Abstract
The diagnosis and management of pancreatic neuroendocrine neoplasms (NENs) have evolved significantly in recent years. There are several diagnostic and therapeutic challenges and controversies regarding the management of these lesions. In this review, we focus on the recent significant changes and controversial issues regarding the diagnosis and management of NENs and discuss the role of imaging in the multidisciplinary team approach.
Keywords: Pancreas, Neuroendocrine tumor, NET, Staging, Management, Multidisciplinary
INTRODUCTION
Pancreatic neuroendocrine neoplasms (NENs) are a diverse group of tumors that show heterogeneity in their pathologic, functional, and clinical features. As a general concept, well-differentiated pancreatic NENs are categorized as neuroendocrine tumors (NETs) and poorly differentiated pancreatic NENs as neuroendocrine carcinomas (NECs) (1). There have been rapid advances in knowledge regarding the pathophysiology and molecular biology of NENs in recent years, which have led to improvements in the diagnosis and management of patients with these lesions (2) and application of new therapeutic strategies for well-differentiated NENs.
In 2017, there were substantial changes in the grading and staging systems for NEN. First, the World Health Organization (WHO) grading system changed the definition of grade 3 NET/NEC. Second, the 8th American Joint Committee on Cancer (AJCC) changed its TNM staging system for NEN. Third, the treatment strategies changed according to the new concepts of grade 3 NET/NEC.
These changes were implemented in order to solve the controversial issues that arose in the 2010 WHO grading system (3) and the 7th AJCC staging system (4). These issues were that the definition of grade 3 NEN in the 2010 WHO grading system was too broad to distinguish NETs from NECs, which is critical in terms of choosing appropriate treatment strategies, and that there was a discrepancy in the T3 stage, which is the most important stage in determining resectability, between the 7th AJCC and European Neuroendocrine Tumor Society (ENETS) staging systems (5,6).
Radiologists should be aware of these recent updates and their rationale in order to give a key role in the multidisciplinary team. In this review, we focus on current issues concerning NENs, provide a comprehensive update on their diagnosis and management, and discuss the role of imaging in the multidisciplinary team approach.
Background to the Updates in the 2017 WHO Grading System
Ambiguity of Grade 3 NEC in the 2010 WHO Grading System
The 2010 WHO classification categorizes NEN as a NET (3) grade 1, NET grade 2, and NEC grade 3 (Table 1). In general, a well-differentiated NEN is composed of cells showing minimal to moderate atypia, lacks necrosis, expresses general markers of neuroendocrine differentiation, i.e., diffuse and intense synaptophysin or chromogranin A staining, and produces hormones. In contrast, poorly differentiated NEN is composed of highly atypical small cells or cells of large to intermediate size that express general markers of neuroendocrine differentiation, i.e., faint synaptophysin or chromogranin A staining. The histologic grading was based on the Ki-67 index or mitotic index that should be counted in tumor hot spots. Ki-67 is an excellent marker of cell proliferation. The fraction of Ki-67-positive tumor cells (the Ki-67 labeling index) is often correlated with the clinical course of cancer and its prognosis (7,8). If there is a discrepancy between the Ki-67 index and the mitotic index, the higher grade should be used.
Table 1. WHO Classification: Comparison to WHO 2010 and 2017.
| WHO 2010 | Mitoses/10 HPF* | Ki-67 Index* | WHO 2017 | Mitoses/10 HPF* | Ki-67 Index* |
|---|---|---|---|---|---|
| Well-differentiated NENs | Well-differentiated NENs | ||||
| NET grade 1 | < 2 | < 3 | NET grade 1 | < 2 | < 3 |
| NET grade 2 | 2–20 | 3–20 | NET grade 2 | 2–20 | 3–20 |
| NET grade 3 | > 20 | > 20 | |||
| Poorly differentiated NENs | Poorly differentiated NENs | ||||
| NEC (small-cell or large-cell), grade 3 | > 20 | > 20 | NEC grade 3 | > 20 | > 20 |
| Small-cell type | |||||
| Large-cell type | |||||
| MANEC | MiNEN* |
*MiNENs may have non-endocrine component other than adenocarcinoma, e.g., squamous cell carcinoma or acinar cell carcinoma. To qualify as MiNEN, each component must be at least 30%. HPF = high power fields, MANEC = mixed adenoneuroendocrine carcinoma, MiNEN = mixed endocrine non-endocrine neoplasm, NEC = neuroendocrine carcinoma, NEN = neuroendocrine neoplasm, NET = neuroendocrine tumor, WHO = World Health Organization
In the 2010 WHO grading system, there was confusion regarding the discrepancy between grade and differentiation. “Differentiation” refers to the morphologic resemblance of the tumor cells to islets of Langerhans. In contrast, “grade” refers to the aggressiveness of the tumor cells in terms of their potential for rapid tumor growth and spread (1,4). Based on the definition in the 2010 WHO classification (9), it was possible that morphologically well-differentiated NETs could show a high Ki-67 level and be technically classified as grade 3 NEC. These well-differentiated NETs, which are technically classified as grade 3 NEC, may not be sensitive to the chemotherapy regimen used in poorly differentiated grade 3 NEC. This finding suggested that the definition of grade 3 NEC based simply on the Ki-67 level and/or mitotic index was too broad to distinguish the differentiation and grade, and could lead to inappropriate treatment and an unsatisfactory clinical outcome (4).
The 2017 WHO Grading System
The WHO grading system was revised in 2017 and the above-mentioned issues have been reflected in the changes (Table 1) (10). In the revised grading system, a new subset of well-differentiated NENs has been recognized, i.e., lesions that are morphologically well-differentiated and often identical to grade 1 or grade 2 NET but have a high Ki-67 index (> 20%) (11). Interestingly, if an adequate number of pathologic specimens are available for an accurate mitotic count, most grade 3 NETs contain a proportion of cells with a mitotic rate fewer than 20 per 10 high-power and lower-grade regions may be present elsewhere with the tumor focus (1). Furthermore, the genomic features of grade 3 NET resemble those of lower-grade NET, i.e., MEN1, DAX, and ATRX mutation, and differ markedly from the genomic alteration in poorly differentiated NEC, i.e., p53 and RB1 mutation.
Another revision to the 2017 WHO grading system is the change in nomenclature for mixed adenoneuroendocrine carcinoma, a neoplasm with components of a nonendocrine carcinoma (mostly ductal adenocarcinoma or acinar cell carcinoma) combined with a NEN, to mixed endocrine non-endocrine neoplasm (MiNEN). This change has been made to reflect the fact that not all MiNENs are high-grade malignant carcinomas; occasionally one or both of the two components may belong to the well-differentiated NEN category and MiNEN may have a non-endocrine component other than adenocarcinoma, e.g., squamous cell carcinoma or acinar cell carcinoma.
Background to the Updates in the 8th AJCC Staging System
The WHO 2010 classification recommended use of the AJCC TNM staging system (7th edition) but also acknowledged use of the ENETS staging system proposed in 2006 (5,6) (Table 2). Historically, the AJCC/Union for International Cancer Control (UICC) system has used the same staging system for NETs and exocrine pancreatic adenocarcinomas (5), while ENETS has developed a dedicated staging system for foregut NETs (6).
Table 2. Differences between AJCC/UICC TNM Staging System and ENETS TNM Staging System (for Well-Differentiated NET of Pancreas).
| T Stage | 7th AJCC/UICC | ENETS | 8th AJCC/UICC |
|---|---|---|---|
| T1 | Confined to pancreas, < 2 cm | Confined to pancreas*, < 2 cm | Confined to pancreas*, < 2 cm |
| T2 | Confined to pancreas, > 2 cm | Confined to pancreas*, 2–4 cm | Confined to pancreas*, 2–4 cm |
| T3 | Peripancreatic spread, without major vascular invasion | Confined to pancreas*, > 4 cm, or invades duodenum or bile duct | Confined to pancreas*, > 4 cm, or invades duodenum or bile duct |
| T4 | Tumor involves coeliac axis or superior mesenteric artery | Invasion of adjacent organs or major vessels | Invasion of adjacent organs or major vessels |
*Confined to pancreas means there is no invasion of adjacent organs or wall of large vessels. Extension of tumor into peripancreatic adipose tissue is NOT basis for staging. AJCC = American Joint Committee on Cancer, ENETS = European Neuroendocrine Tumor Society, UICC = Union for International Cancer Control
In the 7th AJCC/UICC TNM staging, there is a difference in T staging between the AJCC/UICC and ENETS systems that may be a source of confusion for clinicians. Specifically, T3 is controversial in that it is defined in the AJCC/UICC system as peripancreatic tumor spread without major vascular invasion, but in the ENETS system, it is defined as tumor confined to the pancreas, greater than 4 cm in size, or invading the duodenum or bile duct. Recognition of peripancreatic tumor spread in pathological examinations is very complicated because the pancreas has irregular lobules and fatty degeneration/replacement, both of which may hamper the reproducibility of the criteria (12). Furthermore, the majority of NENs, even when small and/or well-differentiated, protrude from the surface of the pancreas, which may lead to false classification of a small and well-marginated tumor as T3 disease according to the AJCC/UICC system, i.e., overestimation of T staging. In contrast, the ENETS system relies primarily on tumor size, which is a factor related to the malignant potential of NENs and is much more reproducible than peripancreatic fat infiltration. Indeed, a previous study of post-surgical NEN showed that the ENETS system was superior to the AJCC/UICC system as a predictor of patient survival (13). In the 7th AJCC/UICC staging system, there was significant overlap of survival between stage II and III disease, with survival in patients who had stage III disease being better in some time periods. The recently modified ENETS system has maintained the ENETS T, N, and M definitions and adopted the AJCC staging definitions, as well as demonstrated a significantly improved ability to stratify the survival outcome based on stage when compared with the 7th AJCC/UICC staging system (14,15).
These inconsistencies and limitations are addressed in the 8th edition of the AJCC/UICC staging system, which is now modified so as to be consistent with the ENETS system for well-differentiated NETs. The criterion of peripancreatic soft tissue invasion has been removed and NETs are now staged mainly on the basis of size (Table 2).
Updates in Strategies for Treatment of Pancreatic NENs
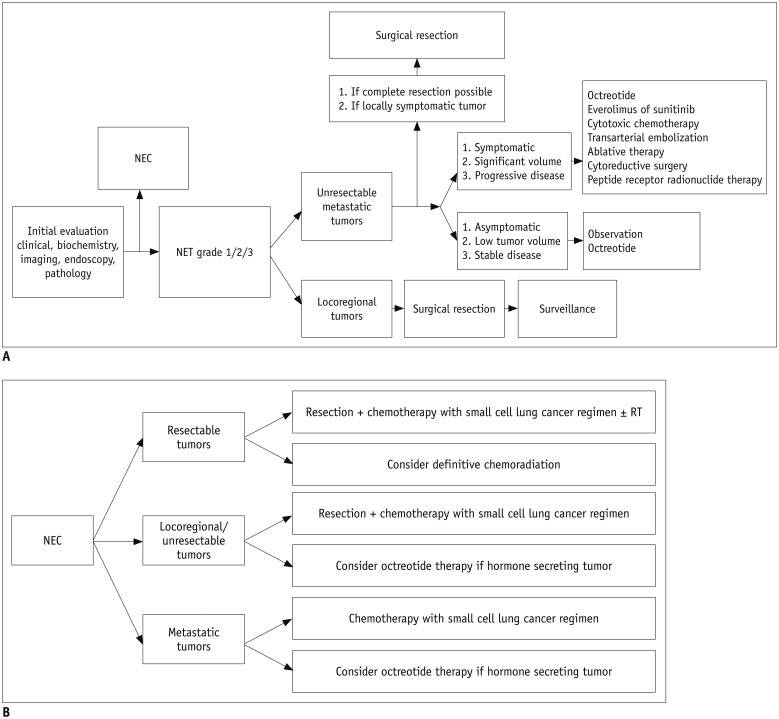
The management of NENs is complex, so assessment and treatment planning should be stepwise and focused (Fig. 1). The first important step is histologic grading and staging of the lesion, which is crucial for optimal decision-making. The management strategies, including both chemotherapy and radiation therapy, for well-differentiated grade 1/2/3 NETs are completely different from those for poorly differentiated grade 3 NEC (9,16,17,18). In the 2010 WHO classification era, the management strategies for well-differentiated grade 3 NET and poorly differentiated NEC were not clearly established. The most important update in the 2017 WHO classification is that the treatment strategies for well-differentiated grade 3 NET and poorly differentiated NEC are clearly separated (19).
Fig. 1. Stepwise management of pancreatic NENs.
Revised from 2017 National Comprehensive Cancer Network guidelines.
A. NET. B. NEC. NEC = neuroendocrine carcinomas, NENs = neuroendocrine neoplasms, NET = neuroendocrine tumor, RT = radiation therapy
Well-Differentiated Grade 1/2/3 NETs
At the time of the initial evaluation, it is important to distinguish resectable locoregional tumors from unresectable or metastatic tumors (20). Complete resection of resectable locoregional NET achieves an excellent outcome (20). The surgical methods used are determined based on the location and size of the tumor as well as its clinicopathologic features. Enucleation may be possible for small (maximum tumor size < 2 cm) peripherally located NETs other than insulinomas, although peripancreatic lymph node dissection should also be performed because of the risk of nodal metastasis (21). For large (> 2-cm) NETs or deep-seated tumors, conventional pancreatectomy should be performed with peripancreatic lymphadenectomy. According to the 2017 National Comprehensive Cancer Network guidelines, appropriate negative margins, including adjacent organs, should be obtained for nonfunctional tumors with a malignant appearance (9). For unresectable and metastatic NENs, the treatment strategy is complex and requires a multidisciplinary team approach. In patients with a resectable primary tumor and resectable metastatic disease, complete surgical resection of both, most commonly by pancreatectomy plus hepatic resection, should be considered first in order to achieve a better survival outcome (22). In patients with unresectable disease, treatment decisions should be based on symptoms, tumor burden, and evidence of disease progression. For asymptomatic, stable, low-volume disease, observation and/or treatment with octreotide is recommended until disease progression. In patients with a significant and symptomatic tumor burden or disease progression, several active treatment options can be considered, including molecular-targeted therapeutic agents such as sunitinib or everolimus (17,23), cytotoxic chemotherapy (9,17), and liver-directed therapies, including transarterial embolization, ablation, or cytoreductive surgery (20). Peptide receptor radionuclide or chemoradionuclide therapy using radionuclide tracers, e.g., 177Lu-dotatate or 90Y-dotatate, is another treatment option for patients with advanced, metastatic, and progressive NET. Most well-differentiated tumors retain high expression of somatostatin receptors that can be identified by molecular imaging and targeted therapeutically by peptide receptor radionuclide therapy. Phase II and III clinical trials of peptide receptor radionuclide therapy have achieved encouraging patient survival rates, radiographic regression, and improvement in symptoms (16,24,25).
Poorly Differentiated Grade 3 NEC
Grade 3 NEC in the pancreas has clinicopathologic features similar to those of small-cell lung cancer, including immunohistochemical findings and aggressive clinical behavior (7). Therefore, NEC is regarded as extrapulmonary small-cell or large-cell carcinoma and its management is similar to that used for small-cell lung cancer (17). A combination of platinum (cisplatin or carboplatin) and etoposide is generally recommended for NEC and is also the preferred chemotherapeutic regimen for small-cell lung cancer (26,27).
Updates Regarding Pancreatic NENs
Imaging of NENs: NETs versus NECs
The distinction between grade 1/2/3 NETs and grade 3 NEC based on imaging features is clinically relevant in view of the difference in patient management. There are a few reports on the imaging findings for NENs according to the 2010 WHO classification (28,29,30,31). These lesions show a range of imaging features depending on their histologic grading (Fig. 2).
Fig. 2. Imaging spectrum of pancreatic NENs according to histologic grade.
A. Low-grade NET is generally small-sized and has well-defined margins on imaging (arrow). B. Higher-grade NET is likely to be larger or more frequently show necrotic change (arrow). C. NEC shows less enhancement than NET, and presents as iso-enhancing or hypoenhancing mass in pancreas. Margin of NEC may be less well demarcated than that of NET (arrow).
In general, well-differentiated NETs are usually smaller than NECs. NETs are well-enhancing when compared with the adjacent pancreatic parenchyma as seen on both arterial and portal venous phase imaging, while most NECs show hypoenhancement in comparison with the adjacent pancreatic parenchyma, particularly on portal venous phase imaging (28). Two studies have investigated the dynamic enhancement pattern of NENs (30,31), and both suggest that the pattern of enhancement is correlated with prognostic factors (30,31) or fibrosis (30). In those studies, tumors that showed an “early enhancement and plateau” or “progressive enhancement” pattern tended to be associated with a worse prognosis and a higher proportion of fibrosis compared with tumors that showed an “early rapid enhancement with washout” pattern. Invasive features, including bile duct dilatation and vascular invasion, were also more common with NECs than with NETs (28).
Functional imaging studies, specifically somatostatin receptor scintigraphy (SRS) and 18F-fluorodeoxyglucose positron-emission tomography (FDG-PET), help to distinguish well-differentiated NETs from poorly differentiated NECs. SRS is based on the biological characteristics of NETs in that somatostatin receptors are highly expressed on the surface of well-differentiated NETs. Octreotide (111In pentetreotide), the most commonly used somatostatin analog, selectively binds to the expressed somatostatin receptor of a NET. Interestingly, well-differentiated NETs frequently present as strongly positive on SRS, while poorly differentiated NECs are usually negative or only weakly positive. In contrast, on FDG-PET scanning, which reflects glucose metabolism in tumors, well-differentiated NETs usually show little or no FDG uptake whereas poorly differentiated NECs show high FDG uptake (32). Based on these observations, the combination of contrast hyperenhancement on computed tomography (CT)/magnetic resonance imaging (MRI), high uptake on SRS, and low FDG avidity on FDG-PET is more suggestive of well-differentiated NET (Fig. 3). Conversely, the combination of hypoenhancement on CT/MRI, low uptake on SRS, and high FDG avidity on FDG-PET is more suggestive of poorly differentiated NEC (Fig. 4) (33).
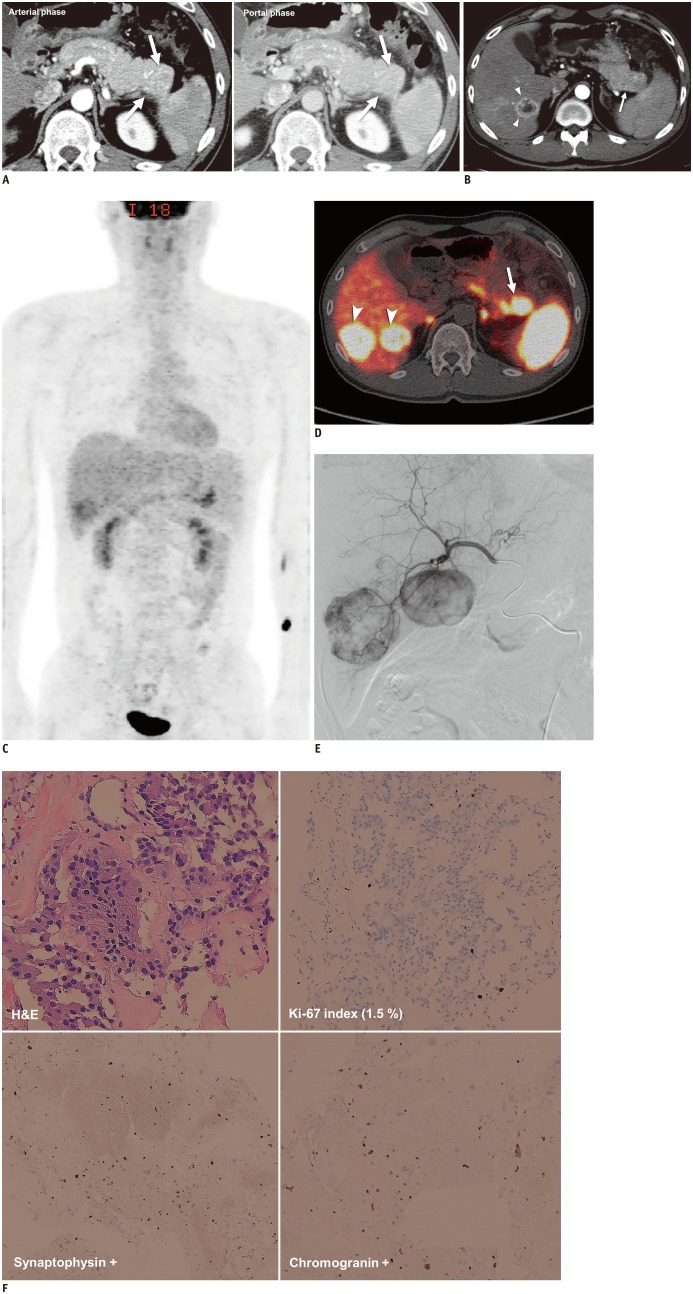
Fig. 3. Grade 1 NET in 42-year-old man.
A. 2.9-cm, arterial enhancing hypervascular mass in tail of pancreas was found on initial contrast-enhanced CT (arrows). B. After seven years, hypervascular mass in tail of pancreas had increased in size (arrow) and there was new hypervascular mass in liver (arrowheads), suggestive of hepatic metastasis. Fluid collection and peripancreatic infiltration were also present because of secondary pancreatitis. C. On 18F-FDG PET/CT, tumors showed negative or weak uptake. D. PET/CT with 68gallium-labeled somatostatin analog revealed 3.3-cm mass with markedly increased uptake (maximum standard uptake value, 40.6) in tail of pancreas (arrow), other foci in body of pancreas, and additional hepatic masses (arrowheads) with increased uptake, suggesting multifocal NET with overexpression of somatostatin receptor. Physiologic uptake was seen in both adrenal glands. E. Patient underwent transarterial embolization for management of hepatic metastases and arteriography showed hypervascular mass in right portion of liver. CT = computed tomography, FDG = fluorodeoxyglucose, PET = positron-emission tomography F. On histologic specimens (× 100) obtained during liver biopsy, tumor appeared to have relatively uniform and round nuclei with abundant cytoplasmic granules on H&E staining, low Ki-67 index (1.5%), and diffuse positivity on synaptophysin and chromogranin staining. These imaging and histologic findings suggested morphologically well-differentiated NET. H&E = hematoxylin and eosin
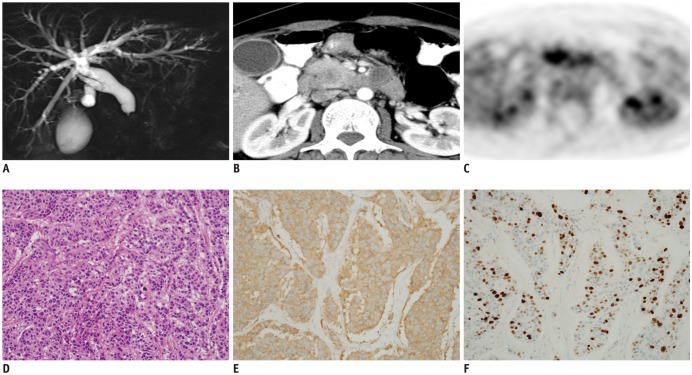
Fig. 4. Grade 3 NEC in 41-year-old woman.
This patient was referred to our hospital with tentative diagnosis of ductal carcinoma of pancreas.
A. Magnetic resonance cholangiopancreatography showed abrupt tapering of intrapancreatic common bile duct with diffuse upstream dilatation. B. Contrast-enhanced CT showed 2.5-cm, relatively well-defined, low attenuating solid mass in head of pancreas. C. Tumor showed high FDG avidity on FDG-PET. Imaging reports suggested possibility of NEC as well as ductal adenocarcinoma of pancreas, indicating need for biopsy. D. H&E staining (× 40) revealed small, round, blue cells in tumor. Immunohistochemistry specimens (× 40) showed positivity on synaptophysin (E) and Ki-67 (F) staining with Ki-67 proliferation index of 70%. These findings confirmed diagnosis of NEC.
In 2016, the US Food and Drug Administration approved the use of a new radioactive tracer, 68gallium dotatate, for PET imaging in order to localize somatostatin receptor-positive NETs. Better results have been achieved using this tracer than those obtained using conventional SRS (34,35,36) because the spatial resolution of PET/CT is better than that of single photon emission computed tomography (SPECT) and its affinity for the receptor is higher than that of octreotide.
Diagnostic Challenges
Although the diagnosis and grading of NENs is primarily determined by histologic examination of biopsy or surgical specimens, several controversies remain in which imaging may have a role. NENs are difficult to diagnose by hematoxylin and eosin staining and are usually confirmed by immunohistochemistry. However, not all pancreatic tumors are evaluated by immunohistochemistry because this is only performed in certain situations, i.e., when clinicians suspect a functioning tumor on the basis of characteristic clinical features or laboratory findings, radiologists suspect NEN on the basis of the imaging features, or pathologists suspect NEN based on the morphology of the lesion. The majority of NENs are non-functioning tumors that are detected incidentally, so it is important for radiologists to have at least a clue on imaging in order to suggest a diagnosis of NEN (37). Some NENs show atypical imaging features, such as cystic change or intraductal growth, which may impede a correct diagnosis. For example, NENs with a cystic appearance have been reported to be misdiagnosed in 43% of patients (38,39). Furthermore, it has recently been reported that serotonin-producing NETs can arise from the wall of the pancreatic duct and cause obstruction/stenosis of the duct as well as ductal dilatation upstream, thus mimicking intraductal papillary mucinous neoplasms (40). Therefore, radiologists should be aware of both the typical and atypical features of NENs (Fig. 5).
Fig. 5. Atypical imaging findings in patients with pancreatic NENs.
A. Intraductal growth in 42-year-old woman. Axial contrast-enhanced CT scan shows large mass (arrow on left) with intraductal growth causing diffuse dilatation of bile duct upstream (arrows on right). First differential diagnosis was invasive cancer arising from intraductal papillary mucinous neoplasm. However, lesion was confirmed to be grade 3 NEC after surgical resection. B. Cystic change in 48-year-old man. Axial contrast-enhanced CT image shows 5.6-cm cystic lesion with relatively thick wall in tail of pancreas. Preoperative imaging diagnosis was pseudocyst or cystic neoplasm of pancreas. However, surgical specimen confirmed that lesion was grade 1 NET with cystic change. C. Vascular invasion with tumor thrombus in 29-year-old man. Axial contrast-enhanced CT image shows large heterogeneous attenuating mass involving body and tail of pancreas and left para-aortic area (arrowhead). Vascular invasion is apparent and there is tumor thrombus extending into splenoportal confluence (arrow). Biopsy confirmed diagnosis of grade 3 NEC.
Another diagnostic challenge occurs when there is a discrepancy between the imaging features and the histology in that NENs, especially NECs, do not always show positive immunohistochemistry markers (41). In some patients diagnosed by biopsy, a small tissue sample might not be positive for immunohistochemistry markers such as chromogranin A or synaptophysin. Moreover, in some patients, the tissue sample may not be representative of the entire tumor, and the sample may underestimate the histologic grading (42). In addition, a morphology suggestive of NEC, e.g., diffuse architecture, irregular nuclei, and less cytoplasmic granularity, may not always correlate with the immunohistologic expression or histologic grading based on the Ki-67 index and mitotic index. Challenging cases are the subject of multidisciplinary discussion by pathologists, oncologists, and radiologists in order to determine the next management steps, including additional immunohistochemistry using other markers, e.g., CD56 and neuron-specific enolase, additional imaging using other modalities, and imaging-guided re-biopsy or treatment based on the imaging and clinical features. Radiologists, as a part of the multidisciplinary team, should provide feedback to oncologists and pathologists regarding the agreement or discrepancy of imaging to/from clinical and histologic findings.
Resectability
Surgery remains the only curative option for NENs, so determining resectability is very important, regardless of the histologic grade. Unlike exocrine pancreatic adenocarcinoma, potential curative resection of NENs should be considered even in patients with metastatic disease. A combined approach that includes surgery and liver ablation is also an option. In patients with NEN and metastatic disease, if it is possible to treat both the primary and metastatic disease by resection and/or ablation, aggressive treatment should be considered first. Radiologists should be knowledgeable regarding the comprehensive treatment plan and provide useful information regarding the resectability/treatability of the disease in addition to the differential diagnosis and TNM staging.
The resectability criteria for NEN and pancreatic ductal adenocarcinoma may be interpreted differently. In pancreatic ductal adenocarcinoma, which is frequently infiltrative, major vascular invasion to the celiac trunk, superior mesenteric artery, common hepatic artery, and portal vein/superior mesenteric vein is the most common cause of tumor unresectability and requires detailed preoperative evaluation. However, NENs are usually well-demarcated tumors rather than infiltrative tumors, so warrant different resectability criteria. Even the pattern of vascular invasion of NEN differs from that of ductal pancreatic adenocarcinoma. NENs tend to penetrate the vessel wall directly and locally and to project into the vessel. A NEN may occasionally form a tumor thrombus, especially in the vein (43). Therefore, at our institution, surgeons are often able to resect a tumor with vascular invasion successfully unless there is extensive tumor thrombus. From this perspective, the resectability of NENs needs to be determined less strictly preoperatively than that of ductal pancreatic adenocarcinoma (Fig. 6). Surprisingly, there are no published studies on the diagnostic accuracy of preoperative CT/MRI for determining the resectability of NENs, and this is an area that warrants further research.
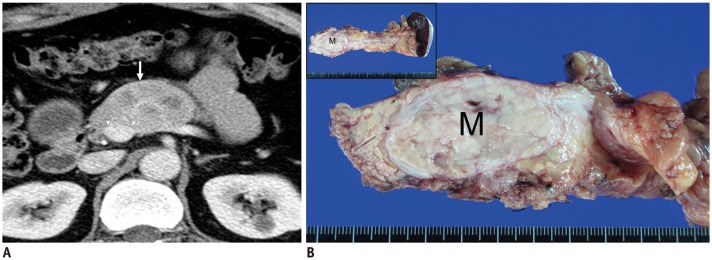
Fig. 6. Discrepancy in determining resectability between imaging and surgery.
A. Contrast-enhanced axial CT scan in 48-year-old man with NET in body of pancreas. Scan shows 5-cm mass (arrow) distortion of contour of main portal vein because of circumferential contact between tumor and portal vein of more than 180 degrees (arrowheads). These CT findings may suggest vessel invasion and indicate unresectability. B. Surgical specimen. Exploratory surgery in this patient confirmed that tumor was resectable in that main portal vein was severely compressed by tumor but without apparent vessel invasion. Therefore, distal pancreatectomy and splenectomy were performed. Gross specimen showed well-defined, ovoid soft-tissue mass (M) in body of pancreas but without vessel invasion.
Future Directions of Imaging for NENs
The imaging, surveillance, and management of NENs are continuing to develop. Novel molecular biomarkers, such as circulating tumor cells, have been identified in patients with NEN and seem to have prognostic value. The ongoing development of agents targeted to particular molecular pathways, including for vascular endothelial growth factor (sunitinib), as well as mammalian target of rapamycin inhibitors (everolimus) and tyrosine kinase inhibitors is also promising. The recent discovery that some somatostatin receptors are truncated (44,45), resulting in aberrant signaling, suggests that more detailed examination of the somatostatin receptor status in tumor tissue may offer the opportunity to customize more selective and effective treatment. In the future, a combination of complementary CT, MR, and nuclear imaging with a novel tracer should have a role in detection and grading of the primary tumor, evaluation of local invasiveness and resectability using more objective morphologic criteria, staging of distant metastasis, and prediction and evaluation of the treatment response after appropriate molecularly targeted treatment or peptide receptor radionuclide therapy. Currently, efforts are being made to differentiate the grade of NEN more accurately using the CT perfusion technique (dynamic contrast-enhanced [DCE] CT), diffusion-weighted imaging, or DCE MRI. In previously published studies, quantitative analysis of enhancement patterns and perfusion parameters using DCE-MRI has been shown to be both objective and helpful for evaluation of malignant diseases with regard to both their diagnosis and monitoring of treatment (46,47). The apparent diffusion coefficient (ADC) value seen on diffusion-weighted imaging for a NEN is moderately correlated with the WHO tumor grade (46,48). Various methods for calculating the ADC are now clinically available; in addition to these, the intravoxel incoherent motion model (49) allows for extraction of additional parameters, such as the slow component of diffusion (representing perfusion-free molecular diffusion), the fast component of diffusion (incoherent microcirculation or pseudodiffusion, representing microcapillary perfusion), and the perfusion fraction, which can provide additional information regarding tumor characteristics and changes after administration of anti-angiogenic agents. Furthermore, with dual-energy CT, by using two different energy levels, i.e., 80 kVp and 140 kVp, instead of a single-energy level as used by conventional CT, materials with different molecular compositions can be differentiated and a variety of datasets obtained, such as iodinated attenuation maps, monochromatic images at various energy levels, and virtual unenhanced images, all of which might add useful tissue information and allow better contrast of tumor images and normal tissue planes (50,51). Functional imaging such as PET-CT with a new tracer that uses an amine precursor, e.g., 18F-dihydroxy-L-phenylalanine, F-DOPA and 11C-5-hydroxy-L-tryptophan, or 11C-5-HTP to reflect the metabolic activity of the tumor and not the receptor state, might have a role in the future for evaluating the treatment response as a complementary tool which seems to be insufficient to rely on the current morphologic criteria.
CONCLUSION
We have reviewed the current concepts regarding the diagnosis and management of pancreatic NENs as well as the updates and controversies related to tumor grading, staging, and resectability. Given the complexity of the management strategies, the importance of the multidisciplinary team approach has increased. Current management systems are based on the best available evidence, although there is a need for more evidence to support best practice in several areas of ongoing controversy. Radiologists, as part of multidisciplinary teams, should be aware of the details of current management as well as the controversies that are not yet resolved so that they can provide practical and helpful information to physicians and avoid unnecessary or futile diagnostic investigations or therapeutic interventions. Future research in pancreatic NEN imaging, including morphologic and functional imaging techniques, will have a role in resolving these controversies in the future in accordance with the development of novel treatment and updates from clinical trials.
Footnotes
This study was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (No. HI18C1216) and a grant from the Asan Institute for Life Sciences, Asan Medical Center (No. 2015-0636).
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Singhi AD, Klimstra DS. Well-differentiated pancreatic neuroendocrine tumours (PanNETs) and poorly differentiated pancreatic neuroendocrine carcinomas (PanNECs): concepts, issues and a practical diagnostic approach to high-grade (G3) cases. Histopathology. 2018;72:168–177. doi: 10.1111/his.13408. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1–18. doi: 10.1016/j.ecl.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer; 2010. [Google Scholar]
- 4.Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–712. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 5.Edge S, Byrd DR, Compton CC, Fritz AG, Greene F, Trotti A. AJCC cancer staging manual. 7th ed. New York, NY: Springer; 2010. pp. 181–190. [Google Scholar]
- 6.Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, et al. all other Frascati Consensus Conference participants; European Neuroendocrine Tumor Society (ENETS) TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klimstra DS, Modlin IR, Adsay NV, Chetty R, Deshpande V, Gönen M, et al. Pathology reporting of neuroendocrine tumors: application of the Delphic consensus process to the development of a minimum pathology data set. Am J Surg Pathol. 2010;34:300–313. doi: 10.1097/PAS.0b013e3181ce1447. [DOI] [PubMed] [Google Scholar]
- 8.Klöppel G, Couvelard A, Perren A, Komminoth P, McNicol AM, Nilsson O, et al. Mallorca Consensus Conference participants; European Neuroendocrine Tumor Society. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology. 2009;90:162–166. doi: 10.1159/000182196. [DOI] [PubMed] [Google Scholar]
- 9.NCCN clinical practice guidelines in oncology. Neuroendocrine tumors. Version 3. National Comprehensive Cancer Network Web site. [Accessed December 16, 2017]. https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf Published June 13, 2017.
- 10.Lloyd RV, Osamura RY, Klöppel G, Rosai J. WHO classification of tumours of endocrine organs. 4th ed. Lyon: International Agency for Research on Cancer; 2017. pp. 209–240. [Google Scholar]
- 11.Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, et al. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol. 2015;39:683–690. doi: 10.1097/PAS.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adsay NV, Bagci P, Tajiri T, Oliva I, Ohike N, Balci S, et al. Pathologic staging of pancreatic, ampullary, biliary, and gallbladder cancers: pitfalls and practical limitations of the current AJCC/UICC TNM staging system and opportunities for improvement. Semin Diagn Pathol. 2012;29:127–141. doi: 10.1053/j.semdp.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Rindi G, Falconi M, Klersy C, Albarello L, Boninsegna L, Buchler MW, et al. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst. 2012;104:764–777. doi: 10.1093/jnci/djs208. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Gou S, Liu Z, Ye Z, Wang C. Assessment of the American Joint Commission on Cancer 8th Edition Staging System for patients with pancreatic neuroendocrine tumors: a surveillance, epidemiology, and end results analysis. Cancer Med. 2018;7:626–634. doi: 10.1002/cam4.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo G, Javed A, Strosberg JR, Jin K, Zhang Y, Liu C, et al. Modified staging classification for pancreatic neuroendocrine tumors on the basis of the American Joint Committee on Cancer and European Neuroendocrine Tumor Society systems. J Clin Oncol. 2017;35:274–280. doi: 10.1200/JCO.2016.67.8193. [DOI] [PubMed] [Google Scholar]
- 16.Bushnell DL, Jr, O'Dorisio TM, O'Dorisio MS, Menda Y, Hicks RJ, Van Cutsem E, et al. 90Y-edotreotide for metastatic carcinoid refractory to octreotide. J Clin Oncol. 2010;28:1652–1659. doi: 10.1200/JCO.2009.22.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramage JK, Ahmed A, Ardill J, Bax N, Breen DJ, Caplin ME, et al. UK and Ireland Neuroendocrine Tumour Society. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs) Gut. 2012;61:6–32. doi: 10.1136/gutjnl-2011-300831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reid MD, Balci S, Saka B, Adsay NV. Neuroendocrine tumors of the pancreas: current concepts and controversies. Endocr Pathol. 2014;25:65–79. doi: 10.1007/s12022-013-9295-2. [DOI] [PubMed] [Google Scholar]
- 19.Raj N, Valentino E, Capanu M, Tang LH, Basturk O, Untch BR, et al. Treatment response and outcomes of grade 3 pancreatic neuroendocrine neoplasms based on morphology: well differentiated versus poorly differentiated. Pancreas. 2017;46:296–301. doi: 10.1097/MPA.0000000000000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burns WR, Edil BH. Neuroendocrine pancreatic tumors: guidelines for management and update. Curr Treat Options Oncol. 2012;13:24–34. doi: 10.1007/s11864-011-0172-2. [DOI] [PubMed] [Google Scholar]
- 21.Parekh JR, Wang SC, Bergsland EK, Venook AP, Warren RS, Kim GE, et al. Lymph node sampling rates and predictors of nodal metastasis in pancreatic neuroendocrine tumor resections: the UCSF experience with 149 patients. Pancreas. 2012;41:840–844. doi: 10.1097/MPA.0b013e31823cdaa0. [DOI] [PubMed] [Google Scholar]
- 22.Hill JS, McPhee JT, McDade TP, Zhou Z, Sullivan ME, Whalen GF, et al. Pancreatic neuroendocrine tumors: the impact of surgical resection on survival. Cancer. 2009;115:741–751. doi: 10.1002/cncr.24065. [DOI] [PubMed] [Google Scholar]
- 23.Kim KW, Krajewski KM, Nishino M, Jagannathan JP, Shinagare AB, Tirumani SH, et al. Update on the management of gastroenteropancreatic neuroendocrine tumors with emphasis on the role of imaging. AJR Am J Roentgenol. 2013;201:811–824. doi: 10.2214/AJR.12.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. NETTER-1 Trial Investigators. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villard L, Romer A, Marincek N, Brunner P, Koller MT, Schindler C, et al. Cohort study of somatostatin-based radiopeptide therapy with [(90)Y-DOTA]-TOC versus [(90)Y-DOTA]-TOC plus [(177)Lu-DOTA]-TOC in neuroendocrine cancers. J Clin Oncol. 2012;30:1100–1106. doi: 10.1200/JCO.2011.37.2151. [DOI] [PubMed] [Google Scholar]
- 26.Terashima T, Morizane C, Hiraoka N, Tsuda H, Tamura T, Shimada Y, et al. Comparison of chemotherapeutic treatment outcomes of advanced extrapulmonary neuroendocrine carcinomas and advanced small-cell lung carcinoma. Neuroendocrinology. 2012;96:324–332. doi: 10.1159/000338794. [DOI] [PubMed] [Google Scholar]
- 27.Sundstrøm S, Bremnes RM, Kaasa S, Aasebø U, Hatlevoll R, Dahle R, et al. Norwegian Lung Cancer Study Group. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years' follow-up. J Clin Oncol. 2002;20:4665–4672. doi: 10.1200/JCO.2002.12.111. [DOI] [PubMed] [Google Scholar]
- 28.Kim DW, Kim HJ, Kim KW, Byun JH, Song KB, Kim JH, et al. Neuroendocrine neoplasms of the pancreas at dynamic enhanced CT: comparison between grade 3 neuroendocrine carcinoma and grade 1/2 neuroendocrine tumour. Eur Radiol. 2015;25:1375–1383. doi: 10.1007/s00330-014-3532-z. [DOI] [PubMed] [Google Scholar]
- 29.Takumi K, Fukukura Y, Higashi M, Ideue J, Umanodan T, Hakamada H, et al. Pancreatic neuroendocrine tumors: correlation between the contrast-enhanced computed tomography features and the pathological tumor grade. Eur J Radiol. 2015;84:1436–1443. doi: 10.1016/j.ejrad.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Kim C, Byun JH, Hong SM, An S, Kim JH, Lee SS, et al. A comparison of enhancement patterns on dynamic enhanced CT and survival between patients with pancreatic neuroendocrine tumors with and without intratumoral fibrosis. Abdom Radiol (NY) 2017;42:2835–2842. doi: 10.1007/s00261-017-1212-6. [DOI] [PubMed] [Google Scholar]
- 31.Cappelli C, Boggi U, Mazzeo S, Cervelli R, Campani D, Funel N, et al. Contrast enhancement pattern on multidetector CT predicts malignancy in pancreatic endocrine tumours. Eur Radiol. 2015;25:751–759. doi: 10.1007/s00330-014-3485-2. [DOI] [PubMed] [Google Scholar]
- 32.Rust E, Hubele F, Marzano E, Goichot B, Pessaux P, Kurtz JE, et al. Nuclear medicine imaging of gastro-entero-pancreatic neuroendocrine tumors. The key role of cellular differentiation and tumor grade: from theory to clinical practice. Cancer Imaging. 2012;12:173–184. doi: 10.1102/1470-7330.2012.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis RB, Lattin GE, Jr, Paal E. Pancreatic endocrine tumors: radiologic-clinicopathologic correlation. Radiographics. 2010;30:1445–1464. doi: 10.1148/rg.306105523. [DOI] [PubMed] [Google Scholar]
- 34.Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–518. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 35.Haug AR, Auernhammer CJ, Wängler B, Schmidt GP, Uebleis C, Göke B, et al. 68Ga-DOTATATE PET/CT for the early prediction of response to somatostatin receptor-mediated radionuclide therapy in patients with well-differentiated neuroendocrine tumors. J Nucl Med. 2010;51:1349–1356. doi: 10.2967/jnumed.110.075002. [DOI] [PubMed] [Google Scholar]
- 36.Sharma P, Singh H, Bal C, Kumar R. PET/CT imaging of neuroendocrine tumors with (68)Gallium-labeled somatostatin analogues: an overview and single institutional experience from India. Indian J Nucl Med. 2014;29:2–12. doi: 10.4103/0972-3919.125760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallotti A, Johnston RP, Bonaffini PA, Ingkakul T, Deshpande V, Fernández-del Castillo C, et al. Incidental neuroendocrine tumors of the pancreas: MDCT findings and features of malignancy. AJR Am J Roentgenol. 2013;200:355–362. doi: 10.2214/AJR.11.8037. [DOI] [PubMed] [Google Scholar]
- 38.Singhi AD, Chu LC, Tatsas AD, Shi C, Ellison TA, Fishman EK, et al. Cystic pancreatic neuroendocrine tumors: a clinicopathologic study. Am J Surg Pathol. 2012;36:1666–1673. doi: 10.1097/PAS.0b013e31826a0048. [DOI] [PubMed] [Google Scholar]
- 39.Lee JH, Byun JH, Kim JH, Lee SS, Kim HJ, Lee MG. Solid pancreatic tumors with unilocular cyst-like appearance on CT: differentiation from unilocular cystic tumors using CT. Korean J Radiol. 2014;15:704–711. doi: 10.3348/kjr.2014.15.6.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi C, Siegelman SS, Kawamoto S, Wolfgang CL, Schulick RD, Maitra A, et al. Pancreatic duct stenosis secondary to small endocrine neoplasms: a manifestation of serotonin production? Radiology. 2010;257:107–114. doi: 10.1148/radiol.10100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lam KY, Lo CY. Pancreatic endocrine tumour: a 22-year clinico-pathological experience with morphological, immunohistochemical observation and a review of the literature. Eur J Surg Oncol. 1997;23:36–42. doi: 10.1016/s0748-7983(97)80140-0. [DOI] [PubMed] [Google Scholar]
- 42.Volante M, Righi L, Berruti A, Rindi G, Papotti M. The pathological diagnosis of neuroendocrine tumors: common questions and tentative answers. Virchows Arch. 2011;458:393–402. doi: 10.1007/s00428-011-1060-7. [DOI] [PubMed] [Google Scholar]
- 43.Balachandran A, Tamm EP, Bhosale PR, Katz MH, Fleming JB, Yao JC, et al. Venous tumor thrombus in nonfunctional pancreatic neuroendocrine tumors. AJR Am J Roentgenol. 2012;199:602–608. doi: 10.2214/AJR.11.7058. [DOI] [PubMed] [Google Scholar]
- 44.Kulke MH, Siu LL, Tepper JE, Fisher G, Jaffe D, Haller DG, et al. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute neuroendocrine tumor clinical trials planning meeting. J Clin Oncol. 2011;29:934–943. doi: 10.1200/JCO.2010.33.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oberg K, Casanovas O, Castaño JP, Chung D, Delle Fave G, Denèfle P, et al. Molecular pathogenesis of neuroendocrine tumors: implications for current and future therapeutic approaches. Clin Cancer Res. 2013;19:2842–2849. doi: 10.1158/1078-0432.CCR-12-3458. [DOI] [PubMed] [Google Scholar]
- 46.Kim JH, Lee JM, Park JH, Kim SC, Joo I, Han JK, et al. Solid pancreatic lesions: characterization by using timing bolus dynamic contrast-enhanced MR imaging assessment--a preliminary study. Radiology. 2013;266:185–196. doi: 10.1148/radiol.12120111. [DOI] [PubMed] [Google Scholar]
- 47.Bali MA, Metens T, Denolin V, Delhaye M, Demetter P, Closset J, et al. Tumoral and nontumoral pancreas: correlation between quantitative dynamic contrast-enhanced MR imaging and histopathologic parameters. Radiology. 2011;261:456–466. doi: 10.1148/radiol.11103515. [DOI] [PubMed] [Google Scholar]
- 48.Hwang EJ, Lee JM, Yoon JH, Kim JH, Han JK, Choi BI, et al. Intravoxel incoherent motion diffusion-weighted imaging of pancreatic neuroendocrine tumors: prediction of the histologic grade using pure diffusion coefficient and tumor size. Invest Radiol. 2014;49:396–402. doi: 10.1097/RLI.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 49.Koh DM, Collins DJ, Orton MR. Intravoxel incoherent motion in body diffusion-weighted MRI: reality and challenges. AJR Am J Roentgenol. 2011;196:1351–1361. doi: 10.2214/AJR.10.5515. [DOI] [PubMed] [Google Scholar]
- 50.De Cecco CN, Darnell A, Rengo M, Muscogiuri G, Bellini D, Ayuso C, et al. Dual-energy CT: oncologic applications. AJR Am J Roentgenol. 2012;199(5 Suppl):S98–S105. doi: 10.2214/AJR.12.9207. [DOI] [PubMed] [Google Scholar]
- 51.Kartalis N, Mucelli RM, Sundin A. Recent developments in imaging of pancreatic neuroendocrine tumors. Ann Gastroenterol. 2015;28:193–202. [PMC free article] [PubMed] [Google Scholar]