Abstract
Background
The prognostic impact of preoperative 18F-FDG PET/CT in advanced gastric cancer (AGC) remains a matter of debate. This study aims to evaluate the prognostic impact of SUVmax in preoperative 18F-FDG PET/CT of AGC according to histologic subtype, with a focus on the differences between tubular adenocarcinoma and signet ring cell (SRC) carcinoma.
Methods
As a discovery set, a total of 727 AGC patients from prospective database were analyzed according to histologic subtype with Cox proportional hazard model and p-spline curves. In addition, another 173 patients from an independent institution was assessed as an external validation set.
Results
In multivariate analysis, high SUVmax in preoperative 18F-FDG PET/CT of AGC was negatively correlated with disease-free survival (DFS) and overall survival (OS) in patients with diffuse type (DFS: HR 2.17, P < 0.001; OS: HR 2.47, P < 0.001) or SRC histology (DFS: HR 2.26, P = 0.005; OS: HR 2.61, P = 0.003). This negative prognostic impact was not observed in patients with intestinal type or well or moderately differentiated histology. These findings have been consistently confirmed in a validation set. The p-spline curves also showed a gradual increase in log HR as SUVmax rises only for SRC histology and for diffuse-type AGC. Finally, a novel predictive model for recurrence of AGC with diffuse type or SRC histology was generated and validated based on the preoperative SUVmax.
Conclusions
Preoperative high SUVmax of AGC is a poor prognostic factor in those with diffuse type or SRC histology. This study is the first to demonstrate the differential prognostic impact of preoperative PET/CT SUVmax in AGC according to histologic subtype and provide a clue to explain previous discrepancies in the prognostic impact of preoperative PET/CT in AGC. Prospective studies are required to validate the role of preoperative SUVmax in AGC.
Electronic supplementary material
The online version of this article (10.1007/s10120-018-0847-5) contains supplementary material, which is available to authorized users.
Keywords: Advanced gastric cancer, PET/CT, Prognostic impact, Signet ring cell carcinoma, Diffuse type
Introduction
F-18 fluoro-2-deoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) has become an indispensable method for the diagnosis, staging, and response evaluation of many malignancies [1–3]. In gastric cancer (GC), 18F-FDG PET/CT is a useful tool for the diagnosis of recurrent disease after curative surgery [4–7]. However, the role of preoperative 18F-FDG PET/CT is not yet fully established. While the National Comprehensive Cancer Network guidelines recommend the use of preoperative 18F-FDG PET/CT in GC patients to rule out distant metastasis, the prognostic impact of preoperative 18F-FDG PET/CT remains a matter of debate. Several reports suggest a potential prognostic role for preoperative 18F-FDG PET/CT, while others argue against this [8–10]. These discrepancies may be due in part to small sample sizes and heterogeneous patient populations in different studies. Notably, 18F-FDG PET/CT has low sensitivity in detecting early GC (EGC) and signet ring cell (SRC) GC, but many previous studies overlooked this, including heterogeneous populations in the studies and analyzing the clinical characteristics of the population as a whole. Because SRC GC is a unique histologic subtype of GC with a distinct tumor biology and bioenergetics, it should be analyzed separately [11–14].
The present study evaluated the prognostic impact of SUVmax in preoperative 18F-FDG PET/CT of advanced GC (AGC) according to histologic subtype, with a focus on the differences between tubular adenocarcinoma and SRC carcinoma.
Materials and methods
Patient selection
Between January 2006 and December 2013, patients with GC who underwent 18F-FDG-PET/CT and subsequent curative surgical resection at Yonsei Cancer Center, Severance Hospital, Seoul, Korea, were enrolled in the study. A predesigned data collection format was utilized to extract data from a prospectively maintained database. The main eligibility criteria were as follows: (1) pathologically confirmed AGC of tubular adenocarcinoma or SRC-histologic subtype; (2) available documented information regarding the primary tumor site, postoperative pathological stage, surgery, recurrence, and survival; and (3) patients who received curative resection including those who presented with enlarged paraaortic lymph nodes having radical surgical resection with a curative aim accompanied by paraaortic lymph node dissection. The main exclusion criteria were as follows: (1) patients with EGC; (2) patients who received neoadjuvant chemo- or radio-therapy; and (3) patients with multiple primary cancers. After applying these criteria, 727 of the original 1605 patients were included in the final analysis (Fig. S1). The pathological stage was classified according to the American Joint Committee on Cancer (AJCC) staging manual (7th edition). This study was approved by the Institutional Review Board of Severance Hospital (#2015-1751-001). For a validation cohort, AGC patients who underwent preoperative 18F-FDG-PET/CT and curative surgical resection at CHA Bundang Medical Center, Seongnam, Korea, between March 2007 and February 2014, were enrolled in the study. Data acquisition and analysis were adopted identically as in the aforementioned institution.
The WHO and the Lauren classifications were used for the histopathological evaluation of surgical specimens. Tubular adenocarcinoma was additionally classified as being well, moderately, or poorly differentiated according to the WHO classification. Accordingly, we divided the patients into three groups for further analyses: well or moderately differentiated (WMD), poorly differentiated (PD), and SRC. In terms of the Lauren classification, the tumors were classified as intestinal, diffuse, or mixed type.
18F-FDG PET/CT and image analyses
All 18F-FDG PET/CT were performed with either the Discovery STe PET/CT (GE Healthcare, Milwaukee, WI, USA) or the Biograph TruePoint 40 PET/CT (Siemens Healthcare, Erlangen, Germany). All patients fasted for at least 6 h before the scan, and the glucose level in the peripheral blood of all patients was confirmed to be 140 mg/dL or less before 18F-FDG injection. Approximately, 5.5 MBq 18F-FDG/kg body weight was administered intravenously 1 hour before image acquisition. After the initial low-dose computed tomography (CT) (Discovery STe: 30 mA, 140 kVp, Biograph TruePoint: 36 mA, 120 kVp), standard PET/CT imaging was performed from the neck to the proximal thighs with acquisition times of 2.5 min/bed position for the Biograph Truepoint 40 PET/CT and 3 min/bed position for the Discovery STe scanner in three-dimensional mode. Images were then reconstructed using ordered subset expectation maximization (2 iterations, 20 subsets).
The images were retrospectively reviewed on a GE AW 4.0 workstation by two experienced nuclear medicine specialists (A.C. and M.Y.) who were unaware of the patients’ clinical information, except for the diagnosis of GC. The evaluation of 18F-FDG PET/CT images was performed in two steps. First, 18F-FDG PET/CT images of all patients were visually assessed and the patients were classified as positive or negative with respect to 18F-FDG uptake in the primary tumor. Lesions showing focally increased 18F-FDG uptake that exceeded the uptake in the surrounding stomach wall and corresponding cancer lesions as observed by contrast-enhanced CT images and gastroduodenoscopy were classified as positive 18F-FDG uptake. Focally or diffusely increased 18F-FDG that was indistinguishable from physiological gastric wall uptake was judged to be negative 18F-FDG uptake. After the visual assessment, the maximum standardized uptake value (SUVmax) of the primary lesion was obtained and recorded for semi-quantitative analysis.
For the validation cohort, the PET/CT imaging for 173 AGC patients from CHA Bundang Medical Center was performed with Biograph mCT 128 scanner (Siemens Medical Solutions, Knoxville, TN, USA). Initial low-dose CT scans for attenuation correction (120 kV, 120 mA, 3 mm section width, 3 mm collimation) and PET/CT scans of same area with three-dimensional mode were acquired consecutively. Images were reconstructed on 400 × 400 matrices using the TrueX algorithm plus time-of-flight (TOF) reconstruction and analyzed using a dedicated workstation and software (Syngo.via, Siemens Medical Solutions, Knoxville, TN, USA). Unless otherwise stated, all other methods applied for image acquisition and data analysis were adopted identically as in the aforementioned institution.
Statistical analysis
The cut-off date was December 31, 2015. The mean SUVmax was compared according to the patients’ basic demographic and clinical characteristics using independent sample t tests or analysis of variance. For pairwise comparisons of each level of categorical variables, the statistical significance was adjusted for inflated type I errors from multiple comparisons using the Bonferroni method.
Relapse-free survival (RFS) was measured from the time of surgery to initial tumor relapse (either local or distant) or death from any cause, and overall survival (OS) was calculated as the time from surgery to death from any cause or to the last follow-up date. Survival outcomes of the group with high SUVmax were compared with survival outcomes of the group with low SUVmax based on the median SUVmax for each histologic subgroup using the log-rank test. The Cox proportional hazards model was used for multivariable analysis of prognostic factors, including age at diagnosis, sex, stage, and PET/CT SUVmax. To determine additional associations between SUVmax and survival outcomes, we examined the Cox regression model using the penalized spline smoothing method as described previously [15]. The performance of prognostic models was measured by Harrell’s c-index. We assessed model calibration by plotting the model-predicted- and actual observed 3- and 5-year RFS probabilities as calculated using the Kaplan–Meier method. The bootstrapping method with 1000 re-samples was used for adjusting bias and checking the interval validation. Statistical significance was set as P < 0.05 for all analyses. All statistical analyses were performed using SPSS version 20.0 (SPSS, Chicago, IL, USA), SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), and R package, version 3.2.4 (http://www.R-project.org).
Results
Patient characteristics
The baseline characteristics of the patients are summarized in Table 1. A total of 727 patients with pathologically confirmed AGC were analyzed. The majority of patients were male (68.0%), and the median age at diagnosis of AGC was 60 years (range 26–94) years. All patients underwent radical gastrectomy; 6.9% were pathological stage I, 26.3% were stage II, 57.4% were stage III, and 9.5% were stage IV. This study included 63 stage IV patients who presented with enlarged paraaortic lymph nodes that were observed by either preoperative PET/CT or CT. These patients underwent radical surgical resection with a curative aim accompanied by paraaortic lymph node dissection. Regarding the WHO classification, 36.9% had WMD histology, 48.0% had PD histology, and the remaining 15.1% had SRC histology. When patients were classified according to the Lauren classification, 46.8% had intestinal-type AGC, 44.6% had diffuse type, and 8.7% had mixed type. Adjuvant chemotherapy was given in 88% of patients, excluding stage I patients (50 patients), those with poor performance after surgery (16 patients), and 21 patients who refused chemotherapy. All patients who had adjuvant chemotherapy received fluorouracil-based chemotherapy.
Table 1.
Clinicopathological features and SUVmax
| Variables | Total (n = 727) n (%) |
SUVmax Mean (SD) |
SUVmax Median (range) |
P value |
|---|---|---|---|---|
| Age (years) | ||||
| Median (range) | 60.0 (26–94) | |||
| Sex | ||||
| Male | 494 (68.0) | 7.8 ± 5.1 | 6.3 (1.7–39.1) | 0.274 |
| Female | 233 (32.0) | 7.4 ± 6.1 | 4.9 (1.3–42.6) | |
| Location of tumor | ||||
| Upper | 144 (19.8) | 7.6 ± 5.0 | 6.0 (1.3–28.6) | 0.250 |
| Middle | 203 (27.9) | 7.5 ± 5.6 | 5.9 (1.9–42.6) | |
| Lower | 365 (50.2) | 7.9 ± 5.7 | 6.1 (1.7–39.1) | |
| Whole | 15 (2.1) | 5.3 ± 2.5 | 4.4 (2.7–10.5) | |
| Histology (WHO) | ||||
| WMD | 268 (36.9) | 9.2 ± 6.1 | 7.8 (1.7–42.6) | < 0.001 |
| PD | 349 (48.0) | 7.5 ± 5.3 | 5.6 (1.3–36.0) | |
| SRC | 110 (15.1) | 4.5 ± 1.9 | 4.1 (1.9–11.8) | |
| Histology (Lauren) | ||||
| Intestinal | 340 (46.8) | 9.2 ± 6.2 | 7.6 (1.7–42.6) | < 0.001 |
| Diffuse | 324 (44.6) | 6.2 ± 4.0 | 4.6 (1.3–26.5) | |
| Mixed | 63 (8.7) | 7.6 ± 5.9 | 5.6 (2.2–28.6) | |
| Stage | ||||
| I | 50 (6.9) | 5.8 ± 3.8 | 4.3 (1.7–18.9) | 0.043 |
| II | 191 (26.3) | 8.3 ± 6.5 | 6.1 (2.1–42.6) | |
| III | 417 (57.4) | 7.7 ± 5.2 | 6.0 (1.9–36.0) | |
| IV | 69 (9.5) | 7.4 ± 4.6 | 5.9 (1.3–22.4) | |
| T stage | ||||
| T2 | 93 (12.8%) | 6.0 ± 3.5 | 4.7 (1.7–18.9) | < 0.001 |
| T3 | 206 (28.3) | 8.9 ± 6.2 | 7.1 (2.0–39.1) | |
| T4 | 428 (58.9) | 7.5 ± 5.3 | 5.7 (1.3–42.6) | |
| N stage | ||||
| N0 | 163 (22.4) | 7.5 ± 6.3 | 4.9 (1.7–42.6) | 0.427 |
| N1 | 135 (18.6) | 8.2 ± 6.0 | 7.0 (2.0–39.1) | |
| N2 | 129 (17.7) | 8.0 ± 5.3 | 6.3 (1.9–29.9) | |
| N3 | 300 (41.3) | 7.4 ± 4.8 | 5.9 (1.3–32.6) | |
WMD adenocarcinoma well to moderately differentiated, PD adenocarcinoma poorly differentiated, SRC signet ring cell carcinoma
SUVmax and histologic subtype
This study only included patients with AGC, and 80% of all patients showed a positive 18F-FDG uptake (Supplementary Figure 1). In terms of WHO classification, 86% of WMD, 81% of PD, and 68% of SRC showed positive 18F-FDG uptake. According to the Lauren classification, 84% of intestinal type and 76% of diffuse type GC showed positive 18F-FDG uptake. In terms of stage, 72% of stage I, 75% of stage II, 82% of stage III, and 94% of stage IV showed positive 18F-FDG uptake. Table 1 shows SUVmax according to various clinicopathologic variables. Notably, SUVmax was significantly correlated with the histologic type of AGC by both the WHO and Lauren classifications (Fig. 1a). The mean SUVmax of AGC patients with SRC histology was 51% lower than that of AGC patients with WMD histology (4.5 ± 1.9 vs. 9.2 ± 6.1, P < 0.001). The majority of patients that had AGC with SRC histology had SUVmax less than 5, whereas the majority of patients that had AGC with WMD histology had SUVmax greater than 5. When the SUVmax was analyzed according to the Lauren classification, patients with diffuse-type AGC, which mostly had SRC histology, had 33% lower SUVmax than those with intestinal-type AGC, which mostly had WMD histology, (6.2 ± 4.0 vs. 9.2 ± 6.2, P < 0.001) (Fig. 1a). Moreover, the SUVmax also correlated with the progression of stage, especially T stage (P < 0.001), but not nodal (N) stage (P = 0.427). Intriguingly, the SUVmax correlated well with the maximal size of the tumor mass in AGC with WMD histology or intestinal type (Fig. 1b). However, the degree of correlation between SUVmax and maximal tumor size was relatively weak in AGC with SRC histology or diffuse type. Collectively, these findings demonstrate the distinct tumor biology of AGC with WMD or SRC histology, especially in terms of glucose metabolism.
Fig. 1.

The preoperative 18F-FDG PET/CT SUVmax of advanced gastric cancer (AGC) according to histologic subtype. a The SUVmax correlates with the histologic type of AGC by both the WHO and Lauren classifications. b The SUVmax correlates well with the maximal size of the tumor in AGC with well to moderately differentiated (WMD) histology or intestinal type. *P < 0.05
The prognostic impact of SUVmax according to histologic subtype
With the median follow-up duration of 32.5 months, 357 (49%) patients recurred and 301 (49%) died. To evaluate the prognostic impact of each histologic subtype, the survival outcomes were compared between the high- and low-SUVmax groups. The cut-off value was the median SUVmax of each histologic group (Table 1). In terms of DFS, AGC patients with high SUVmax had significantly shorter DFS if they had diffuse-type AGC or SRC histology (P < 0.001 and P < 0.001, respectively), while there were no differences in the DFS of AGC patients with intestinal type or WMD histology (Fig. 2a, b). This was also true for OS; high SUVmax only had a negative prognostic impact in AGC with diffuse type or SRC histology (P < 0.001 and P < 0.001, respectively; Fig. 2c, d).
Fig. 2.
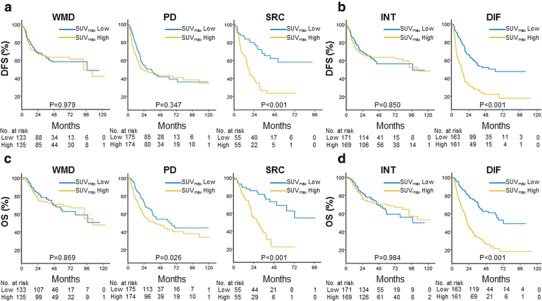
Kaplan–Meier survival curves comparing the high- and low-SUVmax groups in each histologic subtype. a, b High SUVmax only had a negative prognostic impact on disease-free survival (DFS) in AGC with SRC histology or diffuse type. c, d High SUVmax only had a negative prognostic impact on overall survival (OS) in AGC with SRC histology or diffuse type
In the Cox proportional hazard model, which was adjusted for sex, age, T stage and N stage (Table 2), high SUVmax was also negatively correlated with DFS and OS in AGC patients with SRC histology (DFS: HR 2.26, P = 0.005; OS: HR 2.61, P = 0.003). Moreover, high SUVmax was negatively correlated with DFS and OS in AGC patients with diffuse-type AGC (DFS: HR 2.17, P < 0.001; OS: HR 2.47, P < 0.001). This negative prognostic impact was not observed in AGC patients with WMD histology or intestinal type. In addition, even when the Cox regression model was applied with the exception of 16 stage IV patients, high SUVmax was still a poor prognostic factor in SRC and diffuse-type gastric cancer (Supplementary Table 2).
Table 2.
Multivariable cox regression analysis of SUVmax and its predictive impact on clinical outcomes according to histologic type
| WHO classification | WMD (n = 268) | PD (n = 349) | SRC (n = 110) | ||||
|---|---|---|---|---|---|---|---|
| AHR (95% CI) | P value | AHR (95% CI) | AHR (95% CI) | P value | |||
| DFS | |||||||
| Sex | Female vs. male (ref) | 0.97 (0.58–1.62) | 0.916 | 0.94 (0.70–1.27) | 0.686 | 0.78 (0.47–1.31) | 0.354 |
| Age | ≥ 65 vs. < 65 years (ref) | 1.47 (0.98–2.18) | 0.060 | 1.47 (1.09–1.97) | 0.011 | 1.43 (0.83–2.47) | 0.198 |
| T stage | T4 vs. T2 + T3 (ref) | 3.18 (2.08–4.87) | < 0.001 | 2.54 (1.75–3.68) | < 0.001 | 5.44 (1.93–15.34) | 0.001 |
| N stage | N1 + N2 + N3 vs. N0 (ref) | 1.99 (1.14–3.46) | 0.015 | 3.12 (1.75–5.55) | < 0.001 | 2.25 (0.88–5.75) | 0.090 |
| SUVmax | High vs. low (ref) | 0.92 (0.62–1.37) | 0.680 | 1.09 (0.81–1.45) | 0.570 | 2.26 (1.28–4.00) | 0.005 |
| OS | |||||||
| Sex | Female vs. male (ref) | 0.95 (0.53–1.69) | 0.854 | 1.00 (0.73–1.38) | 1.000 | 0.86 (0.50–1.48) | 0.574 |
| Age | ≥ 65 vs. < 65 years (ref) | 1.99 (1.27–3.14) | 0.003 | 1.73 (1.26–2.37) | 0.001 | 1.98 (1.12–3.50) | 0.018 |
| T stage | T4 vs. T2 + T3 (ref) | 3.13 (1.92–5.09) | < 0.001 | 2.87 (1.90–4.34) | < 0.001 | 3.68 (1.29–10.48) | 0.015 |
| N stage | N1 + N2 + N3 vs. N0 (ref) | 1.58 (0.87–2.89) | 0.135 | 3.04 (1.59–5.85) | < 0.001 | 2.22 (0.77–6.35) | 0.138 |
| SUVmax | High vs. low (ref) | 0.90 (0.57–1.41) | 0.638 | 1.39 (1.01–1.90) | 0.043 | 2.61 (1.39–4.91) | 0.003 |
| Lauren classification | Intestinal (n = 340) | Mixed (n = 63) | Diffuse (n = 324) | ||||
|---|---|---|---|---|---|---|---|
| AHR (95% CI) | P value | AHR (95% CI) | AHR (95% CI) | P value | |||
| DFS | |||||||
| Sex | Female vs. male (ref) | 1.02 (0.66–1.59) | 0.922 | 0.65 (0.29–1.42) | 0.277 | 0.97 (0.72–1.29) | 0.814 |
| Age | ≥ 65 vs. < 65 years (ref) | 1.72 (1.21–2.44) | 0.003 | 0.82 (0.38–1.77) | 0.615 | 1.35 (1.00–1.83) | 0.051 |
| T stage | T4 vs. T2 + T3 (ref) | 2.87 (1.96–4.20) | < 0.001 | 3.63 (1.25–10.59) | 0.018 | 3.09 (2.04–4.67) | < 0.001 |
| N stage | N1 + N2 + N3 vs. N0 (ref) | 2.28 (1.37–3.81) | 0.002 | 2.45 (0.67–8.92) | 0.174 | 2.55 (1.44–4.53) | 0.001 |
| SUVmax | High vs. low (ref) | 0.80 (0.56–1.13) | 0.202 | 0.69 (0.32–1.53) | 0.363 | 2.17 (1.60–2.95) | < 0.001 |
| OS | |||||||
| Sex | Female vs. male (ref) | 1.09 (0.67–1.76) | 0.742 | 0.77 (0.34–1.76) | 0.536 | 0.99 (0.72–1.35) | 0.932 |
| Age | ≥ 65 vs. < 65 years (ref) | 2.11 (1.42–3.14) | < 0.001 | 1.27 (0.57–2.84) | 0.558 | 1.74 (1.26–2.41) | < 0.001 |
| T stage | T4 vs. T2 + T3 (ref) | 2.83 (1.84–4.37) | < 0.001 | 4.13 (1.24–13.78) | 0.021 | 3.13 (1.99–4.93) | < 0.001 |
| N stage | N1 + N2 + N3 vs. N0 (ref) | 1.82 (1.05–3.14) | 0.033 | 2.11 (0.57–7.81) | 0.265 | 2.79 (1.41–5.52) | 0.003 |
| SUVmax | High vs. low (ref) | 0.79 (0.53–1.18) | 0.251 | 0.63 (0.27–1.46) | 0.280 | 2.47 (1.77–3.46) | < 0.001 |
WMD adenocarcinoma well to moderately differentiated, PD adenocarcinoma poorly differentiated, SRC signet ring cell carcinoma, DFS disease-free survival, OS overall survival, AHR adjusted hazard ratio, CI confidence interval, ref reference
To externally validate these findings, we also analyzed data from an independent institution. The same results were consistently observed in this validation cohort: (1) diffuse-type AGC with SRC histology had lower SUVmax compared with intestinal-type AGC with WMD histology (Table S1); and (2) higher preoperative SUVmax indicated poorer prognosis in AGC with SRC histology and diffuse type, but not in AGC patients with WMD histology and intestinal type (Fig. S2).
Taken together, these data confirmed that high SUVmax has an independent negative prognostic role in AGC patients with SRC or diffuse-type AGC.
Prognostic implications of SUVmax as a continuous variable (p-spline curve)
To further investigate the role of SUVmax as a continuous variable in survival analysis, p-spline curves for DFS were generated with the R program as described previously [15] after adjusting for sex, age, T stage and N stage (Fig. 3). The results were consistent with those from the Cox regression analysis with dichotomous variables. The p-spline curves showed a gradual increase in log HR as SUVmax rises only for SRC histology (Fig. 3a, right) and for diffuse type (Fig. 3b, right). There was no definite trend for WMD and PD histology (Fig. 3a, left) or intestinal type (Fig. 3b, left). This confirmed that SUVmax is a continuous variable that can predict DFS in AGC patients with SRC histology or diffuse-type AGC.
Fig. 3.
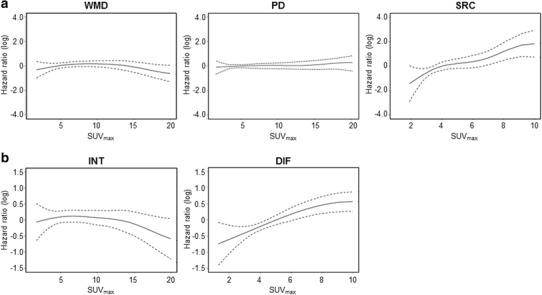
p-spline curves for DFS after adjusting for sex, age, and disease stage. The p-spline curves show a gradual increase in log HR as SUVmax rises only for SRC histology (a, right) and for diffuse type (b, right). There was no definite trend for WMD and PD histology (a, left) or intestinal type (b, left)
Generating a predictive model for recurrence probability based on preoperative SUVmax in SRC or diffuse-type AGC
To predict recurrence after curative surgery more precisely for AGC, we tried to develop a novel predictive model based on preoperative SUVmax. Recurrence-free probabilities at 1, 3, and 5 years were calculated for AGC with SRC histology (Fig. 4a) or diffuse type (Fig. 4b) after adjusting for sex, age, T stage and N stage. The RFS rate gradually decreased as SUVmax increased, and the 5-year RFS rate was less than 20% when the SUVmax was greater than 5. To evaluate the performance of our predictive model, we generated calibration curves (Fig. 5) that showed good agreement between the predicted and actual RFS; the bootstrap-corrected c-indices of the model were 0.751 (95% CI 0.675–0.827) for AGC with SRC histology and 0.687 (95% CI 0.644–0.730) for diffuse-type AGC. Thus, we were able to generate and internally validate our novel predictive model for recurrence in AGC with SRC histology or diffuse-type AGC.
Fig. 4.
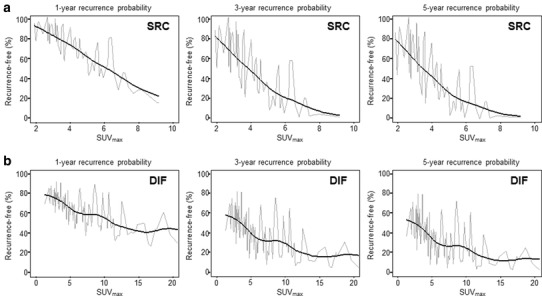
Predictive model for recurrence based on preoperative SUVmax in SRC (a) or diffuse-type AGC (b)
Fig. 5.
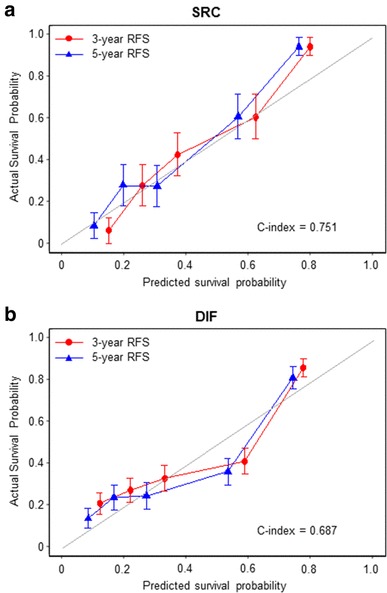
Calibration curves for the performance of predictive model in SRC (a) or diffuse-type AGC (b)
Discussion
Gastric cancer is increasingly recognized as a heterogeneous disease [11, 14, 16, 17]. Classically, it is classified according to its histology, i.e., as intestinal type or diffuse type [18]. Intestinal-type GC is more predominant in older people and in men, whereas diffuse-type GC is more frequently found in younger women. Recently, genomic data is widely utilized to develop molecular classification systems for GC. The TCGA Research Network proposed a classification system to distinguish GC into four subtypes: (1) Epstein–Barr virus (EBV)-positive, with the highest DNA methylation levels; (2) microsatellite instability (MSI), characterized by hypermutated tumors; (3) genomic stability (GS), which represents 20% of GC and comprises the majority of diffuse-type GC, has the most abundant CDH1 mutations and also shows increased RHOA mutations and CLDN18–ARHGAP fusions; and (4) chromosomal instability (CIN), which accounts for 50% of patients and is characterized by frequent TP53 mutations and high percentage of intestinal-type GC [16, 19].
However, current clinical practice does not take this heterogeneity into account; rather, GC is regarded as a single type of malignancy, and a one-size-fits-all approach is applied. Although some previous studies have shown that the SUVmax in PET/CT can differ markedly according to the histologic subtype, most studies still evaluate the prognostic impact of SUVmax by considering GC to be a single disease entity rather than categorizing it into the various histologic subtypes. Not surprisingly, this has resulted in inconsistencies between studies. Furthermore, despite the large number of studies that have already observed poor 18F-FDG PET uptake in patients with EGC only having mucosal or submucosal invasion, most studies still enroll patients with EGC. The largest preoperative study to date was reported by Lee et al., who evaluated the prognostic impact of PET/CT in 271 GC patients [8]. Because approximately half of the enrolled patients had EGC, 45% of the patients had no detectable 18F-FDG uptake, and so only the remaining 149 patients were available for further analysis. Consequently, the subgroup analysis was limited by the small sample size.
To overcome the limitations of previous studies, the present study prospectively collected data from more than 700 patients with AGC while excluding patients with EGC. Moreover, to avoid oversimplification, the patients were divided and analyzed according to their histologic subtypes. Furthermore, the prognostic impact of SUVmax was evaluated not only as a dichotomous variable as determined by the median SUVmax but also as a continuous variable by analyzing p-spline curves. As a result, we were able to reveal the distinct prognostic impact of SUVmax in 18F-FDG PET/CT according to histologic subtype.
First, diffuse-type AGC with SRC histology had lower SUVmax compared with intestinal-type AGC with WMD histology, which is in agreement with previous studies. Second, although the SUVmax of diffuse-type AGC was lower than that of intestinal type, it had a significant prognostic impact in terms of survival outcome. On the other hand, the SUVmax of intestinal-type AGC was more directly correlated with primary tumor size than diffuse-type AGC, but it did not have any prognostic impact. These finding provide a clue to explaining previous discrepancies regarding the prognostic impact of 18F-FDG PET/CT in GC patients. Finally, we established and validated a novel model that utilizes the preoperative SUVmax to predict tumor recurrence after surgery in patients with SRC or diffuse type, which may be a useful tool for clinical application.
Each histologic subtype of GC differs in its biology, especially in its metabolic profiles, which leads to different 18F-FDG uptake patterns [20]. Among the various histologic types of GC, SRC stands out as a unique subtype due to its distinct molecular and metabolic features. In terms of the glucose transporter GLUT-1, SRC is reported to express GLUT-1 at lower levels than WMD adenocarcinoma, leading to reduced 18F-FDG uptake [21, 22]. In addition, SRC has lower levels of the pyruvate kinase M2 isoform (PKM2) compared with other histologic subtypes; PKM2 is responsible for ATP production in the last step of glycolysis [12]. Furthermore, PKM2 expression is correlated with poor prognosis in SRC, while other subtypes are not. These metabolic characteristics help explain the different patterns and prognostic values of 18F-FDG PET/CT in different histologic subtypes of GC, and our data highlight the importance of dividing GC into different histologic subtypes before PET/CT analysis.
The main limitation of our study is the retrospective nature of data collection. Although we verified our findings in two independent cancer centers in Korea, more studies are needed to validate our findings in a prospective cohort. Especially, this study did not include patients who underwent preoperative treatment. Therefore, it is necessary to evaluate the role of PET/CT in Western patients who received preoperative treatment with other studies. In addition, this study only included patients with AGC, thus most patients received adjuvant chemotherapy. There are limitations in analysis of the effect of adjuvant chemotherapy on the prognostic remodeling. Second, volumetric PET parameters were not measured due to the large number of cases. Additional studies are needed to further evaluate the value of volumetric PET parameters rather than SUVmax for predicting clinical outcomes in intestinal-type AGC.
In conclusion, this study demonstrated the differential patterns and prognostic impact of preoperative PET/CT SUVmax in AGC according to histologic type. Although the SUVmax did not have significant prognostic impact in WMD- and intestinal-type AGC, higher preoperative SUVmax indicated poorer prognosis in SRC and diffuse-type AGC. Novel predictive models for recurrence probability can be provided based on the preoperative SUVmax in patients with SRC or diffuse-type AGC. To validate these findings, we are preparing a prospective trial. If the results of this study are confirmed in a prospective trial, SRC or diffuse-type gastric cancer patients with high SUVmax should be stratified in adjuvant chemotherapy. Ultimately, a clinical trial in which novel or more intensive therapy approaches are applied to SRC and diffuse-type AGC patients with high SUVmax should be performed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1. CONSORT diagram (TIF 75 KB)
Supplementary Figure 2. Kaplan–Meier survival curves of validation cohort comparing the high- and low SUVmax groups in each histologic subtype (TIF 129 KB)
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Ministry of Science, ICT and Future Planning (Grant NRF-2016M3A9E8941664 to H.C. NRF-2016R1C1B2014671 to C.K.) and by a Grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare (Grant 1520190 to S.Y.R).
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent to be included in the study, or the equivalent, was obtained from all patients.
Conflict of interest
Research grants from funding agencies: the National Research Foundation of Korea (NRF) Grant funded by the Ministry of Science, ICT and Future Planning (Grant NRF-2016M3A9E8941664 to H.C. NRF-2016R1C1B2014671 to C.K.) and the Grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare (Grant 1520190 to S.Y.R). Honoraria for speaking at symposia: no. Financial support for attending symposia: no. Financial support for educational programs: no. Employment or consultation: no. Support from a project sponsor: no. Position on advisory board or board of directors or other type of management relationships: no. Multiple affiliations: no. Financial relationships, for example equity ownership or investment interest: no. Intellectual property rights (e.g. patents, copyrights and royalties from such rights). Holdings of spouse and/or children that may have financial interest in the work: no.
Contributor Information
Mijin Yun, Phone: +82-2-2228-6068, Email: yunmijin@yuhs.ac.
Sun Young Rha, Phone: +82-2-2228-8050, Email: rha7655@yuhs.ac.
References
- 1.Kostakoglu L, Agress H, Jr, Goldsmith SJ. Clinical role of FDG PET in evaluation of cancer patients 1. Radiographics. 2003;23:315–340. doi: 10.1148/rg.232025705. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum S, Stergar H, Antoch G, Veit P, Bockisch A, Kühl H. Staging and follow-up of gastrointestinal tumors with PET/CT. Abdom Imaging. 2006;31:25–35. doi: 10.1007/s00261-005-0031-3. [DOI] [PubMed] [Google Scholar]
- 3.Bar-Shalom R, Yefremov N, Guralnik L, Gaitini D, Frenkel A, Kuten A, et al. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med. 2003;44:1200–1209. [PubMed] [Google Scholar]
- 4.Bilici A, Ustaalioglu BBO, Şeker M, Kefeli U, Canpolat N, Tekinsoy B, et al. The role of 18F-FDG PET/CT in the assessment of suspected recurrent gastric cancer after initial surgical resection: can the results of FDG PET/CT influence patients’ treatment decision making? Eur J Nucl Med Mol Imaging. 2011;38:64–73. doi: 10.1007/s00259-010-1611-1. [DOI] [PubMed] [Google Scholar]
- 5.De Potter T, Flamen P, Van Cutsem E, Penninckx F, Filez L, Bormans G, et al. Whole-body PET with FDG for the diagnosis of recurrent gastric cancer. Eur J Nucl Med Mol Imaging. 2002;29:525–529. doi: 10.1007/s00259-001-0743-8. [DOI] [PubMed] [Google Scholar]
- 6.Yun M. Imaging of gastric cancer metabolism using 18F-FDG PET/CT. J Gastric Cancer. 2014;14:1–6. doi: 10.5230/jgc.2014.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shan F, Ji J. Updating advances and controversies on the multidisciplinary therapy of gastric cancer. Transl Gastrointest Cancer. 2012;1:151–160. [Google Scholar]
- 8.Lee JW, Lee SM, Lee M-S, Shin HC. Role of 18F-FDG PET/CT in the prediction of gastric cancer recurrence after curative surgical resection. Eur J Nucl Med Mol Med Imaging. 2003;30:288–295. doi: 10.1007/s00259-002-1029-5. [DOI] [PubMed] [Google Scholar]
- 9.Lee JW, Jo K, Cho A, Noh SH, Lee JD, Yun M. Relationship between 18F-FDG uptake on PET and recurrence patterns after curative surgical resection in patients with advanced gastric cancer. J Nucl Med. 2015;56:1494–1500. doi: 10.2967/jnumed.115.160580. [DOI] [PubMed] [Google Scholar]
- 10.Dassen A, Lips D, Hoekstra C, Pruijt J, Bosscha K. FDG-PET has no definite role in preoperative imaging in gastric cancer. Eur J Surg Oncol. 2009;35:449–455. doi: 10.1016/j.ejso.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Nafteux PR, Lerut TE, Villeneuve PJ, Dhaenens JM, De Hertogh G, Moons J, et al. Signet ring cells in esophageal and gastroesophageal junction carcinomas have a more aggressive biological behavior. Ann Surg. 2014;260:1023–1029. doi: 10.1097/SLA.0000000000000689. [DOI] [PubMed] [Google Scholar]
- 12.Lim JY, Yoon SO, Seol SY, Hong SW, Kim JW, Choi SH, et al. Overexpression of the M2 isoform of pyruvate kinase is an adverse prognostic factor for signet ring cell gastric cancer. World J Gastroenterol. 2012;18:4037–4043. doi: 10.3748/wjg.v18.i30.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bamboat ZM, Tang LH, Vinuela E, Kuk D, Shah MA, Brennan MF, et al. Stage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinoma. Ann Surg Oncol. 2014;21:1678. doi: 10.1245/s10434-013-3466-8. [DOI] [PubMed] [Google Scholar]
- 14.Chon HJ, Hyung WJ, Kim C, Park S, Kim J-H, Park CH, et al. Differential prognostic implications of gastric signet ring cell carcinoma: stage adjusted analysis from a single high-volume center in Asia. Ann Surg. 2017;265:946. doi: 10.1097/SLA.0000000000001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meira-Machado L, Cadarso-Suárez C, Gude F, Araújo A. smoothHR: an R package for pointwise nonparametric estimation of hazard ratio curves of continuous predictors. Comput Math Methods Med. 2013;2013:745742. doi: 10.1155/2013/745742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Network CGAR Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chon HJ, Hyung WJ, Kim C, Park S, Kim J-H, Park CH, et al. Differential prognostic implications of gastric signet ring cell carcinoma: stage adjusted analysis from a single high-volume center in Asia. Ann Surg. 2017;265:946–953. doi: 10.1097/SLA.0000000000001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauren P. The two histological main types of gastric carcinoma, an attempt at a histoclinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 19.Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi ST, et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573–582. doi: 10.1038/ng.2983. [DOI] [PubMed] [Google Scholar]
- 20.Yuan L-W, Yamashita H, Seto Y. Glucose metabolism in gastric cancer: the cutting-edge. World J Gastroenterol. 2016;22:2046. doi: 10.3748/wjg.v22.i6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi B-H, Song H-S, An Y-S, Han S-U, Kim J-H, Yoon J-K. Evaluation of 18F-FDG PET/CT in patients with gastric signet ring cell tumor: correlation with GLUT-1 expression, endoscopic and histologic finding. J Nucl Med. 2010;51:65. [Google Scholar]
- 22.Alakus H, Batur M, Schmidt M, Drebber U, Baldus SE, Vallböhmer D, et al. Variable 18F-fluorodeoxyglucose uptake in gastric cancer is associated with different levels of GLUT-1 expression. Nucl Med Commun. 2010;31:532–538. doi: 10.1097/MNM.0b013e32833823ac. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. CONSORT diagram (TIF 75 KB)
Supplementary Figure 2. Kaplan–Meier survival curves of validation cohort comparing the high- and low SUVmax groups in each histologic subtype (TIF 129 KB)


