Abstract
In the present study, an attempt was made to benchmark the hydrolytic potential of cellulase cocktail obtained from stable mutant UV-8 of Talaromyces verruculosus IIPC 324 (NFCCI 4117) with three commercially available cellulases. With two experimental approaches, acid-pretreated sugarcane bagasse was subjected to hydrolysis for 72 h, where all the enzymes were dosed on the basis of common protein or common cellulase activity /g cellulose content. Concentrated fungal enzyme (CFE) of mutant UV-8 resulted in ~ 59% and 55% saccharification of acid-pretreated sugarcane bagasse after 72 h at 55 °C and pH 4.5 with respect to reducing sugar release, when dosed at 25 mg protein/g and 500 IU CMC’ase/g cellulose, respectively. On the other hand, at similar dosages, the performance of Cellic CTec2 was best resulting in 77% and 66% saccharification, respectively. When enzyme desorption studies were undertaken by carrying out cellulase activities in saccharified broth after 72 h CFE of UV-8 emerged as the best cellulase cocktail. A minimum of 90% endoglucanase and 60% cellobiohydrolase I was successfully desorbed from residual biomass, thereby increasing the probability of enzyme recycle and reuse for next round of hydrolysis.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1547-x) contains supplementary material, which is available to authorized users.
Keywords: Talaromyces verruculosus IIPC 324, Enzymatic saccharification, Carboxy methyl cellulase (EG or CMC’ase), Reducing sugars (RS), CFE (concentrated fungal enzyme), Cellobiohydrolase (CBH)
Introduction
Biotechnological interventions form the backbone of the second-generation biofuel sector. In spite of many promising and emerging technologies for cellulose depolymerisation such as mechanocatalysis, non-thermal atmospheric plasma and sonochemistry as reviewed by Jérôme et al., the complete elimination of the enzymes has not been possible till date (2016). The most challenging step during the processing of lignocellulosic feedstock is the production of fermentable sugars at an affordable cost, especially through enzymatic route. The quest to improve the enzyme cocktails in terms of processing multiple feedstocks or similar feedstock obtained after different pretreatment strategies is still ongoing and has already led to a considerable reduction in operating costs of modern bio-refineries.
Cellulases represent a complex group of synergistically acting enzymes comprising principally of endo β-1,4- glucanase (EG), cellobiohydrolyases (CBH) or exo β-1,4, glucanases and β- glucosidase or cellobiase (CBU) enzymes. EG principally attacks the amorphous part of cellulose and randomly cleaves the glycosidic bonds to release cello-oligomers, whereas the CBH processively attack the crystalline cellulose at the terminals of reducing (CBHI) and non reducing (CBH II) end of the chain to finally produce cellobiose. CBU further hydrolyzes cellobiose to release glucose monomers (Müller et al. 2015). However, the recent discovery and characterization of new class of non-hydrolytic auxiliary proteins, especially the copper-dependent lytic polysaccharide monooxygenases (LPMOs), also known as GH61 enzymes, brought a dramatic shift in cellulase research (Harris et al. 2010). Several evidences have been provided by the researchers where LPMOs stimulated biomass saccharification (Harris et al. 2010; Cannella et al. 2012; Jung et al. 2015). The exact mechanistic action of these oxidative enzymes is still enigmatic as some researchers have proposed that aerobic conditions are preferable for their activity (Müller et al. 2015), whereas some have experimentally proven their dependence on hydrogen peroxide (Bissaro et al. 2017) and lignin (Westereng et al. 2015).
Thus, under current scenario, merely conducting preliminary studies on cellulases and their characterization is insufficient for promoting highly promising biofuel cellulases developed by different research groups. To get validation and better acceptance, benchmarking or referencing of these cellulase cocktails against known commercial formulations becomes inevitable.
Several in-house enzymes have been evaluated and compared for hydrolysis of different biomass samples with either using standard strain of Trichoderma reesei RUT C30 or commercial cellulases (Kovacs et al. 2009; de.Castro et al. 2010; Reis et al. 2013; Pensupa et al. 2013; Anasontzis et al. 2017). Even the efficacy and performance of several commercial or pre-commercial enzymes have been compared in the state of the art (Kumar and Wyman 2009; Mc Millan et al. 2011; Sun et al. 2015).
In the present study, we investigated the hydrolysis of dilute acid-pretreated sugarcane bagasse using the cellulases of stable mutant UV-8 obtained from UV mutagenesis of Talaromyces verruculosus IIPC 324 NFCCI 4117, as described earlier (Jain and Agrawal 2018a). Based on the optimum results of enzymatic saccharification, later the hydrolytic potential of its cellulase cocktail was benchmarked with commercial cellulase preparations.
Two different approaches were undertaken for the said study. In the first set of experiments the enzyme loadings were done based on protein content/g cellulose content as described previously (Arantas and Saddler 2014). In the second approach, enzyme loading was done based on fixed cellulase activity/g cellulose content. The cellulase activity chosen was endoglucanase (CMC’ase) activity which represents one of the three principal components of cellulase cocktail. Earlier also Pryor and Nahar (2010) and Sun et al. (2015) have conducted studies wherein enzyme loading was done on common activity (Filter paper units/g cellulose or Cellobiase units/g cellulose content) basis.
Since cellulases are the significant cost contributors affecting overall process economics of the second generation biofuel production (Klein-Marcuschamer et al. 2011), percentage of desorbed active enzyme was also estimated in enzymatic hydrolyzate. Endoglucanase and cellobiohydrolase I (CBH I) activities were performed in the said hydrolyzate as both these enzyme components have carbohydrate-binding module (CBM) and can bind irreversibly with the lignin moieties during hydrolysis. Higher enzyme activities represented higher desorption of cellulases from residual biomass and thus increased likelihood for its recycling and reuse by membrane separation as reported by Qi et al. (2011). Compositional Analysis of the residual biomass after enzymatic saccharification was also conducted to further validate the rate of hydrolysis via material balance closure.
Materials and methods
Chemicals and raw material
All chemicals and media components were either procured from Sigma Aldrich, USA or Hi-Media Laboratories (Mumbai, India) and were of analytical or laboratory grade. Wheat bran was used as substrate for solid-state fermentation (SSF) and was purchased from local market. For enzymatic saccharification studies, raw sugarcane bagasse (SCB) was procured from Doiwala sugar mill, Dehradun, India.
Commercial enzymes
Cellic® CTec2 and Palkonol MBW were kindly gifted by Novozymes A/S (Bagsværd, Denmark) and MAPS Enzymes (Ahmadabad, India), respectively. Sacchari SEB C6 was purchased from Advanced Enzyme Technologies Ltd., Mumbai, India. Cellic CTec2 enzyme is proprietary biofuel cellulase manufactured by Novozymes which has been used by a number of workers (Sun et al. 2015; Rodrigues et al. 2015), whereas Sacchari SEB C6 is commercial cellulase from India which has been previously used for enzymatic saccharification of wheat straw by Agrawal et al. (2015). Palkonol MBW is a specially designed cellulase cocktail previously used for enzymatic hydrolysis of alkali-pretreated denanath grass and napier grass by Mohapatra et al. (2017).
Microorganism
A stable mutant of Talaromyces verruculosus IIPC 324, namely mutant UV-8, was used for the present study. Its parent strain has already been deposited in National Fungal Culture Collection of India (NFCCI), Agharkar Research Institute, Pune (India), with accession number 4117. This mutant was routinely maintained on PDA slants at 4 °C, and simultaneously the glycerol stocks (25% w/v) were also made and stored at − 80 °C. The cellulase production of the said fungus was carried out under SSF conditions by aseptically transferring 24 h grown fungal mycelia in wheat bran supplemented with 1.62% ammonium sulphate incubated at 24 °C and initial moisture content being 61.5 ± 0.5% (Jain and Agrawal 2018a). At the end of 4th day of fermentation, the moldy bran was dried at 45 °C and bottled until use.
Concentration of crude enzyme
For the preparation of the concentrated fungal enzyme (CFE), 120 g of dry moldy bran was extracted with 1500 ml of deionized water and filtered. The spores present in the filtrate were removed by centrifugation at 10,000 rpm for 30 min at 4 °C. The centrifuged filtrate (1300 ml) was subjected to ammonium sulphate precipitation (40–75% saturation) and the precipitated product was reconstituted in 50 mM citrate buffer followed by desalting using 10 kDa membrane (Macrosep Advance Centrifugal devices with Omega Membrane, Pall Make). The final enzyme samples (200 ml) were subjected to cellulase assays, namely endoglucanase, cellobiase and CBH I at pH 4.0 and temperature 60 °C (data not shown) owing to similar profile with its parent strain Talaromyces verruculosus IIPC 324 (Jain and Agrawal 2018b). The protein assay was conducted using Bradford assay with BSA fraction V as the standard (Bradford 1976). This sample was designated as Concentrated Fungal Enzyme (CFE UV-8).
Enzyme assays and protein determination
For all the commercial enzymes, the endoglucanase assay (CMC’ase) was carried out incubating them with 1% sodium salt of carboxymethyl cellulose (Sodium salt of CMC; Fluka- 21,902) in 50 mM citrate buffer at 50 °C for 10 min. Reducing sugars were determined by the 3,5, dinitrosalicylic acid (DNS) method (Miller 1959). One unit of endoglucanase activity was defined as the amount of enzyme which released 1 µmol of glucose/min under the conditions indicated. Cellobiase assay (CBU) was carried out as per IUPAC protocol and one unit of cellobiase activity was defined as the amount of enzyme which hydrolysed 1 µmol of cellobiose or released 2 µmol of glucose/min under the optimized conditions as described previously (Ghosh 1987). CBH assay was carried out at 50 °C with 10 mM p-nitrophenyl β-d-cellobioside (pNPC) as substrate in 50 mM citrate buffer. After 20 min of incubation, the reaction was stopped by adding 1 ml of 2M sodium carbonate solution. The 4-nitrophenol liberated was measured at 405 nm. One unit of enzyme activity was defined as the amount of enzyme required to release 1 µmol of p-nitrophenol/min from pNPC under optimized assay conditions (Despande et al. 1984).
The protein concentration of all the commercial enzymes was measured by the Bradford Assay using bovine serum albumin (BSA) Fraction V as the protein standard (Bradford 1976). The method of Bradford was chosen for all the commercial enzymes based on the results of Mc Millan et al. (2011) where practically no differences in protein content were obtained before and after desalting of pre-commercial enzymes.
Pretreatment of sugarcane bagasse (SCB)
Dilute sulphuric acid (1.25% v/v) pretreatment of SCB was conducted at 140 °C with a holding time of 90 min, with solid: liquid ratio being 1:8, by the method described previously (Ghosh et al. 2015). The compositional analysis and ash content of the acid pretreated SCB were carried out as per the method of NREL described by Sluiter et al. (2008, 2012).
Determination of optimum temperature and pH for saccharification by CFE UV-8
Before benchmarking studies, it was essential to know the optimum temperature and pH of CFE derived from mutant UV-8 of Talaromyces verruculosus IIPC 324 for enzymatic saccharification, though all its cellulase components showed temperature and pH optimum of 60 °C and 4.0, respectively (data not shown). The study undertaken was in line with the protocol developed by Novozymes for assessing the performance of Cellic CTec2 with dilute acid-pretreated corn stover as substrate at 5% total solid loading as described previously (Application Sheet Novozymes 2010).
Three different temperatures, namely 50, 55 and 60 °C were chosen and dosing of CFE UV-8 was done at 25 mg protein/g cellulose content. The procedure involved hydrolysis of 2.5 g of acid-pretreated SCB suspended in 50 ml citrate-buffered medium (pH 4.0) along with addition of 0.15 g PEG 6000/g lignin content for 72 h under agitated conditions (150 rpm). To determine the optimal pH, saccharification studies were conducted at optimum temperature at similar dosage of CFE UV-8 in pH range of 3.5–5.5 at 0.5 intervals. All the experiments were performed in duplicates.
Benchmarking studies
Benchmarking studies of CFE UV-8 with commercial enzymes was conducted using two different approaches. In the first approach the enzyme loading was done at common protein basis (25 mg protein/g cellulose content) and in the other at common cellulase activity basis (500 IU CMC’ase/g cellulose content). The basis of selection on common protein and activity basis relied on the extensive studies carried with these enzymes (data not shown). All the experiments were performed in duplicate.
For all the commercial enzymes the study was performed at 50 °C, whereas the concentrated fungal enzyme (CFE) was conducted at 55 °C for 72 h. The amount of reducing sugars released was determined by 3, 5, Dinitrosalicylic acid (DNS) method using glucose as standard (Miller 1959). Glucose release in the saccharified broth was quantified by GOD–POD kit (Accurex Biomedical Pvt Ltd, India). To assess the complete release of sugars and glucose, after removal of saccharified broth the residual biomass was washed with water and both the sugar assays were conducted in the wash as well.
Percentage saccharification was calculated using the following formula:
The residual biomass left after enzymatic saccharification was subjected to compositional analysis for complete material balance closure. Enzyme desorption studies were conducted by performing CBH and CMC’ase activities in the saccharified broth as they are principle enzymes that get highly adsorbed on biomass due to presence of cellulose-binding domain (CBD) as described earlier (Rocha-Martín et al. 2017). Both the cellulase activities were assessed at the same temperature and pH, which was used during saccharification with their respective controls. Percentage cellulase desorption was calculated by the following formula:
where in cellulase activity referred to CMC’ase activity or CBH I activity.
Results and discussion
Biomass composition
The compositional analysis of acid-pretreated sugarcane bagasse revealed that acid pretreatment removed most of the xylan component of the biomass and after acid treatment the major constituents of the bagasse were cellulose and acid-insoluble lignin comprising 61.2% and 31.9% of the total biomass, respectively, as shown in Table 1.
Table 1.
Compositional analysis of acid-pretreated sugarcane bagasse before enzymatic saccharification
| Biomass composition** | Percentage | Distribution in 2.5 g substrate |
|---|---|---|
| Cellulose | 61.2 | 1.53 |
| Pentosans | 1.5 | 0.0375 |
| Acid-insoluble lignin | 31.9 | 0.7975 |
| Acid-soluble lignin | 1.79 | 0.0445 |
| Ash | 2.61 | 0.06525 |
**Represents the average of triplicates
Determination of optimum temperature and pH for saccharification by CFE UV-8
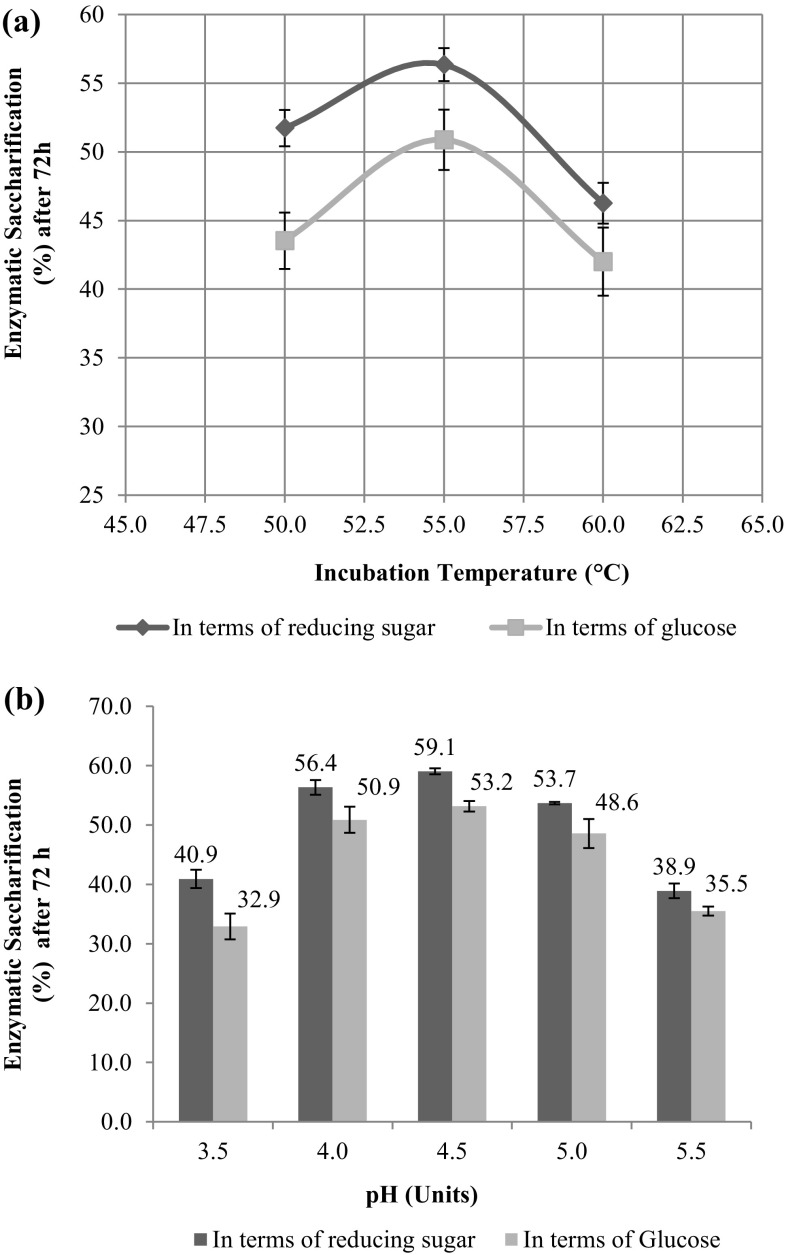
When the enzymatic saccharification studies were conducted with CFE of mutant UV-8 at three different temperatures, 55 °C emerged as the best temperature resulting in ~ 57 and 50% saccharification of acid-pretreated sugarcane bagasse with respect of reducing sugar and glucose, respectively, when added at 25 mg protein/g cellulose content as depicted in Fig. 1a.
Fig. 1.
Effect of temperature (a) and pH (b) on enzymatic saccharification of acid-pretreated sugarcane bagasse by CFE UV-8
There was 21% improvement in the saccharification yields both in terms of reducing sugar and glucose when the temperature was decreased from 60 °C to 55 °C. Further reduction in temperature (50 °C) resulted in lower rate of saccharification. This study revealed that though the optimum assay temperature for all the three cellulase components was 60 °C, optimum temperature for saccharification was 55 °C.
Such behavior of the enzyme cocktail of mutant UV-8 could be attributed to the fact the all the enzyme assays were carried out with shorter incubation time not exceeding beyond 30 min; however, saccharification studies are usually undertaken for 72 h or more. In such cases, the primary aspects, that is, thermostability and unproductive binding on to lignin, cannot be overlooked.
Taking 55 °C as the optimum temperature when enzymatic saccharification was carried out at different pH (3.5–5.5), the best results were obtained at pH 4.5, showing a meager improvement of 4% in hydrolytic potential of CFE UV-8 as shown in Fig. 1b. Thus for all the benchmarking studies the optimum pH and temperature for CFE UV-8 was taken as 4.5 and 55 °C, respectively.
Benchmarking of enzymes on common protein loading basis
When the enzymatic hydrolysis was performed on common protein loading basis (25 mg protein/g cellulose content), depending on the specific activity of each enzyme, the distribution pattern of all the three cellulase components, namely EG, CBHI and CBU, varied significantly as shown in Table 2.
Table 2.
Distribution pattern of cellulase activities (IU) in 2.5 g substrate dosed when at 25 mg protein loading/g cellulose content
| Enzymes | Lot no. | Assay pH and temperature | CMC’ase | CBU | CBH I |
|---|---|---|---|---|---|
| Palkonol MBW | 1202/MBW/984 | 4.5; 50 °C | 4601.2 ± 2.61 | 10.78 ± 0.429 | 55.63 ± 1.99 |
| Sacchari SEB C6 | SCAS031301 | 4.8; 50 °C | 12125.5 ± 19.85 | 150.0 ± 19.85 | 86.46 ± 0.91 |
| Cellic CTec2 | VCSI0009 | 5.2; 50 °C | 4827.06 ± 55.36 | 1666.3 ± 13.02 | 126.48 ± 1.67 |
| CFE UV-8 mutant | 4.7; 55 °C | 959.6 ± 34.4 | 207.74 ± 16.45 | 47.67 ± 2.33 |
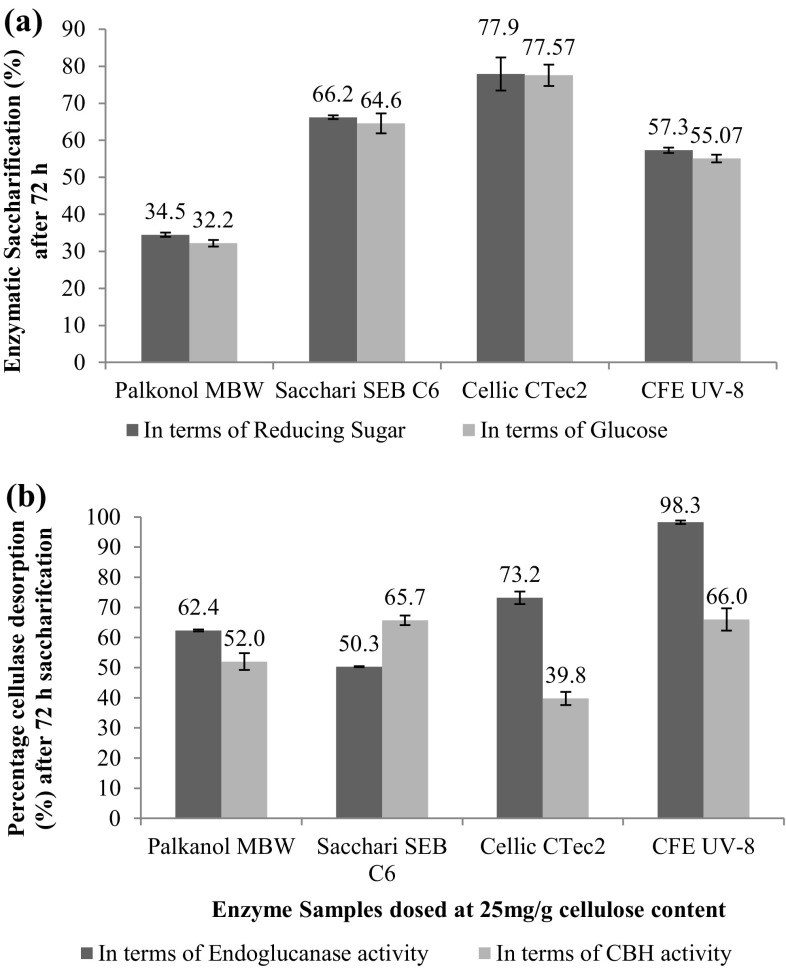
Even though the endoglucanase activities of Sacchari SEB C6 were 2.5 times than Cellic CTec2 (Table 2), yet Cellic CTec2 emerged as the best cellulase for biofuel applications resulting in 77.9% and 77.57% saccharification of acid-pretreated sugarcane bagasse with respect of reducing sugar and glucose released, respectively, as shown in Fig. 2a.
Fig. 2.
Percentage saccharification (a) and cellulase recoveries (b) after 72 h of enzymatic saccharification of acid-pretreated SCB using various cellulases when dosed at 25 mg protein/g cellulose content
Better saccharification efficiency with Cellic CTec2 could be attributed to the presence of LPMO activity in the said preparation (Cannella et al. 2012) and it is likely that the lignin moieties in the acid-pretreated sugarcane bagasse served as electron donors to LPMOs. Cellulose conversion yields have known to be improved by 25% during hydrolysis of hydrothermally pretreated wheat straw with Cellic CTec2, when replaced with Celluclast and Novozym188 combination (Cannella et al. 2012). They studied the action of these oxidative enzymes revealing that in lignin-containing substrates no reducing agent was required for the functioning of these oxidative enzymes and hypothesized that oxidative cleavage of cellulose and redox cycles in lignin were interlinked via electron transport mechanisms.
These results are also in consensus with the results of Ramos et al. where they attained 67.2% glucan conversion from phosphoric acid-impregnated steam-treated sugarcane bagasse (5% solid loading;150 rpm) when Cellic CTec2 was dosed at 0.1 g/g cellulose content (2015). Even Sun et al. (2015) had reported that when Cellic CTec2 was loaded at 20 mg protein/g dry substrate, it resulted in 55% hydrolysis of 2% steam-pretreated sweet sorghum bagasse at the end of 24 h only.
When the performance of cellulase cocktail obtained from mutant UV-8 was compared with commercial enzymes, some interesting results were obtained. When benchmarked against Cellic CTec2, CFE UV-8 was able to release 24.13% lesser reducing sugar from acid-pretreated sugarcane bagasse. The plausible explanation for its lower performance could be lower specific cellulase activities of CFE UV-8 than Cellic CTec2 (Table 2). A similar kind of trend was also observed when comparisons were made between CFE UV-8 and Sacchari SEB C6.
These results are in agreement with the experimental studies conducted with dilute acid-pretreated corn stover where the performance of four different pre-commercial enzymes was evaluated (Mc Millan et al. 2011). In this study, to achieve similar cellulose conversion (75%) in whole slurry, researchers found that the requirement of Enzyme B was minimal (9.1 mg/g cellulose content), whereas requirement of Enzyme C was maximal (28 mg/g cellulose content) due to difference in specific activities. Based on Bradford assay, the specific activities of Enzyme B (1.29 FPU/mg protein; 9.15 IU glucosidase/mg protein) and Enzyme C (0.93 FPU/mg protein; 5.28 IU glucosidase/mg protein) varied significantly and hence their estimated dosages also varied to attain similar cellulose conversions.
This study reaffirmed the findings of Mc Millan et al. (2011) where they raised concern on benchmarking the enzymes based on protein dosages (mg enzyme protein per g cellulose) and showed that adapting different methods of protein estimation may also alter the enzymatic saccharification.
When enzyme desorption studies were carried out by performing cellulase activities in saccharified broth after 72 h of enzymatic hydrolysis, some interesting results were obtained as depicted in Fig. 2b. Among all the enzymes, endoglucanase desorption was found in the order of CFE UV-8 > Cellic CTec2 > Palkonol MBW > Sacchari SEBC6. It is very likely that as the enzymatic hydrolysis proceeded, newer lignin surfaces were exposed and endoglucanases present in Sacchari SEBC6 were lost in residual biomass due to non productive and irreversible lignin binding. On the other hand, 98.3% and 73% desorbed EG in case of CFE UV-8 and Cellic CTec2 activity confirmed that these enzymes had lower lignin-binding properties as compared to other two commercial preparations.
When desorption studies were done by estimating CBH I activities in saccharified broth, Sacchari SEB C6 and CFE UV-8 were best, whereas Cellic CTec2 showed 60% losses (Fig. 2b). The results in the present study are in disagreement with findings of Yarbrough et al. (2015), where they have shown that Cellic CTec2 was able to retain only 4% and 64% endoglucanase and cellobiohydrolase activity, respectively. However, it should be noted that the study in state of art was done with 30% lignin content extracted from corn stover. Unfortunately, cellulase adsorption studies are often conducted by contacting cellulases with isolated or extracted lignin for a limited period at a defined temperature (Li et al. 2016; Lu et al. 2016).
Even when real-time lignocellulosic biomass is used, only the saccharification yields are featured and cellulase desorption are often ignored. Enzyme affinities for lignin-rich biomass is mostly investigated by merely conducting protein assays or performing SDS PAGE and zymography (Girard and Conversa 1993; Zhu et al. 2009; Qi et al. 2011; Rocha-Martín et al. 2017). This study is first of its kind wherein the enzyme desorption studies were calculated by quantitative estimation of two important mono-components of cellulases, namely EG and CBH I.
The present study revealed that addition of PEG 6000 as lignin-blocking additive successfully aided in desorbing the endoglucanase present in CFE UV-8 and Cellic CTec2 significantly but could not work with same efficiacy for CBH. Moreover, PEG 6000 was less effective for Sacchari SEBC6 and Palkonol MBW. It is likely that the sources of enzyme, their structural and surface properties play a pivotal role during lignin binding and further affect its adsorption/desorption.
Similar observations were made by Rodrigues et al. (2015) where they found alkaline washing was highly feasible for cellulase recovery for Cellulclast enzyme but was not effective for Cellic CTec2. They further concluded that recycling strategy must be customized for each enzyme formulation, as different components within cellulase cocktail may behave differently with respect to solid–liquid distribution, stability and cellulose and lignin affinity.
When the compositional analysis of all the residual biomass samples was conducted for mass balance, it verified the authenticity of the results obtained during saccharification experiments as reflected in Table 3. This study suggested that Sacchari SEBC6 and cellulase cocktail CFE UV-8 both harbored side activity of xylanase which resulted in the hydrolysis of pentosans during enzymatic saccharification and subsequent reduction in the pentosan content in the residual biomass (Table 3).
Table 3.
Compositional analysis of the residual acid-pretreated sugarcane bagasse after enzymatic saccharification with various cellulases at loading of 25 mg protein/g cellulose content and percentage cellulose hydrolysis as reflected by residual biomass composition
| Cellulases | Initial biomass | Residual biomass | % Acid insoluble lignin including Ash | % Glucan in residual biomass | % Pentosans in residual biomass | % Glucan Hydrolyzed | % Saccharification in terms of glucose released** |
|---|---|---|---|---|---|---|---|
| Palkonol MBW | 2.5 ± 0.10 | 1.97 ± 0.03 | 45.22 ± 0.75 (0.890 g) | 51.5 ± 0.23 (1.014 g) | 1.74 ± 0.22 (0.034 g) | 33.48 | 32.2 |
| SacchariSEB C6 | 2.5 ± 0.10 | 1.337 ± 0.01 | 61.74 ± 0.10 (0.825 g) | 37.6 ± 0.42 (0.502 g) | 0.15 ± 0.003 (0.002 g) | 66.98 | 64.6 |
| Cellic CTec2 | 2.5 ± 0.10 | 1.30 ± 0.03 | 63.88 ± 0.10 (0.833 g) | 26.5 ± 0.10 (0.345 g) | 2.6 ± 0.003 (0.034 g) | 77.23 | 77.57 |
| CFE UV-8 mutant | 2.5 ± 0.10 | 1.51 ± 0.03 | 53.87 ± 0.03 (0.815 g) | 45.3 ± 0.40 (0.685 g) | 0.45 ± 0.0007 (0.006 g) | 55.01 | 55.07 |
The figures mentioned in the brackets represent the actual components in the residual biomass
**Denotes the percentage saccharification with respect to glucose released in saccharified broths
Benchmarking of enzymes on common cellulase activity basis
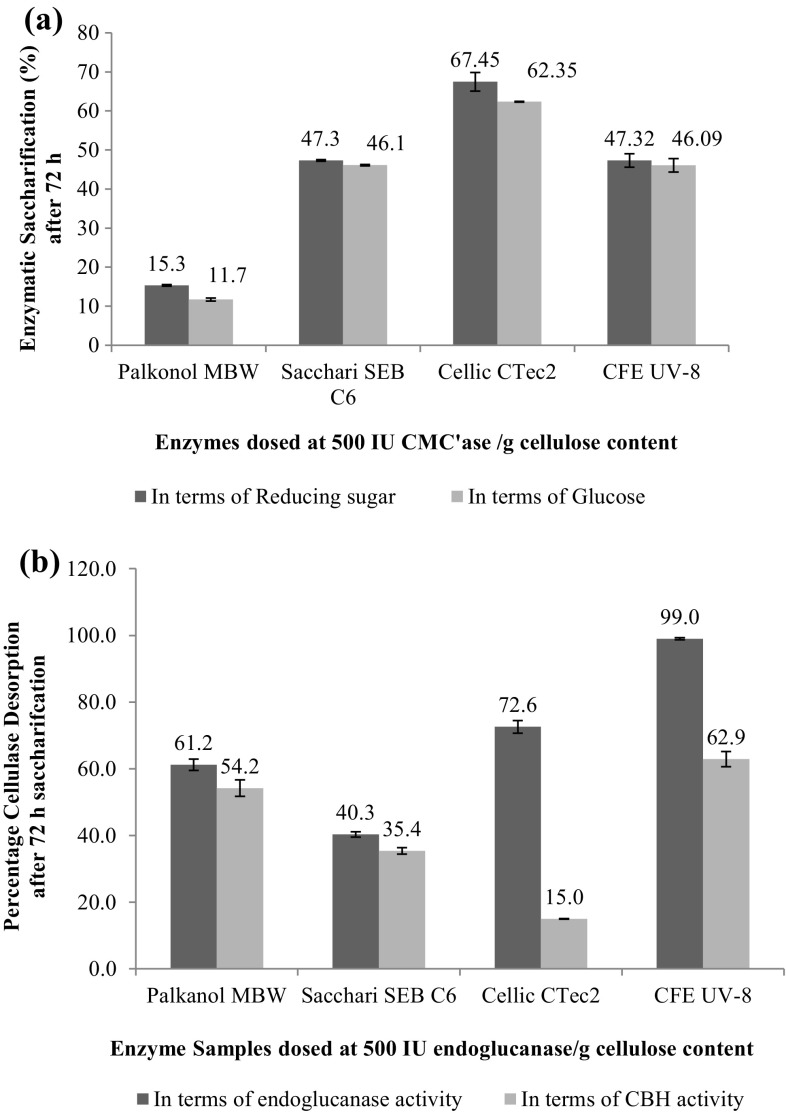
When the studies were conducted on common cellulase activity basis (500 IU/g cellulose content), the hydrolytic potential was in the order of Cellic CTec2 > CFE UV-8 > Sacchari SEB C6 > Palkonol MBW (Fig. 3a). Among all the enzymes Cellic CTec2 was able to release 1.146 ± 0.04 g of reducing sugar from 2.5 g acid-pretreated sugarcane bagasse containing ~ 61% cellulose. Similarly, CFE UV-8 and Sacchari SEB C6 were able to release 0.925 g and 0.80 g of reducing sugars, respectively, from the same substrate.
Fig. 3.
Percentage saccharification (a) and cellulase recoveries (b) after 72 h of enzymatic saccharification of acid-pretreated SCB using various cellulases when dosed at 500 IU CMC’ase /g cellulose content
Thus at common cellulase activity loading, CFE UV-8 was able to reasonably compete with the commercial enzyme Sacchari SEB C6 and released 3 times more reducing sugar when compared with Palkonol MBW.
It should be noted that when the enzyme loading was done on common cellulase activity basis, there was an appreciable reduction in the protein loading (BSA equivalents) as shown in Table S1. Reduction in protein loading led to greater losses of enzyme in residual biomass as depicted in Fig. 3b. The CBH desorption in case of Cellic CTec2 (39.2–15%) and Sacchari SEB C6 (65–35%) were more prominently affected when benchmarking was shifted from protein basis to cellulase activity basis.
A similar trend was observed by Girard and Converse, when diluted acid-pretreated hardwood was subjected to hydrolysis using enzyme Cytolase CL (Genencor) and they recovered higher fraction of enzyme in solution at the end of hydrolysis with increasing protein loading (Girard and Conversa 1993). However, cellulase recoveries of CFE UV-8 and Palkonol MBW were least affected in the present case.
Table 4 depicts the compositional analysis of all the residual samples, representing the actual leftover components in the biomass and percentage cellulose hydrolyzed as per the residual biomass composition. A complete material balance closure was visible in all the samples irrespective of the type of enzymatic treatment. Even at common cellulase loadings, the reduced pentosan content in the residual biomass obtained after CFE UV-8 treatment suggested xylanase side activity in its cellulase cocktail.
Table 4.
Compositional analysis of the residual acid-pretreated sugarcane bagasse after enzymatic saccharification with various cellulases at loading of 500 CMC’ase/g cellulose content and percentage cellulose hydrolysis as reflected by residual biomass composition
| Cellulases | Initial biomass | Residual biomass | % Acid insoluble lignin including Ash | % Glucan in residual biomass | % Pentosan in residual biomass | % Glucan hydrolysed | % Saccharification in terms of glucose released** |
|---|---|---|---|---|---|---|---|
| Palkonol MBW | 2.5 ± 0.10 | 2.20 ± 0.03 | 37.54 ± 0.91 (0.825 g) | 61.4 ± 0.1 (1.349 g) | 1.07 ± 0.25 (0.023 g) | 11.66 | 11.7 |
| SacchariSEB C6 | 2.5 ± 0.10 | 1.70 ± 0.02 | 50.06 ± 0.12 (0.850 g) | 46.8 ± 0.6 (0.807 g) | 1.98 ± 0.1 (0.033 g) | 47.85 | 46.1 |
| Cellic CTec2 | 2.5 ± 0.10 | 1.53 ± 0.03 | 57.66 ± 0.05 (0.883 g) | 33.4 ± 0.5 (0.511 g) | 2.4 ± 0.06 (0.034 g) | 66.40 | 62.35 |
| CFE UV-8 mutant | 2.5 ± 0.10 | 1.642 ± 0.03 | 50.03 ± 0.05 (0.821 g) | 49.62 ± 0.1 (0.814 g) | 0.3 ± 0.0006 (0.004 g) | 46.58 | 46.09 |
Note: The figures mentioned in the brackets represent the actual components in the residual biomass
**Denotes the percentage saccharification with respect to glucose released in saccharified broths
Thus the present study concluded with the fact that CFE UV-8 of Talaromyces verruculosus IIPC 324 was highly competitive when its performance was compared to two commercial cellulase preparations, namely Palkonol MBW and Sacchari SEB C6 at similar cellulase activity basis. However, significantly low yields of glucose by CFE UV-8 when compared to CellicCTec2 irrespective of type of enzyme loading was inevitable. This response can be attributed to the fact that a direct correlation between saccharification yield and LPMO activity of Cellic CTec2 exists, as demonstrated by Müller et al. (2015).
The present investigation suggests that benchmarking of novel cellulase cocktails with commercial enzymes on protein loading basis may be unwise. The downstream processing of these commercial enzymes is well established and therefore during comparative assessment, their higher specific activities give them an edge over novel enzyme cocktails.
The present study took into consideration one of the critical aspects of process economics, namely the enzyme adsorption and its losses in residual biomass. This aspect is usually ignored by most of the researchers, in spite of the fact that enzymes are one of the major cost contributors in entire process chain for production of second-generation biofuels and chemicals. Higher desorption properties of EG and CBHI present in CFE UV-8 gave an advantage over other commercial cellulases including Cellic CTec2, in spite of low saccharification yields. It also reflected the potentiality of this enzyme cocktail, particularly regarding the thermostability and its robustness in the presence of inhibitory lignin component.
In future, studies will be undertaken to know whether addition of copper sulphate during SSF production of biofuel cellulases would trigger or induce the production of LPMO in the mutant UV-8 of Talaromyces verruculosus IIPC 324 or not. Alternatively, second round of mutagenesis can be envisaged in which LPMO activity-based screening can be conducted. Secretome and transcriptome analysis of the said mutant strain is also targeted to know the actual composition of the cellulase cocktail secreted by the said fungus and the hidden potential of the strain at gene level.
Conclusions
The present benchmarking study demonstrated that hydrolytic potential of cellulase cocktail obtained from mutant UV-8 of Talaromyces verruculosus IIPC 324 was highly promising as compared to commercial cellulases, namely Palkonol MBW and Sacchari SEB C6. However, the performance of the said cocktail was 30% and 22% lower than high LPMO containing Cellic CTec2, when dosed on protein and cellulase activity basis, respectively. While assessing the desorption potential of cellulases, CFE UV-8 was superior to all the commercial enzymes with a minimum of 90% endoglucanase and 60% CBHI activity observed in saccharified broth.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors are grateful to Dr Anjan Ray, Director CSIR-Indian Institute of Petroleum for providing necessary facilities to complete this work and constant encouragement. This research was funded by CSIR-IIP as in-house project under OLP- 350919. We would like to thank Dr Debashish Ghosh, Scientist Biofuel Division for kindly providing acid pretreated sugarcane bagasse for the entire study. Senior research fellowship awarded to Ms Lavika Jain by Council of Scientific and Industrial Research, New Delhi, India is greatly acknowledged.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Application Sheet of Novozymes (2010) Cellic® CTec2 and HTec2—enzymes for hydrolysis of lignocellulosic materials. http://www.shinshu-u.ac.jp/faculty/engineering/chair/chem010/manual/Ctec2.pdf. Accessed on 4 July 2018
- Agrawal R, Gaur R, Mathur A, Kumar R, Gupta RP, Tuli DK, Satlewal A. Improved saccharification of pilot-scale acid pretreated wheat straw by exploiting the synergistic behavior of lignocellulose degrading enzymes. RSC Adv. 2015;5:71462–71471. doi: 10.1039/C5RA13360B. [DOI] [Google Scholar]
- Anasontzis GE, Thuy NT, Hang DTM, Huong HT, Thanh DT, Hien DD, Thanh VN, Olsson L. Rice straw hydrolysis using secretomes from novel fungal isolates from Vietnam. Biomass Bioenerg. 2017;99:11–20. doi: 10.1016/j.biombioe.2017.02.008. [DOI] [Google Scholar]
- Arantes V, Saddler JN. Cellulose accessibility limits the effectiveness of minimum cellulase loading on the efficient hydrolysis of pretreated lignocellulosic substrates. Biotechnol Biofuels. 2011;4:3. doi: 10.1186/1754-6834-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissaro B, Røhr AK, Müller G, Chylenski P, Skaugen M, Forsberg Z, Horn SJ, Vaaje-Kolstad G, Eijsink VGH. Oxidative cleavage of polysaccharides by monocopper enzymes depends on H2O2. Nat Chem Biol. 2017;13(10):1123–1128. doi: 10.1038/nchembio.2470. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A Rapid and Sensitive Method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cannella D, Hsieh CC, Felby C, Jørgensen H. Production and effect of aldonic acids during enzymatic hydrolysis of lignocellulose at high dry matter content. Biotechnol Biofuels. 2012;5:26. doi: 10.1186/1754-6834-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Castro AM, Carvalho MLDAD, Leite SGF, Pereira N., Jr Cellulases from Penicillium funiculosum: production, properties and application to cellulose hydrolysis. J Indus Microbiol Biotechnol. 2010;37:151–158. doi: 10.1007/s10295-009-0656-2. [DOI] [PubMed] [Google Scholar]
- Despande MV, Eriksson K, Pettersson LG. An assay for selective determination of exo-1,4,-β-glucanases in a mixture of celluloytic enzymes. Anal Biochem. 1984;138:481–487. doi: 10.1016/0003-2697(84)90843-1. [DOI] [PubMed] [Google Scholar]
- Ghosh TK. Measurement of cellulase activities. Pure Appl Chem. 1987;59:257–268. doi: 10.1351/pac198759020257. [DOI] [Google Scholar]
- Ghosh D, Dasgupta D, Agrawal D, Kaul S, Adhikari DK, Kurmi Ak, Arya PK, Bangwal D, Negi MS. Fuels and chemicals from lignocellulosic biomass: an integrated biorefinery approach. Energ Fuel. 2015;29(5):3149–3157. doi: 10.1021/acs.energyfuels.5b00144. [DOI] [Google Scholar]
- Girard DJ, Conversa AO. Recovery of cellulase from lignaceous hydrolysis residue. Appl Biochem Biotechnol. 1993;39–40:521–533. doi: 10.1007/BF02919015. [DOI] [Google Scholar]
- Harris PV, Welner D, McFarland KC, Re E, Polusen JN, Brown K, Salbo R, Ding H, Vlasenko E, Merino S, Xu F, Cherry J, Larsen S, Leggio LL. Stimulation of lignocellulosic biomass hydrolysis by proteins of glycoside hydrolyase family 61: structure and function of a large, enigmatic family. Biochem. 2010;49:3305–3316. doi: 10.1021/bi100009p. [DOI] [PubMed] [Google Scholar]
- Jain L, Agrawal D. Rational approach for mutant selection of Talaromyces verruculosus IIPC 324 secreting biofuel cellulases-assessing saccharification potential. Ind Crops Prod. 2018;114:93–97. doi: 10.1016/j.indcrop.2018.01.078. [DOI] [Google Scholar]
- Jain L, Agrawal D. Performance evaluation of fungal cellulases with dilute acid pretreated sugarcane bagasse: A robust bioprospecting strategy for biofuel enzymes. Renewe Energ. 2018;115:978–988. doi: 10.1016/j.renene.2017.09.021. [DOI] [Google Scholar]
- Jérôme F, Chatel G, Vigier KDO. Depolymerization of cellulose to processable glucans by non-thermal technologies. Green Chem. 2016;18:3903–3391. doi: 10.1039/C6GC00814C. [DOI] [Google Scholar]
- Jung S, Song Y, Kim HM, Bae H. Enhanced lignocellulosic biomass hydrolysis by oxidative lytic polysaccharide monooxygenase (LPMOs) GH61 from Gleophyllum trabeum. Enzyme Micro Technol. 2015;77:38–45. doi: 10.1016/j.enzmictec.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Klein-Marcuschamer D, Oleskowicz-Popiel P, Simmons BA, Blanch HW. The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol Bioengg. 2012;109(4):1083–1087. doi: 10.1002/bit.24370. [DOI] [PubMed] [Google Scholar]
- Kovacs K, Macrelli S, Szakacs G, Zacchi G. Enzymatic hydrolysis of steam-pretreated lignocellulosic materials with Trichoderma atroviride enzymes produced in-house. Biotechnol Biofuels. 2009;2:14. doi: 10.1186/1754-6834-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Wyman CE. Effect of cellulase and xylanase enzymes on the deconstruction of solids from pretreatment of poplar by leading technologies. Biotechnol Prog. 2009;25(2):302–314. doi: 10.1002/btpr.102. [DOI] [PubMed] [Google Scholar]
- Li YL, Sun Z, Ge X, Zhang J. Effect of lignin and surfactant on adsorption and hydrolysis of cellulases on cellulose. Biotechnol Biofuels. 2016;9:20. doi: 10.1186/s13068-016-0434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Zheng X, Li X, Zhao J. Adsorption and mechanism of cellulase enzymes onto lignin isolated from corn stover pretreated with liquid hot water. Biotechnol Biofuels. 2016;9:118. doi: 10.1186/s13068-016-0531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Millan JD, Jenning EW, Mohagheghi A, Zuccarello M. Comparative performance of pre-commercial cellulases hydrolysing pretreated corn stover. Biotechnol Biofuels. 2011;4:29. doi: 10.1186/1754-6834-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Mohapatra S, Pattathil S, Thatoi H. Structural and functional characterization of two Pennisetum sp. biomass during ultrasono-assisted alkali pretreatment and enzymatic hydrolysis for understanding the mechanism of targeted delignification and enhanced saccharification. ACS Sustain Chem Eng. 2017;5(8):6486–6497. doi: 10.1021/acssuschemeng.7b00596. [DOI] [Google Scholar]
- Müller G, Várnai A, Johansen KS, Eijsink VGH, Horn SJ. Harnessing the potential of LPMO-containing cellulase cocktails poses new demands on processing conditions. Biotechnol Biofuels. 2015;8:187. doi: 10.1186/s13068-015-0376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensupa N, Jin M, Kokolski M, Archer DB, Du C. A solid state fungal fermentation-based strategy for the hydrolysis of wheat straw. Bioresour Technol. 2013;149:261–267. doi: 10.1016/j.biortech.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor SW, Nahar N. Deficiency of cellulase activity measurements for enzyme evaluation. Appl Biochem Biotechnol. 2010;162:1737–1750. doi: 10.1007/s12010-010-8955-7. [DOI] [PubMed] [Google Scholar]
- Qi B, Chen X, Su Y, Wan Y. Enzyme adsorption and recycling during hydrolysis of wheat straw lignocellulose. Bioresour Technol. 2011;102:2881–2889. doi: 10.1016/j.biortech.2010.10.092. [DOI] [PubMed] [Google Scholar]
- Ramos LP, Silva L, Ballem AC, Pitarelo AP, Chiarello LM, Silveira MHL. Enzymatic hydrolysis of steam-exploded sugarcane bagasse using high total solids and low enzyme loadings. Bioresour Technol. 2015;175:195–202. doi: 10.1016/j.biortech.2014.10.087. [DOI] [PubMed] [Google Scholar]
- Reis L, Fontana RC, Delabona PS, Lima DJS, Camassola M, Pradella JGC, Dillon AJP. Increased production of cellulases and xylanases by Penicillium echinulatum S1M29 in batch and fed-batch culture. Bioresour Technol. 2013;146:597–603. doi: 10.1016/j.biortech.2013.07.124. [DOI] [PubMed] [Google Scholar]
- Rocha-Martín J, Martinez-Bernal C, Pérez-Cobas Y, Reyes-Sosa FM, García BD. Additives enhancing enzymatic hydrolysis of lignocellulosic biomass. Bioresour Technol. 2017;244:48–56. doi: 10.1016/j.biortech.2017.06.132. [DOI] [PubMed] [Google Scholar]
- Rodrigues AC, Haven MO, Lindedam J, Felby C, Gama M. Celluclast and cellic® CTec2: saccharification/fermentation of wheat straw, solid–liquid partition and potential of enzyme recycling by alkaline washing. Enzyme Microb Technol. 2015;79–80:70–77. doi: 10.1016/j.enzmictec.2015.06.019. [DOI] [PubMed] [Google Scholar]
- Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2008) Determination of ash in biomass. Technical report. NREL/TP-510-42622
- Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2012) Determination of structural carbohydrates and lignin in biomass laboratory. Technical report. NREL/TP-510-42618
- Sun FF, Hong J, Hu J, Saddler JN, Fang X, Zhang Z, Shen S. Accessory enzymes influence cellulase hydrolysis of the model substrate and the realistic lignocellulosic biomass. Enzyme Microb Technol. 2015;79–80:42–48. doi: 10.1016/j.enzmictec.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Westereng B, Cannella D, Agger JW, Jørgensen H, Anderson ML, Eijsink VGH, Felby C. Enzymatic cellulose oxidation is linked to lignin by long-range electron transfer. Sci Rep. 2015;5:18561. doi: 10.1038/srep18561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarbrough JM, Mittal A, Mansfield E, Taylor IILE, Hobdey SE, Sammond DW, Bomble YJ, Crowley MF, Decker SR, Himmel ME, Vinzant TB. New perspective on glycoside hydrolase binding to lignin from pretreated corn stover. Biotechnol Biofuels. 2015;8:214. doi: 10.1186/s13068-015-0397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Sathitsuksanoh N, Zhang PYH. Direct quantitative determination of adsorbed cellulase on lignocellulosic biomass with its application to study cellulase desorption for potential recycling. Analyst. 2009;134:2267–2272. doi: 10.1039/b906065k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.