Abstract
Identifying disease-associated missense mutations remains a challenge, especially in large-scale sequencing studies. Here we establish an experimentally and computationally integrated approach to investigate the functional impact of missense mutations in the context of the human interactome network and test our approach by analyzing ~2,000 de novo missense (dnMis) mutations found in autism subjects and their unaffected siblings. Interaction-disrupting dnMis mutations are more common in autism probands, these mutations principally affect hub proteins, and they disrupt a significantly higher fraction of hub interactions than in unaffected siblings. Additionally, they tend to disrupt interactions involving genes previously implicated in autism, providing complementary evidence that strengthens previously identified associations and enhances discovery of new ones. Importantly, by analyzing dnMis data from six disorders, we demonstrate that our interactome perturbation approach offers a generalizable framework for identifying and prioritizing missense mutations that contribute risk to human disease.
Introduction
Mutations disrupting the function of proteins are recognized as an important source of risk for developmental disorders (DDs), such as intellectual disability1,2, autism spectrum disorder (ASD)3, and congenital heart defects4. Whole-exome sequencing (WES) has produced a boon of findings linking de novo mutations to risk for DDs5–19. Not all mutations are simple to interpret as causing a loss of gene function. Missense mutations are especially difficult; although there are bioinformatics tools to predict the level of damage20–22, these annotators are far from perfect. This is a critical deficiency because the majority of coding mutations are missense. Here we show that one key feature in evaluating the disruptiveness of mutations is whether they fall in known or predicted protein-protein interaction interfaces and their likelihood to disrupt these interactions.
Large-scale studies of known disease-associated mutations have already reported a strong association with binding interfaces of protein interactions23,24. The major bottleneck for wide application of this feature is limited knowledge about the set of interactions and the binding interfaces of all interactions. To experimentally evaluate the impact of mutations on protein interactions, we establish a high-throughput mutagenesis and interactome-scanning pipeline for generating site-specific mutant clones and testing corresponding mutant protein interactions. Such a pipeline, however, cannot evaluate the impact of missense mutations on many interactions, because high-throughput interaction assays are limited in their coverage25–27. For this reason, we also explore a computational approach for systematically examining the functional impact of missense mutations on protein interactions. This approach builds on our newly-established full-interactome interface predictions28 to computationally predict the impact of all missense mutations on all associated interactions. Here we apply our experimental and computational approaches in tandem, which can be applied to any WES study.
To evaluate the effectiveness of our integrated experimental-computational approach, we focus on 2,821 de novo missense (dnMis) mutations identified from WES of ~2,500 families from the Simons Simplex Collection (SSC)29 (Supplementary Table 1). The SSC targets the study of ASD through a cohort of parent-offspring trios or quads with two unaffected parents, an ASD proband and, for most families, an unaffected sibling30. Previous analyses of the SSC data have reported significantly higher de novo mutation rates in ASD probands versus unaffected siblings across various mutation types, from copy number variants (CNVs)29,31,32, frameshift indels33, to missense mutations12–14,16. While a number of risk de novo copy number29,31,32,34,35 and protein truncating12–14,16,33 variants have been identified, exactly which dnMis mutations play a role and to what extent are open questions. We applied our integrated framework to evaluate the effect of 1,733 dnMis mutations within a protein interactome framework aiming to identify potentially disease-contributing dnMis mutations. Though there are many ways by which a missense mutation can impact a protein’s function, such as by destabilizing protein folding, we evaluate the disruptiveness of a mutation within our framework exclusively on its capacity to disrupt protein interactions, measured experimentally or through prediction. We further compare the network properties of proteins impacted by interaction-disrupting and non-disrupting dnMis mutations, using unaffected siblings as negative controls throughout. While our analyses focus on dnMis mutations in ASD, the integrated experimental-computational approach provides a generalizable framework for investigating the impact of missense mutations uncovered by WES for human diseases.
Results
Proband dnMis mutations are enriched on interaction interfaces
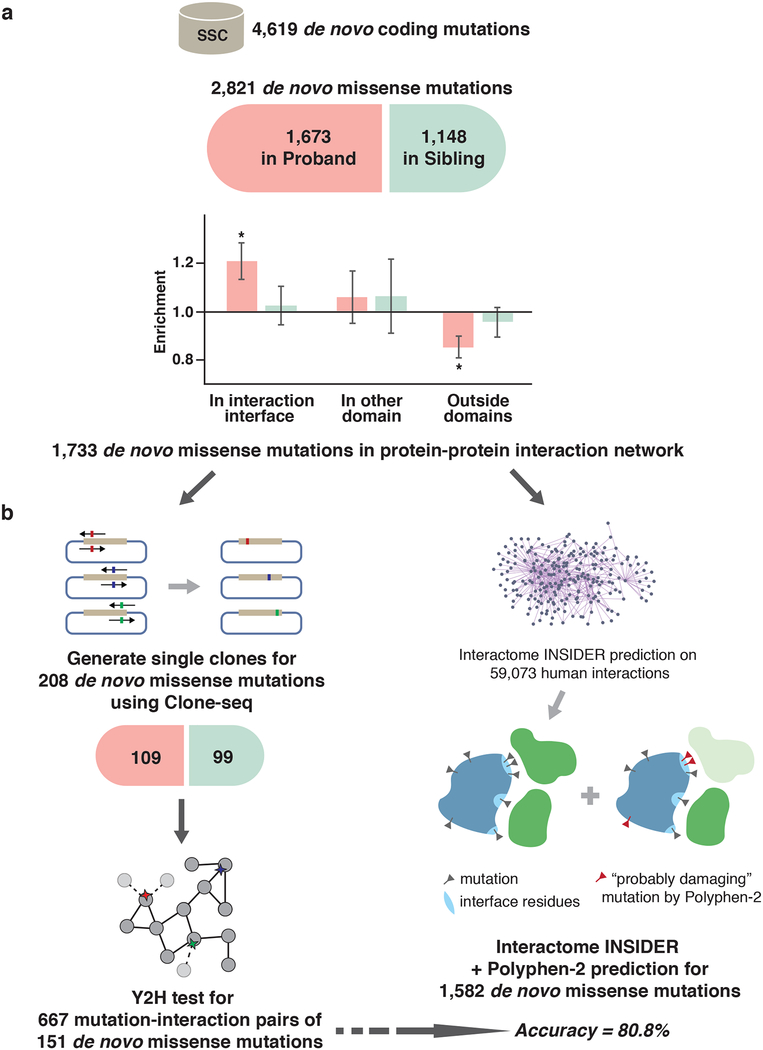
We previously reported that inherited in-frame disease-associated mutations are significantly enriched on protein interaction interfaces and demonstrated that alteration of specific protein interactions is crucial in the pathogenesis of many disease-associated genes36. To explore the relationship between non-inherited dnMis mutations and autism, we used a structurally-resolved 3D human interactome network36,37 to examine where dnMis mutations reside with respect to interaction interfaces. We found that in probands, dnMis mutations are significantly enriched on interaction interfaces: while interaction interfaces cover 30.1% of the proteins harboring these mutations, 38.2% of the mutations fall in interaction interfaces (1.27 fold, P= 2.9×10−3 by two-tail exact binomial test). In contrast, dnMis mutations in siblings fall in interaction interfaces on corresponding proteins at an expected rate (observed 37.6% versus expected 36.5%, 1.03 fold, P = 0.76). Thus, disruption of specific interactions could contribute to ASD etiology for dnMis mutations in probands (Fig. 1a), underscoring the functional significance of dnMis mutations on protein interaction interfaces.
Fig. 1.
Workflow of our integrated experimental-computational interactome perturbation framework. (a) Distribution of de novo missense (dnMis) mutations from SSC across different protein locations. Enrichment was calculated by the ratio of the observed fraction of dnMis mutations that occur on interaction interfaces over the fraction of interface residues on corresponding proteins (expected fraction). P-values were calculated using two-tail exact binomial test (*P < 0.05; Online Methods). Error bars indicate ± standard error. (b) Experimental (left) and computational (right) pipelines for assessing the functional impact of dnMis mutations on protein-protein interactions.
Proband dnMis mutations have elevated disruption rates
We next explored the impact of dnMis mutations on protein interactions by intersecting all 2,821 dnMis mutations with 59,073 human protein interactions from a comprehensive set of high-quality physical interactions compiled from eight widely-used interaction databases38, including BioGRID39, MINT40, iRefWeb41, DIP42, IntAct43, HPRD44, MIPS45, and the PDB46. Of these mutations, 1,733 are on proteins with at least one known interaction within the current human interactome dataset. To experimentally assess the impact of a subset of these mutations, 208 individual clones were generated carrying dnMis mutations – corresponding to 109 in probands and 99 in siblings, respectively – using Clone-seq, a massively parallel site-directed mutagenesis pipeline24. Protein interactions amenable to yeast two-hybrid (Y2H) were then tested, yielding 667 total protein interactions corresponding to 151 of our cloned dnMis mutations (Fig. 1b; Online Methods).
To explore the remaining dnMis mutations and interactions untested by Y2H, we applied a two-tiered computational approach that first predicts whether a particular residue is an interface residue using Interactome INSIDER28, a unified machine-learning framework comprising the first full-interactome map of human interaction interfaces. To determine whether a particular mutation is deleterious, we used PolyPhen-2 (PPH2)20 predictions: if a particular residue is predicted to be an interface residue and its mutation is scored as “probably damaging” by PPH2, that mutation was predicted as interaction-disrupting; if a mutation is unlikely to occur at an interface residue and is scored as “benign” by PPH2, it was predicted as interaction non-disrupting (Fig. 1b; Online Methods).
To evaluate the performance of our computational predictions, we applied this two-tiered prediction approach to our 667 experimentally tested protein interactions and obtained an accuracy of 80.8% (sensitivity: 65.0%, specificity: 82.5%). Additionally, when our approach was applied to a previously published, independent dataset of 204 disease-associated mutations and their impact on protein-protein interactions24, we obtained a similar prediction performance (accuracy: 77.4%, sensitivity: 81.0%, specificity: 75.0%).
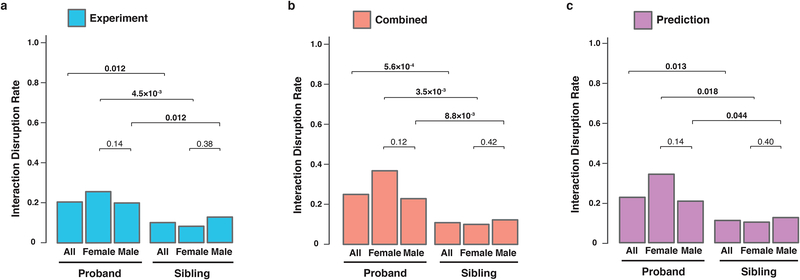
We then analyzed the distribution of disrupted interactions across ASD probands and unaffected siblings. Examining our experimental data revealed that 74/361 (20.5%) tested interactions were disrupted in probands. In contrast, only 21 out of 208 (10.1%) interactions were disrupted in unaffected siblings. Modeling the count of disruptions per subject with a negative binomial model, using case status as the predictor, yielded a 2.54-fold higher rate of disruptions in probands (P = 0.012, Fig. 2a and Supplementary Table 2; Online Methods). This sharp contrast in interaction disruption rate suggests that disruption of the interactome network by dnMis mutations contributes to autism etiology in probands. Combining the experimental data with predictions for all remaining dnMis mutations and interactions, there was again a significant, 2.34-fold higher disruption rate for probands (25.3%) versus unaffected siblings (10.8%, P = 5.6×10−4, Fig. 2b). Furthermore, the predicted disruptions alone showed significantly higher rate of disruption in probands than siblings (2.15 fold, P = 0.013, Fig. 2c). These observations suggest that dnMis mutations in ASD probands are of higher functional consequence than those in unaffected siblings. Therefore, interaction-disrupting mutations identified by our integrated experimental-computational framework could serve as a viable approach for identifying candidate risk variants, which may go undetected by other methodologies. Hereinafter, we shall present results using the combined data. Results using only the Y2H data or predictions are provided in Supplementary Fig. 1.
Fig. 2.
dnMis mutations are more disruptive in ASD probands than in unaffected siblings. Interaction disruption rates of dnMis mutations (a) tested experimentally, (b) by combining experimental results and predictions, and (c) predicted computationally. Probands and unaffected siblings are divided by sex. The count of disruptions per subject was modeled with a negative binomial model (P < 0.05 in bold). Combined: 1,080 out of 4,275 measured interactions were disrupted in ASD probands (25.3%) and 322 out of 2,973 were disrupted in unaffected siblings (10.8%). The interaction disruption rate is significantly higher in ASD probands than that in unaffected siblings (FC = 2.34 [1.44–3.79, 95% CI], P = 5.6×10−4 by two-tail negative binomial test). The trend persists in male and female subgroups: 23.1% disruption rate in male probands versus 12.3% in male siblings (FC = 2.21 [1.15–4.25, 95% CI], P = 8.8×10−3 by one-tail negative binomial test); 37.3% disruption rate in female probands versus 9.9% in female siblings (FC = 3.50 [1.41–8.72, 95% CI], P = 3.5 10−3). Comparing disruption rates between males and females revealed a higher rate, although not quite significant, in females than males in ASD probands (FC = 1.71 [0.71–4.09, 95% CI], P = 0.12 by one-tail negative binomial test), whereas similar rates were observed in female and male siblings (FC = 1.08 [0.52–2.22, 95% CI], P = 0.42).
The female protective effect postulates that females require a larger genetic burden before being diagnosed with ASD7,47. Accordingly, we anticipate dnMis mutations in female probands to be more disruptive than those in male probands, although the 6.5:1 male:female ratio of probands could obscure true differences by limiting power. Indeed, we observed a higher disruption rate in females than in males among ASD probands, fold = 1.71, but the difference is not quite significant (P = 0.12). In contrast, the disruption rate in female versus male siblings is 1.08-fold and does not approach significance (P = 0.42, Fig. 2b).
Disruptive dnMis mutations in probands principally impact network hubs
Previous research has shown that genes harboring known disease-associated mutations differ strongly in their network properties in comparison to non-disease-associated genes48,49. Early studies reported that disease-associated genes often encode for protein hubs that mediate a greater number of protein interactions than their non-disease-associated counterparts as a whole50,51. However, researchers later argued that the observed hub-disease gene correlation might be entirely driven by a handful of hub-encoding essential genes classified within the disease-associated gene class48. Here we investigated whether proteins harboring disruptive dnMis mutations in ASD probands exhibit distinguishable network properties in the human interactome.
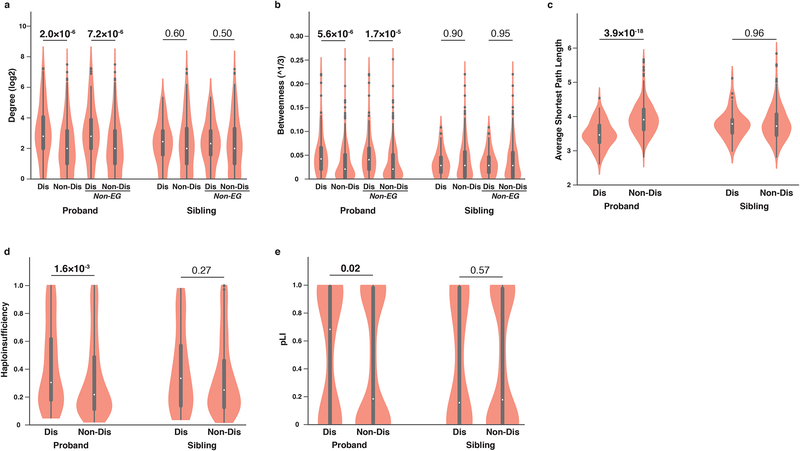
We first compared the degree of all proteins harboring interaction-disrupting dnMis mutations to those harboring non-disrupting dnMis mutations. We found that in ASD probands, proteins with interaction-disrupting dnMis mutations on average have a significantly higher degree than proteins with non-disrupting dnMis mutations (mean±s.e.m: 18.4±2.8 versus 9.3±1.0, fold change [FC] = 1.98, P = 2.0×10−6 by two-tail U-test, Fig. 3a), whereas no significant difference was observed in unaffected siblings (mean±s.e.m: 7.9±1.0 versus 11.4±1.3, FC = 0.69, P = 0.60). This suggests that interaction-disrupting dnMis mutations in ASD probands preferentially impact hub proteins, which play a central role in maintaining the integrity of the human interactome52. Importantly, when we excluded essential human genes53 from our analysis, the correlation between interaction-disrupting dnMis mutations and protein hubs persisted (mean±s.e.m: 17.6±2.9 versus 9.2±1.0, FC = 1.91, P = 7.2±10−6, Fig. 3a). Similarly, no such correlation in unaffected siblings was observed (mean±s.e.m: 8.0±1.0 versus 11.0±1.3, FC = 0.73, P =0.50). Likewise, when we analyzed betweenness, another measure of network centrality based on shortest paths, proteins harboring interaction-disrupting dnMis mutations have a significantly higher betweenness value than proteins harboring non-disrupting dnMis mutations, regardless of whether essential genes were included (Fig. 3b).
Fig. 3.
Disruptive proband dnMis mutations exhibit characteristic network and haploinsufficiency properties. (a) Degree and (b) betweenness distributions of proteins with interaction-disrupting (Dis, n = 109 in probands and n = 68 in siblings) or non-disrupting (Non-Dis, n = 342 in probands and n = 241 in siblings) dnMis mutations across all proteins and across non-essential gene-encoded proteins (Non-EG) in ASD probands (Dis: n = 106; Non-Dis: n = 338) and unaffected siblings (Dis: n = 66; Non-Dis: n = 238). (c) Average shortest path length distributions of proteins with dnMis mutations (in probands, Dis: n = 109, Non-Dis: n = 342; in siblings, Dis: n = 68, Non-Dis: n = 241). (d) Haploinsufficiency and (e) pLI distributions of genes with dnMis mutations. Genes with available haploinsufficiency or pLI scores were included in corresponding analyses (haploinsufficiency: in probands, Dis: n = 95, Non-Dis: n = 304; in siblings, Dis: n = 63, Non-Dis: n = 217; pLI: in probands, Dis: n = 106, Non-Dis: n = 338; in siblings, Dis: n = 63, Non-Dis: n = 237). Genes carrying de novo protein truncating variants (dnPTVs) in SSC data were excluded from all analyses. Violin plots: thick black bar, interquartile range; white dot, median; whiskers, upper and lower limits; points, outliers; while the width of each ‘violin’ is proportional to element abundance. P-values were calculated using two-tail U-test (P < 0.05 in bold).
To further assess whether disruptive dnMis mutations tend to be on essential genes, we analyzed gene essentiality measured by Wang et al. using CRISPR gene knockout screens54. Using this CRISPR score, we observed no significant difference in essentiality between genes with interaction-disrupting and non-disrupting dnMis mutations for probands (mean±s.e.m: −0.43±0.08 versus −0.33±0.04, FC = 1.30, P = 0.28 by two-tail U-test) or for unaffected siblings (mean±s.e.m: −0.37±0.09, versus −0.41±0.05, FC = 0.90, P = 0.39). This confirms that disruptive dnMis mutations have no tendency to be on essential genes while preferentially affecting topologically central positions in the interactome network.
We then investigated whether proteins with dnMis mutations tend to form inter-connected modules within the interactome network. We found that in ASD probands, proteins with interaction-disrupting dnMis mutations on average have a significantly smaller shortest path length to each other than proteins harboring non-disrupting dnMis mutations (mean±s.e.m: 3.48±0.04 versus 3.94±0.03, FC = 0.88, P =3.9×10−18 by two-tail U-test, Fig. 3c). This result indicates that proteins with disruptive dnMis mutations in probands tend to be closely connected to each other in the network and may therefore function as modules with specific roles in ASD etiology. In contrast, no such trend was observed for proteins with disruptive dnMis mutations in unaffected siblings (mean±s.e.m: 3.77±0.05 versus 3.79±0.03, FC = 0.99, P = 0.96), underscoring the functional significance of modules derived from interaction-disrupting dnMis mutations in ASD probands.
Overall, our analyses indicate that network topology should be considered when interpreting the impact of dnMis mutations. In this manner, we can investigate how disruptive missense mutations alter local community structure and how information flow through multiple mutations work together to rewire the whole network that can lead to autism or other disease-associated phenotypes.
Disruptive dnMis mutations in probands target haploinsufficient genes
Disruptive dnMis mutations typically occur only on one copy of the gene. To affect risk, they should occur more frequently on haploinsufficient genes, where a single copy of the wild-type gene is insufficient to carry out its normal function. In probands, genes harboring interaction-disrupting dnMis mutations have a significantly higher probability of being haploinsufficient55 than genes harboring non-disrupting dnMis mutations (mean±s.e.m: 0.42±0.03 versus 0.33±0.02, FC = 1.27, P = 1.6×10−3 by two-tail U-test, Fig. 3d). In contrast, no significant difference was observed in unaffected siblings (mean±s.e.m: 0.39±0.04 versus 0.34±0.02, FC = 1.15, P = 0.27). Reinforcing these findings, we also found that genes with interaction-disrupting dnMis mutations in probands are less tolerant to genetic variation, as indicated by their higher average pLI56 scores in comparison to genes with non-disrupting dnMis mutations (mean±s.e.m: 0.52±0.04 versus 0.43±0.02, FC = 1.21, P = 0.02, Fig. 3e). No such contrast was found in unaffected siblings (mean±s.e.m: 0.44±0.06 versus 0.44±0.03, FC = 1.00, P = 0.57). Collectively, these results demonstrate that interaction-disrupting dnMis mutations in ASD probands tend to affect haploinsufficient genes, for which heterozygous variations are not tolerated, and they may therefore contribute to ASD outcomes through dosage effect57.
Disruptive dnMis mutations in probands cluster closely to known ASD genes
To evaluate whether interaction-disrupting dnMis mutations are associated with ASD risk, we first investigated whether such mutations are enriched in previously reported ASD-associated genes. Using a curated list of 881 genes implicated in ASD in the SFARI database58, we observed a significant enrichment in probands for genes with interaction-disrupting dnMis mutations compared to genes with non-disrupting dnMis mutations (21/109 versus 32/342, OR = 2.3, P = 5.7×10−3 by one-tail Fisher’s exact test, Supplementary Table 3). In contrast, no significant enrichment was observed in unaffected siblings (6/68 versus 17/241, OR = 1.3, P = 0.39). Thus, characterizing interaction perturbation captures new evidence to establish associations of genes with ASD.
Previous studies have reported functional clustering in genes with de novo protein truncating variants (dnPTVs) in ASD individuals13,14,16,59. Here we assessed the network distance within the human interactome between genes harboring interaction-disrupting dnMis mutations (excluding genes with dnPTVs) and seven classes of known ASD-associated genes. These genes (Supplementary Table 4) include: (1) FMRP target genes, with transcripts bound by the fragile X mental retardation protein (FMRP); (2) genes encoding chromatin modifiers (CHM); (3) genes expressed preferentially in embryos (EMB); (4) genes encoding postsynaptic density proteins (PSD); (5) 881 genes in the SFARI database; (6) a high-quality SFARI subset (SFARI-hq, 141 genes scored as syndromic, high confidence, or strong candidate58); and (7) the latest set of 65 ASD genes discovered by de novo mutations (DN65)29. We found that in probands, proteins harboring interaction-disrupting dnMis mutations are significantly closer to proteins from all seven classes in comparison to proteins with non-disrupting dnMis mutations (Table 1 and Supplementary Note; Online Methods). In contrast, no significant differences were observed among unaffected siblings in any category. These findings demonstrate that disruptive dnMis mutations identified by our study are indeed closely related to known ASD genes and functional classes and that they may contribute to ASD etiology by disrupting common pathways shared with dnPTVs.
Table 1.
Distance of genes with interaction-disrupting (Dis) and non-disrupting (Non-Dis) dnMis mutations to seven classes of known ASD-associated genes in a protein interactome network background. Number of genes in each class is indicated in parentheses. Genes carrying de novo protein truncating variants (dnPTVs) in SSC data were excluded from the analyses. P-values were calculated using one-tail U-test (P < 0.05 in bold).
| Proband | Sibling | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dis (109) | Non-Dis (342) | P-value | Dis (68) | Non-Dis (241) | P-value | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| FMRP (794) | 2.61 | 0.38 | 2.85 | 0.46 | 1.5×10−6 | 2.77 | 0.37 | 2.77 | 0.48 | 0.57 |
| CHM (408) | 2.55 | 0.38 | 2.79 | 0.47 | 1.3×10−6 | 2.70 | 0.40 | 2.72 | 0.50 | 0.44 |
| EMB (1,865) | 2.65 | 0.38 | 2.88 | 0.46 | 2.9×10−6 | 2.79 | 0.38 | 2.81 | 0.48 | 0.45 |
| PSD (1,395) | 2.61 | 0.37 | 2.84 | 0.46 | 2.4×10−6 | 2.76 | 0.37 | 2.77 | 0.47 | 0.47 |
| SFARI (881) | 2.69 | 0.38 | 2.92 | 0.46 | 1.8×10−6 | 2.83 | 0.37 | 2.85 | 0.48 | 0.52 |
| SFARI hq (141) | 2.62 | 0.38 | 2.86 | 0.46 | 1.1×10−6 | 2.77 | 0.37 | 2.77 | 0.49 | 0.58 |
| DN65 (65) | 2.70 | 0.39 | 2.94 | 0.46 | 1.0×10−6 | 2.85 | 0.37 | 2.86 | 0.48 | 0.52 |
Identification of candidate ASD genes and mutations
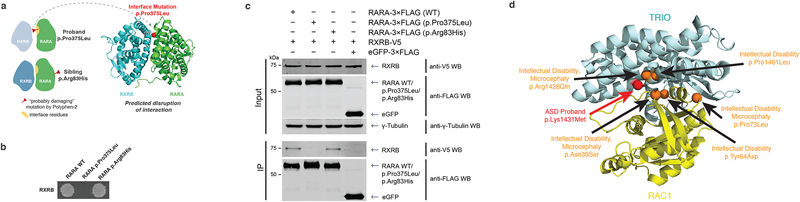
Towards the identification of new candidate ASD-associated genes, we examined mutations on the protein RARA (Supplementary Note). RARA binds with RXRB to form the retinoic acid (RA) receptor complex. When bound to RA, the retinoic acid receptor can then bind RA receptor elements (RAREs) to co-activate transcription of downstream genes. In agreement with our Y2H experiments, our computational approach predicted that a proband mutation p.Pro375Leu on RARA (NP_000955.1) is disruptive, while an unaffected sibling mutation, p.Arg83His, is not (Fig. 4a and Fig. 4b). We note that PPH2 predicts both mutations to be probably damaging and cannot distinguish the two. We further confirmed by co-immunoprecipitation in human cells that the proband mutation p.Pro375Leu disrupts the RARA-RXRB interaction while the sibling mutation p.Arg83His does not (Fig. 4c; Online Methods).
Fig. 4.
Identification of candidate ASD-associated genes and mutations through our interactome perturbation framework. (a) Computational prediction of the effects of RARA p.Pro375Leu and RARA p.Arg83His on the RARA-RXRB interaction. A homology model highlighting the RARA p.Pro375Leu interface mutation is shown. (b) RARA p.Pro375Leu disruption and RARA p.Arg83His non-disruption of RARA-RXRB interaction by Y2H. (c) Co-immunoprecipitation confirming RARA p.Pro375Leu disruption and RARA p.Arg83His non-disruption of RARA-RXRB interaction in HEK 293T cells. See Supplementary Fig. 10 for uncropped gel images. (d) Co-crystal structure of TRIO-RAC1 (PDB ID: 2NZ8) displaying the structural locations of proband ASD (red) and intellectual disability and/or microcephaly (orange) dnMis mutations across the interaction interfaces.
While there is insufficient evidence to directly link mutations on RARA to ASD, there is compelling evidence that mutated RARA does induce ASD risk by affecting RA signaling. Specifically, we would expect the p.Pro375Leu mutation to diminish RA signaling by disrupting its binding to RXRB. Notably, one of the most common genetic risk factors for ASD is maternal duplication of 15q11–q13 and isodicentric chromosome 1560, both of which increase transcription of UBE3A, among other genes. It has recently been shown that UBE3A negatively regulates ALDH1A proteins61, which act as rate-limiting enzymes in RA synthesis. Increased dosage of UBE3A diminishes RA synthesis and RA signaling, altering neuronal development and features such as homeostatic synaptic plasticity61. Moreover, in mice, ASD-like phenotypes are induced by over-expression of UBE3A or by an ALDH1A antagonist, while the wild-type phenotype can be rescued by RA supplementation61. Thus, together with published results regarding the role of UBE3A in RA signaling and autism risk61, our results implicate RARA as an ASD-associated gene, and our experimentally-validated interaction-disrupting prediction for RARA p.Pro375Leu demonstrates how our methodology can be used to identify functional dnMis mutations.
The occurrence of a predicted disruptive mutation near other closely related disease-associated dnMis mutations across interacting proteins can lend strong evidence towards the postulated functionality and shared phenotypic impact of the mutation in question. In this regard, our computational approach predicted an ASD proband mutation, p.Lys1431Met, on the guanine nucleotide exchange factor TRIO (NP_651960.2) that disrupts its interaction with the GTPase RAC1 (Fig. 4d). Of note, two neurodevelopmental disorder dnMis mutations18,62 on TRIO, p.Arg1428Gln and p.Pro1461Leu, occur in structural proximity to the ASD proband interface mutation, p.Lys1431Met, as do three dnMis mutations on RAC1 (NP_008839.2) interface residues, p.Asn39Ser, p.Tyr64Asp, and p.Pro73Leu, which all result in mild to severe intellectual disability63 (Fig. 4d). Moreover, p.Lys1431Met has been recently reported to functionally inhibit synaptic function in human cell lines and statistically postulated to reside within a hotspot for ASD-related de novo mutations in the GEF1 domain of TRIO64. As sequencing data from DD studies becomes more readily available, we anticipate the use of predicted interaction-disrupting mutations to uncover shared molecular pathways between related DDs.
An excess of dnMis mutations in DDs occur on interaction interfaces
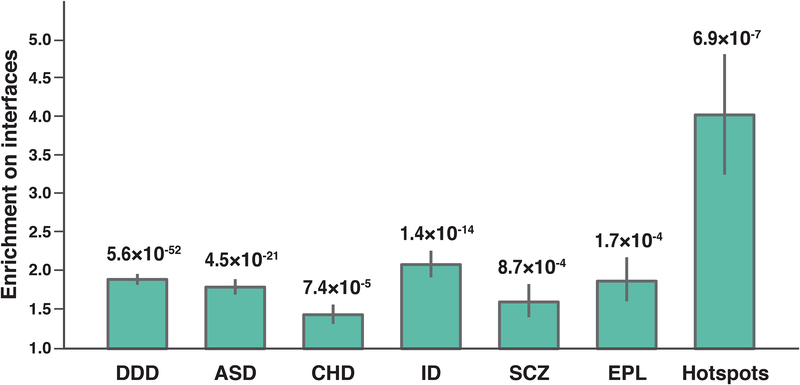
To demonstrate the generalizability of our interactome perturbation approach towards studying the impact of missense mutations in human disease, we investigated how ~10,000 dnMis mutations previously detected in DDs correspond with protein interaction interfaces. The mutation data comprises a collection of 4,565 dnMis mutations from the Deciphering Developmental Disorders project and five lists of dnMis mutations curated from studies of autism, congenital heart disease, intellectual disability, schizophrenia, and epilepsy (denovo-db v.1.5)65. We found that in all six datasets, dnMis mutations occur significantly more frequently on protein interaction interfaces than expected (Fig. 5), indicating that dnMis mutations in DDs can contribute to disease risk by impacting protein interactions. In particular, the strongest signal was observed in intellectual disability: 23.5% of the dnMis mutations occurred on interaction interfaces, resulting in an enrichment of 2.09 (1.77–2.44, 95% CI) in comparison to the fraction of interface residues on corresponding proteins (11.2%, P = 1.4×10−14 by two-tail exact binomial test). In contrast, dnMis mutations in schizophrenia had the weakest significance (Enrichment = 1.61 [1.22–2.06, 95% CI], P = 8.7 10−4), which agrees with previous findings that schizophrenia has a much weaker de novo signal than other DDs66.
Fig. 5.
dnMis mutations are enriched on protein interaction interfaces in developmental disorders. Enrichment was calculated by the ratio of the observed fraction of dnMis mutations that occur on interaction interfaces over the fraction of interface residues on corresponding proteins (expected fraction). Error bars indicate ± standard error. P-values were calculated using two-tail exact binomial test. DDD (Deciphering Developmental Disorders project, n = 2,914 dnMis mutations): Enrichment = 1.90 (1.76–2.04, 95% CI); ASD (autism spectrum disorder, n = 1,512): Enrichment = 1.80 (1.61–2.00, 95% CI); CHD (congenital heart disease, n = 759): Enrichment = 1.44 (1.21–1.70, 95% CI); ID (intellectual disability, n = 498): Enrichment = 2.09 (1.77–2.44, 95% CI); SCZ (schizophrenia, n = 312): Enrichment = 1.61 (1.22–2.06, 95% CI); EPL (epilepsy, n = 181): Enrichment = 1.88 (1.36–2.48, 95% CI); Hotspots (n = 31): Enrichment = 4.03 (2.51–5.58, 95% CI).
Geisheker et al. recently reported 40 dnMis hotspots implicated in neurodevelopmental disorder pathogenesis67. When we examined the 31 corresponding hotspots within the interactome network, we found that they occur on protein interaction interfaces at a very high rate of 48.4% (Enrichment = 4.03 [2.51–5.58, 95% CI], P = 6.9 10−7, Fig. 5). This suggests that interactome perturbations play an important role in the pathogenesis linked with these recurrent events. Taken together, these findings reinforce that our integrated experimental-computational interactome perturbation approach offers a scalable and generalizable framework to identify risk dnMis mutations in human disease.
Discussion
Here we demonstrated that dnMis mutations can contribute to ASD risk by disrupting protein-protein interactions and that our interactome perturbation framework offers a novel and effective way to identify ASD risk dnMis mutations. Because only a small fraction of dnMis mutations found in ASD subjects are believed to be functional12, this framework helps overcome a significant challenge in identifying risk dnMis mutations. Our analyses focused on dnMis mutations from the SSC families because the information on unaffected siblings in the dataset provides robust negative controls. Our results demonstrated that interaction-disrupting dnMis mutations in ASD probands preferentially impact proteins that have many interaction partners in the interactome network (i.e., hubs) and disrupt these interactions at a significantly higher rate than those in unaffected siblings. Our results also lend evidence to previously reported ASD-associated genes and pathways by showing that interaction-disrupting dnMis mutations are closely clustered to proteins in ASD-associated functional classes in the interactome network. Thus, characterizing interactome perturbation provides additional and potentially orthogonal information to strengthen previously identified genetic associations and helps discover new genes that contribute to ASD risk.
Integration of computational predictions with experimental data imbued far more meaning onto missense mutations found in ASD probands and their siblings. Thus the prediction model alone can enhance researchers’ ability to prioritize damaging missense mutations and can be applied across a wide range of human disease studies. We emphasize that the strength of this prediction model is rooted in its integration of PPH2 scores and Interactome INSIDER interface predictions. To demonstrate this, we repeated all analyses using PPH2 and Interactome INSIDER separately. The results show that neither method individually is sufficient to reproduce most signals towards identifying disease-contributing dnMis mutations in ASD (Supplementary Fig. 2 and Supplementary Fig. 3). This confirms that our two-tiered predictor, which evaluates the disruptiveness of a variant on protein interactions, greatly improves the effectiveness of predicting functional missense mutations. We also note that our predictor is robust at different PPH2 score cutoffs (Supplementary Fig. 4). Taken together, we demonstrate that our computational prediction approach can serve as an effective and robust method to identify disease-contributing missense mutations.
Stronger associations between ASD proband mutations and clinical data can be established by filtering out ASD dnMis mutations that are also identified as population variants in the Exome Aggregation Consortium (ExAC)56 since these variants represent standing variations in the population and are less likely to be deleterious as a result68. Adopting this same principle, we found that dnMis mutations in DDs are significantly more enriched on interaction interfaces when mutations coinciding with ExAC are filtered out in comparison to the non-filtered set (Supplementary Fig. 5 and Supplementary Note). Importantly, our results show that the characteristic network and haploinsufficiency properties of disruptive proband dnMis mutations are not unique to ASD but are shared features across different DDs (Supplementary Fig. 6), indicating that our interactome perturbation framework is generalizable to prioritize dnMis mutations across a wide range of DDs (Supplementary Note).
Our analyses indicate that network properties are important in interpreting the functional impact of dnMis mutations and their relevance towards disease etiology. However, we recognize that the human interactome with which these analyses are performed is currently incomplete. As a result, certain classes of protein interactions, for example interactions mediated by membrane-bound proteins, may be under-represented in the current interactome, limiting potential insights from such proteins. Moreover, literature-derived segments of the human interactome are subject to sampling bias present in small-scale studies38,69. Therefore, we re-examined the network topology analyses across a chronologically-ordered series of unbiased high-throughput (HT)-derived human interactomes (Supplementary Note). We show that not only are our results robust across all HT-derived interactomes, more importantly, we also demonstrate that the topological differences between interaction-disrupting and non-disrupting dnMis mutations in probands becomes more significant as the interactome coverage increases (Supplementary Fig. 7). Moreover, we show that all our results remain the same when we expanded our disruptiveness predictions to include all dnMis mutations in the current human interactome (Supplementary Fig. 8 and Supplementary Note). Taken together, we fully expect that as increasingly more human protein-protein interactions and mutations are uncovered, our interactome perturbation framework can be applied to these new interactions and mutations to identify new or currently under-characterized disease-associated mutations and genes.
As large-scale WES studies continue to produce mutation data at ever-increasing scales, our interaction-disruption prediction approach can greatly extend the reach of interactome perturbation studies for investigating complex genotype-phenotype relationships and improving our understanding of how genetic variation affects disease risk through the alteration of topological and community structures of networks.
Online Methods
Enrichment of dnMis mutations on interaction interfaces
The set of 412 proteins with dnMis mutations and containing at least one interaction interface and one known domain was included for calculating dnMis mutation distribution. The sequences were divided into three regions: “in interaction interface”, “in other domain” and “outside domains”. Interaction interfaces were determined by our previously developed human structural interaction network (hSIN36, comprising 4,222 structurally resolved interactions between 2,816 proteins). Other domains were referred to protein domains (obtained from Pfam70 database) that exclude interacting interfaces in hSIN. The rest of residues then were categorized as “outside domains”. If the locations of mutations were not influenced by the domain architecture of the protein, then their relative lengths should determine the frequency of mutations in these three regions. The fraction of mutations expected by chance in each region was calculated by adding the total sequence length of each region in all proteins, and dividing it by the length of all proteins combined; call the probability of falling in an interaction interface p. The number of observed mutations in each region over all proteins was also computed; call the number falling in the interaction interfaces S, and let N be the total number of dnMis missense mutations. An exact binomial test was then computed from p, S, and N. Confidence intervals (CIs) are based on 95% CI for an exact binomial, then transformed to the risk ratio (Enrichment) using the expectation in the denominator and the lower/upper bound in the numerator.
In ASD probands, the total length of 248 proteins is 377,421, which compromises 113,449 residues on interaction interfaces, 69,870 residues on other domains, and 194,102 residues outside domains. The probabilities for a mutation to fall in these regions were computed to be 30.1%, 18.5%, and 51.4%, respectively. The observed distribution of the 296 dnMis mutations on these proteins was 113 on interaction interfaces, 59 on other domains, and 124 outside domains, revealing that dnMis mutations in ASD probands are significantly enriched on protein interaction interfaces (Enrichment = 1.27 [1.09–1.46, 95% CI], P = 2.9×10−3) while occur on other domains with expected rate (Enrichment = 1.08 [0.84–1.35, 95% CI], P = 0.55) and are depleted from regions outside domains (Enrichment = 0.81 [0.70–0.93, 95% CI], P = 1.1×10−3). In contrast, the observed 186 dnMis mutations in unaffected siblings occur on all three regions with expected rates: 70/186 fall on interaction interfaces (37.6% versus expected 36.5%, Enrichment = 1.03 [0.84–1.23, 95% CI], P = 0.76), 32/186 fall on other domains (17.2% versus expected 15.9%, Enrichment = 1.08 [0.76–1.47, 95% CI], P = 0.62), and 84/186 fall outside domains (45.2% versus expected 47.6%, Enrichment = 0.95 [0.79–1.10, 95% CI], P = 0.51).
Cloning of 208 dnMis mutations using our massively-parallel Clone-seq pipeline
Single colony-derived mutant clones were constructed using a high-throughput mutagenesis and next-generation sequencing pipeline called Clone-seq24 (Supplementary Fig. 9). Wild-type clones were picked from hORFeome v8.171 to serve as templates for site-directed mutagenesis (Eurofins). Mutagenesis was performed at 96-well scales using site-specific mutagenesis primers and full-length human ORF templates. PCR product was digested overnight using DpnI (NEB) without a ligation step to maximize throughput then transformed directly into competent cells to isolate single colonies. Four colonies per mutagenesis reaction were then hand-picked and arrayed into 96-well plates. After 21 hrs incubation at 37°C, glycerol stocks were generated then clones were pooled into four respective bacterial pools. Maxiprepped DNAs from each of the four pools were then combined through multiplexing (NEBNext) then sequenced in a single 1×100 single-end Illumina HiSeq run. Properly mutated clones were then identified by next-generation sequencing analysis and recovered from single-colony glycerol stocks. In total, we generated individual clones for 208 dnMis mutations comprising 109 from ASD probands and 99 from unaffected siblings.
Experimental examination of 667 protein-protein interactions using our high-throughput yeast two-hybrid (Y2H) assay
To perform Y2H, pDEST-AD and pDEST-DB plasmid vectors corresponding to the GAL4 activating domain (AD) and DNA-binding (DB) domain, respectively, were used. Full-length Clone-seq identified mutant clones were transferred into Y2H-amenable pDEST-DB and pDEST-AD vectors by Gateway LR reactions then transformed into MATα Y8930 and MATa Y8800, respectively. All DB-ORF MATα transformants, including wild-type ORFs, were then mated against corresponding wild-type (WT) and mutant AD-ORF MATa transformants in a pairwise orientation on YEPD agar plates. After mating, yeast was replica-plated onto selective SC-Leu-Trp-His+ 1 mM of 3-amino-1,2,4-triazole (3AT) as well as SCLeu-Trp-Adenine plates. Interactions were scored after 3 days of incubation and 5 days of incubation for SC-Leu-Trp+3AT and SC-Leu-Trp-Ade plates, respectively. To screen out autoactivating DB-ORFs, all DB-ORF MATα transformants were also mated pairwise against empty pDEST-AD MATa transformants and scored for growth on SC-Leu-Trp+3AT and SC-Leu-Trp-Ade plates. DB-ORFs that trigger reporter activity under this setup were removed from further experiments. We finally examined 667 interactions, of which the WT proteins could be detected with strong Y2H-positive phenotypes in our experiments, for 151 out of the 208 total dnMis mutations that we have successfully generated. The other 57 dnMis mutations corresponded to proteins with no testable interaction partners by Y2H; therefore, they were excluded from Y2H experiments. While on average each of the 151 mutations was tested against 4–5 interaction partners, two proband mutations (Q8TBB1 p.Glu295Lys and Q8TD31 p.Trp337Arg) had >40 interaction partners tested and disrupted >30 of their corresponding interactions. Thus, we excluded these two outliers when comparing the disruption rates of dnMis mutations in ASD probands and unaffected siblings (Fig. 2a).
Computational prediction for protein-protein interaction disruption
For the remaining 1,582 dnMis mutations, we assessed their probabilities to disrupt an interaction based on whether they are likely to be on protein interaction interfaces and whether they tend to have damaging functional effects on the protein. We first applied an ensemble machine learning algorithm to predict interface residues (Interactome INSIDER). For each of these dnMis mutations, on each of its interactions with an interaction-specific partner, we considered a mutation to be an interaction interface residue for this specific interaction if it has a probability score of very high, high or medium in Interactome INSIDER prediction. We next evaluated its deleteriousness using PolyPhen-2 (PPH2). If a mutation predicted as an interface residue also has a “probably damaging” PPH2 score (Interface+ and PPH2+), we considered this mutation to disrupt the interaction. On the other hand, we called a mutation non-disrupting if it was predicted to be unlikely an interaction interface residue (probability below “medium” by Interactome INSIDER) and to be “benign” to the protein by PPH2 (Interface− and PPH2−). Considering that using individual measurements (PPH2 alone or Interactome INSIDER alone) does not provide sufficient signal towards whether a mutation is damaging or not (Supplementary Fig. 2 and Supplementary Fig. 3), mutations that only meet one of these two criteria (Interface+ and PPH2-; Interface- and PPH2+) were excluded from the analyses. Importantly, when we included all the Interface+PPH2− and Interface−PPH2+ mutations as non-disrupting to our analyses, we found that all our results remain the same (Supplementary Fig. 8 and Supplementary Note).
Modeling the number of disrupted interactions as a function of case-control status
Some missense mutations fail to disrupt any interactions, D = 0 disruptions. Other mutations, however, can disrupt D = 1, 2, …, I interactions. To account for the dispersion in D, and to determine whether D is stochastically greater for missense mutations found in ASD probands versus unaffected siblings, we modeled D as a negative binomial distribution and fit it to case-control status. We also evaluated other models for goodness-of-fit, specifically Poisson and zero-inflated versions of Poisson and negative binomial. After accounting for degrees of freedom, none of these models fit the data as well as the negative binomial by the Akaike information criterion.
Construction of plasmids for Western blot and co-immunoprecipitation
Wild-type RARA and RXRB entry clones were obtained from the hORFeome v8.171 collection. Gateway LR reactions were used to transfer bait RARA wild-type, p.Pro375Leu, and p.Arg83His into a pQXIP (ClonTech, 631516) vector modified to include a Gateway cassette featuring a C-terminal 3×FLAG. Prey RXRB was transferred into pcDNA-DEST40 which includes a V5 tag (Invitrogen, 12274–015) also using Gateway LR reactions.
Cell culture, co-immunoprecipitation, and Western blotting
HEK 293T cells were maintained in complete DMEM medium supplemented with 10% FBS. Cells were grown in 6-well dishes to 70–80% confluency then transfected using 1 μg bait construct and 1 μg prey construct with 10 μL of 1mg/mL PEI (Polysciences Inc, 23966) mixed thoroughly with 150 μL OptiMEM (Gibco, 31985–062). After 24 hrs incubation, cells were gently washed three times in 1x PBS and then resuspended in 200 μL cell lysis buffer (10 mM Tris-Cl pH 8.0, 137mM NaCl, 1% Triton X-100, 10% glycerol, 2 mM EDTA, and 1x EDTA-free Complete Protease Inhibitor tablet [Roche)] and incubated on ice for 30 mins. Extracts were cleared by centrifugation for 10 mins at 13,000 rpm at 4 °C. For co-immunoprecipitation, 100 μL cell lysate per sample were incubated with 5 μL EZ view Red Anti-FLAG M2 Affinity Gel (Sigma, F2426) for 2 hrs at 4°C under gentle rotation. After incubation, bound proteins were washed three times in cell lysis buffer then eluted in 50 μL elution buffer (10 mM Tris-Cl pH 8.0, 1% SDS) at 65°C for 10 mins. Cell lysates and co-immunoprecipitated samples were then treated in 6x SDS protein loading buffer (10% SDS, 1 M Tris-Cl pH 6.8, 50% glycerol, 10% β-mercaptoethanol, 0.03% Bromophenol blue) and subjected to SDS-PAGE. Proteins were then transferred from gels onto PVDF (Amersham) membranes. Anti-FLAG (Sigma, F1804), anti-V5 (Invitrogen, R960–25), and anti-γ-Tubulin (Sigma, T5192) at 1:5000, 1:3000, and 1:3000 dilutions, respectively, were used for immunoblotting analysis. Full scans of all the blots are supplied in Supplementary Fig. 10.
Evaluation of the distance between gene sets in the interactome network
We evaluated the distance between two gene sets using the method previously published by Neale et al.59: in an interactome background, the distance between two gene sets (L1and L2) is the average distance of each gene i in L1 to L2, where the distance of a specific gene i in L1 to L2 is the average distance of gene i to each gene j in L2 Let n1 and n2 be the number of genes in L1 and L2,
where .
Then consider i and j as two nodes in the interactome network, the distance between these two nodes) Distance(i,j) here is defined as the minimum number of intermediate nodes that connect i and j in the shortest path.
Life Sciences Reporting Summary
Further information on experimental design is available in the Life Sciences Reporting Summary.
Data availability
dnMis mutations in ASD subjects and their unaffected siblings came from published data in ref. 12 and are available in Supplementary Table 1. Interaction disruption results from Y2H experiments are available in Supplementary Table 2.
Supplementary Material
Acknowledgements
We would like to thank J.F. Beltrán, J. Liang, S. Wierbowski, and other Yu lab members for constructive discussions. This work was supported by National Institute of General Medical Sciences grants (R01 GM104424, R01 GM124559, R01 GM125639); National Cancer Institute grant (R01 CA167824); Eunice Kennedy Shriver National Institute of Child Health and Human Development grant (R01 HD082568); National Human Genome Research Institute grant (UM1 HG009393); National Science Foundation grant (DBI-1661380) to H.Y.; National Institute of Mental Health grant (R37MH057881) to B.D. and K.R.; and Simons Foundation Autism Research Initiative grants (SF367561 to H.Y., B.D., and K.R. and SF402281 to B.D. and K.R.).
We would like to thank the SSC principal investigators (A.L. Beaudet, R. Bernier, J. Constantino, E.H. Cook, Jr, E. Fombonne, D. Geschwind, D.E. Grice, A. Klin, D.H. Ledbetter, C. Lord, C.L. Martin, D.M. Martin, R. Maxim, J. Miles, O. Ousley, B. Peterson, J. Piggot, C. Saulnier, M.W. State, W. Stone, J.S. Sutcliffe, C.A. Walsh, and E. Wijsman) and the coordinators and staff at the SSC clinical sites; the SFARI staff, in particular N. Volfovsky; D. B. Goldstein for contributing to the experimental design; and the Rutgers University Cell and DNA repository for accessing biomaterials.
Footnotes
URLs
Interactome INSIDER predictions, http://interactomeinsider.yulab.org; SFARI database (downloaded on June 6, 2017), https://gene.sfari.org/database/human-gene/; denovo-db (v.1.5), http://denovodb.gs.washington.edu/denovo-db/Download.jsp.
Competing Interests
The authors declare no competing financial interests.
References
- 1.Ropers HH Genetics of early onset cognitive impairment. Annu Rev Genomics Hum Genet 11, 161–87 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Mefford HC, Batshaw ML & Hoffman EP Genomics, intellectual disability, and autism. N Engl J Med 366, 733–43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devlin B & Scherer SW Genetic architecture in autism spectrum disorder. Curr Opin Genet Dev 22, 229–37 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Bruneau BG The developmental genetics of congenital heart disease. Nature 451, 943–8 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Deciphering Developmental Disorders Study. Prevalence and architecture of de novo mutations in developmental disorders. Nature 542, 433–438 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Ligt J et al. Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med 367, 1921–9 (2012). [DOI] [PubMed] [Google Scholar]
- 7.De Rubeis S et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515, 209–15 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epi KC et al. De novo mutations in epileptic encephalopathies. Nature 501, 217–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Euro E-RESC, Epilepsy Phenome/Genome, P. & Epi, K.C. De novo mutations in synaptic transmission genes including DNM1 cause epileptic encephalopathies. Am J Hum Genet 95, 360–70 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fromer M et al. De novo mutations in schizophrenia implicate synaptic networks. Nature 506, 179–84 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilissen C et al. Genome sequencing identifies major causes of severe intellectual disability. Nature 511, 344–7 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Iossifov I et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 515, 216–21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iossifov I et al. De novo gene disruptions in children on the autistic spectrum. Neuron 74, 285–99 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Roak BJ et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485, 246–50 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rauch A et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet 380, 1674–82 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Sanders SJ et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485, 237–41 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaidi S et al. De novo mutations in histone-modifying genes in congenital heart disease. Nature 498, 220–3 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deciphering Developmental Disorders Study. Large-scale discovery of novel genetic causes of developmental disorders. Nature 519, 223–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Ligt J, Veltman JA & Vissers LE Point mutations as a source of de novo genetic disease. Curr Opin Genet Dev 23, 257–63 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Adzhubei IA et al. A method and server for predicting damaging missense mutations. Nat Methods 7, 248–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollard KS, Hubisz MJ, Rosenbloom KR & Siepel A Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res 20, 110–21 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kircher M et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet 46, 310–5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahni N et al. Widespread Macromolecular Interaction Perturbations in Human Genetic Disorders. Cell 161, 647–660 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei X et al. A massively parallel pipeline to clone DNA variants and examine molecular phenotypes of human disease mutations. PLoS Genet 10, e1004819 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu H et al. High-quality binary protein interaction map of the yeast interactome network. Science 322, 104–10 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braun P et al. An experimentally derived confidence score for binary protein-protein interactions. Nat Methods 6, 91–7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venkatesan K et al. An empirical framework for binary interactome mapping. Nat Methods 6, 83–90 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer MJ et al. Interactome INSIDER: a structural interactome browser for genomic studies. Nat Methods (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanders SJ et al. Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron 87, 1215–33 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischbach GD & Lord C The Simons Simplex Collection: a resource for identification of autism genetic risk factors. Neuron 68, 192–5 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Levy D et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron 70, 886–97 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Sanders SJ et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 70, 863–85 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong S et al. De novo insertions and deletions of predominantly paternal origin are associated with autism spectrum disorder. Cell Rep 9, 16–23 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sebat J et al. Strong association of de novo copy number mutations with autism. Science 316, 445–9 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinto D et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature 466, 368–72 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X et al. Three-dimensional reconstruction of protein networks provides insight into human genetic disease. Nat Biotechnol 30, 159–64 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer MJ, Das J, Wang X & Yu H INstruct: a database of high-quality 3D structurally resolved protein interactome networks. Bioinformatics 29, 1577–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Das J & Yu H HINT: High-quality protein interactomes and their applications in understanding human disease. BMC Syst Biol 6, 92 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chatr-Aryamontri A et al. The BioGRID interaction database: 2015 update. Nucleic Acids Res 43, D470–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stelzl U et al. A human protein-protein interaction network: a resource for annotating the proteome. Cell 122, 957–68 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Turner B et al. iRefWeb: interactive analysis of consolidated protein interaction data and their supporting evidence. Database (Oxford) 2010, baq023 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salwinski L et al. The Database of Interacting Proteins: 2004 update. Nucleic Acids Res 32, D449–51 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hermjakob H et al. IntAct: an open source molecular interaction database. Nucleic Acids Res 32, D452–5 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keshava Prasad TS et al. Human Protein Reference Database−−2009 update. Nucleic Acids Res 37, D767–72 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mewes HW et al. MIPS: curated databases and comprehensive secondary data resources in 2010. Nucleic Acids Res 39, D220–4 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berman HM et al. The Protein Data Bank. Nucleic Acids Res 28, 235–42 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang J, Gilman SR, Chiang AH, Sanders SJ & Vitkup D Genotype to phenotype relationships in autism spectrum disorders. Nat Neurosci 18, 191–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goh KI et al. The human disease network. Proc Natl Acad Sci U S A 104, 8685–90 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feldman I, Rzhetsky A & Vitkup D Network properties of genes harboring inherited disease mutations. Proc Natl Acad Sci U S A 105, 4323–8 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu J & Li Y Discovering disease-genes by topological features in human protein-protein interaction network. Bioinformatics 22, 2800–5 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Jonsson PF & Bates PA Global topological features of cancer proteins in the human interactome. Bioinformatics 22, 2291–7 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Albert R, Jeong H & Barabasi AL Error and attack tolerance of complex networks. Nature 406, 378–82 (2000). [DOI] [PubMed] [Google Scholar]
- 53.Chen WH, Lu G, Chen X, Zhao XM & Bork P OGEE v2: an update of the online gene essentiality database with special focus on differentially essential genes in human cancer cell lines. Nucleic Acids Res 45, D940–D944 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang T et al. Identification and characterization of essential genes in the human genome. Science 350, 1096–101 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang N, Lee I, Marcotte EM & Hurles ME Characterising and predicting haploinsufficiency in the human genome. PLoS Genet 6, e1001154 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lek M et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–91 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ronemus M, Iossifov I, Levy D & Wigler M The role of de novo mutations in the genetics of autism spectrum disorders. Nat Rev Genet 15, 133–41 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Basu SN, Kollu R & Banerjee-Basu S AutDB: a gene reference resource for autism research. Nucleic Acids Res 37, D832–6 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neale BM et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485, 242–5 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schanen NC Epigenetics of autism spectrum disorders. Hum Mol Genet 15 Spec No 2, R138–50 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Xu X et al. Excessive UBE3A dosage impairs retinoic acid signaling and synaptic plasticity in autism spectrum disorders. Cell Research (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pengelly RJ et al. Mutations specific to the Rac-GEF domain of TRIO cause intellectual disability and microcephaly. Journal of Medical Genetics 53, 735–742 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reijnders MRF et al. RAC1 Missense Mutations in Developmental Disorders with Diverse Phenotypes. The American Journal of Human Genetics 101, 466–477 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sadybekov A, Tian C, Arnesano C, Katritch V & Herring BE An autism spectrum disorder-related de novo mutation hotspot discovered in the GEF1 domain of Trio. Nature Communications 8, 601 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Turner TN et al. denovo-db: a compendium of human de novo variants. Nucleic Acids Res 45, D804–D811 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Purcell SM et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature 506, 185–90 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Geisheker MR et al. Hotspots of missense mutation identify neurodevelopmental disorder genes and functional domains. Nat Neurosci 20, 1043–1051 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robinson EB et al. Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nature Genetics 48, 552 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rolland T et al. A Proteome-Scale Map of the Human Interactome Network. Cell 159, 1212–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Finn RD et al. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res 44, D279–85 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang X et al. A public genome-scale lentiviral expression library of human ORFs. Nat Methods 8, 659–61 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
dnMis mutations in ASD subjects and their unaffected siblings came from published data in ref. 12 and are available in Supplementary Table 1. Interaction disruption results from Y2H experiments are available in Supplementary Table 2.