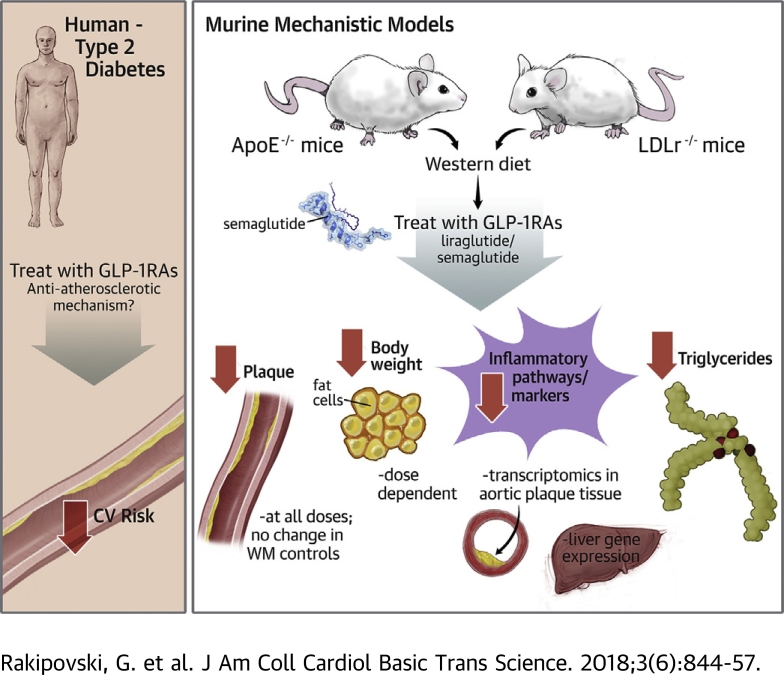
Visual Abstract
Key Words: atherosclerosis, diabetes, GLP-1, inflammation, obesity
Abbreviations and Acronyms: CD163, cluster of differentiation 163 molecule; GLP, glucagon-like peptide; IL, interleukin; IFN, interferon; LDL, low-density lipoprotein; LPS, lipopolysaccharide; MMP, matrix metalloproteinase; NASH, nonalcoholic steatohepatitis; OPN, osteopontin; RNA, ribonucleic acid; TIMP, tissue inhibitor of metalloproteinases; TNF, tumor necrosis factor; WD, Western diet
Highlights
-
•
The GLP-1RAs liraglutide and semaglutide reduce cardiovascular risk in type 2 diabetes patients.
-
•
In ApoE−/− mice and LDLr−/− mice, liraglutide and semaglutide treatment significantly attenuated plaque lesion development, in part independently of body weight and cholesterol lowering.
-
•
Semaglutide decreased levels of plasma markers of systemic inflammation in an acute inflammation model (lipopolysaccharide), and transcriptomic analysis of aortic atherosclerotic tissue revealed that multiple inflammatory pathways were down-regulated by semaglutide.
Summary
The glucagon-like peptide-1 receptor agonists (GLP-1RAs) liraglutide and semaglutide reduce cardiovascular risk in type 2 diabetes patients. The mode of action is suggested to occur through modified atherosclerotic progression. In this study, both of the compounds significantly attenuated plaque lesion development in apolipoprotein E-deficient (ApoE−/−) mice and low-density lipoprotein receptor-deficient (LDLr−/−) mice. This attenuation was partly independent of weight and cholesterol lowering. In aortic tissue, exposure to a Western diet alters expression of genes in pathways relevant to the pathogenesis of atherosclerosis, including leukocyte recruitment, leukocyte rolling, adhesion/extravasation, cholesterol metabolism, lipid-mediated signaling, extracellular matrix protein turnover, and plaque hemorrhage. Treatment with semaglutide significantly reversed these changes. These data suggest GLP-1RAs affect atherosclerosis through an anti-inflammatory mechanism.
Liraglutide (1) and semaglutide (2) are long-acting analogs of the human glucagon like peptide (GLP)-1 incretin hormone, with 97% and 94% amino acid homology, respectively, and were engineered using fatty acid acylation to facilitate serum albumin binding to increase their plasma half-life. The half-life of liraglutide is 13 h in humans and provides a once-daily dosing frequency. Semaglutide is an improved, highly potent GLP-1 receptor agonist (GLP-1RA) that is protected from dipeptidyl peptidase-4 cleavage and is further optimized for high-affinity albumin binding, which increases its human plasma half-life to 160 h, allowing for once-weekly administration (3). Liraglutide is approved for the treatment of both diabetes and obesity, whereas semaglutide is approved for diabetes.
Recently, 4 cardiovascular outcome trials with GLP-1RAs have been reported 4, 5, 6, 7. The LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) (6) and SUSTAIN-6 (Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes) (7) trials, using liraglutide and semaglutide, respectively, demonstrated a significant reduction in major adverse cardiac events in high-risk cardiovascular (CV) disease patients with diabetes. These reductions in major adverse cardiac events with liraglutide and semaglutide have been described as antiatherosclerotic effects potentially driven by anti-inflammatory mechanisms 6, 7, 8, 9, 10. Reduced inflammation is well documented in humans treated with liraglutide 11, 12, 13, associated with lower circulating levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and cluster of differentiation 163 (CD163) (14). Specifically, the well-validated inflammation marker high-sensitivity C-reactive protein (hsCRP) was reduced by approximately 35% from a baseline of approximately 3.5 mg/dl in a large randomized clinical study with liraglutide (12). In pre-clinical studies, liraglutide attenuated development of atherosclerosis and improved plaque stability in proatherogenic apolipoprotein E-deficient (ApoE−/−) mice 15, 16. Liraglutide has also been shown to directly suppress foam cell formation through a reduced uptake of oxidized low-density lipoprotein (LDL), potentially caused by a downregulation of the scavenger receptor CD36 (17). Furthermore in a model of myocardial infarction, liraglutide reduced infarction size (18) associated with reduced TNF-α in cardiac tissue (19).
This study investigated the effects of liraglutide and semaglutide in 2 mouse models of atherosclerosis, the ApoE−/− mouse model and the low-density lipoprotein receptor-deficient (LDLr−/−) mouse model. Both models are well characterized to develop atherosclerotic lesions similar to those in humans, with the exception of thrombosis 16, 20.
The aim of these studies was to specifically evaluate antiatherosclerotic effects of liraglutide and semaglutide and to investigate the mode of action and specifically connection to the degree of inflammation in the aorta. GLP-1RAs have also been proposed as treatments for nonalcoholic-steatohepatitis (NASH) (21), and because of the overlap between NASH and CV disease (22), we also investigated the role of semaglutide in prevention of the development of NASH.
Methods
Animal husbandry
The care and use of mice in these studies were conducted according to national regulations in Denmark and with experimental licenses granted by the Danish Ministry of Justice. Mice were housed under 12:12 light-dark cycle in humidity- and temperature-controlled rooms with free access to standard chow (catalog 1324, Altromin, Brogaarden, Denmark) and water. Mice were identified by subcutaneously (SC) implanted chips (Pico ID transponder, UNO, OPEND, Denmark).
Atherosclerosis in vivo study design
A total of 126 LDLr−/− male mice 6 to 8 weeks of age (stock 02207, Jackson Laboratory, Bar Harbor, Maine) and 180 ApoE−/− 7 mice 10 weeks of age (Taconic, Denmark) were used. Animals were allocated to groups according to body weight (BW). Animals were switched to Western diet (WD) (catalog RD12047; Research Diets, New Brunswick, New Jersey) prior to initiation of dosing. Animals were given SC daily doses of liraglutide, 1 mg/kg, or daily doses of semaglutide at 4.0, 12.0, or 60.0 μg/kg, or vehicle control for 12 to 14 weeks in ApoE−/− mice or for 17 weeks in LDLr−/− mice. Mice receiving liraglutide were compared to a weight-matched group in the comparator study. The weight match was obtained by doses of a food intake-reducing agent with dose frequency similar to that of liraglutide.
Ultrasonography imaging
After the ApoE−/− mice received liraglutide for 14 weeks, the animals were anesthetized with isoflurane, and fur was removed on the upper part of the thorax. Still under anesthesia, each mouse was placed on a scanning platform. Transmission gel (Echophonic, Pharmaceutical Innovation Inc., Newark, New Jersey) was placed on the thorax. The transducer (704 RMV Scanhead; VisualSonics, Toronto, Ontario, Canada) was placed on the thorax, and the aortic sinus was visualized. Two pictures in the b-mode in long- and short-axis views were recorded for each animal.
Termination
Terminal blood samples were collected from the sinus orbital vein in 1,000-μl K3-EDTA-coated tubes (Sarsted, North Rhine-Westphalia, Germany). Plasma was separated (at 4°C; 3,500 rpm; 10 min) and used for total cholesterol (T-chol) and triglyceride(s) (TG) analyses. Subsequently, the animals were perfused with 10 ml of ice-cold saline, and the thoracic aorta from the heart to the 8th rib was excised for measurement of plaque. The aorta was dissected longitudinally and placed on glass plates for en face analyses (Visiomorph, Visiopharm A/S, Hørsholm, Denmark). After en face analysis, the aorta section was snap-frozen in liquid nitrogen and kept at −80°C for gene expression analysis.
Lipid analysis
Livers were rinsed in ice-cold phosphate-buffered saline (140 mM NaCl, 10 mM phosphate, 3 mM KCl, pH 7.4, Millipore, Billerica, Massachusetts) and weighed. TG were analyzed on homogenates from the left lateral lobe. Briefly, 1 ml of buffer (0.15 M sodium acetate and 0.75% Triton X-100) was added to frozen samples and subsequently homogenized. Samples were heated to 100oC for 2 min before being cooled on ice. Five-hundred microliters of homogenate was then centrifuged (4°C; 5,500 rpm; 10 min). The supernatant was analyzed for TG by using the COBAS 6000 multianalyzer (reagent 20767107322, Roche Diagnostics, Rotkreuz ZG, Switzerland). Plasma TG levels were analyzed in 25-μl K3-EDTA-stabilized plasma samples.
RNA purification from aorta
Aortas were homogenized in RLT buffer (Qiagen, Gaithersburg, Maryland) containing β-mercaptoethanol in a TissueLyserII (Qiagen) for 3 min at 30 Hz.
RNA was extracted using an RNeasy 96 kit (Qiagen) on a BIOMEK FXP robot (Beckman Coulter, Brea, California) according to the manufacturer's protocol.
The quality of the purified RNA from the tissue samples was tested by measuring RNA concentration and 260:280 ratio on a Nanodrop instrument (Thermo Fisher Scientific, Waltham, Massachusetts). RNA integrity was confirmed using an Agilent 2100 Bioanalyzer (Agilent, Glostrup, Denmark) with Agilent 6000 Nano chips and reagents for total eukaryotic RNA.
NanoString assay
NanoString gene analysis was performed using a custom-made code set consisting of probes for 275 genes of interest (catalog GXA-P1CS-576, AME Bioscience, Thurleigh, United Kingdom) and a master kit containing all necessary buffers and reagents (catalog NAA-AKIT-192, AME Bioscience). Following hybridization, excess probes were removed, and probe/target complexes were aligned and immobilized on a cartridge by the GEN2 nCounter PrepStation (NanoString, Seattle, Washington), using high-sensitivity settings and finally scanned using the GEN2 nCounter digital analyzer (NanoString), using maximum screening intensity (555 fields of view).
NanoString data processing
Using the proprietary software for the instrument (nSolver analysis software 2.5, NanoString), all samples were subjected to technical normalization to the positive spike-in RNA (present in the CodeSet). A lane-specific value representative of positive control counts was calculated, that is, a sum of positive control counts. The geometric means of these calculated values across all lanes were used as the references against which each lane was normalized. A scaling factor was then calculated for each of the lanes based on the calculated value for the positive controls in each lane relative to the average of this value for the positive controls across all lanes. This normalization factor was used to adjust the counts for each gene target and negative controls in the associated lane. Data were further normalized to a set of selected housekeeping genes (Actb, B2m, Gapdh, Hprt, Gusb, Ppia, and Rps18). The geometric mean of the count for these genes across all lanes was used as the reference against which each lane was normalized. A scaling factor was then calculated for each of the lanes based on the calculated value for the housekeeping genes in each lane relative to the geometric average of this value for the house keeping genes across all lanes.
Acute inflammation in vivo study design
C57BL/6J lean male mice at 12 weeks of age (Taconic, Denmark) were given SC doses of semaglutide (60.0 μg/kg) or vehicle control 1 hour prior to receiving an intraperitoneal (IP) dose of lipopolysaccharide (LPS) (0.05 mg/kg, Escherichia coli O55:B5, Sigma Aldrich, St. Louis, Missouri) or vehicle control. Blood samples were collected from the sinus orbital vein at 1 and 4 h after LPS dose. Blood samples were collected in K3-EDTA-coated tubes, and plasma was isolated as previous described. Plasma levels of TNF-α and interferon (IFN)-gamma were analyzed using a Meso scale Discovery platform (Meso Scale Diagnostics, Rockville, Maryland) according to the manufacturer’s instructions. Plasma levels of osteopontin (OPN) were analyzed by enzyme-linked immunosorbent assay (Quantikine; R&D Systems, Minneapolis, Minnesota).
Data analysis
All data are presented as mean ± SEM. Data were analyzed using Prism version 6.05 software (Graph Pad, San Diego, California). Statistical analysis was performed using 1- or 2-way ANOVA followed by Dunnett post hoc test for multiple group comparison. Statistical significance was defined as a p value <0.05 or FDR ≤ 5%.
Statistical analysis and data processing of NanoString data
Technical and housekeeping gene normalized expression values derived from the proprietary software (described above) were log2 transformed, and transformed values were used throughout the statistical analysis. An expression level filter was applied to filter out genes exhibiting no or very low expression levels of detection. Specifically, any gene where the maximal group mean expression was below 4.5 (log2 normalized counts) was excluded from further analysis.
A principal component analysis of data was performed using Omics Explorer version 3.2 software (Qlucore AB, Lund, Sweden). Differentially expressed genes comparing treatment with the maximum dose of semaglutide (60 μg/kg) versus that of vehicle control were used for Ingenuity pathway analysis (Qiagen).
Results
Liraglutide and semaglutide prevent body weight gain and plaque lesion development in LDLr−/− and ApoE−/− mice
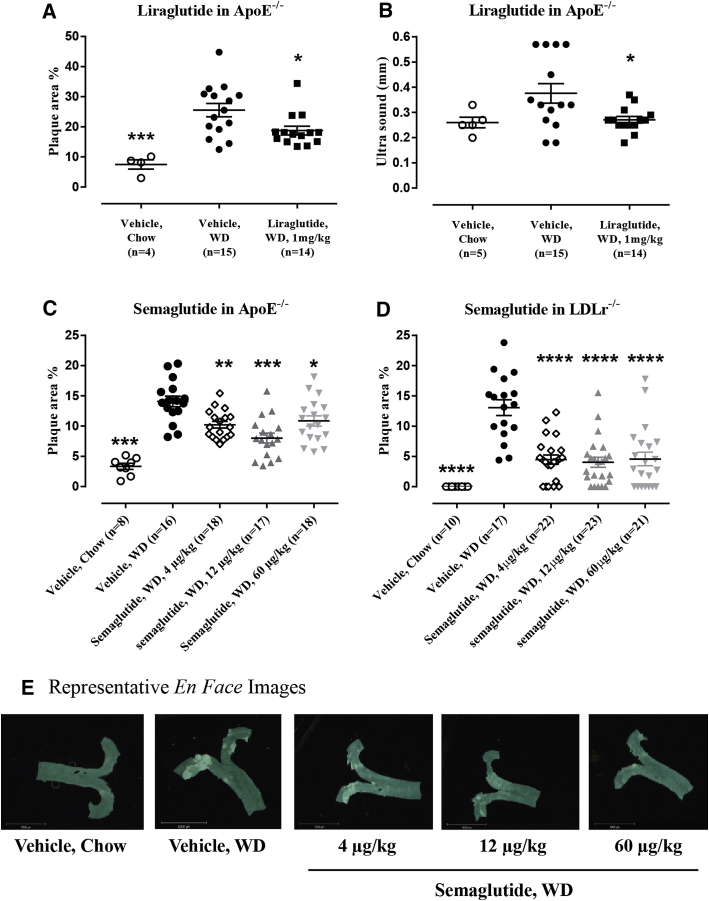
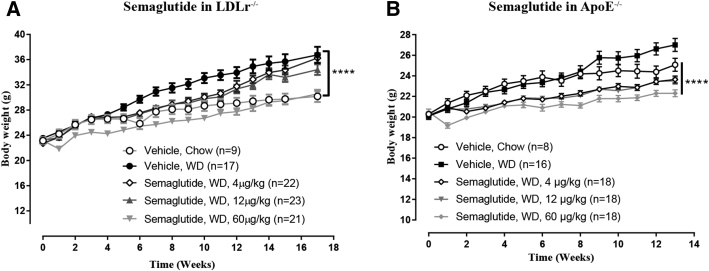
Liraglutide administration in ApoE−/− mice prevented aortic plaque progression, resulting in a plaque area of 18.8 ± 1.5% compared to that in the vehicle control group of 25.3 ± 2.2% (p = 0.0383) (Figure 1A). Liraglutide furthermore significantly prevented aorta intima thickening (0.38 ± 0.04 mm with vehicle vs. 0.27 ± 0.01 mm with liraglutide; p = 0.0279), a surrogate marker for subclinical atherosclerosis (10) (Figure 1B). All dose levels of semaglutide resulted in significantly lower levels of plaque area in ApoE−/− mice, with the middle dose (12 μg/kg) showing the strongest effect (Figure 1C). In LDLr−/− mice, all doses of semaglutide significantly attenuated aortic plaque lesion development (WD group, 13.1% ± 1.3% with vehicle vs. 4.5% ± 0.8% with dose of 4 μg/kg vs. 4.0% ± 0.8% with dose of 12 μg/kg vs. 4.6% ± 1.1% with a dose of 60 μg/kg; p < 0.0001) (Figure 1D). In both mouse models, semaglutide administration resulted in a significant dose-dependent decrease in BW (Figure 2), whereas the attenuating effect on aortic plaque lesions was similar at all dose levels (Figure 1D) and thus did not correlate with the degree of BW lowering.
Figure 1.
Aortic Plaque Lesion Development Is Attenuated by Semaglutide (LDLr−/− and ApoE−/− Mice), and Liraglutide (ApoE−/− Mice), Together With a Significant Reduction in Carotid Intima Thickening
(A) Liraglutide in ApoE-/- mice significantly decreased plaque lesion development: ∗p = 0.0383, ∗∗∗p = 0.0002 versus vehicle WD, and (B) carotid intima thickening: ∗p = 0.0279 vs vehicle WD. (C) Semaglutide significantly decreased WD induced plaque lesion development at all dose levels in ApoE-/- mice: ∗p = 0.0266, ∗∗p = 0.0046, ∗∗∗∗p < 0.0001 versus vehicle WD, and (D) LDLr-/- mice: ∗∗∗∗p < 0.0001 versus vehicle WD. (E) Representive en face images from aortas. Resolution: 0.0677 pixels/μm. ApoE = apolipoprotein E; LDLr = low-density lipoprotein receptor; WD = Western diet.
Figure 2.
Dose-Dependent Effect on Body Weight Gain by Semaglutide in LDLr−/− and ApoE−/− Mice
WD-induced increases in body weight were significantly decreased by semaglutide in LDLr−/−(A) and ApoE−/−(B) mice (****p < 0.0001, vehicle, WD). Abbreviations as in Figure 1.
Liraglutide and semaglutide attenuate plaque lesion development independently of cholesterol lowering
Semaglutide had no significant effects on T-chol; however, at the highest dose (60 μg/kg), T-chol was reduced by 25% (not reaching significance) in LDLr−/− mice. Plasma TG was significantly reduced at 60 μg/kg (11.5 ± 0.7 mM with vehicle vs. 7.1 ± 1.0 mM with semaglutide; p = 0.0342) (Table 1). In ApoE−/− mice, liraglutide had no effect on plasma lipids (data not shown), and semaglutide showed a small but significant rise in total plasma T-chol level compared to that in vehicle controls at the highest dose (p = 0.0125) (Table 1).
Table 1.
Semaglutide Significantly Lowered Plasma TG in LDLr−/− Mice
| LDLr−/− |
ApoE−/− |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vehicle, WD (n = 20) | Semaglutide, 4 μg/kg (n = 21) | Semaglutide, 12 μg/kg (n = 23) | Semaglutide, 60 μg/kg (n = 21) | Vehicle, Chow (n = 9) | Vehicle, WD (n = 16) | Semaglutide, 4 μg/kg (n = 18) | Semaglutide, 12 μg/kg (n = 18) | Semaglutide, 60 μg/kg (n = 18) | Vehicle, Chow (n = 8) | |
| T-chol (mM) | 27.4 ± 2.8 | 23.3 ± 2.1 | 23.9 ± 2.1 | 19.9 ± 1.5 | 5.9 ± 0.3∗∗∗∗ | 25.1 ± 0.9 | 26.6 ± 1.3 | 30.9 ± 1.6 | 33.0 ± 1.3∗∗ | 8.5 ± 0.4∗∗∗∗ |
| TG (mM) | 6.8 ± 0.8 | 6.8 ± 0.6 | 7.1 ± 0.7 | 4.4 ± 0.4∗ | 1.4 ± 0.1∗∗∗∗ | 1.5 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.2∗∗∗∗ |
T-chol = total cholesterol; TG = triglyceride; WD = Western diet.
p Values (comparison vs. Vehicle, WD):
p < 0.00001.
p = 0.0125.
p = 0.0342. 1-way ANOVA, Dunnett’s post hoc test.
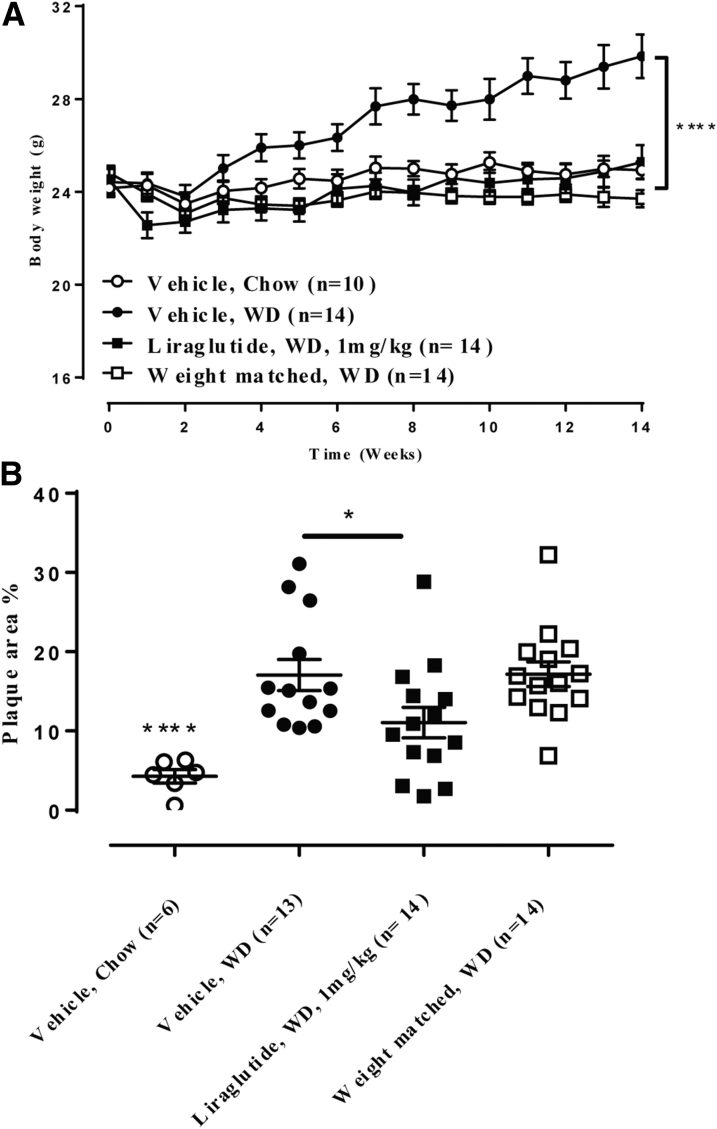
Because GLP-1RAs lower BW by suppressing food intake, we compared liraglutide to a weight-matched control group (Figure 3). Even though both the liraglutide group and the weight-matched groups had differences in BW similar to those in vehicle-treated mice (p < 0.0001 vs. vehicle) (Figure 3A), only liraglutide prevented aortic plaque progression, resulting in a plaque area of 11.1 ± 1.9% compared to the vehicle group of 17.1 ± 1.9% (p = 0.0448) (Figure 3B).
Figure 3.
Liraglutide Decreased Aortic Plaque Lesion Development in ApoE−/− Mice Whereas a Weight-Matched Comparator Did Not
(A) WD-induced increases in body weight were significantly lowered by liraglutide and in the weight matched comparator: ****p < 0.0001 versus vehicle, WD. (B) Liraglutide significantly attenuated WD-induced plaque lesion development, whereas the weight-matched comparator group did not: *p = 0.0448, ****p <0.0001 versus vehicle, WD; liraglutide, WD versus weight-matched, WD, p = 0.06. Abbreviations as in Figure 1.
Semaglutide affects inflammatory genes in atherosclerotic aortas and acute systemic inflammation
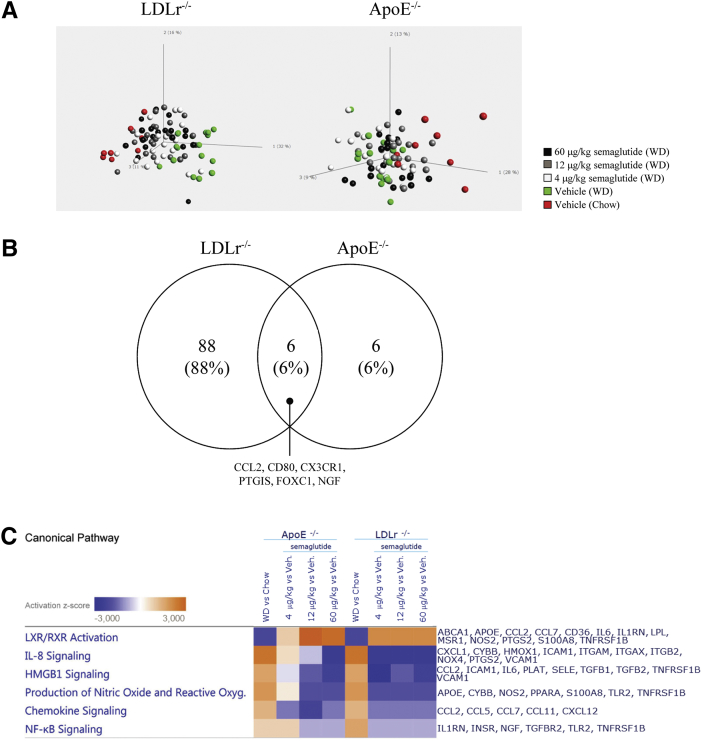
To further evaluate the effects of semaglutide on aortic plaque formation in the LDLr−/− and ApoE−/− mouse models, gene expression profiling of aorta samples was performed. The selected genes consisted of 275 genes relevant to the pathogenesis of atherosclerosis (list of genes is shown in Supplemental Table S1). Principal component analysis of the entire dataset (Figure 4A) revealed the untreated chow-fed and WD-fed animals displayed the clearest segregation, whereas the semaglutide groups were positioned between these groups without clear dose relationships. When we compared the highest dose of semaglutide (60 μg/kg) with that of vehicle in both models, 94 genes in the LDLr−/− mice and 12 genes in the ApoE−/− mice were differentially expressed compared to those in WD-fed animals given vehicle (FDR = 5%) (Figure 4B). A full list of differentially expressed genes is presented in Supplemental Table S2. Semaglutide partially prevented the WD-induced changes in gene expression, as demonstrated through pathway analysis of differentially expressed genes from both mouse models (Figure 4C). The opposite directions of the diet and treatment effects are also exemplified by the representative genes (Figure 4D) for processes relevant to the pathogenesis of atherosclerosis, such as leukocyte recruitment (IL-6, IL-1 receptor antagonist [IL-1RN], chemokine [C-C motif] ligand 2 [CCL2], leukocyte rolling, adhesion and extravasation (SELE, VCAM-1), cholesterol metabolism and lipid-mediated signaling (ATP-binding cassette transporter 1 [ABCA 1], prostaglandin I2 synthase [PTGIS], extracellular matrix protein turnover [MMP-3 and MMP-13], and plaque hemorrhage [CD163]).
Figure 4.
Semaglutide Affected Local (Aortic) and Systemic Inflammation
(A) Principal component analysis illustrates the total variance of the 275 genes analyzed. (B) Venn diagram of significant gene expression changes (60 μg/kg vs. vehicle; FDR = 5%) in LDLr−/− and ApoE−/− models. (C) Directions (activation z-scores) are shown for significant changes in represented pathways of IPA. (D) Gene expression changes by WD and semaglutide (compared to vehicle-dosed chow-fed animals), exemplifying genes that represent pathways with well described relevance to plaque formation and the pathophysiology of atherosclerosis. Benjamini-Hochberg-corrected p values: ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05 versus LDLr−/−, WD; ####p < 0.0001, ###p < 0.001; ##p < 0.01; #p < 0.05 versus ApoE−/−, WD (uncorrected and Benjamini-Hochberg-corrected p values can be found in Supplemental Table S3). Subcutaneous administration of semaglutide in an LPS inflammation model reduced plasma levels of (E) TNFα: **p = 0.0024 versus vehicle at 1 h and p = 0.048 versus vehicle at 4 h, (F) IFNg: ** p = 0.0050 versus vehicle at 4 h, and (G) OPN: **p = 0.0014 versus vehicle at 4 h. FDR = false discovery rate; IPA = ingenuity pathway analysis; LPS = lipopolysaccharide; OPN = osteopontin; other abbreviations as in Figure 1.
Because semaglutide reduced gene expression of inflammatory markers in atherosclerotic aortas, we further evaluated its anti-inflammatory properties in an acute in vivo inflammation model. Lean C57BL/6J mice were challenged with a single dose of LPS (0.05 mg/kg), and the systemic inflammatory profile was examined by analyzing plasma levels of the inflammatory cytokines TNF-α and IFN-γ. Administration of semaglutide (60 μg/kg) prior to LPS reduced the TNF-α response at both 1 and 4 h after LPS exposure (p = 0.0024 vs. vehicle at 1 h and p = 0.048 vs. vehicle at 4 h) (Figure 4E), whereas IFN-γ was reduced by semaglutide after 4 h (p = 0.005 vs. vehicle at 4 h) (Figure 4F). In addition, immune cell recruitment was reduced by semaglutide after 4 h, as assessed by the circulating levels of the chemoattractant OPN (p = 0.0014 vs. vehicle at 4 h) (Figure 4G).
Semaglutide ameliorates markers of liver inflammation and reduces genes related to liver fibrosis and liver fat content
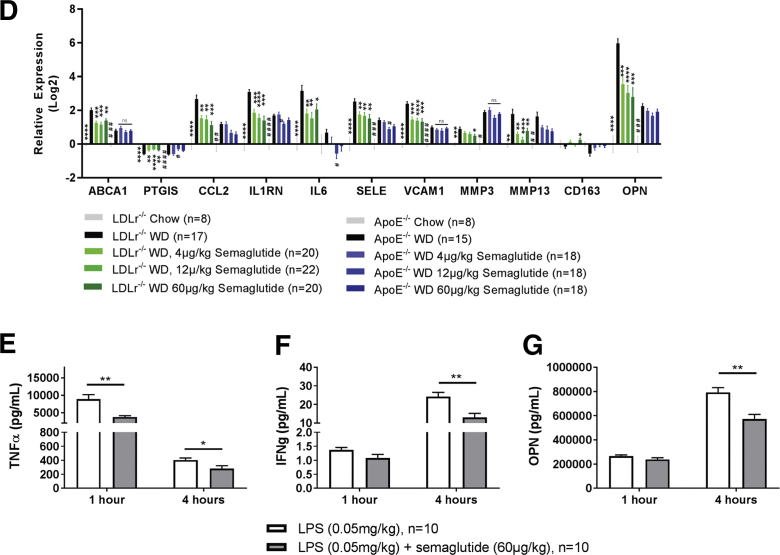
The LDLr−/− mouse model was also used as the model for NASH (23). WD feeding produced a 7-fold increase in liver TG content compared to that in chow-fed animals, and this was accompanied by a significant increase in collagen types I, II, III, and IV and expression of several inflammation-related genes. Semaglutide significantly reduced liver TG content for the 2 highest doses (Figure 5A). Additionally, expression levels of 3 of 5 collagen genes were reduced at all dose levels suggesting reduced generation of liver fibrosis (Figure 5B). Inflammation has been proposed to play a key role in the development of NASH (23), and semaglutide prevented the WD-induced changes in genes related to inflammatory markers at all dose levels (Figure 5C).
Figure 5.
Semaglutide Lowered TG and Prevented WD-Induced Transcriptional Changes in Liver of LDLr−/− Mice
(A) WD-induced increases of liver triglycerides were dose-dependently reduced by semaglutide: $$p = 0.0017, **p = 0.0045, ****p <0.0001 versus vehicle WD. (B) Semaglutide partially prevented WD induced increases in collagen gene expression: Benjamini-Hochberg-corrected p values: **p < 0.01; *p < 0.05 versus vehicle, WD. (C) Graphic representation of relative gene expression changes by WD and semaglutide compared to vehicle, chow. Benjamini-Hochberg-corrected p values: ****p < 0.0001, ***p < 0.001, **p < 0.01; *p < 0.05 versus vehicle, WD. Uncorrected and Benjamini-Hochberg-corrected p values for Figures 5B and 5C can be found in Supplemental Table S4. TG = triglyceride.
Discussion
The CV risk reduction (RR) observed using the GLP-1RAs liraglutide and semaglutide in the LEADER and SUSTAIN-6 trials is proposed to be mediated through antiatherosclerotic mechanisms 6, 7, 9. GLP-1RAs are potent regulators of BW, hyperglycemia, and to some extent dyslipidemia (10). In the studies presented here, semaglutide exerted antiatherosclerotic efficacy at doses that did not lower BW significantly (Figures 1 and 2), indicating that the antiatherosclerotic effects cannot be attributed solely to prevention of gain in BW. These observations were further supported by the weight-matched comparator study in ApoE−/− mice, where only liraglutide decreased the plaque lesion development (Figure 3), and in an acute inflammation study in lean mice, where semaglutide reduced circulating levels of TNF-α, IFN-γ, and OPN in response to an LPS challenge (Figure 4). In line with our findings, continuous infusion of native GLP-1 at weight-neutral doses in ApoE−/− mice lowered the amount of atherosclerotic lesions accompanied with reduced macrophage infiltration in the vasculature (24). Similar observations of atherosclerosis and reduced systemic inflammation using exendin-4 were reported by Wang et al. (25) and Yanay et al. (26).
Recently, a proteomics-based comparison of carotid specimens from symptomatic and asymptomatic patients identified a molecular signature with increased inflammatory markers in the symptomatic patient group, including MMP-9, OPN, and cathepsin D, thus suggesting that inflammatory markers in vascular tissue may be a more specific measurement of plaque instability (27). OPN is an important proinflammatory cytokine which also plays a role in immune cell recruitment (28), and elevated circulating OPN levels have been associated with increased CV disease risk in type 2 diabetes patients (29). In the present studies, semaglutide decreased plasma OPN levels following LPS challenge, and OPN expression was decreased in aortic tissue with semaglutide treatment. Gene expression analysis of the aorta further demonstrated that semaglutide partially prevented WD-induced changes for transcripts associated with pathways relevant to the pathogenesis of atherosclerosis (Figure 4C). In LDLr−/− mice, the group-wise comparison suggests that all doses of semaglutide were equally effective in preventing the WD-induced changes in gene expression. In ApoE−/− mice, smaller effect sizes were observed both for the WD and the semaglutide treatment, which could be explained by differences in the metabolic phenotype between the 2 mouse models, where LDLr−/− mice are more prone to developing obesity and insulin resistance (23). The genes exemplified in Figure 4 comprise markers of inflammation and plaque stability that are associated with leukocyte recruitment (e.g., IL-1RN, IL-6, CCL2, OPN), leukocyte adhesion (SELE, VCAM-1), leukocyte extravasation, and plaque stability (e.g., MMP-3, MMP-13, CCL2), and plaque rupture and hemorrhage (CD163). A clinical relevance for CV disease is suggested, for example, for IL-1RN, MMP-3, CD163, and IL-6 and its receptor (30). The endogenous antagonist of IL-1R, IL-1RN, is upregulated in atherosclerosis, and circulating levels are increased in patients with unstable angina (31). Furthermore the CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) trial demonstrated that neutralizing IL-1β resulted in reduced CV risk (32). MMPs affect the turnover of extracellular matrix proteins such as collagens and elastins and have been implicated in CV disease affecting plaque stability and rupture (33), and circulating MMP3 concentrations are of predictive value for CV disease in patients with type 2 diabetes (34). Clinical studies with native GLP-1 or GLP-1RAs have demonstrated effects on soluble biomarkers that are implicated in the pathogenesis of atherosclerosis. In a case control study of 10 obese type 2 diabetes patients, liraglutide significantly lowered serum CD163 levels after 8 weeks of treatment (14). In another randomized control study in type 2 diabetes patients, liraglutide treatment added to metformin was associated with significant changes for SELE and PAI-1, whereas no changes in VCAM-1, CRP, and CCL-2 were observed (35).
Previous cell-based studies also support a role for GLP-1RAs in inflammation, demonstrating effects directly on vascular endothelial cells or monocytes. Although these results may be controversial due to lack of GLP-1R expression 8, 36, studies have indicated improved endothelial function in both rodents 15, 16 and humans (37). In the present study, GLP-1R mRNA expression levels in aortic tissue were below the level of quantification in both animal models, suggesting that GLP-1Rs in other tissues contribute to the mechanism rather than direct action of liraglutide and semaglutide on the vascular bed. In the gastrointestinal system, the GLP-1R has restricted expression in several locations, including mucus-secreting Brunner glands, and these glands have high GLP-1R expression in both rodents (38) and humans (39). Recently, liraglutide has been shown to upregulate genes encoding mucins and other barrier protective molecules including IL-33 in Brunner glands of mice (40). This may add to gut defense mechanisms and reduce intestinal permeability, which is expected to improve systemic inflammation (41). Another location for GLP-1R expression is intestinal intraepithelial lymphocytes (IELs) (42). Yusta et al. (42) showed that exendin-4 in IELs improved gut barrier function and significantly attenuated induction of mRNA activity and protein expression of proinflammatory cytokines. A role for liraglutide or semaglutide in improving the gut barrier function and consequently reducing inflammation may be one of the mechanisms involved in CV risk reduction.
In the LEAN (liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis) trial, liraglutide treatment (48 weeks) in NASH patients protected against further worsening of liver fibrosis (43). The beneficial effect on liver fibrosis was suggested to be mediated through decreased lipotoxicity beyond BW or blood glucose-lowering effects (44). In the present study in LDLr−/− mice, liver fat content as well as genes related to liver fibrosis and inflammation were significantly decreased by semaglutide. Of particular translational relevance to human treatment, semaglutide partially prevented the WD-induced expression of TIMP-1 and the S100 calcium-binding proteins A8 and A9 (S100A8 and S100A9) at all dose levels. Serum TIMP-1 levels have been used to detect fibrosis (45), whereas S100A8/-A9 are biomarkers for cirrhosis in chronic hepatitis C infection and various other diseases (46). Similar to findings in the vascular bed, we did not identify GLP-1R expression in the liver (data not shown), concordant with previous reports 38, 39, 47. Interestingly, for both aorta and liver, a local GLP-1R-independent effect to reduce inflammation was seen, highlighting the possibility that this is mediated by GLP-1RAs in other locations.
The structural properties of GLP-1RAs may drive some of the differences in their clinical effect to reduce major adverse cardiac events. Exenatide, a short-acting GLP-1-RA has shown beneficial effects on systemic inflammation in clinical studies, both acutely and by chronic treatment 8, 36, but whether those effects translate into CV RR remains to be demonstrated in a CV outcome trial. Lixisenatide, another short-acting GLP-1RA, did not provide evidence of CV RR in the ELIXA (The Evaluation of Lixisenatide in Acute Coronary Syndrome) trial (4). Differences in patient populations and trial duration could explain the differences in outcome, but the duration of action by these different GLP-1RAs might also be relevant for the CV RR (48). It is well documented that the actions of GLP-1RAs have a strict pharmacokinetic-to-pharmacodynamic relationship (49). Interestingly and in contrast to the clinical setting, lixisenatide given as an infusion to rodents affected the plaque progression and stability (50). In the EXCSEL (Exenatide Study of Cardiovascular Event Lowering) trial, the once weekly exenatide (Bydureon) did not show CV RR (5). However, there was a statistically significant difference on all-cause mortality, and all endpoints trended in the same direction as in the LEADER trial. It may be speculated that the lack of significant CV effect in the EXSCEL trial could be due to the high rate of treatment discontinuation and the patients' low adherence (5). Dulaglutide, another long-acting GLP-1RA, is currently undergoing a cardiovascular outcome trial (REWIND [Researching Cardiovascular Events With a Weekly Incretin in Diabetes]) (51). Whether CV protection is a class effect for all long-acting GLP-1RAs will thus be further clarified when the REWIND trial is reported.
Study limitations
Investigating interventions affecting the development of atherosclerosis in murine models often leads to discussions of the validity and translational value of the findings to humans. The lack of plaque rupture and thrombosis and the subsequent effect on the heart or cerebral ischemia have often led to questions of the relevance of these models for validation of novel pharmaceuticals in the field of CV disease (52). A recent study by Pasterkamp et al. (53) reported discrepancies in comparing regulation of murine versus human genes in the development of atherosclerosis, and it was suggested that a sensible way to improve the translational value was to group genes related to specific pathways. In the studies presented here, there seems to be an overlap in the pathways identified in the transcriptomic analysis compared to what has been shown in human pathophysiology. This overlap and the CV outcome trials for liraglutide and semaglutide show an effect consistent with a reduced underlying atherosclerotic burden, suggesting that the results here are more likely to translate to the human situation. A limitation to the present work, however, is that compounds were not evaluated at multiple time points; future clinical studies evaluating transcriptomic and proteomic changes at a number of time points would thus be mechanistically informative. Furthermore, although semaglutide decreased levels of plasma markers of systemic inflammation in an acute inflammation model (LPS), the transcriptomic changes observed in aortic tissue were not validated at the protein level in this study.
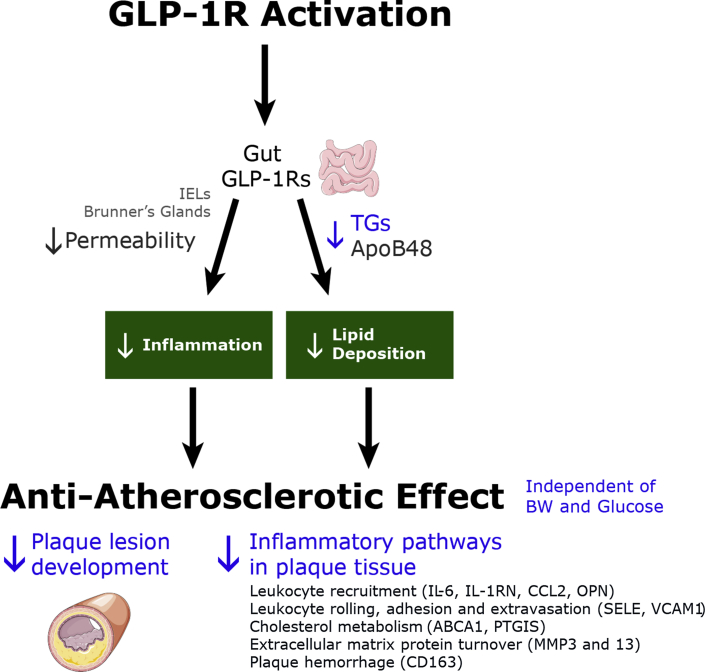
Conclusions
A proposed model illustrating how long-acting GLP-1RAs could reduce the atherosclerotic burden is shown in Figure 6. The antiatherosclerotic effect of GLP-1RAs or GLP-1 is most likely not mediated through direct actions on the vasculature due to lack of GLP-1R expression. The pancreas and brain are organs with abundant GLP-1R expression and well-characterized roles in blood glucose and BW regulation. However, the effect on atherosclerosis presented here is in nondiabetic models and appears independent from weight loss. Inflammation may serve as an important mechanism, which together with reduced post-prandial lipids and reduced oxidative stress may be the effective combination mediating the antiatherosclerotic effect of liraglutide and semaglutide.
Figure 6.
Proposed Mechanisms for a GLP-1RA-Induced Anti-Atherosclerotic Effect
Proposed model illustrating how long-acting GLP-1RAs could reduce atherosclerotic burden. Illustration elements courtesy of Servier Medical Art.
In 2 murine models of atherosclerosis (ApoE−/− and LDLr−/−), liraglutide and semaglutide showed significant reductions of aortic plaque areas, at least partially independent of changes in body weight. Several changes in gene expression in the aorta were related to proteins representing inflammatory pathways associated with leucocyte recruitment, adhesion, and migration. Semaglutide additionally reduced acute systemic inflammation in a lean mouse model. These findings support roles for liraglutide and semaglutide in anti-inflammatory processes, thus providing a mechanistic hypothesis for the significant prevention of WD- induced aortic plaque formation. Collectively, our results with liraglutide and semaglutide show that long-acting GLP-1RAs have a role in the protection against atherosclerosis, mediated by a reduction in inflammatory pathways.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Six GLP-1RAs are currently approved as diabetes treatments. One GLP-1RA, liraglutide, is approved for treatment of obesity, and 2 GLP-1RAs, liraglutide and semaglutide, have been shown to reduce CV risk in diabetes. The cardiovascular findings were reported in the LEADER and SUSTAIN-6 studies, where the effects were consistent across the individual major adverse cardiac events endpoints and were hypothesized to be consistent with an underlying effect on atherosclerosis. We investigated this hypothesis in rodent models of atherosclerosis and found that liraglutide and semaglutide reduced markers of inflammation in aortic plaque tissue.
TRANSLATIONAL OUTLOOK: Future clinical studies should explore whether treatment with GLP-1RAs affects inflammation in atherosclerotic plaques.
Acknowledgments
The authors thank Lene Takla, Nikolaj Hansen, Mathilde Bonde, Louise Justesen, Malene Pedersen, Caroline Kvist, and Steen Kryger (Novo Nordisk A/S, Global Research, Denmark) for expert technical assistance with conducting the animal studies, and Christina Rye Underwood for assistance with protein assays.
Footnotes
All authors are or have been shareholders and/or employees of Novo Nordisk. Novo Nordisk markets liraglutide for the treatment of diabetes and obesity, and semaglutide for the treatment of diabetes.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Knudsen L.B., Nielsen P.F., Huusfeldt P.O. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem. 2000;43:1664–1669. doi: 10.1021/jm9909645. [DOI] [PubMed] [Google Scholar]
- 2.Lau J., Bloch P., Schaffer L. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J Med Chem. 2015;58:7370–7380. doi: 10.1021/acs.jmedchem.5b00726. [DOI] [PubMed] [Google Scholar]
- 3.Kapitza C., Dahl K., Jacobsen J.B., Axelsen M.B., Flint A. Effects of semaglutide on beta cell function and glycaemic control in participants with type 2 diabetes: a randomised, double-blind, placebo-controlled trial. Diabetologia. 2017;60:1390–1399. doi: 10.1007/s00125-017-4289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfeffer M.A., Claggett B., Diaz R. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 5.Holman R.R., Bethel M.A., Mentz R.J. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marso S.P., Daniels G.H., Brown-Frandsen K. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marso S.P., Bain S.C., Consoli A. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 8.Drucker D.J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 2016;24:15–30. doi: 10.1016/j.cmet.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Kaul S. Mitigating cardiovascular risk in type 2 diabetes with antidiabetes drugs: a review of principal cardiovascular outcome results of EMPA-REG OUTCOME, LEADER, and SUSTAIN-6 trials. Diabetes Care. 2017;40:821–831. doi: 10.2337/dc17-0291. [DOI] [PubMed] [Google Scholar]
- 10.Rizzo M., Rizvi A.A., Patti A.M. Liraglutide improves metabolic parameters and carotid intima-media thickness in diabetic patients with the metabolic syndrome: an 18-month prospective study. Cardiovasc Diabetol. 2016;15:162. doi: 10.1186/s12933-016-0480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouchi R., Nakano Y., Fukuda T. Reduction of visceral fat by liraglutide is associated with ameliorations of hepatic steatosis, albuminuria, and micro-inflammation in type 2 diabetic patients with insulin treatment: a randomized control trial. Endocr J. 2017;64:269–281. doi: 10.1507/endocrj.EJ16-0449. [DOI] [PubMed] [Google Scholar]
- 12.Pi-Sunyer X., Astrup A., Fujioka K. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 13.von Scholten B.J., Persson F., Rosenlund S. Effects of liraglutide on cardiovascular risk biomarkers in patients with type 2 diabetes and albuminuria: a sub-analysis of a randomized, placebo-controlled, double-blind, crossover trial. Diabetes Obes Metab. 2017;19:901–905. doi: 10.1111/dom.12884. [DOI] [PubMed] [Google Scholar]
- 14.Hogan A.E., Gaoatswe G., Lynch L. Glucagon-like peptide 1 analogue therapy directly modulates innate immune-mediated inflammation in individuals with type 2 diabetes mellitus. Diabetologia. 2014;57:781–784. doi: 10.1007/s00125-013-3145-0. [DOI] [PubMed] [Google Scholar]
- 15.Gaspari T., Liu H., Welungoda I. A GLP-1 receptor agonist liraglutide inhibits endothelial cell dysfunction and vascular adhesion molecule expression in an ApoE−/− mouse model. Diab Vasc Dis Res. 2011;8:117–124. doi: 10.1177/1479164111404257. [DOI] [PubMed] [Google Scholar]
- 16.Gaspari T., Welungoda I., Widdop R.E., Simpson R.W., Dear A.E. The GLP-1 receptor agonist liraglutide inhibits progression of vascular disease via effects on atherogenesis, plaque stability and endothelial function in an ApoE(−/−) mouse model. Diab Vasc Dis Res. 2013;10:353–360. doi: 10.1177/1479164113481817. [DOI] [PubMed] [Google Scholar]
- 17.Tashiro Y., Sato K., Watanabe T. A glucagon-like peptide-1 analog liraglutide suppresses macrophage foam cell formation and atherosclerosis. Peptides. 2014;54:19–26. doi: 10.1016/j.peptides.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Noyan-Ashraf M.H., Momen M.A., Ban K. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–983. doi: 10.2337/db08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noyan-Ashraf M.H., Shikatani E.A., Schuiki I. A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation. 2013;127:74–85. doi: 10.1161/CIRCULATIONAHA.112.091215. [DOI] [PubMed] [Google Scholar]
- 20.Stary H.C., Chandler A.B., Dinsmore R.E. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15:1512–1531. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 21.Blaslov K., Bulum T., Zibar K., Duvnjak L. Incretin based therapies: a novel treatment approach for non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:7356–7365. doi: 10.3748/wjg.v20.i23.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim S., Oh T.J., Koh K.K. Mechanistic link between nonalcoholic fatty liver disease and cardiometabolic disorders. Int J Cardiol. 2015;201:408–414. doi: 10.1016/j.ijcard.2015.08.107. [DOI] [PubMed] [Google Scholar]
- 23.Bieghs V., Van Gorp P.J., Wouters K. LDL receptor knock-out mice are a physiological model particularly vulnerable to study the onset of inflammation in non-alcoholic fatty liver disease. PLoS One. 2012;7:e30668. doi: 10.1371/journal.pone.0030668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagashima M., Watanabe T., Terasaki M. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia. 2011;54:2649–2659. doi: 10.1007/s00125-011-2241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y., Parlevliet E.T., Geerling J.J. Exendin-4 decreases liver inflammation and atherosclerosis development simultaneously by reducing macrophage infiltration. Br J Pharmacol. 2014;171:723–734. doi: 10.1111/bph.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yanay O., Bailey A.L., Kernan K., Zimmerman J.J., Osborne W.R. Effects of exendin-4, a glucagon like peptide-1 receptor agonist, on neutrophil count and inflammatory cytokines in a rat model of endotoxemia. J Inflam Res. 2015;8:129–135. doi: 10.2147/JIR.S84993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langley S.R., Willeit K., Didangelos A. Extracellular matrix proteomics identifies molecular signature of symptomatic carotid plaques. J Clin Invest. 2017;127:1546–1560. doi: 10.1172/JCI86924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zwakenberg S.R., van der Schouw Y.T., Schalkwijk C.G., Spijkerman A.M.W., Beulens J.W.J. Bone markers and cardiovascular risk in type 2 diabetes patients. Cardiovascular Diabetology. 2018;17:45. doi: 10.1186/s12933-018-0691-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giachelli C.M., Lombardi D., Johnson R.J., Murry C.E., Almeida M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am J Pathol. 1998;152:353–358. [PMC free article] [PubMed] [Google Scholar]
- 30.Collaboration IRGCERF. Sarwar N., Butterworth A.S. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–1213. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patti G., D'Ambrosio A., Dobrina A. Interleukin-1 receptor antagonist: a sensitive marker of instability in patients with coronary artery disease. J Thromb Thrombolysis. 2002;14:139–143. doi: 10.1023/a:1023284912712. [DOI] [PubMed] [Google Scholar]
- 32.Ridker P.M., Everett B.M., Thuren T. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 33.Koenig W., Khuseyinova N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol. 2007;27:15–26. doi: 10.1161/01.ATV.0000251503.35795.4f. [DOI] [PubMed] [Google Scholar]
- 34.van der Leeuw J., Beulens J.W., van Dieren S. Novel biomarkers to improve the prediction of cardiovascular event risk in type 2 diabetes mellitus. J Am Heart Assoc. 2016 May 31;5(6) doi: 10.1161/JAHA.115.003048. https://doi.org/10.1161/JAHA.115.003048 pii: e003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forst T., Michelson G., Ratter F. Addition of liraglutide in patients with Type 2 diabetes well controlled on metformin monotherapy improves several markers of vascular function. Diabet Med. 2012;29:1115–1118. doi: 10.1111/j.1464-5491.2012.03589.x. [DOI] [PubMed] [Google Scholar]
- 36.Ussher J.R., Drucker D.J. Cardiovascular actions of incretin-based therapies. Circ Res. 2014;114:1788–1803. doi: 10.1161/CIRCRESAHA.114.301958. [DOI] [PubMed] [Google Scholar]
- 37.Wei R., Ma S., Wang C. Exenatide exerts direct protective effects on endothelial cells through the AMPK/Akt/eNOS pathway in a GLP-1 receptor-dependent manner. Am J Physiol Endocrinol Metab. 2016;310:E947–E957. doi: 10.1152/ajpendo.00400.2015. [DOI] [PubMed] [Google Scholar]
- 38.Pyke C., Heller R.S., Kirk R.K. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155:1280–1290. doi: 10.1210/en.2013-1934. [DOI] [PubMed] [Google Scholar]
- 39.Korner M., Stockli M., Waser B., Reubi J.C. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med. 2007;48:736–743. doi: 10.2967/jnumed.106.038679. [DOI] [PubMed] [Google Scholar]
- 40.Bang-Berthelsen C.H., Holm T.L., Pyke C. GLP-1 induces barrier protective expression in brunner's glands and regulates colonic inflammation. Inflamm Bowel Dis. 2016;22:2078–2097. doi: 10.1097/MIB.0000000000000847. [DOI] [PubMed] [Google Scholar]
- 41.Cani P.D., Delzenne N.M. Interplay between obesity and associated metabolic disorders: new insights into the gut microbiota. Curr Opin Pharmacol. 2009;9:737–743. doi: 10.1016/j.coph.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 42.Yusta B., Baggio L.L., Koehler J. GLP-1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP-1R. Diabetes. 2015;64:2537–2549. doi: 10.2337/db14-1577. [DOI] [PubMed] [Google Scholar]
- 43.Armstrong M.J., Gaunt P., Aithal G.P. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 44.Armstrong M.J., Hull D., Guo K. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J Hepatol. 2016;64:399–408. doi: 10.1016/j.jhep.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdelaziz R., Elbasel M., Esmat S., Essam K., Abdelaaty S. Tissue Inhibitors of metalloproteinase-1 and 2 and obesity related non-alcoholic fatty liver disease: is there a relationship? Digestion. 2015;92:130–137. doi: 10.1159/000439083. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Zhang Z., Zhang L. S100A8 promotes migration and infiltration of inflammatory cells in acute anterior uveitis. Sci Rep. 2016;6:36140. doi: 10.1038/srep36140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Panjwani N., Mulvihill E.E., Longuet C. GLP-1 Receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(−/−) mice. Endocrinology. 2013;154:127–139. doi: 10.1210/en.2012-1937. [DOI] [PubMed] [Google Scholar]
- 48.Ying Y.L., Chen Y.C., Jandeleit-Dahm K., Peter K. GLP-1 receptor agonists: an example of the challenge for animal models to predict plaque instability/rupture and cardiovascular outcomes. Atherosclerosis. 2017;265:250–252. doi: 10.1016/j.atherosclerosis.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 49.Meier J.J., Rosenstock J., Hincelin-Mery A. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open-label trial. Diabetes Care. 2015;38:1263–1273. doi: 10.2337/dc14-1984. [DOI] [PubMed] [Google Scholar]
- 50.Vinue A., Navarro J., Herrero-Cervera A. The GLP-1 analogue lixisenatide decreases atherosclerosis in insulin-resistant mice by modulating macrophage phenotype. Diabetologia. 2017;60:1801–1812. doi: 10.1007/s00125-017-4330-3. [DOI] [PubMed] [Google Scholar]
- 51.Gerstein H.C., Colhoun H.M., Dagenais G.R. Design and baseline characteristics of participants in the Researching cardiovascular Events with a Weekly INcretin in Diabetes (REWIND) trial on the cardiovascular effects of dulaglutide. Diabetes Obes Metab. 2018;20:42–49. doi: 10.1111/dom.13028. [DOI] [PubMed] [Google Scholar]
- 52.Whitman S.C. A practical approach to using mice in atherosclerosis research. Clin Biochem Rev. 2004;25:81–93. [PMC free article] [PubMed] [Google Scholar]
- 53.Pasterkamp G., van der Laan S.W., Haitjema S. Human validation of genes associated with a murine atherosclerotic phenotype. Arterioscler Thromb Vasc Biol. 2016;36:1240–1246. doi: 10.1161/ATVBAHA.115.306958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.