Abstract
In this study, Gelidium elegans is investigated for ethanol production. A combination of factors including different temperatures, acid concentration and incubation time was evaluated to determine the suitable saccharification conditions. The combination of 2.5% (w/v) H2SO4 at 120 °C for 40 min was selected for hydrolysis of the seaweed biomass, followed by purification, and fermentation to yield ethanol. The galactose and glucose were dominant reducing sugars in the G. elegans hydrolysate and under optimum condition of dilute acid hydrolysis, 39.42% of reducing sugars was produced and fermentation resulted in ethanol concentration of 13.27 ± 0.47 g/L. A modified method was evaluated for sample preparation for gas chromatography (GC) analysis of the ethanol content. A solvent mixture of acetonitrile and iso-butanol precipitated dissolved organic residues and reduced water content in GC samples at least by 90%. Results showed that this method could be successfully used for bioethanol production from seaweed.
Keywords: G. elegans, Seaweed, Sample preparation, Gas chromatography, Bioethanol
Introduction
Biofuels have received great attention as sustainable alternative fuels (Sudhakar et al. 2018). In Brazil, bioethanol is being extracted from the agriculture crops and used as biofuel in the transportation sector (Salles-Filho et al. 2017). Conflicts on the use of land for cultivation of biofuel crops instead of agriculture crops have arisen (Adams et al. 2009). The cultivation of algae to produce feedstock for biofuel production, using non-agricultural land, can avoid this conflict. Algae-based biofuels have high impact, unlike the use of corn or sugarcane (Searchinger et al. 2008; Chia et al. 2018). Algae have potential to reduce greenhouse gases by consumption of CO2 emitted from power plants and natural gas operations, as indicated by life-cycle assessments (Maranduba et al. 2015; Vuppaladadiyam et al. 2018). An advantage of using seaweed is the low lignin content which improves the enzymatic hydrolysis of cellulose. The carbohydrate content in the red seaweed G. elegans was high compared to lipid and protein contents; the amount of carbohydrate was increased to 72.6% from 53.4% by pre-processing, and showed its potential use as feedstock for bioethanol production (Wi et al. 2009). Adams et al. (2009) reported that sugarcane is the most productive terrestrial crop and can produce 6756 L/ha for bioethanol production. However, the bioethanol yield from seaweeds interestingly could reach 23,400 L/ha. Use of seaweeds as feedstocks will not compete with their use as food, and there will be no conflicts with other land uses such as urban development or other agricultural and industrial usage.
Therefore, in the present study we selected the red seaweed species, G. elegans, which is cultivated in East Asia mostly to produce agar. Another advantage of using G. elegans that it possesses high-quality fibres that can be used as an alternative feedstock for the pulp and paper industry (Yoon et al. 2012). Therefore, there is a potential for the commercial cultivation and use of G. elegans as a feedstock for high-value products including agar, paper and biofuel. In the present study, dilute acid treatment was optimised to increase reducing sugar content, followed by fermentation of the reducing sugars to bioethanol, with the use of acclimated yeast, Saccharomyces cerevisiae, under anaerobic condition. In addition, a novel technique was applied in the sample preparation for ethanol analysis by gas chromatography.
Materials and methods
Source of chemicals
High-performance liquid chromatography-grade solvents such as iso-butanol, acetonitrile, ethanol absolute, and trifluoroacetic acid (TFA) were purchased from F.S. Chemicals Ltd, India. Sodium borohydride, 1-methylimidazole, standard glucose, galactose, mannose, fucose, rhamnose, xylose and arabinose were obtained from Sigma-Aldrich, USA. Acetic anhydride, dimethyl sulfoxide, and Milli-Q-purified water were purchased from Merck, Germany. Analytical-grade chemicals such as sulphuric acid, sodium hydroxide, and calcium hydroxide were procured from, Labchem Inc. and Chemolab supplies Inc., Malaysia.
Seaweed collection and sample preparation
The G. elegans was obtained from a local market in Geelong, South Korea. Voucher specimens were prepared as dried herbarium specimens and deposited at University of Malaya Seaweeds and Sea grasses Herbarium. The seaweeds were first cleaned with diluted seawater, debris and epiphytes were removed carefully, followed by drying in the oven at 75 °C to reach constant weight. The dried specimen was preserved in a sealed plastic bag for further use.
Yeast pre-culture and adaptation
Saccharomyces cerevisiae (NBRC 10217) was purchased from the National Institute of Technology and Evaluation (NITE), Japan, and it was maintained on yeast potato dextrose (YPD) agar plate. Two days before the fermentation study, it was cultured in YPD broth media (20 g/L glucose, 20 g/L peptone, 10 g/L yeast extract), and kept on a rotary shaker with 150 rpm at 30 °C. To reduce the influence of yeast seed media, the desired volume (5% v/v) was washed with normal saline and concentrated by centrifugation (2500g, Hettich EBA 20, Germany) followed by addition of the prepared media to obtain 1.5 × 107 CFU/mL (Hessami et al. 2018a).
Optimization of saccharification of the seaweed
The seaweed sample was incubated in different temperatures 80, 100, 120 and 140 °C with various sulphuric acid concentrations 0.5, 1, 2.5, 5 and 10% (w/v) at different time intervals 10, 20, 40 and 60 min for obtaining the best saccharification method. A 0.5 g seaweed sample was mixed with 10 mL of diluted sulfuric acid in a 15-mL centrifuge tube and incubated in the above-mentioned temperatures for different time intervals. The sample was then centrifuged (2500×g) for 15 min and the reducing sugar content in the supernatant was analysed using the 3,5-dinitrosalicylic acid method (Miller 1959).
Preparation of hydrolysate for fermentation study
A seaweed sample (20 g) was treated with diluted seawater to remove excessive pigments, and then soaked in 2% (w/v) sodium hydroxide for 2 h at 80 °C. The coloured solution was removed from the alkaline-treated sample, and the leaned seaweed was washed with 0.25% (w/v) H2SO4 and soaked in 0.5% (w/v) H2SO4 for 3 h in room temperature to ensure removal of the alkaline agent. The seaweed was washed again and treated with 200 mL of 2.5% (w/v) H2SO4 for 40 min in an autoclave at 121 °C. The acid hydrolysate was subjected to over-liming. For that, the seaweed hydrolysate was filtered using Whatman filtre paper no. 1, followed by adding calcium hydroxide with stirring, until the pH of the hydrolysate reached to 11 and kept for 30 min at 30 °C. The samples were then immediately centrifuged and the pH was adjusted to 6 using concentrated H2SO4 (Yadav et al. 2011; Hessami et al. 2018b).
Monosaccharide profile by gas chromatography (GC)
The cleaned G. elegans biomass was hydrolysed with 2M TFA at 120 °C for 1 h. The supernatant was collected and derived to alditol acetate compounds (Kim et al. 1967). Gas chromatography analysis of the sugar molecules was performed with an HP-7820A gas chromatograph, Agilent, USA, equipped with a flame ionisation detector (FID), using a fused silica capillary column (30 m × 0.32 mm) as per the procedure of Kim et al. (1967). Monosaccharides such as galactose, glucose, fucose, rhamnose, mannose, arabinose and xylose were used as standards, and allose used as internal standard.
Analysis of bioethanol by GC
Frozen samples were defrosted and centrifuged for 10 min at 10,000×g. The clear brown supernatant was collected and 200 µL of each sample was added to 1800 µL of solvent mixture (1% w/v, iso-butanol in acetonitrile), shaken vigorously for 15 s, followed by centrifugation at 5000×g for 3 min at 5 °C. The bioethanol concentration was analysed by GC (Agilent 5820-A, Agilent Inc., USA) equipped with a split/splitless inlet, a flame ionisation detector (FID) and a capillary column (HP-Innowax 30 m, 0.32 mm, 0.15 µm) according to the procedure of Lin et al. (2014).
A standard curve of ethanol was plotted using different levels of ethanol concentration (0.01–5% w/v). A standard curve was prepared for different ethanol concentrations and the amount of ethanol was corrected by internal standard value according to the equation below:
| 1 |
The yield of ethanol was calculated using
| 2 |
where YEtOH is the yield of ethanol (g/g), [EtOH]max the maximum ethanol titre attained during fermentation (g/L), and [Sugar]ini is the total initial fermentable sugar equivalent to both glucose and galactose contents (g/L).
For evaluating the accuracy of sample preparation method and standard curve using solvent mixture, three ethanol concentrations (1.05, 0.55 and 0.30%, w/v) were prepared (as known concentration in Table 2) and the amount of ethanol concentration was calculated based on the standard curve; the variation of concentration was defined as error% and extracted by
| 3 |
Table 2.
Evaluating the solvents mixture method using three known bioethanol concentration samples
| Known sample conc. (w/v) | Calculated conc. (w/v) | Error (%) | |
|---|---|---|---|
| (i) | 1.050a | 1.068 ± 0.043c | 1.745a |
| (ii) | 0.550b | 0.546 ± 0.019a | 0.739b |
| (iii) | 0.300c | 0.299 ± 0.012b | 0.460c |
Values are represented as mean ± SD. Values not sharing a similar superscript within the same column are significantly different (p < 0.05) as determined by ANOVA
Data were represented as mean ± standard error (SE), and the differences in results were tested by analysis of variance. Standard curve was prepared by 12 different ethanol concentrations, and ethanol concentration was corrected with internal standard value according this equation:
| 4 |
Statistical analysis
The experiments were performed in triplicates and the data were presented as the average of mean of three independent experiments with standard deviation. The data were analysed by one-way analysis of variance using Microsoft Excel XP (Microsoft Corp., Redmond, WA, USA), and the post hoc mean separation was performed by Duncan’s multiple test at p < 0.05 (Harter 1960).
Results and discussion
Monosaccharide composition by GC
The composition of monosaccharide in the seaweed species is presented in Table 1. The glucose, galactose, mannose and xylose were detected in the sample. The major reducing sugars such as galactose (0.243 g/g) and glucose (0.238 g/g) were found in G. elegans. In the previous studies, the galactose content in G. amansii was reported as 0.23–0.39 g/g (Meinita et al. 2013). Xylose and mannose were detected in very lower content, whereas arabinose and rhamnose were not detected in G. elegans. Park et al. (2012) reported that only galactose and glucose were detected in G. amansii but no other sugars, including mannose, rhamnose or xylose were detected. Song et al. (2010) reported that the monosaccharide composition of Bryopsis sp. such as galactose (2.38–43%), arabinose (4.36–31%), and glucose (4.62–90.30%) of total sugars was varied in different samples.
Table 1.
Monosaccharide composition of hydrolysate of G. elegans
| Monosaccharides | (g/g, DW) |
|---|---|
| Glucose | 0.238 ± 0.013b |
| Galactose | 0.243 ± 0.009a |
| Mannose | 0.005 ± 0.001d |
| Xylose | 0.010 ± 0.002c |
| Arabinose | ND |
| Rhamnose | ND |
Values are represented as mean ± SE
Values not sharing a similar superscript within the same column are significantly different (p < 0.05) as determined by ANOVA
ND not detected, DW dry weight
Seaweed hydrolysis by dilute acid treatment
The G. elegans is an important agarophyte seaweed that has been reported to have soft tissue due to lack of lignin and crystalline cellulose (Park et al. 2012; Kim et al. 2015), allowing a milder treatment to be applied for converting their polysaccharides into fermentable sugars. Figure 1a–d shows the reducing sugar content obtained by dilute acid treatment of G. elegans biomass. Figure 1a shows seaweed hydrolysed at 80 °C where reducing sugar content was increased with increasing acid concentration at different time intervals. Therefore, reducing sugar content (21.48 ± 5.56% DW) was found high in 10% (w/v) sulphuric acid treatment with 60-min incubation. Similarly, the same pattern was observed in hydrolysis of seaweed at 100 °C, the maximum reducing sugar content (26.20 ± 0.69% DW) was observed in 10% acid concentration for 60-min incubation (Fig. 1b). Figure 1c shows hydrolysis of G. elegans at 120 °C where highest sugar content (40.52 ± 6.49% DW) was found after 20 min and in 10% sulphuric acid. However, significant differences (p ≤ 0.05) was not observed in 5% acid treatment at 20 min, 2.5% acid treatment at 40 min, 5% acid treatment at 40 min, 10% acid treatment at 40 min and also 5% acid treatment at 60 min. Further, the reducing sugar content was not increased at 140 °C incubation temperature (Fig. 1d).
Fig. 1.
Reducing sugar content obtained under different conditions during thermal-acidic treatment of G. elegans. Sample treatment at 80 °C (a); 100 °C (b); 120 °C (c); and 140 °C (d)
In the present study, we optimised dilute acid treatment to find the suitable condition of monosaccharide production from seaweed biomass. Based on high content of total carbohydrate and reduced sugar, G. elegans is selected for biofuel applications. The hydrolysate form this seaweed, which is mainly due to degradation of agar, consists of 3,6-anhydro-l-galactose and galactose where the latter cannot be metabolised by common microorganisms, thus precluding it from producing ethanol (Yun et al. 2014).
The hydrolysis kinetic factor depends on the biomass concentration, incubation time, acid concentration and temperature (Lenihan et al. 2010). G. elegans was hydrolysed at different acid concentrations, temperatures, and time intervals. Based on the results of this experiment, the condition of 2.5% w/v H2SO4 at 120 °C for 40-min incubation was selected as the suitable condition, which is regarded as milder, but still effective. The hydrolysis treatment yielded 39.42% on the dry weight basis from the G. elegans. Previously, Meinita et al. (2013) reported that the sugar yield 30.5 g/L with 25.6 g/L galactose and the bioethanol yield (1.7 g/L) were observed in K. alvarezii by acid hydrolysis treatment with condition 0.2 M sulphuric acid at 130 °C for 15-min incubation. Another study by Khambhaty et al. (2012) reported the sugar yield of 30.6% on the dry weight basis in K. alvarezii with 2.5% w/v sulphuric acid at 100 °C for 60-min incubation. In another study, G. salicornia was incubated in 120 °C with 2% w/v sulphuric acid at 30-min incubation (Wang et al. 2011).
Higher sugar yield (38.09%) and lower fermentation inhibitors were obtained in G. amansii with continuous acid hydrolysis treatment in a reactor (Park et al. 2012). Despite the fact that acid hydrolysis is an inexpensive process compared to enzymatic process, the generation of fermentation inhibitors is one of its main drawbacks (Taherzadeh and Karimi 2007). Generally, the sugar yield will decrease with increase in the hydrolysis processing time due to the degradation of fermentation inhibitors such as 5-HMF and levulinic acid (Ra et al. 2013).
Bioethanol fermentation using S. cerevisiae
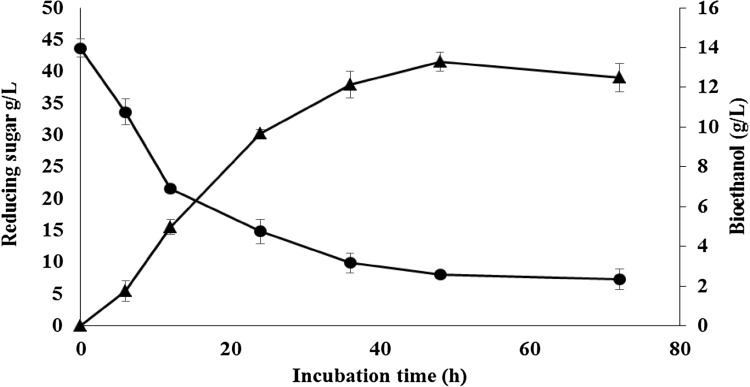
The production of ethanol in relation to consumption of reducing sugar at different incubation time intervals during the fermentation of hydrolysates of G. elegans by S. cerevisiae is shown in Fig. 2. Whereas initial reducing sugar concentration hydrolysate was 43.69 ± 1.41 g/L and maximum ethanol production (13.27 ± 0.47 g/L) was achieved at 48 h corresponding to 63.30% of theoretical fermentation yield, the reducing sugar in G. elegans hydrolysate was not fully consumed after 72 h and it was 7.34 ± 1.63 g/L. Previously, higher bioethanol concentration (55.0 g/L) was produced from G. elegans by Yanagisawa et al. (2011). In that study, they used both chemical and enzymatic approaches to produce high-concentrated reducing sugar.
Fig. 2.
Fermentation with dilute acid hydrolysate of G. elegans hydrolysate using, S. cerevisiae
In fermentation of seaweed hydrolysates, yeast or bacteria consume reduced sugar to yield bioethanol under an anaerobic condition. S. cerevisiae has been proven to be highly vigorous and well fitted for conversion of reducing sugars in cellulosic hydrolysates into bioethanol (Galbe and Zacchi 2002). Park et al. (2012) used Brettanomyces custersii to ferment Gelidium amansii and bioethanol concentration of 27 g/L was reported while Ra et al. (2013) used Scheffersomyces stipites to ferment the same seaweed species and achieved 20 g/L bioethanol concentration; however, the yield of fermentation was highly different with 38% and 91%, respectively. S. cerevisiae was used to convert G. amansii hydrolyzate, producing 0.66 g/L ethanol, with a fermentation efficiency of only 12% of theoretical yield (Meinita et al. 2013). In the present study, the ethanol production rate and fermentation efficiency were low, due to the presence of the galactose as the main reducing sugar in the dilute acid hydrolysate, and the fact that ethanol yield from galactose is lower than glucose (Hong et al. 2011; Lee et al. 2011). d-Galactose undergoes conversion via the Leloir pathway; basically, in this pathway, a five-step enzymatic pathway converts d-galactose to glucose-6-phosphate (Timson 2007). All these processes lead to higher energy consumption in galactose metabolism, thus lower fermentation yield compared with glucose.
Analysis of bioethanol with novel sample preparation
In this study, a mixture of two solvents, acetonitrile and iso-butanol, was used. In GC analysis, these two solvents peaks can be separated from the ethanol’s peak (Canfield et al. 1998). Figure 3a, b shows the effect of solvent mixture (acetonitrile/iso-butanol) on a sample by which adding the solvent mixture to samples which caused a precipitation of the water-soluble complex organic compounds (Fig. 3c) and followed by centrifugation, water-soluble compounds become precipitated at the bottom of the vial and a clear yellowish supernatant was achieved (Fig. 3d). This clear supernatant would be safely injected into the GC and the GC chromatogram of this treated sample is illustrated in Fig. 4. Ethanol is the first detected in GC after 2.30 min, followed by acetonitrile (2.66 min) and internal standard, iso-butanol (3.06 min). For evaluating the accuracy of this sample preparation method, triplicate samples of three known ethanol concentrations were prepared and the errors (%) were calculated (Table 2). A high accuracy was achieved using this method, whereas the lowest error (0.460%) was observed in sample with ethanol concentration of 0.3% w/v. Maximum error (1.765%) was found in sample with 1.050% w/v ethanol content.
Fig. 3.
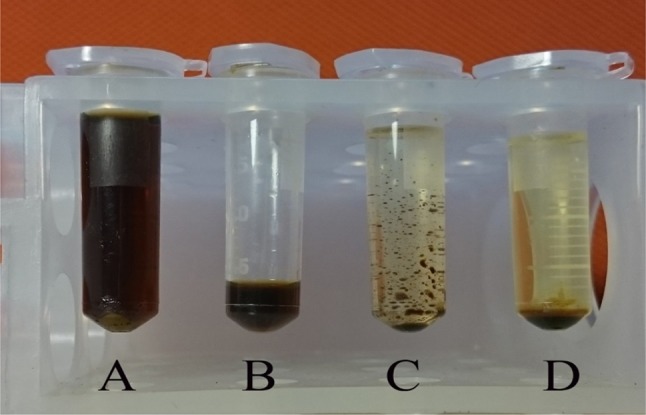
The steps involved in sample preparation by iso-butanol/acetonitrile solvent mixture. Centrifuged fermented sample (a), 200 µL of clear supernatant was transferred to new tube (b), 1800 µL of solvent mixture was added to tube (c), mixture of sample and solvent was centrifuged for by 10,000×g (d)
Fig. 4.
The GC analysis of ethanol (a), acetonitrile (b), and iso-butanol (c)
Regardless of the developments in GC techniques, injecting aqueous samples for GC analysis is a topic of great interest, since the presence of water is hazardous to not only gas chromatograph machine and capillary column, but it is also capable of interrupting the results of the analysis. Minimisation of labour cost and time consumption of sample preparation is desired (Santos and Galceran 2002). For achieving fast, simple and solvent-free operations, an effective, robust, reliable sample preparation needs to be developed. The main issue with water in GC samples is its large expansion volume. It starts in the injector, where the samples are vaporised so that the analyses can be swept into the column by the carrier gas, a problem known as back-flash (Kuhn 2002). Another concern regarding the presence of high amount of water in the samples is the interacting and bonding with the stationary phase, consequently reducing the capillary column efficiency (de Zeeuw and Luong 2002). The presence of water-soluble compounds in fermented samples such as plant pigments, proteins, lipids (Palmqvist and Hahn-Hagerdal 2000; Hou et al. 2015) and fermentation additives (yeast extract, meat peptone, vitamins, enzymes, etc.) are matters of concern in the context of GC analysis. Generally, these compounds are non-volatile and are retained in GC units, mostly the injection chamber, column, and even detectors.
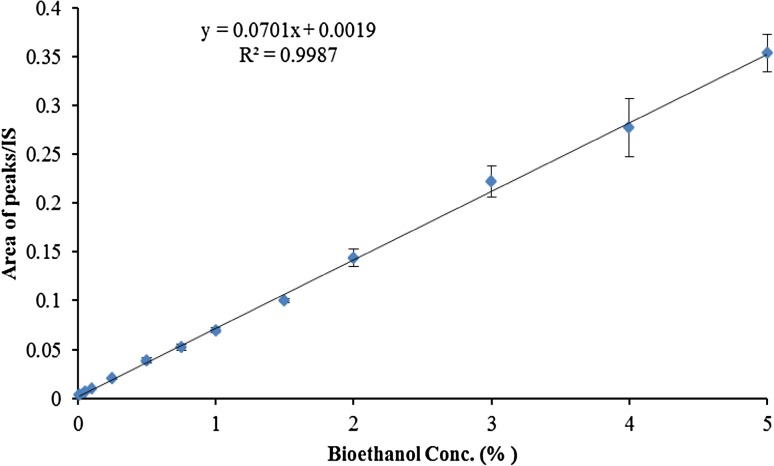
Applying this method resulted in the reduction of water content to 10% of its initial content. Thus, with 1 µL sample injection and a split ratio of 100:1 in split/splitless injector, a maximum amount of 1 nL of water could enter the injection chamber and capillary column, which is ten times lower compared to direct sample injection. Also, applying this simple approach, fewer unwanted compounds would pass through the GC machine, including the path of injection part, column, and detector. Thus, it would lead to the increased life-span of capillary column and maintenance of the system’s cleaner over injections. Last but not least, the accuracy of measuring ethanol by this method is within an acceptable range of R2 in a standard plot (Fig. 5).
Fig. 5.
The standard curve of ethanol (data presented as mean ± SD: n = 4)
Conclusion
The agarophyte G. elegans has potential for use in bioethanol production. However, in terms of economic viability, more improvement in the fermentation technology must be achieved to make it an industrially feasible feedstock for bioethanol production. A novel approach to sample preparation for analysing ethanol in the fermented sample was evaluated successfully in the present study, which enhances the accuracy of measurement and increased the life-span of capillary column and gas chromatograph components. The results showed that applying this method might gain several advantages for analysis of bioethanol content in the fermented mash by GC equipped with a capillary column. This advantage is based on the decrease in amount of water that passes through the capillary column, while still keeping the accuracy of measurement, and also based on decreasing the organic nutrients in the gas chromatographic samples by sedimentation approach.
Acknowledgements
Authors acknowledge University of Malaya for providing University of Malaya Research Grant (Project no: PV026-2012), GC002B-15SBS and HICoE Grant IOES-2014F. We are grateful to the Algae Research Laboratory, University of Malaya members, specifically my good friends Dr. Bahram Barati, Dr. Vejeysri Vello and Mr. Hamed Nassrolahi who assisted us in this research work.
Abbreviations
- GC
Gas chromatography
- CO2
Carbon dioxide
- NITE
National institute of technology evaluation
- YPD
Yeast potato dextrose
- CFU
Colony-forming unit
- H2SO4
Sulphuric acid
- FID
Flame ionisation detector
- EtOH
Ethanol
- Conc
Concentration
- ND
Not detected
- DW
Dry weight
Compliance with ethical standards
Conflict of interest
Authors declare no conflict of interest.
References
- Adams JM, Gallagher JA, Donnison IS. Fermentation study on Saccharina latissima for bioethanol production considering variable pre-treatments. J Appl Phycol. 2009;21(5):569–574. doi: 10.1007/s10811-008-9384-7. [DOI] [Google Scholar]
- Canfield DV, Smith MD, Adams HJ, Houston ER. Selection of an Internal Standard for postmortem ethanol analysis. Oklahama: Civil Aeromedical Institute, Federal Aviation Administration; 1998. [Google Scholar]
- Chia SR, Chew KW, Show PL, Yap YJ, Ong HC, Ling TC, Chang JS. Analysis of economic and environmental aspects of microalgae bio-refinery for biofuels production: a review. Biotechnol J. 2018;13(6):1700618. doi: 10.1002/biot.201700618. [DOI] [PubMed] [Google Scholar]
- De Zeeuw J, Luong J. Developments in stationary phase technology for gas chromatography. Trends Anal Chem. 2002;21(9):594–607. doi: 10.1016/S0165-9936(02)00809-9. [DOI] [Google Scholar]
- Galbe M, Zacchi G. A review of the production of ethanol from softwood. Appl Microbiol Biotechnol. 2002;59(6):618–628. doi: 10.1007/s00253-002-1058-9. [DOI] [PubMed] [Google Scholar]
- Harter LN. Critical values for Duncan’s new multiple range test. Biometrics. 1960;16:671–685. doi: 10.2307/2527770. [DOI] [Google Scholar]
- Hessami MJ, Phang SM, Salleh A, Rabiei R. Evaluation of tropical seaweeds as feedstock for bioethanol production. Int J Environ Sci Technol. 2018;15(5):977–992. doi: 10.1007/s13762-017-1455-3. [DOI] [Google Scholar]
- Hessami MJ, Aishah S, Phang SM. Bioethanol a by-product of agar and carrageenan production industry from the tropical red seaweeds, Gracilaria manilaensis and Kappaphycus alvarezii. Iran J Fish Sci. 2018 doi: 10.22092/ijfs.2018.117104. [DOI] [Google Scholar]
- Hong KK, Vongsangnk W, Vemuri GN, Nielsen J. Unravelling evolutionary strategies of yeast for improving galactose utilization through integrated systems level analysis. Proc Natl Acad Sci. 2011;108(29):12179–12184. doi: 10.1073/pnas.1103219108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Hansen JH, Bjerre AB. Integrated bioethanol and protein production from brown seaweed Laminaria digitata. Bioresour Technol. 2015;197:310–317. doi: 10.1016/j.biortech.2015.08.091. [DOI] [PubMed] [Google Scholar]
- Khambhaty Y, Mody K, Gandhi MR, Thampy S, Maiti P, Brahmbhatt H, Eswaran K, Pushpito K, Ghosh PK. Kappaphycus alvarezii as a source of bioethanol. Bioresour Technol. 2012;103(1):180–185. doi: 10.1016/j.biortech.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Kim JH, Shome B, Liao TH, Pierce JG. Analysis of neutral sugars by gas liquid chromatography of alditol acetates: application to thyrotropic hormone and other glycoproteins. Anal Biochem. 1967;20(2):258–274. doi: 10.1016/0003-2697(67)90031-0. [DOI] [PubMed] [Google Scholar]
- Kim HM, Wi SG, Jung S, Song Y, Bae HJ. Efficient approach for bioethanol production from red seaweed Gelidium amansii. Bioresour Technol. 2015;175:128–134. doi: 10.1016/j.biortech.2014.10.050. [DOI] [PubMed] [Google Scholar]
- Kuhn ER. Water injections in GC how wet can you get? LCGC Asia Pac. 2002;5(3):30–32. [Google Scholar]
- Lee KS, Hong ME, Jung SC, Ha SJ, Yu BJ, Koo HM, Park SM, Seo JH, Kweon DH, Park JC, Jin YS. Improved galactose fermentation of Saccharomyces cerevisiae through inverse metabolic engineering. Biotechnol Bioeng. 2011;108(3):621–631. doi: 10.1002/bit.22988. [DOI] [PubMed] [Google Scholar]
- Lenihan P, Orozco A, O’neill E, Ahmad M, Rooney D, Walker G. Dilute acid hydrolysis of lignocellulosic biomass. Chem Eng J. 2010;156(2):395–403. doi: 10.1016/j.cej.2009.10.061. [DOI] [Google Scholar]
- Lin X, Fan J, Wen Q, Li R, Jin X, Wu J, Qian W, Liu D, Xie J, Bai J, Ying H. Optimization and validation of a GC–FID method for the determination of acetone-butanol-ethanol fermentation products. J Chromatogr Sci. 2014;52(3):264–270. doi: 10.1093/chromsci/bmt022. [DOI] [PubMed] [Google Scholar]
- Maranduba HL, Robra S, NascimentoI A, da Cruz RS, Rodrigues LB, de Almeida Neto JA. Reducing the life cycle GHG emissions of microalgal biodiesel through integration with ethanol production system. Bioresour Technol. 2015;194:21–27. doi: 10.1016/j.biortech.2015.06.113. [DOI] [PubMed] [Google Scholar]
- Meinita MDN, Marhaeni B, Winanto T, Jeong GT, Khan MNA, Hong YK. Comparison of agarophytes (Gelidium, Gracilaria, and Gracilariopsis) as potential resources for bioethanol production. J Appl Phycol. 2013;25(6):1957–1961. doi: 10.1007/s10811-013-0041-4. [DOI] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Palmqvist E, Hahn-Hagerdal B. Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour Technol. 2000;74(1):25–33. doi: 10.1016/S0960-8524(99)00161-3. [DOI] [Google Scholar]
- Park JH, Hong JY, Jang HC, Oh SG, Kim SH, Yoon JJ, Kim YJ. Use of Gelidium amansii as a promising resource for bioethanol: a practical approach for continuous dilute-acid hydrolysis and fermentation. Bioresour Technol. 2012;108:83–88. doi: 10.1016/j.biortech.2011.12.065. [DOI] [PubMed] [Google Scholar]
- Ra CH, Jeong GT, Shin MK, Kim SK. Biotransformation of 5-hydroxymethylfurfural (HMF) by Scheffersomyces stipitis during ethanol fermentation of hydrolysate of the seaweed Gelidium amansii. Bioresour Technol. 2013;140:421–425. doi: 10.1016/j.biortech.2013.04.122. [DOI] [PubMed] [Google Scholar]
- Salles-Filho SLM, de Castro PFD, Bin A, Edquist C, Ferro AFP, Corder S. Perspectives for the Brazilian bioethanol sector: the innovation driver. Energy Policy. 2017;108:70–77. doi: 10.1016/j.enpol.2017.05.037. [DOI] [Google Scholar]
- Santos FJ, Galceran MT. The application of gas chromatography to environmental analysis. Trends Anal Chem. 2002;21(9):672–685. doi: 10.1016/S0165-9936(02)00813-0. [DOI] [Google Scholar]
- Searchinger T, Heimlich R, Houghton RA, Dong F, Elobeid A, Fabiosa J, Tokgoz S, Hayes D, Yu TH. Use of US croplands for biofuels increases greenhouse gases through emissions from land-use change. Science. 2008;319(5867):1238–1240. doi: 10.1126/science.1151861. [DOI] [PubMed] [Google Scholar]
- Song H, Zhang Q, Zhang Z, Wang J. In vitro antioxidant activity of polysaccharides extracted from Bryopsis plumosa. Carbohydr Polym. 2010;80(4):1057–1061. doi: 10.1016/j.carbpol.2010.01.024. [DOI] [Google Scholar]
- Sudhakar K, Mamat R, Samykano M, Azmi WH, Ishak WFW, Yusaf T. An overview of marine macroalgae as bioresource. Renew Sustain Energy Rev. 2018;91:165–179. doi: 10.1016/j.rser.2018.03.100. [DOI] [Google Scholar]
- Taherzadeh MJ, Karimi K. Acid-based hydrolysis processes for ethanol from lignocellulosic materials: a review. BioResources. 2007;2(3):472–499. [Google Scholar]
- Timson DJ. Galactose metabolism in Saccharomyces cerevisiae. Dyn Biochem Process Biotechnol Mol Biol. 2007;1(1):63–73. [Google Scholar]
- Vuppaladadiyam A, Yao JG, Florin N, George A, Wang X, Labeeuw L, Jiang Y, Davis RW, Abbas A, Ralph P, Fennell PS, Zhao M. Impact of flue gas compounds on microalgae and mechanisms for carbon assimilation and utilization. ChemSusChem. 2018;11(2):334–355. doi: 10.1002/cssc.201701611. [DOI] [PubMed] [Google Scholar]
- Wang X, Liu X, Wang G. Two stage hydrolysis of invasive algal feedstock for ethanol fermentation. J Integr Plant Biol. 2011;53(3):246–252. doi: 10.1111/j.1744-7909.2010.01024.x. [DOI] [PubMed] [Google Scholar]
- Wi SG, Kim HJ, Mahadevan SA, Yang DJ, Bae HJ. The potential value of the seaweed Ceylon moss (Gelidium amansii) as an alternative bioenergy resource. Bioresour Technol. 2009;100(24):6658–6660. doi: 10.1016/j.biortech.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Yadav KS, Naseeruddin S, Prashanthi GS, Sateesh L, Rao LV. Bioethanol fermentation of concentrated rice straw hydrolysate using co-culture of Saccharomyces cerevisiae and Pichia stipitis. Bioresour Technol. 2011;102(11):6473–6478. doi: 10.1016/j.biortech.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Nakamura K, Ariga O, Nakasaki K. Production of high concentrations of bioethanol from seaweeds that contain easily hydrolyzable polysaccharides. Proc Biochem. 2011;46:2111–2116. doi: 10.1016/j.procbio.2011.08.001. [DOI] [Google Scholar]
- Yoon MH, Lee YW, Lee CH, Seo YB. Simultaneous production of bio-ethanol and bleached pulp from red algae. Bioresour Technol. 2012;126:198–201. doi: 10.1016/j.biortech.2012.08.102. [DOI] [PubMed] [Google Scholar]
- Yun EJ, Lee S, Kim HT, Pelton JG, Kim S, Ko HJ, Choi G, Kim KH. The novel catabolic pathway of 3,6-anhydro-l-galactose, the main component of red macroalgae, in a marine bacterium. Environ Microbiol. 2014;17(5):1677–1688. doi: 10.1111/1462-2920.12607. [DOI] [PubMed] [Google Scholar]