Summary
The clinical use of doxorubicin in cancer is limited by cardiotoxic effects that can lead to heart failure. Whereas earlier work focused on the direct impact of doxorubicin on cardiomyocytes, recent studies have turned to the endothelium, because doxorubicin-damaged endothelial cells can trigger the development and progression of cardiomyopathy by decreasing the release and activity of key endothelial factors and inducing endothelial cell death. Thus, the endothelium represents a novel target for improving the detection, management, and prevention of doxorubicin-induced cardiomyopathy.
Key Words: cardiomyopathy, doxorubicin, endothelium, heart failure
Abbreviations and Acronyms: AKT, protein kinase B; Bcl-2, B-cell lymphoma-2; DNA, deoxyribonucleic acid; ERK1/2, extracellular signal-regulated kinase 1/2; ET, endothelin; LV, left ventricular; MRP, multidrug resistance protein; NADPH, nicotinamide adenine dinucleotide phosphate; NO, nitric oxide; NOS, nitric oxide synthase; NRG-1, neuregulin-1; PGI2, prostaglandin I2; PI3K, phosphoinositide 3-kinase; RNS, reactive nitrogen species; ROS, reactive oxygen species; ZO, zona occludens
Central Illustration
The anthracycline doxorubicin is an antineoplastic agent widely used in the treatment of breast, lung, ovarian, thyroid, and gastric cancers (1). Because it is amphoteric, doxorubicin is able to translocate into a variety of subcellular compartments, where it disrupts the integrity of intracellular deoxyribonucleic acid (DNA), proteins, and lipid molecules (2). These toxic insults are not restricted to cancer cells, and doxorubicin-associated cardiotoxicity is, in fact, a result of the damage it exerts on noncancerous cells, especially cardiomyocytes (2). Because doxorubicin is administered into the systemic circulation, the first cellular contact the drug makes is with the endothelium (2). Accordingly, doxorubicin therapy can first trigger detrimental changes to endothelial cells before it travels into other tissues such as the heart. This review will detail the importance of the endothelium in maintaining the health and function of cardiomyocytes and discuss how doxorubicin-mediated endothelial cell death and dysfunction contribute to the development and progression of cardiomyopathy.
Doxorubicin therapy is associated with both acute and chronic cardiotoxicity. Acute cardiotoxicity has an incidence of approximately 11% and typically manifests within days after doxorubicin treatment has been initiated (3). Acute cardiotoxicity is usually reversible and often presents as myopericarditis, cardiac dysrhythmias, and left ventricular (LV) dysfunction 4, 5. Conversely, chronic cardiotoxicity, although significantly less prevalent than acute cardiotoxicity, is currently irreversible (6), has an appreciably poorer prognosis (7), and usually presents months or even years after treatment is completed (8). In the initial stages, chronic cardiotoxicity typically presents as LV dysfunction with progression to cardiomyopathy 9, 10. Some patients with chronic cardiotoxicity can go on to develop heart failure, a severe condition associated with a 1-year mortality rate of 50% (7). In view of the severe implications of chronic cardiotoxicity, patients treated with doxorubicin should be longitudinally monitored for features that suggest the reduction of LV function and cardiomyopathy.
What Risk Factors Contribute to Doxorubicin-Mediated Cardiotoxicity?
A myriad of factors have been postulated to elevate the risk of doxorubicin-induced cardiomyopathy. Polymorphisms of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase or multidrug resistance protein (MRP) are clearly associated with doxorubicin-mediated heart disease (11). Membrane-bound NADPH oxidases represent a major generator of damaging reactive oxygen species (ROS) (12). Doxorubicin exposure induces NADPH oxidase to produce excessive amounts of ROS, which can, under prolonged duration, exhaust antioxidant defense mechanisms that culminate in cardiac myocyte apoptosis (13). Within the same system, cell surface MRPs such as MRP1 serve to protect cells from cytotoxic agents by actively transporting doxorubicin out of the intracellular space (11). Thus, individuals with increased or decreased expression or activity of NADPH oxidases or MRPs, respectively, will sustain greater cellular damage after doxorubicin therapy.
Patients with diabetes mellitus, liver disease, or a history of cardiovascular disease are at a greater risk of developing doxorubicin-induced cardiotoxicity (14). Several mechanisms have been proposed to explain how diabetes mellitus increases the risk of developing cardiomyopathy (15). One is via hyperglycemia-mediated activation of protein kinase C signaling, which can culminate in cardiac dysfunction through changes in protein kinase B (AKT)/endothelial nitric oxide synthase (NOS) signaling and caveolin-3 expression (16). Because the liver is a major site of doxorubicin clearance, any alteration in doxorubicin metabolism caused by liver disease or concurrent medications would be expected to result in elevated levels of doxorubicin and increased exposure to toxic concentrations of the drug (17). As such, previous use or coadministration of mediastinal radiation and other cardiotoxic antineoplastic agents with doxorubicin can increase the risk of adverse events 18, 19. Finally, previous cardiac disease(s) can also predispose the heart to damage by doxorubicin exposure.
Other risk factors for doxorubicin-induced cardiotoxicity include sex- and age-related factors. The sex-related risk is complex, because prepubescent girls are more likely to develop complications than boys, whereas women are at lower risk for doxorubicin-mediated heart disease than age-matched men 20, 21. Two opposing factors could account for these observations. Doxorubicin is amphoteric and distributes poorly into adipose tissue (22). Although women tend to have greater concentrations of doxorubicin in heart tissue because of proportionately higher body fat composition (23), women also have higher circulating levels of the cardioprotective hormone estrogen (24). In general, therefore, patients <4 years of age and those 65 years and older are more likely to develop cardiomyopathy after doxorubicin therapy (14). These observations can be attributed in part to the immature liver function in young children and declining liver activity among older adults, both of which slow doxorubicin clearance and prolong exposure to circulating doxorubicin 25, 26, 27, 28.
Of all the risk factors, the most significant is the cumulative dose of doxorubicin (3). Too low a doxorubicin dosage can lead to suboptimal efficacy against cancer, but a small uptitration can drastically elevate the risk of cardiotoxicity. Current evidence indicates that cardiomyopathy incidence rates are 4% at 500 to 550 mg/m2, 18% at 551 to 600 mg/m2, and 36% at >600 mg/m2 (all cumulative doses) (3). Therefore, a safe and effective dose needs to be identified through careful titration for each patient based on risk factor profile. Personalized dosing would position patients with cancer at lower risk of developing cardiotoxicity with minimal compromise to treatment efficacy.
Detection and management of doxorubicin-induced cardiotoxicity: How can treatment strategies be improved?
Early diagnosis is critical for preventing progression and improving recovery from doxorubicin-mediated heart damage. A variety of diagnostic tools are currently used to detect doxorubicin-mediated cardiomyopathy. The gold standard is endomyocardial biopsy, which will reveal vacuolization of the cytoplasm, loss of myofibrils, and enlargement of the sarcoplasm reticulum in cardiomyocytes if myopathy is present 6, 14. However, this procedure requires intensive training and is invasive (6). Echocardiography and radionuclide ventriculography are more commonly used but often fail to detect early doxorubicin-induced cardiac damage 14, 29. This is likely because a significant change in LV systolic function, which these techniques assess, does not occur until the heart has undergone substantial damage (30). Clearly, the technologies currently available for early detection of doxorubicin-induced cardiotoxicity have room for improvement.
To date, there is no definitive treatment indicated to prevent or reverse doxorubicin-induced chronic cardiotoxicity (6). Once chronic cardiomyopathy is established, the symptoms can be managed with heart failure medications such as β-blockers and angiotensin II inhibitors, none of which significantly improve the long-term prognosis (6). The strategy most commonly used to lessen doxorubicin-elicited cardiotoxicity is coadministration of the iron chelator dexrazoxane, which reduces the formation of iron-doxorubicin complexes and subsequent ROS generation (2). This treatment plan, however, also diminishes the efficacy of doxorubicin against tumor cells and therefore might not be the best therapeutic choice for patients who are being managed with a low dosage of doxorubicin (2).
Doxorubicin-mediated cardiac damage has become more prevalent in recent years because cancer survival rates are increasing (31). This underscores the importance of identifying effective treatments for doxorubicin-mediated cardiotoxicity. More comprehensive understanding regarding the mechanism(s) underlying doxorubicin-induced cardiomyopathy is needed to improve the prevention and management of doxorubicin-mediated cardiotoxicity (32).
Doxorubicin-Mediated Endothelial Cell Damage
The endothelial lining of coronary blood vessels forms a protective barrier (33) for cardiomyocytes, permits the delivery of nourishment (34), and releases paracrine factors (35) to maintain cardiac myocyte health and function. Predictably, doxorubicin disrupts these beneficial endothelium-regulated processes by damaging endothelial cells, which leads to the development of severe chronic vascular diseases such as atherosclerosis (36). Indeed, children who receive doxorubicin therapy often develop severe vascular disease pathology as adults. Because the initial endothelial cell insult is likely asymptomatic, there is often a long delay between the termination of doxorubicin therapy and the onset of vascular disorders. However, with time, the declining health of the endothelium progressively renders endothelial cells more vulnerable to chronic inflammatory stressors and hyperlipidemia insults. In response to the concept that compromised endothelial cells can have a negative impact on cardiomyocyte health and function, there has been a strategic shift in research paradigm from how doxorubicin directly affects cardiomyocytes to how it alters endothelial cell health and function upstream of observed cardiomyopathy 14, 37.
Doxorubicin induces oxidative stress in endothelial cells
Numerous studies imply a central role for increased ROS production in the mechanism of doxorubicin-mediated endothelial cell toxicity (Central Illustration) 38, 39. The mechanisms of ROS production and how ROS exerts damage are similar in endothelial cells and cardiomyocytes (38). In brief, doxorubicin is first converted to a semiquinone radical via receipt of a single electron through catalytic pathways involving mitochondrial nicotinamide adenine dinucleotide dehydrogenase, cytosolic xanthine oxidase, endoplasmic reticular cytochrome p450 NADPH-reductase, or NOS 40, 41, 42. The semiquinone anion then passes the electron to molecular oxygen, forming a superoxide radical (41). Under normal rates of superoxide production, superoxide dismutase will convert the radical to hydrogen peroxide, which is further detoxified by catalase and glutathione peroxidase 43, 44. However, when doxorubicin-induced oxidative stress exceeds endothelial cell antioxidant capacity, toxic metabolites will begin to accumulate (43). The superoxide radical is also produced from the complex of doxorubicin and iron (45). Notably, the drug-iron complex converts hydrogen peroxide to hydroxyl radicals, which are a more damaging form of ROS (45). Doxorubicin can also increase intracellular iron concentrations through multiple pathways, including the release of iron from aconitase (46) and ferritin (47), the decrease of ferritin synthesis, and the increase of transferrin receptors on the plasma membrane (48). Finally, superoxide radicals have been observed to react with nitric oxide (NO) to form peroxynitrite, a reactive nitrogen species (RNS) (49). This highly cytotoxic compound can rapidly diffuse across phospholipid membranes and gain access to lipids, nucleic acids, and protein targets (50). Thus, by increasing oxidative and nitrosative stress, doxorubicin can progressively damage vital intracellular components of endothelial cells.
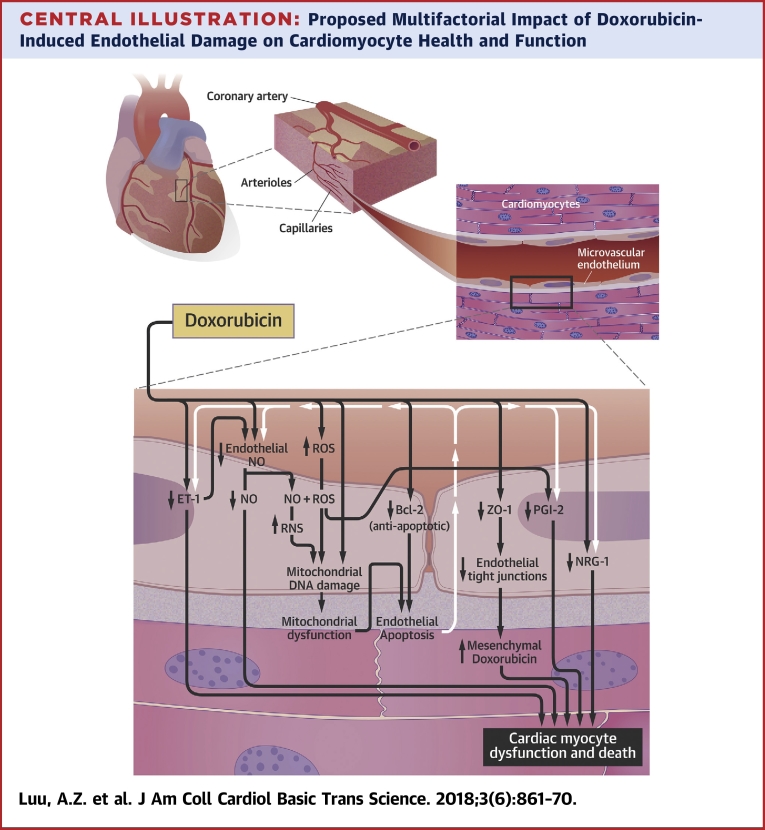
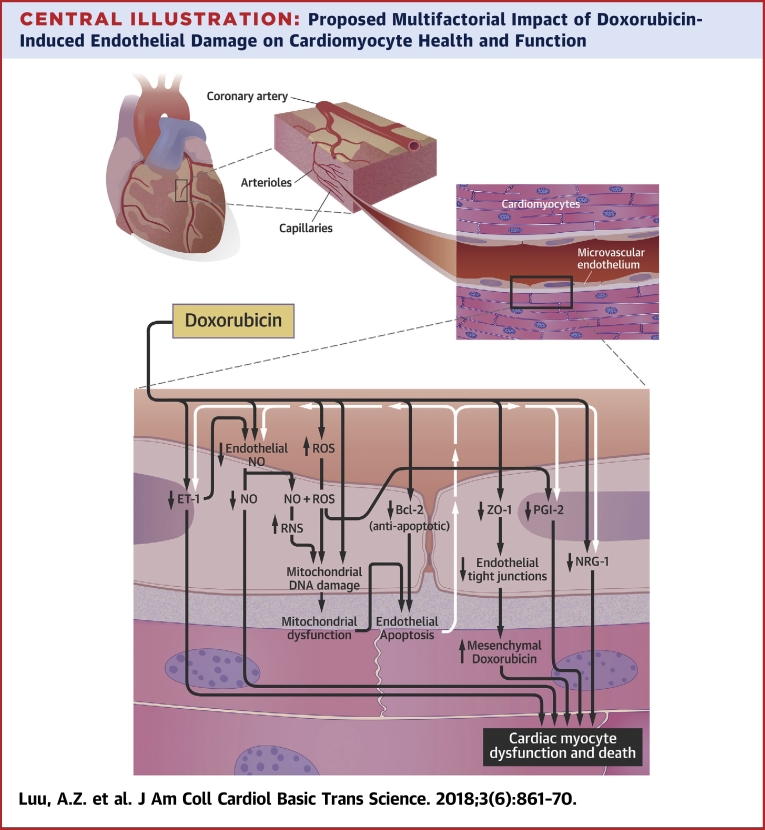
Central Illustration.
Proposed Multifactorial Impact of Doxorubicin-Induced Endothelial Damage on Cardiomyocyte Health and Function
The endothelium-mediated cardiomyocyte supportive functions occur at the capillaries. Doxorubicin likely reduces tight junction formation by lowering the expression of zona occludens (ZO)-1, which can increase the levels of doxorubicin in cardiac tissues. Doxorubicin can decrease the levels of endothelium-derived neuregulin (NRG)-1 through an unknown mechanism and prostaglandin I2 (PGI2) via reactive oxygen species (ROS) generation. Doxorubicin is suggested to directly decrease the levels of nitric oxide (NO) in endothelial cells via enzymatic inhibition. There might also be an indirect decrease of NO through the reduction in endothelin (ET)-1 and elevation in ROS levels. Reactive nitrogen species (RNS) forms from the reaction between ROS and NO. RNS and ROS damage mitochondrial deoxyribonucleic acid (DNA). It is well established that doxorubicin induces mitochondrial DNA damage in an RNS/ROS-independent manner. The resulting mitochondrial dysfunction, along with a possible doxorubicin-mediated decrease in B-cell lymphoma (Bcl)-2, leads to apoptosis of the endothelial cells. The death of these cells can further reduce the availability of NO, ET-1, PGI2, and NRG-1 to the cardiomyocytes. The decline in NO, ET-1, PGI2, and NRG-1 release from endothelial cells and elevation in mesenchymal doxorubicin concentrations are postulated to increase the death and dysfunction of cardiac myocytes.
Oxidative stress leads to endothelial cell death
Increased ROS and RNS production can induce cumulative damage toward mitochondrial DNA, proteins, and lipids, leading to severe metabolic dysfunction in endothelial cells (51). Subsequently, mitochondria release cytochrome C and activate proapoptotic factors such as caspases, central components of the apoptotic response 51, 52, 53, 54. Doxorubicin has been reported to down-regulate B-cell lymphoma (Bcl)-2 protein expression. Bcl-2 is generally considered be an antiapoptotic member of the Bcl-2 homology domain-containing family of proteins that includes both proapoptotic and antiapoptotic members 55, 56. Thus, doxorubicin-initiated oxidative and nitrosative stress induce mitochondrial damage, which can lead to endothelial cell loss via apoptosis. Antiapoptotic strategies relevant to endothelial cells might, therefore, reduce doxorubicin-mediated toxicity. For example, the active metabolite of zofenopril, an angiotensin-converting enzyme inhibitor, diminished doxorubicin-induced endothelial cell apoptosis by preventing the activation of p53 and caspase-3 (57). Additionally, prodrug zofenopril treatment was associated with decreased doxorubicin-mediated myocardial damage in rats (58).
Doxorubicin induces direct DNA damage in endothelial cells
In addition to oxidative and nitrosative stress, doxorubicin can directly damage DNA through DNA topoisomerase II inhibition and DNA intercalation, which are also well-established antitumor mechanisms of the drug 39, 59. Topoisomerases are DNA-unwinding enzymes necessary for DNA repair, replication, and transcription because they alleviate torsional stress in the DNA (60). In general, topoisomerase enzymes bind and induce DNA breakage, allowing DNA to rotate before re-ligation (61). The beta isoenzyme of topoisomerase II is elevated in terminally differentiated cells, such as endothelial cells of the coronary microvessels 62, 63. On the other hand, topoisomerase IIα is increased in dividing cells, such as cancer cells (64). Doxorubicin binds and stabilizes the cleavable complex of both topoisomerase IIα and β, which leads to unrepaired double-strand DNA breaks in both cancer and endothelial cells (65). Notably, pixantrone, an antineoplastic agent that is structurally related to doxorubicin, shows significantly less cardiotoxicity in mice because it has a greater affinity for the topoisomerase IIα relative to the IIβ isoform (66). Thus, the promiscuity of doxorubicin for both topoisomerase II isoforms results in off-target DNA damage in both cancer cells and the more slowly dividing endothelial cells.
Majzner et al. (59) observed dose-dependent and preferential DNA binding of doxorubicin at CG-rich sequences. At high concentrations of doxorubicin, this phenomenon led to DNA aggregation, entangling, and breakages (67). These effects were absent at low concentrations of the drug (67). It has hence been suggested that therapeutic dosages of doxorubicin expose endothelial cells to high concentrations of the drug, which induces significant DNA damage (68). Thus, topoisomerase IIβ inhibition and direct DNA binding by doxorubicin are nonoxidative and non-nitrosative stress-dependent mechanisms that induce direct endothelial cell DNA damage and downstream apoptosis.
Disruption of the Endothelial Cell–Cardiomyocyte Signaling Axis
Perfusion of the heart is mainly driven by the left and right coronary arteries. The larger branches of the coronary artery system generally run superficially in the epicardium and the arteriole branches penetrate perpendicularly to the myocardium. Because cardiac muscle is the most aerobic organ in the body, the vasculature is very rich in capillaries. Muscle to capillary ratio in the heart is approximately 1:1, which translates to a capillary density that is about 10-fold greater than that of skeletal muscle. The distance between a capillary endothelial cell and the closest cardiomyocyte is approximately 1 μm, which suggests close intimacy between the 2 cell types and their dependence on each other (69). Numerous studies have demonstrated that doxorubicin disrupts the supporting processes that endothelial cells provide for cardiac myocytes (Central Illustration). The additive effects of doxorubicin-mediated ROS–dependent and direct endothelial cell death can therefore promote a decline in cardiomyocyte health and function over time as a consequence of the interdependence between the 2 cell types. Although doxorubicin-induced endothelial cell damage can occur in many tissues throughout the body, endothelial cell death within the heart results in greater damage to heart muscle than other tissues because of the unique structure of the coronary vasculature.
Doxorubicin perturbs endothelium barrier function
The relative impermeability of the cardiac endothelium, related to the presence of tight junctions, prevents the exposure of cardiomyocytes to harmful compounds 33, 70. Circulating doxorubicin, however, can compromise the integrity of these tight junctions in the coronary microvasculature. Indeed, Wilkinson et al. (71) found reduced expression of the tight junction protein zona occludens (ZO)-1 in doxorubicin-treated human cardiac microvascular endothelial cells, which was associated with increased microvascular permeability and longer exposure of the cardiomyocytes to doxorubicin. Cardiolipin, a mitochondrial phospholipid important for energy production, has a high affinity for doxorubicin (72). The dominant presence of cardiolipin in cardiomyocytes is why cardiomyocytes are particularly sensitive to doxorubicin toxicity (73). How doxorubicin decreases the expression of ZO-1 in the absence of endothelial cell apoptosis is unknown; however, endothelial cell loss can increase the permeability of the vasculature, and strategies to maintain vessel integrity could represent a novel therapeutic opportunity. Indeed, pretreatment of human cardiac microvascular endothelial cells with endothelium-acting, cholesterol-lowering simvastatin prevented doxorubicin-induced microvascular permeability because of increased localization of ZO-1 to the plasma membrane and subsequent tight junction formation 74, 75.
Doxorubicin disrupts endothelial cell paracrine signaling to cardiomyocytes
Cumulative data suggest that doxorubicin reduces the secretion of endothelial cell–derived paracrine molecules that act directly on cardiomyocytes to influence the survival, function, and adaptation of the myocardium to environmental stressors (76). The following sections of this review will specifically discuss how doxorubicin dysregulates endothelium-derived endothelin (ET)-1, NO, prostaglandin I2 (PGI2), and neuregulin (NRG)-1, all of which have been well characterized.
Endothelin-1
ET-1 is a 21-amino acid vasoconstrictive peptide that has a critical role in regulating the function, size, and survival of cardiomyocytes that are under stress (77). The binding of ET-1 to the G-coupled protein receptors ETA and ETB on the plasma membrane of cardiomyocytes activates Gq proteins (78). Gq activation increases sarcoplasmic reticulum calcium release, which can intensify the contractility of myocytes 78, 79. An association between activation of cardiac ETA and ETB receptors and cardiac myocyte hypertrophy has been speculated 80, 81. Zhao et al. (82) demonstrated that ET-1 diminishes tissue necrosis factor–induced programmed death of cultured rat neonatal cardiomyocytes through up-regulation of nuclear factor-κB signaling. Doxorubicin was observed to reduce ET-1 mitochondrial ribonucleic acid and protein via an unknown mechanism in cultured human umbilical vein endothelial cells (83), although it is likely that endothelial cell death contributed in part to the decline in ET-1 levels. Thus, doxorubicin therapy could diminish endothelium-mediated ET-1 signaling, decreasing the survival of cardiomyocytes during inflammation.
Nitric oxide
Coronary microvessel-derived NO also modulates the contractility of cardiomyocytes. NO is produced from L-arginine in endothelial cells via the catalytic activity of the enzyme NOS (84). Once synthesized, NO diffuses across lipid membranes to arrive at the target cells (35) and activate soluble guanylate cyclases, which produce cyclic guanosine monophosphate (84). Elevated levels of cyclic guanosine monophosphate have been associated with earlier relaxation of cardiomyocytes and increased ventricular compliance (85). Endothelial-derived NO could also increase mechanical efficiency through inhibition of some components of the electron transport chain (85). Although exposure of cultured bovine aortic endothelial cells to doxorubicin up-regulated the expression of endothelial NOS, reportedly because of elevated levels of intracellular calcium and hydrogen peroxide (86), direct binding of doxorubicin to NOS has been predicted to inhibit NO production and release (40). Doxorubicin binding to NOS also redirects the electron flow away from the NO-generating oxygenase domain and toward the reductase domain, which reduces doxorubicin and increases reactivity (40). Increased ROS produced by the reduced form of doxorubicin could further decrease NO availability 40, 87. In addition, endothelial cell–derived ET-1 increases NO synthesis through the ET-B receptor–mediated autocrine loop (88). Thus, endothelial cell NO release can be further diminished as a result of the doxorubicin-mediated decrease in endothelial cell ET-1 production. Finally, a decrease in NO levels was observed in cultured human umbilical vein endothelial cells and cardiac tissues of mice after doxorubicin exposure 89, 90, and restoration of cardiac NO levels with folic acid preserved cardiac function in doxorubicin-treated mice (90). Therefore, strategies to bolster NO signaling in cardiomyocytes are expected to decrease doxorubicin-induced cardiotoxicity.
NO and ET-1 are also vasoactive molecules and critical regulators of blood pressure and flow to cardiomyocytes (91). Reductions in endothelial NO could account in part for the increased vascular stiffness observed in patients treated with doxorubicin (92). Notably, angiotensin-converting enzyme inhibitors decreased vascular stiffness and improved LV ejection fraction in patients who had undergone doxorubicin therapy 32, 93. Thus, changes in hemodynamics during doxorubicin therapy could stress cardiac myocytes and contribute to the progression of cardiomyopathy.
Prostaglandin I2
PGI2 is a physiologically active lipid compound that has also been demonstrated to regulate cardiomyocyte morphology and survival (94). PGI2 is synthesized from arachidonic acid via the sequential enzymatic activity of cyclooxygenase and prostacyclin synthase (94). Treatment with the PGI2 mimetic cicaprost reduced ET-1–induced hypertrophy in cultured adult rat cardiomyocytes (95). The reduction was postulated to have occurred through binding and activation of the IP prostanoid receptor and the subsequent cyclic adenosine monophosphate–dependent signaling in cardiomyocytes (95). Using cultured adult rat cardiomyocytes, Shinmura et al. (96) showed that the PGI2 analogue carbaprostacyclin bound to EP3 receptors, which opened mitochondrial adenosine triphosphate–sensitive potassium channels. They proposed that the opening of these channels protected the cardiomyocytes from oxidative stress (96). However, high intracellular concentrations of ROS from doxorubicin metabolism can inhibit the cyclooxygenase step of PGI2 synthesis in cultured endothelial cells 38, 97. Endothelial cell apoptosis will likely also reduce the amount of endothelium-derived PGI2 available to cardiac myocytes. Administration of iloprost, a synthetic analogue of PGI2, in mice decreased cardiac dysfunction through the attenuation of cardiomyocyte apoptosis without compromising the anticancer efficacy of doxorubicin (98). Thus, strategies to bolster the cardioprotective effects of endothelial cell–derived PGI2 are warranted for prevention of doxorubicin-induced toxicity.
Neuregulin-1
Finally, coronary endothelial cell–derived NRG-1 is a growth factor (35) that also regulates the size, structure, and survival of cardiomyocytes. NRG-1 binds to the tyrosine kinase receptor erythroblastic leukemia viral oncogene homolog 4 on the cellular membranes of the myocytes. This activates the extracellular signal–regulated kinase 1/2 (ERK1/2) and phosphoinositide 3-kinase (PI3K)/AKT signaling pathways 35, 99, both of which can be involved in enhancing cardiomyocyte hypertrophy (100). The ERK1/2 network could modulate myocyte structure, and the PI3K/AKT pathway can promote myocyte survival (35). NRG-1 levels are profoundly reduced in individuals using anthracycline or human epidermal growth factor receptor 2–targeted therapy for breast cancer (101). Because the cardiac microvascular endothelium is a prominent source of NRG-1, a decrease in circulating NRG-1 levels suggests reduced endothelial cell release of the protein (102). How doxorubicin controls the levels of endothelial cell–derived NRG-1 is not well understood, but endothelial cell apoptosis is a likely causal factor. Recombinant human NRG-1 improved cardiac function and survival rates, although whether this was through attenuation of cardiomyocyte death after doxorubicin treatment remains unknown (103). Thus, reversing doxorubicin-induced NRG-1 deficiency is postulated to restore morphology and promote the survival of cardiomyocytes after doxorubicin therapy.
Collectively, direct activities of ET-1, NO, PGI2, and NRG-1 on cardiomyocytes suggest paracrine molecules from the coronary endothelium play an important role in cardiomyocyte homeostasis, from survival to contractile function. Thus, doxorubicin-mediated decrease of endothelial cell–derived factors decreases the adaptability and survivability of cardiomyocytes.
Bringing Endothelium Protection From the Bench to the Bedside
The endothelium plays a critical role in the development and progression of doxorubicin-induced cardiomyopathy. As such, it is important that during doxorubicin therapy, appropriate measures are taken to protect the endothelium while ensuring that the efficacy of doxorubicin is maximized. A comprehensive understanding of the mechanisms of action underlying the detrimental doxorubicin effects on endothelial cells is essential to optimally preserve endothelium-mediated support of cardiomyocytes. Doxorubicin-induced endothelial cell death and dysregulation are complex and involve multiple networks that might or might not act in concert. Current published data suggests ROS generation has a central role in doxorubicin-induced endothelium dysfunction; however, which pathways are affected and how these molecular mechanisms challenge endothelial cell–mediated cardiomyocyte support functions remain unknown. Uncovering this information will be critical for the identification and design of novel therapeutic targets to prevent doxorubicin-induced endothelium damage, maximizing endothelial cell–mediated protection of cardiomyocytes during doxorubicin treatment.
In summary, despite being a versatile and effective anticancer drug, doxorubicin can cause cardiomyopathy, which can evolve into heart failure. Cumulative evidence supports the notion that the detrimental cardiac effects observed with doxorubicin might in part be mediated through the deleterious effects doxorubicin exerts on endothelial cells. Doxorubicin increases the permeability of the endothelium, thus prolonging the duration that cardiomyocytes are exposed to doxorubicin and allowing for more direct damage to the myocytes. ROS is a major effector molecule of doxorubicin. It disrupts endothelium-based cardiac myocyte supportive functions and promotes pathological release of endothelial cell–derived ET-1, PGI2, NO, and NRG-1. The biochemical imbalance reduces the survivability and adaptability of cardiac myocytes. We propose that investigations designed to better understand the mechanisms of doxorubicin-induced endothelial cell damage are urgently required to provide insights for the identification of novel treatment targets and development of innovative strategies to circumvent or reduce doxorubicin-associated cardiomyopathy.
Footnotes
Mr. Luu has received a studentship from the Department of Pharmacology and Toxicology, University of Toronto. Dr. Hess has received a grant from the Canadian Institutes of Health Research (MOP#378189). Dr. Verma has received grants from the Canadian Institutes of Health Research and the Heart & Stroke Foundation. All other authors have reported that they have no relationships relevant to the content of this paper to report.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Contributor Information
David A. Hess, Email: dhess@robarts.ca.
Subodh Verma, Email: vermasu@smh.ca.
References
- 1.Thorn C.F., Oshiro C., Marsh S. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics. 2011;21:440–446. doi: 10.1097/FPC.0b013e32833ffb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitry M.A., Edwards J.G. Doxorubicin induced heart failure: phenotype and molecular mechanisms. Int J Cardiol Heart Vasc. 2016;10:17–24. doi: 10.1016/j.ijcha.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lefrak E.A., Pitha J., Rosenheim S., Gottlieb J.A. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302–314. doi: 10.1002/1097-0142(197308)32:2<302::aid-cncr2820320205>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Gaudin P.B., Hruban R.H., Beschorner W.E. Myocarditis associated with doxorubicin cardiotoxicity. Am J Clin Pathol. 1993;100:158–163. doi: 10.1093/ajcp/100.2.158. [DOI] [PubMed] [Google Scholar]
- 5.Takemura G., Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330–352. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee K., Zhang J., Honbo N., Karliner J.S. Doxorubicin cardiomyopathy. Cardiology. 2010;115:155–162. doi: 10.1159/000265166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Von Hoff D.D., Layard M.W., Basa P. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 8.Appel J.M., Nielsen D., Zerahn B., Jensen B.V., Skagen K. Anthracycline-induced chronic cardiotoxicity and heart failure. Acta Oncol. 2007;46:576–580. doi: 10.1080/02841860601156165. [DOI] [PubMed] [Google Scholar]
- 9.Shakir D.K., Rasul K.I. Chemotherapy induced cardiomyopathy: pathogenesis, monitoring and management. J Clin Med Res. 2009;1:8–12. doi: 10.4021/jocmr2009.02.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipshultz S.E., Colan S.D., Gelber R.D., Perez-Atayde A.R., Sallan S.E., Sanders S.P. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 11.Wojnowski L., Kulle B., Schirmer M. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation. 2005;112:3754–3762. doi: 10.1161/CIRCULATIONAHA.105.576850. [DOI] [PubMed] [Google Scholar]
- 12.Cave A., Grieve D., Johar S., Zhang M., Shah A.M. NADPH oxidase-derived reactive oxygen species in cardiac pathophysiology. Philos Trans R Soc Lond B Biol Sci. 2005;360:2327–2334. doi: 10.1098/rstb.2005.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilleron M., Marechal X., Montaigne D., Franczak J., Neviere R., Lancel S. NADPH oxidases participate to doxorubicin-induced cardiac myocyte apoptosis. Biochem Biophys Res Commun. 2009;388:727–731. doi: 10.1016/j.bbrc.2009.08.085. [DOI] [PubMed] [Google Scholar]
- 14.Octavia Y., Tocchetti C.G., Gabrielson K.L., Janssens S., Crijns H.J., Moens A.L. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52:1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Jia G., Hill M.A., Sowers J.R. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122:624–638. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei S., Li H., Xu J. Hyperglycemia-induced protein kinase C β2 activation induces diastolic cardiac dysfunction in diabetic rats by impairing caveolin-3 expression and Akt/eNOS signaling. Diabetes. 2013;62:2318–2328. doi: 10.2337/db12-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koren G., Beatty K., Seto A., Einarson T.R., Lishner M. The effects of impaired liver function on the elimination of antineoplastic agents. Ann Pharmacother. 1992;26:363–371. doi: 10.1177/106002809202600311. [DOI] [PubMed] [Google Scholar]
- 18.Jain D. Cardiotoxicity of doxorubicin and other anthracycline derivatives. J Nucl Cardiol. 2000;7:53–62. doi: 10.1067/mnc.2000.103324. [DOI] [PubMed] [Google Scholar]
- 19.Byrd B.F., 3rd, Mendes L.A. Cardiac complications of mediastinal radiotherapy: the other side of the coin. J Am Coll Cardiol. 2003;42:750–751. doi: 10.1016/s0735-1097(03)00760-5. [DOI] [PubMed] [Google Scholar]
- 20.Lipshultz S.E., Sambatakos P., Maguire M. Cardiotoxicity and cardioprotection in childhood cancer. Acta Haematol. 2014;132:391–399. doi: 10.1159/000360238. [DOI] [PubMed] [Google Scholar]
- 21.Myrehaug S., Pintilie M., Yun L. A population-based study of cardiac morbidity among Hodgkin lymphoma patients with preexisting heart disease. Blood. 2010;116:2237–2240. doi: 10.1182/blood-2010-01-263764. [DOI] [PubMed] [Google Scholar]
- 22.Rossi C., Gasparini G., Canobbio L. Doxorubicin distribution in human breast cancer. Cancer Treat Rep. 1987;71:1221–1226. [PubMed] [Google Scholar]
- 23.Blaak E. Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care. 2001;4:499–502. doi: 10.1097/00075197-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Murphy E., Lagranha C., Deschamps A. Mechanism of cardioprotection: what can we learn from females? Pediatr Cardiol. 2011;32:354–359. doi: 10.1007/s00246-010-9877-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Völler S., Boos J., Krischke M. Age-dependent pharmacokinetics of doxorubicin in children with cancer. Clin Pharmacokinet. 2015;54:1139–1149. doi: 10.1007/s40262-015-0272-4. [DOI] [PubMed] [Google Scholar]
- 26.Li J., Gwilt P.R. The effect of age on the early disposition of doxorubicin. Cancer Chemother Pharmacol. 2003;51:395–402. doi: 10.1007/s00280-002-0554-z. [DOI] [PubMed] [Google Scholar]
- 27.Lu H., Rosenbaum S. Developmental pharmacokinetics in pediatric populations. J Pediatr Pharmacol Ther. 2014;19:262–276. doi: 10.5863/1551-6776-19.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangoni A.A., Jackson S.H.D. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57:6–14. doi: 10.1046/j.1365-2125.2003.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahman A.M., Yusuf S.W., Ewer M.S. Anthracycline-induced cardiotoxicity and the cardiac-sparing effect of liposomal formulation. Int J Nanomedicine. 2007;2:567–583. [PMC free article] [PubMed] [Google Scholar]
- 30.Tjeerdsma G., Meinardi M.T., van Der Graaf W.T. Early detection of anthracycline induced cardiotoxicity in asymptomatic patients with normal left ventricular systolic function: autonomic versus echocardiographic variables. Heart. 1999;81:419–423. doi: 10.1136/hrt.81.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeSantis C.E., Lin C.C., Mariotto A.B. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 32.Vejpongsa P., Yeh E.T. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol. 2014;64:938–945. doi: 10.1016/j.jacc.2014.06.1167. [DOI] [PubMed] [Google Scholar]
- 33.Wallez Y., Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2008;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Narmoneva D.A., Vukmirovic R., Davis M.E., Kamm R.D., Lee R.T. Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation. 2004;110:962–968. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim S.L., Lam C.S., Segers V.F., Brutsaert D.L., De Keulenaer G.W. Cardiac endothelium-myocyte interaction: clinical opportunities for new heart failure therapies regardless of ejection fraction. Eur Heart J. 2015;36:2050–2060. doi: 10.1093/eurheartj/ehv132. [DOI] [PubMed] [Google Scholar]
- 36.Bar-Joseph H., Ben-Aharon I., Tzabari M., Tsarfaty G., Stemmer S.M., Shalgi R. In vivo bioimaging as a novel strategy to detect doxorubicin-induced damage to gonadal blood vessels. PLoS One. 2011;6:e23492. doi: 10.1371/journal.pone.0023492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeyaseelan R., Poizat C., Wu H.Y., Kedes L. Molecular mechanisms of doxorubicin-induced cardiomyopathy: selective suppression of Reiske iron-sulfur protein, ADP/ATP translocase, and phosphofructokinase genes is associated with ATP depletion in rat cardiomyocytes. J Biol Chem. 1997;272:5828–5832. doi: 10.1074/jbc.272.9.5828. [DOI] [PubMed] [Google Scholar]
- 38.Kotamraju S., Konorev E.A., Joseph J., Kalyanaraman B. Doxorubicin-induced apoptosis in endothelial cells and cardiomyocytes is ameliorated by nitrone spin traps and ebselen: role of reactive oxygen and nitrogen species. J Biol Chem. 2000;275:33585–33592. doi: 10.1074/jbc.M003890200. [DOI] [PubMed] [Google Scholar]
- 39.Gewirtz D.A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 40.Vasquez-Vivar J., Martasek P., Hogg N., Masters B.S., Pritchard K.A., Jr., Kalyanaraman B. Endothelial nitric oxide synthase-dependent superoxide generation from adriamycin. Biochemistry. 1997;36:11293–11297. doi: 10.1021/bi971475e. [DOI] [PubMed] [Google Scholar]
- 41.Dudka J., Burdan F., Korga A. Intensification of doxorubicin-related oxidative stress in the heart by hypothyroidism is not related to the expression of cytochrome P450 NADPH-reductase and inducible nitric oxide synthase, as well as activity of xanthine oxidase. Oxid Med Cell Longev. 2012;2012:139327. doi: 10.1155/2012/139327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brodsky J.L. The use of in vitro assays to measure endoplasmic reticulum-associated degradation. Methods Enzymol. 2010;470:661–679. doi: 10.1016/S0076-6879(10)70027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yarana C., St Clair D.K. Chemotherapy-induced tissue injury: an insight into the role of extracellular vesicles-mediated oxidative stress responses. Antioxidants (Basel) 2017;6:E75. doi: 10.3390/antiox6040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Day B.J. Catalase and glutathione peroxidase mimics. Biochem Pharmacol. 2009;77:285–296. doi: 10.1016/j.bcp.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keizer H.G., Pinedo H.M., Schuurhuis G.J., Joenje H. Doxorubicin (adriamycin): a critical review of free radical-dependent mechanisms of cytotoxicity. Pharmacol Ther. 1990;47:219–231. doi: 10.1016/0163-7258(90)90088-j. [DOI] [PubMed] [Google Scholar]
- 46.Minotti G., Cairo G., Monti E. Role of iron in anthracycline cardiotoxicity: new tunes for an old song? FASEB J. 1999;13:199–212. [published correction appears in FASEB J 1999;13:594] [PubMed] [Google Scholar]
- 47.Thomas C.E., Aust S.D. Release of iron from ferritin by cardiotoxic anthracycline antibiotics. Arch Biochem Biophys. 1986;248:684–689. doi: 10.1016/0003-9861(86)90523-0. [DOI] [PubMed] [Google Scholar]
- 48.Kotamraju S., Chitambar C.R., Kalivendi S.V., Joseph J., Kalyanaraman B. Transferrin receptor-dependent iron uptake is responsible for doxorubicin-mediated apoptosis in endothelial cells: role of oxidant-induced iron signaling in apoptosis. J Biol Chem. 2002;277:17179–17187. doi: 10.1074/jbc.M111604200. [DOI] [PubMed] [Google Scholar]
- 49.Mihm M.J., Bauer J.A. Peroxynitrite-induced inhibition and nitration of cardiac myofibrillar creatine kinase. Biochimie. 2002;84:1013–1019. doi: 10.1016/s0300-9084(02)00005-6. [DOI] [PubMed] [Google Scholar]
- 50.Marla S.S., Lee J., Groves J.T. Peroxynitrite rapidly permeates phospholipid membranes. Proc Natl Acad Sci U S A. 1997;94:14243–14248. doi: 10.1073/pnas.94.26.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kannan K., Jain S.K. Oxidative stress and apoptosis. Pathophysiology. 2000;7:153–163. doi: 10.1016/s0928-4680(00)00053-5. [DOI] [PubMed] [Google Scholar]
- 52.Turk B., Stoka V. Protease signalling in cell death: caspases versus cysteine cathepsins. FEBS Lett. 2007;581:2761–2767. doi: 10.1016/j.febslet.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 53.Zou H., Li Y., Liu X., Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 54.Porter A.G., Janicke R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 55.Lorenzo E., Ruiz-Ruiz C., Quesada A.J. Doxorubicin induces apoptosis and CD95 gene expression in human primary endothelial cells through a p53-dependent mechanism. J Biol Chem. 2002;277:10883–10892. doi: 10.1074/jbc.M107442200. [DOI] [PubMed] [Google Scholar]
- 56.Martinou J.-C., Youle R.J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monti M., Terzuoli E., Ziche M., Morbidelli L. The sulphydryl containing ACE inhibitor Zofenoprilat protects coronary endothelium from doxorubicin-induced apoptosis. Pharmacol Res. 2013;76:171–181. doi: 10.1016/j.phrs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 58.Sacco G., Mario B., Lopez G., Evangelista S., Manzini S., Maggi C.A. ACE inhibition and protection from doxorubicin-induced cardiotoxicity in the rat. Vascul Pharmacol. 2009;50:166–170. doi: 10.1016/j.vph.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 59.Majzner K., Wojcik T., Szafraniec E. Nuclear accumulation of anthracyclines in the endothelium studied by bimodal imaging: fluorescence and Raman microscopy. Analyst. 2015;140:2302–2310. doi: 10.1039/c4an01882f. [DOI] [PubMed] [Google Scholar]
- 60.Yang F., Teves S.S., Kemp C.J., Henikoff S. Doxorubicin, DNA torsion, and chromatin dynamics. Biochim Biophys Acta. 2014;1845:84–89. doi: 10.1016/j.bbcan.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pommier Y., Sun Y., Huang S.-Y.N., Nitiss J.L. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat Rev Mol Cell Biol. 2016;17:703–721. doi: 10.1038/nrm.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Capranico G., Tinelli S., Austin C.A., Fisher M.L., Zunino F. Different patterns of gene expression of topoisomerase II isoforms in differentiated tissues during murine development. Biochim Biophys Acta. 1992;1132:43–48. doi: 10.1016/0167-4781(92)90050-a. [DOI] [PubMed] [Google Scholar]
- 63.Sriram G., Tan J.Y., Islam I., Rufaihah A.J., Cao T. Efficient differentiation of human embryonic stem cells to arterial and venous endothelial cells under feeder- and serum-free conditions. Stem Cell Res Ther. 2015;6:261. doi: 10.1186/s13287-015-0260-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lynch B.J., Guinee D.G.J., Holden J.A. Human DNA topoisomerase II-alpha: a new marker of cell proliferation in invasive breast cancer. Hum Pathol. 1997;28:1180–1188. doi: 10.1016/s0046-8177(97)90256-2. [DOI] [PubMed] [Google Scholar]
- 65.Pavillard V., Kherfellah D., Richard S., Robert J., Montaudon D. Effects of the combination of camptothecin and doxorubicin or etoposide on rat glioma cells and camptothecin-resistant variants. Br J Cancer. 2001;85:1077–1083. doi: 10.1054/bjoc.2001.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hasinoff B.B., Wu X., Patel D., Kanagasabai R., Karmahapatra S., Yalowich J.C. Mechanisms of action and reduced cardiotoxicity of pixantrone: a topoisomerase II targeting agent with cellular selectivity for the topoisomerase IIalpha isoform. J Pharmacol Exp Ther. 2016;356:397–409. doi: 10.1124/jpet.115.228650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cassina V., Seruggia D., Beretta G.L. Atomic force microscopy study of DNA conformation in the presence of drugs. Eur Biophys J. 2011;40:59–68. doi: 10.1007/s00249-010-0627-6. [DOI] [PubMed] [Google Scholar]
- 68.Wojcik T., Buczek E., Majzner K. Comparative endothelial profiling of doxorubicin and daunorubicin in cultured endothelial cells. Toxicol Vitr. 2015;29:512–521. doi: 10.1016/j.tiv.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 69.Aird W.C. Phenotypic heterogeneity of the endothelium, II: representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 70.Brutsaert D.L., De Keulenaer G.W., Fransen P. The cardiac endothelium: functional morphology, development, and physiology. Prog Cardiovasc Dis. 1996;39:239–262. doi: 10.1016/s0033-0620(96)80004-1. [DOI] [PubMed] [Google Scholar]
- 71.Wilkinson E.L., Sidaway J.E., Cross M.J. Cardiotoxic drugs Herceptin and doxorubicin inhibit cardiac microvascular endothelial cell barrier formation resulting in increased drug permeability. Biol Open. 2016;5:1362–1370. doi: 10.1242/bio.020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aryal B., Rao V.A. Deficiency in cardiolipin reduces doxorubicin-induced oxidative stress and mitochondrial damage in human B-lymphocytes. PLoS One. 2016;11:e0158376. doi: 10.1371/journal.pone.0158376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hatch G.M. Regulation of cardiolipin biosynthesis in the heart. Mol Cell Biochem. 1996;159:139–148. doi: 10.1007/BF00420916. [DOI] [PubMed] [Google Scholar]
- 74.Ratchford E.V., Martin S.S. Statins. Vasc Med. 2017;22:442–445. doi: 10.1177/1358863X17722212. [DOI] [PubMed] [Google Scholar]
- 75.Wilkinson E.L., Sidaway J.E., Cross M.J. Statin regulated ERK5 stimulates tight junction formation and reduces permeability in human cardiac endothelial cells. J Cell Physiol. 2018;233:186–200. doi: 10.1002/jcp.26064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hsieh P.C.H., Davis M.E., Lisowski L.K., Lee R.T. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu Rev Physiol. 2006;68:51–66. doi: 10.1146/annurev.physiol.68.040104.124629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yanagisawa M., Kurihara H., Kimura S. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 78.Kedzierski R.M., Grayburn P.A., Kisanuki Y.Y. Cardiomyocyte-specific endothelin A receptor knockout mice have normal cardiac function and an unaltered hypertrophic response to angiotensin II and isoproterenol. Mol Cell Biol. 2003;23:8226–8232. doi: 10.1128/MCB.23.22.8226-8232.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ishikawa T., Yanagisawa M., Kimura S., Goto K., Masaki T. Positive inotropic action of novel vasoconstrictor peptide endothelin on guinea pig atria. Am J Physiol. 1988;255:H970–H973. doi: 10.1152/ajpheart.1988.255.4.H970. [DOI] [PubMed] [Google Scholar]
- 80.Cheng T.-H., Shih N.-L., Chen C.-H. Role of mitogen-activated protein kinase pathway in reactive oxygen species-mediated endothelin-1-induced beta-myosin heavy chain gene expression and cardiomyocyte hypertrophy. J Biomed Sci. 2005;12:123–133. doi: 10.1007/s11373-004-8168-6. [DOI] [PubMed] [Google Scholar]
- 81.Lee G.R., Bell D., Kelso E.J., Argent C.C.H., McDermott B.J. Evidence for altered ETB receptor characteristics during development and progression of ventricular cardiomyocyte hypertrophy. Am J Physiol Heart Circ Physiol. 2004;287:H425–H432. doi: 10.1152/ajpheart.00461.2003. [DOI] [PubMed] [Google Scholar]
- 82.Zhao X.S., Pan W., Bekeredjian R., Shohet R.V. Endogenous endothelin-1 is required for cardiomyocyte survival in vivo. Circulation. 2006;114:830–837. doi: 10.1161/CIRCULATIONAHA.105.577288. [DOI] [PubMed] [Google Scholar]
- 83.Keltai K., Cervenak L., Mako V., Doleschall Z., Zsary A., Karadi I. Doxorubicin selectively suppresses mRNA expression and production of endothelin-1 in endothelial cells. Vasc Pharmacol. 2010;53:209–214. doi: 10.1016/j.vph.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 84.Förstermann U., Sessa W.C. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seddon M., Shah A.M., Casadei B. Cardiomyocytes as effectors of nitric oxide signalling. Cardiovasc Res. 2007;75:315–326. doi: 10.1016/j.cardiores.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 86.Kalivendi S.V., Kotamraju S., Zhao H., Joseph J., Kalyanaraman B. Doxorubicin-induced apoptosis is associated with increased transcription of endothelial nitric-oxide synthase: effect of antiapoptotic antioxidants and calcium. J Biol Chem. 2001;276:47266–47276. doi: 10.1074/jbc.M106829200. [DOI] [PubMed] [Google Scholar]
- 87.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hirata Y., Emori T., Eguchi S. Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. J Clin Invest. 1993;91:1367–1373. doi: 10.1172/JCI116338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yin Z., Zhao Y., Li H. miR-320a mediates doxorubicin-induced cardiotoxicity by targeting VEGF signal pathway. Aging (Albany NY) 2016;8:192–207. doi: 10.18632/aging.100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Octavia Y., Kararigas G., de Boer M. Folic acid reduces doxorubicin-induced cardiomyopathy by modulating endothelial nitric oxide synthase. J Cell Mol Med. 2017;21:3277–3287. doi: 10.1111/jcmm.13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.El-Boghdady N.A. Increased cardiac endothelin-1 and nitric oxide in adriamycin-induced acute cardiotoxicity: protective effect of Ginkgo biloba extract. Indian J Biochem Biophys. 2013;50:202–209. [PubMed] [Google Scholar]
- 92.Chaosuwannakit N., D’Agostino R.J., Hamilton C.A. Aortic stiffness increases upon receipt of anthracycline chemotherapy. J Clin Oncol. 2010;28:166–172. doi: 10.1200/JCO.2009.23.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shahin Y., Khan J.A., Chetter I. Angiotensin converting enzyme inhibitors effect on arterial stiffness and wave reflections: a meta-analysis and meta-regression of randomised controlled trials. Atherosclerosis. 2012;221:18–33. doi: 10.1016/j.atherosclerosis.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 94.Ricciotti E., FitzGerald G.A. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ritchie R.H., Rosenkranz A.C., Huynh L.P., Stephenson T., Kaye D.M., Dusting G.J. Activation of IP prostanoid receptors prevents cardiomyocyte hypertrophy via cAMP-dependent signaling. Am J Physiol Heart Circ Physiol. 2004;287:H1179–H1185. doi: 10.1152/ajpheart.00725.2003. [DOI] [PubMed] [Google Scholar]
- 96.Shinmura K., Tamaki K., Sato T., Ishida H., Bolli R. Prostacyclin attenuates oxidative damage of myocytes by opening mitochondrial ATP-sensitive K+ channels via the EP3 receptor. Am J Physiol Heart Circ Physiol. 2005;288:H2093–H2101. doi: 10.1152/ajpheart.01003.2004. [DOI] [PubMed] [Google Scholar]
- 97.Whorton A.R., Montgomery M.E., Kent R.S. Effect of hydrogen peroxide on prostaglandin production and cellular integrity in cultured porcine aortic endothelial cells. J Clin Invest. 1985;76:295–302. doi: 10.1172/JCI111960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Neilan T.G., Jassal D.S., Scully M.F. Iloprost attenuates doxorubicin-induced cardiac injury in a murine model without compromising tumour suppression. Eur Heart J. 2006;27:1251–1256. doi: 10.1093/eurheartj/ehl003. [DOI] [PubMed] [Google Scholar]
- 99.Sanchez-Soria P., Camenisch T.D. ErbB signaling in cardiac development and disease. Semin Cell Dev Biol. 2010;21:929–935. doi: 10.1016/j.semcdb.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heineke J., Molkentin J.D. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 101.Geisberg C.A., Abdallah W.M., da Silva M. Circulating neuregulin during the transition from stage A to stage B/C heart failure in a breast cancer cohort. J Card Fail. 2013;19:10–15. doi: 10.1016/j.cardfail.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lemmens K., Doggen K., De Keulenaer G.W. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation. 2007;116:954–960. doi: 10.1161/CIRCULATIONAHA.107.690487. [DOI] [PubMed] [Google Scholar]
- 103.An T., Zhang Y., Huang Y. Neuregulin-1 protects against doxorubicin-induced apoptosis in cardiomyocytes through an Akt-dependent pathway. Physiol Res. 2013;62:379–385. doi: 10.33549/physiolres.932516. [DOI] [PubMed] [Google Scholar]