Abstract
Triclosan (TCS) is a phenolic antimicrobial chemical used in consumer products and medical devices. Evidence from in vitro and in vivo animal studies has linked TCS to numerous health problems, including allergic, cardiovascular, and neurodegenerative disease. Using Caenorhabditis elegans as a model system, we here show that short-term TCS treatment (LC50: ~0.2 mM) significantly induced mortality in a dose-dependent manner. Notably, TCS-induced mortality was dramatically suppressed by co-treatment with non-ionic surfactants (NISs: e.g., Tween 20, Tween 80, NP-40, and Triton X-100), but not with anionic surfactants (e.g., sodium dodecyl sulfate). To identify the range of compounds susceptible to NIS inhibition, other structurally related chemical compounds were also examined. Of the compounds tested, only the toxicity of phenolic compounds (bisphenol A and benzyl 4-hydroxybenzoic acid) was significantly abrogated by NISs. Mechanistic analyses using TCS revealed that NISs appear to interfere with TCS-mediated mortality by micellar solubilization. Once internalized, the TCS-micelle complex is inefficiently exported in worms lacking PMP-3 (encoding an ATP-binding cassette (ABC) transporter) transmembrane protein, resulting in overt toxicity. Since many EDCs and surfactants are extensively used in commercial products, findings from this study provide valuable insights to devise safer pharmaceutical and nutritional preparations.
Keywords: Caenorhabditis elegans, endocrine-disrupting chemicals, micelle, non-ionic surfactants, phenolic compound, PMP-3/ABC transporter, triclosan
INTRODUCTION
Endocrine-disrupting chemicals (EDCs) are exogenous compounds that perturb the physiology of the endocrine glandular tissue (Swedenborg et al., 2009). These compounds can disturb hormone production, release, transport, and metabolism (Kabir et al., 2015). Routes of human exposure are varied owing to the wide array of applications and sources rich in EDCs. Transdermal absorption from cosmetics and personal hygiene products, ingestion in drinking water and food packaging material, and inhalation in dust represent the major and most common forms of exposure that carry the greatest risk potential (Diamanti-Kandarakis et al., 2009). Furthermore, the developing neuroendocrine tissue of neonates is constantly being exposed to high concentrations of EDCs in breast milk and infant formula (Azzouz et al., 2016; Fang et al., 2010), implicating these xenobiotics in developmental, neurological, and reproductive anomalies (Schug et al., 2011).
Classification of EDCs is complicated as the number of newly identified, and erroneously recognized compounds, continues to steadily grow. Although many remain insufficiently characterized, phenolic EDCs are among the most common and well-studied classes. A prominent example is triclosan (TCS); an antimicrobial extensively used in the manufacture of plastics, toys, cosmetics, and kitchenware (Fig. 1A). TCS has also been used for decades in hospital settings as an antiseptic and a disinfectant (Dann and Hontela, 2011; Rodricks et al., 2010). The antimicrobial activity of TCS is attributed to the compound’s interference with the enzyme enoyl-acyl carrier protein reductase (FabI), which is required for fatty acid and biotin biosynthesis (Rodricks et al., 2010). Beside its antimicrobial properties, TCS toxicity has been studied in various living systems including humans, and the chemical has been shown to build up in body fluids including blood, urine, and breast milk (Fang et al., 2010; Rodricks et al., 2010). Due to its widespread use and high chlorine content, TCS and its derivatives are ubiquitous in soil and aquatic environments, and have been detected in wastewater treatment systems as well as drinking water sources (Benotti et al., 2009; Escalada et al., 2005; Li et al., 2010; McAvoy et al., 2002).
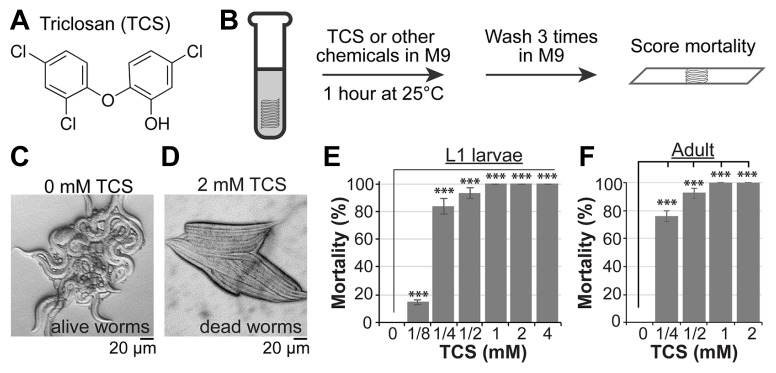
Fig. 1. TCS induces mortality of wild-type worms.
(A) Chemical structure of TCS. (B) Strategy for chemical treatment. (C and D) DIC pictures of wild-type worms in the absence or presence of TCS. (E and F) Percent mortality in TCS-treated wild-type L1 larvae. Standard deviation bars were calculated from at least three independent experiments (n>300). *P < 0.05; **P < 0.01; ***P < 0.001; N.S. (Not statistically significant).
The nematode Caenorhabditis elegans (C. elegans) has emerged as an attractive model animal for functional analysis of various bioactive compounds (Honnen, 2017; Hunt, 2017; Tejeda-Benitez and Olivero-Verbel, 2016). Recent reports have shown that TCS exposure increased the mortality and infertility of wild-type C. elegans worms in a dose-dependent manner (Garcia-Espineira et al., 2018; Lenz et al., 2017; Vingskes and Spann, 2018; Yoon et al., 2017). To date, although significant progress has been made in our understanding of TCS toxicity, studies devoted to the identification of clinically or industrially relevant TCS inhibitors are extremely scarce. In this study, we demonstrate that nonionic surfactants (NISs), such as Tween 20 (Tw20), Tween 80 (Tw80), NP-40, and Triton X-100 (TX100), act as potent antagonists of phenolic EDCs, including TCS, bisphenol A (BPA), and benzyl 4-hydroxybenzoic acid (B4HB). Mechanistic analyses revealed that NISs inhibit TCS-induced mortality by micellar solubilization, and that internalized TCS-micelle complex appears to be exported by PMP-3 (encoding an ATP-binding cassette (ABC) transporter) protein. Given the concerns surrounding TCS exposure, our findings may provide an innovative approach to reduce the burden of TCS on ecosystems and human health alike.
MATERIALS AND METHODS
Chemicals and reagents
All chemicals used in this study were purchased from Sigma Aldrich (MO, USA) and were of analytical grade. TCS and benzyl 4-hydroxybenzoic acid (B4HB) were prepared in ethanol as 0.1 M stock solutions. Bisphenol A (BPA) was dissolved in methanol to obtain a 0.1 M stock solution, while 0.1 M stock solutions of sodium dodecyl sulfate (SDS) and sodium azide (NaN3) were made in distilled water.
Strains and maintenance
C. elegans wild-type Bristol isolate (N2) and pmp-3(ok1087) mutant worms were obtained from the Caenorhabditis Genetics Center (CGC). All strains were cultured at 20°C in nematode growth medium (NGM) as previously described (Brenner, 1974).
Toxicity assays
Embryos were obtained by sodium hypochlorite (0.5 M NaOH and 1.2% NaClO) treatment of gravid hermaphrodites and incubated in M9 buffer (22 mM KH2PO4, 42 mM Na2HPO, 86 mM NaCl, and 1 mM MgSO4) at 20°C overnight, as described elsewhere (Yoon et al., 2016). Hatched L1 animals were either exposed to chemicals or were allowed to grow to adults on NGM plates for 3 days at 20°C before exposure. All chemicals were diluted in M9 or M9/0.1% NISs to the final testing concentrations. Treatment groups were compared to the vehicle control, which did not exceed 0.2% in each case. The mortality rate was calculated visually by counting live and dead worms using a bright field microscope (Fig. 1B). Live worms exhibited normal locomotive behavior (Fig. 1C), whereas dead worms were nonmotile and appeared rod-like in shape (Fig. 1D).
Antimicrobial susceptibility testing
E. coli OP50 bacteria were grown at 37°C for 5 h in Lysogeny Broth (LB) medium. Exposure was conducted in the same medium supplemented with TCS ranging from 0.001 mM to 0.05 mM with or without 0.1% Tw20. The optical density (OD600) was measured spectrophotometrically every two hours as an indicator of bacterial growth.
Pharyngeal pumping rate
Wild-type adult worms were incubated for 1 h at 25°C in M9 buffer with or without 0.1% Tw20, before they were plated on NGM and examined for pumping using a dissecting microscope. Grinder movements were monitored for one minute, and the number of pumps per minute (ppm) was recorded.
Disruption of intracellular micelles
NIS micelles were heat-disrupted at 35°C. Following TCS treatment with or without 0.1% Tw20, two approaches were followed for micelle disruption (Fig. 4A). In method I, worms were immediately incubated at 35°C for an additional hour, whereas in method II, removal of extracellular TCS-Tw20 complexes by sequential washing in M9 buffer preceded incubation at 35°C.
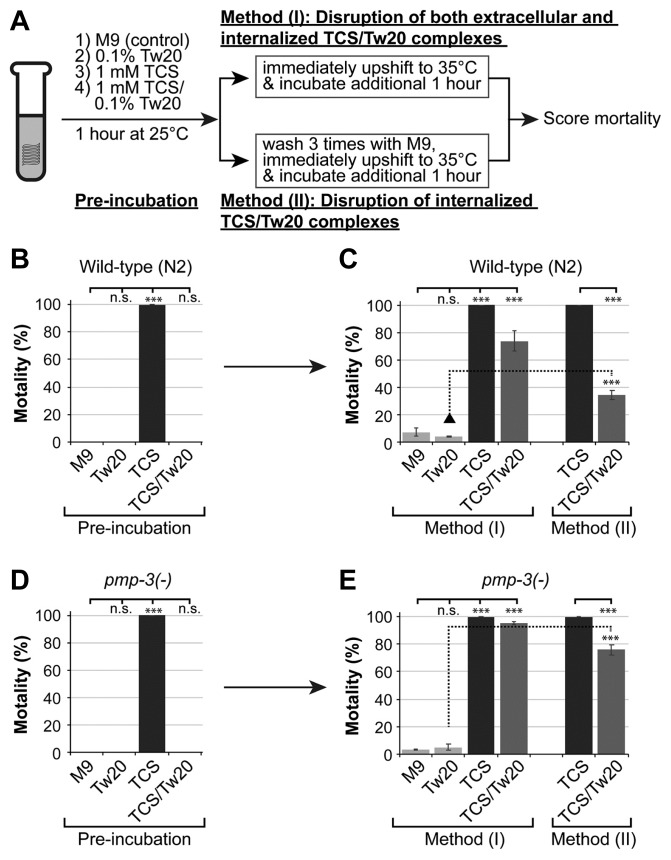
Fig. 4. Tw20 inhibits TCS-induced mortality via micelle formation.
(A) Exposure strategy. (B, C) Effects of Tw20 micelle formation on TCS-induced mortality in wild-type worms. (D, E) Role of PMP-3 in the export of TCS-Tw20 micellar complex. Standard deviation bars were calculated from at least three independent experiments (n > 300). *P < 0.05; **P < 0.01; ***P < 0.001; N.S. (Not statistically significant).
Statistical analysis
Results are expressed as arithmetic means ± SD of at least three independent replicates (n > 300). Comparative assessments between control and treatment groups were conducted using the paired t-student test. Statistical significance was determined by a p value of less than 0.05.
RESULTS
TCS increases mortality dose-dependently
Amongst phenolic EDCs, we initially investigated TCS due to its widespread occurrence and well-documented toxicity (Rodricks et al., 2010) (Fig. 1A). In eukaryotes, TCS disrupts mitochondrial oxidative phosphorylation and leads to profoundly increased reactive oxygen species (ROS) (Weatherly et al., 2016). Also, we have recently reported that TCS induces toxicity, at least in part, by disrupting the SKN-1 (SKiNhead-1)/NRF2 (erythroid-2-related factor 2)-mediated oxidative stress response in both C. elegans and human stem cells (Yoon et al., 2017).
To initially determine the effect of TCS on C. elegans mortality, synchronized L1 larvae were treated with varying concentrations of TCS (0, 0.125, 0.25, 0.5, 1, 2, and 4 mM) for 1 h at 25°C. As shown in Fig. 1E, the mortality rate of L1 worms increased in a dose-dependent manner with a TCS concentration of half maximal response (EC50) of ~0.2 mM. A similar dose-dependent pattern of increased mortality was also observed in adult worms (Fig. 1F). These results suggest that TCS significantly increased the mortality of wild-type (N2) worms in a dose-dependent manner.
NISs abrogate TCS-induced mortality
Hydrophobic substances can be emulsified in micelles formed by NISs such as Tw20 (Lu and Park, 2013), and the benzene ring in TCS imparts a hydrophobic nature to the antiseptic (Petersen, 2016). To test the hypothesis that TCS-induced mortality could be neutralized by NISs, both L1 larvae and adult worms were treated with 0.125–8 mM TCS in the absence or presence of 0.1% Tw20 for 1 h at 25°C, and the mortality rate of both groups was calculated. Interestingly, co-treatment of TCS and Tw20 led to a profound decrease in the mortality of both stages when compared to TCS alone (Figs. 2A and Supplementary Fig. S1A). The highest TCS concentration susceptible to 0.1% Tw20 was around 8 mM and 4 mM for L1 larvae and adult worms, respectively (Figs. 2A and Supplementary Fig. S1A).
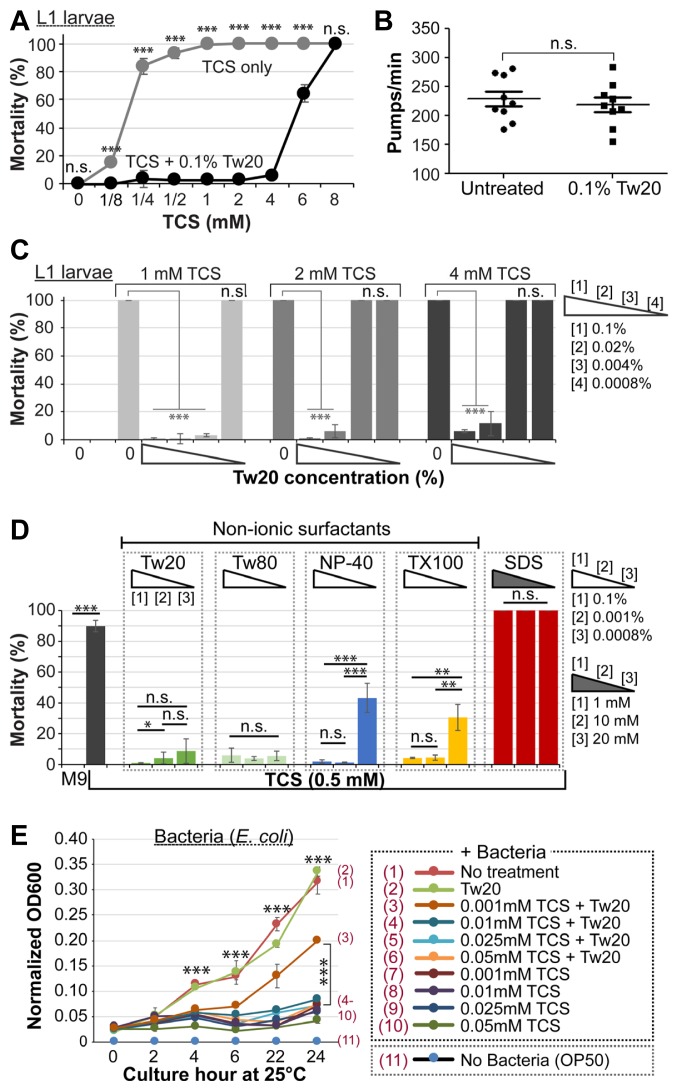
Fig. 2. Protective role of NISs against TCS.
(A) Effect of Tw20 on TCS-induced mortality in L1 larvae. (B) Effect of Tw20 on pharyngeal pumping. (C) Dose-dependent effect of TCS and Tw20 on mortality. (D) Effect of NISs on TCS-induced mortality. (E) Effect of Tw20 on the antimicrobial activity of TCS. Standard deviation bars were calculated from at least three independent experiments (n > 300). *P < 0.05; **P < 0.01; ***P < 0.001; N.S. (Not statistically significant).
Ingestion in C. elegans is accomplished through the pharynx, and requires two sequential events; pumping and peristalsis. Pharyngeal movement is related to food intake, and is influenced by environmental conditions in the immediate vicinity of the worm (Song and Avery, 2013). Thus, we examined if the protective role of Tw20 is related to restricted pharyngeal pumping. Which would hinder TCS uptake. To this end, adults were exposed to 0.1% Tw20 or left untreated for 1 h at 25°C. Worms were then plated and grinder movements (number of pumps per minute) were recorded under a differential interference contrast (DIC) microscope. Our results showed no significant difference in the rate of pharyngeal pumping in the absence or presence of Tw20 (Fig. 2B), which indicates that Tw20 does not neutralize TCS toxicity by reducing its physical intake.
To determine the minimum effective concentration of Tw20 required to confer protection against lethal concentrations of TCS, L1 larvae were incubated for 1 h with 1, 2, and 4 mM TCS in the absence or presence of 0.1, 0.02, 0.004, and 0.0008 % Tw20. As for L1 larvae, at least 0.02% Tw20 was sufficient to protect against 4 mM TCS, while 0.004% Tw20 was sufficient against 1 mM TCS (Fig. 2C). Parallel to L1 larvae, the mortality rate of adult worms at 1 and 2 mM TCS was significantly abrogated with at least 0.004% and 0.02% Tw20, respectively (Supplementary Fig. S1B).
Next, we tested the effects of other NISs, including Tw80, NP-40, and TX100 on TCS-induced mortality. To this end, L1 staged wild-type worms were incubated with or without 0.5 mM TCS (a minimum concentration with >80% mortality) in the absence or presence of 0.1–0.0008% Tw80, NP-40, or TX100 for 1 h at 25°C, and the mortality rate was calculated as described earlier. As seen in Fig. 2D, all NISs tested significantly protected the worms against 0.5 mM TCS dose-dependently. In industrial settings, anionic surfactants such as sodium dodecyl sulfate (SDS) are also added to commercial products to solubilize TCS (Babich and Babich, 1997). In order to determine if SDS is also capable of antagonizing TCS toxicity, we incubated L1 larvae with 0.5 mM TCS in the presence of 1, 10, and 20 mM SDS for 1 h at 25°C and scored the mortality rate. SDS was found to be lethal to worms at 10 and 20 mM (Fig. 2D). Although 1 mM SDS was not toxic, it nonetheless failed to protect against TCS (Fig. 2D). This suggests that TCS-induced mortality is abrogated by co-treatment of NISs (i.e. Tw20, Tw80, NP-40, and TX100), but not by SDS.
We then sought to inquire whether Tw20 could also neutralize the antimicrobial activity of TCS. To this end, E. coli bacteria were cultured with or without 0.001–0.2 mM TCS in the absence or presence of 0.1% Tw20 for 24 h at 25°C. We specifically cultured E. coli bacteria at 25°C, instead of 37°C, to preserve the micellar state of Tw20. Optical density at 2-hour intervals was measured as a function of bacterial growth. Although TCS unsurprisingly inhibited bacterial growth at all concentrations tested, significant resistance was observed under conditions of a combination of 0.001 mM TCS and 0.1% Tw20 (Fig. 2E, compare (3) and (7)). These findings suggest that the inhibitory action of NISs against TCS is also effective in bacteria at a certain concentration.
NISs mitigate the toxicity induced by other phenolic EDCs: BPA and B4HB
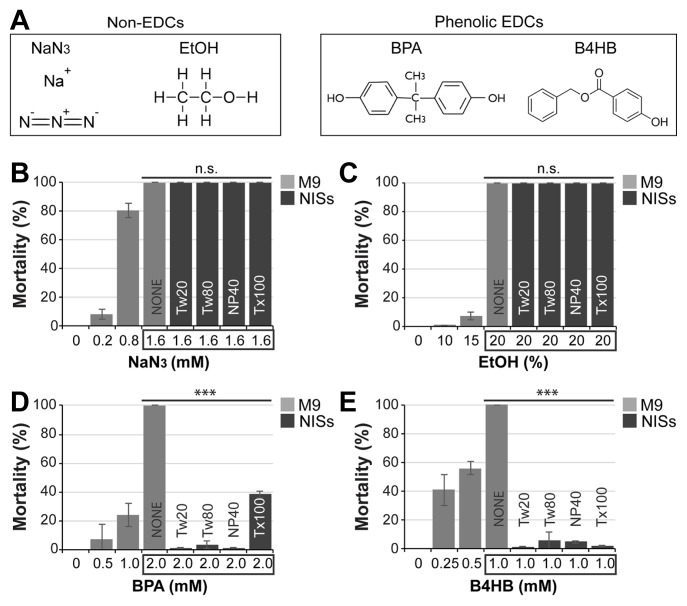
To assess the range of compounds sensitive to NIS interference, we tested the effect of NISs on other toxicants that are not recognized as EDCs (Fig. 3A, left). Sodium azide (NaN3) is a polar, ionic salt commonly used as a solution preservative owing to its biocidal properties (Ishikawa et al., 2006). It interferes with mitochondrial oxidative phosphorylation by chelating iron ions required for cytochrome oxidase activity (Ishikawa et al., 2006). We evaluated the ability of NISs to subvert sodium azide toxicity by incubating L1 larvae with or without 0.2–1.6 mM sodium azide, in the absence or presence of 0.1% NISs for 24 h at 25°C. Fig. 3B shows that the dose-responsive increase in mortality was not nullified by co-treatment with NISs. Ethanol (EtOH) is another polar compound with disruptive behavioral effects on C. elegans (Davies et al., 2004) (Fig. 3A, left). To test whether NISs could protect against EtOH toxicity, L1 larvae were incubated with or without 10%–20% EtOH in the absence or presence of 0.1% NISs for 1 h at 25°C, and the mortality rate was subsequently scored. Our data show that 20% EtOH resulted in 100% mortality in wild-type worms which was not reversed by co-treatment with 0.1% NISs (Fig. 3C).
Fig. 3. NISs suppress the mortality induced by other phenolic EDCs.
(A) Chemical structures of non-EDCs and phenolic EDCs. (B–E) Effects of NISs on chemical toxicant-induced mortality. For NaN3 and BPA, total exposure period was 24 h at 25°C. For EtOH and B4HB, worms were treated for 1 h at 25°C. Standard deviation bars were calculated from at least three independent experiments (n > 300). *P < 0.05; **P < 0.01; ***P < 0.001; N.S. (Not statistically significant).
Although we cannot exclude all other possibilities, these results indicate that molecular similarity among compounds may be a determining factor in their susceptibility to NISs. Hence, we examined two other chemicals that are closely related to TCS in terms of both their chemical nature (a common phenol ring) and activity (endocrine disruption) – Bisphenol A (BPA) and Benzyl 4-hydroxybenzoic acid (B4HB) (Fig. 3A, right). The xenoestrogenic activity of BPA is associated with increased proliferation of ovarian and breast cancer cells (Dong et al., 2011), genotoxicity (Pupo et al., 2012), and elevated prolactin, estradiol, and progesterone levels in females (Miao et al., 2015). B4HB is a paraben widely used as a preservative in cosmetics and food processing (Ye et al., 2006). Exposure to parabens has been strongly linked to human health concerns mainly due to their estrogenicity and proliferative stimulation of breast cancer cells (Byford et al., 2002). Moreover, butylparaben has been shown to cause DNA damage in human sperm (Meeker et al., 2011). To test if NISs could protect against BPA-induced mortality, L1 larvae were incubated with or without 0.5–2.0 mM BPA in the absence or presence of 0.1% NISs for 24 h at 25°C. In agreement with previous reports (Watanabe et al., 2005), BPA caused a dose-dependent increase in mortality and, interestingly, co-treatment with 0.1% NISs significantly ablated BPA-induced mortality (Fig. 3D). We also determined the mortality of B4HB and its sensitivity to NIS inhibition. To this end, L1 larvae were incubated with or without 0.25–1.0 mM B4HB in the absence or presence of 0.1% NISs for 1 h at 25°C. As shown in Fig. 3E, B4HB resulted in a significant, dose-dependent increase in mortality at all concentrations tested. Interestingly, a similar pattern of inhibition to TCS and BPA was also observed in worms co-treated with B4HB and NISs (Fig. 3E). Taken together, these results suggest that NISs may protect against phenolic EDCs that share structural similarity to TCS.
Micellar solubilization is required for NIS-mediated protection
We next tested if NISs could inhibit the toxicity of TCS via micelle formation. Tw20 was chosen as a representative NIS as it showed potent inhibitory action against TCS concentration with 100% mortality (Fig. 2A). To this end, L1 larvae were incubated with or without 1 mM TCS in the absence or presence of 0.1% Tw20 for 1 h at 25°C (optimal temperature for micelle formation) (Fig. 4A). As observed earlier, 1 mM TCS resulted in 100% mortality, which was reversed by co-treatment with 0.1% Tw20 (Fig. 4B, Pre-incubation). To evaluate the importance of micellar solubilization for the anti-toxic activity of Tw20, micelles were heat-disrupted by upshifting exposure temperature to 35°C (Markovic-Housley and Garavito, 1986) for an additional hour (Fig. 4A, Method (I)). Notably, the mortality of worms co-treated with TCS and Tw20 significantly increased following the temperature upshift (Fig. 4C, Method (I)). This seems to indicate that sequestered TCS molecules within Tw20 micelles were released upon temperature-mediated micelle disruption and regained their lethal activity. Furthermore, to rule out the contribution of extracellular TCS in the observed mortality following the upshift, extracellular TCS-Tw20 complexes were removed by repeated washing and L1 larvae were then upshifted to 35°C for an additional hour (Fig. 4A, Method (II)). Under these conditions, up to ~30% increase in mortality in comparison to TW20-treated worms (Fig. 4C, Method (II)) was observed, which is apparently attributed to the internalized TCS-Tw20 complexes.
The PMP-3/ABC transporter modulates the absorption, metabolism, and cytotoxicity of pharmacological agents (Das et al., 2006). Of recent, we have reported that lack of PMP-3 increases susceptibility to TCS (Yoon et al., 2017). A reporter gene analysis showed that pmp-3 (promoter)::GFP is expressed in the pharynx, muscles, intestine, and stem cell niche (Supplementary Fig. S2). To ask if internalized TCS-Tw20 micelle complexes are exported out of the worms’ bodies through PMP-3, L1 stage pmp-3(ok1087) loss-of-function mutant worms (pmp-3(−)) were treated as described in Fig. 4A. As is the case with wild-type worms (Fig. 4B), 1 mM TCS caused 100% mortality in pmp-3(−) mutant worms, which was significantly ameliorated in the presence of 0.1% of Tw20 (Fig. 4D, Pre-incubation). Importantly, the mortality of pmp-3(−) mutants was significantly enhanced following heat-mediated micelle disruption, an effect that was significantly higher than that of their wild-type counterparts (p < 0.01, compare Figs. 4C and 4E, Method (I)). Next, to test if accumulated intracellular TCS could increase the mortality in pmp-3(−) mutant worms, we co-treated L1 staged pmp-3(−) mutant worms with 1 mM TCS and 0.1% Tw20 for 1 h at 25°C, before the temperature was upshifted to 35°C following washing for three times (Fig. 4A, Method (II)). Interestingly, TCS molecules released from Tw20 micelles at 35°C significantly increased the mortality (76 ± 3.7%) of pmp-3(−) mutant worms more than that (34 ± 3.6%) seen in wild-type worms (p < 0.01, compare Method( II) in Figs. 4C and 4E).
Taken together, these results point at two possible conclusions: First, Tw20 may inhibit the toxicity of TCS by micellar solubilization. Second, export of internalized TCS-Tw20 micellar complexes may be facilitated, at least in part, through a PMP-3-mediated detoxification mechanism. Although only TCS was evaluated under these conditions, it is reasonable to suggest that a similar pattern is likely mirrored by other phenolic EDCs and NISs.
DISCUSSION
EDCs are ubiquitous in the environment and pose a global threat to human and wildlife health. To date, studies elucidating the toxicity of EDCs have received greater attention from researchers, while investigations devoted to the identification of EDC inhibitors are only recently emerging. For instance, just a few years ago, Sengupta et al. reported that atrazine inhibits TCS toxicity by activating the nuclear receptor HR96 (an ortholog of CAR/PXP/VDR) in Daphnia magna (Sengupta et al., 2015). In addition, the interaction of surfactants with other antibacterials such as amoxicillin and moxifloxacin has previously been studied (Schwameis et al., 2013). However, no studies have investigated the protective role of NISs against EDC toxicity in eukaryotic model systems.
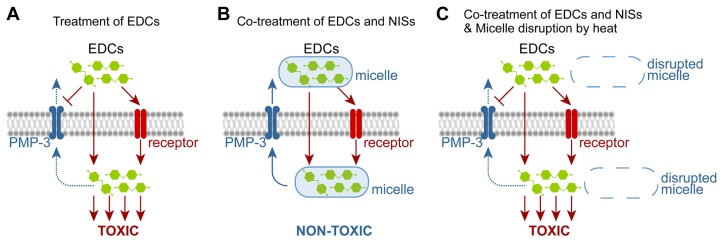
This work establishes the nematode C. elegans as a model for studying the toxicity of phenolic EDCs and also demonstrates the ameliorative potential of NISs against the mortality of TCS and other phenolic EDCs, facilitated through micellar solubilization. The TCS-micelle complex appears to be exported out of the worms’ bodies at least in part through a PMP-3/ABC transporter (Figs. 5A and 5B). However, following micelle disruption, released TCS seems to regain its activity and in turn perturbs the survival of worms (Fig. 5C). In pharmaceutical and nutritional preparations, NISs have been used as solubilizers and stabilizers, but their potential effects on the detoxification of EDCs have largely been overlooked. Therefore, our findings present broad insights into EDC intoxication, detoxification, and drug formulation strategies.
Fig. 5. A working model for NIS amelioration of EDC-induced mortality.
(A) EDCs can act via receptor-based mechanism, but at high doses, EDCs may employ receptor-independent mechanisms. EDCs (e.g., TCS) may also inhibit PMP-3-mediated detoxification mechanisms (Yoon et al., 2017). (B) EDCs could be inactivated in vivo by NIS-mediated micellar solubilization and the EDC-NIS complex may be exported at least in part by PMP-3/ABC transporters. (C) Upon micelle disruption, liberated EDC molecules regain their toxicity and may inhibit PMP-3-mediated detoxification.
The activity of phenolic compounds is influenced by their percent saturation in solution (Ogata and Shibata, 2000). Micelle aggregates are formed when surfactants are dissolved in solutions at or above their critical micelle concentration (CMC). Surfactants can solubilize phenolics in the micellar phase and thus reduce their thermodynamic activity (Allawala and Riegelman, 1953). To put things into perspective, a saturated water solution of chloroxylenol, a phenolic disinfectant, was shown to exhibit comparable biocidal efficacy to a saturated surfactant solution with concentrations of many orders of magnitude higher (Mitchell, 1964). Moreover, Taylor et al. compared the efficacy of TCS against E. coli at 100% saturation in ammonium lauryl sulfate (ALS) solutions of varying concentrations (Taylor et al., 2004). Interestingly, the degree of bacterial growth reduction when ALS was increased was similar to that observed when less ALS and twice as much TCS were used (Taylor et al., 2004). This indicates that surfactant to EDC ratio, but not EDC concentration, determines the overall fate of EDC activity. Similarly, other phenolic antimicrobial agents, most notably rifampicin and isoeugenol, were found to be highly susceptible to inactivation by Tw80 (Nielsen et al., 2016). In E. coli, our data show that TCS retains its antibacterial activity at a minimum concentration of 0.001 mM when co-administered with 0.1% Tw20, suggesting that the bioavailable portion of TCS was sufficient to exhibit its bactericidal effect under these saturation conditions (see Fig. 2E). This is corroborated by the contrasting synergistic effect of Tw80 on water-soluble antimicrobials such as polymyxin B and benzalkonium chloride (Toutain-Kidd et al., 2009). It is important to note that, because TCS in prokaryotes inhibits the synthesis of fatty acids, which are abundant in surfactants, it has been surmised that TCS-resistant Staphylococcus aureus compensate for TCS-induced anti-lipogenic effect by utilizing exogenous fatty acids presumably provided by the Tween surfactants (Morvan et al., 2016). In C. elegans, manipulating the NIS-TCS ratio showed that NISs are potent inhibitors of phenolic EDCs at very low concentrations. Our results revealed that NIS concentrations as low as 0.0008% significantly reduced the mortality caused by a lethal TCS dose of 0.5 mM (see Fig. 2C). This remarkable inhibitory efficiency, compared to that seen in E. coli, could be ascribed to the outer cuticle that encapsulates the worms and imparts environmental and anti-toxic protection.
In conclusion, the current study identifies NISs as potent inhibitors of phenolic EDCs in an eukaryotic model organism. The findings presented herein may pave the way for devising and developing potentially effective preventive and therapeutic strategies to control the widespread dissemination of phenolic EDCs, while still maintaining their beneficial antimicrobial properties. The observations presented here, along with those from previous studies, mandate further investigations based on a multidisciplinary approach, combining physicochemical and biological aspects, to fully characterize the direct interaction between NISs and EDCs. Future efforts should be directed toward investigating the complex interplay between NIS solubilization and its net effect on drug digestion, absorption, and overall activity in highly relevant vertebrate model systems.
Supplementary data
ACKNOWLEDGEMENTS
We thank the members of the Lee laboratory for helpful advice and discussion during this work. This work was supported in part by the Brody Brothers Grant (21602-664261) to M-H.L. and the Saudi Government Graduate Scholarship (from King Saud University) to M.A.A. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Footnotes
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Allawala N.A., Riegelman S. The release of antimicrobial agents from solutions of surface-active agents. J Am Pharm Assoc Am Pharm Assoc. 1953;42:267–275. doi: 10.1002/jps.3030420502. [DOI] [PubMed] [Google Scholar]
- Azzouz A., Rascon A.J., Ballesteros E. Simultaneous determination of parabens, alkylphenols, phenylphenols, bisphenol A and triclosan in human urine, blood and breast milk by continuous solid-phase extraction and gas chromatography-mass spectrometry. J Pharm Biomed Anal. 2016;119:16–26. doi: 10.1016/j.jpba.2015.11.024. [DOI] [PubMed] [Google Scholar]
- Babich H., Babich J.P. Sodium lauryl sulfate and triclosan: in vitro cytotoxicity studies with gingival cells. Toxicol Lett. 1997;91:189–196. doi: 10.1016/s0378-4274(97)00022-2. [DOI] [PubMed] [Google Scholar]
- Benotti M.J., Trenholm R.A., Vanderford B.J., Holady J.C., Stanford B.D., Snyder S.A. Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water. Environ Sci Technol. 2009;43:597–603. doi: 10.1021/es801845a. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byford J.R., Shaw L.E., Drew M.G., Pope G.S., Sauer M.J., Darbre P.D. Oestrogenic activity of parabens in MCF7 human breast cancer cells. J Steroid Biochem Mol Biol. 2002;80:49–60. doi: 10.1016/s0960-0760(01)00174-1. [DOI] [PubMed] [Google Scholar]
- Dann A.B., Hontela A. Triclosan: environmental exposure, toxicity and mechanisms of action. J Appl Toxicol. 2011;31:285–311. doi: 10.1002/jat.1660. [DOI] [PubMed] [Google Scholar]
- Das G.C., Bacsi A., Shrivastav M., Hazra T.K., Boldogh I. Enhanced gamma-glutamylcysteine synthetase activity decreases drug-induced oxidative stress levels and cytotoxicity. Mol Carcinog. 2006;45:635–647. doi: 10.1002/mc.20184. [DOI] [PubMed] [Google Scholar]
- Davies A.G., Bettinger J.C., Thiele T.R., Judy M.E., McIntire S.L. Natural variation in the npr-1 gene modifies ethanol responses of wild strains of C. elegans. Neuron. 2004;42:731–743. doi: 10.1016/j.neuron.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E., Bourguignon J.P., Giudice L.C., Hauser R., Prins G.S., Soto A.M., Zoeller R.T., Gore A.C. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S., Terasaka S., Kiyama R. Bisphenol A induces a rapid activation of Erk1/2 through GPR30 in human breast cancer cells. Environ Pollut. 2011;159:212–218. doi: 10.1016/j.envpol.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Escalada M.G., Harwood J.L., Maillard J.Y., Ochs D. Triclosan inhibition of fatty acid synthesis and its effect on growth of Escherichia coli and Pseudomonas aeruginosa. J Antimicrob Chemother. 2005;55:879–882. doi: 10.1093/jac/dki123. [DOI] [PubMed] [Google Scholar]
- Fang J.L., Stingley R.L., Beland F.A., Harrouk W., Lumpkins D.L., Howard P. Occurrence, efficacy, metabolism, and toxicity of triclosan. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2010;28:147–171. doi: 10.1080/10590501.2010.504978. [DOI] [PubMed] [Google Scholar]
- Garcia-Espineira M.C., Tejeda-Benitez L.P., Olivero-Verbel J. Toxic effects of bisphenol a, propyl paraben, and triclosan on Caenorhabditis elegans. Int J Environ Res Public Health. 2018;15 doi: 10.3390/ijerph15040684. pii: E684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honnen S. Caenorhabditis elegans as a powerful alternative model organism to promote research in genetic toxicology and biomedicine. Arch Toxicol. 2017;91:2029–2044. doi: 10.1007/s00204-017-1944-7. [DOI] [PubMed] [Google Scholar]
- Hunt P.R. The C. elegans model in toxicity testing. J Appl Toxicol. 2017;37:50–59. doi: 10.1002/jat.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T., Zhu B.L., Maeda H. Effect of sodium azide on the metabolic activity of cultured fetal cells. Toxicol Ind Health. 2006;22:337–341. doi: 10.1177/0748233706071737. [DOI] [PubMed] [Google Scholar]
- Kabir E.R., Rahman M.S., Rahman I. A review on endocrine disruptors and their possible impacts on human health. Environ Toxicol Pharmacol. 2015;40:241–258. doi: 10.1016/j.etap.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Lenz K.A., Pattison C., Ma H. Triclosan (TCS) and triclocarban (TCC) induce systemic toxic effects in a model organism the nematode Caenorhabditis elegans. Environ Pollut. 2017;231:462–470. doi: 10.1016/j.envpol.2017.08.036. [DOI] [PubMed] [Google Scholar]
- Li X., Ying G.G., Su H.C., Yang X.B., Wang L. Simultaneous determination and assessment of 4-nonylphenol, bisphenol A and triclosan in tap water, bottled water and baby bottles. Environ Int. 2010;36:557–562. doi: 10.1016/j.envint.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Lu Y., Park K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int J Pharm. 2013;453:198–214. doi: 10.1016/j.ijpharm.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic-Housley Z., Garavito R.M. Effect of temperature and low pH on structure and stability of matrix porin in micellar detergent solutions. Biochim Biophys Acta. 1986;869:158–170. doi: 10.1016/0167-4838(86)90290-6. [DOI] [PubMed] [Google Scholar]
- McAvoy D.C., Schatowitz B., Jacob M., Hauk A., Eckhoff W.S. Measurement of triclosan in wastewater treatment systems. Environ Toxicol Chem. 2002;21:1323–1329. [PubMed] [Google Scholar]
- Meeker J.D., Yang T., Ye X., Calafat A.M., Hauser R. Urinary concentrations of parabens and serum hormone levels, semen quality parameters, and sperm DNA damage. Environ Health Perspect. 2011;119:252–257. doi: 10.1289/ehp.1002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao M., Yuan W., Yang F., Liang H., Zhou Z., Li R., Gao E., Li D.K. Associations between bisphenol A exposure and reproductive hormones among female workers. Int J Environ Res Public Health. 2015;12:13240–13250. doi: 10.3390/ijerph121013240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A.G. Bactericidal activity of chloroxylenol in aqueous solutions of cetomacrogol. J Pharm Pharmacol. 1964;16:533–537. doi: 10.1111/j.2042-7158.1964.tb07509.x. [DOI] [PubMed] [Google Scholar]
- Morvan C., Halpern D., Kenanian G., Hays C., Anba-Mondoloni J., Brinster S., Kennedy S., Trieu-Cuot P., Poyart C., Lamberet G., et al. Environmental fatty acids enable emergence of infectious Staphylococcus aureus resistant to FASII-targeted antimicrobials. Nat Commun. 2016;7:12944. doi: 10.1038/ncomms12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen C.K., Kjems J., Mygind T., Snabe T., Meyer R.L. Effects of tween 80 on growth and biofilm formation in laboratory media. Front Microbiol. 2016;7:1878. doi: 10.3389/fmicb.2016.01878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N., Shibata T. Binding of alkyl- and alkoxy-substituted simple phenolic compounds to human serum proteins. Res Commun Mol Pathol Pharmacol. 2000;107:167–173. [PubMed] [Google Scholar]
- Petersen R.C. Triclosan antimicrobial polymers. AIMS Mol Sci. 2016;3:88–103. doi: 10.3934/molsci.2016.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupo M., Pisano A., Lappano R., Santolla M.F., De Francesco E.M., Abonante S., Rosano C., Maggiolini M. Bisphenol A induces gene expression changes and proliferative effects through GPER in breast cancer cells and cancer-associated fibroblasts. Environ Health Perspect. 2012;120:1177–1182. doi: 10.1289/ehp.1104526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodricks J.V., Swenberg J.A., Borzelleca J.F., Maronpot R.R., Shipp A.M. Triclosan: a critical review of the experimental data and development of margins of safety for consumer products. Crit Rev Toxicol. 2010;40:422–484. doi: 10.3109/10408441003667514. [DOI] [PubMed] [Google Scholar]
- Schug T.T., Janesick A., Blumberg B., Heindel J.J. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. 2011;127:204–215. doi: 10.1016/j.jsbmb.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwameis R., Erdogan-Yildirim Z., Manafi M., Zeitlinger M.A., Strommer S., Sauermann R. Effect of pulmonary surfactant on antimicrobial activity in vitro. Antimicrob Agents Chemother. 2013;57:5151–5154. doi: 10.1128/AAC.00778-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta N., Litoff E.J., Baldwin W.S. The HR96 activator, atrazine, reduces sensitivity of D. magna to triclosan and DHA. Chemosphere. 2015;128:299–306. doi: 10.1016/j.chemosphere.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B.M., Avery L. The pharynx of the nematode C. elegans: A model system for the study of motor control. Worm. 2013;2:e21833. doi: 10.4161/worm.21833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedenborg E., Ruegg J., Makela S., Pongratz I. Endocrine disruptive chemicals: mechanisms of action and involvement in metabolic disorders. J Mol Endocrinol. 2009;43:1–10. doi: 10.1677/JME-08-0132. [DOI] [PubMed] [Google Scholar]
- Taylor T.J., Seitz E.P., Fox P., Fischler G.E., Fuls J.L., Weidner P.L. Physicochemical factors affecting the rapid bactericidal efficacy of the phenolic antibacterial triclosan. Int J Cosmet Sci. 2004;26:111–116. doi: 10.1111/j.1467-2494.2004.00205.x. [DOI] [PubMed] [Google Scholar]
- Tejeda-Benitez L., Olivero-Verbel J. Caenorhabditis elegans, a biological model for research in toxicology. Rev Environ Contam Toxicol. 2016;237:1–35. doi: 10.1007/978-3-319-23573-8_1. [DOI] [PubMed] [Google Scholar]
- Toutain-Kidd C.M., Kadivar S.C., Bramante C.T., Bobin S.A., Zegans M.E. Polysorbate 80 inhibition of Pseudomonas aeruginosa biofilm formation and its cleavage by the secreted lipase LipA. Antimicrob Agents Chemother. 2009;53:136–145. doi: 10.1128/AAC.00500-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingskes A.K., Spann N. The toxicity of a mixture of two antiseptics, triclosan and triclocarban, on reproduction and growth of the nematode Caenorhabditis elegans. Ecotoxicology. 2018;27:420–429. doi: 10.1007/s10646-018-1905-9. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Mitani N., Ishii N., Miki K. A mutation in a cuticle collagen causes hypersensitivity to the endocrine disrupting chemical, bisphenol A, in Caenorhabditis elegans. Mutat Res. 2005;570:71–80. doi: 10.1016/j.mrfmmm.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Weatherly L.M., Shim J., Hashmi H.N., Kennedy R.H., Hess S.T., Gosse J.A. Antimicrobial agent triclosan is a proton ionophore uncoupler of mitochondria in living rat and human mast cells and in primary human keratinocytes. J Appl Toxicol. 2016;36:777–789. doi: 10.1002/jat.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X., Bishop A.M., Reidy J.A., Needham L.L., Calafat A.M. Parabens as urinary biomarkers of exposure in humans. Environ Health Perspect. 2006;114:1843–1846. doi: 10.1289/ehp.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon D.S., Choi Y., Cha D.S., Zhang P., Choi S.M., Alfhili M.A., Polli J.R., Pendergrass D., Taki F.A., Kapalavavi B., et al. Triclosan disrupts SKN-1/Nrf2-mediated oxidative stress response in C. elegans and human mesenchymal stem cells. Sci Rep. 2017;7:12592. doi: 10.1038/s41598-017-12719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon D.S., Pendergrass D.L., Lee M.H. A simple and rapid method for combining fluorescent in situ RNA hybridization (FISH) and immunofluorescence in the C. elegans germline. MethodsX. 2016;3:378–385. doi: 10.1016/j.mex.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.