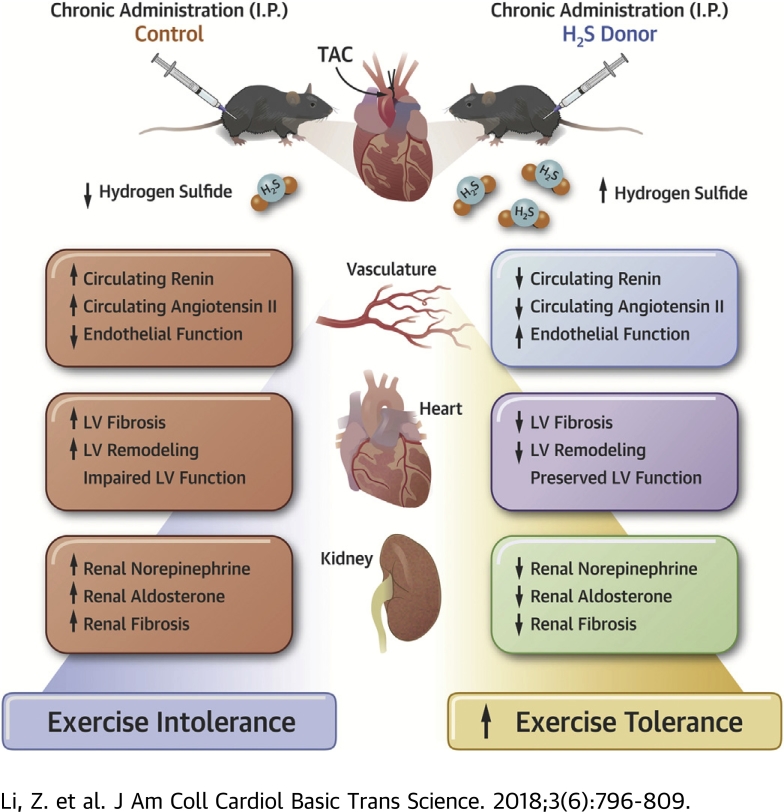
Visual Abstract
Key Words: cardiorenal syndrome, exercise tolerance, heart failure, hydrogen sulfide, RAAS
Abbreviations and Acronyms: BNP, B-type natriuretic peptide; cGMP, cyclic guanosine monophosphate; HF, heart failure; LVEF, left ventricular ejection fraction; RAAS, renin-angiotensin-aldosterone system; SNS, sympathetic nervous system; TAC, transverse aortic constriction
Highlights
-
•
Pressure overload induced by transverse aortic constriction led to severe heart failure accompanied by renal fibrosis and dysfunction, endothelial dysfunction, and exercise intolerance in mice.
-
•
3-week but not 10-week-delayed H2S therapy preserved left ventricular ejection fraction and attenuated cardiac fibrosis.
-
•
H2S therapy attenuated renal fibrosis and dysfunction, preserved endothelium, and enhanced treadmill exercise capacity.
-
•
H2S therapy blunted the maladaptive overactivation of the sympathetic nervous system, resulting in reduced renal tissue norepinephrine levels as well as attenuated circulating levels of renin, angiotensin II, and aldosterone.
Summary
Cardioprotective effects of H2S have been well documented. However, the lack of evidence supporting the benefits afforded by delayed H2S therapy warrants further investigation. Using a murine model of transverse aortic constriction-induced heart failure, this study showed that delayed H2S therapy protects multiple organs including the heart, kidney, and blood-vessel; reduces oxidative stress; attenuates renal sympathetic and renin-angiotensin-aldosterone system pathological activation; and ultimately improves exercise capacity. These findings provide further insights into H2S-mediated cardiovascular protection and implicate the benefits of using H2S-based therapies clinically for the treatment of heart failure.
Heart failure (HF) is a heterogeneous disease resulting from a number of common pathological stimuli including, but not limited to, long-standing hypertension, profound inflammation, and overactivation of neurohumoral pathways (1). HF is a major public health problem in the United States, with a prevalence of 7 million people, and it is projected to affect more than 9 million people by 2030 (2). Due to its heterogeneous nature, the consequences of HF are not limited to cardiac pathology. Noncardiac comorbidities such as renal dysfunction and vascular dysfunction frequently accompany HF and further decrease patients’ quality of life and clinical outcomes 3, 4. For example, HF patients with renal dysfunction have greater chance for readmission, longer hospital stay, and worse prognosis, and vascular injury has been linked to reduced exercise capacity, leading to decreased quality of life 5, 6.
Neurohumoral dysregulation is a hallmark feature in many cardiovascular diseases including heart failure 7, 8, 9, 10. Specifically, overactivation of the sympathetic nervous system and the renin-angiotensin-aldosterone system (RAAS) exacerbates HF symptoms and contributes to the development of comorbidities 4, 11, 12. In fact, efficacious first-line medications for HF such as beta-blockers, mineralocorticoid receptor antagonists, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs) target the sympathetic nervous system (SNS) and the RAAS overactivity. However, these medications are imperfect and have several limitations. For example, beta-blockers, mineralocorticoid receptor antagonists, and ARBs are receptor antagonists designed to compete with endogenous ligands without altering or decreasing the levels of endogenous ligands, which potentially limits their vascular protective effects 13, 14. Distinct from receptor antagonists, ACE inhibitors reduce the endogenous production of angiotensin II (Ang II), but its use is contraindicated in patients with certain cardiorenal syndromes due to unwanted side effects (15). Current treatments inadequately manage the diverse population of HF patients in a manner that effectively extends longevity and quality of life. Therefore, examination of novel pharmacotherapies that have the ability to protect multiple organ systems in heart failure is warranted.
H2S plays a key role in cardiovascular homeostasis and cardioprotection 16, 17, 18, 19, 20. H2S wields a wide range of biological functions in the cardiovascular system, including mitochondria biogenesis, metabolic modulation, angiogenesis, vasodilation and blood pressure regulation, inhibition of inflammation, antioxidant, and antiapoptosis (21). Furthermore, H2S reportedly exerts protective effects in multiple organ systems including vasculature and the kidney 22, 23, 24, both of which are involved in the pathogenesis and progression of HF. Taken together, H2S represents a promising therapeutic target for the treatment of HF and its comorbidities.
JK-1 is a novel, phosphorothioate-based synthetic H2S donor that releases H2S upon hydrolysis. Recently we reported that administration of JK-1 at the time of reperfusion significantly reduced infarct size and circulating troponin-I levels in a murine model of myocardial ischemia-reperfusion injury (20). In the present study, using a well-established pressure overload HF model, the efficacy of delayed therapy with JK-1 on cardiac, renal, and vascular injury and dysfunction in the setting of HF was investigated.
Methods
Experimental compounds
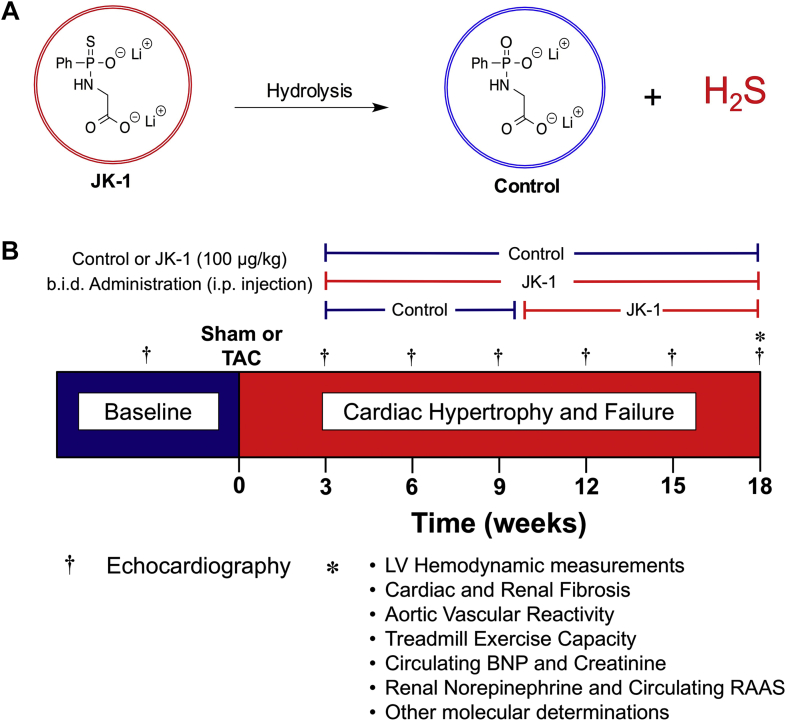
JK-1 and its control compound were designed and synthesized by Dr. Ming Xian et al. (20). The optimal dose of JK-1 was selected based on the results of a previous study investigating the infarct-sparing effects of JK-1 (20). The structures of JK-1 and control are described in Figure 1A.
Figure 1.
Experimental Compounds and Experimental Protocol
(A) Chemical structures of JK-1 and control. Hydrolysis of JK-1 yields H2S and the chemical backbone of JK-1, which was selected as Control. The control compound is devoid of sulfur and does not release H2S. (B) Experimental design and protocol. At 1 week following baseline echocardiography, mice were subjected to TAC or sham TAC surgery. Mice were studied for a period of 18 weeks following TAC. The H2S donor, JK, was administered at either 3 or 10 weeks following TAC at a dose of 100 μg/kg b.i.d. through i.p. injection. This study involved 4 groups: sham TAC, HF plus Control compound, HF plus JK-1 administered at 3 weeks following TAC, or HF plus JK-1 administered starting at 10 weeks post TAC. b.i.d. = twice daily; BNP = B-type natriuretic peptide; HF = heart failure; LV = left ventricle; RAAS = renin-angiotensin-aldosterone system; TAC = transverse aortic constriction.
Experimental animals
Male C57BL/6J mice (Jackson Laboratory, Bar Harbor, Maine) were 9 weeks of age at the time of study initiation. All experimental protocols were approved by the Institute for Animal Care and Use Committee at LSU Health Sciences Center and conformed to the Guide for the Care and Use of Laboratory Animals. All experimental animals received humane care in accordance with National Society of Medical Research and National Institutes of Health tenets.
Transverse aortic constriction protocol
Cardiac hypertrophy and heart failure were induced through transverse aortic constriction (TAC) as previously described 19, 25, 26.
Experimental design and treatment regimen
Sham or TAC procedures were performed 1 week after baseline echocardiography. All mice subjected to the TAC procedure were randomly assigned to 1 of 3 study groups. One group received control treatment starting at 3 weeks post TAC and continued until 18 weeks post TAC. The second group was treated with JK-1 starting at 3 weeks post TAC. The third group started with control at 3 weeks post TAC and were then switched to JK-1 at 10 weeks post TAC. All treatments were administered twice a day at a dosage of 100 μg/kg (200 μg/kg/day) through i.p. injection. A detailed experimental protocol is shown in Figure 1B.
Echocardiography
Echocardiography was performed at baseline and once every 3 weeks (model Vevo-2100 ultrasonography system; Visual Sonic, Baltimore, Maryland) as previously described (25). M-mode long-axis views were used to measure the thickness of interventricular septal walls at systole and diastole and left ventricle (LV) posterior wall at systole and diastole. LV chamber diameters at end systole and end diastole and LV ejection fraction (LVEF) were also measured using M-mode long-axis images.
LV hemodynamics assessment
Invasive hemodynamic measurement was performed at the 18-week endpoint using a 1.2-F transonic pressure catheter. The pressure catheter was inserted into the right common carotid and advanced into the LV. Systolic and diastolic arterial blood pressure, LV end diastolic pressure, and relaxation constant Tau values were recorded. Equally elevated systolic blood pressure across groups indicates a successful TAC procedure (Supplemental Figure S3A).
Histological assessment
Cardiac and renal fibrosis were assessed at the 18-week endpoint as previously described (26). To quantify global fibrosis from the Picrosirius red-stained slides, fluorescent images were obtained (original magnification ×20) using an Olympus (model BX53-DP80, Tokyo, Japan) system and analyzed using ImageJ software (U.S. National Institutes of Health, Bethesda, Maryland).
Quantitative PCR
RNA was isolated from the heart and kidney tissues of control and JK-1-treated mice at 18 weeks post TAC. One milligram of RNA was transcribed using I-script cDNA synthesis kit (Bio-Rad, Portland, Maine). TaqMan primers for collagen 1a1 (Col1a1), collagen 3a1 (Col3a1), interleukin (IL)-6, connective tissue growth factor (CTGF), and fibronectin (FN1) were used to amplify by quantitative polymerase chain reaction (qPCR). For real-time qPCR experiments, 18S ribosomal RNA was used as a housekeeping gene, and 2-delta-deltaCT values corrected to 18S rRNA were used for data analysis.
Circulating B-type natriuretic peptide quantification
Circulating B-type natriuretic peptide (BNP) levels were measured at 18 weeks post TAC using an enzyme immunoassay (catalog number EK-011-23; Phoenix Pharmaceuticals, Burlingame, California).
Plasma creatinine quantification
Creatinine levels were measured at 18 weeks post TAC using a commercially available assay kit (catalog number ab65340, Abcam, Cambridge, Massachusetts).
Aortic vascular reactivity assessment
Vascular function was measured in isolated segments (3 mm in length) of thoracic aorta as previously described (27) at 18 weeks following TAC in all study groups. Vascular rings were equilibrated for 60 min and then pre-contracted with phenylephrine (1 μM). The vascular rings were then challenged with increasing concentrations of acetylcholine (10−9 to 10−5) and sodium nitroprusside (SNP) at concentrations from 10−10.5 to 10−6.5. Data are reported as percent of relaxation from maximum contraction to phenylephrine.
Exercise capacity assessment
Treadmill exercise capacity was measured using an rodent treadmill (IITC, Woodland Hills, California) at 18 weeks post TAC as previously described with modifications (28). Briefly, immediately before the test, mice were put on the treadmill to acclimate for 15 min. Then, the mice are subjected to a slow walk/run period to be familiarized with treadmill running. In this period, the treadmill is set to start at 0 m/min speed and increased by 1 m/min every min for 10 min. Finally, in the test phase, the treadmill was set to a 30° incline, and the starting speed was set to 10 m/min and programed to increase by 2.67 m/min every min for 3 min, at which time the treadmill reached and maintained its top speed at 18 m/s. All mice were run at this setting until they reach a state of exhaustion. Duration and distance of the running and shocks per minute were recorded. Shocks per minute is a measurement of endurance. As mice felt tired and fell behind the speed of the treadmill, they received an electrical shock by the shocking grid. Higher shocks per min indicated lower endurance. A mouse was deemed to be exhausted when it spent more than 5 consecutive s at the back of the treadmill despite receiving electrical shocks repeatedly.
Circulating 8-isoprostane measurement
Plasma, myocardial, and renal 8-isoprostane levels were measured at 18 weeks post TAC by using an enzyme- linked immunosorbent assay kit (catalog number 516351, Cayman Chemical, Ann Arbor, Michigan). Tissue levels of 8-isoprostane were adjusted based on protein concentration (catalog number 23225; Thermo Fisher Scientific, Halethorpe, Maryland).
SNS and RAAS overactivation assessment
Renal norepinephrine levels were measured at 18 weeks post TAC, using an enzyme-linked immunosorbent assay kit (catalog number KA3836, Abnova, Taipei, Taiwan). Renin (catalog number EMREN1, Thermo Fisher Scientific), Ang II (catalog number ADI-900-204, Enzo Clinical Labs, Farmingdale, New York), and aldosterone (catalog number ADI-900-173, Enzo) levels were measured at 18 weeks post TAC using commercially available enzyme immunoassays. Renal levels of norepinephrine and aldosterone were adjusted based on protein concentration.
H2S and NO measurements
Plasma, myocardial, and renal H2S and nitric oxide (NO) levels were measured at 18 weeks post TAC as previously described 18, 29, 30. Tissue levels of H2S and NO were adjusted based on protein concentration.
cGMP measurement
Cardiac and renal cyclic guanosine monophosphate (cGMP) levels were measured at 18 weeks post TAC as previously described (31).
Statistical analysis
All data in this study are mean ± SEM. Differences in data among the groups were compared using 1-way ANOVA analysis or 2-way ANOVA analysis, using Prism 6 software (GraphPad, San Diego, California) followed by a Bonferroni multiple comparison test. A p value of <0.05 was considered statistically significant. Presented data may have different numbers of animals per endpoint due to procedural complications, limited sample collection (i.e., plasma volume), or lack of participation in involuntary treadmill running. Prior to conducting statistical analysis, an outlier test was performed using “ROUT” method developed by Prism 6 to identify and remove any outlier in the data set.
Results
Delayed treatment with JK-1 attenuates cardiac dilation, preserves LV ejection fraction, improves LV hemodynamics, and reduces cardiac fibrosis after TAC
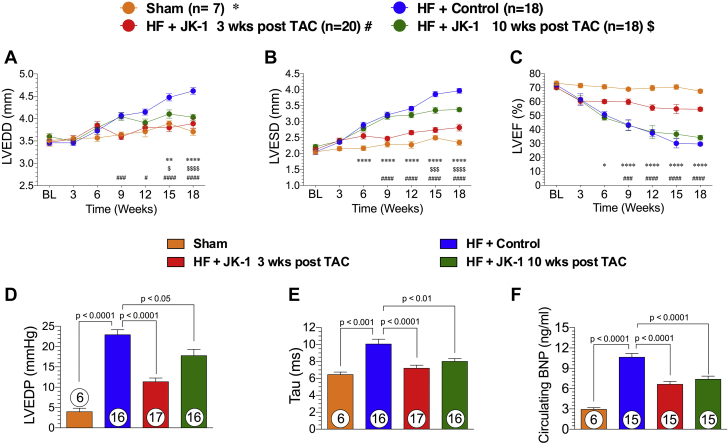
The effects of delayed therapy with JK-1 on the failing heart were evaluated using a TAC model of HF (Figure 1). Administration of JK-1 was initiated at 3 or 10 weeks post TAC and continued until 18 weeks post TAC. Mice treated with JK-1 initiated at 3 weeks or 10 weeks post TAC displayed significant reductions in LV chamber dimensions at end-diastole and end-systole compared to control mice (Figures 2A and 2B). In contrast, only the mice from 3-week post-TAC-treated group had preserved LVEF (Figure 2C), and there was no effect on LVEF in those that received JK-1 starting at 10 weeks following TAC. Invasive hemodynamic measurements revealed that delayed JK-1 treatment significantly decreased LV end-diastolic pressure and shortened relaxation constant Tau (Figures 2D and 2E). In addition, circulating BNP levels were significantly reduced in mice that received delayed JK-1 treatment (Figure 2F). Furthermore, the TAC-induced global LV hypertrophic response and subsequent wall thinning were attenuated in both of the JK-1 treatment groups (Supplemental Figures S1A to S1D). We also demonstrated that, at the 18-week endpoint, the whole heart weight and left atrial weight-to-tibia length ratio were significantly lower in JK-1-treated mice than in control mice, indicating that JK-1 reduced pathological remodeling in pressure overload HF (Supplemental Figures S2A to S2C).
Figure 2.
LV Structure, Function, and Circulating BNP Levels at 18 Weeks Post TAC
(A) (B) LVEDD and LVESD (mm) throughout the experimental protocol. (C) Percent of LVEF. (D) Left ventricular end-diastolic pressure (mm Hg) at 18 weeks following TAC. (E) LV relaxation constant Tau (ms) at 18 weeks post TAC. (F) Circulating BNP levels (ng/ml) in mice from Sham, HF + Control, HF + 3-week-delayed JK-1 treatment, and HF + 10-week-delayed JK-1 treatment. *p < 0.05 between Sham vs. HF + Control; **p < 0.01 between Sham vs. HF + Control; ****p < 0.0001 between Sham vs. HF + Control; #p < 0.05 between HF + JK-1 3 weeks post TAC vs. HF + Control; ###p < 0.001 between HF + JK-1 3 weeks post TAC vs. HF + Control; ####p < 0.0001 between HF + JK-1 3 weeks post TAC vs. HF + Control; $p < 0.05 between HF + JK-1 10 weeks post TAC; $$$p < 0.001 between HF + JK-1 10 weeks post TAC; $$$$p < 0.0001 between HF + JK-1 10weeks post TAC. LVEDD, LVESD, and LVEF data were analyzed with 2-way-ANOVA, whereas LVEDP, Tau, and circulating BNP data were analyzed with 1-way ANOVA. Circles inside bars denote sample size. LVEDD = left ventricular end-diastolic dimensions; LVEF = left ventricular ejection fraction; LVESD = left ventricular end-systolic dimensions; other abbreviations as in Figure 1.
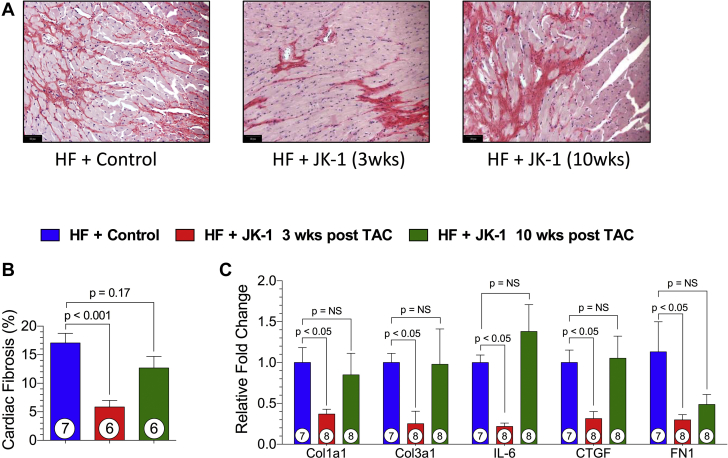
Next, we evaluated the effects of delayed JK-1 treatment on cardiac fibrosis with PicroSirius red staining at 18 weeks post TAC. Representative photomicrographs of heart sections are shown in Figure 3A. Treatment with JK-1 initiated at 3 weeks post TAC significantly reduced cardiac fibrosis whereas there was no significant change when JK-1 treatment was initiated at 10 weeks post TAC (Figure 3B). Furthermore, 3-week-delayed JK-1 treatment mitigated the transcription of pro-fibrotic genes such as collagen 1a1, collagen 3a1, interleukin-6, connective tissue growth factor, and fibronectin in the heart (Figure 3C).
Figure 3.
Cardiac Fibrosis at 18 Weeks Post TAC
(A) Representative images (original magnification ×20) of Picrosirius red-stained cardiac tissue sections from HF + Control and HF + JK-1 (3 weeks and 10 weeks, respectively) delayed treatment. (B) Quantified cardiac fibrosis. (C) Relative fold changes in myocardial mRNA levels of a panel of fibrosis-related genes in mice from HF + Control, HF + JK-1 3 weeks post TAC, and HF + JK-1 10 weeks post TAC groups at 18 weeks post TAC. All data in Figure 3 were analyzed with 1-way ANOVA. Circles inside bars denote sample size. Abbreviations as in Figure 1.
Delayed treatment with JK-1 ameliorates renal dysfunction and reduces renal fibrosis in HF
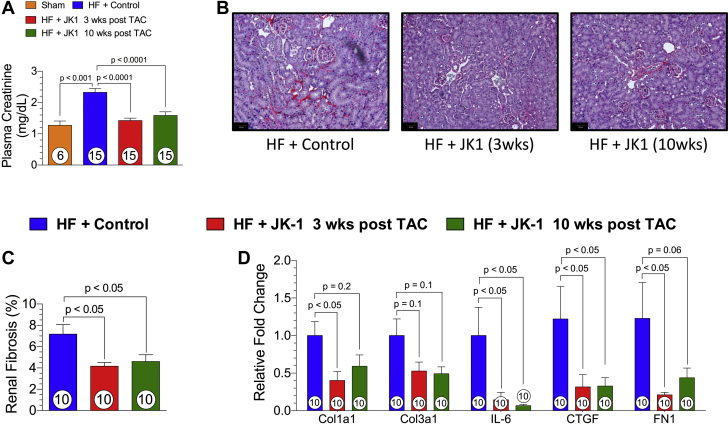
The effect of JK-1 on renal function in HF was assessed at 18 weeks post TAC by plasma creatinine measurements. Mice treated with Control exhibited impaired renal function as shown by elevated plasma creatinine levels that were compared to Sham mice. Mice treated with JK-1 initiated at 3 weeks post TAC and 10 weeks post TAC displayed significant reductions in plasma creatinine levels, indicating preserved renal function (Figure 4D). In addition, histological assessment of PicroSirius red-stained kidney sections revealed increased renal fibrosis in mice subjected to the TAC procedure and those that received Control treatment. Contrary to our observation in cardiac fibrosis, we saw significantly lessened amounts of renal fibrosis in mice that received 3-week-delayed or 10-week-delayed JK-1 treatment (Figure 4A and 4B). Furthermore, both 3-week- and 10-week-delayed JK-1 treatment significantly mitigated the transcription of interleukin-6 and connective tissue growth factor in the kidney, whereas only 3-week-delayed treatment significantly reduced the transcription of collagen 1a1 and fibronectin (Figure 4C).
Figure 4.
Renal Fibrosis and Plasma Creatinine Levels at 18 Weeks Post TAC
(A) Plasma creatinine levels. (B) Representative images (original magnification ×20) of Picrosirius red- stained renal tissue sections. (C) Quantified global renal fibrosis. (D) Relative fold changes in renal mRNA levels of fibrosis-related genes in mice from HF + Control, HF + JK-1 3 weeks post TAC, and HF + JK-1 10 weeks post TAC groups at 18 weeks post TAC. All data were analyzed with 1-way ANOVA. Circles inside bars denote the sample size. Abbreviations as in Figure 1.
Delayed treatment with JK-1 attenuates endothelial dysfunction in HF
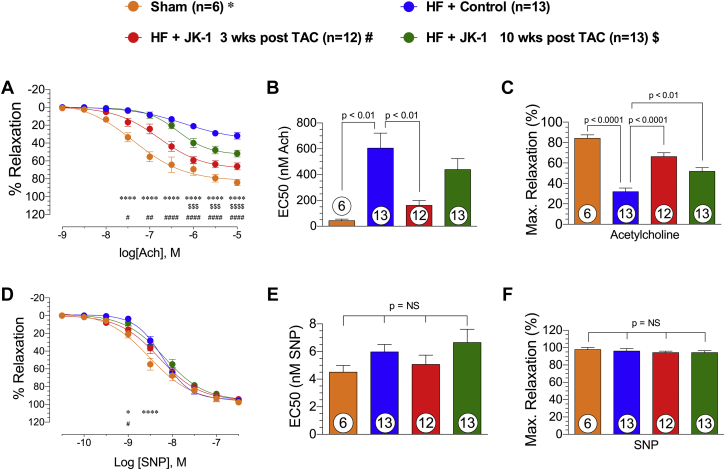
Vascular reactivity of isolated thoracic aortas to acetylcholine and SNP is shown in Figure 5. Aortas from mice that received the Control compound displayed significantly worsened responses to acetylcholine, as shown in concentration-response curve, maximal relaxation reached, and Half maximal effective concentration (EC50) values. Interestingly, delayed treatment with JK-1 (3 and 10 weeks delayed) resulted in better relaxation responses to acetylcholine (Figures 5A to 5C).
Figure 5.
Aortic Vasorelaxation at 18 Weeks Post TAC
Freshly isolated thoracic aortic rings were allowed to equilibrate, and pre-contracted with 1 μM phenylephrine. (A) Concentration-response curve (% relaxation from 10−9 to 10−5). (B) EC50 (nM). (C) Maximal relaxation (%) achieved for acetylcholine (Ach)-mediated vasorelaxation in mice from Sham, HF + Control, HF + JK-1 3 weeks post TAC, and HF + JK-1 10 weeks post TAC groups at 18 weeks post TAC. (D) Concentration-response curve (% relaxation from 10−10.5 to 10−6.5) (E) EC50 (nM), and (F) maximal relaxation (%) achieved for SNP mediated vasorelaxation in mice from Sham, HF + Control, HF + JK-1 3 weeks post TAC, and HF + JK-1 10 weeks post TAC groups at 18 weeks post TAC. *p < 0.05 between Sham vs. HF + Control; ****p < 0.0001 between Sham vs. HF + Control; # p < 0.05 between HF + JK-1 3 weeks post TAC vs. HF + Control; ##p < 0.01 between HF + JK-1 3 weeks post TAC vs. HF + Control; ####p < 0.0001 between HF + JK-1 3 weeks post TAC vs. HF + Control; $$$p < 0.001 between HF + JK-1 10weeks post TAC; $$$$p < 0.0001 between HF + JK-1 10weeks post TAC. Concentration-response curve were generated using a model of nonlinear regression with variable slope followed by 2-way-ANOVA analysis. EC50 and maximal relaxation data were analyzed with 1-way ANOVA. Circles inside bars denote the sample size. EC50 = half maximal effective concentration; SNP = sodium nitroprusside; other abbreviations as in Figure 1.
We also evaluated vascular relaxation responses of isolated thoracic aorta to SNP. Aortas from mice that received the Control compound displayed worsened vasodilation only at concentrations of 1 and 10 nM SNP, and no significant differences were observed in maximal relaxation and EC50 (Figures 5D to 5F). Taken together, these data suggest that TAC-induced HF resulted in severe endothelial dysfunction, and delayed treatment with JK-1 partially corrected this dysfunction.
Delayed treatment with JK-1 enhanced treadmill exercise performance
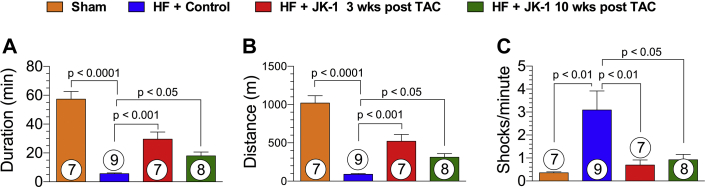
Reduced exercise capacity is one of the hallmark symptoms of HF in patients. We examined the effects of delayed therapy with JK-1 on exercise capacity using a rodent treadmill at 18 weeks post TAC (Figure 6). Mice from the HF + Control group displayed greatly reduced time to exhaustion and distance of running and experienced higher numbers of shocks per min than Sham mice, suggesting that TAC-induced HF resulted in reduced exercise capacity and lower endurance. Mice that received JK-1 treatment initiated at 3 or 10 weeks post TAC showed significantly improved exercise capacity as they had better treadmill running duration and distance and received fewer shocks per min.
Figure 6.
Treadmill Exercise Capacity at 18 Weeks Post TAC
Exercise capacity was evaluated in all study groups at 18 weeks following TAC surgery. (A) Duration (min) of the running before exhaustion. (B) Distance (m) of the running before exhaustion. (C) Shocks per minute in mice from Sham, HF + Control, HF + JK-1 3 weeks post TAC, and HF + JK-1 10 weeks post TAC groups at 18 weeks post TAC. All data were analyzed with 1-way ANOVA. Circles inside bars denote the sample size. Abbreviations as in Figure 1.
Delayed treatment with JK-1 augments H2S and NO bioavailability, and reduced systemic and tissue levels of oxidative stress
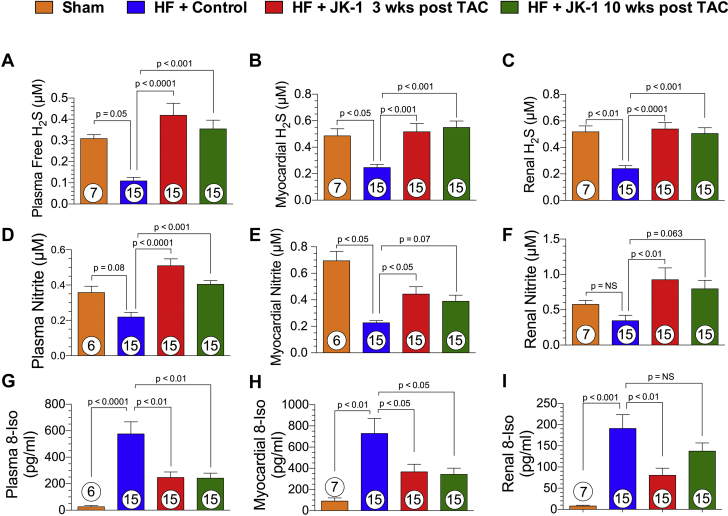
Next, we examined the effects of delayed JK-1 treatment on the bioavailability of H2S, NO, and levels of oxidative stress at 18 weeks post TAC (Figure 7). Mice treated with JK-1 starting at 3 or 10 weeks post TAC had significantly elevated levels of circulating H2S (Figure 7A), myocardial tissue H2S (Figure 7B), and renal tissue H2S (Figure 7C), further verifying the ability of JK-1 to donate pharmacologically relevant concentrations of H2S.
Figure 7.
H2S and NO Bioavailability and 8-Isoprostane Levels at 18 Weeks Post TAC
H2S levels (μM) in plasma (A), myocardial tissue (B), and renal tissue (C). Nitrite levels (μM) in plasma (D), myocardial tissue (E), and renal tissue (F). 8-Isoprostane (8-Iso) levels (pg/ml) in circulation (G), myocardial tissue (H), and renal tissue (I) in mice from Sham, HF + Control, HF + JK-1 3 weeks post TAC, and HF + JK-1 10 weeks post TAC groups at 18 weeks post TAC. All data were analyzed with 1-way ANOVA. Circles inside bars denote the sample size. Abbreviations as in Figure 1.
Multiple studies have previously reported that H2S augments the bioavailability and signaling of NO 18, 31, 32, 33. The current study found that JK-1 treatment initiated at 3 or 10 weeks post TAC resulted in significantly elevated levels of nitrite in circulation, heart, and kidney (Figures 7D to 7F). In addition, significantly elevated levels of cGMP were also observed in cardiac and renal tissue at 18 weeks post TAC in mice receiving JK-1 treatment (Supplemental Figures S4A and S4B).
In addition, it has been reported that both H2S and NO are powerful antioxidants 31, 34. Therefore, we examined the levels of oxidative stress by using 8-isoprostane as a biomarker (Figures 7G to 7I). TAC-induced HF resulted in elevated oxidative stress as the mice receiving the Control compound exhibited significantly higher levels of 8-isoprostane in circulation, heart, and kidney. Such elevation in oxidative stress was ameliorated by both the 3- and 10-week-delayed JK-1 treatment.
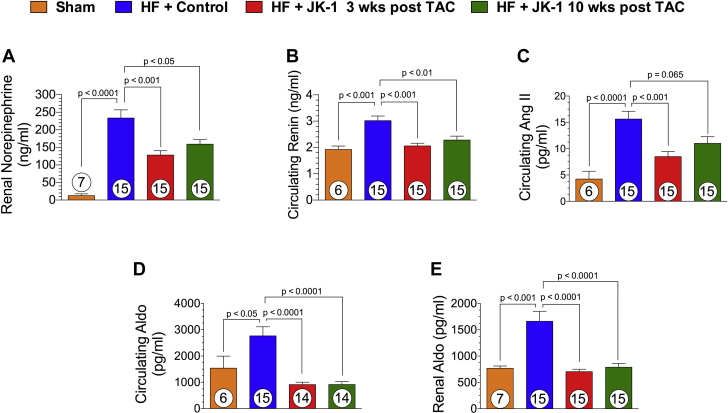
Delayed treatment with JK-1 reduced renal norepinephrine contents, attenuated RAAS signaling in HF
Neurohumoral dysregulation is a significant contributor to the pathogenesis of HF and its co-morbidities 14, 35. Specifically, overactivation of the SNS and the RAAS significantly exacerbates the severity of HF (4). In the current study, we evaluated the extent of SNS and RAAS overactivation by measuring renal or circulating levels of norepinephrine, renin, Ang II, and aldosterone (Figure 8). We observed that TAC-induced HF significantly activated the SNS as mice receiving the Control compound displayed nearly 20-fold increase in renal norepinephrine content. JK-1 treatment initiated at 3 or 10 weeks post TAC significantly attenuated renal norepinephrine content compared to mice receiving Control (Figure 8A). Furthermore, delayed JK-1 treatment ameliorated RAAS activation as mice that received 3-week- and 10-week-delayed JK-1 treatment had significantly reduced levels of circulating renin (Figure 8B), circulating Ang II (Figure 8C), and circulating aldosterone (Figure 8D) compared to those that received Control. Similarly, renal aldosterone content was significantly reduced in mice that received either 3-week- or 10-week-delayed JK-1 therapy (Figure 8E).
Figure 8.
Renal Norepinephrine Content, Circulating Renin, Ang II, and Aldosterone Levels at 18 Weeks Post TAC
(A) Renal tissue norepinephrine content (ng/ml). (B) Plasma renin (ng/ml). (C) Plasma Ang II (pg/ml). (D) Circulating Aldo levels (pg/ml). (E) Renal Aldo levels (pg/ml) in mice from Sham, HF + Control, HF + JK-1 3 weeks post TAC, and HF + JK-1 10 weeks post TAC groups at 18 weeks post TAC. All data were analyzed with 1-way ANOVA. Circles inside bars denote the sample size. Aldo = aldosterone; Ang II = angiotensin II; other abbreviations as in Figure 1.
Discussion
It was previously shown that pharmacological H2S therapy ameliorates TAC-induced HF severity 19, 25, 36, 37. However, one major limitation of those studies is that the experimental H2S therapies were administered prior to or right after the induction of HF by TAC. Such treatment regimens do not reflect clinical scenarios where medications are given to patients after the establishment of cardiac dysfunction or failure 8, 38. The current study examined the efficacy of a novel H2S donor, JK-1, in TAC-induced HF with a delayed treatment approach. To better simulate the clinical situation, we initiated the H2S therapy with JK-1, either at 3 or 10 weeks post TAC. At 3 weeks post TAC, echocardiographic assessment revealed that all mice subjected to TAC started to develop cardiac hypertrophy. At 10 weeks following TAC, all mice that were subjected to TAC and received the Control compound had LVEFs below 45%.
We observed significant preservation of LVEF in mice treated with JK-1 starting at 3 weeks post TAC but not in those started at 10 weeks post TAC. This preservation in LVEF was accompanied by reduction in cardiac fibrosis, which was also seen only in mice starting to receive H2S therapy at 3 weeks post TAC. These data suggest that 10-week-delayed H2S therapy fails to reverse established cardiac fibrosis and pathological remodeling in the failing heart. Interestingly, despite its neutral effects in LVEF and cardiac fibrosis, 10-week-delayed JK-1 therapy ameliorated other aspects of cardiac dysfunction. We observed significantly attenuated LV chamber dilatation, lower LVEDP, and shorter relaxation constant Tau with both the 3-week- and the 10-week-delayed treatment compared to Control mice. These improvements in LV chamber size translated into significant reductions in circulating BNP levels for both the 3-week- and the 10-week-delayed JK-1 treatments. Taken together, these data demonstrate that delayed H2S therapy is efficacious in TAC-induced HF with greater improvements in LV function when treatment is initiated early.
HF is a systemic condition that deteriorates the function of multiple organ systems including the kidney and the vasculature 3, 4, 5, 6, 15, 39, 40. As chronic HF progresses, cardiac pathologies such as overactive SNS and RAAS, inflammation, and excessive oxidative stress induces renal injury and impairs normal renal function. Such phenomenon is defined as type II cardiorenal syndrome and often results in poorer prognosis and survival 5, 35. To date, no pharmacological therapeutic has been developed to simultaneously target both the failing heart and the injured kidney. Previous studies have reported that H2S therapies effectively ameliorated renal dysfunction in several animal models of kidney diseases through differential mechanisms, suggesting that H2S-based therapies may be efficacious in treating both the heart and the kidney in HF 14, 38, 41, 42, 43, 44, 45, 46, 47. In consistent with previous literature, we observed that the mice received Control developed type-II cardiorenal syndrome as evidenced by elevated levels of plasma creatinine and renal fibrosis at 18 weeks post TAC (39). Delayed treatment with JK-1 started at 3 or 10 weeks post TAC significantly lowered plasma creatinine levels, renal fibrosis, as well as the transcriptional levels of pro-fibrotic genes such as collagen 1a1, collagen 3a1, interleukin 6, connective tissue growth factor, and fibronectin. Taken together, our data provides the first evidence that H2S therapy is efficacious for the treatment of cardiorenal syndrome induced by TAC.
In addition to cardiorenal syndrome, endothelial injury and dysfunction is another commonly seen comorbidities in HF 3, 4. Endothelium is a monolayer of cells covering the inner surface of blood vessels that mediates the vascular hemostasis through providing NO, antiproliferative and anti-inflammatory actions. Endothelial dysfunction results in the inactivation of endothelial NOS (eNOS) and attenuated NO bioavailability, over-produced inflammatory cytokines, and increased oxidative stress, which further exacerbate the progression of HF 6, 48, 49. Multiple studies have reported that H2S augments endothelial function via reducing oxidative stress, promoting eNOS activity, and other mechanisms 16, 33, 50, 51; However, there is a lack of information regarding the endothelium-protective actions of H2S in the setting of HF. In the current study, we observed that the aortic rings of isolated from HF + Control mice exhibited diminished response to acetylcholine, verifying that TAC- induced HF indeed caused severe endothelium dysfunction. More importantly, delayed H2S treatment with JK-1 attenuated endothelium dysfunction as shown by improved concentration-response curve, achieving greater maximal relaxation, and resulting in lower EC50. This improvement in the vasodilatory response to acetylcholine was also reflected in enhanced NO bioavailability and signaling following JK-1 treatment.
Severe exercise intolerance is a primary chronic symptom associated with decreased quality of life in patients with HF, even when stable and well compensated (52). More recently, the endothelium has emerged as a therapeutic target for exercise intolerance in HF 6, 53. Given the prominent endothelium-protective effects of JK-1 in HF, we measured the exercise capacity and we observed that TAC- induced HF significantly impaired the exercise capacity of mice treated with control, whereas JK-1 treatment initiated at 3 and 10 weeks post TAC significantly increased the duration and distance of treadmill running. Interestingly, despite the neutral effect on LVEF, the mice received 10-week-delayed JK-1 treatment displayed 3-fold increase in duration and distance of running. This suggests that vascular endothelial function plays an independent role from cardiac function in H2S-mediated improvement in exercise capacity in TAC- induced HF.
Additionally, we examined the effects of delayed JK-1 treatment on the levels of SNS and RAAS activation. In the pathogenesis of HF, maladaptive SNS signaling increases in response to decreased cardiac output, which in turn, activates RAAS (8). SNS and RAAS overactivation are major maladaptive neurohumoral alterations in HF that accelerate the exacerbation of HF and its comorbidities 8, 9. Previously, it was reported that NO exerts sympathoinhibitory effects via attenuating the release of norepinephrine from neural terminals (54). More recently, it was discovered that elevation in oxidative stress in central nervous system resulted in systemic sympathoexcitation (40). Given the powerful antioxidant and NO-promoting effects of H2S, we propose that delayed therapy with JK-1 down regulates SNS and RAAS activation. We observed that renal norepinephrine content was significantly reduced in kidneys from mice received 3-week- and 10-week-delayed JK-1 treatment when compared to HF + Control. Reduction in renal norepinephrine content was accompanied by reduced levels of renin, Ang II, and aldosterone in the circulation.
Although we observed similar degree of reductions in plasma creatinine levels, renal fibrosis, renal norepinephrine content, renin, and aldosterone levels for both 3-week- and 10-week-delayed JK-1-treated groups, the 3-week-delayed treatment offered greater reductions in circulating Ang II, and greater increases in NO bioavailability and signaling. This resulted in greater improvements in cardiac and vascular function as well as exercise capacity. This observation suggests that although H2S therapy is efficacious in both early and late stages of the diseases, earlier intervention may offer better outcomes.
Study limitations
We demonstrated the differential effects of 3-week-delayed versus 10-week-delayed JK-1 treatment in the context of pressure overload-induced heart failure; however, in order to verify the efficacy of JK-1 and fully understand its mechanism of action, further investigations using other heart failure models are warranted. In addition, although we showed that JK-1 treatment significantly reduced the plasma creatinine levels, a more clinically relevant index of kidney function would be glomerular filtration rate or urine creatinine-to-albumin ratio. Furthermore, the pharmacokinetics and effects of JK-1 treatment on naïve mice were not investigated in the current study. Such information would provide strong basis for the translation of JK-1 as a therapeutic agent.
Conclusions
We have shown that delayed treatment with a novel and potent H2S donor, JK-1, protects multiple organs including the heart, peripheral arteries, and the kidneys against injury and dysfunctions in the setting of TAC- induced heart failure. Such protection in multiple organs translated into improved exercise capacity. Furthermore, our results demonstrate that delayed treatment with JK-1 reduced the overactivation of the SNS and RAAS, possibly through enhancing NO bioavailability and signaling, and reducing oxidative stress. We conclude that delayed H2S therapy is efficacious in HF, and is worthy of further development as a promising therapeutic for the treatment of HF and its comorbidities. Future studies will investigate the cellular and molecular mechanism by which H2S mitigates the overactivation of SNS and RAAS in HF.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In the setting of heart failure, the lack of proper perfusion and the activation of maladaptive signaling pathways such as RAAS lead to renal and endothelial dysfunction. Similar to NO, H2S is a endogenous gaseous molecule that exerts protective effects on cardiovascular system. Here we used JK-1, a novel synthetic H2S donor, to simultaneously protect the heart, the kidneys, the vessels, and to improved exercise performance in mice subjected to heart failure.
TRANSLATIONAL OUTLOOK: H2S-based therapeutics have been tested in heart failure patients and proven to be safe and bio-active. Our study identified that JK-1, a novel H2S donor, protects the heart against pressure overload-induced heart failure and reduces the collateral damages to the kidney and the endothelium. These findings support further clinical investigation of the efficacy of H2S-based therapies for treating heart failure and its comorbidities.
Acknowledgements
The authors thank Ms. Jean Carnal for technical support and Dr. Luis Marrero and the Morphology and Imaging Core at LSUHSC for performing PicroSirius red staining of cardiac and renal tissues.
Footnotes
Supported by National Heart, Lung, and Blood Institute/National Institutes of Health grants 5R01HL092141, 5R01HL093579, and 1U24 HL094373 to Dr. Lefer; and grant 1R01HL116571 to Drs. Xian and Lefer. Funding was also received from the Louisiana State University Medical School Foundation and LSU Medical School Alumni Association. All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Heineke J., Molkentin J.D. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 2.Voigt J., Sasha John M., Taylor A., Krucoff M., Reynolds M.R., Michael Gibson C. A reevaluation of the costs of heart failure and its implications for allocation of health resources in the United States. Clin Cardiol. 2014;37:312–321. doi: 10.1002/clc.22260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Deursen V.M., Urso R., Laroche C. Co-morbidities in patients with heart failure: an analysis of the European heart failure pilot survey. Eur J Heart Fail. 2014;16:103–111. doi: 10.1002/ejhf.30. [DOI] [PubMed] [Google Scholar]
- 4.van Deursen V.M., Damman K., van der Meer P. Co-morbidities in heart failure. Heart Fail Rev. 2014;19:163–172. doi: 10.1007/s10741-012-9370-7. [DOI] [PubMed] [Google Scholar]
- 5.Ronco C., Di Lullo L. Cardiorenal syndrome. Heart Fail Clin. 2014;10:251–280. doi: 10.1016/j.hfc.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Van Craenenbroeck E.M., Conraads V.M. Mending injured endothelium in chronic heart failure: a new target for exercise training. Int J Cardiol. 2013;166:310–314. doi: 10.1016/j.ijcard.2012.04.106. [DOI] [PubMed] [Google Scholar]
- 7.Pagani M., Lucini D. Autonomic dysregulation in essential hypertension: insight from heart rate and arterial pressure variability. Auton Neurosci. 2001;90:76–82. doi: 10.1016/S1566-0702(01)00270-3. [DOI] [PubMed] [Google Scholar]
- 8.Muiesan M.L., Paini A., Agabiti Rosei C., Bertacchini F., Stassaldi D., Salvetti M. Current pharmacological therapies in heart failure patients. High Blood Press Cardiovasc Prev. 2017;24:107–114. doi: 10.1007/s40292-017-0194-3. [DOI] [PubMed] [Google Scholar]
- 9.Grippo A.J., Johnson A.K. Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress. 2009;12:1–21. doi: 10.1080/10253890802046281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grippo A.J. Mechanisms underlying altered mood and cardiovascular dysfunction: the value of neurobiological and behavioral research with animal models. Neurosci Biobehav Rev. 2009;33:171–180. doi: 10.1016/j.neubiorev.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabbah H.N. Biologic rationale for the use of beta-blockers in the treatment of heart failure. Heart Fail Rev. 2004;9:91–97. doi: 10.1023/B:HREV.0000046363.59374.23. [DOI] [PubMed] [Google Scholar]
- 12.Grassi G., Seravalle G., Dell'Oro R., Mancia G. Sympathetic mechanisms, organ damage, and antihypertensive treatment. Curr Hypertens Rep. 2011;13:303–308. doi: 10.1007/s11906-011-0200-4. [DOI] [PubMed] [Google Scholar]
- 13.Levy B.I. How to explain the differences between renin angiotensin system modulators. Am J Hypertens. 2005;18:134S–141S. doi: 10.1016/j.amjhyper.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Polhemus D.J., Trivedi R.K., Gao J. Renal sympathetic denervation protects the failing heart via inhibition of neprilysin activity in the kidney. J Am Coll Cardiol. 2017;70:2139–2153. doi: 10.1016/j.jacc.2017.08.056. [DOI] [PubMed] [Google Scholar]
- 15.Koniari K., Nikolaou M., Paraskevaidis I., Parissis J. Therapeutic options for the management of the cardiorenal syndrome. Int J Nephrol. 2010;2011:194910. doi: 10.4061/2011/194910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polhemus D.J., Lefer D.J. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res. 2014;114:730–737. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polhemus D.J., Calvert J.W., Butler J., Lefer D.J. The cardioprotective actions of hydrogen sulfide in acute myocardial infarction and heart failure. Scientifica. 2014;2014:768607. doi: 10.1155/2014/768607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Predmore B.L., Kondo K., Bhushan S. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am J Physiol Heart Circ Physiol. 2012;302:H2410–H2418. doi: 10.1152/ajpheart.00044.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polhemus D., Kondo K., Bhushan S. Hydrogen sulfide attenuates cardiac dysfunction after heart failure via induction of angiogenesis. Circ Heart Fail. 2013;6:1077–1086. doi: 10.1161/CIRCHEARTFAILURE.113.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang J., Li Z., Organ C.L. pH-Controlled hydrogen sulfide release for myocardial ischemia-reperfusion injury. J Am Chem Soc. 2016;138:6336–6339. doi: 10.1021/jacs.6b01373. [DOI] [PubMed] [Google Scholar]
- 21.Predmore B.L., Lefer D.J. Development of hydrogen sulfide-based therapeutics for cardiovascular disease. J Cardiovasc Transl Res. 2010;3:487–498. doi: 10.1007/s12265-010-9201-y. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Z., Martin E., Sharina I. Regulation of soluble guanylyl cyclase redox state by hydrogen sulfide. Pharmacol Res. 2016;111:556–562. doi: 10.1016/j.phrs.2016.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andreadou I., Iliodromitis E.K., Rassaf T., Schulz R., Papapetropoulos A., Ferdinandy P. The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br J Pharmacol. 2015;172:1587–1606. doi: 10.1111/bph.12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao X., Bian J.S. The role of hydrogen sulfide in renal system. Front Pharmacol. 2016;7:385. doi: 10.3389/fphar.2016.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kondo K., Bhushan S., King A.L. H(2)S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation. 2013;127:1116–1127. doi: 10.1161/CIRCULATIONAHA.112.000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradley J.M., Li Z., Organ C.L. A novel mtDNA repair fusion protein attenuates maladaptive remodeling and preserves cardiac function in heart failure. Am J Physiol Heart Circ Physiol. 2018;314:H311–H321. doi: 10.1152/ajpheart.00515.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trivedi R.K., Polhemus D.J., Li Z. Combined angiotensin receptor-neprilysin inhibitors improve cardiac and vascular function via increased no bioavailability in heart failure. J Am Heart Assoc. 2018 Mar 3;7(5) doi: 10.1161/JAHA.117.008268. https://doi.org/10.1161/JAHA.117.008268 pii: e008268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sung M.M., Byrne N.J., Robertson I.M. Resveratrol improves exercise performance and skeletal muscle oxidative capacity in heart failure. Am J Physiol Heart Circ Physiol. 2017;312:H842–H853. doi: 10.1152/ajpheart.00455.2016. [DOI] [PubMed] [Google Scholar]
- 29.Polhemus D.J., Li Z., Pattillo C.B. A novel hydrogen sulfide prodrug, SG1002, promotes hydrogen sulfide and nitric oxide bioavailability in heart failure patients. Cardiovasc Ther. 2015;33:216–226. doi: 10.1111/1755-5922.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimizu Y., Nicholson C.K., Lambert J.P. Sodium sulfide attenuates ischemic-induced heart failure by enhancing proteasomal function in an Nrf2-dependent manner. Circ Heart Fail. 2016;9:e002368. doi: 10.1161/CIRCHEARTFAILURE.115.002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King A.L., Polhemus D.J., Bhushan S. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci U S A. 2014;111:3182–3187. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katsouda A., Bibli S.I., Pyriochou A., Szabo C., Papapetropoulos A. Regulation and role of endogenously produced hydrogen sulfide in angiogenesis. Pharmacol Res. 2016;113:175–185. doi: 10.1016/j.phrs.2016.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bibli S.I., Szabo C., Chatzianastasiou A. Hydrogen sulfide preserves endothelial nitric oxide synthase function by inhibiting proline-rich kinase 2: implications for cardiomyocyte survival and cardioprotection. Mol Pharmacol. 2017;92:718–730. doi: 10.1124/mol.117.109645. [DOI] [PubMed] [Google Scholar]
- 34.Islam K.N., Polhemus D.J., Donnarumma E., Brewster L.P., Lefer D.J. Hydrogen sulfide levels and nuclear factor-erythroid 2-related factor 2 (NRF2) activity are attenuated in the setting of critical limb ischemia (CLI) J Am Heart Assoc. 2015 May 14;4(5) doi: 10.1161/JAHA.115.001986. pii: e001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smilde T.D., Hillege H.L., Navis G., Boomsma F., de Zeeuw D., van Veldhuisen D.J. Impaired renal function in patients with ischemic and nonischemic chronic heart failure: association with neurohormonal activation and survival. Am Heart J. 2004;148:165–172. doi: 10.1016/j.ahj.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Calvert J.W., Jha S., Gundewar S. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng G., Liu J., Liu S. Hydrogen sulfide pretreatment improves mitochondrial function in myocardial hypertrophy via a SIRT3-dependent manner. Br J Pharmacol. 2018;175:1126–1145. doi: 10.1111/bph.13861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Z., Polhemus D.J., Lefer D.J. Evolution of hydrogen sulfide therapeutics to treat cardiovascular disease. Circ Res. 2018;123 doi: 10.1161/CIRCRESAHA.118.311134. [DOI] [PubMed] [Google Scholar]
- 39.Kamal F.A., Travers J.G., Schafer A.E., Ma Q., Devarajan P., Blaxall B.C. G protein-coupled receptor-G-protein betagamma-subunit signaling mediates renal dysfunction and fibrosis in heart failure. J Am Soc Nephrol. 2017;28:197–208. doi: 10.1681/ASN.2015080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao L., Zimmerman M.C., Biswal S., Zucker I.H. Selective Nrf2 gene deletion in the rostral ventrolateral medulla evokes hypertension and sympathoexcitation in mice. Hypertension. 2017;69:1198–1206. doi: 10.1161/HYPERTENSIONAHA.117.09123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xue R., Hao D.D., Sun J.P. Hydrogen sulfide treatment promotes glucose uptake by increasing insulin receptor sensitivity and ameliorates kidney lesions in type 2 diabetes. Antioxid Redox Signal. 2013;19:5–23. doi: 10.1089/ars.2012.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu D., Gao B., Li M. Hydrogen sulfide mitigates kidney injury in high fat diet-induced obese mice. Oxid Med Cell Longev. 2016;2016:2715718. doi: 10.1155/2016/2715718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber G.J., Pushpakumar S.B., Sen U. Hydrogen sulfide alleviates hypertensive kidney dysfunction through an epigenetic mechanism. Am J Physiol Heart Circ Physiol. 2017;312:H874–H885. doi: 10.1152/ajpheart.00637.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.John A., Kundu S., Pushpakumar S. GYY4137, a hydrogen sulfide donor modulates miR194-dependent collagen realignment in diabetic kidney. Sci Rep. 2017;7:10924. doi: 10.1038/s41598-017-11256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang D., Zhang Y., Yang M., Wang S., Jiang Z., Li Z. Exogenous hydrogen sulfide prevents kidney damage following unilateral ureteral obstruction. Neurourol Urodyn. 2014;33:538–543. doi: 10.1002/nau.22450. [DOI] [PubMed] [Google Scholar]
- 46.Hsu C.N., Tain Y.L. Hydrogen Sulfide in hypertension and kidney disease of developmental origins. Int J Mol Sci. 2018 May 11;19(5) doi: 10.3390/ijms19051438. pii: E1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hou C.L., Wang M.J., Sun C. Protective effects of hydrogen sulfide in the ageing kidney. Oxid Med Cell Longev. 2016;2016:7570489. doi: 10.1155/2016/7570489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piepoli M.F., Guazzi M., Boriani G. Exercise intolerance in chronic heart failure: mechanisms and therapies. Part I. Eur J Cardiovasc Prev Rehabil. 2010;17:637–642. doi: 10.1097/HJR.0b013e3283361dc5. [DOI] [PubMed] [Google Scholar]
- 49.Piepoli M.F., Guazzi M., Boriani G. Exercise intolerance in chronic heart failure: mechanisms and therapies. Part II. Eur J Cardiovasc Prev Rehabil. 2010;17:643–648. doi: 10.1097/HJR.0b013e32833f3aa5. [DOI] [PubMed] [Google Scholar]
- 50.Cheng Z., Garikipati V.N., Nickoloff E. Restoration of hydrogen sulfide production in diabetic mice improves reparative function of bone marrow cells. Circulation. 2016;134:1467–1483. doi: 10.1161/CIRCULATIONAHA.116.022967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanagy N.L., Szabo C., Papapetropoulos A. Vascular biology of hydrogen sulfide. Am J Physiol Cell Physiol. 2017;312:C537–C549. doi: 10.1152/ajpcell.00329.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Esposito F., Mathieu-Costello O., Shabetai R., Wagner P.D., Richardson R.S. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol. 2010;55:1945–1954. doi: 10.1016/j.jacc.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marti C.N., Gheorghiade M., Kalogeropoulos A.P., Georgiopoulou V.V., Quyyumi A.A., Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol. 2012;60:1455–1469. doi: 10.1016/j.jacc.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 54.Seilicovich A., Lasaga M., Befumo M. Nitric oxide inhibits the release of norepinephrine and dopamine from the medial basal hypothalamus of the rat. Proc Natl Acad Sci U S A. 1995;92:11299–11302. doi: 10.1073/pnas.92.24.11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.