Abstract
Introduction
Polydrug use involving heroin and benzodiazepines is common. The potential risk of additive pharmacological effects may be associated with poorer outcomes in patients who use benzodiazepines together with heroin. The aim of this study was to determine the clinical picture of patients presenting to the emergency department following acute drug toxicity involving heroin and benzodiazepines.
Methods
Exposure information, clinical data and outcome of acute drug toxicity presentations were collected between 1 October 2013 and 30 September 2014 as part of the European Drug Emergencies Network (Euro-DEN) project. The database was interrogated to identify patients who had taken heroin with or without benzodiazepine(s).
Results
A total of 1345 presentations involving acute heroin toxicity were identified: 492 had used one or more non-heroin/benzodiazepine drug and were not further considered in this study; 662 were lone heroin users and 191 had co-used heroin with one or more benzodiazepines. Co-users were more likely than lone heroin users to have reduced respiratory rate at presentation 12.7 ± 4.9 vs 13.6 ± 4.4 (p = 0.02) and require admission to hospital 18.3 vs 9.8% (p < 0.01). There were no differences in critical care admission rates 3.1 vs 3.9% (p = 0.83) or length of stay 4 h 59 min vs 5 h 32 min (p = 0.23). The 3 most common benzodiazepines were clonazepam, diazepam, and alprazolam. No differences were observed for clinical features between the three benzodiazepines.
Conclusion
This study shows that co-use of heroin and benzodiazepines is common, although the overall outcomes between co-users of heroin and benzodiazepines and heroin-only users were similar.
Keywords: Heroin, Benzodiazepines, Acute toxicity, Euro-DEN, Emergency department
Background
The concurrent use of benzodiazepines is common amongst heroin users, with 91% reporting life-time use of a benzodiazepine and 41% of heroin users surveyed reported using a benzodiazepines more than once a week in the 6 months prior to the survey [1–7]. The desire for ‘intoxication’ and the management of heroin withdrawal and sleep disorders are some of the reasons for benzodiazepine use in this group [2, 8]. However, this practice is not without its risk, and benzodiazepine use has been implicated in many cases of overdoses and fatalities amongst heroin users [3, 9]. Harm may be from the risk of combined sedative effects, development of benzodiazepine dependence and withdrawal and/or exposure to the high-risk behaviour of sharing of needles [7, 10–13]. In addition, there are associated negative social impacts with increased associations with criminal activity and increased utilisation of health services in this population compared to other heroin users [4, 14].
The choice of benzodiazepine used is likely affected by availability and also the pharmacological property of the drug. Benzodiazepines in general are considered readily available on the illicit market and the Internet, but a significant proportion of users have been able to obtain benzodiazepines solely through a prescription from a doctor and the phenomenon of ‘doctor shopping’ has been described [2, 15, 16]. Benzodiazepines with a quick onset of action and increased sedative/hypnotic effects are likely to be favoured with alprazolam, diazepam, flunitrazepam and oxazepam being identified as some of the most commonly used [6, 17–21].
In 2010 and 2011, there was a recognised shortage of heroin supplies in some countries across the European market, leading to increased prices and decreased purity of the drug [22, 23]. The reasons for the shortage were thought to be multifactorial, ranging from reduced production in Afghanistan through to disruption of the main trafficking networks and record seizures in Europe [22]. The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) reported that this shortfall in availability had led to heroin users seeking to ‘fill the vacuum’ by other means, one of which was to increase the use of benzodiazepines [22].
Past studies on the effects of benzodiazepine use in heroin users have looked into patterns of use, user characteristics and risk factors and psychosocial effects of concurrent use [2, 4, 5, 7, 13, 14, 24]. In this paper, we describe a case series of heroin toxicity presentations to Euro-DEN centres over a 12-month period to compare the clinical symptoms/signs, treatments and outcomes between those presentations involving lone heroin use and those presentations involving heroin co-use with one or more benzodiazepine.
Methods
Data for this study was collected through the European Drug Emergencies Network (Euro-DEN) [25, 26]. The Euro-DEN project was initially established a network of 16 sentinel emergency departments (EDs) in 10 European countries to collect data on all acute recreational drug toxicity presentations (this includes the use of new psychoactive substances and prescription/over the counter medicines). Lone alcohol presentations, along with those not related to acute toxicity, are excluded (e.g. drug withdrawal, intentional self-harm). Data is collected within each centre using a standardised data collection tool, and then, all of the data from the participating centres is collated by the lead centre in London, UK. After the initial year of data collection, the project has continued as the expanded Euro-DEN Plus project (by May 2018, there were 31 sentinel EDs in 21 European and neighbouring countries). Ethical approval was obtained by each centre to collect the data as part of Euro-DEN project.
The Euro-DEN dataset was searched to identify heroin toxicity presentations to the emergency department (ED) between 1 October 2013 and 31 September 2014 to the original 16 sentinel EDs in 10 European countries. Cases were included if they had (a) lone heroin use or (b) heroin and benzodiazepine co-use with no other drug. The following data extracted for these cases: (i) demographics (age, sex, city), (ii) benzodiazepine(s) used, (iii) clinical observations at presentation to the ED, (iv) presence of the Euro-DEN pre-defined clinical features (vomiting, dyspnoea, hyperthermia, headache, anxiety, hallucinations, agitation/aggression, psychosis, seizures, cerebellar features, palpitations, chest pain, hypertension, hypotension, arrhythmias) prior to and/or during the hospital admission, (v) initial disposition from the ED and (vi) overall length of hospital admission (including the time spent in the ED).
Extracted information was analysed using Excel® 2013 and SPSS® version 24. Data are presented as frequency, percentages and mean ± standard deviation (SD) or median (interquartile range (IQR)) as appropriate. For continuous variables, t test or one-way ANOVA was used to compare means and Mann-Whitney U test or Kruskal-Wallis test used to compare medians. Pearson’s chi-square test or Fisher’s exact test was used for any categorical variables. Significance was defined as p < 0.05.
Results
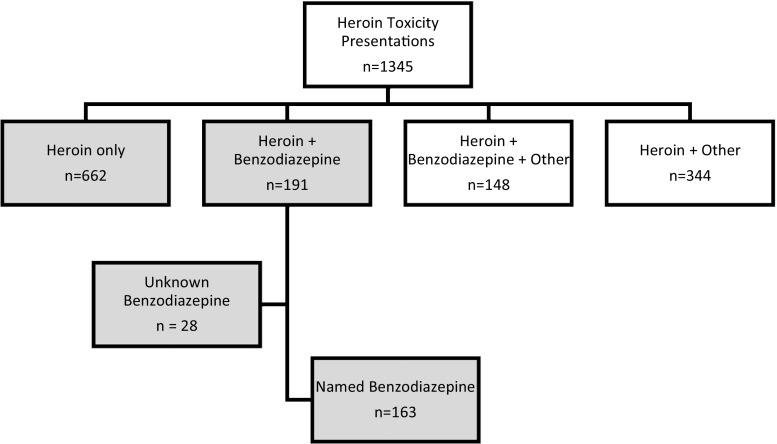
In the 12-month period from 1 October 2013 to 30 September 2014, there were 5529 ED presentations reported to the Euro-DEN project. Heroin use was reported in 1345 (24.3%) of these presentations, of which 853 (63.4%) were included for analysis. This consisted of 662 (49.2%) lone heroin users (heroin group) and 191 (14.2%) combined heroin and benzodiazepine users (heroin-BZD group) (Fig. 1). The remaining 492 presentations reported additional use of a non-benzodiazepine drug in combination with heroin (n = 344, 25.6%) or heroin and benzodiazepine (148, 11.0%) (Fig. 1) and therefore were excluded from the analysis. The centres that had the most number of presentations were Oslo (n = 528; 61.9%), Dublin (133; 15.6%), London (n = 88; 10.3%) and York (n = 68; 7.9%).
Fig. 1.
Study population (shaded areas indicate the groups included in the study)
Patient Demographics
The median (IQR, range) age for the patients included was 36 (29–44, 15–67) years and 697 (81.6%) were male (Table 1). The median age of males was 36 (IQR 29.5–45) years and females 35 (IQR 26–44) years. There were no differences between the heroin group and the heroin-BZD for age [36 (29–45, 17–67) vs 35 (28–44, 15–62) years, p = 0.12] and gender [542 males (81.9%) vs 155 females (81.2%), Mann-Whitney U Test p = 0.82].
Table 1.
Heroin vs heroin-benzodiazepine group
| Heroin alone (n = 662) | Heroin-BZD (n = 191) | |
|---|---|---|
| Age (n) | 655 | 190 |
| Median (IQR) | 36 (29–45) | 35 (28–44) |
| Range | 17–67 | 15–62 |
| Gender (n) | 662 | 191 |
| Male (%) | 542 (81.9) | 155 (81.2) |
| Top 3 cities | ||
| 1. | Oslo (381) | Oslo (147) |
| 2. | Dublin (116) | Dublin (17) |
| 3. | London (62) | York (11) |
| Level of consciousness (n) | 651 | 190 |
| Alert (GCS = 15) | 189 (29.0%) | 50 (26.3%) |
| Drowsy (GCS9–14) | 404 (62.1%) | 124 (65.3%) |
| Coma (GCS3–8) | 58 (8.9%) | 16 (8.4%) |
| Temp (n) | 575 | 175 |
| Mean, °C (SD) | 36.1 ± 0.83 | 36.2 ± 0.84 |
| Heart rate (n) | 634 | 188 |
| Mean, bpm (SD) | 83.4 ± 18.4 | 82.9 ± 18.6 |
| Blood pressure (n) | 583 | 169 |
| Mean systolic, mmHg (SD) | 117.8 ± 18.8 | 117.3 ± 16.4 |
| Mean diastolic, mmHg (SD) | 72.3 ± 14.5 | 73.3 ± 13.8 |
| Respiratory rate (n) | 608 | 178 |
| Mean, breaths/min (SD) | 13.6 ± 4.4 | 12.7 ± 4.9* |
| Respiratory rate, < 12/min | 166 (27.3%) | 66 (36.9%)** |
| Clinical features | ||
| Vomiting | 3.5% | 3.7% |
| Dyspnoea | 5.0% | 6.3% |
| Hyperthermia | 1.1% | 1.6% |
| Headache | 0.6% | 0.5% |
| Anxiety | 2.6% | 2.1% |
| Hallucinations | 0.9% | 0.0% |
| Agitation/aggression | 15.0% | 7.9%^ |
| Psychosis | 1.4% | 0.0% |
| Seizures | 2.1% | 2.1% |
| Cerebellar features | 0.5% | 0.0% |
| Palpitations | 1.1% | 0.0% |
| Chest pain | 2.0% | 2.0% |
| Hypertension | 0.5% | 0.0% |
| Hypotension | 5.9% | 7.3% |
| Arrhythmias | 0.7% | 0.0% |
| Treatments (n) | 662 | 191 |
| Naloxone | 263 (39.7%) | 74 (38.7%) |
| Flumazenil | 7 (1.1%) | 4 (2.1%) |
| Intubated | 14 (2.1%) | 2 (1.0%) |
| Disposition from ED (n) | 662 | 191 |
| Discharged/self-discharged | 564 (85.2%) | 149 (78.0%)+ |
| Admission to other bed | 65 (9.8%) | 35(18.3%)++ |
| Admit to critical care | 26 (3.9%) | 6 (3.1%) |
| Admit to psychiatry | 5 (0.8%) | 1 (0.5%) |
| Died | 2 (0.3%) | 0 (0%) |
*p = 0.02 by independent t test; **p = 0.02 by Fisher’s exact test; ^p = 0.01 by Fisher’s exact test; +p = 0.02 by Pearson’s chi-square test; ++p < 0.02 by Pearson’s chi-square test
Symptoms and Clinical Features at Presentation
The majority of patients (62.8%) presented with drowsiness (defined as a Glasgow Coma Score (GCS) of 9–14) and a further 8.8% presented in a coma (GCS ≤ 8). 29.6% had a low respiratory rate (defined as RR < 12 breaths per minute), 5.9% had low blood pressure (defined as systolic BP ≤ 90 mmHg) and 8.4% were bradycardic (defined as heart rate (HR) < 60 bpm). Conversely, 0.2% had high blood pressure (defined as systolic BP ≥ 180 mmHg) and 2.7% were tachycardic (defined as HR > 120 bpm).
Comparing the heroin and heroin-BZD groups, there were no differences in the proportion of patients presenting with drowsiness (defined as GCS 9-14; heroin 62.1% vs heroin-BZD 65.3%) or in a coma (defined as GCs ≤ 8; 8.9 vs 8.4%). There was no difference found between the two groups in terms of mean temperature, heart rate, systolic blood pressure, or diastolic blood pressure on presentation (Table 1). Although the heroin-BZD group compared to the lone heroin group had a higher proportion of patients with a respiratory rate of less than 12 (36.9 vs 27.4%, p = 0.02) and a lower mean respiratory rate (12.7 ± 4.9 vs 13.6 ± 4.3 breaths per minute, p = 0.02), there was no difference in the proportion of patients who were intubated (1.0 vs 2.1%).
Of the 15 pre-defined clinical features, at least one was observed in 214 (32.3%) of the heroin group and 49 (25.7%) of the heroin-BZD group (p = 0.09). The frequency of each pre-defined clinical feature is listed in Table 1; only agitation/aggression was seen more commonly in the heroin group compared to that in the heroin-BZD group (15.0 vs 7.9%, p = 0.01).
Treatments Given
In all, 412 (48.3%) patients received treatment. Naloxone was the most common treatment given in 337 (39.3%) (183 given pre-hospital), followed by intubation in 16 (1.9%) (4 intubated pre-hospital), flumazenil in 11 (1.3%; 4 given pre-hospital) and inotropic support in 13 (1.5%).
There were no differences in the proportion of patients who received treatment in the heroin group and heroin-BZD group (heroin 49.2% vs heroin BZD 45.0%, Pearson chi-square test p = 0.30). No differences could be observed between the two groups for the number of patients receiving naloxone (39.7 vs 38.7%, Pearson chi-square test p = 0.87), flumazenil (1.1 vs 2.1%, p = 0.27) or intubation rates (2.1 vs 1.0%, Fischer’s exact test p = 0.54).
Disposition from the ED and Outcome
The majority of patients (n = 713, 83.6%) were discharged (medically or self-discharged) directly from the ED; of the remaining patients, 138 (16.2%) were admitted to hospital (including 32 (3.8%) who were admitted to critical care (high dependency unit or intensive care unit)) and there were 2 (0.2%) deaths in ED.
In terms of initial disposition from the ED, the lone heroin group of patients were more likely to be discharged directly from the ED (85.2 vs 78.0%, p = 0.02) (Table 1). There was no difference in the proportion of either group that were admitted to critical care or an inpatient psychiatry bed (Table 1). The overall median (IQR) overall length of stay (from time of ED presentation to final discharge from hospital) was 5 h 6 min (3 h 8 min to 7 h 45 min), and 93.3% of patients were discharged within 24 h of presentation to the ED; there was no difference in the length of stay between the two groups [heroin 4 h 59 min (2 h 56 min to 7 h 46 min) vs heroin-BZD 5 h 32 min (3 h 35 min to 7 h 41 min), Mann-Whitney U Test p = 0.23].
In total, there were six deaths (two deaths in the ED and four after admission to hospital) recorded in this study. All deaths were in the lone heroin group. Further information surrounding the circumstances of death was available for five of these deaths (Table 2).
Table 2.
Details and causes of deaths of patients who died following presentation to hospital
| Patient | Circumstances |
|---|---|
| Female, 34 years old | Found in cardiorespiratory arrest pre-hospital; declared dead in the ED |
| Male, 36 years old | Found in cardiorespiratory arrest pre-hospital, successfully resuscitated; died of hypoxic brain injury in the ED |
| Male, 43 years old | Found in cardiorespiratory arrest pre-hospital, successfully resuscitated and admitted to ICU; died in hospital 10 days later from hypoxic brain injury |
| Male, 27 years old | Found in cardiorespiratory arrest pre-hospital, successfully resuscitated and admitted to ICU; died in hospital 2 days later from hypoxic brain injury |
| Male, 48 years old | Presented to ED with GCS 10, HR70, BP111/81, and RR24; symptoms of vomiting and dyspnoea; died in hospital 1 day later from perforation of ulcer and haemorrhagic shock and disseminated intravascular coagulation |
| Male, 36 years old | Found in cardiorespiratory arrest pre-hospital, successfully resuscitated and admitted to ICU; cause of death not recorded |
Benzodiazepines Involved in Heroin-BDZ Presentations
The most common reported benzodiazepine used in the 191 heroin-BZD presentations was clonazepam (seen in 93 presentations; 48.7%); others reported were the following: alprazolam (27 presentations; 14.1%), diazepam (26 presentations; 13.6%), zopiclone (9, 4.7%), oxazepam (6, 3.1%), flunitrazepam (5, 2.6%), nitrazepam (4, 2.1%) and etizolam (1, 0.5%). In 28 presentations (14.7%), the exact benzodiazepine was not known. There were no differences (by one-way ANOVA) in the pattern of clinical features at presentation between these three most common benzodiazepines: level of consciousness (p = 0.35), temperature (p = 0.29), heart rate (p = 0.25), systolic blood pressure (p = 0.20), diastolic blood pressure (p = 0.68), respiratory rate (p = 0.27); additionally, length of stay was not different between the groups (Kruskal-Wallis, p = 0.78) (Table 3). Heroin-diazepam presentations had a higher rate of admission compared to heroin-alprazolam presentations (37.5 vs 8.7%; Fisher’s exact test, p = 0.03) and compared to heroin-clonazepam although this was not significant (37.5 vs 17.4%, p = 0.06). There was no difference in the proportion of patients admitted between the heroin-alprazolam and heroin-clonazepam groups (8.7 vs 17.4%, p = 0.35). Poly-benzodiazepine use was found to be an uncommon practice when no other classes of drugs were involved with only 6 cases seen (3.1%).
Table 3.
Clinical features of the top 3 most popular reported used benzodiazepines. Cases where the drugs were used in combination with other benzodiazepines have not been included
| Clonazepam (n = 87) | Diazepam (n = 24) | Alprazolam (n = 23) | |
|---|---|---|---|
| Demographics | |||
| Age (n) | 87 | 24 | 23 |
| Median (IQR) | 38 (26–44.5) | 35 (31.75–37.75) | 36 (28.5–43) |
| Range | 15–62 | 20–53 | 24–53 |
| Gender (n) | 87 | 24 | 23 |
| Male (%) | 74 (85.1%) | 20 (83.3%) | 17 (73.9%) |
| Top 3 cities (n) | |||
| 1. | Oslo (86) | York (9) | Oslo (16) |
| 2. | London (1) | Oslo (8) | Mallorca (6) |
| 3. | Dublin (4) | London (1) | |
| Presenting clinical observations | |||
| Level of consciousness (n) | 86 | 24 | 23 |
| Alert (GCS = 15) | 18 (21%) | 8 (33%) | 10 (43%) |
| Drowsy (GCS9–14) | 63 (73%) | 12 (50%) | 13 (57%) |
| Coma (GCS3–8) | 5 (6%) | 4 (17%) | 0 (0%) |
| Temp (n) | 82 | 23 | 16 |
| Mean, °C (SD) | 36.3 (0.74) | 36.0 (1.1) | 36.2 (0.71) |
| Heart rate (n) | 86 | 24 | 22 |
| Mean, bpm (SD) | 79 ± 18.8 | 86 ± 18.2 | 82 ± 19.4 |
| Blood pressure (n) | 73 | 24 | 20 |
| Mean systolic, mmHg (SD) | 115 ± 16.6 | 122 ± 17.8 | 117 ± 9.2 |
| Mean diastolic, mmHg (SD) | 72 ± 13.3 | 74 ± 14.7 | 74 ± 11.7 |
| Respiratory rate (n) | 73 | 24 | 19 |
| Mean, breaths/min (SD) | 12 ± 5.2 | 14 ± 5.9 | 13 ± 4.0 |
| Intubated (n) | 87 | 24 | 23 |
| Yes | 1 (1.1%) | 1 (4.2%) | 0 (0%) |
| Outcomes | |||
| Disposition from ED (n) | 87 | 24 | 23 |
| Discharged/self-discharged | 71 (82.6%) | 15 (62.5%) | 21 (91.3%) |
| Admission to other bed | 14 (16.1%) | 8 (33.3%) | 0 (0%) |
| Admit to critical care | 2 (2.3%) | 1 (4.2%) | 1 (4.3%) |
| Admit to psychiatry | 0 (0%) | 0 (0%) | 1 (4.3%) |
| Died | 0 (0%) | 0 (0%) | 0 (0%) |
| Length of hospital stay (n) | 87 | 24 | 23 |
| Median (IQR) | 5:38 (3:48–7:22) | 5:27 (3:49–8:22) | 5:51(3:25–6:53) |
p = 0.02 when admission rates for diazepam and alprazolam are compared
Drug Use by City
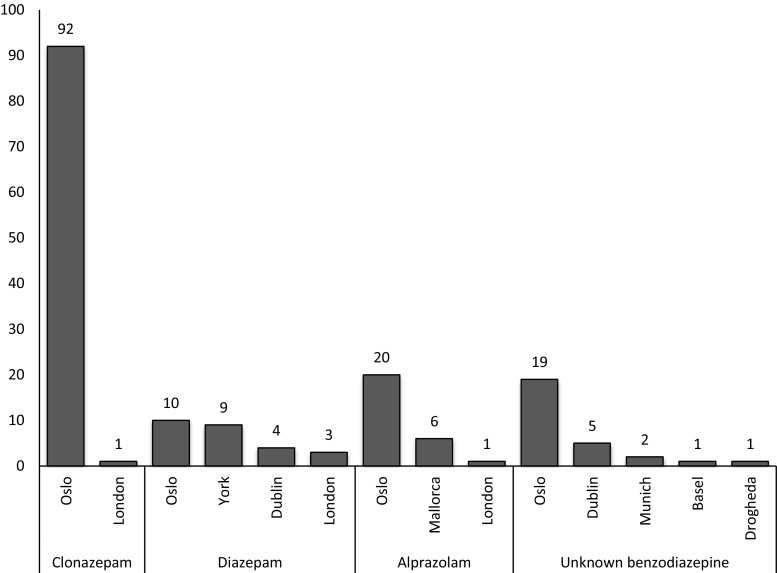
In this study, the majority of the data (n = 729, 85.4%) came from five sentinel centres that encompassed just three cities (Table 1). The largest number came from Oslo, where two sentinel centres located in the city accounted for 528 (61.9%) of all presentations. The most common benzodiazepines in the top 3 cities were the following: Oslo—clonazepam (92 cases, 17.4% of presentations to the city); Dublin—zopiclone (8, 6.0%); London—diazepam (3, 4.4%) (Fig. 2).
Fig. 2.
Frequency of the most common benzodiazepines used by city
Analytical Confirmation
Analysis of biological samples (urine and/or blood) to provide supportive evidence of use was only available in 28 cases. In this group, 22 (78.6%) were reported as lone heroin users, and 6 (21.4%) had reported co-use of a benzodiazepine. All patients who reported use of benzodiazepines had a positive analysis for that benzodiazepine.
Discussion
This study describes the clinical pattern of toxicity in lone heroin and co-used heroin-benzodiazepine acute toxicity presentations to the emergency department. The prevalence of co-use was 25.2% which is in line with previous studies. However, this is likely to be an overestimation of the true number of co-users in the community as it is likely that this group presents more frequently to ED [14]. Amongst co-users, 56.3% used benzodiazepines as the sole additional drug. Excluding alcohol, which was not included as a drug in the current analysis, benzodiazepines were the biggest class of drugs co-used in this cohort presenting to the ED with acute heroin toxicity. Despite the many harm users can potentially experience from the co-use of heroin and benzodiazepines, the current study was only able to identify reduced respiratory rate as an indicator of additional acute toxicity in the heroin-BZD group [4, 10, 11]. Although those who co-used heroin with a benzodiazepine had reduced respiratory rate, the actual difference is unlikely to be of clinical significance and this is supported by the fact there was no difference in the need for intubation between the two groups. It is likely that other factors, such as respiratory effort and vomiting, in addition to respiratory rate may impact on the decision whether to intubate an individual patient. Furthermore, although the heroin-BZD group was more likely to require admission to hospital, there was no difference in terms of outcomes as measured by the need for admission to critical care or length of stay in hospital. All deaths that were observed in the current study were in the group who reported lone use of heroin.
Choice of Benzodiazepines
Diazepam and alprazolam have been previously reported as common benzodiazepines used by opioid users, and our study confirms their popularity amongst heroin users [6, 18, 19]. Another key finding is the continuing emergence of clonazepam and decline of flunitrazepam as a benzodiazepine of misuse in heroin users. Clonazepam was found to be the most common benzodiazepine, although this is largely due to the fact that a large proportion of the study data originated from Oslo, where clonazepam was found to be the most commonly used benzodiazepine [27]. In addition, the use of flunitrazepam was rarely reported by patients in this study.
There were concerns that the 2010/2011 shortage of heroin in European markets was associated with some of heroin users replacing heroin with an increased use of benzodiazepine amongst other drugs, leading to worse clinical outcomes [4, 22]. However, this heroin shortage was recovering by 2013 when the data for Euro-DEN was collected [10]. It is therefore possible that users who had shifted to benzodiazepines at the time of shortage may have reverted back to heroin by the time of the study. The lack of systematic analytical confirmation in the large majority of cases in this as well as previous studies does not allow inferences into the influence of different benzodiazepines or any drugs used that were not reported by the patient.
Limitations
Data on the drugs used is based on the patient’s self-report and is therefore reliant on the patient’s ability to self-report accurate information on drug use. Systematic analytical confirmation was not undertaken in this study; however, this is consistent with routine clinical care of patients with illicit drug toxicity in which patients are managed on the basis of the reported drugs used and/or their clinical picture rather than based on the results of analytical toxicology testing. Furthermore, due to the patchy recording of history of alcohol ingestion, which in itself has central nervous system depressant effects, some of the clinical effects seen could be related to co-used alcohol. The Euro-DEN project uses a sentinel-based approach meaning the data does not necessarily provide a national representative picture nor a European-wide picture; however, it should be noted that the sentinel centres involved are in areas of high illicit recreational drug use. It is interesting to note there are regional differences in the benzodiazepines that individuals report co-using, and work is needed to understand the reasons behind this and whether general prescribing and use impacts on these regional differences.
Conclusion
Despite concerns about the co-use of heroin with benzodiazepines could increase the acute harms, through synergistic sedative actions, in this study, we were unable to detect any difference in clinical features, severity of acute toxicity (need for intubation and/or admission to a critical care facility) or outcomes between those patients presenting with acute toxicity related to lone heroin use and those who had co-used benzodiazepines with heroin. Further work is need to understand whether users alter the pattern of their heroin use when co-using with benzodiazepines to prevent inadvertent acute toxicity/overdose and what are the impacts of these alterations on not only the desired effects, but also the acute harms associated with use.
Acknowledgments
Euro-DEN Research Group Collaborators: Chevillard L, Eyer F, Galicia M, Homar C, Jürgens G, Kabata PM, Liakoni E, Liechti ME, Markey G, Mégarbane B, Miro O, Moughty A, O’ Connor N, Paasma P, Persett PS, Pold K, Puiguriguer J, Shields G, Vallersnes OM, Waring WS, Waldman W, Yeung S-J.
Sources of Funding
This study received a financial support from the DPIP/ISEC Programme of the European Union.
Compliance with Ethical Standards
Ethical approval was obtained by each centre to collect the data as part of Euro-DEN project.
Conflicts of Interest
PID and DMW work with the European Monitoring Centre for Drugs and Drug Addiction and the UK Advisory Council for the Misuse of Drugs. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Contributor Information
D. M. Wood, Email: David.Wood@gstt.nhs.uk
On behalf of the Euro-DEN Research Group:
L Chevillard, F Eyer, M Galicia, C Homar, G Jürgens, PM Kabata, E Liakoni, ME Liechti, G Markey, B Mégarbane, O Miro, A Moughty, N O’ Connor, P Paasma, PS Persett, K Pold, J Puiguriguer, G Shields, OM Vallersnes, WS Waring, W Waldman, and S-J Yeung
References
- 1.Zador D, Sunjic S, Darke S. Heroin-related deaths in New South Wales, 1992: toxicological findings and circumstances. Med J Aust. 1996;164:204–207. doi: 10.5694/j.1326-5377.1996.tb94136.x. [DOI] [PubMed] [Google Scholar]
- 2.Ross J, Darke S, Hall W. Benzodiazepine use among heroin users in Sydney: patterns of use, availability and procurement. Drug Alcohol Rev. 1996;15(3):237–243. doi: 10.1080/09595239600185971. [DOI] [PubMed] [Google Scholar]
- 3.Darke S, Ross J, Hall W. Overdose among heroin users in Sydney, Australia: I. Prevalence and correlates of non-fatal overdose. Addiction. 1996;91:405–411. doi: 10.1111/j.1360-0443.1996.tb02289.x. [DOI] [PubMed] [Google Scholar]
- 4.Darke S, Ross J, Mills K, Teesson M, Williamson A, Havard A. Benzodiazepine use among heroin users: baseline use, current use and clinical outcome. Drug Alcohol Rev. 2010;29:250–255. doi: 10.1111/j.1465-3362.2009.00101.x. [DOI] [PubMed] [Google Scholar]
- 5.Darke S, Marel C, Mills KL, Ross J, Slade T, Burns L, Teesson M. Patterns and correlates of non-fatal heroin overdose at 11-year follow-up: findings from the Australian Treatment Outcome Study. Drug Alcohol Depend. 2014;144:148–152. doi: 10.1016/j.drugalcdep.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Iguchi MY, Handelsman L, Bickel WK, Griffiths RR. Benzodiazepine and sedative use/abuse by methadone maintenance clients. Drug Alcohol Depend. 1993;32:257–266. doi: 10.1016/0376-8716(93)90090-D. [DOI] [PubMed] [Google Scholar]
- 7.Ross J, Darke S. The nature of benzodiazepine dependence among heroin users in Sydney, Australia. Addiction. 2000;95:1785–1793. doi: 10.1046/j.1360-0443.2000.951217858.x. [DOI] [PubMed] [Google Scholar]
- 8.Vogel M, Knöpfli B, Schmid O, Prica M, Strasser J, Prieto L, Wiesbeck GA, Dürsteler-MacFarland KM. Treatment or “high”: benzodiazepine use in patients on injectable heroin or oral opioids. Addict Behav. 2013;38:2477–2484. doi: 10.1016/j.addbeh.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Darke S, Ross J, Zador D, Sunjic S. Heroin-related deaths in New South Wales, Australia, 1992–1996. Drug Alcohol Depend. 2000;60:141–150. doi: 10.1016/S0376-8716(99)00147-7. [DOI] [PubMed] [Google Scholar]
- 10.EMCDDA, Perspectives on drugs. Opioid trafficking routes from Asia to Europe, in European Monitoring Centre for Drugs and Drug Addiction. 2015. http://www.emcdda.europa.eu/topics/pods/opioid-trafficking-routes [last accessed 27th April 2018].
- 11.White JM, Irvine RJ. Mechanisms of fatal opioid overdose. Addiction. 1999;94:961–972. doi: 10.1046/j.1360-0443.1999.9479612.x. [DOI] [PubMed] [Google Scholar]
- 12.Puntillo K, Casella V, Reid M. Opioid and benzodiazepine tolerance and dependence: application of theory to critical care practice. Heart Lung: J Acute Crit Care. 1997;26:317–324. doi: 10.1016/S0147-9563(97)90089-3. [DOI] [PubMed] [Google Scholar]
- 13.Rooney S, Kelly G, Bamford L, Sloan D, O’Connor JJ. Co-abuse of opiates and benzodiazepines. Ir J Med Sci. 1999;168:36–41. doi: 10.1007/BF02939579. [DOI] [PubMed] [Google Scholar]
- 14.Darke S, Ross J, Teesson M, Lynskey M. Health service utilization and benzodiazepine use among heroin users: findings from the Australian Treatment Outcome Study (ATOS) Addiction. 2003;98:1129–1135. doi: 10.1046/j.1360-0443.2003.00430.x. [DOI] [PubMed] [Google Scholar]
- 15.EMCDDA, The misuse of benzodiazepines among high-risk opioid users in Europe, in European Monitoring Centre for Drugs and Drug Addiction. 2015. http://www.emcdda.europa.eu/topics/pods/benzodiazepines [last accessed 27th April 2018].
- 16.Frauger E, Pauly V, Pradel V, Rouby F, Arditti J, Thirion X, Lapeyre Mestre M, Micallef J. Evidence of clonazepam abuse liability: results of the tools developed by the French Centers for Evaluation and Information on Pharmacodependence (CEIP) network. Fundam Clin Pharmacol. 2011;25:633–641. doi: 10.1111/j.1472-8206.2010.00882.x. [DOI] [PubMed] [Google Scholar]
- 17.Barnas C, et al. Benzodiazepines and other psychotropic drugs abused by patients in a methadone maintenance program: familiarity and preference. J Clin Psychopharmacol. 1992;12:397–402. doi: 10.1097/00004714-199212000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez Sobrino AM, Fernandez Rodriguez V, Lopez Castro J. Benzodiazepine use in a sample of patients on a treatment program with opiate derivatives (PTDO) Adicciones. 2009;21:143–146. doi: 10.20882/adicciones.241. [DOI] [PubMed] [Google Scholar]
- 19.Gelkopf M, Bleich A, Hayward R, Bodner G, Adelson M. Characteristics of benzodiazepine abuse in methadone maintenance treatment patients: a 1 year prospective study in an Israeli clinic. Drug Alcohol Depend. 1999;55:63–68. doi: 10.1016/S0376-8716(98)00175-6. [DOI] [PubMed] [Google Scholar]
- 20.Navaratnam V, Foong K. Adjunctive drug use among opiate addicts. Curr Med Res Opin. 1990;11:611–619. doi: 10.1185/03007999009112687. [DOI] [PubMed] [Google Scholar]
- 21.Bramness JG, Kornor H. Benzodiazepine prescription for patients in opioid maintenance treatment in Norway. Drug Alcohol Depend. 2007;90(2–3):203–209. doi: 10.1016/j.drugalcdep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 22.EMCDDA, Trendspotter summary report, in European Monitoring Centre for Drugs and Drug Addiction. 2011. http://www.emcdda.europa.eu/scientific-studies/2011/trendspotters-report [Last accessed 27th April 2018].
- 23.United Nations Office of Drugs and Crime, World drug report 2012. 2012. https://www.unodc.org/unodc/en/about-unodc/index.html?ref=menutop [Last accessed 27th April 2018].
- 24.Backmund M, Meyer K, Henkel C, Soyka M, Reimer J, Schütz CG. Co-consumption of benzodiazepines in heroin users, methadone-substituted and codeine-substituted patients. J Addict Dis. 2005;24:17–29. doi: 10.1300/J069v24n04_02. [DOI] [PubMed] [Google Scholar]
- 25.Wood DM, Heyerdahl F, Yates CB, Dines AM, Giraudon I, Hovda KE, Dargan PI. The European Drug Emergencies Network (Euro-DEN) Clin Toxicol (Phila) 2014;52:239–241. doi: 10.3109/15563650.2014.898771. [DOI] [PubMed] [Google Scholar]
- 26.Dines AM, Wood DM, Yates C, Heyerdahl F, Hovda KE, Giraudon I, Sedefov R, Dargan PI, Euro-DEN Research Group Acute recreational drug and new psychoactive substance toxicity in Europe: 12 months data collection from the European Drug Emergencies Network (Euro-DEN) Clin Toxicol (Phila). 2015;53:893–900. doi: 10.3109/15563650.2015.1088157. [DOI] [PubMed] [Google Scholar]
- 27.Hoiseth G, et al. Has previous abuse of flunitrazepam been replaced by clonazepam? Eur Addict Res. 2015;21:217–221. doi: 10.1159/000377628. [DOI] [PubMed] [Google Scholar]