Abstract
Introduction
Gadolinium-based contrast agents (GBCAs) have been increasingly used in clinical practice since their introduction in the 1980s. Recently, increased public attention has been given to patients who report new symptoms following GBCA exposure. This review details the current knowledge surrounding GBCAs, with a focus on the known and proposed disease states that may be associated with GBCAs. Recommendations for the appropriate clinical workup of a patient suspected of having symptoms attributable to gadolinium exposure are included.
Discussion
GBCAs are known to precipitate the disease state nephrogenic systemic fibrosis (NSF), a syndrome characterized by skin thickening in patients with preexisting renal disease. An additional syndrome, termed gadolinium deposition disease, has been proposed to describe patients with normal renal function who develop an array of symptoms following GBCA exposure. While there is a potential physiologic basis for the development of this condition, there is no conclusive evidence to support a causal relationship between GBCA administration and the reported symptoms yet. Clinical evaluation revolves around focused history-taking and physical examination, given the absence of a reliable link between patient symptoms and measured gadolinium levels. There are no recommended treatments for suspected gadolinium deposition disease. Chelation therapy, which is not approved for this indication, carries undue risk without documented efficacy.
Conclusions
The extent to which GBCAs contribute to clinically relevant adverse effects remains an important and evolving field of study. NSF remains the only proven disease state associated with GBCA exposure. Additional data are required to evaluate whether other symptoms should be attributed to GBCAs.
Keywords: Gadolinium, Gadolinium deposition disease, Nephrogenic systemic fibrosis, Gadolinium-based contrast, Magnetic resonance imaging
Introduction
Gadolinium-based contrast agents (GBCAs) first became available for commercial use in 1988 [1]. Since that time, the safety profile of these agents has been established through formal clinical trials and institutional research initiatives. With increasing experience surrounding GBCAs, the scope of safety data continues to evolve. A disease state associated with GBCA exposure was described in the year 2000—nephrogenic systemic fibrosis (NSF). Recently published retrospective case series involving MRI data [2–4], bone composition analyses [5–7], and human autopsy studies [8, 9] have provided insight into potential mechanisms of toxicity contributing to other novel, proposed disease states, without establishing causation. Given the increased attention provided to gadolinium exposure, patients who have been exposed to GBCAs may associate their symptoms with GBCA exposure and seek advice from both the lay and medical community. This poses a conundrum for toxicologists evaluating patients who are concerned for GBCA toxicity but present with symptoms whose etiology is not yet firmly founded in scientific fact. This review summarizes the physiologic aspects of gadolinium toxicity that are relevant to the practicing toxicologist, and provides recommendations for the appropriate evaluation and counseling of patients who are concerned that their symptoms are attributable to gadolinium exposure.
Background
Magnetic resonance imaging (MRI) relies on the ability of hydrogen atoms to absorb and emit radiofrequencies when exposed to strong magnetic fields, thus allowing tissues to be differentiated based on their hydrogen composition [10]. Magnetic resonance contrast agents augment this modality by enhancing local magnetic fields and interacting with hydrogen nuclei to shorten their relaxation time, allowing for increased signal intensity on the resulting images.
Gadolinium is a rare earth metal that was found to be superior to other agents in its ability to enhance MRIs [11]. Free gadolinium is known to be toxic in vivo via multiple mechanisms, including calcium channel blockade, enzyme inhibition, reactive oxygen species formation, and altered cytokine expression [12, 13]. The chelation of gadolinium to various ligands was introduced as a method to mitigate the toxicity of free gadolinium. While there are no studies identifying the effects of pure free gadolinium exposure, one study that evaluated the morbidity of subchronic gadodiamide (Gd[DTPA-BMA]) exposure found that rats exposed to high concentrations of gadodiamide clinically developed skin lesions, hair loss, and loss of testicular mass, with histopathologic evaluation identifying cutaneous mineralization and ulceration, gastric mineralization and inflammation, and testicular giant cell degeneration [14]. A strongly paramagnetic atom due to its seven unpaired electrons, gadolinium maintains a strong magnetic moment despite chelation, making it an effective contrast agent [15].
The volume of MRI scans continues to increase throughout the USA and globally. In 2013, there were 106.8 scans per 1000 US inhabitants, up from 34 scans per 1000 in 1995 [16]. While the exact number and proportion of MRIs requiring contrast is unknown, a review of one quarternary institution’s MRI ordering practices found that 70% of scans over a 1-year period were contrast-enhanced [17].
This narrative review summarizes the physiologic aspects of gadolinium toxicity that are relevant to the practicing toxicologist and provides recommendations for the appropriate evaluation and counseling of patients who are concerned that their symptoms are attributable to gadolinium exposure.
Articles for this review were retrieved from MEDLINE and PubMed through the search terms “gadolinium” AND “toxicity,” and “gadolinium” AND “mechanism.” Reference lists of retrieved articles were reviewed for additional studies not found by the above search method. Manuscripts were limited to those published in the English language; those pertaining to human studies were preferred over animal studies. No date range was set in the search engine.
Pharmacology
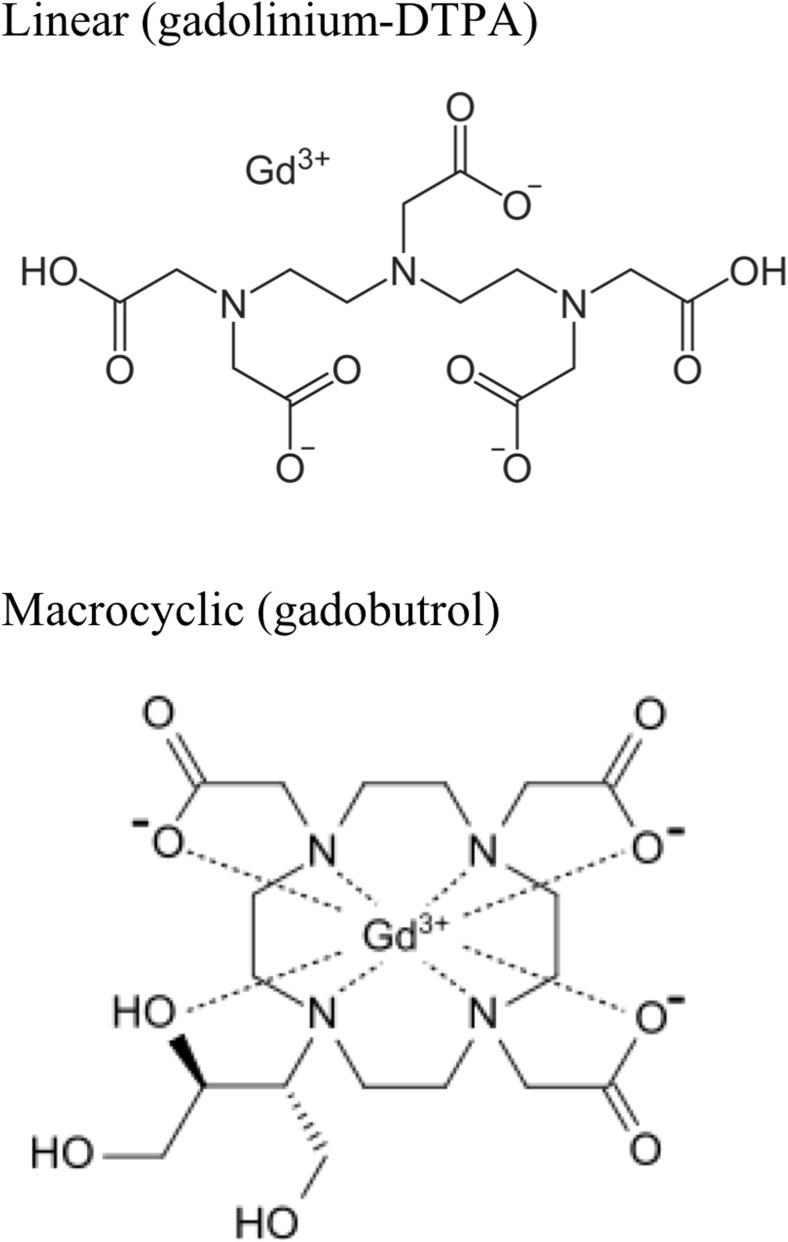
Free gadolinium (Gd3+) has a similar ionic radius to calcium. As such, free gadolinium is a competitive inhibitor of calcium-based processes [18]. All GBCAs contain a ligand to minimize the physiologic effects of free gadolinium. The pharmacology of gadolinium-based contrast agents, and the imaging indication, varies based on the ligand (see Table 1). Linear, or open chain, contrast agents are long molecules containing gadolinium. Macrocyclic, or caged, contrast agents contain a central gadolinium atom (see Fig. 1). Both linear and macrocyclic contrast agents can be ionic or nonionic. The selection of an individual GBCA for a particular study weighs pharmacologic properties, relaxivity (referring to the gadolinium ion’s ability to change the magnetic properties of surrounding water molecules, allowing for enhanced image acquisition), and cost against risks (such as allergy, nephrogenic systemic fibrosis, and gadolinium retention) [19].
Table 1.
List of FDA-approved gadolinium-based contrast agents
| Brand name | Chemical name | Structure | FDA indicated to visualize |
|---|---|---|---|
| Linear | |||
| Omniscan® | Gadodiamide | Non-ionic | Lesions with abnormal vascularity in the brain, spine, thoracic, abdominal, pelvic cavities, and the retroperitoneal space |
| Magnevist® | Gadopentetate | Ionic | Lesions and abnormal vascularity in the central nervous system, extracranial/extraspinal tissues, head, neck, and body |
| MultiHance® | Gadobenate | Ionic | Lesions with abnormal blood–brain barrier or abnormal vascularity of the brain, spine, and associated tissues |
| Eovist® | Gadoxetate | Non-ionic | Lesions in adults with known or suspected focal liver disease |
| Macrocyclic | |||
| ProHance ® | Gadoteridol | Non-ionic | Abnormal vascularity in the brain (intracranial lesions), spine, head, neck, and associated tissues |
| Gadavist ® | Gadobutrol | Non-ionic | Areas with disrupted blood–brain barrier and/or abnormal vascularity of the central nervous system; presence and extent of malignant breast disease; known or suspected supra-aortic or renal artery disease |
| Dotarem ® | Gadoterate | Ionic | Areas with disruption of the blood–brain barrier (BBB) and/or abnormal vascularity in the brain (intracranial), spine, and associated tissues |
Fig. 1.
Representative GBCA structures
In vivo, gadolinium ions eventually dissociate from the ligand; the macrocylic ligands dissociate more slowly than the linear compounds. Absorption is rapid after intravenous administration, and these agents achieve a volume of distribution matching extracellular fluid. GBCAs are primarily excreted via the kidneys, with a short elimination half-life of 1.5–2 h, after intravenous injection. By 24–48 h, GBCAs are almost completely eliminated, though trace amounts of urinary gadolinium are still detectable after 48 h. There are also several hepatobiliary-specific formulations, such as gadoxetic acid, which are excreted in feces as well as urine [20].
Headache, nausea, a cold sensation at the injection site, and dizziness are commonly identified adverse events after GBCA administration. These latter events tend to be self-limited. Serious adverse events associated with GBCA administration include hypersensitivity reactions, acute renal failure, nephrogenic systemic fibrosis, and extravasation with local tissue injury [21–23]. Through post-marketing studies, certain unique adverse effects have been reported for individual agents. For example, gadopentetate and gadobenate have been reported to cause QTc changes [21, 22]. Gadodiamide, gadoxetate, and gadobenate may cause transient, asymptomatic changes in serum iron concentrations and interfere with calcium measurement, depending on the analytical method being used [22–24]. However, because of the non-standardized way that post-marketing reporting is completed, it cannot be concluded that the aforementioned effects are truly unique to specific agents, as these effects may have simply not yet been reported for other agents. Additionally, GBCAs have the potential to interact with other pharmaceuticals. For example, gadobenate competes for the canalicular multispecific organic anion transporter (cMOAT), an ATP-dependent system that transports hydrophobic anionic compounds across various tissue membranes. In the presence of gadobenate, the actions of drugs like cisplatin, anthracyclines, vincristine, methotrexate, and etoposide can be prolonged [22].
Free gadolinium that has dissociated from the ligand can remain in tissues and organs for months to years. At equivalent doses, linear agents lead to more free gadolinium retention than the macrocyclic agents due to their greater dissociation. The likelihood of free gadolinium retention varies within individual classes. Among the linear agents, gadodiamide and gadoversetamide demonstrate the most retention [25]. Once dissociated, retained gadolinium is most highly concentrated in bone, followed by organs including the brain, skin, kidney, liver, and spleen. The latest product labeling for gadobenate does include information on gadolinium retention. [22] Patients at higher risk for adverse effects potentially related to GBCA retention include the following: pregnant women, children, individuals with inflammatory conditions, and those who have had multiple GBCA-enhanced studies [22]. However, the significance of retention is unknown, and retention itself is not diagnostic of toxicity.
Known Disease States
For over a decade after the introduction of GBCAs, the primary adverse events associated with GBCAs were related to immediate, self-limited allergic type, or vasogenic reactions [26]. In a review of 28,340 incidences of gadolinium administration over a 14-year period at one institution, investigators found an adverse reaction rate of 0.07% in patients evaluated soon after GBCA administration, including urticaria/itching (n = 14), nausea/vomiting (n = 4), and bronchospasm (n = 1) [27]. In contrast, rates of adverse reactions related to iodinated contrast media range from 1 to 12%, of which 0.01–0.2% are considered serious adverse reactions [28]. Another review of 21,000 instances of gadolinium administration at a single institution over a 4-year period documented an adverse reaction rate of 0.17% with nausea/vomiting (n = 15), skin changes (hives, irritation, erythema; n = 12), respiratory symptoms (n = 7), and life-threatening allergic reactions (periorbital edema, respiratory distress, severe chest pain; n = 2) [29]. In patient and provider voluntary reporting of Gd-DTPA-related adverse events regarding over 45 million administration instances through 2002, 0.018% of instances were associated with adverse events, with 90.7% of these including non-serious symptoms. The most common adverse events were subjective symptoms including pain, dizziness, and malaise, followed by urticaria, mucosal reaction, and vomiting [1]. Rates of adverse reactions that were captured by these early studies occurred more frequently in individuals with prior history of allergy or asthma, history of adverse reaction to GBCAs, and in those who had received the GBCA injection more quickly [30]. These aforementioned studies were limited in their scope as their focus was on allergic type symptoms or other events occurring soon after GBCA administration. Patients were not followed up to identify delayed reactions.
In 2000, a new adverse effect of GBCA administration was described: nephrogenic systemic fibrosis. This syndrome was initially identified in 15 dialysis patients who presented with similar cutaneous abnormalities including skin thickening, hardening, and hyperpigmentation [31]. The association of NSF with gadolinium exposure became apparent in 2006 following a case series of nine patients receiving hemodialysis who underwent MRI studies and subsequently developed symptoms [32]. Diagnostic criteria for NSF include suggestive skin changes, histologic abnormalities, renal dysfunction, and exposure to GBCAs [33]. The full pathogenesis of NSF has not been defined; it is suspected that NSF occurs in patients with renal dysfunction due to the increased elimination half-life of GBCAs in this population. In one study of patients with CKD, the median elimination half-life of gadodiamide was found to be 34 h relative to 1.3 h in healthy patients [34]. Experts postulate that the increased time of GBCA exposure in vivo creates more opportunity for gadolinium dissociation and transmetallation, a process by which organometallic compounds exchange their metal components with other organometals or metals [12, 35]. Transmetallation has been shown to occur in vivo with several cations, including zinc, calcium, phosphorous, and iron [13, 36]. There are, however, numerous hypotheses regarding the pathogenesis of NSF, many of which are not yet supported by data. Some of the hypothesis that currently have some support in the data include the following: gadolinium transmetallation with ferric iron, deposition of gadolinium phosphate, and ferric iron-induced oxidative stress; macrophage phagocytosis of free gadolinium with subsequent stimulation of fibrocyte infiltration of the dermis; GBCAs acting as a stimulus to the immune response with downstream effects of activated dendritic cells and transforming growth factor beta (TGFb) synthesis; and GBCA activation of transglutaminases [12].
All GBCA formulations have been associated with NSF, but exposure to linear gadolinium chelates (such as gadodiamide) has been found with greater frequency among NSF patients than exposure to macrocyclic chelates [37]. In response to the data linking GBCAs to NSF, the FDA introduced GBCA black box warnings and published safety guidelines in conjunction with the American College of Radiology restricting use of GBCAs in individuals with impaired kidney function [38]. Following implementation of these guidelines, there have been no new cases of NSF identified in the US since 2009 [39–41].
Proposed Gadolinium-Associated Disease States
In recent years, individuals with normal kidney function have reported developing a chronic disease state following GBCA exposure. Semelka et al. coined the term “gadolinium deposition disease” to describe the suspected disease state of individuals who develop a range of symptoms from hours to up to 2 months following gadolinium exposure. Support for this categorization is based primarily based on data from several small qualitative and quantitative investigations [42]. In one investigation by Semelka et al., four individuals with normal renal function who presented for outpatient evaluation following exposure to one to four GBCA injections and underwent focused histories, physical exams, and urinalyses [43]. All patients reported body pain, three reported skin thickening and rubbery subcutaneous tissue in the extremities, and two endorsed “clouded mentation.” All patients had urine concentrations of gadolinium in excess of reference standards and reported physical abnormalities that were corroborated on physical exam. Objective evaluation of dermal complaints, such as skin biopsy, was not performed in this study.
A case report by Roberts et al. identified a patient with normal renal function who developed symptomatic joint contracture after undergoing 61 contrast-enhanced brain MRIs over 11 years. Skin biopsy demonstrated an elevated concentration of gadolinium, as well as gadolinium deposition in deep tissue layers [44]. A separate case by Miller et al. identified a patient who had undergone 36 contrast-enhanced brain MRIs between ages 5 and 21. Neuropsychological testing identified difficulties in executive function, reading and math performance, visual memory, and reasoning. Review of MRI studies identified increased signal ratios in the dentate nuclei, pons, thalamus, globus pallidus, and caudate nuclei following increased exposure to GBCAs over time [45]. Finally, a qualitative study by Semelka et al. invited members of two online gadolinium toxicity support groups to respond to a survey aimed at describing the patients’ symptoms. Among the 42 participants in the study, the most commonly reported symptoms were headache (n = 28), bone pain (n = 26), peripheral pain (n = 26), central pain (n = 15), and skin thickening (n = 22). [46]
A causative relationship between patient-reported symptoms and GBCA exposure has not yet been established, but the presence of retained gadolinium in brain tissue is used to support a theoretical basis for long term symptoms. Numerous studies have identified evidence of gadolinium deposition in the brain and in the bone in individuals with normal kidney function, a condition coined “gadolinium storage condition” by Semelka et al. [42]. Multiple retrospective studies evaluated patients who had numerous contrast-enhanced brain MRIs and identified increased T1 signal intensity in the dentate nucleus with increasing numbers of contrast enhanced MRI studies [2–4]. The patients in these studies had a significant exposure to contrast medium, having received at least six contrast-enhanced MRIs over a 1-year period (mean 7.1) [2], five consecutive contrast-enhanced MRIs over a 10-month period (mean 7.7) [3], and having undergone a range of 2–21 contrast-enhanced MRIs over a 5-year period [4]. Autopsy studies have strengthened the association between GBCAs and radiologically evident abnormalities by demonstrating that patients with prior GBCA exposure have higher than average gadolinium concentration in multiple brain regions [8, 9]. The decedents in these studies also had a moderate exposure to GBCAs, with those in McDonald et al.’s study having undergone a range of 4–29 contrast-enhanced MRIs over a 15-year period [8] and those in Kanda et al.’s study having undergone 2–4 contrast-enhanced MRIs over a 14-year period [9]. Gadolinium also accumulates in bone in patients with GBCA exposure in concentrations greater than that of unexposed controls [5–7].
The clinical significance of increased T1 signal intensity on brain MRI has not been established, but there is some evidence that these changes are associated with patient symptoms in other conditions. A retrospective review of data and imaging of 119 patients with multiple sclerosis (MS) found that a hyperintense T1 dentate nucleus signal was more frequently found in patients with secondary progressive MS (defined by initially relapsing–remitting symptoms that transition to progressive symptom worsening) versus relapsing–remitting or primary progressive MS. These MRI changes were also found more frequently in patients with higher scores on the expanded disability status scale [47]. The cause of the observed hyperintense T1 dentate nucleus signaling is not known. However, a separate study aiming to correlate patient-level factors among those with MS with increased T1 signal intensity identified a history of prior brain imaging, which often involves undergoing multiple contrast-enhanced MRIs to evaluate response, as the only associated variable; the number of prior MRIs obtained was not directly examined [48]. This study does not establish GBCA exposure as a cause of the abnormalities seen on later imaging, but it does provide an interesting association that requires further investigation.
Importantly, GBCA association with any disease state other than NSF remains hypothetical, and the associations that are drawn are based on data extrapolation rather than discrete evidence. According to the Bradford Hill Criteria, the following nine characteristics need to be met in order to judge an observed association as causal: strength of association, consistency, specificity, temporality, biologic gradient, plausibility, coherence, experiment, and analogy [49]. The available evidence does not meet the Bradford Hill Criteria and does not support a conclusion that gadolinium exposure causes any disease states other than NSF. At this time, any correlation between gadolinium deposition and disease states remains speculative. Even among patients with NSF, the degree of gadolinium retention in tissues does not correlate with disease burden, suggesting that gadolinium deposition is not the sole driver of symptomatology [50]. The existing studies evaluating gadolinium deposition disease do not include data regarding patients who were exposed to GBCAs but remained asymptomatic, making it difficult to convincingly suggest a cause and effect between GBCA exposure and future side effects. As of May 2017, the FDA issued a summary statement on the clinical significance of gadolinium retention: “All GBCAs may be associated with some gadolinium retention in the brain and other body tissues. However, because we identified no evidence to date that gadolinium retention in the brain from any of the GBCAs, including GBCAs associated with higher retention of gadolinium, is harmful, restricting GBCA use is not warranted at this time” [51].
Evaluation in the Outpatient Toxicology Setting
Assessment of the patient presenting with symptoms purported to be caused by GBCA exposure relies primarily on completing a detailed and thorough history and physical exam. The healthcare provider must obtain a timeline of the patient’s symptoms, being sure to document the original condition that required evaluation via contrast-enhanced MRI. The patient’s self-reported history should be supplemented with a review of the patient’s previous medical records, when available, as this can aid in determining a definitive timeline of symptom onset or progression that is not biased by recall. This step is essential to understand the true temporality of medical complaints. There is a gap in the available literature describing the indications for multiple MRIs in this patient population (e.g., diagnosis of a suspected demyelinating condition due to non-specific neurologic symptoms).
A complete physical evaluation should be performed, with a specific focus on dermatological changes associated with NSF, such as epidermal thickening and induration. A dermatologist should be consulted if there is a concern for NSF as diagnosis requires confirmation by skin biopsy [33, 52]. Laboratory testing should, at a minimum, include a serum creatinine level to evaluate renal function.
Many patients will report non-specific, subjective symptoms that do not have correlating physical findings. These patients often provide previous laboratory testing by other providers that was non-diagnostic. [53] Providers must seek objective documentation of symptoms whenever possible (e.g., audiometry, EMG), and alternative causes of the patient’s symptoms should be excluded.
A systematic approach must be used if gadolinium deposition disease is being considered. Clinicians can use a framework like the Naranjo criteria to assess the likelihood that symptoms are due to gadolinium exposure [54]. Example realms for exploration are outlined in Table 2.
Table 2.
Topics for review during patient evaluation
| Sample inquiry | |
|---|---|
| Did the symptoms appear after the gadolinium administration? | |
| Did the adverse event reappear when gadolinium was re-administered? | |
| Are there alternative causes (other than gadolinium) that could have caused these symptoms? | |
| Were the adverse symptoms confirmed by any objective evidence? | |
| Are the symptoms consistent with previous reported symptoms after gadolinium administration? | |
| Did the symptoms improve after gadolinium was discontinued? |
Analytical Testing for Gadolinium Toxicity
The most accurate way to identify in vivo gadolinium deposition is to obtain tissue or bone biopsy, and submit these specimens for plasma atomic emission spectroscopy testing or histological examination [20]. However, this method is both invasive and expensive and cannot be used to identify deposition in critical areas like neural tissue. Further, the mere detection of gadolinium in analytical testing does not define toxicity. The majority of studies on gadolinium toxicity have made the diagnosis clinically, based on the presence of symptoms following known exposure to a GBCA [55]. Although multiple studies have demonstrated that gadolinium deposits in the tissues and in bone [52], to date, no studies have described the gadolinium concentration thresholds required to produce toxic effects or clinically significant symptoms in humans. Studies of gadolinium deposition in NSF have found that both affected and unaffected skin have gadolinium present; however, there were significantly larger concentrations in affected skin, suggesting a threshold level at which symptoms occur [56]. Gadolinium is also deposited in the bones of unaffected individuals [20], demonstrating the inutility of simple gadolinium concentration measurements.
Since GBCAs undergo primary renal elimination, Ramalho et al. suggest that a 24-h urine collection can be used to evaluate for gadolinium toxicity for at least 30 days after the administration of contrast [20]. One case report of four individuals with suspected gadolinium deposition disease and normal renal function included one individual with a gadolinium concentration of 82 μg in a 24-h urine collection at 28 days following the last MRI (reference range 0.0–0.4 μg). This value trended down to 3.3 μg at 103 days following exposure; however, the patient’s symptoms persisted despite this trend towards normalization of measured urine gadolinium concentration [43]. Gadolinium serum measurements are unlikely to be useful as they represent both complexed and free forms, and only free gadolinium is thought to be the etiology for NSF and postulated to be responsible for gadolinium deposition disease [57]. Well-designed studies showing a clinical correlation between measured gadolinium concentrations and toxicity must be performed before clear interpretation of analytical testing in this setting.
Urine and serum gadolinium levels can be obtained from reference laboratories and must be collected in a trace metal-free fashion. Different laboratories provide different reference ranges, with some citing a normal range being less than 0.7 mcg/24 h for urine gadolinium. Gadolinium can be found in regional water sources, which may account for gadolinium identification in individuals who have never received a GBCA [58, 59]. The available literature regarding urine gadolinium concentrations is further complicated by the availability of provoked urine testing. Based on the limitations of testing, we do not recommend that clinicians routinely obtain urine gadolinium concentrations on all patients reporting symptoms outside of a research setting. However, if urine gadolinium concentrations are warranted, the collection should occur over 24 h, in a trace metal-free fashion, in an unprovoked manner, and through a reputable analytical laboratory. In pharmacokinetic studies, GBCAs were detectable in the urine of subjects with normal and moderate renal impairment for up to 7 days after exposure [57]. If patients present with elevated urinary gadolinium concentrations performed prior to the visit, adherence to these collection requirements must be assessed.
Previous studies have shown increased T1-weighted signal intensity in posterior fossa and basal ganglia after exposure to GBCAs [4, 8, 9]. However, this increase in signal intensity is not specific to gadolinium deposition and can be seen in several other disease states [20]. Despite a correlation between increased T1 signaling in the posterior fossa and basal ganglia and tissue gadolinium concentration on autopsy, these measures should not be used to determine severity of disease.
Role of Chelation
There is no consistently effective means to completely remove gadolinium from the body after exposure. Among NSF patients, treatment remains elusive. Successive hemodialysis sessions remove the majority of free gadolinium in the blood, but hemodialysis is not effective at removing complexed gadolinium in circulation. It remains unclear whether hemodialysis has an effect on gadolinium retained in tissues [60]. Recovery of renal function, whether by renal transplant or by other means, is accepted as a potential avenue to achieve symptom improvement; these interventions are not equally effective in all patients [61]. Other therapies have been suggested from experience with small numbers of patients, including plasmapheresis, UVA therapy, extracorporeal photopheresis (a procedure involving leukapheresis, photoactivation, and reinfusion of treated cells), and high-dose steroids. Unfortunately, none of these therapies have been shown to be consistently effective [62].
For patients who suspect that their symptoms are caused by gadolinium deposition disease, few treatment options are validated in the medical literature. However, multiple online resources tout the benefits of various modalities, including chelation therapy, leading some patients to request this therapy to alleviate their symptoms. According to one prominent online gadolinium toxicity education and support group, “The most obvious treatment is to try to remove the Gadolinium from your body with Chelation.” [63].
One study evaluating the impact of lanthanides (including lanthanium, gadolinium, and ytterbium) on mitochondria found that mitochondria exposed to lanthanides showed less swelling following exposure to ethylene glycol tetraacetic acid (EGTA), a chelating agent [64]. A recent study on chelation therapy in mice models found that the chelator 3,4,3-LI(1,2-HOPO) (HOPO) was more effective than diethylenetriaminepentaacetic acid (DTPA) in removing radiolabeled gadolinium from mice tissue up to 48 h postexposure, though chelation did not result in complete removal [65].
The only human data on the effect of chelation therapy following gadolinium exposure stems from two case reports. One, published in 2009, describes a 65-year-old woman with NSF resistant to renal transplant who underwent chelation therapy with deferoxamine at doses of 500 mg IM daily for 7 days, followed by 1000 mg IM daily for 5 days. This therapy resulted in increased urine gadolinium excretion, from 6 to 13 μg/day; however, the effect on serum gadolinium levels was insignificant (falling from 1.7 to 1.4 ng/ml). There was no improvement in the patient’s symptoms over the long term [66]. The second case report describes a 55-year-old man with zinc toxicity who underwent 6 months of oral therapy with 2,3,-dimercaptosuccinic acid (DMSA) and ethylenediaminetetraacetic acid (EDTA) and received 22 treatments with intravenous infusions of edetate disodium or edetate calcium disodium. The man underwent two contrast-enhanced MRI studies during this time. Urine testing intended to monitor zinc excretion incidentally found that urine gadolinium excretion increased to a maximum of 89 μg/day during chelation therapy relative to 0.8 μg/day prior to initiation of chelation. The patient had no associated symptoms of gadolinium toxicity [67].
While patients may perceive chelation therapy to be a cure for their ailments, they are rarely informed of the minimal scientific basis for chelation therapy in general, or of the significant risks associated with chelator use. There have also been multiple fatalities directly attributed to chelation therapy through hypocalcemia-induced cardiac arrest [68]. The package insert of Ca-DTPA, a commonly used chelating agent, lists “depletion of endogenous trace metals,” exacerbation of asthma, and deaths in patients with hemochromatosis as significant side effects; it also states that “the likelihood that single dose or multiple doses of Ca-DTPA is teratogenic in humans cannot be ruled out” [69].
With the known risks of chelation and the absence of evidence supporting benefit regarding chelation for subjective gadolinium-associated symptoms, we do not support the use of chelators in this population.
Conclusions
The understanding of GBCA impact on patients following exposure continues to evolve. While GBCAs are known to induce NSF in susceptible patients, the available evidence does not conclusively support the existence of other diseases stemming from GBCA exposure in those with normal renal function. However, the absence of data regarding the association between patient symptoms and prior GBCA exposure necessitates further robust scientific inquiry. Patient evaluation is primarily limited to history-taking and physical examination, with a limited role at this time for analytical testing. Treatment options (e.g., chelation) for those suspected of having symptoms attributable to GBCA exposure are not founded in the literature and cannot be supported due to risk of patient injury.
Funding Source
The authors received no sources of funding for this research.
Previous Presentations
This research has not previously been presented in any form.
Conflict of Interest
None
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Knopp MV, Balzer T, Esser M, Kashanian FK, Paul P, Niendorf HP. Assessment of utilization and pharmacovigilance based on spontaneous adverse event reporting of gadopentetate dimeglumine as a magnetic resonance contrast agent after 45 million administrations and 15 years of clinical use. Investig Radiol. 2006;41(6):491–499. doi: 10.1097/01.rli.0000209657.16115.42. [DOI] [PubMed] [Google Scholar]
- 2.Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834–841. doi: 10.1148/radiol.13131669. [DOI] [PubMed] [Google Scholar]
- 3.Weberling LD, Kieslich PJ, Kickingereder P, Wick W, Bendszus M, Schlemmer H-P, Radbruch A. Increased signal intensity in the dentate nucleus on unenhanced T1-weighted images after gadobenate dimeglumine administration. Investig Radiol. 2015;50(11):743–748. doi: 10.1097/RLI.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 4.Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC. Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with Normal renal function, suggesting dechelation. Investig Radiol. 2014;49(10):685–690. doi: 10.1097/RLI.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 5.Darrah TH, Prutsman-Pfeiffer JJ, Poreda RJ, Ellen Campbell M, Hauschka PV, Hannigan RE. Incorporation of excess gadolinium into human bone from medical contrast agents. Metallomics. 2009;1(6):479–488. doi: 10.1039/b905145g. [DOI] [PubMed] [Google Scholar]
- 6.White GW, Gibby WA, Tweedle MF. Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-DO3A) (ProHance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Investig Radiol. 2006;41(3):272–278. doi: 10.1097/01.rli.0000186569.32408.95. [DOI] [PubMed] [Google Scholar]
- 7.Murata N, Gonzalez-Cuyar LF, Murata K, Fligner C, Dills R, Hippe D, Maravilla KR. Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Investig Radiol. 2016;51(7):447–453. doi: 10.1097/RLI.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 8.McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015;275(3):772–782. doi: 10.1148/radiol.15150025. [DOI] [PubMed] [Google Scholar]
- 9.Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology. 2015;276(1):228–232. doi: 10.1148/radiol.2015142690. [DOI] [PubMed] [Google Scholar]
- 10.Jensen EC. Technical review, types of imaging, part 4-magnetic resonance imaging. Anat Rec. 2014;297(6):973–978. doi: 10.1002/ar.22927. [DOI] [PubMed] [Google Scholar]
- 11.Zamora CA, Castillo M. Historical perspective of imaging contrast agents. Magn Reson Imaging Clin N Am. 2017;25(4):685–696. doi: 10.1016/j.mric.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Idée J-M, Port M, Raynal I, Schaefer M, Le Greneur S, Corot C. Clinical and biological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: a review. Fundam Clin Pharmacol. 2006;20(6):563–576. doi: 10.1111/j.1472-8206.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 13.Rogosnitzky M, Branch S. Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. Biometals. 2016;29(3):365–376. doi: 10.1007/s10534-016-9931-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cacheris WP, Quay SC, Rocklage SM. The relationship between thermodynamics and the toxicity of gadolinium complexes. Magn Reson Imaging. 1990;8(4):467–481. doi: 10.1016/0730-725x(90)90055-7. [DOI] [PubMed] [Google Scholar]
- 15.Runge VM, Clanton JA, Lukehart CM, Partain CL, James AE. Paramagnetic agents for contrast-enhanced NMR imaging: a review. AJR Am J Roentgenol. 1983;141(6):1209–1215. doi: 10.2214/ajr.141.6.1209. [DOI] [PubMed] [Google Scholar]
- 16.OECD . OECD Health Data: Health care resources. Paris: OECD Publishing; 2016. pp. 1–1. [Google Scholar]
- 17.Shankar PR, Parikh K, Davenport MS. Financial implications of revised ACR guidelines for estimated glomerular filtration rate testing before contrast-enhanced MRI. J Am Coll Radiol. 2018;15(2):250–257. doi: 10.1016/j.jacr.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Bellin M-F, Van Der Molen AJ. Extracellular gadolinium-based contrast media: an overview. Eur J Radiol. 2008;66(2):160–167. doi: 10.1016/j.ejrad.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 19.Davenport MS. Choosing the safest gadolinium-based contrast medium for MR imaging: not so simple after all. Radiology. 2018;286(2):483–485. doi: 10.1148/radiol.2017172224. [DOI] [PubMed] [Google Scholar]
- 20.Ramalho J, Semelka RC, Ramalho M, Nunes RH, AlObaidy M, Castillo M. Gadolinium-based contrast agent accumulation and toxicity: an update. AJNR Am J Neuroradiol. 2016;37(7):1192–1198. doi: 10.3174/ajnr.A4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Food and Drug Administration. Magnevist (gadopentetate dimeglumine) injection label. 2010;:1–10.
- 22.Food and Drug Administration. MultiHance (gadobenate dimeglumine) Injection and MultiHance Multipack (gadobenate dimeglumine) Injection. 2018;:1–34.
- 23.Food and Drug Administration. Omniscan (gadodiamide) injection label. 2010;:1–8.
- 24.Anderson A. NDA 022090 Eovist Gadolinium Warning 27Dec2017 USPI DRAFT. 2018;:1–15.
- 25.Ersoy H, Rybicki FJ. Biochemical safety profiles of gadolinium-based extracellular contrast agents and nephrogenic systemic fibrosis. J Magn Reson Imaging. 2007;26(5):1190–1197. doi: 10.1002/jmri.21135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haneder S, Kucharczyk W, Schoenberg SO, Michaely HJ. Safety of magnetic resonance contrast media: a review with special focus on nephrogenic systemic fibrosis. Top Magn Reson Imaging. 2015;24(1):57–65. doi: 10.1097/RMR.0b013e3182a14e79. [DOI] [PubMed] [Google Scholar]
- 27.Cochran ST, Bomyea K, Sayre JW. Trends in adverse events after IV administration of contrast media. AJR Am J Roentgenol. 2001;176(6):1385–1388. doi: 10.2214/ajr.176.6.1761385. [DOI] [PubMed] [Google Scholar]
- 28.Bottinor W, Polkampally P, Jovin I. Adverse reactions to iodinated contrast media. Int J Angiol. 2013;22(3):149–154. doi: 10.1055/s-0033-1348885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy KJ, Brunberg JA, Cohan RH. Adverse reactions to gadolinium contrast media: a review of 36 cases. AJR Am J Roentgenol. 1996;167(4):847–849. doi: 10.2214/ajr.167.4.8819369. [DOI] [PubMed] [Google Scholar]
- 30.Nelson KL, Gifford LM, Lauber-Huber C, Gross CA, Lasser TA. Clinical safety of gadopentetate dimeglumine. Radiology. 1995;196(2):439–443. doi: 10.1148/radiology.196.2.7617858. [DOI] [PubMed] [Google Scholar]
- 31.Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356(9234):1000–1001. doi: 10.1016/S0140-6736(00)02694-5. [DOI] [PubMed] [Google Scholar]
- 32.Grobner T. Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104–1108. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 33.Kribben A, Witzke O, Hillen U, Barkhausen J, Daul AE, Erbel R. Nephrogenic systemic fibrosis: pathogenesis, diagnosis, and therapy. J Am Coll Cardiol. 2009;53(18):1621–1628. doi: 10.1016/j.jacc.2008.12.061. [DOI] [PubMed] [Google Scholar]
- 34.Joffe P, Thomsen HS, Meusel M. Pharmacokinetics of gadodiamide injection in patients with severe renal insufficiency and patients undergoing hemodialysis or continuous ambulatory peritoneal dialysis. Acad Radiol. 1998;5(7):491–502. doi: 10.1016/s1076-6332(98)80191-8. [DOI] [PubMed] [Google Scholar]
- 35.Frenzel T, Lengsfeld P, Schirmer H, Hütter J, Weinmann H-J. Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Investig Radiol. 2008;43(12):817–828. doi: 10.1097/RLI.0b013e3181852171. [DOI] [PubMed] [Google Scholar]
- 36.Abraham JL, Thakral C, Skov L, Rossen K, Marckmann P. Dermal inorganic gadolinium concentrations: evidence for in vivo transmetallation and long-term persistence in nephrogenic systemic fibrosis. Br J Dermatol Wiley/Blackwell (10.1111); 2007 Dec 7;158(2):273–80. [DOI] [PubMed]
- 37.Broome DR. Nephrogenic systemic fibrosis associated with gadolinium based contrast agents: a summary of the medical literature reporting. Eur J Radiol. 2008;66(2):230–234. doi: 10.1016/j.ejrad.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Leiner T, Kucharczyk W. NSF prevention in clinical practice: summary of recommendations and guidelines in the United States, Canada, and Europe. J Magn Reson Imaging. 2009;30(6):1357–1363. doi: 10.1002/jmri.22021. [DOI] [PubMed] [Google Scholar]
- 39.Zou Z, Zhang HL, Roditi GH, Leiner T, Kucharczyk W, Prince MR. Nephrogenic systemic fibrosis. JACC Cardiovasc Imaging. 2011;4(11):1206–1216. doi: 10.1016/j.jcmg.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 40.Bennett CL, Qureshi ZP, Sartor AO, Norris LB, Murday A, Xirasagar S, Thomsen HS. Gadolinium-induced nephrogenic systemic fibrosis: the rise and fall of an iatrogenic disease. Clin Kidney J. 2012;5(1):82–88. doi: 10.1093/ckj/sfr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altun E, Martin DR, Wertman R, Lugo-Somolinos A, Fuller ER, Semelka RC. Nephrogenic systemic fibrosis: change in incidence following a switch in gadolinium agents and adoption of a gadolinium policy--report from two U.S. universities. Radiology. 2009;253(3):689–696. doi: 10.1148/radiol.2533090649. [DOI] [PubMed] [Google Scholar]
- 42.Semelka RC, Ramalho J, Vakharia A, AlObaidy M, Burke LM, Jay M, Ramalho M. Gadolinium deposition disease: initial description of a disease that has been around for a while. Magn Reson Imaging. 2016;34(10):1383–1390. doi: 10.1016/j.mri.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 43.Semelka RC, Commander CW, Jay M, Burke LMB, Ramalho M. Presumed gadolinium toxicity in subjects with Normal renal function. Investig Radiol. 2016;51(10):661–665. doi: 10.1097/RLI.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 44.Roberts DR, Lindhorst SM, Welsh CT, Maravilla KR, Herring MN, Adam Braun K, et al. High levels of gadolinium deposition in the skin of a patient with Normal renal function. Investig Radiol. 2016;51(1):280–289. doi: 10.1097/RLI.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 45.Miller JH, Hu HH, Pokorney A, Cornejo P, Towbin R. MRI brain signal intensity changes of a child during the course of 35 gadolinium contrast examinations. Pediatrics. 2015;136(6):e1637–e1640. doi: 10.1542/peds.2015-2222. [DOI] [PubMed] [Google Scholar]
- 46.Semelka RC, Ramalho M, AlObaidy M, Ramalho J. Gadolinium in humans: a family of disorders. AJR Am J Roentgenol. 2016;207(2):229–233. doi: 10.2214/AJR.15.15842. [DOI] [PubMed] [Google Scholar]
- 47.Roccatagliata L, Vuolo L, Bonzano L, Pichiecchio A, Mancardi GL. Multiple sclerosis: hyperintense dentate nucleus on unenhanced T1-weighted MR images is associated with the secondary progressive subtype. Radiology. 2009;251(2):503–510. doi: 10.1148/radiol.2511081269. [DOI] [PubMed] [Google Scholar]
- 48.Kasahara S, Miki Y, Kanagaki M, Yamamoto A, Mori N, Sawada T, Taoka T, Okada T, Togashi K. Hyperintense dentate nucleus on unenhanced T1-weighted MR images is associated with a history of brain irradiation. Radiology. 2011;258(1):222–228. doi: 10.1148/radiol.10100508. [DOI] [PubMed] [Google Scholar]
- 49.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58(5):295–300. [PMC free article] [PubMed] [Google Scholar]
- 50.Thakral C, Abraham JL. Nephrogenic systemic fibrosis: histology and gadolinium detection. Radiol Clin N Am. 2009;47(5):841–853. doi: 10.1016/j.rcl.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 51.Food and Drug Administration. FDA identifies no harmful effects to date with brain retention of gadolinium-based contrast agents for MRIs; review to continue. 2017;:1–4.
- 52.Girardi M, Kay J, Elston DM, LeBoit PE, Abu-Alfa A, Cowper SE. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol. 2011;65(6):1095–1097. doi: 10.1016/j.jaad.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 53.Leikin JB, Mycyk MB, Bryant S, Cumpston K, Hurwitz S. Characteristics of patients with no underlying toxicologic syndrome evaluated in a toxicology clinic. J Toxicol Clin Toxicol. 2004;42(5):643–648. doi: 10.1081/clt-200026960. [DOI] [PubMed] [Google Scholar]
- 54.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 55.Burke LMB, Ramalho M, AlObaidy M, Chang E, Jay M, Semelka RC. Self-reported gadolinium toxicity: a survey of patients with chronic symptoms. Magn Reson Imaging. 2016;34(8):1078–1080. doi: 10.1016/j.mri.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 56.Christensen KN, Lee CU, Hanley MM, Leung N, Moyer TP, Pittelkow MR. Quantification of gadolinium in fresh skin and serum samples from patients with nephrogenic systemic fibrosis. J Am Acad Dermatol. 2011;64(1):91–96. doi: 10.1016/j.jaad.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 57.Aime S, Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging. 2009;30(6):1259–1267. doi: 10.1002/jmri.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.TEST ID: GDU [Internet]. mayomedicallaboratories.com. [cited 2018 Jun 3]. Available from: https://www.mayomedicallaboratories.com/test-catalog/2011/Clinical+and+Interpretive/89301. Accessed 3 June 2018.
- 59.Telgmann L, Sperling M, Karst U. Determination of gadolinium-based MRI contrast agents in biological and environmental samples: a review. Anal Chim Acta. 2013;764:1–16. doi: 10.1016/j.aca.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Silberzweig JI, Chung M. Removal of gadolinium by dialysis: review of different strategies and techniques. J Magn Reson Imaging. 2009;30(6):1347–1349. doi: 10.1002/jmri.21981. [DOI] [PubMed] [Google Scholar]
- 61.Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35(4):238–249. doi: 10.1016/j.semarthrit.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Basak P, Jesmajian S. Nephrogenic systemic fibrosis: current concepts. Indian J Dermatol. 2011;56(1):59–64. doi: 10.4103/0019-5154.77555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams S, Grimm H, editors. Treatment possibilities for gadolinium toxicity. 2014. Available from: https://gadoliniumtoxicity.com/help/treatments/. Accessed 3 June 2018.
- 64.Liu H, Yuan L, Yang X, Wang K. La3+, Gd3+ and Yb3+ induced changes in mitochondrial structure, membrane permeability, cytochrome c release and intracellular ROS level. Chem Biol Interact. 2003;146(1):27–37. doi: 10.1016/s0009-2797(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 65.Rees JA, Deblonde GJP, An DD, Ansoborlo C, Gauny SS, Abergel RJ. Evaluating the potential of chelation therapy to prevent and treat gadolinium deposition from MRI contrast agents. Sci Rep. 2018;8(1):4419. doi: 10.1038/s41598-018-22511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leung N, Pittelkow MR, Lee CU, Good JA, Hanley MM, Moyer TP. Chelation of gadolinium with deferoxamine in a patient with nephrogenic systemic fibrosis. NDT Plus. 2009;2(4):309–311. doi: 10.1093/ndtplus/sfp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greenberg SA. Zinc transmetallation and gadolinium retention after MR imaging: case report. Radiology. 2010;257(3):670–673. doi: 10.1148/radiol.10100560. [DOI] [PubMed] [Google Scholar]
- 68.Centers for Disease Control and Prevention (CDC) Deaths associated with hypocalcemia from chelation therapy—Texas, Pennsylvania, and Oregon, 2003-2005. MMWR Morb Mortal Wkly Rep. 2006;55(8):204–207. [PubMed] [Google Scholar]
- 69.Hamenl Pharmaceuticals Package Insert - Instructions for use: Pentetate calcium trisodium injection [Internet]. [cited 2018 Jun 16]. Available from: https://orise.orau.gov/reacts/documents/calcium-dtpa-package-insert.pdf. Accessed 3 June 2018.