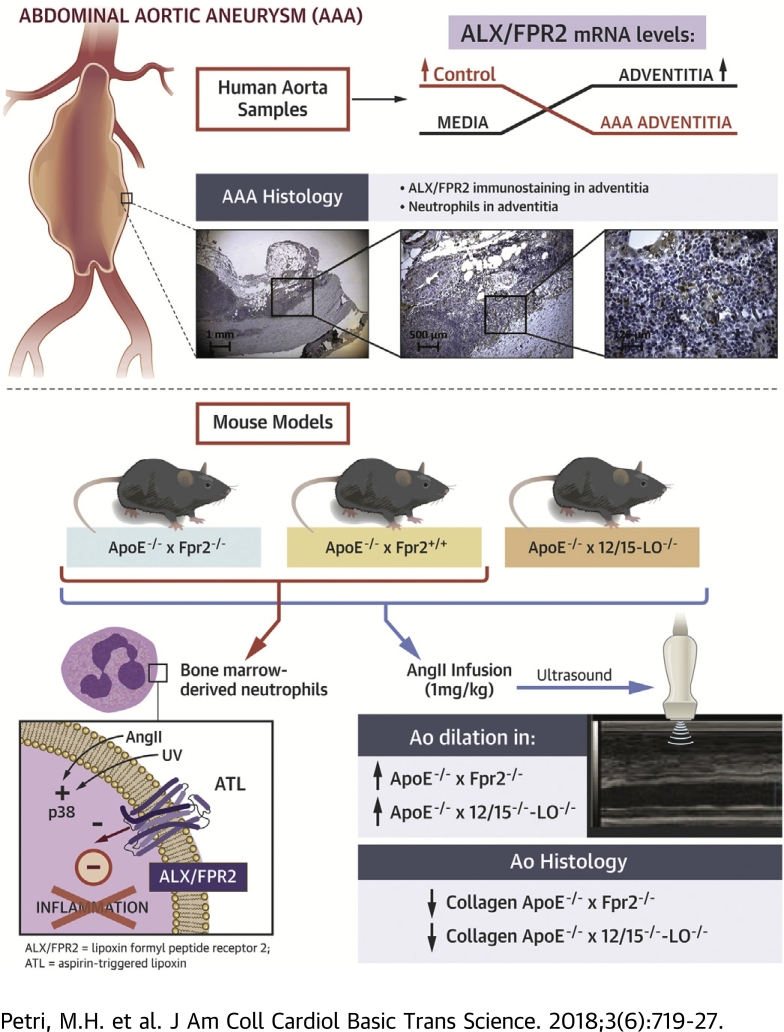
Visual Abstract
Key Words: abdominal aortic aneurysms, cardiovascular disease, eicosanoids, inflammation, lipoxygenase
Abbreviations and Acronyms: AAA, abdominal aortic aneurysm; ALX/FPR2, A lipoxin/formyl peptide receptor 2; Ang II, angiotensin II; ATL, aspirin-triggered lipoxin; LO, lipoxygenase; LX, lipoxin; Rv, resolvin; SPM, specialized pro-resolving mediators
Highlights
-
•
Specialized lipid mediators transduce the resolution of inflammation by means of the ALX/FPR2.
-
•
Human AAA exhibited decreased ALX/FPR2 expression.
-
•
Genetic disruption of the murine ALX/FPR2 ortholog exacerbated AAA and increased inflammation.
-
•
The ALX/FPR2 agonist ATL induced pro-resolving signaling in bone marrow-derived murine cells.
-
•
Pro-resolving signaling by means of the ALX/FPR2 receptor may decrease the progression of AAA.
Summary
An abdominal aortic aneurysm (AAA) is a progressive aortic dilation that may lead to rupture, which is usually lethal. This study identifies the state of failure in the resolution of inflammation by means of decreased expression of the pro-resolving receptor A lipoxin/formyl peptide receptor 2 (ALX/FPR2) in the adventitia of human AAA lesions. Mimicking this condition by genetic deletion of the murine ALX/FPR2 ortholog in hyperlipidemic mice exacerbated the aortic dilation induced by angiotensin II infusion, associated with decreased vascular collagen and increased inflammation. The authors also identified key roles of lipoxin formation through 12/15-lipoxygenase and neutrophil p38 mitogen-activated protein kinase. In conclusion, this study established pro-resolving signaling by means of the ALX/FPR2 receptor in aneurysms and vascular inflammation.
Abdominal aortic aneurysm (AAA) is characterized by a progressive aortic dilation and weakening of the vascular wall (1). Biomechanical factors contribute to both the initiation and the progression of AAA and may eventually also provoke an aortic rupture, which most commonly is fatal (2). AAA most often originates in atherosclerotic segments of the aorta (1). Morphologically, the aneurysmal aortic wall is characterized by loss of smooth muscle cells and remodeling of the extracellular matrix (3). In addition, inflammatory infiltrates overlook the aneurysmal segments from both sides of the aortic wall, with a strong component of adventitial inflammation as well as a key inflammatory activity in the intraluminal thrombus commonly covering the AAA lesions (1).
Typical neutrophil chemoattractants, such as interleukin-8, RANTES (regulated on activation, normal T-cell expressed and secreted), and the lipid mediator leukotriene B4, as well as a number of neutrophil-derived proteolytic enzymes (e.g., matrix metalloproteinases, neutrophil elastase, myeloperoxidase) are closely related to AAA expansion, and those findings have underlined the key role of neutrophils in this disease 4, 5, 6. Importantly, neutrophil recruitment at a site of inflammation is not only regulated by stimulatory factors but can also be actively terminated by specific “stop signals” mediating a resolution of inflammation. Initially considered a passive phenomenon, the resolution of inflammation is now recognized as an active process mediated by specialized pro-resolving mediators (SPM) (7).
Lipoxins (LX) and resolvins (Rv) are pro-resolving lipid mediators that limit neutrophil infiltration by means of promoting, for example, phagocytosis-induced neutrophil apoptosis and egress of neutrophils from sites of inflammation (7). The A lipoxin and formyl peptide receptor 2 (ALX/FPR2) ligates LXA4 (8), aspirin-triggered LX (ATL) (9), and resolvin D1 (RvD1) (10) to transduce their pro-resolving effects. Also, this receptor is activated by amyloidogenic and antibacterial peptides (11) and the pro-resolving protein annexin A1 (12). Previous studies indicate that, in the absence of SPMs (i.e., in a state of failure in the resolution of inflammation), signaling through the ALX/FPR2 receptor may be predominantly pro-inflammatory (13). The authors recently generated apolipoprotein E (ApoE)-deficient mice, which also lack the murine ortholog of the human ALX/FPR2 receptor termed formyl peptide receptor 2 (Fpr2). These mice are insensitive to the antiatherogenic effects of ATL infusion observed in ApoE−/−×Fpr2+/+ mice, indicating beneficial pro-resolving signaling through ALX/FPR2 in experimental atherosclerosis (14). However, the effects of genetic deletion of this receptor in a model of AAA have not previously been explored.
A recent mass spectrometry lipidomics analysis revealed that LXA4, ATL, and RvD1 are increased in patients undergoing surgical AAA repair (15). In addition, D-series Rvs inhibit aortic dilation in experimental murine models of AAA formation (16). However, the mechanisms mediating those effects remain unknown. The present study therefore attempted to decipher the role of potential pro-resolving signaling by means of ALX/FPR2 in angiotensin II (Ang II)-induced aortic dilation in hyperlipidemic mice and to provide the translational implications of these findings in samples derived from patients undergoing surgery for AAA.
Methods
For an expanded Methods section, please see the Supplemental Material.
Human abdominal aneurysmal tissue and microarray analysis
For gene expression studies, human aortic samples of control aortas derived from organ donors and aneurysmal aortas derived from patients undergoing elective open surgery for AAA were obtained. Aortic tissues were divided into intimal/medial parts and an adventitial part and stratified according to presence or absence of an intraluminal thrombus. Tissue RNA was extracted, and gene expression analysis was performed for 76 thrombus-covered AAA walls, 34 nonthrombus-covered walls, and 13 control aortas, by using HTA 2.0 Genechip arrays (Affymetrix, Santa Clara, California) as described in the Supplemental Material. The study was approved by the Regional Ethical Review Board in Stockholm (Dnr 2009/9-31/4, Dnr 2013/615-31/:4).
Animals
ApoE−/−×Fpr2−/− mice were generated as previously described (14). ApoE−/−×12/15-lipoxygenase (12/15-LO)−/− mice were a gift from Dr. Stephen Malin (Karolinska Institutet, Stockholm, Sweden). Experimental protocols are described in the Supplemental Material. All experiments were performed according to animal regulation and guidelines of Karolinska Institutet and were approved by the local ethics committee (permits N138/12 and N646/12).
Results
Human aortic ALX/FPR2 expression predominates in the adventitia and is decreased in abdominal aortic aneurysms
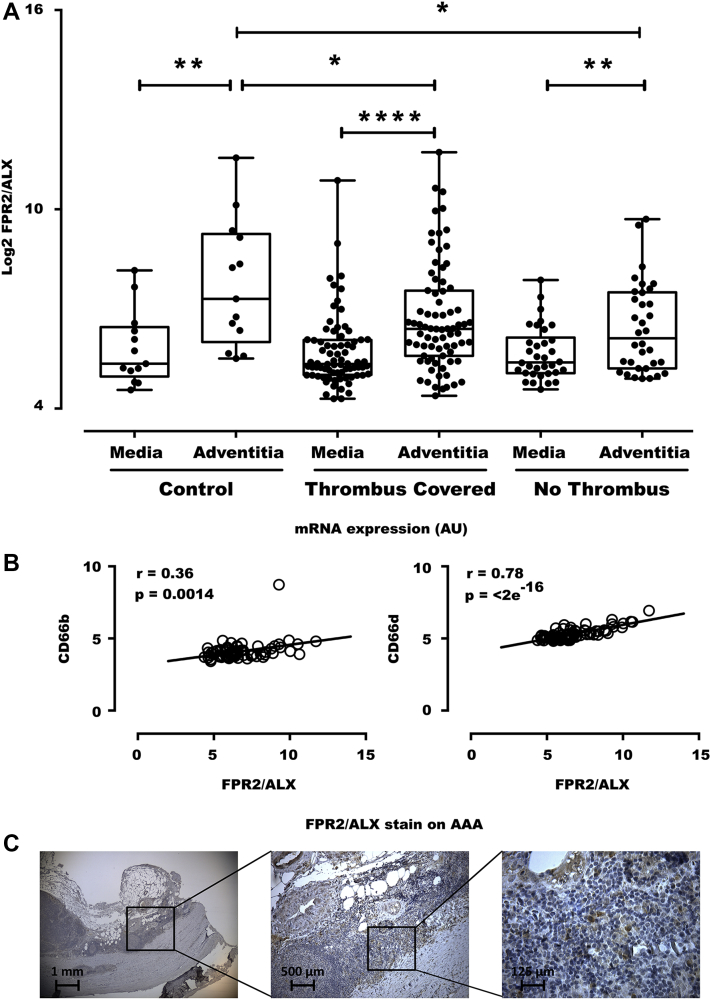
In human aortic samples, significantly higher mRNA levels of ALX/FPR2 were observed in the adventitia than in the media (Figure 1A). Furthermore, these adventitial ALX/FPR2 levels were significantly lower in the adventitia derived from patients with abdominal aortic aneurysms than in adventitia from healthy aortae, both in the presence and in the absence of a thrombus covering the aneurysmal lesion (Figure 1A, Table 1). There was a strong and highly significant correlation between ALX/FPR2 expression and expression levels of the neutrophil markers CD66b and CD66d in AAA samples (n = 76) (Figure 1B). Immunohistochemical analysis confirmed predominant adventitial ALX/FPR2 expression in human abdominal aortic aneurysms (Figure 1C). Although the adventitia was populated mainly by mononuclear cells, polymorphonuclear cells that were positive for ALX/FPR2 were also identified (Figure 1C).
Figure 1.
Decreased Adventitial ALX/FPR2 Expression in Human AAA
(A) ALX/FPR2 expression in the media and adventitia derived from 13 control aortas from organ donors, 76 thrombus-covered AAA, and 34 nonthrombus-covered AAA segments. (B) Correlations between the neutrophil markers CD66b and CD66d in AAA samples (n = 76). (C) Immunohistochemical analysis of ALX/FPR2 revealed predominant adventitial expression in AAA samples, and at higher magnification, polymorphonuclear cells positive for ALX/FPR2 were identified. AAA = abdominal aortic aneurysms.
Table 1.
Clinical Characteristics of Aortic Tissue Donors
| AAA (n = 76) |
Controls (n = 13) |
p Value | |
|---|---|---|---|
| Age, yrs | 69 (65–75) | 53 (44–68) | <0.001 |
| Females | 16 (21) | 6 (46) | 0.08 |
| Current/previous/never smoker, % | 31/45 (41/59) | 3/10 (23/77) | 0.36 |
| Aneurysm diameter, mm | 64 (58–75) | NA | – |
| Hypertension | 55 (72) | NA | – |
| Stroke | 12 (16) | NA | – |
| Previous myocardial infarction | 17 (22) | NA | – |
| Peripheral arterial disease | 11 (14) | NA | – |
| Diabetes | 5 (7) | NA | – |
| Aspirin | 47 (62) | NA | – |
| Clopidogrel | 2 (3) | NA | – |
| ACE inhibitors | 20 (26) | NA | – |
| Angiotensin II receptor blockers | 14 (18) | NA | – |
| Beta-blockers | 29 (38) | NA | – |
| Statins | 55 (72) | NA | – |
Values are median (Interquartile range) or n (%).
Either Student t-test or Fisher exact test was used to compare continuous and categorical data, respectively.
AAA = abdominal aortic aneurysm; ACE = angiotensin-converting enzyme; NA = not available.
Genetic disruption of the lipoxin receptor Fpr2 or the lipoxin-synthesizing 12/15-lipoxygenase enhances abdominal aortic aneurysms in mice
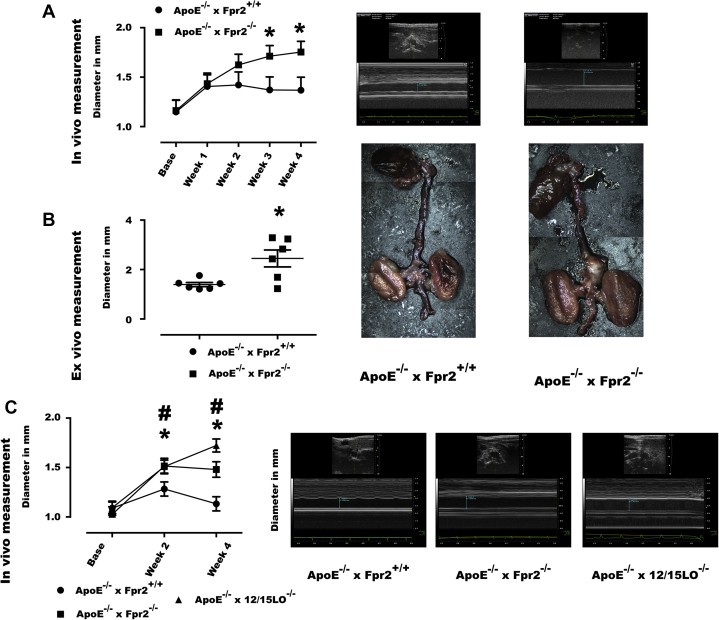
ApoE−/−×Fpr2−/− mice exhibited significantly larger aortic diameters after Ang II infusion than ApoE−/−×Fpr2+/+ mice, as determined by both repeated ultrasonography measurements (Figure 2A) and ex vivo evaluation (Figure 2B). Use of Ang II is also known to induce aortic dissection and rupture. In ex vivo analyses, aortic dissections were observed in 3 of 6 ApoE−/−×Fpr2−/− mice, whereas signs of dissections were observed in none of the 6 ApoE−/−×Fpr2+/+ mice (0 of 6), and this difference was not statistically significant. There were also no significant differences between the 2 groups in terms of cholesterol and triglyceride levels or blood cell counts (Supplemental Table S1). In a second series of experiments, disruption of the 12/15-LO enzyme in ApoE−/− mice, Ang II-induced aortic dilation was observed to an extent similar to that in ApoE−/−×Fpr2−/− mice (Figure 2C).
Figure 2.
Fpr2 and LO Deficiencies Exacerbate Ang II-induced Aortic Dilation
(A) Aortic dimensions in ApoE−/−×Fpr2+/+(circles) and ApoE−/−×Fpr2−/−(squares) mice, implanted with osmotic pumps containing Ang II (1 μg/kg/min). (Left) Ultrasonography measurements of the inner edges of the suprarenal abdominal aorta before implantation (Base) and every week until sacrifice (4 weeks). (Right) Echocardiographic images at week 4. (B) Ex-vivo measurements of aortic dilation from the outer edges of the abdominal segments. (Right) Representative images of each genotype. (C) Ultrasonographic measurements of aortic dimensions in a second series of ApoE−/−×Fpr2+/+(circles), ApoE−/−×Fpr2−/−(squares), and ApoE−/−×12/15LO−/−(triangles) mice treated with Ang II (1 μg/kg/min). Ultrasonography measurements of the inner edges of the suprarenal abdominal aorta before implantation (Base) and every 2 weeks until sacrifice (4 weeks). Representative echocardiographic images show measurements by genotype at week 4. At least n = 6 in each group and graphs represent mean ± SEM. *p < 0.05 of ApoE−/−×Fpr2+/+ versus ApoE−/−×Fpr2−/−; #p < 0.05 of ApoE−/−×Fpr2+/+ and ApoE−/−×12/15LO−/−. Ang II = angiotensin II; ApoE = apolipoprotein E; LO = 12/15-lipoxygenase.
Fpr2 deficiency reduces aortic collagen and increases MMP-9 mRNA levels
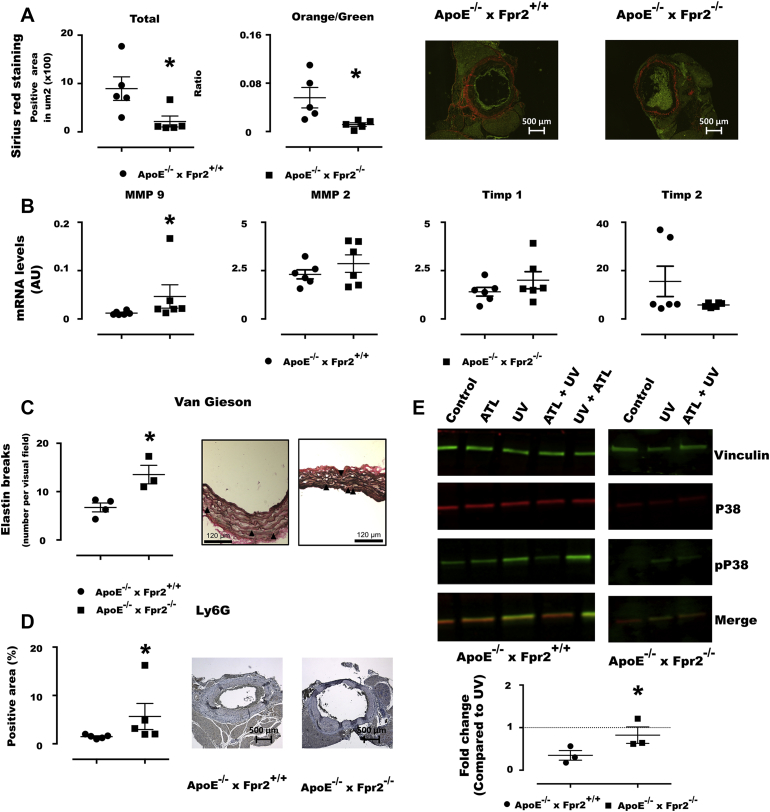
ApoE−/−×Fpr2−/− mice exhibited significantly lower total collagen content with thin collagen fibers than ApoE−/−×Fpr2+/+ mice, and similar results were obtained in ApoE−/−×12/15LO−/− mice (orange [thick] fiber-to-green [thin] fiber ratio of 0.02 ± 0.0099; p > 0.05 vs. ApoE−/−×Fpr2−/− mice). This difference was associated with upregulated MMP-9 in aneurysmal lesions (Figure 3B) and increased elastin breaks in the media of ApoE−/−×Fpr2−/− aortae (Figure 3C).
Figure 3.
Decreased Aortic Collagen and Increased Inflammatory Infiltrates in ApoE−/−×Fpr2−/− Mice and Reduced Neutrophil p38 Phosphorylation by the ALX/FPR2 Agonist ATL
(A) Total collagen of aortic aneurysmal lesions by Sirius red stain and orange (thick fibers)-to-green (thin fibers) ratio in ApoE−/−×Fpr2+/+(circles) and ApoE−/−×Fpr2−/−(squares) implanted with osmotic pumps containing Ang II (1 μg/kg/min). (Right) Micrographs of each genotype. (B) Levels of mRNA for MMP-9, MMP-2, Timp-1, and Timp-2 in aortae derived from either ApoE−/−×Fpr2+/+(circles) or ApoE−/−×Fpr2−/−(squares) mice. Six mice in each group, and graphs represent mean ± SEM. (C) Van Gieson elastic stain shows increased elastin breaks in ApoE−/−×Fpr2−/−. (D) Immunohistochemical quantifications of the neutrophil marker Ly6G in sections from abdominal aortas derived from either ApoE−/−×Fpr2+/+(circles) or ApoE−/−×Fpr2−/−(squares) mice treated with Ang II (1 μg/kg/min). n = 5 in each group, and graphs represent mean ± SEM; *p < 0.05. (E) Western blot analysis of bone marrow-derived neutrophils from either ApoE−/−×Fpr2+/+(filled circles) or ApoE−/−×Fpr2−/−(filled squares) mice. Cells were pretreated in the presence or absence of ATL (100 nM) before exposure to UV-C light to induce p38 phosphorylation. UV-induced p38 phosphorylation was significantly inhibited by pretreatment with ATL (100 nM) before UV exposure in neutrophils derived from ApoE−/−×Fpr2+/+(filled circles) but not in those derived from ApoE−/−×Fpr2−/−(filled squares). (Lower panel) Quantification of phosphorylated p38 in relation to total p38 was compared between UV-treated neutrophils in the absence and presence of ATL. Vinculin was used as a loading control. n = 3 in each group, and graphs represent mean ± SEM; *p < 0.05. ATL = aspirin-triggered lipoxin; AU = arbitrary unit; MMP = matrix metalloproteinase; UV = ultraviolet.
Fpr2 deficiency increases aneurysmal leukocyte infiltration
Significantly higher numbers of both neutrophil granulocytes (Ly6G; p = 0.029) (Figure 3D) and macrophages (Mac-2; p = 0.033 [data not shown]) were quantified by immunohistochemical staining of aneurysmal lesions from ApoE−/−×Fpr2−/− mice compared with ApoE−/−×Fpr2+/+ mice.
The ALX/FPR2 agonist ATL reduces neutrophil p38 phosphorylation
To specifically address the potential suppressive signaling through ALX/FPR2 in neutrophils, p38 phosphorylation was assessed in murine bone marrow-derived neutrophils. Under basal conditions, p38 but not the phosphorylated form of p38 was detected (Figure 3E). UV light exposure induced a readily detectable p38 phosphorylation. Pretreatment of ApoE−/−×Fpr2+/+ neutrophils with ATL (100 nM) before exposure to UV light significantly reduced detection of phosphorylated p38 by more than 80% (Figure 3E). Importantly, the latter treatment did not significantly alter the UV light-induced p38 phosphorylation in neutrophils derived from Fpr2−/− mice (Figure 3E).
Discussion
When exploring human aortas in the present study, a striking finding was the significantly higher mRNA levels of ALX/FPR2 in the adventitia than in the media. Furthermore, these adventitial ALX/FPR2 levels were significantly lower in the adventitia derived from patients with abdominal aortic aneurysms than in adventitia from healthy aortas. The similar results observed in both the presence and absence of a thrombus covering the aneurysmal lesion indicate that ALX/FPR2 levels marked an adventitial response rather than activity in the intraluminal thrombus. The strong correlation between adventitial FPR2/ALX expression with CD66b and CD66d expression supports neutrophils as the major targets for ALX/FPR2 activation in human AAA. Although mononuclear cell aggregates prevailed in the adventitia of human AAA lesions, neutrophils contribute to both the adventitial inflammation (1) and the chemotactic activity of the intraluminal thrombus covering the AAA lesion 4, 5. Adventitial neutrophil infiltration also increased in Ang II-induced murine aneurysmal lesions (17).
Previous studies of a genetically targeted Fpr2 (the murine ortholog of ALX/FPR2) in different hyperlipidemic murine models have generated conflicting results as to reduced 14, 18, increased (19), and neutral (20) effects on atherosclerotic lesion size. One possible reason for this controversy may be differences in the balance between proinflammatory and pro-resolving ALX/FPR2 agonists in the different models used (13). Also, differential receptor expression in the cell types targeted in the different experimental settings might have influenced these contrasting results. The authors note how in vivo administration of pro-resolving ALX/FPR2 agonists, such as either ATL (14) or annexin-peptides (20), has revealed beneficial effects of murine Fpr2 signaling in terms of limiting development of atherosclerosis and reducing macrophage recruitment and activation. The present study is, to these authors' knowledge, the first to implicate ALX/FPR2 signaling as protection against aneurysm formation. This conclusion is based on several observations as discussed below.
In support of a causal involvement of ALX/FPR2 in aortic dilation, hyperlipidemic ApoE−/− mice lacking the murine ALX/FPR2 ortholog Fpr2 exhibited increased abdominal aortic dilation in response to AngII infusion. In a second series of experiments, disruption of the 12/15-LO enzyme in ApoE−/− mice, which is a necessary step in the production of the ALX/FPR2 agonists LXA4 and RvD1, exacerbated Ang II-induced aortic dilation to an extent similar to that observed in ApoE−/−×Fpr2−/− mice. This latter observation is important because, first, it replicated the phenotype of Fpr2 deficiency in a second set of mice in the present study and, second, implied that endogenous lipoxygenase products serve to limit Ang II-induced aortic dilation. Indeed, Ang II is known to upregulate 12-LO activity (21), and Ang II-induced 12 and or 15 LO activity may therefore serve to limit its effects in the context of aneurysm formation by means of lipoxin formation and downstream Fpr2 signaling.
Also, another LO, namely 5-LO, may be involved in lipoxin formation (13). However, in contrast to the exacerbated aneurysm in ApoE−/−×12/15LO−/− mice in the present study, 5-LO disruption reduces aneurysm formation in different murine models of aortic aneurysms (22). It should be pointed out, however, that 5-LO (but not 12/15-LO) also is necessary for the production of proinflammatory leukotrienes, which are detected in human AAA (4), and signal through other specific receptors to enhance aneurysm formation in different experimental models (23).
Human and murine smooth muscle cells express ALX/FPR2 (18), which transduces beneficial effects induced by ATL in intimal hyperplasia (23) and atherosclerosis (14). Fpr2-deficient smooth muscle cells exhibit a distinct phenotype with, for example, an enhanced migration (23). Changes in extracellular matrix in the present study, in terms of thin collagen, increased MMP-9, and more elastin breaks are consistent with the reduced collagen content with a larger proportion of thin collagen fibers observed in the aortic root of Ldlr−/−×Fpr2−/− mice (18). In the latter study, those Fpr2 genotype-dependent differences were not reproduced by Fpr2−/− bone marrow transplantation, hence supporting direct effects of Fpr2 signaling in smooth muscle cells, which may, in addition to stabilization of atherosclerotic lesions (18), also be involved in protection against Ang II-induced aortic dilation.
In addition to LXA4's direct effects on smooth muscle cells, it also ligates ALX/FPR2 on leukocytes, for example, neutrophil granulocytes, which have been associated with important pathophysiological features of human AAA. In line with the fact that lipoxins limit inflammation by means of stimulating, for example, granulocyte apoptosis and egress from sites of inflammation (7), aneurysmal lesions in ApoE−/−×Fpr2−/− mice exhibited more neutrophil granulocytes than in ApoE−/−×Fpr2+/+ mice in the present study.
One of the established downstream signaling pathways following ALX/FPR2 activation by SPMs is the suppression of calcium-sensing kinase calcium-calmodulin-dependent protein kinase and subsequent inhibition of p38 mitogen-activated protein kinase phosphorylation in murine bone marrow-derived cells (24). The latter pathway could potentially be of particular importance for the present model, because p38 is activated by high cholesterol (25) and Ang II (26) and has been shown to play a key role in AAA (27). Furthermore, p38 inhibition diminishes lipopolysaccharide-induced neutrophil activation (28). The Fpr2-dependent suppression of neutrophil p38 phosphorylation by ATL in the present study, therefore, provides an example that reinforces the importance of inflammation suppression by ATL signaling through ALX/FPR2.
Study limitations
The differences in characteristics between the subjects in the groups presented in Table 1 should be acknowledged as a limitation for the comparison between control aortas and AAA. However, ALX/FPR2 expression was not significantly associated with sex in either control or AAA samples (data not shown).
Conclusions
This study showed that ALX/FPR2 signaling confers protection against Ang II-induced aortic dilation, an outcome associated with beneficial effects in terms of increased collagen content and decreased neutrophil infiltration into aneurysmal lesions. The study also demonstrated that disruption of LX production mimicked the Fpr2-deficient aortic phenotype and that ATL signaled through ALX/FPR2 to evoke protective effects in neutrophils. Finally, this study provides translational implications of these findings by identifying predominant adventitial ALX/FPR2 expression in human aortic samples and its association with neutrophil markers, as well as decreased ALX/FPR2 expression in the adventitia derived from AAA patients. These results unveil a previously unknown protective role of lipoxin signaling through FPR2/ALX in the limitation of aortic dilation and indicates a key role of this pathway in promoting the resolution of inflammation for protection against AAA.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Inflammation emerges as a therapeutic target in atherosclerosis. Results of the present study identify a state of failure in the resolution of inflammation by means of decreased expression of the pro-resolving receptor ALX/FPR2 in the adventitia of human AAA lesions.
TRANSLATIONAL OUTLOOK: Mimicking this condition by genetic deletion of the murine ALX/FPR2 ortholog in hyperlipidemic mice exacerbated the aortic dilation induced by angiotensin II infusion, associated with increased inflammation. Stimulating an active resolution of inflammation may represent a novel strategy in prevention of cardiovascular disease.
Footnotes
Supported by Swedish Research Council grant 2014-2312, Swedish Heart and Lung Foundation grants 20150600 and 20150683, Marianne and Marcus Wallenberg Foundation grant 2015.0104, King Gustaf V’s and Queen Victoria’s Freemason Foundation, and Stockholm County Council grants 20150869 and 20170365. Dr. Petri was supported by a KID PhD fellowship from Karolinska Institutet. Dr. Thul was supported by the Deutsche Forschungsgemeinschaft through research fellowship award MU 3851/1-1. Dr. Caidahl was supported by Swedish Research Council grant 2011-3579, Swedish Heart and Lung Foundation grant 20150423, and Stockholm County Council grant 20150517. Dr. Perretti was supported by Wellcome Trust grant 086867/Z/08/Z. Dr. Roy was supported by Stockholm County Council grants 20150906 and SLL-HMT:20160861. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Michel J.B., Martin-Ventura J.L., Egido J. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc Res. 2011;90:18–27. doi: 10.1093/cvr/cvq337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bäck M., Gasser T.C., Michel J.B., Caligiuri G. Biomechanical factors in the biology of aortic wall and aortic valve diseases. Cardiovasc Res. 2013;99:232–241. doi: 10.1093/cvr/cvt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michel J.B., Li Z., Lacolley P. Smooth muscle cells and vascular diseases. Cardiovascular Res. 2012;95:135–137. doi: 10.1093/cvr/cvs172. [DOI] [PubMed] [Google Scholar]
- 4.Houard X., Ollivier V., Louedec L., Michel J.B., Bäck M. Differential inflammatory activity across human abdominal aortic aneurysms reveals neutrophil-derived leukotriene B4 as a major chemotactic factor released from the intraluminal thrombus. FASEB J. 2009;23:1376–1383. doi: 10.1096/fj.08-116202. [DOI] [PubMed] [Google Scholar]
- 5.Houard X., Touat Z., Ollivier V. Mediators of neutrophil recruitment in human abdominal aortic aneurysms. Cardiovasc Res. 2009;82:532–541. doi: 10.1093/cvr/cvp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umeda H., Aikawa M., Libby P. Liberation of desmosine and isodesmosine as amino acids from insoluble elastin by elastolytic proteases. Biochem Biophys Res Commun. 2011;411:281–286. doi: 10.1016/j.bbrc.2011.06.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang N., Serhan C.N., Dahlén S.E. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 9.Petri M.H., Laguna-Fernandez A., Tseng C.N., Hedin U., Perretti M., Bäck M. Aspirin-triggered 15-epi-lipoxin A(4) signals through FPR2/ALX in vascular smooth muscle cells and protects against intimal hyperplasia after carotid ligation. Int J Cardiol. 2015;179:370–372. doi: 10.1016/j.ijcard.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnamoorthy S., Recchiuti A., Chiang N., Fredman G., Serhan C.N. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am J Pathol. 2012;180:2018–2027. doi: 10.1016/j.ajpath.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye R.D., Boulay F., Wang J.M. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayhoe R.P., Kamal A.M., Solito E., Flower R.J., Cooper D., Perretti M. Annexin 1 and its bioactive peptide inhibit neutrophil-endothelium interactions under flow: indication of distinct receptor involvement. Blood. 2006;107:2123–2130. doi: 10.1182/blood-2005-08-3099. [DOI] [PubMed] [Google Scholar]
- 13.Bäck M., Powell W.S., Dahlén S.E. Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR review 7. Brit J Pharmacol. 2014;171:3551–3574. doi: 10.1111/bph.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petri M.H., Laguna-Fernandez A., Arnardottir H. Aspirin-triggered lipoxin A4 inhibits atherosclerosis progression in apolipoprotein E−/− mice. Brit J Pharmacol. 2017;174:4043–4054. doi: 10.1111/bph.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pillai P.S., Leeson S., Porter T.F. Chemical mediators of inflammation and resolution in post-operative abdominal aortic aneurysm patients. Inflammation. 2012;35:98–113. doi: 10.1007/s10753-011-9294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pope N.H., Salmon M., Davis J.P. D-series resolvins inhibit murine abdominal aortic aneurysm formation and increase M2 macrophage polarization. FASEB J. 2016;30:4192–4201. doi: 10.1096/fj.201600144RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wales K.M., Kavazos K., Nataatmadja M., Brooks P.R., Williams C., Russell F.D. N-3 PUFAs protect against aortic inflammation and oxidative stress in angiotensin II-infused apolipoprotein E−/− mice. PLoS One. 2014;9:e112816. doi: 10.1371/journal.pone.0112816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petri M.H., Laguna-Fernandez A., Gonzalez-Diez M., Paulsson-Berne G., Hansson G.K., Bäck M. The role of the FPR2/ALX receptor in atherosclerosis development and plaque stability. Cardiovasc Res. 2015;105:65–74. doi: 10.1093/cvr/cvu224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drechsler M., de Jong R., Rossaint J. Annexin A1 counteracts chemokine-induced arterial myeloid cell recruitment. Circ Res. 2015;116:827–835. doi: 10.1161/CIRCRESAHA.116.305825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredman G., Kamaly N., Spolitu S. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci Transl Med. 2015;7:275ra20. doi: 10.1126/scitranslmed.aaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natarajan R., Gu J.L., Rossi J. Elevated glucose and angiotensin II increase 12-lipoxygenase activity and expression in porcine aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1993;90:4947–4951. doi: 10.1073/pnas.90.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefevre M., Kris-Etherton P.M., Zhao G., Tracy R.P. J Am Diet Assoc. 2004;104:410–419. doi: 10.1016/j.jada.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 23.Ahluwalia N., Lin A.Y., Tager A.M. Inhibited aortic aneurysm formation in BLT1-deficient mice. Journal of immunology. 2007;179:691–697. doi: 10.4049/jimmunol.179.1.691. [DOI] [PubMed] [Google Scholar]
- 24.Fredman G., Ozcan L., Spolitu S. Resolvin D1 limits 5-lipoxygenase nuclear localization and leukotriene B4 synthesis by inhibiting a calcium-activated kinase pathway. Proc Natl Acad Sci U S A. 2014;111:14530–14535. doi: 10.1073/pnas.1410851111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y., Ishibashi M., Seimon T. Free cholesterol accumulation in macrophage membranes activates Toll-like receptors and p38 mitogen-activated protein kinase and induces cathepsin K. Circ Res. 2009;104:455–465. doi: 10.1161/CIRCRESAHA.108.182568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martorell S., Hueso L., Gonzalez-Navarro H., Collado A., Sanz M.J., Piqueras L. Vitamin D receptor activation reduces angiotensin-II-induced dissecting abdominal aortic aneurysm in apolipoprotein E-knockout mice. Arterioscler Thromb Vasc Biol. 2016;36:1587–1597. doi: 10.1161/ATVBAHA.116.307530. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh A., DiMusto P.D., Ehrlichman L.K. The role of extracellular signal-related kinase during abdominal aortic aneurysm formation. J Am Coll Surg. 2012;215:668–680 e1. doi: 10.1016/j.jamcollsurg.2012.06.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senger K., Pham V.C., Varfolomeev E. The kinase TPL2 activates ERK and p38 signaling to promote neutrophilic inflammation. Sci Signal. 2017 Apr 18;10(475) doi: 10.1126/scisignal.aah4273. pii: eaah4273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.