Yao K, Yin YL, Chu W, Liu Z, Deng D, Li T, Huang R, Zhang J, Tan B, Wang W, and Wu G.’Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J Nutr. 2008; 138: 867–72. [DOI] [PubMed] [Google Scholar]
- Zanchi NE, Gerlinger-Romero F, Guimarães-Ferreira L, de Siqueira Filho MA, Felitti V, Lira FS, Seelaender M, and Lancha AH Jr. HMB supplementation: clinical and athletic performance-related effects and mechanisms of action. Amino Acids. Apr;40(4):1015–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.