Abstract
The pharmacological treatment of knee osteoarthritis (OA) is a purely symptomatic therapy, which often ensures that the mobility of the patient is successfully retained. This article refers to the recommendations and opinions regarding the pharmacotherapy of knee OA contained in the new guideline of the Association of the Scientific Medical Societies in Germany (AWMF), highlighting several important aspects and describing the considerations underlying the decision-making process. With this article it is hoped that therapeutic effectiveness can be realistically estimated, that any risks of medication errors and avoidable side effects can be reduced, and that further helpful measures can be taken into consideration.
Key words: Knee, Osteoarthritis, Guideline, pharmacotherapy, NSAIDs
Introduction
A number of non-pharmaceutical and pharmaceutical measures are available, either individually or in combination, for treating the widespread disease and public health issue that is osteoarthritis (OA).1-3 Only a part of patients with radiologically detectable OA will actually suffer clinically relevant symptoms.2,3 However, when the arthritis becomes symptomatic and painful, drug therapy can then become useful. Pharmaceuticals represent an essential pillar of therapy, whereby a wide range of very different drugs, especially non-steroidal anti-inflammatory drugs (NSAIDS) in combination with other medications (e.g. proton pump inhibitors), opioid analgesics, potentially cartilage active agents and phytopharmaceuticals, have all found use. Topical agents are also popular since they exert fewer systemic side effects and enjoy a high level of acceptance amongst patients. In addition, glucocorticoids and hyaluronic acid preparations number amongst those medications used for intra-articular OA therapy.
A guideline-compliant treatment of a multimorbid and usually elderly patient represents a particular challenge, since in many of the studies that the guidelines were based upon, no attempt was made to investigate elderly, multiply pre-afflicted patients. This is often because multi-morbidity in its many forms can often complicate study design by introducing excessive variability. However, there are also age-related differences in pharmacokinetics and dynamics which also make it difficult to draw comparisons between older and younger study cohorts. Recent studies have shown that the number of prescribed medicines more than doubles from the 65th year of life, and that after the 74th year it increases by a further 50%.4 The guideline-compliant treatment of each individual disease in a multi-morbid elderly patient demands that a larger number of medicines need to be prescribed. This can result in potentially serious interactions and side effects.4,5 A single disease in a multimorbid patient should therefore not be treated just for itself alone, and instead the various medications used to treat the different diseases must all be mutually compatible. A close coordination between general practitioners and specialists, as well as knowledge of all (including non-prescription) medications taken by the patient should reduce the risk of medication errors and any avoidable side effects. This, in turn, should increase the safety of the drug therapy. In addition, a number of other measures, such as dose adaptation to compensate for reduced renal function, or the use of checklists such as the PRISCUS-list,5 should all help to achieve an optimized medication management in old age. Since October 1st 2016, in accordance with the German EHealth Law, patients who receive three or more drugs have been able to request a medication plan from their general practitioner which facilitates the exchange of information between doctors involved in treating the patient.
Chronic diseases without any prospect of healing combined with the understandable desire to maintain full mobility present a particularly major challenge, and an assessment of the various pharmacological therapies according to the standards of evidence- based medicine would be particularly helpful. The new guideline for knee OA of the Association of the Scientific Medical Societies in Germany (AWMF),6 under the aegis of the German Society for Orthopedics and Orthopedic Surgery, is designed to provide clear practical recommendations and opinions based on the currently available literature. The five authors were members of the committee that decided to present the new S2k-guideline as the current, completely newly developed revision of the old guideline published in 2002. Medical guidelines are intended to be transparent and practical, but are not binding, meaning that each individual case will still demand a critical personal assessment. The present article on the medical therapy of knee OA is oriented towards this new guideline and brings to light some particularly important and revealing aspects. This publication has been also recently published in German.1
Analgesics
Acetaminophen
Acetaminophen is the most commonly used and freely available analgesic on the market, and it is also used to treat OA. The extent to which acetaminophen leads to any symptom improvement in knee OA at all has been unclear until now, since conclusive studies in this area have been lacking. Some guidelines7,8 have recommended the use of acetaminophen as a first-line analgesic for OA, although current meta-analyzes9-11 have led to another evidence-based conclusion. Three recent meta-analyzes independently concluded that acetaminophen even at high daily doses of up to 4 g only exerts a small and not clinically relevant analgesic effect in knee OA. For this reason, the current AWMF guideline for knee OA no longer recommends the use of acetaminophen for knee OA.
Metamizole
As a prescription analgesic with an additional antipyretic activity, metamizole has an analgesic potency within the order of magnitude of a weakly effective opioid such as tramadol, tilidine or codeine. OA is not an indication for metamizole per se, because it does not exert any anti-inflammatory effect. Metamizole is generally well tolerated, but in very rare cases serious lifethreatening complications such as agranulocytosis or allergic reactions can occur, which in the worst case can culminate in anaphylactic shock.12 The Drug Commission of the German Medical Association (AkdA) recommends that the application can only be made within the scope of its approval and only after extensive clarification of the patient about the risks and possible adverse effects such as fever/chills, fatigue, sore throat and inflammation in the area of the oral mucous membranes. In addition, it recommends that wherever there is a suspicion of agranulocytosis, or wherever the agent has been taken for a long time, blood counts should be performed. 12,13 Due to its potentially life-threatening complications, the indications of metamizole are restricted to acute and chronic pain when other therapeutic measures are not indicated.14 As such there are only a few cases where a short-term use of metamizole for the treatment of OA pain can actually be considered. The new AWMF guideline for knee OA6 therefore contains no recommendation for the use of metamizole.
Opioids
The restrictive legal regulations applicable in Germany in combination with existing guidelines have prevented a drastic increase in opioid-dependency here, unlike the situation seen in the USA in 2017 when a health emergency had to be declared. The addiction potential for opioids is very high, especially when they flood in quickly or when not given in prolonged release form. The current AWMF guideline for Long- Term Opioid-Use in Non-Cancer Pain (LONTS) recommends that in the case of non-tumor-related pain they should only be given when strictly indicated, in prolongedrelease form, according to a fixed time scheme, at low doses, for a restricted period of time, and in a controlled manner.15 The German Society of Pain Medicine (DGS) noted that the prescription of opioids is restrictively regulated and that patients only receive opioids when the strict rules of the German Narcotic Drugs Prescription Ordinance (BtmVV) and the German Narcotic Drugs Act (BtmG) are complied with. Despite these restrictions, it is estimated that with long-term therapy, approximately 1-3% of pain patients treated with opioids will develop dependency symptoms. For OA, opioids are neither used over the long term nor routinely. However, for a short-term therapy they may be indicated when other therapeutic measures have been exhausted, are not possible, or are contraindicated (Figure 1). In accordance with the LONTS guideline, one should only use WHO level 2 opioids such as tramadol where with a short-term, 1 to 3-month duration of therapy a reliable analgesic effect has been confirmed.15 To treat the patient optimally and clarify any potential interactions and side effects when prescribing opioids (e.g. interactions with other drugs, addiction history, cognitive impairment), an interdisciplinary collaboration between general practitioners/orthopaedists and pain therapists may be fruitful. A Cochrane review published in 2014 showed that with other non-tramadol opioids such as oxycodone, codeine, morphine, tapentadol and buprenorphine, only a small and barely clinically relevant analgesic effect can be achieved.16 In a direct comparison with nonsteroidal anti-inflammatory drugs (NSAIDs), tramadol was even inferior in terms of its analgesic effect.17
Figure 1.
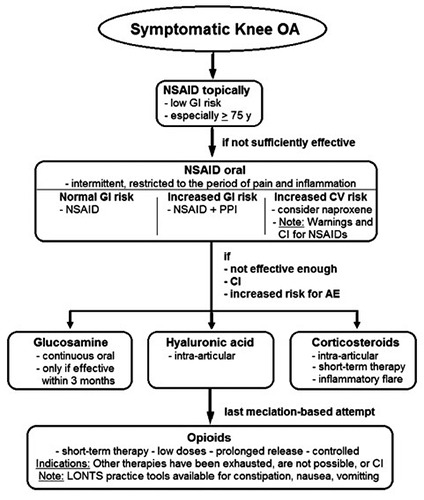
Algorithm of medical treatment for knee OA in accordance with the new German guidelines. GI, gastrointestinal; Y, years, CI, contraindication(s); NSAID non steroidal anti-inflammatory drug = traditional NSAID and cyclooxygenase-2 inhibitor (COX-2 inhibitor); PPI, proton pump inhibitor; AE, adverse effects.
Also, as a result of central nervous system effects including fatigue, an increased tendency to fall, dizziness and loss of equilibrium, opioids pose more problems than NSAIDs.18 As such the rate of fracture incidence, hospitalization and mortality is 2-4 times higher than is the case with administration of traditional NSAIDs.18 In addition, opioids cause constipation in every second patient, whereby there is no development of tolerance, meaning that this adverse effect does not disappear over the course of therapy. This effect is due to the μ-opioid receptors in the enteric nervous system, the activation of which reduces intestinal peristalsis and leads to an increased absorption of water as well as an inhibition of gastrointestinal secretion. The specific mode of administration appears to be a less important factor here. Before starting an opioid therapy, it is therefore necessary to inform the patients about the frequent incidence of constipation, and in most patients to prescribe a prophylactic laxative such as a macrogol containing preparation. Because fiber-rich foods alone are often insufficient to relieve an opioid-induced constipation, laxatives are also frequently used.19 An antiemetic therapy may also be required at the beginning of treatment, whereby after 2-4 weeks the indication can be reviewed due to the development of tolerance. For the treatment of common side effects such as constipation, nausea and vomiting, the LONTS guideline provides a number of helpful and practical tools.15
Non-Steroidal Anti- Inflammatory Drugs
Non-steroidal anti-inflammatory drugs (NSAIDs), which include both the “traditional” NSAIDs and the COX-2 selective inhibitors (coxibs), are not only analgesic, but also anti-inflammatory. NSAIDs are therefore particularly effective with inflammation- induced arthritis pain for which they are often used to treat.3,9,10 However, the oral administration of NSAIDs carries additional gastrointestinal and cardiovascular risks.20 In the case of long-term application, these risks are more prevalent and can entail significant health consequences. In the new German AWMF guideline for knee OA,6 the recommendations to protect the patient are based on this fact. Risk minimization is the approach that leads effectively to a reduction in undesirable complications and in so doing protects the patient (Table 1).
Table 1.
Contraindications and precautions relating in particular to the gastrointestinal and cardiovascular risks of traditional NSAIDs and COX-2 inhibitors.
| Naproxene | Ibuprofen | Diclofenac | Etoricoxib | Celecoxib | |
|---|---|---|---|---|---|
| Gastrointestinal disorders | CI: Existing or a history of recurrent peptic ulceration/bleeding; GI bleeding or perforation under NSAIDs in the history | CI: Existing or a history of recurrent peptic ulceration/bleeding; GI bleeding or perforation under NSAIDs in the history | CI: Existing or a history of recurrent peptic ulceration/bleeding; GI bleeding or perforation under NSAIDs in the history | CI: active peptic ulceration or active GI bleeding; Inflammatory bowel disease | Celecoxib |
| Cardiac insufficiency | CI: NYHA class III-IV | CI: NYHA class III-IV | CI: NYHA class III-IV | CI: NYHA II-IV | CI: active peptic ulceration or active GI bleeding; Inflammatory bowel disease |
| Cardiovascular diseases | - | - | CI: CHD, PAOD, cerebrovascular disease, not adequately controlled hypertension | CI: CHD, PAOD, cerebrovascular disease, not adequately controlled hypertension | CI: NYHA II-IV |
| Renal insufficiency | CI: severe RI (CrCl <30 ml/min); no DA: mild to moderate RI | CI: CHD, PAOD, cerebrovascular disease, not adequately controlled hypertension | CI: CHD, PAOD, cerebrovascular disease, not adequately controlled hypertension | ||
| Lungs | CI: Asthma | ||||
| Dosage, duration | Precaution: Sufficient, as low as possible dose until inflammation symptoms recede, where there is an increased GI risk a proton pump inhibitor should be given where needed. |
CrCl: Creatinine clearance; DA: Dose adjustment; GI: Gastrointestinal; CI: Contraindication; RI: Renal insufficiency; NYHA: New York Heart Association; CHD: Coronary heart disease; PAOD: Peripheral arterial occlusive disorder.
Topically used NSAIDs
A simple and effective way to reduce the risk of gastrointestinal side effects is to apply NSAIDs topically. Recent studies have shown that systemic side effects, including gastrointestinal complaints under topical application of diclofenac or ketoprofen. occur no more frequently than they do with placebo.21 Blood plasma concentrations of NSAIDs after topical application lie at between 5-15% of the values seen after oral administration. In contrast, however, a higher prevalence of usually weak local skin reactions such as dry skin, redness and itching occurs after topical administration of diclofenac, but not ketoprofen, compared to topically administered placebo.21 A 50% reduced pain was seen in 60% of OA patients after topical application of diclofenac or ketoprofen over a period of 8-12 weeks, although the topical application of placebo also proved effective in at least 50% of cases.21 For the drug to exert an anti-inflammatory effect, it needs to be present at concentrations in the pain-causing structures, such as the subcutaneous tissue, the muscles, the tendons, the joint capsule and the synovium, that are sufficient for inhibiting cyclooxygenase-2. The actual attainable concentrations at this site of action depend on the chemical structure of the compound, the concentration of the preparation, the formulation, and the dosage. The correct dosage should therefore be read off from the package leaflet and is often a 3-5 cm long strand of gel or cream.
The topical application of an NSAID is the safer option not just for older patients, but also for all other patients, and especially those with an increased risk of suffering gastrointestinal side effects (Figure 1). The new AWMF guideline for knee OA6 therefore recommends that topical application of NSAIDs should be considered before any oral application. This recommendation is therefore compliant with a range of other current recommendations from guidelines produced by NICE,7 AAOS,23 OARSI,8 and ACR.22 The American College of Rheumatology (ACR) recommends a preferential application of topical NSAIDs especially in elderly patients (>75 years).
Orally applied NSAIDs
If topical application does not provide a sufficient analgesic effect, an oral administration of NSAIDs may be prescribed once the risk factors and contraindications are taken into account. A number of placebocontrolled studies have shown good efficacy against OA-related pain which has been attributed to the anti-inflammatory effect.3,9,10 Despite equieffective dosing, this effect may be differently pronounced due to inter-individual differences between patients regarding drug bioavailability and metabolism.24 There is also evidence that NSAIDs differ regarding their adverse gastrointestinal, cardiovascular and renal effects.25,26 The individual risk of adverse effects as well as the co-morbidities of the usually elderly patients must therefore be taken into account when deciding whether and when specific NSAIDs should be prescribed. Individual risk factors for NSAIDinduced gastrointestinal complications include, among others, an age over 60 years, a history of gastrointestinal disorders, high dosage, long duration of therapy, administration of 2 or more NSAIDs, Helicobacter pylori infection, irregular eating, and alcoholism.3,24,26-29 Some of these risk factors can be easily minimized. The new AWMF guideline for knee OA6 recommends a range of measures for reducing risks. Individual doses should on the one hand be sufficient, but on the other be as low as possible, with only one NSAID being applied until the inflammation symptoms including resting pain, swelling and fever recede (usually up to 2 weeks). Where there is an increased risk, a proton pump inhibitor (PPI) combined with a COX-2 inhibitor30,31 should be prescribed.
To protect themselves against NSAIDinduced gastrointestinal complications, elderly patients should take a PPI to inhibit gastric acid production.6,32 In addition, for those patients over 60 years of age, NSAIDs with a short half-life in combination with an age adapted reduction of the daily dose are recommended along with monitoring of the gastrointestinal tract, blood pressure and renal function. The patient must also be informed about the possibility of gastrointestinal symptoms such as abdominal pain, heartburn, dyspepsia or tarred stool occurring, since when any of these do occur the therapy will have to be discontinued, and the physician treating the patient will have to be consulted. This will demand further clarification and, wherever necessary, a treatment of the gastrointestinal symptoms, and it shall also involve seeking out an alternative therapy for the OA. It is important to stress here that PPIs offer only limited protection against gastrointestinal complications, since they exert no protective effect in the small or large intestine.27 A blanket prescription of PPI when administering NSAIDs is not recommended, since PPIs themselves can cause adverse effects.
An increased cardiovascular risk has also been described for NSAIDs. Even though their benefits outweigh their risks, the incidence of myocardial infarction and stroke slightly increases depending on the dose and duration of application.20,25,33 Diclofenac at long-term high doses (150 mg per day) shows a cardiovascular risk comparable to that of COX-2- selective inhibitors. For this reason, the European Medicines Agency (EMA) has recommended that diclofenac should not be administered to patients with severe cardiovascular diseases (e.g. heart failure, heart attack, or stroke in the anamnesis); it should also only be offered with special care to patients with cardiovascular risk factors in general.35,36 A more recent study interestingly revealed that the use of naproxene entails no increased risk of acute myocardial infarction. 20,26,36 Naproxene, however, is associated with an increased risk of gastrointestinal complaints or complications and has rarely been prescribed in Germany up until now. The new AWMF guideline for knee OA6 recommends that patients with cardiovascular risk factors such as smoking, hyperlipidemia, diabetes mellitus or arterial hypertension should only receive NSAIDs if strictly indicated and in as low a dose and as briefly as necessary. In this case, prescription of naproxene, if necessary together with a PPI, should also be considered. Alternatively, application of hyaluronic acid or even a weakly effective opioid can be considered in these patients as a preferred therapeutic option.
Intra-articular therapy
The need for an intra-articular injection to treat knee OA requires critical consideration given its invasive nature. Prerequisites for implementing an intra-articular injection include a reliable mastering of the atraumatic injection technique and the observance of all hygiene rules described in the AWMF guidelines for intra-articular punction and injection.37 Despite even the most careful implementation, each intra-articular injection carries the risk of an iatrogenic infection, where this usually involves staphylococci. Glucocorticoids and hyaluronic acid preparations in particular have to be named among the medications used for intra-articular OA therapy.
Glucocorticoids
Since the 1950s and even more so in the 1960s, intra-articular glucocorticoid injections found widespread use. Initially, it was only applied for rheumatoid arthritis, but today it is applied for almost all types of non-infectious joint swelling. Despite the initial high expectations and its widespread application, their indication is now more restricted.
An intra-articular injection of glucocorticoids into acute inflammation of an activated knee OA might represent a rational approach. The aim of treatment is to reduce the pain and restore mobility. Randomized and placebo-controlled studies have shown that the intra-articular injection of a glucocorticoid into an OA knee joint can significantly reduce joint symptoms for at least one week.38-40 Interestingly enough, even when placebo was given intra-articularly, a marked alleviation of pain could be achieved, although a greater reduction of pain was seen in the group treated with glucocorticoids. A simultaneously conducted joint puncture removes mediators of inflammation and cartilage detritus from the joint, and probably contributes to the therapeutic success of the intra-articular glucocorticoid injection. Occasionally, however, a longer lasting effect of 16-24 weeks duration is seen in practice after an intra-articular glucocorticoid application.39,40 This suggests that other (e.g. functional and psychosocial) factors as well as disease-related symptoms alter the response.41
A detailed listing and comparison of the potency of each intra-articularly applied glucocorticoid (similar to the “equivalence doses” with systemically applied corticoids) is complicated by the lack of controlled comparative studies performed on each of the individual preparations. The frequency of the earlier, occasionally observed crystalinduced side effects, such as acute crystal synovitis, periarticular soft tissue calcification, and soft tissue atrophy, is markedly reduced when micro-crystalline substances and lipid microspheres are used. Intra-articularly injected glucocorticoids at high doses can inhibit cartilage metabolism and even reduce the cartilage mass.42 The current AWMF guideline of knee OA6 therefore recommends that intra-articular glucocorticoids be applied in a low as possible but nevertheless effective dosage over the short term for painful knee OA refractory to other therapeutic measures. This might be advisable in the case of acute pain exacerbation that can occur with inflammatory OA (Figure 1). While the new AWMF guideline for knee OA6 considers a restricted use of glucocorticoids to be appropriate in the same way as the OARSI guideline,8 the AAOS guideline23 has made neither a positive nor a negative recommendation in this respect.
Hyaluronic acid
The synovial fluid of a healthy joint has a hyaluronic acid (HA) concentration of 2-4 mg/ml, the molecular weight of which ranges widely up to a maximum of 4-6 MDa.43 The treatment of OA with intraarticular HA is given with the intention of substituting the pathologically altered synovial HA which is reduced both in size and concentration by the OA process. Synovial HA, along with other components, contributes to the lubrication of joint surfaces as well as shock absorption. The concept of supplementing physiological HA, also referred to as “viscous supplementation”, is now approved in many countries including Germany as a drug, or more commonly as a medical product.
Individual HA products differ with regard to their manufacture (e.g. from cockscomb, or produced by fermentation), their molecular weight (0.5-6 MDa), their degree of cross-linking, their viscosity and their frequency of application (1-5 intraarticular injections per series). The half-life of the HA preparations depends on their molecular mass and lies within a range of 17 to 60 hours. The heterogeneity of the clinical trials in which HA preparations of high or low molecular weight were compared does not allow any preferential recommendation for any one particular preparation.
Despite the large number of scientific studies, the effectiveness of this form of therapy remains a matter of debate in the literature. Although preclinical results and a number of initial exploratory studies produced encouraging results, no extensive studies with a generally accepted and standardized methodology have been carried out that have confirmed a structure-modifying or chondroprotective effect. A relevant pain inhibition has been described in more recent, high-evidence-level meta-analyzes. 9,44,45 The analgesic effect is delayed and after a maximum at two months can last up to six months.44 Also, because of its invasive nature, the new AWMF guideline for knee OA6 considers that intra-articular HA injection should only be indicated wherever application of NSAIDs is not possible due to side effects or contraindications, or wherever they are not sufficiently effective.
While the AAOS23 and NICE7 do not recommend the use of HA products, the new AWMF guideline for knee OA5 has made recommendations consistent with the practice-oriented argumentation of the ESCEO-group.46,47 They refer to the fact that intra-articular HA induces other, often less serious adverse effects than NSAIDs, opioids or corticosteroids, and that HA should therefore be considered for a differentiated application. The undesirable effects of HA include joint reactions which are usually mild and moderate, like minor knee pain, redness, and swelling around the joint area. These can be easily and adequately treated by protecting the joint area from stress, application of an ice compress for five to ten minutes, and analgesics. The symptoms only usually persist for a few days. Local or general hypersensitivity reactions are rare.
Slow Acting Drugs in Osteoarthritis (SADOAs)
Glucosamine and chondroitin sulphate belong to the group of slow-acting drugs in osteoarthritis (SADOAs). Due to the slow onset of symptomatic relief, the two SADOAs are also referred to as Symptomatic Slow Acting Drugs in Osteo- Arthritis (SYSADOAs).3 While the body of evidence for the symptom-reducing effect of both substances is still contradictory, the new AWMF guideline for knee OA has for the first time issued a recommendation for glucosamine.6
A number of guidelines recommend or provide positive statements regarding treatment with glucosamine, although reference is made to the still rather contradictory body of evidence concerning its use.7,8,22,23,46,49 The application of glucosamine is therefore only recommended by the ACR under certain circumstances,22 while neither the British NICE7 nor the AAOS23 recommend the use of glucosamine for knee OA. The guideline of the OARSI from 2014,8 in turn, considered its recommendation on the use of glucosamine as “uncertain” due to the small effect size and the heterogeneity observed between the various studies.
Despite the contradictory information on the symptom-relieving effect, according to the new AWMF guideline for knee OA,6 and other recent publications, there are a few indications for which the application of glucosamine may be considered.46,49 In patients with contraindications for NSAIDs or with an increased gastrointestinal and/or cardiovascular risk, orally applied glucosamine can be explored as a treatment before any more invasive therapies afflicted with more adverse effects and complications are carried out. This can of course be of special concern for elderly patients. Also, any wish of a patient to undergo a reduced side effect trial therapy should also be taken into account. If no improvement occurs, the therapy should be interrupted, although no later than after three months of therapy. In this respect, the new AWMF guideline for knee OA6 does not recommend a general “background therapy”, but instead recommends at least considering a differentiated use of glucosamine.
The extent to which glucosamine and chondroitin sulfate number amongst the disease- modifying osteoarthritis drugs (DMOADs) and which therefore act by modifying the structure respectively chondroprotectively is a matter of debate. The results are in part contradictory and there is no clinical proof beyond doubt for their effectiveness. While some studies and meta-analyzes for glucosamine and/or chondroitin sulphate alone or in combination reported a structure-modifying effect,50-53 others could not confirm this.54,55
Outlook
OA is no longer considered as an inevitable “war wound” for the aged, and today the ageing individual prefers to keep staying mobile and physically fit well into his or her old age. For the drug therapy of OA, this understandable desire means that many challenges need to be met: Amongst the many medications that must be applied to an individual patient, an optimally suited drug needs to be prescribed that works causally while at the same time inducing only a few or at best no side effects.
Over the last few decades many academia- based, although for the most part industry- based efforts have been expended on developing better tolerated anti-inflammatory agents as well as new and indeed effective disease-modifying drugs. However, up until now, no chondroprotectively active drug has been approved either in Europe or the USA. A possible reason for this is the usually late diagnosis of OA, whereby because of the already existing cartilage injury, any effective chondroprotection afforded by a pharmaceutical compound is counteracted by mechanical destruction. The diagnosis of the onset of OA for determining the early symptomless phases of the degenerative process as well as the intensity and progression of OA are still proving to be problematic issues. Also, any quantitative objective evaluation of the OA cartilage damage poses its own problems. These issues that are all in urgent need of research must be resolved before any causal therapy for OA can be established.
In order to optimize the therapeutic options that are still possible today, further studies need to be carried out. Studies in which elderly patients are also preferentially investigated, given the high level of drug consumption among these patients, should also now be the rule rather than the exception. Studies in which the salt glucosamine sulphate is compared directly with glucosamine hydrochloride are also necessary. For prescription practice, it would also be very helpful to know the intra-articular injection frequency of HA that would actually be necessary, and for which patients. It would also be very interesting to study how strongly and how long various HA preparations act as a direct comparison within a single study. The implementation of direct comparative studies should only be financed through public funding, as has been the case with the GAIT study by the NIH,56 so that scientific and public acceptance of the results can also be fostered.
Preparations for the treatment of OA are available that contain devil’s claw, herbaceous nettle extract or indeed frankincense. 57 The currently available, sometimes open label and/or small studies and observational studies cannot be considered as proof of efficacy due to their methodological shortcomings.57 There is still considerable need for research here. The new AWMF guideline for knee OA6 could only find an adequate evidence base for comphrey extract gel regarding its analgesic effect. In view of the great popularity of herbal medicinal products and their potential for reducing the consumption of NSAIDs, further, high-quality, publicly funded clinical studies are urgently needed. This will allow an objective clinical evaluation of their clinical pharmacology, so that a solid evidence base can be provided for the inclusion of phytopharmaceuticals into the current pharmacotherapeutic algorithm for OA.
Funding Statement
Funding: none
References
- 1.Steinmeyer J, Bock F, Stöve J, Jerosch J, Flechtenmacher J. Medikamentöse Therapie der Gonarthrose – besondere Aspekte der neuen Leitlinie. OUP 2018;7:374-80. [Google Scholar]
- 2.Fuchs J, Rabenberg M, Scheidt-Nave C. Prävalenz ausgewählter muskuloskelettaler Erkrankungen. Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1). Bundesge - sundheitsbl 2013;56:678-86. [DOI] [PubMed] [Google Scholar]
- 3.Steinmeyer J, Konttinen YT. Oral treatment options for degenerative joint disease--presence and future. Adv Drug Deliv Rev 2006;58:168-211. [DOI] [PubMed] [Google Scholar]
- 4.Grandt D, Schubert I. Arzneimittelreport 2016. Analysen zur Arzneimitteltherapie und Arzneimittel - therapiesicherheit. In: Barmer GEK. (Ed.): Schriftenreihe zur Gesundheitsanalyse. Berlin: Asgard Verlagsservice GmbH, 2016, Vol.39. [Google Scholar]
- 5.Holt S, Schmiedl S, Thürmann PA. Potentially inappropriate medications in the elderly: The PRISCUS List. Dtsch Arztebl Int 2010;107:543-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Association of the Scientific Medical Societies in Germany (AWMF). S2k guideline for gonarthrosis. Register number 033-004, current as of 18/01/2018, under: http://www.awmf.org/leitlinien/detail/ll/033-004.html (accessed on 20/03/2018). [Google Scholar]
- 7.NICE National Institute for Health and Care Excellence. Osteoarthritis: care and management. Clinical guideline. Published on 12/02/2014, at: http://www.nice.org.uk/guidance/cg177/resources/osteoarthritis-care-andmanagement-35109757272517 (accessed on 20/03/2018). [Google Scholar]
- 8.McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthrit Cartilage 2014;22:363-88. [DOI] [PubMed] [Google Scholar]
- 9.Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis. A systematic review and network meta-analysis. Ann Intern Med 2015;152:46-54. [DOI] [PubMed] [Google Scholar]
- 10.da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet 2016;387:2093-105. [DOI] [PubMed] [Google Scholar]
- 11.Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ 2014;350:1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stamer UM, Gundert-Remy U, Biermann E, et al. Metamizol - Überlegungen zum Monitoring zur frühzeitigen Diagnose einer Agranulo - zytose. Schmerz 2017;31:5-13. [DOI] [PubMed] [Google Scholar]
- 13.Jerosch J, Breil-Wirth A. Worauf müssen wir beim Einsatz von Metamizol achten? OUP 2017;6:577-81. [Google Scholar]
- 14.Federal Institute for Drugs and Medical Products. Metamizol (Novalgin, Berlosin, Novaminsulfon, etc.): BfArM weist auf richtige Indikationsstellung und Beachtung von Vorsichtsma - ßnahmen und Warnhinweisen hin. Published 28/05/2009, at: https://www.-bfarm.de/SharedDocs/Risikoinfor-mationen/Pharmakovigilanz/DE/RI/2009/RI-metamizol.html (accessed on 20/03/2018). [Google Scholar]
- 15.Association of the Scientific Medical Societies in Germany (AWMF). Empfehlungen der S3-Leitlinie - Langzeitanwendung von Opioiden bei nicht tumorbedingten Schmerzen - "LONTS". Register number 145/003, version 09/2014, revision 01/2015, at: http://www.awmf.org/uploads/tx_szleitlinien/145-003l_S3_LONTS_2015-01.pdf (retrieved on 20/03/2018). [Google Scholar]
- 16.da Costa BR, Nüesch E, Kasteler R, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev 2014;CD003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welsch P, Sommer C, Schiltenwolf M, Häuser W. Opioids in chronic non-cancer pain: Are opioids superior to nonopioid analgesics? A systematic review and metaanalysis of efficacy and harms of randomized head-to-head comparisons of opioids versus non-opioid analgesics in studies of at least four weeks duration. Schmerz 2015;29:85-95. [DOI] [PubMed] [Google Scholar]
- 18.Miller M, Sturmer T, Azrael D, et al. Opioid analgesics and the risk of fractures in older adults with arthritis. J Am Geriatr Soc 2011;59:430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andresen V, Wedel T. Opioidinduzierte Obstipation. Arzneiverordnung in der Praxis 2016;43:21-9.. [Google Scholar]
- 20.Coxib and traditional NSAID Trialists’ (CNT) Collaboration. Vascular and upper gastrointestinal effects of nonsteroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013;382:769-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derry S, Conaghan P, Da Silva JAP, et al. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev 2016;CD007400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res 2012;64:465-74. [DOI] [PubMed] [Google Scholar]
- 23.AAOS American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee, evidencebased guideline, 2nd edition. Published on 18/05/2013, at: http://www.aaos.org/uploadedFiles/PreProduction/Quality/Guidelines_and_Reviews/Osteoarthritis%20of%20the%20Knee%20-%20non-arthroplasty.pdf (accessed on 20/03/2018). [Google Scholar]
- 24.Fischbach W, Baerwald C, Darius H, et al. Schmerztherapie mit traditionellen NSAR und Coxiben – eine interdisziplinäre Betrachtung. Dtsch Med Wochenschr 2013;138:91-6. [DOI] [PubMed] [Google Scholar]
- 25.Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med 2016;375:2519-29. [DOI] [PubMed] [Google Scholar]
- 26.Drug Committee the German Medical Association (AkdÄ): Nichtsteroidale Antirheumatika (NSAR) im Vergleich: Risiko von Komplikationen im oberen Gastrointestinaltrakt, Herzinfarkt und Schlaganfall. Dtsch Ärztebl 2013;110: A1447-8 [Google Scholar]
- 27.Hippisley-Cox J, Coupland C, Logan R. Risk of adverse gastrointestinal outcomes in patients taking cyclo-oxygenase- 2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ 2005;331:1310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis SC, Langman MJS, Laporte JR, et al. Dose-response relationships between individual nonaspirin nonsteroidal anti-inflammatory drugs (NANS AIDs) and serious upper gastrointestinal bleeding: a meta-analysis based on individual patient data. Brit J Clin Pharm 2002;54:320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castellsague J, Riera-Guardia N, Calingaert B, et al. Individual NSAIDs and upper gastrointestinal complications: a systematic review and meta-analysis of observational studies (the SOS project). Drug Saf 2012;35:1127-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rostom A, Muir K, Dube C, et al. Prevention of NSAID-related upper gastrointestinal toxicity: a meta-analysis of traditional NSAIDs with gastroprotection and COX-2 inhibitors. Drug Healthc Patient Saf 2009;1:47-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spiegel BM, Farid M, Dulai GS, et al. Comparing rates of dyspepsia with Coxibs vs NSAID+PPI: a meta-analysis. Am J Med 2006;119:448. [DOI] [PubMed] [Google Scholar]
- 32.Bundesärztekammer (BÄK) Kassenärztliche Bundesvereinigung (KBV) Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF): Nationale Versorgungsleitlinie Nicht-spezifischer Kreuzschmerz – Langfassung, 2nd edition. Version 1, 2017. Under: http://www.awmf.org/uploads/tx_szleitlinien/nvl-007l_S3_Kreuzschmerz_2017-03.pdf (accessed on 20/03/ 2018). [Google Scholar]
- 33.European Medicines Evaluation Agency (EMEA): Public assessment report for medicinal products containing non-selective non steroidal antiinflammatory drugs (NSAIDs). Published on 07/11/2006, at: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2010/01/WC500054344.pdf (accessed on 20.03.2018) [Google Scholar]
- 34.EMEA: PRAC recommends the same cardiovascular precautions for diclofenac as for selective COX-2 inhibitors. EMA/353084/2013. Published on 14/06/2013, at: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2013/06/WC500144451.pdf (accessed on 20/03/2018) [Google Scholar]
- 35.Drug Commission of the German Medical Association (AkdA). Diclofenac - Neue Kontraindikationen und Warnhinweise nach europaweiter Überprüfung der kardiovaskulären Sicherheit. Red hand letter dated 15/07/2013, at: https://www.akdae.de/Arzneimittelsicherheit/RHB/Archiv/2013/20130715.pdf (accessed on 20/03/2018) [Google Scholar]
- 36.Varas-Lorenzo C, Riera-Guardia N, Calingaert B, et al. Myocardial infarction and individual nonsteroidal antiinflammatory drugs meta-analysis of observational studies. Pharmaco - epidemiol Drug Saf 2013;22:559-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Association of the Scientific Medical Societies in Germany (AWMF). S1- Leitlinie Intraartikuläre Punktionen und Injektionen: Hygienemaßnahmen. Register number 029/006, last update 08/2015, under: http://www.awmf.org/uploads/tx_szleitlinien/029-006l_S1_Hygiene_intraartikulaere_Punktionen_und_Injektionen_2015-08_01.pdf (accessed on 20/03/2018). [Google Scholar]
- 38.Hepper CT, Halvorson JJ, Duncan ST, et al. The efficacy and duration of intraarticular corticosteroid injection for knee osteoarthritis: a systematic review of level I studies. J Am Acad Orthop Surg 2009;17:638-66. [DOI] [PubMed] [Google Scholar]
- 39.Arden NK, Reading IC, Jordan KM, et al. A randomised controlled trial of tidal irrigation vs corticosteroid injection in knee osteoarthritis: the KIVIS Study. Osteoarthritis Cartilage 2008;16:733-9. [DOI] [PubMed] [Google Scholar]
- 40.Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: metaanalysis. BMJ 2004;328:869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirsch G, Kitas G, Klocke R. Intra-articular corticosteroid injection in osteoarthritis of the knee and hip: factors predicting pain relief--a systematic review. Semin Arthritis Rheum 2013;42:451-73. [DOI] [PubMed] [Google Scholar]
- 42.McAlindon TE, LaValley MP, Harvey WF, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: A randomized clinical trial. JAMA 2017;317:1967-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kosinska MK, Ludwig TE, Liebisch G, et al. Articular joint lubricants during osteoarthritis and rheumatoid arthritis display altered levels and molecular species. PLoS One 2015;10:e0125192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bannuru RR, Natov NS, Dasi UR, et al. Therapeutic trajectory following intraarticular hyaluronic acid injection in knee osteoarthritis-meta-analysis. Osteoarthritis Cartilage 2011;19:611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller LE, Block JE. US-approved intra-articular hyaluronic acid injections are safe and effective in patients with knee osteoarthritis: Systematic review and meta-analysis of randomized saline-controlled trials. Clin Med Insights Arthritis Musculoskelet Disord 2013;6:57-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruyere O, Cooper C, Pelletier JP, et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum 2014;44:253-63. [DOI] [PubMed] [Google Scholar]
- 47.Bruyere O, Cooper C, Pelletier JP, et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis- From evidence-based medicine to the real-life setting. Semin Arthritis Rheum 2016;45:S3-11. [DOI] [PubMed] [Google Scholar]
- 48.Wandel S, Juni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ 2010;341:c4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henrotin Y, Marty M, Mobasheri A. What is the current status of chondroitin sulfate and glucosamine for the treatment of knee osteoarthritis? Maturitas 2014;78:184-7. [DOI] [PubMed] [Google Scholar]
- 50.Hochberg MC, Zhan M, Langenberg P. The rate of decline of joint space width in patients with osteoarthritis of the knee: a systematic review and metaanalysis of randomized placebo-controlled trials of chondroitin sulfate. Curr Med Res Opin 2008;24:3029-35. [DOI] [PubMed] [Google Scholar]
- 51.Lee YH, Woo JH, Choi SJ, et al. Effect of glucosamine or chondroitin sulfate on the osteoarthritis progression: a meta-analysis. Rheumatol Int 2010;30: 357-63. [DOI] [PubMed] [Google Scholar]
- 52.Martel-Pelletier J, Roubille C, Abram F, et al. First-line analysis of the effects of treatment on progression of structural changes in knee osteoarthritis over 24 months: data from the osteoarthritis initiative progression cohort. Ann Rheum Dis 2015;74:547-56. [DOI] [PubMed] [Google Scholar]
- 53.Raynauld JP, Pelletier JP, Abram F, et al. Long-term effects of glucosamine and chondroitin sulfate on the progression of structural changes in knee osteoarthritis: Six-year followup data from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 2016;68:1560-6. [DOI] [PubMed] [Google Scholar]
- 54.Sawitzke AD, Shi H, Finco MF, et al. The effect of glucosamine and/or chondroitin sulfate on the progression of knee osteoarthritis: a report from the glucosamine/chondroitin arthritis intervention trial. Arthritis Rheum 2008;58:3183-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang S, Eaton CB, McAlindon TE, Lapane KL. Effects of glucosamine and chondroitin supplementation on knee osteoarthritis: an analysis with marginal structural models. Arthritis Rheumatol 2015;67:714-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulphate, and the two in combination for painful knee osteoarthritis. New Engl J Med 2006;354:795-808. [DOI] [PubMed] [Google Scholar]
- 57.Steinmeyer J. Weihrauch zur Behandlung der Arthrose? Arzneiveror - dnung in der Praxis 2017;44:132-4. [Google Scholar]


