Abstract
A causal relationship between cell metabolism and the fate of pluripotent stem cells through epigenome regulation is emerging. A recent study shows that the tricarboxylic acid cycle intermediate alpha‐ketoglutarate (αKG) can both sustain naïve mouse embryonic stem cell pluripotency and promote primordial germ cell differentiation. This observation together with other studies provides intriguing possibilities for stabilizing ephemeral embryonic cell states and enhancing desired fate transitions through specific metabolite manipulations.
Subject Categories: Development & Differentiation, Metabolism, Stem Cells
Primordial germ cells (PGCs) are precursors of gametes that connect one generation to the next. Modeling early mouse germ cell development in vitro begins with the transition of naïve mouse embryonic stem cells (mESCs) into transient, primed mouse epiblast‐like cells (EpiLCs) (Fig 1). This is followed by conversion of EpiLCs into PGC‐like cells (PGCLCs), which can produce sperm and oocytes. mESCs and PGCs are similar in expressing pluripotency regulators Oct4, Nanog, and Sox2, but are unique from epigenome and transcriptome changes that yield cell states with distinct developmental potentials (Hayashi et al, 2011). A key question under study in many lineage‐specific developmental systems is what is the role for metabolism in cell fate transitions and outcomes?
Figure 1. αKG effects on PGC competency and primordial germ cell development.
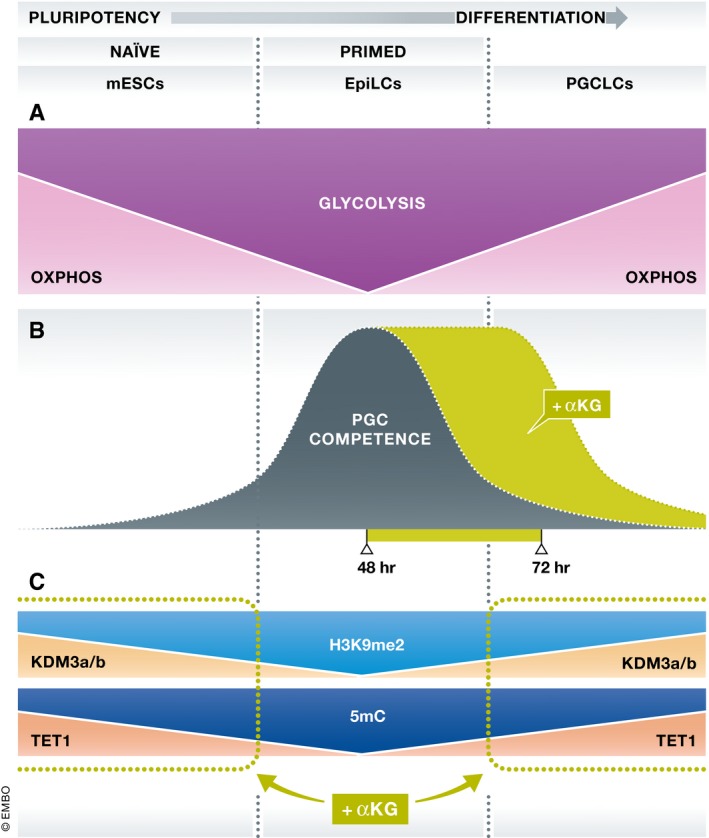
(A) A transition from naïve mESCs to primed EpiLCs coincides with reduced OXPHOS and increased glycolysis that reverses when EpiLCs convert to PGCLCs. (B,C) Addition of αKG prolongs PGC competency from 48 to 72 h, potentially by locking‐in an epigenetic pattern regulated by KDM3a/b and TET1 enzyme activities. mESC, mouse embryonic stem cells; EpiLCs, epiblast‐like cells; PGCLCs, primordial germ cell‐like cells; PGC, primordial germ cell; αKG, alpha‐ketoglutarate.
Now, Tischler et al (2019) identify roles for alpha‐ketoglutarate (αKG) in maintaining mESC pluripotency and enhancing PGCLC differentiation. Using single‐cell RNA‐Seq over a developmental time course referred to as a “pseudotime analysis”, they show a transition from mainly oxidative (OXPHOS) to glycolytic metabolism with progression from mESCs to EpiLCs. On conversion of EPiLCs to PGCLCs, OXPHOS increases and glycolysis decreases, consistent with a prior study (Hayashi et al, 2017). Repressing glycolysis or elevating αKG levels favors retention of mESC self‐renewal over exit from naïve pluripotency. Adding αKG boosts PGCLC production by ~50%, and added αKG can replace 2i/Lif medium to maintain mESC self‐renewal or can substitute for BMP4/8 in PGCLC fate induction. Remarkably, αKG addition also extends EpiLC competency to form PGCLCs without affecting cell quality, with H3K9me2 and H3K27me3 histone marks stabilized.
Alpha‐ketoglutarate is a cofactor for Jumonji C (JmjC)‐domain‐containing histone demethylases (JHDMs) and ten‐eleven translocation (TET) DNA demethylases. Cell‐permeable dimethyl‐αKG (dm‐αKG) used to elevate αKG also has non‐epigenome activities and can stabilize hypoxia‐inducible factor 1α (HIF1α) to induce a pseudo‐hypoxic state with altered gene expression (Hou et al, 2014). αKG can also inhibit the ATP synthase and mTOR (Chin et al, 2014), although added αKG did not affect the ATP synthase and could substitute for dm‐αKG to promote human ESC differentiation in a prior study (TeSlaa et al, 2016). Consistent with an epigenome effect, dm‐αKG altered H3K9me3 and H3K27me3 levels to promote self‐renewal of naïve mESCs in another study (Carey et al, 2015). Added αKG also enhanced spontaneous mESC differentiation (Hwang et al, 2016) and directed human neuroectoderm differentiation (TeSlaa et al, 2016) with altered histone and DNA methylation levels.
Using pseudotime modeling, the current RNA‐Seq analysis revealed cell heterogeneity in differentiation capacity on EpiLC induction, with added αKG increasing PGC competency. Also, αKG preserved histone methylation patterns and prolonged a transient state for PGC competency. Whether succinate, a product and inhibitor of dioxygenase epigenome modifying enzyme reactions, would impair PGC competency was not examined. Also, the epigenetic state resulting from increased αKG may be physiologic or aberrant and requires further comparison studies. These results further suggest that αKG or other metabolites could prolong (or shorten) other transitory intermediate cell states. For example, cancer cells use glutaminolysis to generate αKG as an anapleurotic fuel for the TCA cycle and drug resistance may occur by induction of a transient transcriptional state via epigenome remodeling (Shaffer et al, 2017). Manipulating αKG levels by glutamine reduction could deplete TCA cycle intermediates and block transient drug‐resistant cancer cell states.
In vivo studies of αKG and other metabolites on cell fate are largely unexplored, yielding questions about how diet and environment influence germ cell metabolite levels and outcomes. Most epigenetic marks shaped by short‐term environmental conditions are not inherited even with known imprinting (Heard & Martienssen, 2014). Caloric restriction, high‐fat diet, and malnourishment in parents can impact the epigenome and small noncoding RNAs transmitted by gametes to offspring (Sharma & Rando, 2017). Emerging genome editing technologies may abet in vivo manipulations of metabolite levels at specific times within a developing embryo to start addressing the role of metabolites in vivo. Indeed, large knowledge gaps exist for (i) how to assess the contribution of diet to key metabolite levels in both somatic and germ cells, (ii) the responsiveness of epigenetic regulators and cofactors to specific metabolite levels, and (iii) whether metabolite‐influenced epigenome modifications persist and manifest into adulthood and across generations. An impediment to early embryo studies is the paucity of starting materials, with advances in single‐cell methods holding promise to bridge the gap between metabolism, epigenetics, and stem cell fates (Zhang et al, 2018). Advancing this emerging opportunity in vitro at first, Tischler et al (2019) deploy single‐cell technologies to reveal how targeted metabolic manipulations augment (or potentially retard) dynamic cell state conversions during PCG development.
The EMBO Journal (2019) 38: e100615
See also: J Tischler et al (January 2019)
References
- Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB (2015) Intracellular alphaketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518: 413–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin RM, Fu X, Pai MY, Vergnes L, Hwang H, Deng G, Diep S, Lomenick B, Meli VS, Monsalve GC, Hu E, Whelan SA, Wang JX, Jung G, Solis GM, Fazlollahi F, Kaweeteerawat C, Quach A, Nili M, Krall AS et al (2014) The metabolite alphaketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature 510: 397–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M (2011) Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 146: 519–532 [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Otsuka K, Ebina M, Igarashi K, Takehara A, Matsumoto M, Kanai A, Igarashi K, Soga T, Matsui Y (2017) Distinct requirements for energy metabolism in mouse primordial germ cells and their reprogramming to embryonic germ cells. Proc Natl Acad Sci USA 114: 8289–8294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard E, Martienssen RA (2014) Transgenerational epigenetic inheritance: myths and mechanisms. Cell 157: 95–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou P, Kuo CY, Cheng CT, Liou JP, Ann DK, Chen Q (2014) Intermediary metabolite precursor dimethyl‐2‐ketoglutarate stabilizes hypoxia‐inducible factor‐1alpha by inhibiting prolyl‐4‐hydroxylase PHD2. PLoS ONE 9: e113865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang IY, Kwak S, Lee S, Kim H, Lee SE, Kim JH, Kim YA, Jeon YK, Chung DH, Jin X, Park S, Jang H, Cho EJ, Youn HD (2016) Psat1‐dependent fluctuations in alpha‐ ketoglutarate affect the timing of ESC differentiation. Cell Metab 24: 494–501 [DOI] [PubMed] [Google Scholar]
- Shaffer SM, Dunagin MC, Torborg SR, Torre EA, Emert B, Krepler C, Beqiri M, Sproesser K, Brafford PA, Xiao M, Eggan E, Anastopoulos IN, Vargas‐Garcia CA, Singh A, Nathanson KL, Herlyn M, Raj A (2017) Rare cell variability and drug‐induced reprogramming as a mode of cancer drug resistance. Nature 546: 431–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma U, Rando OJ (2017) Metabolic inputs into the epigenome. Cell Metab 25: 544–558 [DOI] [PubMed] [Google Scholar]
- TeSlaa T, Chaikovsky AC, Lipchina I, Escobar SL, Hochedlinger K, Huang J, Graeber TG, Braas D, Teitell MA (2016) alpha‐ketoglutarate accelerates the initial differentiation of primed human pluripotent stem cells. Cell Metab 24: 485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler J, Gruhn WH, Reid R, Allgeyer E, Buettner F, Marr C, Theis F, Simons BD, Lorenz Wernisch L, Surani MA (2019) Metabolic regulation of pluripotency and germ cell fate through alpha–ketoglutarate. EMBO J 38: e99518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhao J, Dahan P, Lu V, Zhang C, Li H, Teitell MA (2018) Metabolism in pluripotent stem cells and early mammalian development. Cell Metab 27: 332–338 [DOI] [PubMed] [Google Scholar]


