Abstract
Causative species of Harmful Algal Bloom (HAB) and toxins in commercially exploited molluscan shellfish species are monitored weekly from four classified shellfish production areas in Perú (three in the north and one in the south). Okadaic acid (OA) and pectenotoxins (PTXs) were detected in hand-picked cells of Dinophysis (D. acuminata-complex and D. caudata) and in scallops (Argopecten purpuratus), the most important commercial bivalve species in Perú. LC-MS analyses revealed two different toxin profiles associated with species of the D. acuminata-complex: (a) one with OA (0.3–8.0 pg cell−1) and PTX2 (1.5–11.1 pg cell−1) and (b) another with only PTX2 which included populations with different toxin cell quota (9.3–9.6 pg cell−1 and 5.8–9.2 pg cell−1). Toxin results suggest the likely presence of two morphotypes of the D. acuminata-complex in the north, and only one of them in the south. Likewise, shellfish toxin analyses revealed the presence of PTX2 in all samples (10.3–34.8 µg kg−1), but OA (7.7–15.2 µg kg−1) only in the northern samples. Toxin levels were below the regulatory limits established for diarrhetic shellfish poisoning (DSP) and PTXs (160 µg OA kg−1) in Perú, in all samples analyzed. This is the first report confirming the presence of OA and PTX in Dinophysis cells and in shellfish from Peruvian coastal waters.
Keywords: okadaic acid, pectenotoxins, Dinophysis, D. acuminata-complex, D. caudata, Argopecten purpuratus
1. Introduction
Diarrhetic shellfish poisoning (DSP) toxins cause a gastrointestinal human health syndrome with the main symptoms being nausea, diarrhea, vomiting, and gastrointestinal pain [1,2]. Okadaic acid (OA) and its congeners, dinophysistoxins (DTX1, DTX2), their high-polarity precursors (DTX4, DTX5), and their 7-O-acyl-derivatives (“DTX3”) are liposoluble polyethers that have been designated as diarrhetic shellfish toxins [3,4,5]. Pectenotoxins are liposoluble, non-diarrheogenic, polyether lactones which may co-occur with DSP toxins and can be coeluted with them [6] using the usual extraction procedures. The two groups of toxins have been found in different species of Dinophysis (D. acuminata, D. acuta, D. caudata, D. fortii, D. infundibula, D. miles, D. norvegica, D. ovum, D. sacculus, D. tripos) and two species of Phalacroma (P. mitra, P. rotundatum) [5]. In addition, OA and its congeners have been found in several benthic species from the genus Prorocentrum (P. concavum, P. texanum, P. arenarium, P. lima) [5,7,8].
Filter-feeding bivalves accumulate algal toxins and are the main vectors transferring them to humans through the food web. DSP toxins and pectenotoxins (PTXs) pose a global threat to public health and aquaculture [9,10,11,12]. The analysis of DSP toxins for monitoring programmes in Perú have been carried out only by mouse bioassay [13], and there is no information on the toxin profiles of either the potentially toxic species of Dinophysis or of the contaminated molluscan shellfish. Nevertheless, D. acuminata, D. caudata, D. tripos, and Phalacroma rotundatum (=Dinophysis rotundata) have been reported in Peruvian coastal waters [14], and their toxins associated with positive results for lipophilic toxins in mouse bioassays [13]. There have also been reports on the occurrence of the benthic dinoflagellate Prorocentrum lima [13], but to date this species has not been associated with DSP events in Perú.
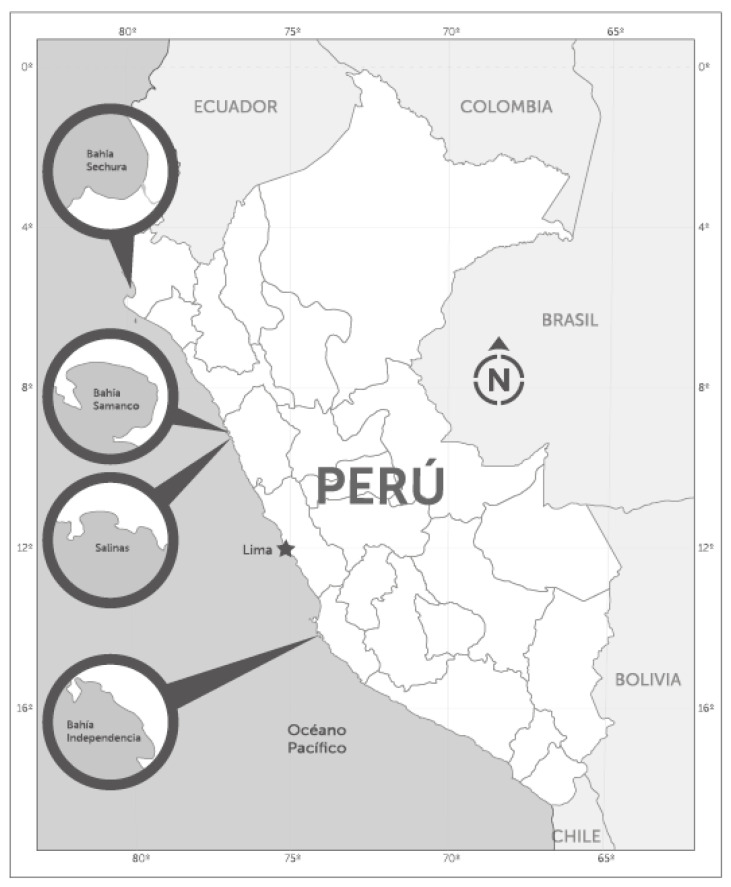
During 2017 and 2018, seawater and shellfish samples were collected from classified shellfish production areas (Figure 1), in the framework of the Molluscan Shellfish Safety Programme (PCMB) of the National Fisheries Health Organization of Perú (SANIPES), to establish the relationship between the toxic profiles detected in shellfish and the occurrence of potentially toxic dinoflagellates.
Figure 1.
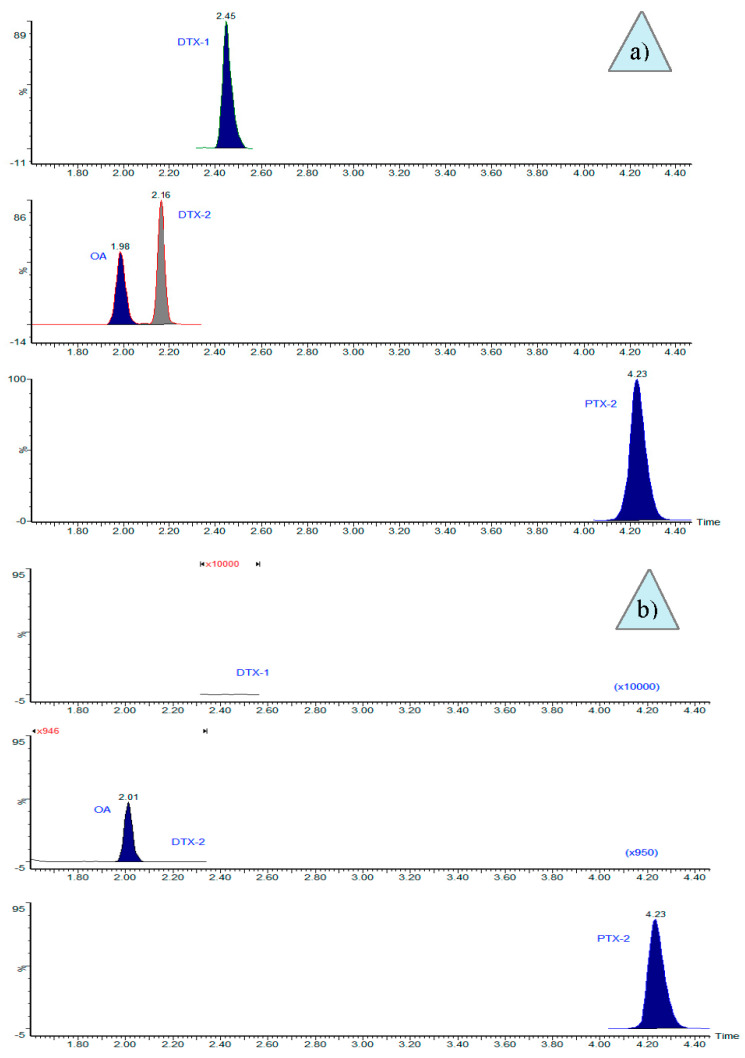
Selective ion chromatograms from the LC-MS/MS analyses of isolated cells of Dinophysis acuminata-complex in: (a) multitoxin standard, 3 ng mL−1; and (b) chromatogram of the sample from Bahía de Sechura-Vichayo, where the absence of DTX1 (increase ×10,000) and DTX2 (increase ×950) was observed.
2. Results
Chromatograms from the LC-MS/MS analyses of hand-picked cells showed that the toxin profile of Dinophysis acuminata-complex cells from the northernmost production area (Sechura Bay) included OA and PTX2 (Figure 1b).
Other toxins, such as DTX1 and DTX2, were not found (LOD = 0.16 ng mL−1). In contrast, only PTX2 was detected in the analyses of picked cells of Dinophysis cf. acuminata and D. caudate from the other three areas (i.e., Independencia Bay, Samanco Bay, and Salinas), with an average toxin content of 9.3 pg PTX2 cell−1 (Table 1).
Table 1.
Toxin content in isolated cells of Dinophysis.
| Date (d/m/y) | Location | Sampling Depth (m) | Species | Picked Cells (No.) | OA (pg Cell−1) |
PTX2 (pg Cell−1) |
|---|---|---|---|---|---|---|
| 27/01/2017 | Independencia Bay | 0–8 | D. acuminata-complex | 150 | <LOD * | 9.6 |
| 09/02/2017 | Samanco Bay | 0–15 | D. caudata | 92 | <LOD * | 9.2 |
| 10/04/2017 | Salinas | 0–15 | D. acuminata-complex | 204 | <LOD * | 9.3 |
| 24/03/2018 | Sechura Bay (Vichayo) |
0–15 | D. acuminata-complex | 440 | 0.8 | 11.1 |
| 24/03/2018 | Sechura Bay (Puerto Rico) |
0–15 | D. acuminata-complex | 400 | 0.3 | 1.5 |
| 14/04/2018 | Sechura Bay (Parachique) |
0–15 | D. caudata | 400 | <LOD * | 5.8 |
* LOD: 0.16 ng mL−1. OA: okadaic acid; PTX: pectenotoxin.
The same profile of toxins present in the cells was detected in the shellfish Argopecten purpuratus from the same production areas (Table 2).
Table 2.
Toxin content in Argopecten purpuratus (whole flesh).
| Date d/m/y |
Place | OA µg kg−1 Post-Hydrolysis |
PTX2 µg kg−1 |
|---|---|---|---|
| 27/01/2017 | Independencia Bay-El Queso | <LOD * | 22.2 |
| 09/02/2017 | Samanco Bay | <LOD * | 20.3 |
| 10/04/2017 | Salinas | <LOD * | 10.3 |
| 14/04/2018 | Sechura Bay-Puerto Rico | 10.4 | 34.8 |
| 14/04/2018 | Sechura Bay-Barrancos | 8.6 | 20.8 |
| 14/04/2018 | Sechura Bay-San Pedro | 15.2 | 27.6 |
| 05/05/2018 | Sechura Bay-Las Delicias | 7.7 | 10.7 |
* LOD: 1.6 µg kg−1.
3. Discussion
Okadaic acid and PTX2 were detected in both Dinophysis cells and scallops. In some cases, both toxins were present but in others only PTX2 was detected. There are previous reports on Dinophysis species with a toxin profile constituted by only PTX2. That was the case with D. acuminata cells isolated from Inglesa Bay [15] and from Reloncaví estuary [4], both from Chile, as well as with D. caudata from the Galician Rías Bajas, northwest Spain [16] and D. acuminata from Danish waters [17]. Likewise, our analyses of shellfish meat from the same areas in Perú were in agreement with the toxin profiles of the dinoflagellates, that is, just PTX-2 (10.3–22.2 µg PTX2 kg−1 meat) and no traces of OA in the areas where Dinophysis species had the same profile.
The Peruvian strains of Dinophysis seemed to contain much lower amounts of toxin per cell than those reported from Galicia, Spain (D. caudata: 100.0–127.4 pg PTX2 cell−1) [16] and from Chile (D. acuminata: 180 pg PTX2 cell−1), which had a toxin content one order of magnitude higher than the Peruvian strains (Table 2).
Cells of Dinophysis acuminata-complex around Sechura Bay showed a higher variability in their PTX2 content, with values ranging from 1.5 to 11.1 pg cell−1 and to a lesser extent in their OA cell quota (from 0.3 to 0.8 pg cell−1). We do not know if this variability is due to the co-occurrence of different species of the D. cf. acuminata in the same area. Nevertheless, previous laboratory experiments and field data have shown a large variability of toxin content per cell of Dinophysis associated with different phases of the population growth and their interaction with environmental conditions [18]. More studies, including physiological and genetic factors affecting toxin profiles and content, are needed to clarify these questions. The presence of OA and PTX2 has been also reported in D. acuminata from Lake Orbetello, Italy [19] and in New Zealand [20], USA [4], and Japan [21], where other toxins (e.g., DTX1 and some PTX analogues) were also reported.
D. caudata from the same bay had 5.8 pg PTX2 cell−1. Therefore, there was a co-occurrence of two toxic Dinophysis species in this area. Scallop samples (“concha de abanico”) during the occurrence of these species reached toxin levels ranging from 7.7 to 15.2 µg OA kg−1 and from 10.7 to 34.8 µg PTX2 kg−1 (Table 2).
The low toxin content found in Dinophysis cells in Perú suggests a low risk of DSP toxins accumulation in shellfish above the regulatory levels. That was the case during the 2 years (maximum levels in Independencia Bay: <LOD OA and 22.2 µg kg−1 PTX2; Samanco Bay: <LODOA and 16.4 µg kg−1 PTX2; Salinas: <LOD OA and 12.2 µg kg−1 PTX2; Sechura Bay-Puerto Rico: 10.4 µg kg−1 OA and 48.2 µg kg−1 PTX2; Sechura Bay-Barrancos: 8.6 µg kg−1 OA and 21.0 µg kg−1; Sechura Bay-San Pedro: 15.2 µg kg−1 OA and 43.7 µg kg−1 PTX2; and Sechura Bay-Las Delicias: 7.7 µg kg−1 OA y 19.6 µg kg−1, June 2016–May 2018) of toxin monitoring of scallops, A. purpuratus, by LC-MS/MS. During this period, toxin levels never reached regulatory limits, although Dinophysis densities above 104·cells L−1 were recorded. Dinophysis densities of around 103 cells L−1 are considered a bloom, and have often been related to toxic outbreaks in other parts of the world [5].
4. Conclusions
The toxin profiles, including OA and PTX2, of several species of Dinophysis and of scallops (Argopecten purpuratus) from shellfish production areas in Perú were characterized. Different species included in the Dinophysis acuminata-complex and D. caudata, producers of toxins regulated by the EU, co-occur in northern Perú. The toxic species of Dinophysis from Sechura Bay, Samanco Bay, Salinas, and Independencia Bay showed low cell-toxin content (pg cell−1) in comparison with those reported for the same species in other parts of the world, although more studies, including physiological and genetic factors affecting toxin profiles and content, are needed. Dinophysis acuminata-complex and D. caudate are most likely the main species concerning molluscan shellfish safety in Perú.
5. Materials and Methods
5.1. Field Sampling
Seawater and shellfish samples for the analyses of potentially toxic phytoplankton and shellfish toxins were collected weekly in the framework of the National Molluscan Shellfish Safety Programme (PCMB) of the National Fisheries Health Organization of Perú (SANIPES), which is the national competent authority for the control of seafood safety. Samples from classified shellfish production areas were analyzed at the SANIPES official laboratory. During 2017 and the first half of 2018, seawater and scallops (Argopecten purpuratus “concha de abanico”) samples were collected at the fixed monitoring stations in Sechura Bay, Samanco Bay, Salinas, and Independencia Bay (Figure 2) for analyses. The objective was to determine the toxin profiles in the plankton and shellfish at the time of detection of lipophilic shellfish toxins. Two kinds of water samples were collected at each station for phytoplankton analyses: (i) vertical net-hauls (10 µm mesh size), with no fixatives added, for the identification of the species in vivo and for single cell isolations; (ii) depth-integrated hose-samples (hose length 15 m), which were immediately fixed with acid Lugol’s solution, for quantitative analyses by the standard Utermöhl method [22].
Figure 2.
Location of sampling stations.
5.2. Single Cell Isolations
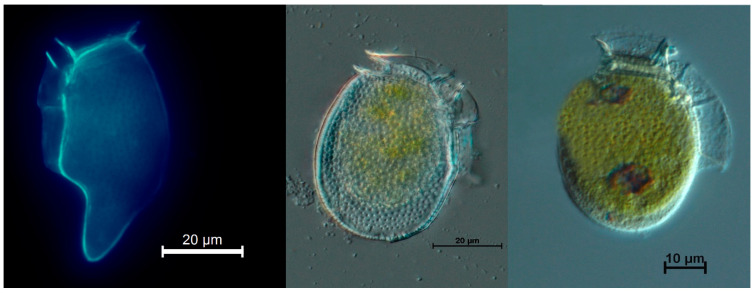
Cells of Dinophysis caudata and D. acuminata-complex (two different morphotypes D. cf. acuminata and D. cf. ovum) (Figure 3) were isolated one by one from the plankton net-haul concentrates with a microcapillary pipette under an inverted microscope Olympus IX71, at 200× magnification. Each picked cell was transferred three times through drops of sterile seawater and finally placed (with as little seawater as possible) in a 1.5 mL Eppendorf tube with 500 µL methanol, and kept at −20 °C until analysis.
Figure 3.
Dinophysis cells isolated from Samanco Bay, Perú. (Left) Epifluorescence of Dinophysis caudata; (middle) and (right) DIC micrographs of cells of the Dinophysis acuminata-complex.
5.3. Standards and Reagents
LC-MS grade methanol (MeOH) and acetonitrile (CH3CN) were used for the extraction and analyses of toxins by liquid chromatography coupled to mass spectrometry (LC-MS). Analytical-grade ammonium hydroxide, sodium hydroxide (NaOH), and hydrochloric acid (HCl) were used for the mobile phase and hydrolysis. Ultrapure water was obtained with a Sartorius (Arium Pro) purification system. Certified reference solutions for okadaic acid CRM-OA-d (batch #20141119), dinophysistoxin-2 CRM-DTX2-b (batch #20150819), dinophysistoxin-1 CRM-DTX1-b (batch #20151209), and pectenotoxin-2 CRM-PTX2-b (batch #20120516) were obtained from NRC-CNRC.
5.4. Toxins Extraction
5.4.1. From Isolated Cells of Dinophysis
To prepare for LC/MS analysis, samples kept frozen in Eppendorf tubes were transferred to a 2-mL microtube, the remains in the Eppendorf tube were washed twice with 200 µL of methanol, incorporated to the microtube, and mixed in a vortex prior to being dried at 40 °C under a flow of nitrogen gas. The dried toxin extract was re-suspended in 500 µL of methanol, mixed in a vortex, and filtered through 0.2 µm pore size nylon filters (Sterlitech, 13 mm) as described in [23].
5.4.2. From Shellfish Meat
Whole flesh samples of 12–15 scallops, Argopecten purpuratus, were homogenized and a 2 ± 0.05 g subsample, weighed on an analytical scale (Precisa, LX 220A), was placed in a 50 mL centrifuge tube and extracted twice with 9 mL methanol, stirred with a vortex (Thermo, maxi mix II) for 3 min, and centrifuged at 2000× g (Thermo, Sorvall ST 16R) for 10 min at a temperature of 20 °C. The supernatants were transferred and mixed in a volumetric flask and made up to 20 mL with methanol. To explore the presence of esterified derivatives of OA and DTXs, an aliquot of the methanolic extract was taken for alkaline hydrolysis.
The alkaline hydrolysis was carried out by adding 125 µL of 2.5 N NaOH to 1 mL of the methanolic extract, vortexing the mixture for 0.5 min, heating it for 40 min at 76 °C, and finally neutralizing the added NaOH with an equivalent amount of 2.5 N HCl.
Finally, all extracts (raw and hydrolyzed) were filtered through a 0.2 µm pore size nylon filter (Chromafil®Xtra, 25 mm) following the recommended protocols from the European Union Reference Laboratory for Marine Biotoxins [24].
5.5. LC-MS/MS Analyses
For LC-MS/MS analysis of the lipophilic toxins, a Waters Acquity I Class chromatograph coupled to a triple quadrupole mass spectrometer Waters XEVO-TQS by means of an electrospray interface (ESI) was used. Analytical separation was performed following a modification of the method developed by Gerssen et al. [25], with an Acquity UPLC® BEH C18 (1.7 µm, 2.1 × 100 mm) column kept at 40 °C. A binary gradient elution was used, with phase A consisting of H2O and phase B of 90% CH3CN, both containing ammonium hydroxide 6.7 mM (approximate pH of 11). The gradient started with 30% B, that proportion was kept for 1 min and then linearly increased to 90% B in 4 min. It was maintained at that proportion for 1 min, returned to the initial proportion in 0.1 min, and maintained for equilibration during 1.5 min before the next injection. The flow rate was 0.4 mL min−1 and the injection volume was 2 µL. The mass spectrometer was operated in both ESI positive and negative modes, the cone voltage was 3.0 kV, desolvation gas temperature was 500 °C with an N2 flow of 1000 L h−1 and a source temperature of 150 °C. Voltage parameters of the cone and collision energy were optimized during the tuning phase by direct infusion in alkaline medium. Product ions used for the quantification of each toxin in microalgae and shellfish and the MS/MS conditions for the multiple reaction monitoring (MRM) for each molecule are shown in Table 3.
Table 3.
Multiple reaction monitoring (MRM) and MS/MS of each toxin from the shellfish analysis.
| Toxins | ESI Mode | Ion | Cone Voltage (V) | Collision Energy (CE) (eV) |
Dwell (s) |
|
|---|---|---|---|---|---|---|
| Precursor (m/z) | Product (m/z) | |||||
| OA | ESI− | 803.5 | 255.1 * | 30 | 50 | 0.05 |
| 113.0 | 60 | |||||
| DTX1 | ESI− | 817.5 | 255.1 * | 30 | 50 | 0.05 |
| 113.0 | 60 | |||||
| DTX2 | ESI− | 803.5 | 255.1 * | 30 | 50 | 0.05 |
| 113.0 | 60 | |||||
| PTX1 | ESI+ | 892.5 | 821.5 * | 30 | 30 | 0.02 |
| 213.3 | 40 | |||||
| PTX2 | ESI+ | 876.6 | 823.5 * | 30 | 20 | 0.20 |
| 213.1 | 40 | |||||
* Transitions used for the phytoplankton analyses. ESI: electrospray ionization.
Acknowledgments
The authors acknowledge the Shellfish Monitoring Program of Bivalve Molluscs of the National Fisheries Health Organization of Perú for supplying shellfish and seawater samples for this study. We also thank Beatriz Reguera (Spanish Institute of Oceanography (IEO), Oceanographic Centre of Vigo) and Juan Blanco (Marine Research Centre—CIMA, Pontevedra 36620, Spain) for reviewing and translating this work.
Author Contributions
Conceptualization, A.A.-R., V.B.-M. and O.F.-S.; Investigation, A.A.-R., V.B.-M., M.R.-P., K.M.-A., A.R.-V. and L.V.-T.; Project administration, A.A.-R.; Visualization, A.A.-R., V.B.-M. and O.F.-S.; Writing—original draft, A.A.-R., V.B.-M. and O.F.-S.; Writing—review & editing, A.A.-R., V.B.-M., O.F.-S. and R.E.-J.
Funding
This work was funded by the National Programme of Bivalve Molluscs of the National Fisheries Health Organization of Perú, an autonomous entity attached to the Ministry of Production.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Key Contribution
This study shows that the D. acuminata-complex in northern Peru produces OA and PTX2 and in the southern zone only PTX2. D. caudata only produces PTX2. No DTXs were detected in the analyzed cells. The same profile was observed in the scallop Argopecten purpuratus, suggesting that only these toxins are produced by the phytoplankton species analyzed in these areas.
References
- 1.Yasumoto T., Murata M., Oshima Y., Sano M., Matsumoto G.K., Clardy J. Diarrhetic shellfish toxins. Tetrahedron. 1985;41:1019–1025. doi: 10.1016/S0040-4020(01)96469-5. [DOI] [Google Scholar]
- 2.Yasumoto T., Oshima Y., Yamaguchi M. Occurrence of a new type of shellfish poisoning in the Tohoku district. NIPPON SUISAN GAKKAISHI. 1978;44:1249–1255. doi: 10.2331/suisan.44.1249. [DOI] [Google Scholar]
- 3.Yasumoto T., Murata M. Polyether Toxins Involved in Seafood Poisoning. In: Hall S., Strichartz G., editors. Marine Toxins. Volume 418. American Chemical Society; Washington, DC, USA: 1990. pp. 120–132. [Google Scholar]
- 4.Fux E., Smith J.L., Tong M., Guzmán L., Anderson D.M. Toxin profiles of five geographical isolates of Dinophysis spp. from North and South America. Toxicon. 2011;57:275–287. doi: 10.1016/j.toxicon.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Reguera B., Riobó P., Rodríguez F., Díaz P., Pizarro G., Paz B., Franco J., Blanco J. Dinophysis Toxins: Causative Organisms, Distribution and Fate in Shellfish. Mar. Drugs. 2014;12:394–461. doi: 10.3390/md12010394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco J., Moroño Á., Fernández M.L. Toxic episodes in shellfish, produced by lipophilic phycotoxins: An overview. Galician J. Mar. Resour. 2005;1:1–70. [Google Scholar]
- 7.Van Dolah F.M. Marine Algal Toxins: Origins, Health Effects, and Their Increased Occurrence. Environ. Health Perspect. 2000;108:9. doi: 10.1289/ehp.00108s1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reguera B. Biología, Autoecología y Toxinología de las Principales Especies del Género Dinophysis Asociadas a Episodios de Intoxicación Diarreogénica por Bivalvos (DSP) Universidad de Barcelona; Barcelona, España: 2003. [Google Scholar]
- 9.Jørgensen K., Andersen P. Relation between the concentration of Dinophysis acuminata and diarrheic shellfish poisoning toxins in blue mussels (Mytilus edulis) during a toxic episode in the Limfjord (DENMARK), 2006. J. Shellfish Res. 2007;26:1081–1087. doi: 10.2983/0730-8000(2007)26[1081:RBTCOD]2.0.CO;2. [DOI] [Google Scholar]
- 10.Nincevic-Gladan Z., Skejic S., Arapov J., Buzancic M., Bojanic N., Ujevic I., Kuspilic G., Grbec B., Vidjack O. Seasonal variability in Dinophysis spp. abundances and diarrhetic shellfish poisoning outbreaks along the eastern Adriatic coast. Botanica Mar. 2008;51:449–463. doi: 10.1515/BOT.2008.067. [DOI] [Google Scholar]
- 11.Hossen V., Jourdan-da Silva N., Guillois-Bécel Y., Marchal J., Krys S. Food poisoning outbreaks linked to mussels contaminated with okadaic acid and ester dinophysistoxin-3 in France, June 2009. Eurosurveillance. 2011:16. doi: 10.2807/ese.16.46.20020-en. [DOI] [PubMed] [Google Scholar]
- 12.Reguera B., Velo-Suárez L., Raine R., Park M.G. Harmful Dinophysis species: A review. Harmful Algae. 2012;14:87–106. doi: 10.1016/j.hal.2011.10.016. [DOI] [Google Scholar]
- 13.Sanchez S., Bernales A., Delgado E., Carmen Chang F.D., Jacobo N., Quispe J. Variability and Biogeographical Distribution of Harmful Algal Blooms in Bays of High Productivity Off Peruvian Coast (2012–2015) J. Environ. Anal.Toxicol. 2017;7 doi: 10.4172/2161-0525.1000530. [DOI] [Google Scholar]
- 14.Ochoa N., Gómez O., Sánchez S., Delgado E. Diversidad de Diatomeas y Dinoflagelados Marinos del Perú. Bol. Inst. Mar. Perú. 1999;18:1–14. [Google Scholar]
- 15.Blanco J., Álvarez G., Uribe E. Identification of pectenotoxins in plankton, filter feeders, and isolated cells of a Dinophysis acuminata with an atypical toxin profile, from Chile. Toxicon. 2007;49:710–716. doi: 10.1016/j.toxicon.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Fernández M.L., Reguera B., González-Gil S., Míguez A. Pectenotoxin-2 in single-cell isolates of Dinophysis caudata and Dinophysis acuta from the Galician Rías (NW Spain) Toxicon. 2006;48:477–490. doi: 10.1016/j.toxicon.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen L.T., Krock B., Hansen P.J. Production and excretion of okadaic acid, pectenotoxin-2 and a novel dinophysistoxin from the DSP-causing marine dinoflagellate Dinophysis acuta—Effects of light, food availability and growth phase. Harmful Algae. 2013;23:34–45. doi: 10.1016/j.hal.2012.12.004. [DOI] [Google Scholar]
- 18.Pizarro G., Paz B., Gonzalez-Gil S., Franco J.M., Reguera B. Seasonal variability of lipophilic toxins during a Dinophysis acuta bloom in Western Iberia: Differences between picked cells and plankton concentrates. Harmful Algae. 2009;8:926–937. doi: 10.1016/j.hal.2009.05.004. [DOI] [Google Scholar]
- 19.Pigozzi S., Ceredi A., Milandri A., Pompei M., Buzzichelli S., Macori G., Susini F., Forletta R. Pectenotoxin and okadaic acid-based toxin profiles in phytoplankton and shellfish from Orbetello Lagoon, Italy; Proceedings of the 7th International Conference on Molluscan Shellfish Safety; Nantes, France. 14–19 June 2009; [(accessed on 17 November 2018)]. Available online: https://www.researchgate.net/profile/Guerrino_Macori2/publication/304776353_Pectenotoxin_and_okadaic_acid-based_toxin_profiles_in_phytoplankton_and_shellfish_from_Orbetello_Lagoon_Italy/links/577a35e608ae355e74f05c29/Pectenotoxin-and-okadaic-acid-based-toxin-profiles-in-phytoplankton-and-shellfish-from-Orbetello-Lagoon-Italy.pdf. [DOI] [Google Scholar]
- 20.MacKenzie L., Beuzenberg V., Holland P., McNabb P., Suzuki T., Selwood A. Pectenotoxin and okadaic acid-based toxin profiles in Dinophysis acuta and Dinophysis acuminata from New Zealand. Harmful Algae. 2005;4:75–85. doi: 10.1016/j.hal.2003.12.001. [DOI] [Google Scholar]
- 21.Nagai S., Suzuki T., Nishikawa T., Kamiyama T. Differences in the production and excretion kinetics of okadaic acid, dinophysistoxin-1, and pectenotoxin-2 between cultures of Dinophysis acuminata and Dinophysis fortii isolated from western Japan. J.Phycol. 2011;47:1326–1337. doi: 10.1111/j.1529-8817.2011.01076.x. [DOI] [PubMed] [Google Scholar]
- 22.Utermöhl H. Zur Vervollkommung der quantitativen phytoplankton-methodik. Mitt Int. Ver Limnol. 1958;9:38. [Google Scholar]
- 23.Raho N., Pizarro G., Escalera L., Reguera B., Marín I. Morphology, toxin composition and molecular analysis of Dinophysis ovum Schütt, a dinoflagellate of the “Dinophysis acuminata complex”. Harmful Algae. 2008;7:839–848. doi: 10.1016/j.hal.2008.04.006. [DOI] [Google Scholar]
- 24.European Union Reference Laboratory for Marine Biotoxins EU-Harmonised Standard Operating Procedure for Determination of Lipophilic Marine Biotoxins in Molluscs by LC-MS/MS, V.5. [(accessed on 15 March 2017)]. Available online: http://aesan.msssi.gob.es/en/CRLMB/web/home.shtml.
- 25.Gerssen A., Mulder P.P.J., McElhinney M.A., de Boer J. Liquid chromatography–tandem mass spectrometry method for the detection of marine lipophilic toxins under alkaline conditions. J. Chromatogr. A. 2009;1216:1421–1430. doi: 10.1016/j.chroma.2008.12.099. [DOI] [PubMed] [Google Scholar]