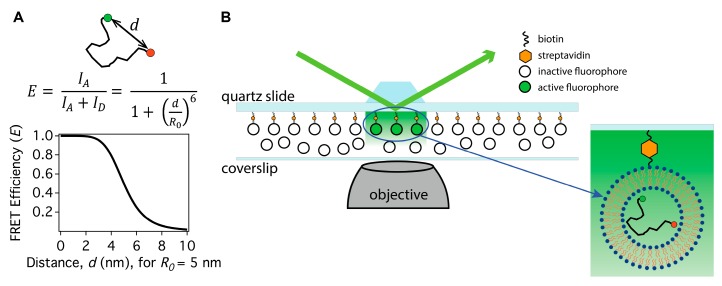
Figure 2.
(A) The fluorescence (or Förster) resonance energy transfer (FRET) efficiency (E) is calculated by the relationship between donor (D, green circle) and acceptor (A, red circle) intensities (ID and IA). FRET efficiency has a strongly non-linear dependence (sixth power) on the distance between the donor and acceptor molecules (d) attached to an intrinsically disordered protein. R0 is the Förster radius, which determines the length scale of the FRET coupling and is the value where the transfer efficiency is 50%. The donor and acceptor fluorescent dye properties determine R0, which usually is around 4–7 nm. (B) A schematic of prism-type total internal reflection (TIR) illumination for smFRET. The green arrow shows an incident laser beam that is totally internally reflected at the quartz-water interface of a fluidic channel, producing an evanescent wave that excites fluorophores near the surface. The zoomed detail shows an IDP labeled with a D–A pair that is encapsulated in a liposome (not to scale). The liposome is attached to the quartz surface via a biotin-streptavidin linkage while additional liposomes are shown in solution in the flowcell diagram.