Corresponding Author

Key Words: cardiomyocytes, cardiomyopathy, disease model, genetics, heart disease, induced pluripotent stem cells, MYH7, stem cells
Autosomal dominant missense mutations in MYH7 contribute to approximately 30% to 40% of identified mutations in adults with familial hypertrophic cardiomyopathy (HCM) (1). MYH7 encodes β-myosin heavy chain protein (MHC), which is the major MHC in human adult ventricular tissue. In the sarcomere, MHC is part of the thick filament and is responsible for the mechanochemical cycle that powers muscle contraction. MHC exists as a dimer with an amino-terminal globular head (called S1), a neck and/or hinge region (called S2), followed by a long α-helical tail domain. The globular S1 heads project laterally and are responsible for adenosine triphosphate and/or actin binding and contractile force generation in the heart. The 2 tail domains dimerize into a coiled-coiled motif to form the thick filament rod. MYBPC3 encodes cardiac myosin binding protein C (cMyBP-C), which binds β-MHC in the S2 region. The interaction of cMyBP-C with the S2 region β-MHC regulates the actin-myosin cross bridge cycle and cardiac contraction (2). Currently, >400 different mutations have been identified in MYH7 (3). Both hypertrophic and dilated cardiomyopathy (DCM) are associated with heart failure and arrhythmias, and the mechanisms by which position-specific MYH7 variants elicit distinct cardiomyopathies has been studied using in vitro motility assays with purified motor proteins. HCM-associated MYH7 missense changes cluster into distinct regions of the myosin head, often falling into the mesa and converter domains where they alter the super-relaxed state of myosin 4, 5, 6.
DCM-associated MYH7 mutations have been harder to classify, in part, because of a lack of suitable models. Using mice to study Myh7 is compromised by the fact that Myh6, which encodes the α-MHC, is the major myosin of the adult mouse left ventricle. Human-induced pluripotent stem cell−derived cardiomyocytes (IPSC-CMs) are an attractive alternative. However, multiple analyses have reported differences between IPSC-CMs and adult primary cardiomyocytes in gene expression, action potentials, calcium handling, and metabolism 7, 8, 9. Although the relative immaturity of iPSC-CMs limits their ability to model adult heart diseases, bioengineering techniques, including nanopatterned surfaces and engineered heart tissues (EHTs) promote IPSC-CM maturity and therefore improve IPSC-CMs as a model.
The work of Yang et al. (10) in this issue of JACC: Basic to Translational Science brings new insights not only into the molecular pathogenesis of MYH7 mutations, but also to using IPSC-CMs as a model of genetic cardiomyopathy. The investigators identified a 44-year-old man after he survived sudden cardiac arrest. An echocardiogram showed normal ventricular wall thickness, a nondilated ventricle, and a reduced ejection fraction of 35%. Targeted gene sequencing identified the MYH7 E848G variant. The proband’s sister also presented with heart failure symptoms at age 63 years. On echocardiography, her heart showed a similar pattern, with a reduced ejection fraction of 31% and no evidence of ventricular wall thickening or chamber dilation. Neither the index patient nor his sister met echocardiographic criteria for hypertrophic cardiomyopathy or DCM. The 2 children of the index patient were examined, and they carried the E848G mutation and had preserved left ventricular ejection fractions. Both had decreased systolic wall thickening of the interventricular septum, a marker of regional systolic dysfunction (11). MYH7 E848G falls within the S2 region of β-MHC in the domain that binds cMyBP-C, and E848G impaired binding to cMyBP-C. The binding partner, cMyBP-C, was previously described with a E258K change that also impairs this same interaction between β-MHC and cMyBP-C. This mutation frequently results in reduced systolic function (12).
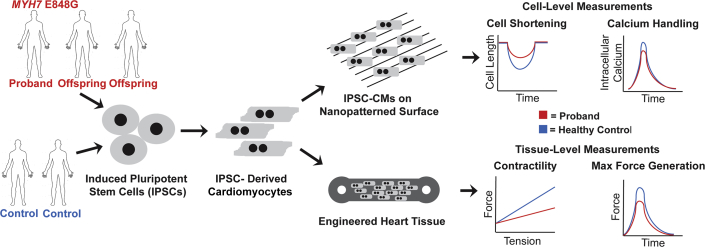
To enhance the usefulness of IPSC-CMs as a model, the investigators applied 2 different bioengineering techniques (Figure 1). When grown in normal conditions, IPSC-CMs often display a circular, rather than elongated, myofibril alignment. By growing IPSC-CMs on nanopatterned surfaces with 800-nm parallel grooves, the investigators observed elongated myofibril alignment that permitted measuring of fractional cell shortening. After 30 days in culture, E848G IPSC-CMs were indistinguishable from healthy control cardiomyocytes. The patient-derived iPSC-CMs were allowed to mature for 50 days, and then demonstrated a time-dependent reduction in fractional shortening similar to the age-dependent penetrance seen in human hearts. In EHTs, E848G IPSC-CMs were co-cultured with human marrow stromal cells in 3-dimensional molds 13, 14, 15. After 2 to 3 weeks, during which cells were exposed to constant external stress, E848G EHTs showed significant contractile dysfunction with a 4-fold reduction in the Starling curve slope and a >75% reduction in maximal active twitch force per area. Mutant EHTs displayed no defects in passive stiffness or relaxation, as would be seen with hypertrophic cardiomyopathy, recapitulating the E848G clinical phenotype of systolic dysfunction.
Figure 1.
Methodology to Model Cardiomyopathy-Associated Mutations at the Cell and Engineered Tissue Level
Cells from the index proband and his offspring, as well as related and unrelated healthy controls were reprogramed into induced pluripotent stem cells (IPSCs) and differentiated into cardiomyocytes (IPSC-CMs). IPSC-CMs are plated on nanopatterned surfaces to improve sarcomere alignment. Engineered heart tissues allowed for measurement of force−tension relationships and maximum force generation.
The age-dependent penetrance seen in iPSC-CM models reflects what occurs in human hearts, whereas cardiac phenotypes may not be expressed until relatively late in life. Intriguingly, iPSC-CMs derived from the oldest and most severely affected E848G gene carrier had the most clear cut defects in culture compared with the cells in the 2 younger E848G gene carriers. The more severe cellular phenotype in the index case may reflect additional genetic variants and/or epigenetic changes. The investigators generated iPSC lines from multiple carriers and used gene mutation−negative controls from unrelated subjects. This approach, rather than using isogenic lines, which was generated through gene editing, may provide a more representative view of human disease. Many investigators choose gene-edited isogenic controls for iPSC studies; however, this approach introduced variability due to gene editing off-target events, as well as the stress of additional rounds of culture selection. Deriving cell lines from unique patients each carrying the same primary mutations more accurately mirrors what occurs in human patients.
Footnotes
Mr. Gacita was supported by National Institutes of Health (NIH) grant HL142187. Drs. Puckelwartz and McNally were supported by NIH grant R01 HL128075. Dr. Puckelwartz was supported by American Heart Association grant 18CDA34110460.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and US Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Ho C.Y., Day S.M., Ashley E.A. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe) Circulation. 2018;138:1387–1398. doi: 10.1161/CIRCULATIONAHA.117.033200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barefield D., Sadayappan S. Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J Mol Cell Cardiol. 2010;48:866–875. doi: 10.1016/j.yjmcc.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colegrave M., Peckham M. Structural Implications of β-cardiac myosin heavy chain mutations in human disease. Anatom Rec. 2014;297:1670–1680. doi: 10.1002/ar.22973. [DOI] [PubMed] [Google Scholar]
- 4.Homburger J.R., Green E.M., Caleshu C. Multidimensional structure-function relationships in human beta-cardiac myosin from population-scale genetic variation. Proc Natl Acad Sci U S A. 2016;113:6701–6706. doi: 10.1073/pnas.1606950113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alamo L., Ware J.S., Pinto A. Effects of myosin variants on interacting-heads motif explain distinct hypertrophic and dilated cardiomyopathy phenotypes. Elife. 2017;6 doi: 10.7554/eLife.24634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson R.L., Trivedi D.V., Sarkar S.S. Deciphering the super relaxed state of human beta-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc Natl Acad Sci U S A. 2018;115:E8143–E8152. doi: 10.1073/pnas.1809540115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fijnvandraat A.C., van Ginneken A.C., de Boer P.A. Cardiomyocytes derived from embryonic stem cells resemble cardiomyocytes of the embryonic heart tube. Cardiovasc Res. 2003;58:399–409. doi: 10.1016/s0008-6363(03)00282-7. [DOI] [PubMed] [Google Scholar]
- 8.Ma J., Guo L., Fiene S.J. High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am J Physiol Heart Circ Physiol. 2011;301:H2006–H2017. doi: 10.1152/ajpheart.00694.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhute V.J., Bao X., Dunn K.K. Metabolomics identifies metabolic markers of maturation in human pluripotent stem cell-derived cardiomyocytes. Theranostics. 2017;7:2078–2091. doi: 10.7150/thno.19390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang K.-C., Breitbart A., De Lange W.J. Novel adult-onset systolic cardiomyopathy due to MYH7 E848G mutation in patient-derived induced pluripotent stem cells. J Am Coll Cardiol Basic Trans Science. 2018;3:728–740. doi: 10.1016/j.jacbts.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong S.-J., MacGregor J.H., Crawley A.P. Left ventricular wall thickness and regional systolic function in patients with hypertrophic cardiomyopathy. A three-dimensional tagged magnetic resonance imaging study. Circulation. 1994;90:1200–1209. doi: 10.1161/01.cir.90.3.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Lange W.J., Grimes A.C., Hegge L.F., Spring A.M., Brost T.M., Ralphe J.C. E258K HCM-causing mutation in cardiac MyBP-C reduces contractile force and accelerates twitch kinetics by disrupting the cMyBP-C and myosin S2 interaction. J Gen Physiol. 2013;142:241–255. doi: 10.1085/jgp.201311018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruan J.L., Tulloch N.L., Razumova M.V. Mechanical stress conditioning and electrical stimulation promote contractility and force maturation of induced pluripotent stem cell-derived human cardiac tissue. Circulation. 2016;134:1557–1567. doi: 10.1161/CIRCULATIONAHA.114.014998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tulloch N.L., Murry C.E. Trends in cardiovascular engineering: organizing the human heart. Trends Cardiovasc Med. 2013;23:282–286. doi: 10.1016/j.tcm.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinberger F., Mannhardt I., Eschenhagen T. Engineering cardiac muscle tissue: a maturating field of research. Circ Res. 2017;120:1487–1500. doi: 10.1161/CIRCRESAHA.117.310738. [DOI] [PubMed] [Google Scholar]