Abstract
Objectives
The purpose of this study is to shorten the decellularization time of trachea by using combination of physical, chemical, and enzymatic techniques.
Methods
Approximately 3.5-cm-long tracheal segments from 42 New Zealand rabbits (3.5±0.5 kg) were separated into seven groups according to decellularization protocols. After decellularization, cellular regions, matrix and strength and endurance of the scaffold were followed up.
Results
DNA content in all groups was measured under 50 ng/mg and there was no significant difference for the glycosaminoglycan content between group 3 (lyophilization+deoxycholic acid+de-oxyribonuclease method) and control group (P=0.46). None of the decellularized groups was different than the normal trachea in tensile stress values (P>0.05). Glucose consumption and lactic acid levels measured from supernatants of all decellularized groups were close to group with cells only (76 mg/dL and 53 mg/L).
Conclusion
Using combination methods may reduce exposure to chemicals, prevent the excessive influence of the matrix, and shorten the decellularization time.
Keywords: Trachea, Scaffold, Freeze Drying, Tissue Engineering, Deoxycholic Acid
INTRODUCTION
Using conventional techniques such as end to end trachea anastomosis, it is not possible to reconstruct a functioning trachea that had lost more than half of its length as a result of stenosis, infection, cancer or congenital anomalies. Although trachea transplantation is technically possible, its clinical applications are limited because of two-stage surgery and the need for a life-long immune suppression use [1]. Tissue engineering that is used in many cases of organ failure has recently started to come into question for trachea reconstruction as well [2].
The choice of scaffold on which the cells will be seeded is as important as the choice of stem cells in tissue engineering. Cadaveric trachea is a good option for reconstruction because of its three-dimensional structure, biomechanical properties, flexibility, air-tightness and endurance to collapse [3]. Decellularized trachea is the best scaffold up to day, due to its non-immunological extracellular matrix (ECM) that does not carry major histocompatibility complex class I (MHC-I) and class II (MHC-II) and its pro-angiogenic properties [4]. At the same time, retain matrices provide mechanical and chemical signals to aid stem cell differentiation [5] and regeneration without additional biological additives [6].
Effective purification of an organ from its cells depends on the origin of the would-be decellularized tissue and the method to be used. Cartilage tissue decellularization is equally challenging. The dense ECM makes full decellularization difficult due to limitations in diffusion [7]. The tissue is often mechanically disrupted to increase the efficacy of chemical decellularization; thereupon mechanical properties of the matrix are deteriorated [8]. The dense nature of the cartilage also restricts cell migration into the matrix [9]. On the other hand, physically devitalized cartilage particles do not exhibit a chondrogenic response to the extent that chemically decellularized cartilage and have greater down regulation of collagen [10].
The previous studies mostly employed detergent enzymatic method (DEM) and its modifications for the purpose of maintaining the structural integrity of trachea [1,11-13] in contrast to chemical fixation, cryopreservation, or lyophilization [12]. In this study, we investigated the effects of lyophilization and DEM combinations on the structure, composition and biocompatibility of tracheal matrix for the purpose of preventing the effects of detergent on the matrix by reducing duration of exposure to chemicals and for shortening decellularization time. And we tried to decide which chemicals can be combined with lyophilization method.
MATERIALS AND METHODS
The study was conducted in Acibadem Labcell Laboratories with the approval of Local Ethical Board for Animal Studies. Approximately 3.5-cm-long tracheas from 42 New Zealand rabbits (3.5±0.5 kg) were separated into seven groups each containing six tracheas. The tracheas in the first six groups were decellularized using six different methods. The seventh group was control that contained untreated tracheal segments. During the removal of tracheas from rabbits, a 2-cm incision was made to the inguinal area reaching the adipose pads and 100 mg of fat tissue was removed for the isolation of stem cells.
Tissue follow-up and decellularization
Rabbit tracheal segments were transported via cold-chain in phosphate buffered saline (PBS; Biological Industries, Beit Haemek, Israel) containing penicillin/streptomycin (Biological Industries)/gentamicine (I.E Ulugay, Istanbul, Turkey)/Fungizone (Bristol-Myers Squibb, New York, NY, USA) and were brought to Acibadem Labcell Laboratories and washed. Tracheas stored at 2°C–8°C were washed in 1% povidone iodine (Kimpa Ilac, Istanbul, Turkey) containing PBS for 5 minutes and later washed twice in 1% penicillin/streptomycin/gentamicine/Fungizone containing PBS in order to get rid of povidone iodine. This process was repeated twice. Washed tracheas were randomly separated into six groups to start decellularization processes.
After freezing all samples at –80°C for 4 hours, Lyophilizator (FreeZone 2.5 Liter Benchtop Freeze Dry System; Labconco, Kansas City, MO, USA) cabin temperature was decreased to –50°C and vacuuming was initiated. Vacuuming process was performed for 24 hours. Decellularization protocols summarized in Table 1.
Table 1.
Decellularization protocols
| Group | Decellularization protocol |
|---|---|
| Group 1 | LYP |
| Group 2 | LYP+DNase (150 U/mL)+MgSO4 (50 mmol)+LYP |
| Group 3 | LYP+deoxycholic acid+DNase+MgSO4+LYP |
| Group 4 | LYP+deoxycholic acid+Triton X-100+DNase+MgSO4+LYP |
| Group 5 | LYP+SDS+1% (w/v) Triton X-100+LYP |
| Group 6 | LYP+SDS+PBS+DNase+LYP |
| Group 7 | Control:untreated trachea |
Approximately 3.5-cm-long tracheas from 42 New Zealand rabbits were separated into seven groups each containing six tracheas.
LYP, lyophilization; DNase, deoxyribonuclease; SDS, sodium dodecyl sulfate; PBS, phosphate buffered saline.
Group 1 (n=6)
Tracheas were processed with lyophilization for 24 hours only.
Group 2 (n=6)
After 24 hours of lyophilization, tracheas were washed for 2 hours at 37°C in deoxyribonuclease (DNase; Roche Diagnostic, Indianapolis, IN, USA; 150 U/mL)+MgSO4 (Galen Ilac, Istanbul, Turkey; 50 mmol) containing physiological saline solution (PSS; Eczacıbası Baxter, Istanbul, Turkey) and later washed for 2 hours at 4°C in antibiotic containing cell culture medium. Tracheas were then processed with lyophilization overnight and obtained decellularized tracheas were stored at –20°C.
Group 3 (n=6)
Tracheas were processed for 24 hours using lyophilization method, incubated for 4 hours at 37°C in 0.25% deoxycholic acid (Sigma Aldrich, St. Louis, MO, USA) containing PSS and washed for 2 hours at 4°C in 1% penicillin/streptomycin/gentamicine/Fungizone containing cell culture medium (Biological Industries; antibiotic containing cell culture medium). Washed tracheas were treated in PSS containing DNase (150 U/mL)+ MgSO4 (50 mmol) at 37°C for 3 hours and then washed overnight at 4°C in antibiotic containing cell culture medium again. After repeating the lyophilization process overnight, decellularized tracheas were stored at –20°C.
Group 4 (n=6)
Tracheas were processed for 24 hours using lyophilization method and treated for 4 hours at 37°C in 0.25% Triton X-100 (Sigma Aldrich)+0.25% deoxycholic acid+DNase (150 U/mL)+ MgSO4 (50 mmol) containing PSS. After 4 hours of washing at 4°C in antibiotic containing cell culture medium, lyophilization process was performed overnight and decellularized tracheas were stored at –20°C.
Group 5 (n=6)
After 24 hours of lyophilization process, tracheas were treated with 0.01% (w/v, weight to volume) sodium dodecyl sulfate (SDS; Merck Millipore, Darmstadt, Germany) containing distilled water for 5 minutes. Tracheas were washed with PSS once and the process was repeated three times with changing time periods to 10, 15, and 20 minutes. After treatment with 0.01% (w/v) and 0.1% (w/v) SDS for 24 hours each, 0.2% and 0.1% SDS was used for 3 hours of treatment each. The 1% (w/v) Triton X-100 containing distilled water was used for removing nucleic acids for 30 minutes and the tracheas were washed for 2 hours in PSS. After the washing step, tissue graft was processed with lyophilization for 24 hours and decellularized tracheas were stored at –20°C.
Group 6 (n=6)
After 24 hours of lyophilization process, tracheas were treated with 0.01% (w/v) SDS containing distilled water for 5 minutes. Tracheas were washed in PSS once and the treatment was repeated three times with changing time periods to 10, 15, and 20 minutes. Later, tracheas were perfused with PBS for 1 hour. Decellularized tissue was washed in 1% penicillin/streptomycin/gentamicine/Fungizone and 2,000 U DNase containing PSS for 24 hours. After the washing step, tissue graft was processed with lyophilization for 24 hours. Decellularized tracheas were stored at –20°C.
Evaluation of decellularization
Measurement of DNA concentration
DNA isolation was performed from 0.007 g pieces of decellularized, lyophilized tracheas using QIAamp DNA Mini Kit (Qiagen, Venlo, the Netherlands). The concentration and purity of isolated DNA were measured using NanoPhotometer Pearl device (Implen, Westlake Village, CA, USA). DNA samples to be measured were put into quartz cuvettes to minimize light reflection and measurements were done in nanophotometer. At the end of the measurement, total DNA concentration in tissue was calculated in terms of nanograms. DNA purity was calculated at 260 nm/280 nm wavelength range.
Glycosaminoglycan assay
Pieces from decellularized, lyophilized tracheas were obtained and treated with collagenase (Sigma, St. Louis, MO, USA) for 3 hours to fully dissolve the tissue. At the end of this process, to inhibit the collagenase activity, 100 μL human serum was added onto the suspension and vortexed. Onto 100 μL sample from this suspension, 1,000 μL dimethylene blue (Sigma) was added and was analyzed at 520 nm in Synergy HT Multi-Detection Microplate Reader (BioTek, Winooski, VT, USA) device.
Histopathological analysis
Paraffin blocks were prepared from samples and put in 10% formaldehyde solution (Merck, Darmstadt, Germany). The 5 μm sections were stained with hematoxylin and eosin (H&E; Merck), reticulin (Merck), alcian blue (Merck) and toluidine blue (Merck) and examined under light microscope for number and quality of epithelial and mesenchymal components. During the examination, ciliated surface epithelia was recognized as epithelial component, basal membrane under the epithelia, collagen fibers, vessels and fibroblasts were recognized as primary mesenchymal zone and hyaline cartilage and chondroid matrix were recognized as secondary mesenchymal zone.
Scanning electron microscopy
Specimens of trachea were fixed by immersion in 4% glutaraldehyde (Merck) in 0.1 M Sorensen phosphate buffer solution (SPBS; pH 7.2) for 4 hours and post-fixed in 1% SPBS buffered osmium tetroxide (OsO4) for 2 hours. Samples were dehydrated in an ascending graded ethanol series and were embedded in Araldite CY212 (Merck). Semithin sections were prepared using an ultramicrotome (Ultratome NOVA-LKB), counterstained with 1% toluidine blue, and examined with a Zeiss Axioplan light microscope (Jena, Germany). To qualitatively evaluate decellularized matrix structure, matrices were fixed with 4% (v/v) glutaraldehyde in a buffered solution of 0.1 M SPBS (pH 7.2). After rinsing in Sorensen phosphate, specimens were dehydrated thr-ough an ethanol gradient, critical point dried (Polaron, E3000; Quorum Technologies, West Sussex, UK), sputter coated (Polaron, SC7620) with gold and analyzed using Zeiss LEO 1430 sca-nning electron microscope (SEM; Oberkochen, Germany).
Biomechanical tests
Four tracheal segments from each of the six groups and six fresh tracheas obtained from rabbits sacrificed on the day of the test were vertically cut to two; they were shaped as a dog bone and were bound to the universal test device using custom made hooks for soft tissue. A total of 60 samples were prepared accordingly for the test. Biomechanical tests were performed in Faculty of Mechanical Engineering Biomechanical Laboratory of Istanbul Technical University using MTS 858 Mini Bionix II universal test device (model: 359.XX, part no: 100-146-714, rev: A, serial no: 10189576; Eden Prairie, MN, USA). Designing a loading scenario, an axial pulling test program was developed. Pulling experiments were performed with ESIT Load Cell (serial no: 592, model: SPA-10 kg, output: 2.0 mV/V; Istanbul, Turkey). During the pulling experiment the velocity of the mobile jaw of the test device was 15 mm/min and sampling rate was 10 Hz. Time, axial displacement and axial force values were recorded during the pulling experiment. The experiments were continued until the samples were damaged. Load-displacement curves were plotted using Excel (Microsoft, Redmond, WA, USA) and MATLAB (MathWorks, Natick, MA, USA) programs (Fig. 1). Elasticity modules were calculated for each sample using the loads and tensions at the time of breaking from these curves.
Fig. 1.
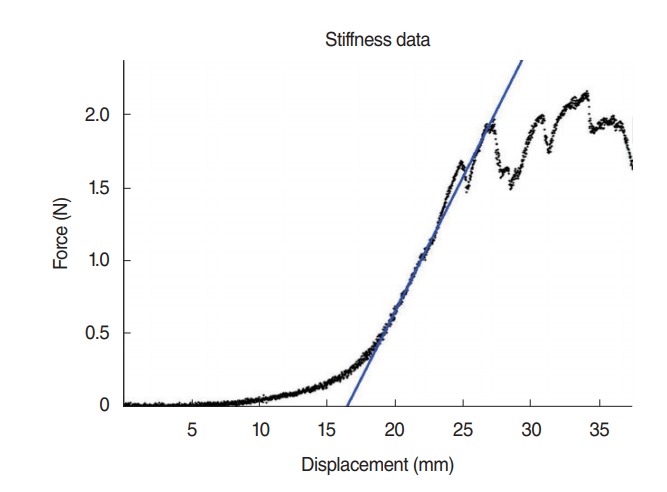
Yield load force testing. Graph shows a representative force (N) versus displacement (mm) profile obtained from yield load testing. As outward displacement (tracheal rupture) proceeds, the force increases in linear fashion until tissue failure. The yield load was calculated using a fixed displacement offset (blue line) measured from the point where the load-displacement curve (black line) transitions from the linear response region to enter the plateau region.
Vitality test
Autologous cell isolation and culture
The 100 mg of rabbit adipose tissue samples were carried to the laboratory in sterile transfer medium. Adipose tissue was deprived of transfer medium, transferred into petri dishes and washed three times with PSS for mesenchymal stem cell production. After the removal of PSS, adipose tissue pieces were incubated in 0.075% type 2 collagenase containing 3 mL of PBS at 37°C for 2 hours. At the end of 3 hours, the Falcon tube was centrifuged at 800 g until the adipocytes were removed.
Obtained cells were seeded into T-150 flasks containing 1% penicillin/streptomycin, 10% fetal bovine serum (FBS; HyClone, Thermo Scientific, Waltham, MA, USA) containing DMEM-LG (Biological Industries). The culture medium was refreshed every 3 days and the cells were cultured until 70% density was reached in the flasks. Following primary culture, the cells were detached using trypsin (Biological Industries), cultured in the same medium and the cells collected from the first passage by trypsinization were resuspended in 1% penicillin/streptomycin, 10% FBS containing DMEM-LG.
Seeding cells onto decellularized trachea
Equal sizes of tissue samples taken from decellularized tracheas from each group were put on 12-well plates. Each tissue sample was seeded with a density of 1×106 cells and 1% penicillin/streptomycin, 10% FBS containing DMEM-LG was added onto the cells.
Vitality test
Twenty-four hours after refreshing the medium on day 3, glucose and lactic acid levels were examined in the supernatants using Integra (Zizers, Switzerland) and Cobas 8000 (Roche, Basel, Switzerland) systems. The experiments were repeated three times and the average of these three trials were used for analyses.
Statistical analyses
IBM SPSS ver. 19.0 (IBM Corp., Armonk, NY, USA) was used for statistical analyses. Definitive statistics were given as average and standard deviation for numerical variables. For comparison of variables Mann-Whitney U-test was used as a nonparametric test with Bonferroni rectification. Statistical significance was accepted at P<0.05.
RESULTS
Evaluation of decellularization
DNA concentration
DNA content of tracheal tissues in all methods were observed to be under 50 ng/mg which is the decellularization criteria of the tissue given by Gilbert [14] (Table 2).
Table 2.
DNA and GAG content of decellularized cartilage matrix
| Group | Control | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 |
|---|---|---|---|---|---|---|---|
| DNA content/dry weight (ng/mg) | - | 5.15 | 24.28 | 1.64 | 5.67 | 10.32 | 15.85 |
| GAG content/wet weight (µg/mg) | 16.37 | 5.51 | 6.84 | 13.82 | 4.87 | 11.11 | 6.25 |
GAG, glycosaminoglycan.
Glycosaminoglycan assay
Glycosaminoglycan (GAG) content of the cartilage matrix of the group 1, 2, 4, 5, and 6 was decreased significantly after decellularization (P<0.001, P<0.001, P<0.001, P=0.01, P< 0.001, respectively) but there was no significant difference between GAG content in the cartilage matrix of group 3 and control untreated trachea (P=0.46) (Table 2).
Histopathological analysis
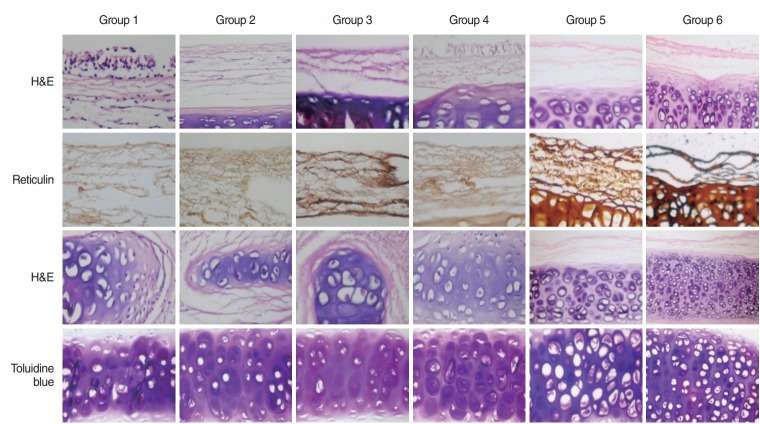
Epithelial structure and many cellular components in the primary mesenchymal zone were visible in group 1 decellularization technique as shown in H&E stained sections, although not as clear, this is also the case for groups 4 and 6. Vascular structures and cellular remnants were visible in primary mesenchymal zone of groups 2 and 4. Reticulin stained sections revealed that the basal membrane structure was preserved in all groups, whereas collagen fibers loosen in group 6. H&E and toluidine blue stained sections showed that matrix structure was generally preserved; however, in groups 1 and 5 the morphological cellular structures were lost maximally (Fig. 2).
Fig. 2.
Sections of decellularized tracheas from groups 1–6. First two rows depict H&E and reticulin staining of epithelial and primary mesenchymal zones (basal membrane, collagen fibers, vesicles, and fibroblasts); third and fourth rows depict H&E and toluidine blue staining of secondary mesenchymal zone (matrix). Magnification, ×200, respectively.
Scanning electron microscopy
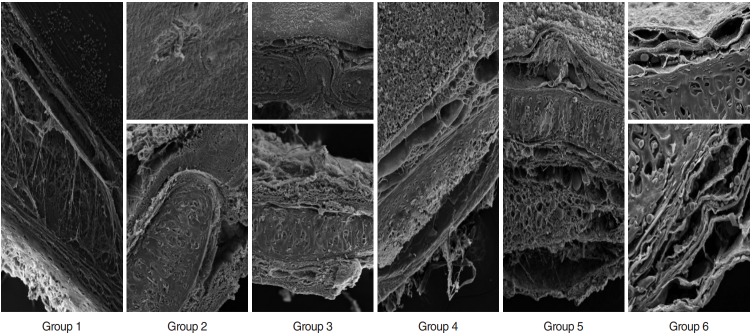
In scanning electron microscopy, no cellular components were observed on the luminal surface of groups 2 and 6, whereas other groups contained a cellular layer on the surface. Basal membrane of group 2 was denticulated on the surface, but it was preserved normally in other groups. Collagen structure in mesenchymal zone was rather compact in groups 2, 3, and 5, whereas it was loose and fibrous in groups 1, 4, and 6. Except for group 1, this zone contained cellular components in all groups. Matrices (territorial and inter-territorial matrices) were preserved in groups 1, 3, and 5 with deformed cells; however, isogenic groups were deformed in groups 2 and 4, no cells were observed in group 2 and preserved cartilage matrix and cells were observed in group 6 (Fig. 3).
Fig. 3.
Scanning electron microscopy examination of sections from decellularized tracheas of groups 1–6. Group 1: deformed cells on luminal surface, preserved basal membrain (BM), dispersed collagen fibers; preserved matrix, deformed chondrocytes (magnification, ×500). Group 2: no cells on luminal surface; affected BM, compact collagens, deformed isogenic groups, acellular (magnification, ×350 and ×600). Group 3: cellular remnants are observed on luminal surface; BM, collagen integrity, and cartilage matrix are preserved, but chondrocytes are deformed (magnification, ×350 and ×500). Group 4: large deformed cell layer on luminal surface; preserved BM, separation in collagen fibers, deformed isogenic groups and chondrocytes (magnification, ×350). Group 5: luminal surface covered with deformed cells; preserved BM, collagen fibers and matrix, deformed chondrocytes (magnification, ×350). Group 6: no cells on luminal surface; preserved BM, loose collagens; preserved matrix and chondrocytes (magnification, ×1.45 K).
Biomechanical tests
After the decellularization process, there were no changes or collapse in the tubular structure of the trachea. Table 3 shows the average breaking force of the decellularized tracheas as experimented using a dog bone model in tension test. However, the diameters and thicknesses of the tracheas were different from each other, thus the forces were not used for comparison. The average values of the rupture force at the breaking point which shows how much weight can the trachea resist, also called the tensile strength, and the rigidity which shows how enduring and firm the trachea is, also called the elasticity module, were compared with normal trachea as shown in Table 3. According to this, none of the groups had a tensile strength different than the normal trachea (P>0.05 for all groups), but the rigidities of groups 1, 3, and 5 were higher than normal (P=0.003, P=0.021, and P=0.011, respectively).
Table 3.
Tension properties of normal trachea and decellularized tracheal segments
| Variable | Control (n=12) | Group 1 (n=8) | Group 2 (n=8) | Group 3 (n=8) | Group 4 (n=8) | Group 5 (n=8) | Group 6 (n=8) |
|---|---|---|---|---|---|---|---|
| Rupture force (N) | 2.16±0.53 | 3.09±0.80 | 2.64±0.73 | 3.08±0.83 | 2.40±0.56 | 2.95±0.62 | 2.96±0.53 |
| Stress (MPa) | 0.96±0.23 | 0.93±0.12 | 0.83±0.13 | 0.91±0.21 | 0.81±0.13 | 0.85±0.24 | 0.94±0.23 |
| Elasticity module-rigidity (N/mm) | 0.24±0.09 | 0.45±0.18 | 0.35±0.14 | 0.41±0.18 | 0.30±0.13 | 0.33±0.05 | 0.30±0.08 |
Values are presented as mean±standard deviation.
Vitality test
For the examination of the vitality of seeded adipose-derived stem cells on decellularized trachea, 24 hours after refreshing the culture medium on day 3, samples from supernatants were analyzed for glucose consumption. Glucose consumption of all groups (after stem cell seeding) were similar to the group with cells only (76 mg/dL), whereas the glucose level in empty trachea sample remained highest (101 mg/dL) as no consumption was expected. There were no significant differences between glucose level of cell only supernatants and all cell seeded decellularized tracheal groups (P=0.4, P=0.58, P=1, P=0.07, P= 0.06, and P=1, respectively) and there was significant difference between glucose level of empty trachea sample supernatants and all cell seeded decellularized tracheal groups (P=0.011, P= 0.012, P=0.02, P=0.03, P=0.03, and P=0.04, respectively). Lactic acid, which is the metabolite of anaerobic glycolysis, was measured to be 23.5 mg/L in cell-free medium, but as lactic acid levels of all groups (after stem cell seeding) were similar to the only cell containing medium (53 mg/L), this suggests lactic acid was produced in all groups. There was no significant difference between lactose level of cell only supernatants and all cell seeded decellularized tracheal groups (P=0.1, P=0.51, P=0.69, P=0.07, P=0.08, and P=0.65, respectively).
DISCUSSION
Tissue engineering studies generally involve cells that make up the relevant tissue, the structure that these cells will attach to (scaffold-matrix) and growth factors that control cellular activities [15]. As the mesenchymal stem cells are multipotent, they are able to differentiate into adipocytes, chondrocytes, osteocytes, and endothelial cells [16]. Thus, a failed organ can be reconstructed in vivo with tissue engineering by using the mesenchymal stem cells of the patient and placing them to the failed area on a proper tissue scaffold.
An ideal scaffold needs to be three-dimensional and biocompatible and also requires proper mechanical and physical properties that allow cellular attachment, reproduction and differentiation [9]. Decellularization of trachea results in loss of all cellular and nuclear materials (becomes nonimmunogenic and the recipient does not receive any immune-suppressive medication) without losing its supportive scaffold properties. During the process, preserving structural contents such as ECM and basal membrain (BM) provides attachment and proliferation of cells and protects the scaffold’s morphological and biomechanical properties. Also, preserving basic fibroblast growth factor and transforming growth factor-β [17] which affect angiogenesis in ECM increases chondrogenesis (differentiation signal for MSCs into adult cartilage) and provides a living and functional trachea [4,9,18].
Optimal effectiveness in decellularization can be achieved by removing maximum amount of cellular components with minimum damage and loss of ECM [14]. Decellularization methods can be categorized into physical, chemical, and enzymatic techniques, as well as their combinations. The decellularization method to be used depends on the properties of the tissue [14]. Physical methods such as thermal shock, ultrasonic, and mechanical breaking cause break-up of cell membrane and remove cellular components from the tissue with agitation and perfusion [14,19]. In chemical methods, detergents, solvents, acidic or alkaline solutions or ionic solutions are used [14]. Decellularization of complex tissues is usually done by short-term use of many different chemicals to increase the effectiveness and to avoid the negative effects of the chemicals on tissue by decreasing contact period. Ionic solutions work by breaking up the cells as in physical methods. Detergents are the most used materials in chemical decellularization and work by dissolving cell membranes [19,20]. Detergents are categorized as ionic, non-ionic and zwitterionic. Non-ionic detergents such as Triton X-100 have very mild affects on protein structure. Ionic detergents such as SDS and sodium deoxycholate affect protein structure severely, but they also cause loss of matrix. Zwitterionic detergents such as 3-[(3cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS) have an effect range between ionic and non-ionic detergents. The use of different concentrations of these detergents in combination and the use of these detergents in different techniques make the comparison of different studies fairly difficult. Alkaline and acidic solutions such as peracetic acid are not sufficient for decellularization of complex tissues, but they are useful in sterilization [14,21]. Several enzymes such as trypsin (a protease), DNase (a nuclease), lipase and a-galactosidase are used frequently for decellularization. However, prolonged use of trypsin to break cell-matrix interactions can cause loss of collagen and thus loss of mechanical strength [22].
For decellularization of trachea, DEM and its modifications are the most used methods up to day, as they are believed to protect tissue-matrix integration and biomechanical properties and provide best decellularization [1,11,12,22,23]. Remlinger et al. [13] used xenogenic decellularization for trachea in the study in 2010. However, even the studies that employ DEM differ among each other and there is no consensus on how many cycles are required for best decellularization while scaffold would be less damaged. Zang et al. [1] reported five cycles of DEM were sufficient for decellularization and matrix preservation, whereas Jungebluth et al. [12] reported this to be 17 cycles in a pig experiment and 25 cycles have been recommended for human tracheas by Baiguera et al. [11]. The length of periods for which the chemicals are used affects ECM negatively [11]. Although ionic detergents in trachea decellularization are very successful in removing cellular components, they affect ECM integration and damage the natural structure [1,12,13,19]. Besides, the detergents used for decellularization may also have an effect on the compatibility of tracheal ECM and the cells to be seeded [1].
In our previous study, we have decellularized rabbit tracheas using DEM and transplanted the allogenic tracheas seeded with adipose-derived mesenchymal stem cells onto the rabbits and managed to observe consequences of DEM in vivo [24]. In that study, narrowing of trachea was observed due to fibrosis. Therefore, we designed a study to investigate the combinations of lyophilization and DEM to prevent the excessive effects of chemicals and physical methods.
Lyophilization or freeze-drying is a method in which water content of a material is snap-frozen first, followed by removal of ice-crystals by sublimation initially (primary drying) and then by desorption (secondary drying) [25]. Intracellular ice crystal formation, osmotic dehydration and mechanical forces during rapid freezing cause disruption of cellular membranes fragmentation of genetic material and cell lysis [19]. However, the risk of damaging biomolecules and the ECM as well, and the requirement of another process to remove cellular debris are the disadvantages [20]. Addition of lyoprotectant such as sucrose or trehalose may eliminate the adverse effects on scaffold ultrastructure [26]. During rehydration after freeze-drying, tissue tends to absorb fluids more. Thus, this absorption force might be the reason to use this method to promote the better infusion of other decellularization agents [27]. Therefore in our study we thought that combining this method with DEM will assist cell lysis and removal of cellular debris while minimizing the amounts of chemical agents required for effective decellularization and their toxic effect.
The first study that combined physical and chemical decellularization methods (DEM and lyophilization combination) for cartilage decellularization was performed by Sutherland et al. [10] in 2015 on articular cartilage. The author showed that freezing, lyophilization, and cryo-grinding had no significant effect on GAG and DNA content in the tissue. Chemical decellularization and cryo-grinding (lyophilization) provided 86% reduction in DNA content and 55% reduction in GAG content. It was also demonstrated that the combined method has a better chondrogenic potential compared to physical methods and suggested that partial reduction in GAG content might be beneficial to create a less dense matrix that allows for cell infiltration and migration [7]. Indeed, retention of GAG within the matrix is beneficial for chondroinduction based on previous studies citing that GAGs such as chondroitin sulfate and aggrecan may have chondroinductive effects in vitro [10,28].
In the histopathological analysis of decellularized rabbit tracheas, epithelial structure and many cellular components in the primary mesenchymal zone were visible in group 1 in which trachea was lyophilized only. It is possible that the cells were not removed from the environment after lyophilization although degenerated since the tracheas were not treated with detergents and enzymes. However, this group was one of the groups with the lowest DNA content. Similarly in the histopathological analysis of groups 4 and 6, primary mesenchymal zone remnants were observed. Group 6 showed more DNA content than other groups, but the content was low enough to satisfy decellularization criteria. In the SEM investigations, cellular remnants were observed on the surfaces of groups 1, 3, 4, and 5. BM and matrix were found to be preserved in all groups (collagen fibers were loose in group 6) in the light microscope analysis and although matrix was preserved in all groups, collagen structure in mesenchymal zone was found to be loose in groups 1, 4, and 6 in SEM analysis. Since very small areas can be imaged in histopathological analysis, it is not very accurate to discuss cellular contents; however, these analyses are valuable to observe general morphological structure, cell depletion and the preservation of the framework.
On the other hand, DNA content was low enough to satisfy decellularization criteria of Gilbert [14] in all groups, but only group 3 had GAG content similar to untreated rabbit trachea. GAG is the main substance of tracheal cartilage and combined with its water storage capacity, provides the trachea with its mechanical strength to resist compressive forces [29]. GAG loss also explained the early reduction in compressive strength after decellularization. Decellularized trachea showed decreased stiffness and tensile strength compared with native tracheas. In this study, none of the groups had a different tensile strength than the normal trachea but the rigidities of groups 1, 3, and 5 were higher than untreated rabbit trachea. Chemicals used in this decellularization process are inherently damaging to cells. Therefore, matrix scaffolds with high concentrations of residual chemicals are likely to be toxic to cells [1]. Glucose consumption and lactic acid production levels of all groups (after stem cell seeding) were similar to the only cell-containing medium in this study. This revealed that stem cells seeded on all decellularized trachea groups were able to survive.
We investigated the effects of combined decellularization techniques on the structure, composition, mechanical properties and biocompatibility of tracheal matrix in vitro. According to the results, lyophilization+deoxycholic acid+de-oxyribonuclease method is the best technique with good decellularization with protected matrix and it takes only 3 days to perform the procedure. A limitation to this study is the lack of comparison between combined methods and DEM because we prioritized investigation of these methods to make sure they work properly. Furthermore, a future study was designed to compare the combined decellularization method with DEM for human trachea.
HIGHLIGHTS
▪ The decellularization time of trachea was shortened by using combination of physical, chemical and enzymatic techniques.
▪ The combination methods included lyophilization, deoxycholic acid, and deoxyribonuclease.
▪ The combination methods were undergone within only 3 days without excessive influence of matrix.
Acknowledgments
This study was presented at the 36th National ENT and Head and Neck Surgery Congress in Belek-Antalya, Turkey, on November 5–9, 2014.
This study was supported by Istanbul University Scientific Research Project Unit (BAP, project no. 398688), Istanbul, Turkey.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Zang M, Zhang Q, Chang EI, Mathur AB, Yu P. Decellularized tracheal matrix scaffold for tissue engineering. Plast Reconstr Surg. 2012 Sep;130(3):532–40. doi: 10.1097/PRS.0b013e31825dc084. [DOI] [PubMed] [Google Scholar]
- 2.Macchiarini P, Walles T, Biancosino C, Mertsching H. First human transplantation of a bioengineered airway tissue. J Thorac Cardiovasc Surg. 2004 Oct;128(4):638–41. doi: 10.1016/j.jtcvs.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 3.Go T, Jungebluth P, Baiguero S, Asnaghi A, Martorell J, Ostertag H, et al. Both epithelial cells and mesenchymal stem cell-derived chondrocytes contribute to the survival of tissue-engineered airway transplants in pigs. J Thorac Cardiovasc Surg. 2010 Feb;139(2):437–43. doi: 10.1016/j.jtcvs.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Jungebluth P, Moll G, Baiguera S, Macchiarini P. Tissue-engineered airway: a regenerative solution. Clin Pharmacol Ther. 2012 Jan;91(1):81–93. doi: 10.1038/clpt.2011.270. [DOI] [PubMed] [Google Scholar]
- 5.Benders KE, van Weeren PR, Badylak SF, Saris DB, Dhert WJ, Malda J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013 Mar;31(3):169–76. doi: 10.1016/j.tibtech.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Schwarz S, Elsaesser AF, Koerber L, Goldberg-Bockhorn E, Seitz AM, Bermueller C, et al. Processed xenogenic cartilage as innovative biomatrix for cartilage tissue engineering: effects on chondrocyte differentiation and function. J Tissue Eng Regen Med. 2015 Dec;9(12):E239–51. doi: 10.1002/term.1650. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz S, Koerber L, Elsaesser AF, Goldberg-Bockhorn E, Seitz AM, Dürselen L, et al. Decellularized cartilage matrix as a novel biomatrix for cartilage tissue-engineering applications. Tissue Eng Part A. 2012 Nov;18(21-22):2195–209. doi: 10.1089/ten.TEA.2011.0705. [DOI] [PubMed] [Google Scholar]
- 8.Yang Z, Shi Y, Wei X, He J, Yang S, Dickson G, et al. Fabrication and repair of cartilage defects with a novel acellular cartilage matrix scaffold. Tissue Eng Part C Methods. 2010 Oct;16(5):865–76. doi: 10.1089/ten.TEC.2009.0444. [DOI] [PubMed] [Google Scholar]
- 9.Kalathur M, Baiguera S, Macchiarini P. Translating tissue-engineered tracheal replacement from bench to bedside. Cell Mol Life Sci. 2010 Dec;67(24):4185–96. doi: 10.1007/s00018-010-0499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutherland AJ, Beck EC, Dennis SC, Converse GL, Hopkins RA, Berkland CJ, et al. Decellularized cartilage may be a chondroinductive material for osteochondral tissue engineering. PLoS One. 2015 May;10(5):e0121966. doi: 10.1371/journal.pone.0121966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baiguera S, Jungebluth P, Burns A, Mavilia C, Haag J, De Coppi P, et al. Tissue engineered human tracheas for in vivo implantation. Biomaterials. 2010 Dec;31(34):8931–8. doi: 10.1016/j.biomaterials.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Jungebluth P, Go T, Asnaghi A, Bellini S, Martorell J, Calore C, et al. Structural and morphologic evaluation of a novel detergent-enzymatic tissue-engineered tracheal tubular matrix. J Thorac Cardiovasc Surg. 2009 Sep;138(3):586–93. doi: 10.1016/j.jtcvs.2008.09.085. [DOI] [PubMed] [Google Scholar]
- 13.Remlinger NT, Czajka CA, Juhas ME, Vorp DA, Stolz DB, Badylak SF, et al. Hydrated xenogeneic decellularized tracheal matrix as a scaffold for tracheal reconstruction. Biomaterials. 2010 May;31(13):3520–6. doi: 10.1016/j.biomaterials.2010.01.067. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert TW. Strategies for tissue and organ decellularization. J Cell Biochem. 2012 Jul;113(7):2217–22. doi: 10.1002/jcb.24130. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki T, Kobayashi K, Tada Y, Suzuki Y, Wada I, Nakamura T, et al. Regeneration of the trachea using a bioengineered scaffold with adipose-derived stem cells. Ann Otol Rhinol Laryngol. 2008 Jun;117(6):453–63. doi: 10.1177/000348940811700609. [DOI] [PubMed] [Google Scholar]
- 16.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997 Apr;276(5309):71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 17.Kafienah W, Mistry S, Williams C, Hollander AP. Nucleostemin is a marker of proliferating stromal stem cells in adult human bone marrow. Stem Cells. 2006 Apr;24(4):1113–20. doi: 10.1634/stemcells.2005-0416. [DOI] [PubMed] [Google Scholar]
- 18.Tsutsumi S, Shimazu A, Miyazaki K, Pan H, Koike C, Yoshida E, et al. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun. 2001 Oct;288(2):413–9. doi: 10.1006/bbrc.2001.5777. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006 Jul;27(19):3675–83. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011 Apr;32(12):3233–43. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodde J, Hiles M. Virus safety of a porcine-derived medical device: evaluation of a viral inactivation method. Biotechnol Bioeng. 2002 Jul;79(2):211–6. doi: 10.1002/bit.10281. [DOI] [PubMed] [Google Scholar]
- 22.Yang M, Chen CZ, Wang XN, Zhu YB, Gu YJ. Favorable effects of the detergent and enzyme extraction method for preparing decellularized bovine pericardium scaffold for tissue engineered heart valves. J Biomed Mater Res B Appl Biomater. 2009 Oct;91(1):354–61. doi: 10.1002/jbm.b.31409. [DOI] [PubMed] [Google Scholar]
- 23.Meezan E, Hjelle JT, Brendel K, Carlson EC. A simple, versatile, nondisruptive method for the isolation of morphologically and chemically pure basement membranes from several tissues. Life Sci. 1975 Dec;17(11):1721–32. doi: 10.1016/0024-3205(75)90119-8. [DOI] [PubMed] [Google Scholar]
- 24.Batioglu-Karaaltin A, Karaaltin MV, Ovali E, Yigit O, Kongur M, Inan O, et al. In vivo tissue-engineered allogenic trachea transplantation in rabbits: a preliminary report. Stem Cell Rev. 2015 Apr;11(2):347–56. doi: 10.1007/s12015-014-9570-8. [DOI] [PubMed] [Google Scholar]
- 25.Nireesha GR, Divya L, Sowmya C, Venkateshan N, Babu MN, Lavakumar V. Lyophilization/freeze drying: an review. Int J Nov Trends Pharm Sci. 2013 Oct;3(4):87–98. [Google Scholar]
- 26.Wang S, Goecke T, Meixner C, Haverich A, Hilfiker A, Wolkers WF. Freeze-dried heart valve scaffolds. Tissue Eng Part C Methods. 2012 Jul;18(7):517–25. doi: 10.1089/ten.TEC.2011.0398. [DOI] [PubMed] [Google Scholar]
- 27.Hung SH, Su CH, Lee FP, Tseng H. Larynx decellularization: combining freeze-drying and sonication as an effective method. J Voice. 2013 May;27(3):289–94. doi: 10.1016/j.jvoice.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Ingavle GC, Frei AW, Gehrke SH, Detamore MS. Incorporation of aggrecan in interpenetrating network hydrogels to improve cellular performance for cartilage tissue engineering. Tissue Eng Part A. 2013 Jun;19(11-12):1349–59. doi: 10.1089/ten.tea.2012.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts CR, Rains JK, Pare PD, Walker DC, Wiggs B, Bert JL. Ultrastructure and tensile properties of human tracheal cartilage. J Biomech. 1998 Jan;31(1):81–6. doi: 10.1016/s0021-9290(97)00112-7. [DOI] [PubMed] [Google Scholar]