Abstract
Ivosidenib (Tibsovo®) is a small molecule, orally available inhibitor of mutated cytosolic isocitrate dehydrogenase 1 (IDH1) that is being developed by Agios Pharmaceuticals for the treatment of cancer in patients with IDH1 mutations. The mutated form of the IDH1 enzyme produces a metabolite, 2-hydroxyglutarate (2-HG), which is thought to play a role in the formation and progression of acute myeloid leukaemia (AML), gliomas and other cancers. Elevated 2-HG levels interfere with cellular metabolism and epigenetic regulation, thereby contributing to oncogenesis. Ivosidenib targets the IDH1 metabolic pathway to prevent a build-up of the oncometabolite 2-HG. This article summarizes the milestones in the development of ivosidenib leading to this first approval in the USA for the treatment of patients with relapsed or refractory AML with a susceptible IDH1 mutation. Clinical development for AML, cholangiocarcinoma, glioma, myelodysplastic syndromes and solid tumours is ongoing worldwide.
Introduction
Acute myeloid leukaemia (AML) is a heterogeneous group of haematological malignancies and the most common acute leukaemia in adults, particularly in the elderly (aged ≥ 65 years) who account for > 50% of cases [1]. Because of patient (e.g. comorbidities) and disease (e.g. cytogenetic and molecular abnormalities) characteristics, treatment outcomes in the elderly are very poor, with complete remission (CR) rates of 40–50% and shorter duration of remission [1, 2]. A better understanding of the pathophysiology of the disease in the elderly in recent years has led to the development of targeted therapies.
One such agent ivosidenib (Tibsovo®) is being developed by Agios Pharmaceuticals. Ivosidenib is a small molecule, orally available inhibitor of mutated cytosolic isocitrate dehydrogenase 1 (IDH1). IDH1 and IDH2 are key metabolic enzymes catalyzing the conversion of isocitrate to α-ketoglutarate (αKG), while reducing nicotinamide adenine dinucleotide phosphate (NADP) to NADPH [3, 4]. Recurrent mutations in IDH1 and IDH2 genes have been identified in several cancers, including AML, myelodysplastic syndromes and myeloproliferative neoplasms, and cholangiocarcinoma. Mutated IDH enzymes acquire gain-of-function activity, converting NADPH and αKG to NADP+ and the oncometabolite D-2-hydroxyglutarate (2-HG). Elevated 2-HG levels interfere with cellular metabolism and epigenetic regulation, thereby contributing to oncogenesis [3, 4]. Ivosidenib targets the IDH1 metabolic pathway to prevent a build-up of the oncometabolite 2-HG. Ivosidenib has been approved by the US FDA for the treatment of patients with relapsed or refractory AML with a susceptible IDH1 mutation [5]. The recommended dosage of ivosidenib is 500 mg administered orally once daily with or without food until disease progression or unacceptable toxicity. This article summarizes the milestones in the development of ivosidenib leading to this first approval for patients with relapsed/refractory AML.
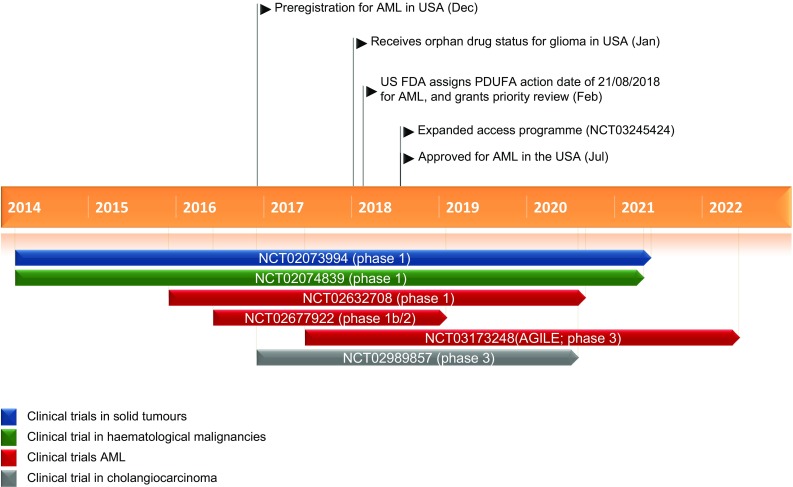
Key milestones in the development of ivosidenib for the treatment of patients with relapsed or refractory acute myeloid leukaemia with a susceptible IDH1 mutation. AML acute myeloid leukaemia, IDH1 isocitrate dehydrogenase 1
Company Agreements
In April 2010, Agios Pharmaceuticals and Celgene Corporation entered into a three year global collaboration to develop therapeutics targeting cancer metabolism. Under the terms of the agreement, Agios was to receive an upfront payment of $US130 million, including an equity investment, and was entitled to milestone payments and royalties [6]. Agios was to lead discovery and early development in the cancer metabolism research programme. Celgene had an exclusive option to license any resulting clinical candidates at the end of phase 1, and lead and fund the global development and commercialisation of licensed programmes [6]. Agios and Celgene agreed to extend this programme by one year in October 2011 for which Agios received $US20 million from Celgene [7]. In December 2013, the agreement was extended for another year, under similar terms [8]. Celgene retained the ability to extend this exclusive collaboration further for additional payments [8]. In February 2014, Agios exercised its option for US development and commercialisation rights for the IDH1 programme, including ivosidenib, under the terms of the collaboration with Celgene [9]. Celgene was eligible to receive royalties on US sales of the product, whereas Agios was to receive up to $US120 million in milestone payments as well as royalties on sales outside the US. In June 2014, Agios Pharmaceuticals and Celgene entered into an agreement, wherein the latter exclusively exercised the option of licensing ivosidenib outside the US [10]. In May 2016, Agios Pharmaceuticals gained the option to conduct the development and commercialisation of ivosidenib worldwide, including in the US. Previously, Agios held US rights for ivosidenib from Celgene [11].
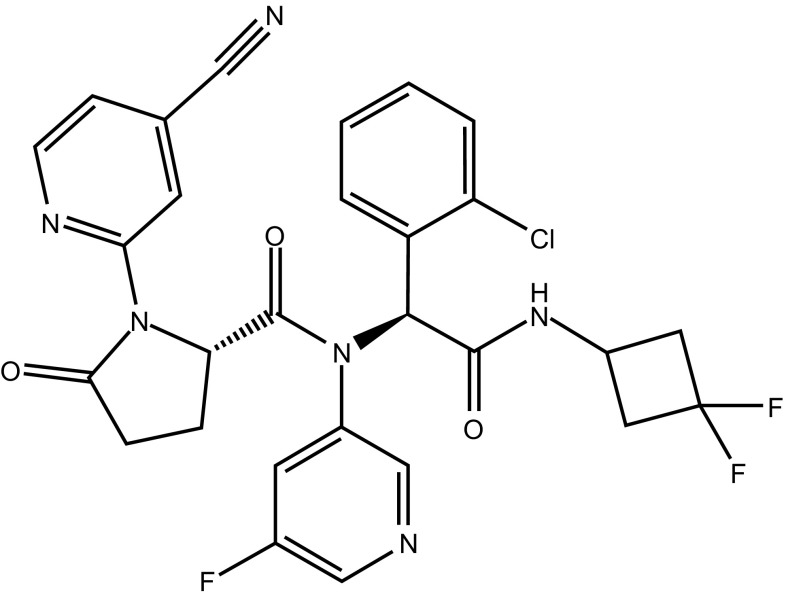
Chemical structure of ivosidenib
In June 2018, Agios Pharmaceuticals and CStone Pharmaceuticals entered into an exclusive collaboration and license agreement for the development and commercialisation of ivosidenib (Tibsovo®) in Mainland China, Hong Kong, Macau and Taiwan (“Territory”), either as monotherapy or in combination with other therapies [12]. CStone Pharmaceuticals will be responsible for conducting the development and commercialisation activities for ivosidenib in haematological and solid tumour indications in the territory, with an initial focus on AML and cholangiocarcinoma, and will be responsible for all costs associated with the development and commercialisation activities for ivosidenib conducted in the territory as per the agreement. Agios will retain all rights to ivosidenib in the rest of the world. Under the terms of the agreement, Agios will receive an upfront payment of $US12 million and will be eligible to receive up to $US412 million in milestone payments, of which $US147 million are related to development and regulatory events, and $US265 million to the achievement of certain sales levels. Approximately half of the milestone payments are related to the development and commercialization of ivosidenib in AML, cholangiocarcinoma and other potential indications. The other half are payable only if development and commercialization of ivosidenib in brain cancer indications, including glioma, are pursued as part of the collaboration at a later date. In addition, CStone Pharmaceuticals will pay Agios tiered royalties ranging from the mid to high-teens as a percentage of annual net sales of ivosidenib in the territory [12].
Scientific Summary
Pharmacodynamics
In vitro ivosidenib inhibited selected IDH1 R132 mutants at much lower concentrations than wild-type IDH1 [13]. Ivosidenib was shown to be highly selective for IDH1 mutants and did not inhibit wild-type or mutant isoforms of IDH2 [14]. Ivosidenib rapidly reduced 2-HG levels and induced cellular differentiation in preclinical models [14]. In vitro, ivosidenib inhibited invasion and migration of chondrosarcoma cells bearing an IDH1 mutation [15]. In a mouse xenograft model of human IDH1-mutated glioma, ivosidenib dose-dependently reduced 2-HG levels in brain tumours (maximal inhibition 85%), inhibited tumour growth in vivo and did not antagonize the efficacy of radiation therapy [16]. In vitro, ivosidenib plus azacitidine enhanced cell differentiation (46-fold versus 25-fold with ivosidenib alone and 1-fold with azacitidine alone) and potentiated cell death compared with either agent alone [17].
In phase 1, open-label studies of patients with IDH1 mutation-bearing advanced haematological malignancies (NCT02074839) [18] or advanced solid tumours (including cholangiocarcinoma; NCT02073994) [19], ivosidenib 500 mg/day reduced plasma 2-HG levels by > 90%, and to levels similar to those in healthy subjects. 2-HG levels in the bone marrow were also substantially reduced following ivosidenib therapy [18, 19]. Ivosidenib-induced inhibition of mutant IDH1 and 2-HG levels in the plasma were positively correlated with those in tumour biopsies from patients with cholangiocarcinoma [19]. Tumour biopsies from patients with cholangiocarcinoma participating in a phase 1 study (NCT02073994) showed that ivosidenib treatment promoted morphological changes and upregulated liver-specific genes [20]. The increased cholangiolar histology following ivosidenib therapy appeared to be associated with increased progression-free survival (PFS) [20].
Differentiation syndrome has been associated with ivosidenib in patients with relapsed or refractory AML, which is thought to be due to the rapid increase in differentiation of neutrophils following the removal of differentiation block in the malignant myeloid clone [21].
Pharmacokinetics
Oral ivosidenib is rapidly absorbed [22] and reaches steady state within 14 days of administration [13, 22]. A less than dose proportional increase in plasma exposure is seen with ivosidenib 200–1200 mg/day (0.4–2.4 times the recommended dosage) [13, 22]. Following administration of ivosidenib 500 mg/day, at steady-state, the mean peak plasma concentration (Cmax) of ivosidenib was 6551 ng/ml and the mean area under the concentration-time-curve (AUC) was 117,348 ng·h/mL [13, 22]; Cmax was reached in ≈ 3 h (median) [13]. Over 1 month of treatment, the accumulation ratio of ivosidenib was ≈ 1.9-fold for AUC and ≈ 1.5-fold for Cmax [13, 22]. Ivosidenib can be administered with or without food; however, ivosidenib must not be administered with a high-fat meal. In healthy patients, a high-fat meal prior to the administration of a single dose of ivosidenib 500 mg was associated with a 98% increase in the ivosidenib Cmax and an ≈ 25% increase in AUC [13, 23].
The steady-state mean apparent volume of distribution of ivosidenib is 234 L; in vitro, ivosidenib is 92–96% protein bound [13]. Ivosidenib accounts for > 92% of total radioactivity in the plasma. Ivosidenib metabolism is primarily mediated by CYP3A4, with N-dealkylation and hydrolytic pathways playing minor roles. Following a single 500 mg dose in healthy subjects, ivosidenib was largely (77%) excreted in the faeces as unchanged drug (67% of a dose), with 17% of the dose eliminated in the urine (10% as unchanged drug). The terminal elimination half-life of ivosidenib is 93 h and the apparent clearance is 4.3 L/h [13].
Age (18–89 years), sex, race, bodyweight (38–150 kg), Eastern Cooperative Oncology Group performance status (ECOG PS), mild or moderate renal impairment [estimated glomerular filtration rate (eGFR) ≥ 30 mL/min/1.73 m2] and mild hepatic impairment had no clinically meaningful effects on the pharmacokinetics of ivosidenib. The effects of severe renal impairment (eGFR < 30 mL/min/1.73 m2), renal impairment requiring dialysis, or moderate or severe hepatic impairment on the pharmacokinetics of ivosidenib are unknown.
Coadministration of ivosidenib with strong or moderate CYP3A4 inhibitors (resulting in increased ivosidenib concentrations) or corrected QT (QTc) prolonging drugs may increase the risk of QTc interval prolongation [13]. Therefore, alternative therapies should be considered; if concomitant use of ivosidenib with these agents is unavoidable, ivosidenib dosage should be reduced and patients should be monitored for increased risk of QTc prolongation. Coadministration of ivosidenib with strong CYP3A4 inducers decreased ivosidenib plasma concentrations; therefore, concomitant use of these agents must be avoided. Coadministration of ivosidenib with drugs that are sensitive substrates of CYP3A4 or CYP2C9 will decrease the plasma concentrations of the CYP3A4 sensitive drugs and may decrease the plasma concentrations of the CYP2C9 sensitive drugs, resulting in the loss of therapeutic effect of these drugs. Use of alternative therapies is recommended, and if concomitant use of ivosidenib with these agents is unavoidable, patients should be monitored for the loss of therapeutic effect of these drugs [13].
Features and properties of Ivosidenib
| Alternative names | AG-120; Tibsovo® |
| Class | Antineoplastics, cyclobutanes, nitriles, pyridines, pyrrolidines, small molecules |
| Mechanism of Action | Isocitrate dehydrogenase 1 inhibitor |
| Route of Administration | Oral |
| Pharmacodynamics | Selective, small molecule inhibitor of isocitrate dehydrogenase R132 mutants, resulting in the inhibition of mutant isocitrate dehydrogenase enzyme and decreased 2-hydroxyglutarate levels |
| Pharmacokinetics | Rapidly absorbed (median time to peak plasma concentration ≈ 3 h) |
| Terminal elimination half-life 93 h | |
| Adverse events in patients with acute myeloid leukaemia | |
| Any-grade treatment-emergent | Diarrhoea, leucocytosis, febrile neutropenia, nausea, fatigue, dyspnoea, prolongation of the QT interval, peripheral oedema, anaemia, pyrexia, cough |
| Grade ≥ 3 treatment-related | Prolongation of QT interval, isocitrate dehydrogenase differentiation syndrome |
| ATC codes | |
| WHO ATC code | L01X-X (Other antineoplastic agents) |
| EphMRA ATC code | L1X9 (All other antineoplastics) |
| Chemical Name | (2S)-N-[(1S)-1-(2-Chlorophenyl)-2-[(3,3-difluorocyclobutyl)amino]-2-oxoethyl]-1-(4-cyanopyridin-2-yl)-N-(5-fluoropyridin-3-yl)-5-oxopyrrolidine-2-carboxamide |
Therapeutic Trials
Acute Myeloid Leukaemia
Oral ivosidenib monotherapy was associated with durable remissions in 179 patients with IDH1-mutated, relapsed or refractory AML in a phase 1 dose-escalation and dose-expansion study (NCT02074839; analysis cut-off date 12 May 2017) [22]. Patients aged ≥ 18 years, with an ECOG PS of 0–2 and documented IDH1-mutated haematological cancer received ivosidenib in 28-day cycles (100 mg twice daily or 300–1200 mg once daily in the dose escalation phase, and 500 mg once daily in the dose expansion phase). In the primary efficacy population (n = 125), 30% of patients receiving ivosidenib 500 mg/day had investigator-assessed CR or sponsor-assessed complete remission with partial haematological recovery (CRh) (primary efficacy endpoint) for a median duration of 8 months; the median time to CR or CRh was 3 months. The CR rate and median duration of CR in this population was 22% and 9 months, respectively, and the overall response rate (ORR) and median duration of response was 42% and 7 months. The median overall survival (OS) was 9 months after a median follow-up of 15 months. Among 34 ivosidenib recipients with CR or CRh, the mean levels of IDH1 mutations in bone marrow mononuclear cells and neutrophils decreased over time, and 7 (21%) patients had no residual detectable IDH1 mutations on digital polymerase-chain-reaction assay (p = 0.003 for the association between clearance of mutation and CR or CRh). In addition, transfusion independence for ≥ 56 days was achieved by 35% (29/84) of patients who were dependent on red-blood cell and/or platelet transfusion at baseline; 56% (23/41) of the patients who were transfusion-independent at baseline remained transfusion-independent for ≥ 56 days [22].
An ongoing, open-label phase 1b/2 study (NCT02677922) is assessing the efficacy of ivosidenib 500 mg/day or enasidenib 100 mg or 200 mg once daily in combination with subcutaneous azacitidine 75 mg/m2/day for 7 days/cycle in patients aged ≥ 18 years with newly diagnosed (de novo or secondary) untreated AML with IDH1 or IDH2 mutation who were ineligible for intensive chemotherapy [24]. Interim results (data cut-off 15 March 2018) showed that in patients with IDH1 mutation, ivosidenib 500 mg/day in combination with subcutaneous azacitidine was associated with an ORR of 78% (18/23), and in patients with IDH2 mutation, enasidenib (100 mg once daily) plus azacitidine was associated with an ORR of 67% (4/6 patients). In the ivosidenib and enasidenib groups, 44% (10/23) and 50% (3/6) of patients had CR, 22% (5/23) and 0% of patients had CR with incomplete haematological/platelet recovery (CRi/CRp), and 13% (3/23) and 17% (1/6) of patients had a morphological leukaemia-free state (MLFS). Four (17%) patients in the ivosidenib group and one (17%) patient in the enasidenib group had stable disease; no patient in the ivosidenib group and one patient in the enasidenib group had progressive disease [24].
Another ongoing, open-label, multicentre, phase 1 study (NCT02632708) is assessing the efficacy of ivosidenib or enasidenib in combination with standard induction chemotherapy in patients aged ≥ 18 years with previously untreated AML (de novo or secondary) with locally documented IDH1 and/or IDH2 mutation [25]. Initial results (data cut-off 1 August 2017) showed that 77% (23/30) of ivosidenib and 62% (31/50) of enasidenib recipients had CR or CRi/CRp. The CR rates with ivosidenib and enasidenib were 63% and 50%, respectively, CRi/CRP rates were 13% and 12%, MLFS rates were 3% and 20%, partial response rates were 7% and 0%, and 7% and 10% of patients had persistent disease. The treatment regimen is 1–2 cycles of induction therapy (daunorubicin 60 mg/m2/day, or idarubicin 12 mg/m2/day for 3 days with cytarabine 200 mg/m2/day for 7 days) in combination with either ivosidenib 500 mg once daily or enasidenib 100 mg once daily, after which patients can receive ≤ 4 cycles of consolidation chemotherapy while continuing treatment with the mutated IDH inhibitor, and those who complete or are ineligible for consolidation may continue maintenance treatment with ivosidenib or enasidenib for ≤ 2 years from the start of induction [25].
In addition to these studies, a pharmacoeconomic analysis suggested that the potential for improved survival and a more favourable tolerability profile of ivosidenib relative to other therapies may result in better health-related quality of life in patients with relapsed/refractory AML receiving ivosidenib [26].
Advanced Solid Tumours
Oral ivosidenib demonstrated clinical activity in an open-label, multicentre, phase 1 study in 168 patients (as off 10 March 2017) with advanced solid tumours (NCT02073994), including intrahepatic cholangiocarcinoma, chondrosarcoma and glioma [27]. Eligible patients had measurable disease (based on RECIST 1.1) that had recurred or progressed after standard therapy, ECOG PS of 0 or 1 and documented IDH1 mutated advanced solid tumours. Patients received ivosidenib in 28-day cycles at doses of 100 mg twice daily or 300–1200 mg once daily in the dose escalation phase and 500 mg once daily in the dose expansion phase of the study [27].
In patients with pretreated intrahepatic cholangiocarcinoma (n = 73), the ORR was 5% (4/73; all partial responses) and stable disease was reported in 56% (41/73) of patients following up to 80 weeks’ treatment with ivosidenib in the dose escalation and dose expansion phases of the study [27]. The median PFS was 4 months, and the 6- and 12-month PFS rates were 39 and 21%, respectively; the OS data were immature.
In patients with pretreated chondrosarcoma (n = 21, as of 23 September 2016), 55% (11/20) of evaluable patients had stable disease and 30% (6/20) of patients had disease progression after median 2.6 months’ treatment with ivosidenib [28]. The 3-month PFS rate with ivosidenib was 58%.
Following median treatment of 16 months (data cut-off 12 May 2017) in 35 patients with non-enhancing glioma, two (5.7%) patients had minor responses, 29 (82.9%) patients had stable disease and four (11.4%) patients had progressive disease [29]. The median PFS with ivosidenib was 13 months.
Key clinical trials of Ivosidenib
| Drug(s) | Indication | Phase | Status | Location(s) | Identifier | Sponsor |
|---|---|---|---|---|---|---|
| Ivosidenib, placebo | Untreated AML | 3 | Recruiting | Multnational | NCT03173248; AGILE; AG120-C-009 | Agios Pharmaceuticals, Inc |
| Ivosidenib, placebo | Advanced cholangiocarcinoma | 3 | Recruiting | Multinational | NCT02989857; A G120-C-005 | Agios Pharmaceuticals, Inc |
| Ivosidenib | R/R AML | EA | Approved for marketing | NA | NCT03245424; AG120-C-010 | Agios Pharmaceuticals, Inc |
| Ivosidenib | Myelodysplastic syndrome | 2 | Planned | NA | NCT03503409; IDIOME-STUDY | Groupe Francophone des Myelodysplasies |
| Ivosidenib, other targeted agents | Untreated AML | 1b/2 | Recruiting | USA | NCT03013998; BAML-16-001 | Beat AML, LLC |
| Ivosidenib/enasidenib (with azacitidine) | Newly diagnosed AML | 1b/2 | Recruiting | Multinational | NCT02677922; AG-221-AML-005 | Celgene |
| Ivosidenib, venetoclax | Haematologic malignancies | 1b/2 | Recruiting | NA | NCT03471260; 2017-0490 | MD Anderson Cancer Center |
| Ivosidenib | Myeloid neoplasms | 1 | Planned | USA | NCT03564821; 18-123 | Massachusetts General Hospital |
| Ivosidenib, enasidenib (with chemotherapy) | Newly diagnosed AML | 1 | Recruiting | USA, Germany, Netherlands | NCT02632708; AG120-221-C-001 | Agios Pharmaceuticals, Inc |
| Ivosidenib | Advanced haematological malignancies (including R/R AML) | 1 | Ongoing | USA, France | NCT02074839; AG120-C-001 | Agios Pharmaceuticals, Inc |
| Ivosidenib | Advanced solid tumors | 1 | Ongoing | USA, France | NCT02073994; AG120-C-002 | Agios Pharmaceuticals, Inc |
| Ivosidenib, vorasidenib | Recurrent, non-enhancing, low-grade glioma | 1 | Recruiting | USA | NCT03343197; AG120-881-C-001 | Agios Pharmaceuticals, Inc |
AML acute myeloid leukaemia, EA expanded access, NA not available, R/R relapsed or refractory
Adverse Events
Acute Myeloid Leukaemia
Oral ivosidenib as monotherapy had a generally acceptable tolerability profile in patients with relapsed or refractory IDH1-mutated AML in the phase 1 dose-escalation and dose-expansion study (NCT02074839) [22]. In patients receiving ivosidenib 500 mg/day (n = 179) for a median duration of 3.5 months, treatment-emergent adverse events (AEs) were reported in 99% of patients, with diarrhoea (31%), leucocytosis (30%), febrile neutropenia (29%), nausea (28%), fatigue (26%), dyspnoea (25%), prolongation of the QT interval (25%), peripheral oedema and anaemia (22% each), and pyrexia and cough (21% each) reported most frequently (incidence ≥ 20%). Treatment-related grade ≥ 3 adverse events (AEs) were reported in 21% of patients, with the most common (incidence > 2%) AEs being prolongation of QT interval (8%) and IDH differentiation syndrome (4%). There were no deaths due to treatment-related AEs in patients receiving ivosidenib 500 mg/day; one AE-related death (grade 5 cardiac tamponade) was assessed as being possibly related to treatment with ivosidenib 800 mg/day [22].
Adverse events of special interest with ivosidenib were IDH differentiation syndrome, leucocytosis (including the preferred terms of leukocytosis, hyperleukocytosis and white blood cell count increased) and QT prolongation. Investigator-reported IDH differentiation syndrome (median time of onset 29 days) occurred in 11% of ivosidenib 500 mg/day recipients, with grade ≥ 3 AEs reported in 5% of patients; no patient had grade ≥ 4 IDH differentiation syndrome or required permanent discontinuation of therapy [22]. Thirty-seven percent of the patients with IDH differentiation syndrome also had grade 2 or 3 leukocytosis. Leukocytosis of any grade occurred in 36% of patients receiving ivosidenib, with 65% of patients having first onset within the first 30 days of treatment. Dose interruptions were required in 3% of patients, but no patient needed dose reductions or permanent discontinuation of therapy because of leukocytosis. Grade ≥ 3 leukocytosis occurred in 8% of ivosidenib recipients. Prolongation of QT interval occurred in 25% of patients receiving ivosidenib, with grade ≥ 3 AEs reported in 10% of patients, 7% of patients required dose interruptions and 1% of patients needed dose reductions; no patient required permanent discontinuation of therapy. Serious AEs of QT prolongation occurred in 7% of patients, which were not fatal [22].
In a phase 1b/2 study, the tolerability profile of ivosidenib in combination with azacitidine in patients with IDH1-mutated AML (NCT02677922) was generally similar to that of ivosidenib alone. There were no unexpected safety findings with ivosidenib combination therapy in patients receiving ivosidenib 500 mg/day plus azacitidine (n = 23) [24]. The most common any-grade treatment-emergent AEs with ivosidenib plus azacitidine were gastrointestinal AEs (including nausea in 14 patients, and constipation and diarrhoea in 9 patients each) and the most common grade 3 or 4 treatment-emergent AEs were haematological AEs (including anaemia and thrombocytopenia in 10 patients each). Treatment-related AEs occurred in 96% of patients receiving combination therapy, with nausea (12 patients), vomiting (seven patients), prolongation of QT interval (six patients) and fatigue (five patients) being the most common AEs. Serious treatment-emergent AEs occurring in more than two patients receiving ivosidenib plus azacitidine were febrile neutropenia (eight patients) and IDH differentiation syndrome (three patients) [24].
In another phase 1 study (NCT02632708) in patients with previously untreated IDH1- and/or IDH2-mutated AML who were receiving ivosidenib or enasidenib in combination with standard induction therapy, grade ≥ 3 treatment-emergent non-haematological AEs occurred in 94% (30/32) and 91% (51/56) of patients in the respective groups, with the most common AEs being febrile neutropenia in 60 and 63% of patients respectively [25].
Advanced Solid Tumours
Ivosidenib monotherapy had a generally acceptable tolerability profile in a phase 1 study in patients with advanced solid tumours (NCT02073994; AG120-C-002), including intrahepatic cholangiocarcinoma, chondrosarcoma and glioma [27]. In patients with intrahepatic cholangiocarcinoma receiving ivosidenib 500 mg/day (n = 62), any-grade treatment-emergent AEs were reported in all patients, with fatigue (45%) and nausea (37%) reported most commonly. Grade ≥ 3 treatment-emergent AEs occurred in 35% (22/62) of patients, with ascites (3 patients), abdominal distension (2 patients) and anaemia (2 patients) occurring most frequently [27]. In patients with chondrosarcoma receiving ivosidenib in the dose-escalation and dose-expansion phases (n = 21), grade ≥ 3 treatment-emergent AEs occurred in 52% of patients, with one possibly treatment-related event (hypophosphatemia) [28]. In patients with non-enhancing glioma receiving ivosidenib in the dose-escalation and dose-expansion phases (n = 35), treatment-emergent AEs occurred in 91% of patients, with grade ≥ 3 AEs reported in 20% of patients [29]. The most common (incidence > 20%) any-grade treatment-emergent AEs included headache (34%) and diarrhoea (26%). Grade ≥ 3 hypophosphatemia was reported in 2 patients; all other grade ≥ 3 AEs were reported in no more than one patient [29].
Companion Diagnostic
The US FDA has approved the Abbott RealTime IDH1 polymerase chain reaction (PCR) assay, developed collaboratively by Agios Pharmaceuticals and Abbott as a companion diagnostic for the detection of IDH1 mutations in the blood or bone marrow of patients with AML [13, 30]. The PCR assay, when used with the Abbott m2000rt System, qualitatively detects single nucleotide variants (SNVs) coding five IDH1 R132 mutations (R132C, R132H, R132G, R132S and R132L) in DNA extracted from human blood (EDTA) or bone marrow (EDTA) [30]. It is recommended that patients negative for IDH1 mutations at diagnosis should be retested at relapse, as IDH1 mutations may emerge during treatment and at relapse [13].
Ongoing Clinical Trials
A phase 1, multicentre, dose-escalation and dose-expansion study is assessing the safety, pharmacokinetics, pharmacodynamics and efficacy of ivosidenib in advanced haematological malignancies with an IDH1 mutation (NCT02074839). Results from an analysis conducted on 12 May 2017 are available (Sect. 2.3.1). Another open-label, multicentre, phase 1 study (NCT02632708) is determining the safety of ivosidenib and enasidenib, in combination with standard induction and consolidation chemotherapy, in patients aged ≥ 18 years with previously untreated AML with locally documented IDH1 and/or IDH2 mutation [25]. The study plans to enrol 144 patients and initial results (data cut-off 1 August 2017) from 88 patients are available (Sect. 2.3.1). Another open-label phase 1b/2 study (NCT02677922) is assessing the safety and efficacy of ivosidenib plus azacitidine and enasidenib plus azacitidine in patients with newly diagnosed AML with an IDH1- or IDH2-mutation who are ineligible for intensive induction chemotherapy. Interim safety and efficacy results from this study are available (Sect. 2.3.1). Based on these results, a randomized, double-blind, placebo-controlled, global, phase 3 study AGILE (NCT03173248) is underway to evaluate the efficacy and safety of ivosidenib plus azacitidine versus placebo plus azacitidine in adults with previously untreated IDH1-mutated AML who are ineligible for intensive therapy [31]. The study plans to enrol 392 patients; the primary endpoint is OS and the secondary endpoints include event-free survival, ORR and the rates of CR and CR/CRh [31].
A multicentre, phase 1 dose-escalation and dose-expansion study is evaluating the safety, pharmacokinetics, pharmacodynamics and efficacy of ivosidenib in patients with advanced solid tumours with IDH1 mutations. Initial results from patients with intrahepatic cholangiocarcinoma, chondrosarcoma and glioma are available (NCT02073994) (Sect. 2.3.1) [27]. A randomized, double-blind, multicentre, placebo-controlled phase 3 study is underway that will assess the efficacy and safety of ivosidenib in 186 previously-treated patients with nonresectable or metastatic cholangiocarcinoma with an IDH1 mutation (NCT02989857) [32]. The primary endpoint of the study is PFS, with secondary endpoints including OS, ORR and safety.
Several other studies are planned/underway, including a phase 1b/2 dose escalation and dose expansion study of ivosidenib and venetoclax in patients with haematological malignancies (NCT03471260), a phase 1b/2 study of biomarker-based treatment of AML sponsored by the Leukemia and Lymphoma Society (Beat AML; NCT03013998) and an expanded access programme (NCT03245424) that aims to provide patients with relapsed or refractory, IDH1-mutated AML access to ivosidenib monotherapy.
Current Status
On the 20 July 2018, ivosidenib received its first global approval in the USA for the treatment of adults with relapsed or refractory AML with a susceptible IDH1 mutation, as detected by an FDA-approved test.
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sohita Dhillon is a salaried/ contracted employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Footnotes
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
The original version of this article was revised due to a retrospective Open Access request.
Change history
1/4/2019
IThe article Ivosidenib: First Global Approval, written by Sohita Dhillon, was originally published Online First without open access.
References
- 1.Buege MJ, DiPippo AJ, DiNardo CD. Evolving treatment strategies for elderly leukemia patients with IDH mutations. Cancers (Basel). 2018;10(6). [DOI] [PMC free article] [PubMed]
- 2.Almeida AM, Ramos F. Acute myeloid leukemia in the older adults. Leuk Res Rep. 2016;6:1–7. doi: 10.1016/j.lrr.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dang L, Yen K, Attar EC. IDH mutations in cancer and progress toward development of targeted therapeutics. Ann Oncol. 2016;27(4):599–608. doi: 10.1093/annonc/mdw013. [DOI] [PubMed] [Google Scholar]
- 4.Mondesir J, Willekens C, Touat M, et al. IDH1 and IDH2 mutations as novel therapeutic targets: current perspectives. J Blood Med. 2016;7:171–180. doi: 10.2147/JBM.S70716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Food & Drug Administration. FDA approves first targeted treatment for patients with relapsed or refractory acute myeloid leukemia who have a certain genetic mutation [media release]. 20 July 2018. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm614115.htm.
- 6.Celgene Corporation, Agios Pharmaceuticals. Celgene Corporation and Agios Pharmaceuticals announce global strategic collaboration to advance unique science of cancer metabolism [media release]. 15 Apr 2010. http://investor.agios.com/news-releases/news-release-details/celgene-corporation-and-agios-pharmaceuticals-announce-global.
- 7.Agios Pharmaceuticals. Celgene and Agios extend cancer metabolism collaboration [media release]. 5 Oct 2011. http://investor.agios.com/news-releases/news-release-details/celgene-and-agios-extend-cancer-metabolism-collaboration.
- 8.Agios Pharmaceuticals. Agios advances cancer metabolism collaboration with Celgene [media release]. 11 Dec 2013. http://investor.agios.com/news-releases/news-release-details/agios-advances-cancer-metabolism-collaboration-celgene.
- 9.Agios Pharmaceuticals. Agios Pharmaceuticals exercises option to US development and commercialization rights for IDH1 program under Celgene collaboration [media release]. 3 Feb 2014. http://investor.agios.com/news-releases/news-release-details/agios-pharmaceuticals-exercises-option-us-development-and.
- 10.Celgene Corporation. Celgene reports second quarter 2015 operating and financial results [media release]. 23 July 2015. http://ir.celgene.com/releasedetail.cfm?releaseid=923485.
- 11.Agios Pharmaceuticals. Agios and Celgene establish new collaboration in metabolic immuno-oncology and amend certain rights from 2010 agreement [media release]. 17 May 2016. http://ir.celgene.com/releasedetail.cfm?releaseid=971425.
- 12.Agios Pharmaceuticals, CStone Pharmaceuticals. Agios and CStone Pharmaceuticals announce exclusive collaboration and license agreement to develop and commercialize ivosidenib in Greater China [media release]. 26 June 2018. http://investor.agios.com/news-releases/news-release-details/agios-and-cstone-pharmaceuticals-announce-exclusive.
- 13.Agios Pharmaceuticals. TIBSOVO® (Ivosidenib): US prescribing Information. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211192s000lbl.pdf. Accessed 11 Aug 2018.
- 14.Popovici-Muller J, Lemieux RM, Artin E, et al. Discovery of AG-120 (ivosidenib): a first-in-class mutant IDH1 inhibitor for the treatment of IDH1 mutant cancers. ACS Med Chem Lett. 2018;9(4):300–305. doi: 10.1021/acsmedchemlett.7b00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heredia V, Mendiola M, Ortiz E, et al. AG-120, a novel IDH1 targeted molecule, inhibits invasion and migration of chondrosarcoma cells in vitro [abstract no. 1524P] Ann Oncol. 2017;28(Suppl. 5):v538. [Google Scholar]
- 16.Nicolay B, Narayanaswamy R, Aguado E, et al. The IDH1 mutant inhibitor AG-120 shows strong inhibition of 2-HG production in an orthotopic IDH1 mutant glioma model in vivo [abstract no. EXTH-59 plus poster] Neuro-oncology. 2017;19(Suppl. 6):vi86. doi: 10.1093/neuonc/nox168.351. [DOI] [Google Scholar]
- 17.Yen K, Chopra V, Tobin E, et al. Functional characterization of the ivosidenib (AG-120) and azacitidine combination in a mutant IDH1 AML cell model [abstract no. 4956]. Cancer Res. 2018;78(13 Suppl.).
- 18.Dai D, Dinardo CD, Stein E, et al. Clinical pharmacokinetics/pharmacodynamics (PK/PD) of ivosidenib in patients with IDH1-mutant advanced hematologic malignancies from a phase 1 study [abstract no. 2581 plus poster]. J Clin Oncol. 2018;36(Suppl).
- 19.Fan B, Goyal L, Lowery MA, et al. Pharmacokinetic/pharmacodynamic (PK/PD) profile of AG-120 in patients with IDH1-mutant cholangiocarcinoma from a phase 1 study of advanced solid tumors [abstract no. 4082 plus poster]. J Clin Oncol. 2017;35(15 Suppl.).
- 20.Ishii Y, Sigel C, Lowery MA, et al. AG-120 (ivosidenib), a first-in-class mutant IDH1 inhibitor, promotes morphologic changes and upregulates liver-specific genes in IDH1 mutant cholangiocarcinoma [abstract no. A071 plus poster]. Mol Cancer Ther. 2017;17(1 Suppl.).
- 21.Birendra KC, DiNardo CD. Evidence for clinical differentiation and differentiation syndrome in patients with acute myeloid leukemia and IDH1 mutations treated with the targeted mutant IDH1 inhibitor, AG-120. Clin Lymphoma Myeloma Leuk. 2016;16(8):460–465. doi: 10.1016/j.clml.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386–2398. doi: 10.1056/NEJMoa1716984. [DOI] [PubMed] [Google Scholar]
- 23.Fan B, Dai D, Connor G, et al. Evaluation of food effect on pharmacokinetics of ivosidenib (AG-120), an oral, potent, targeted, small molecule inhibitor of mutant IDH1, in healthy subjects [abstract no. PF256 plus poster]. In: 23rd congress of the European Hematology Association. 2018.
- 24.Dinardo CD, Stein AS, Stein EM, et al. Mutant IDH (mIDH) inhibitors, ivosidenib or enasidenib, with azacitidine (AZA) in patients with acute myeloid leukemia (AML) [abstract no. 7042 plus poster]. J Clin Oncol. 2018;36(15 Suppl.).
- 25.Stein EM, DiNardo CD, Mims AS, et al. Ivosidenib or enasidenib combined with standard induction chemotherapy is well tolerated and active in patients with newly diagnosed aml with an IDH1 or IDH2 mutation: initial results from a phase 1 trial [abstract no. 726 plus oral presentation]. Blood. 2017;130(Suppl. 1).
- 26.Wehler E, Storm M, Kowal S. A health state utility model estimating the impact of ivosidenib on quality of life in patients with relapsed/refractory acute myeloid leukemia [abstract no. PS1442 plus poster]. In: 23rd congress of the European Haematology Association. 2018.
- 27.Lowery MA, Abou-Alfa GK, Burris HA, et al. Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: results from the cholangiocarcinoma dose escalation and expansion cohorts [abstract no. 4015 plus poster]. J Clin Oncol. 2017;35(15 Suppl.).
- 28.Tap W, Villalobos V, Cote G, et al. A phase 1 study of AG-120, an IDH1 mutant enzyme inhibitor: results from the chondrosarcoma dose escalation and expansion cohorts [abstract no. P1-138 plus poster]. In: Connective Tissue Oncology Society (CTOS) annual meeting. 2016.
- 29.Mellinghoff IK, Touat M, Maher E, et al. AG-120, a first-in-class mutant IDH1 inhibitor in patients with recurrent or progressive IDH1 mutant glioma: updated results from the phase 1 non-enhancing glioma population [abstract no. ACTR-46 plus oral presentation] Neuro-oncol. 2017;19(Suppl. 6):vi10–vi11. doi: 10.1093/neuonc/nox168.037. [DOI] [Google Scholar]
- 30.US FDA. List of cleared or approved companion diagnostic devices (in vitro and imaging tools). 2018. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm301431.htm. Accessed 10 Aug 2018.
- 31.Stein E, Dinardo CD, Jang JH, et al. AGILE: a phase 3, multicenter, randomized, placebo-controlled study of ivosidenib in combination with azacitidine in adult patients with previously untreated acute myeloid leukemia with an IDH1 mutation [abstract no. TPS7074 plus poster]. J Clin Oncol. 2018;36(15 Suppl.).
- 32.Lowery MA, Abou-Alfa GK, Valle JW, et al. ClarIDHy: A phase 3, multicenter, randomized, double-blind study of AG-120 vs placebo in patients with an advanced cholangiocarcinoma with an IDH1 mutation [abstract no. TPS4142 plus poster]. J Clin Oncol. 2017;35(15 Suppl.).