Abstract
DRAIC (also known as LOC145837 and RP11-279F6.1), is a long non-coding RNA associated with several types of cancer including prostate cancer, lung cancer, and breast cancer. Its expression is elevated in tumor tissues compared to adjacent benign tissues in breast cancer patients and is regulated by estrogen treatment in breast cancer cells. In addition, expression analysis of DRAIC in more than 100 cell lines showed that DRAIC expression is high in luminal and basal subtypes compared to claudin low subtype, suggesting a prognostic value of DRAIC expression in breast cancer. In the present study, we analyzed DRAIC expression in 828 invasive breast carcinomas and 105 normal samples of RNA sequencing datasets from The Cancer Genome Atlas (TCGA) and found that DRAIC expression was correlated with estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status, and is increased in cancerous tissues. Additionally, higher DRAIC expression was associated with poorer survival of patients, especially in ER positive breast cancer. DRAIC was also investigated in the Oncomine database and we found that DRAIC expression predicted patients’ response to paclitaxel and FEC as well as lapatinib, which are commonly used therapy options for breast cancer. Finally, DRAIC expression in breast cancer was negatively correlated with immune cell infiltration. These results reinforce the importance of DRAIC in breast cancer.
Keywords: lncRNA, DRAIC, LOC145837, RP11-279F6.1, breast cancer
1. Introduction
Over the past few years, there has been an increasing widespread interest in long non-coding RNAs (lncRNAs), which are non-protein coding transcripts more than 200 nucleotides in length [1,2]. They are now known as important players in human diseases including various types of cancers [3,4,5,6]. They were reported to mediate important cellular functions like genomic imprinting, X-chromosome inactivation, and RNA splicing. They modulate gene expression at the epigenetic, transcriptional, and post-transcriptional levels through different mechanisms like enhancer-associating RNAs, chromatin looping, transcription factor trapping, and acting as competing endogenous RNA (ceRNA, a.k.a. RNA sponges) interacting and sequestering microRNAs (miRNAs) [7,8].
DRAIC (a.k.a. LOC145837 and RP11-279F6.1) is a 1.7 kb long noncoding RNA. It, together with another noncoding RNA gene PCAT29, locates at 15q23. This locus was first found to be a tumor suppressor in prostate cancer [9]. In breast cancer, Lee reported that DRAIC was a potential oncogene and using more than 100 breast cancer cell lines they showed that DRAIC was high in luminal and basal subtypes compared to the claudin low subtype [10]. They carried out in vitro studies and showed that knockdown DRAIC significantly inhibited cell proliferation by regulating cell cycle and E2-dependent signaling. They also confirmed their finding in patients’ samples and included 135 tumor tissues and 27 benign tissues, showing DRAIC mRNA expression is indeed higher in tumor tissues [10]. However, they failed to examine DRAIC expression in different subtypes and correlate it to clinically prognostic and pathological parameters, possibly due to the small number of samples analyzed.
In the present study, we investigated DRAIC expression in 828 invasive breast carcinoma samples and 105 normal samples from The Cancer Genome Atlas (TCGA) and confirmed DRAIC expression was higher in tumor tissues than normal tissues. We also described that DRAIC expression is significantly higher in estrogen receptor (ER) positive, progesterone receptor (PR) positive, and human epidermal growth factor receptor 2 (HER2) positive patients compared to their negative counterparts. DRAIC expression correlated with tumor stage and lymph node metastasis. Higher expression of DRAIC also predicts poorer overall survival and disease specific survival of breast cancer patients especially in ER positive subtypes. Additionally, we investigated DRAIC expression in Oncomine database and found DRAIC expression also predicts patients’ response to anticancer drugs like paclitaxel and FEC (Fluorouracil, Epirubicin, and Cyclophosphamide, which are commonly used drugs in chemotherapy) and lapatinib (which is a used to treat women with HER2 positive breast cancer). Finally, DRAIC is investigated in TIMER and interestingly its expression predicts immune cell infiltration levels in breast cancer. These results indicate DRAIC plays an important role in breast cancer and may be a potential therapeutic target or a prognostic marker.
2. Results
2.1. DRAIC Expression Increased in Breast Cancer and Correlated with Estrogen Receptor, Progesterone Receptor, and Human Epidermal Growth Factor 2 and Tumor Stages and Lymph Node Metastasis
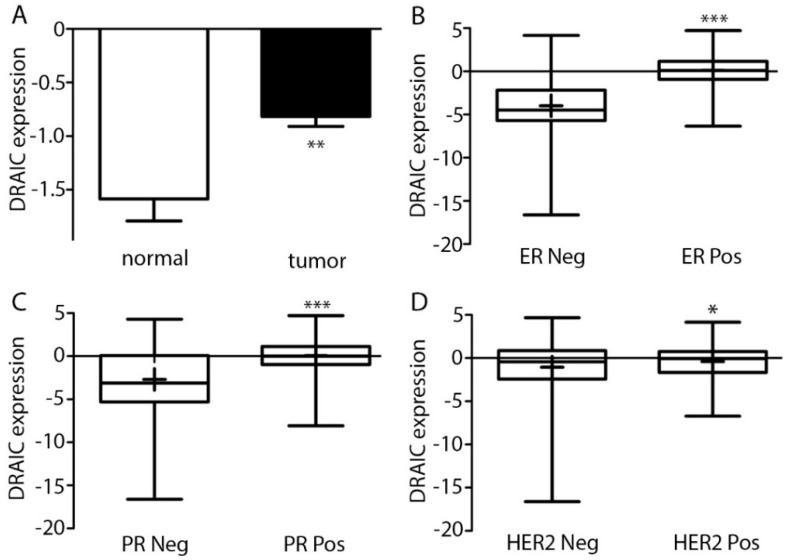
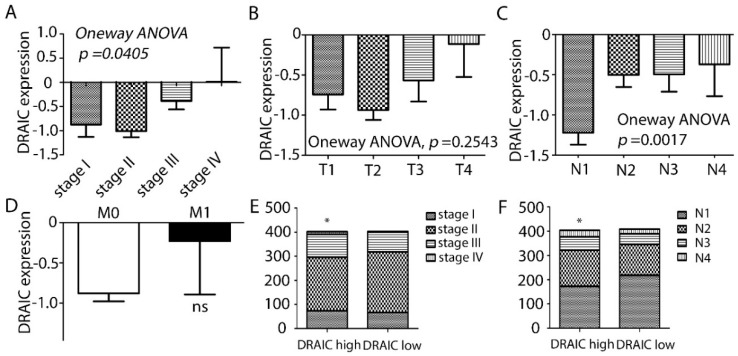
The expression of DRAIC was investigated in 828 tumor tissues and 105 normal breast tissues. We showed that DRAIC expression was significantly higher in cancer tissues than normal tissues (Figure 1A), consistent with a previous report [10]. Breast cancer is closely related to hormone signaling and EGF signaling and could be divided into several subtypes based on the expression of ER, PR, and HER2 [11]. We divided the 828 samples according to their ER, PR, and HER2 status and expression of DRAIC in these subgroups showed that DRAIC expression is higher in ER, PR, and HER2 positive patients compared to their negative counterparts (Figure 1B–D). We found DRAIC expression was higher in late stage tumors compared to early stages and higher expression of DRAIC is detected in lymph node positive patients compared to lymph node negative patients, indicating DRAIC may be involved also in the metastatic progression of breast cancer. When only tumor size (the T stage) or long-distance metastasis (M stage) were considered, a trend was also found that higher DRAIC indeed was bad for the patients (Figure 2A–D). We divided the 828 patients into DRAIC high and DRAIC low and we found that in DRAIC high group, there is a significant higher proportion of late stage and lymph node metastasis patients (Figure 2E,F).
Figure 1.
DRAIC expression is high in breast cancer compared with normal tissues and its expression correlates with important breast cancer marker genes estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor (HER2). (A) DRAIC expression was analyzed in 828 breast cancer patients and 105 normal breast tissue samples, the data shown are mean ± SEM (standard error of the mean). (B–D) The breast cancer patients were divided according to their ER, PR, and HER2 status, respectively. Shown is the Whisker plots: Min to max and for each group, the mean value was shown as “+”. Statistical significance was calculated by two-tailed student’s t-test, * indicates p < 0.05, ** indicates p < 0.01, and *** indicates p < 0.001. DRAIC expression was shown as log2 value.
Figure 2.
High DRAIC expression in breast cancer correlates with certain clinical pathological parameters. (A–D) The 828 breast cancer patients were divided according to their neoplasm disease stages (A), cancer tumor stages (B), neoplasm disease lymph node stages (C), and cancer metastasis stages (D). DRAIC expression in each different group of stages was shown as mean ± SEM (standard error of the mean). Statistical significance was calculated by either one-way ANOVA (for A–C) or two-tailed student’s t-test (for D). (E,F) The 828 breast cancer patients were divided into DRAIC high and DRAIC low group based on the median value of DRAIC expression. In each group, a stacked bar chart was created to show the distribution of patients in different neoplasm disease stages (E) or lymph node stages (F). Chi-square tests were used to test the differences. * indicates p < 0.05; ns, not significant. DRAIC expression was shown as log2 value.
2.2. DRAIC Expression Impact Patients Survival
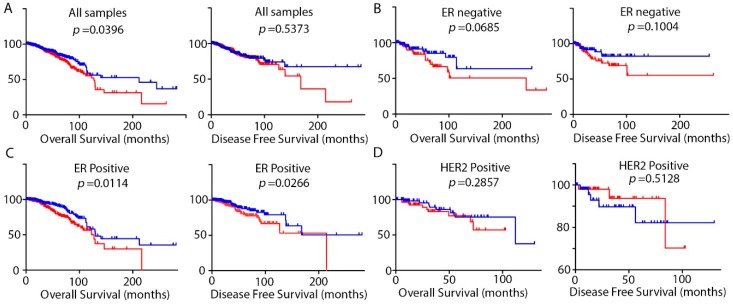
Then we investigated the impact of the expression of DRAIC on the overall survival and disease specific survival of the patients. We found that in general, higher DRAIC predicts shorter overall survival of patients (Figure 3A). Detailed analysis showed that higher DRAIC significantly predicts shorter overall survival and disease specific survival in ER positive patients but not in ER negative patients (Figure 3B,C), which is consistent with our finding that DRAIC is higher in ER positive patients than ER negative patients (Figure 1B). We also found that higher DRAIC in HER2 positive patients, in the contrary, showed better disease-free survival although no statistical significance was reached, suggesting a subtype specific impact (Figure 3D).
Figure 3.
Overall survival or disease-free survival determined by Kaplan–Meier plots and the log-rank test according to DRAIC expression. The patients were divided as DRAIC high (red) or DRAIC low (blue) according to the median value of DRAIC expression. Kaplan–Meier plots were created using GraphPad Prism 5 software using data for all samples (A) or only ER negative patients (B), ER positive patients (C), and HER2 positive patients (D). Log-rank tests were used to calculate the differences between groups.
2.3. DRAIC Expression Predicts Patients’ Response to Chemotherapy Treatments
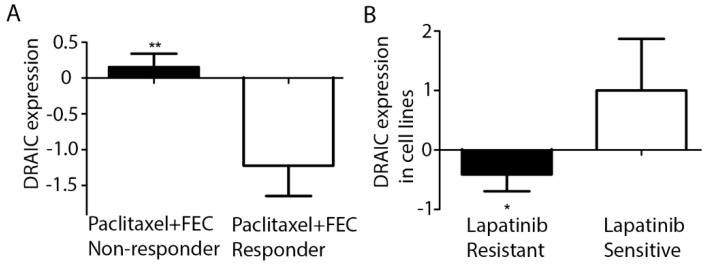
We investigated DRAIC and its expression in the Oncomine database [12]. Interestingly, we found that DRAIC expression is higher in patients who are resistant to paclitaxel and FEC (F, Fluorouracil; E, Epirubicin Hydrochloride; C, Cyclophosphamide) (Figure 4A) [13]. All these drugs are commonly used chemotherapy drugs for treatment of breast cancer. We also found one report regarding breast cancer cell lines showing that DRAIC expression is higher in lapatinib sensitive breast cancer cell lines compared to Lap resistant cell lines (Figure 4B) [14]. Lapatinib is a commonly used anti-HER2 therapy drug in HER2 overexpressing breast cancer patients. These results indicate that DRAIC expression may help guide the treatment options for breast cancer patients. Its higher expression may render patients resistant to certain chemotherapy treatments while sensitize patients to other drugs.
Figure 4.
DRAIC expression correlates to chemotherapy treatment response. (A) DRAIC expression in 88 paclitaxel and FEC (fluorouracil/epirubicin/cyclophosphamide) non-responder patients and 27 paclitaxel and FEC responder patients’ samples (Miyake 2012) were shown as mean ± SEM. (B) DRAIC expression in 17 lapatinib resistant cell lines and 5 sensitive cell lines (Barretina 2012) were shown as mean ± SEM. Statistical significance was calculated by two-tailed student’s t-test, * indicates p < 0.05 and ** indicates p < 0.01.
3. Discussion
The lncRNA DRAIC was first identified in prostate cancer cell lines and showed higher expression in human LNCaP cells (which is androgen-dependent) compared with LNCaP-derived C4-2B cells (which is androgen independent). It was located in the 15q23 region, together with another lncRNA PCAT29 [9]. Further, they discovered that DRAIC was downregulated by androgen and found androgen receptor (AR) and FOXA1 and NKX3-1 directly bound DRAIC promoters. AR inhibits DRAIC expression and FOXA1 and NKX3-1 upregulated DRAIC expression. Functional assays through overexpression or knockdown studies showed that DRAIC promoted proliferation but inhibited migration and invasion of LNCaP cells while PCAT29 inhibited LNCaP proliferation. In a more recent paper, the authors have shown that a specific metabolite named 5-alpha-Abi of Abiraterone, which is a steroidgenesis enzyme inhibitor for the treatment of metastatic castration resistant prostate cancer, could induce DRAIC expression, indicating DRAIC’s involvement in anti-androgen therapy resistance for prostate cancer [15]. DRAIC expression was repressed by androgen treatment and antiandrogen treatment induced DRAIC expression [16]. It was also found DRAIC expression was upregulated in IR-resistant PCs through expression analyses of human prostate cancer xenografts with predetermined radioresistant/sensitive phenotypes [17].
Aberrant expression of DRAIC was also related to other types of cancers. For example, in one multiple type cancer study, the authors obtained 132 samples of paired tumor and normal adjacent tissues for four types of cancers (bladder, prostate, lung, and ER positive breast cancer), analyses of the RNA-seq data revealed DRAIC to be significantly upregulated in three of the four cancer types except bladder cancer [18]. In another study, the authors focused on gastrointestinal cancers (pancreas, liver, stomach, esophagus, and colorectal cancers), and used data from Oncomine database to identify genes that are dysregulated, among which DRAIC was discovered as one of the 28 downregulated genes unique to the pancreas cancers [19]. In colorectal cancers, DRAIC expression was higher in chemotherapy responding versus non-responding patients [20]. In lung cancer, DRAIC was identified as one of the 71 lncRNAs in non-small-cell lung carcinomas that could distinguish squamous cell carcinomas from adenocarcinomas [21] and was found to be one of the most significantly (top 5) downregulated lncRNAs in squamous lung cancer [22].
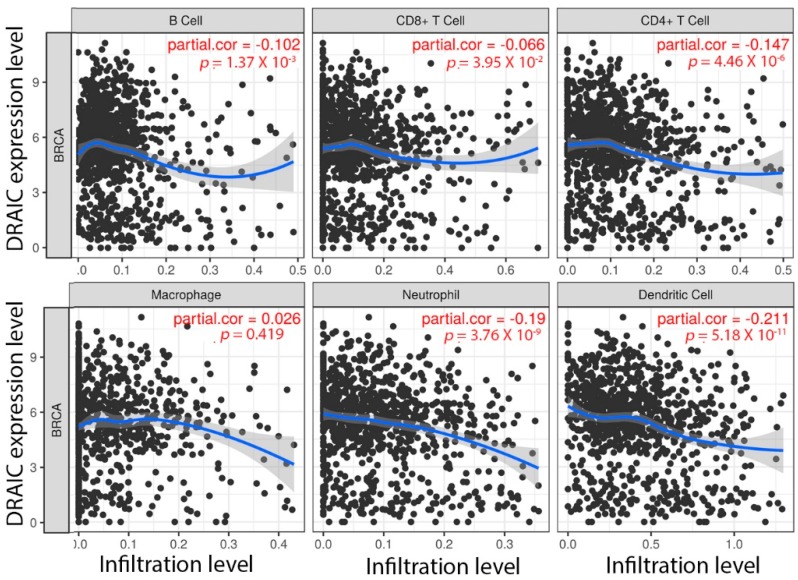
In breast cancer, DRAIC expression was identified as a potential onco-lncRNA based on its elevated expression in tumor tissues compared to the adjacent normal tissues [23]. It was shown to be higher in ER positive breast cancer patients from 4 different studies [23,24,25,26]. However, while DRAIC knockdown in MCF7 cells inhibited E2-regulated gene expression, the expression of DRAIC itself was also inhibited by E2 treatment and it was speculated that DRAIC may be important for both E2-dependent and the basal growth of cancer cells [10]. By analyzing ChIP-seq data of ER and 26 known transcription factors, it was shown that there is direct ER binding on the genomic region of DRAIC and DRAIC expression also could be regulated by key transcription factors like GATA3, E2F1, MYC, and RAD21, which are key regulators in breast tumorigenesis [23]. DRAIC was annotated to be one of the 38 lncRNAs associated with ER and based on the “guilt-by-association” analyses, researchers revealed DRAIC to be potentially involved in inhibition of immunity, indicating DRAIC to be involved in both ER signaling and other processes beyond ER [25]. We explored DRAIC in TIMER [27], a web server developed by Harvard to explore the correlation between its expression and abundance of immune infiltrates. We found that higher DRAIC expression in breast cancer significantly negatively correlates with immune cell infiltration especially dendritic cells and neutrophils (Figure 5). Further studies are needed to investigate whether or not DRAIC really participates in immune responses of breast cancer patients and if it acts through cooperation with ER signaling (or HER2 signaling) or not. In another study, researchers constructed lncRNA-miRNA-mRNA ceRNA networks based on data obtained from 116 ER positive breast cancer samples and proposed that DRAIC may function as miRNA sponge and exert its effects through mir-296-3p, mir-432-5p, and mir-149-5p [24].
Figure 5.
DRAIC expression negatively correlates with immune cell infiltration levels in breast cancer. The correlation between DRAIC expression and abundance of immune infiltrates is investigated through TIMER [27]. Correlation between DRAIC expression and the abundances of six immune infiltrates (B cells, CD4+ T cells, CD8+ T cells, Neutrophils, Macrophages, and Dendritic cells) are displayed. The purity-corrected partial Spearman correlation and statistical significance are shown on the top right corners.
In this study, we included expression analysis of DRAIC in 828 tumor samples and 105 normal breast samples from TCGA. This large number of samples enables broad analyses and allowed us to analyze DRAIC expression in more detail, for example, in only ER positive patients.
High expression of DRAIC in breast cancer tissues compared to normal breast tissues was validated in the present study, confirming its role as a potential onco-lncRNA in breast cancer (Figure 1A). The present study, which was based on TCGA analysis, also described high DRAIC expression in ER positive, PR positive, and HER2 positive patients compared to their receptor negative counterparts (Figure 1). Detailed analyses revealed high DRAIC expression to be correlated to tumor stages and lymph node metastasis of the patients (Figure 2). Survival analysis considering all patients with breast cancer revealed high expression of DRAIC was significantly associated with poor overall survival. Detailed analyses revealed that high DRAIC expression was associated with poor survival only in ER positive patients but not in ER negative or HER2 positive patients (Figure 3), which could be related to its involvement in estrogen and ER regulated gene expression as reported previously [10]. Very interestingly, in HER2 positive patients, 5-year DFS is better in DRAIC high patients (93.7% in DRAIC high group compared to 82.2% in DRAIC low group, Figure 3D). Although no significance was reached, which could be due to smaller number (only 129) of HER2 positive patients in this study, this may indicate a subtype specific effect of DRAIC in breast cancer and its function in HER2 positive patients need to be investigated further.
DRAIC was also investigated in the Oncomine database and very intriguingly, we found its high expression may indicate resistance to paclitaxel and FEC while on the contrary, sensitivity to lapatinib, which inhibits HER2 and is widely used as anti-HER2 therapy in HER2 overexpressing breast cancer patients. This may partially explain why its high expression in HER2 positive patients showed better (although not significant) disease-free survival. In another study [24], researchers have found DRAIC to be lower in HER2 positive (which is contrary to our results) and higher in ER positive (which is similar to our findings) patients’ samples, but DRAIC didn’t show an effect on survival in their researches. This could be due to much smaller cohort size of their study compared to ours (148 vs 828), especially in HER2 positive patients (38 vs 129). Further studies with larger patients number especially in HER2 positive samples are needed to address DRAIC function in HER2+ breast cancer.
In conclusion, DRAIC expression was found to be higher in breast cancer patients, especially in ER, PR, and HER2 positive patients. Its expression correlates with tumor stages and lymph node metastases and patients’ survival especially in ER positive patients. DRAIC expression may also predict responsiveness to chemotherapy and anti-HER2 therapy and immune therapy. Additional studies are needed to better understand how DRAIC is involved in breast cancer and what molecular pathways are involved.
4. Materials and Methods
DRAIC expression for 828 invasive breast carcinomas and 105 normal breast tissues was collected by the open-access web resource TANRIC [28], which assessed and analyzed the breast cancer TCGA data. Clinical data for the patients were obtained from the open access cBioportal database [29,30]. Statistical analyses were carried out similar to a previous paper [31], briefly, two-tailed student’s t-test was used to assess statistical differences between two groups (for example, negative and positive ER, PR, HER2 status). One-way ANOVA followed by Bonferroni test was used to assess statistical differences for more than three groups.
Survival analyses were carried out similar to a previous paper [32], patients were divided into two groups (one above the median and the other below). The Kaplan–Meier curves and log-rank tests were used to estimate overall and disease-free survival of the patients.
Datamining in Oncomine database was performed by using DRAIC or LOC145837 or RP11-279F6 as keywords and related breast cancer studies were chosen for further look in details.
Data were analyzed using the GraphPad Prism 5 software and was considered to be significantly different when p was less than 0.05.
Acknowledgments
The authors thank all Dong lab member for all their support.
Author Contributions
D.Z. and J.-T.D. conceived and designed the experiments, D.Z. analyzed all data and drafted the manuscript. D.Z. and J.-T.D. read and revised the manuscript.
Funding
This work was funded by Grant 81472464 from the National Natural Science Foundation of China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ponting C.P., Oliver P.L., Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Wilusz J.E., Sunwoo H., Spector D.L. Long noncoding RNAs: Functional surprises from the RNA world. Genes Dev. 2009;23:1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsai M.C., Spitale R.C., Chang H.Y. Long intergenic noncoding RNAs: New links in cancer progression. Cancer Res. 2011;71:3–7. doi: 10.1158/0008-5472.CAN-10-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wapinski O., Chang H.Y. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Schmitt A.M., Chang H.Y. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29:452–463. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X., Tudoran O.M., Calin G.A., Ivan M. The many faces of long noncoding RNAs in cancer. Antioxid. Redox Signal. 2017 doi: 10.1089/ars.2017.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang K.C., Chang H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagano T., Fraser P. No-nonsense functions for long noncoding RNAs. Cell. 2011;145:178–181. doi: 10.1016/j.cell.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Sakurai K., Reon B.J., Anaya J., Dutta A. The lncRNA DRAIC/PCAT29 locus constitutes a tumor-suppressive nexus. Mol. Cancer Res. 2015;13:828–838. doi: 10.1158/1541-7786.MCR-15-0016-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun M., Gadad S.S., Kim D.S., Kraus W.L. Discovery, annotation, and functional analysis of long noncoding RNAs controlling cell-cycle gene expression and proliferation in breast cancer cells. Mol. Cell. 2015;59:698–711. doi: 10.1016/j.molcel.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eroles P., Bosch A., Perez-Fidalgo J.A., Lluch A. Molecular biology in breast cancer: Intrinsic subtypes and signaling pathways. Cancer Treat. Rev. 2012;38:698–707. doi: 10.1016/j.ctrv.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Rhodes D.R., Yu J.J., Shanker K., Deshpande N., Varambally R., Ghosh D., Barrette T., Pandey A., Chinnaiyan A.M. Oncomine: A cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/S1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyake T., Nakayama T., Naoi Y., Yamamoto N., Otani Y., Kim S.J., Shimazu K., Shimomura A., Maruyama N., Tamaki Y., et al. GSTP1 expression predicts poor pathological complete response to neoadjuvant chemotherapy in ER-negative breast cancer. Cancer Sci. 2012;103:913–920. doi: 10.1111/j.1349-7006.2012.02231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barretina J., Caponigro G., Stransky N., Venkatesan K., Margolin A.A., Kim S., Wilson C.J., Lehar J., Kryukov G.V., Sonkin D., et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z.F., Alyamani M., Li J.N., Rogacki K., Abazeed M., Upadhyay S.K., Balk S.P., Taplin M.E., Auchus R.J., Sharifi N. Redirecting abiraterone metabolism to fine-tune prostate cancer anti-androgen therapy. Nature. 2016;533:547. doi: 10.1038/nature17954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colditz J., Rupf B., Maiwald C., Baniahmad A. Androgens induce a distinct response of epithelial-mesenchymal transition factors in human prostate cancer cells. Mol. Cell Biochem. 2016;421:139–147. doi: 10.1007/s11010-016-2794-y. [DOI] [PubMed] [Google Scholar]
- 17.Agemy L., Kela I., Waks T., Pfeffer R.M., Bar-Shira A., Orr-Urtreger A., Domany E., Eshhar Z. Gene expression profiles predict sensitivity of prostate cancer to radiotherapy. J. Cancer Ther. 2013;4:11. doi: 10.4236/jct.2013.44A003. [DOI] [Google Scholar]
- 18.Li S.Q., Li B., Zheng Y.T., Li M.L., Shi L.M., Pu X.M. Exploring functions of long noncoding RNAs across multiple cancers through co-expression network. Sci. Rep. 2017;7:754. doi: 10.1038/s41598-017-00856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakraborty S. In silico analysis identifies genes common between five primary gastrointestinal cancer sites with potential clinical applications. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2014;27:231. [PMC free article] [PubMed] [Google Scholar]
- 20.Li L.X., Shang J., Zhang Y.P., Liu S., Peng Y.N., Zhou Z., Pan H.Q., Wang X.B., Chen L.P., Zhao Q. MEG3 is a prognostic factor for CRC and promotes chemosensitivity by enhancing oxaliplatin-induced cell apoptosis. Oncol. Rep. 2017;38:1383–1392. doi: 10.3892/or.2017.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H., Xu Q.H., Liu F., Ye X., Wang J.L., Meng X. Identification and validation of long noncoding RNA biomarkers in human non-small-cell lung carcinomas. J. Thorac. Oncol. 2015;10:645–654. doi: 10.1097/JTO.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 22.Molina-Pinelo S., Gutierrez G., Pastor M.D., Hergueta M., Moreno-Bueno G., Garcia-Carbonero R., Nogal A., Suarez R., Salinas A., Pozo-Rodriguez F., et al. MicroRNA-dependent regulation of transcription in non-small cell lung cancer. PLoS ONE. 2014;9:e90524. doi: 10.1371/journal.pone.0090524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y., Wagner E.K., Guo X., May I., Cai Q., Zheng W., He C., Long J. Long intergenic non-coding RNA expression signature in human breast cancer. Sci. Rep. 2016;6:37821. doi: 10.1038/srep37821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian C., Guan M., Si C., Shen H., Jin T., Zhang T. Identification of differentially expressed profiles of lncRNAs and mRNAs in ER-negative and HER-2 positive breast cancer. Arch. Med. Sci.-Civ. Dis. 2017;2:148–160. doi: 10.5114/amscd.2017.71413. [DOI] [Google Scholar]
- 25.Van Grembergen O., Bizet M., de Bony E.J., Calonne E., Putmans P., Brohee S., Olsen C., Guo M.Z., Bontempi G., Sotiriou C., et al. Portraying breast cancers with long noncoding RNAs. Sci. Adv. 2016;2:e1600220. doi: 10.1126/sciadv.1600220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varley K.E., Gertz J., Roberts B.S., Davis N.S., Bowling K.M., Kirby M.K., Nesmith A.S., Oliver P.G., Grizzle W.E., Forero A., et al. Recurrent read-through fusion transcripts in breast cancer. Breast Cancer Res. Treat. 2014;146:287–297. doi: 10.1007/s10549-014-3019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li T., Fan J., Wang B., Traugh N., Chen Q., Liu J.S., Li B., Liu X.S. Timer: A web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77:e108–e110. doi: 10.1158/0008-5472.CAN-17-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J., Han L., Roebuck P., Diao L.X., Liu L.X., Yuan Y., Weinstein J.N., Liang H. Tanric: An interactive open platform to explore the function of lncRNAs in cancer. Cancer Res. 2015;75:3728–3737. doi: 10.1158/0008-5472.CAN-15-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A., Jacobsen A., Byrne C.J., Heuer M.L., Larsson E., et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao J.J., Aksoy B.A., Dogrusoz U., Dresdner G., Gross B., Sumer S.O., Sun Y.C., Jacobsen A., Sinha R., Larsson E., et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioportal. Sci. Signal. 2013;6 doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao D., Ma G., Zhang X., He Y., Li M., Han X., Fu L., Dong X.Y., Nagy T., Zhao Q., et al. Zinc finger homeodomain factor Zfhx3 is essential for mammary lactogenic differentiation by maintaining prolactin signaling activity. J. Biol. Chem. 2016;291:12809–12820. doi: 10.1074/jbc.M116.719377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y.G., Leonard M., Zhang Y.J., Zhao D., Mahmoud C., Khan S., Wang J., Lower E.E., Zhang X.T. HER2-driven breast tumorigenesis relies upon interactions of the estrogen receptor with coactivator MED1. Cancer Res. 2018;78:422–435. doi: 10.1158/0008-5472.CAN-17-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]