Abstract
The effectiveness of regenerated chicken bone char (CBC) in fluoride removal was investigated in the present study. Heat treatment was studied as the regeneration method. Results revealed that the CBC regenerated at 673 K yielded the highest fluoride adsorption capacity, hence, 673 K was the best regenerating temperature. The study continued up to five regeneration cycles at the best regenerating temperature; 673 K. The CBC accounted to 16.1 mg F/g CBC as the total adsorption capacity after five regeneration cycles. The recovery percentage of CBC reduced from 79% at the first regeneration to 4% after five regeneration cycles. The hydroxyapatite structure of CBC was not changed during the fluoride adsorption by five regeneration cycles. The ion exchange incorporated with the chemical precipitation occurred during the fluoride adsorption. The repeated regeneration of CBC is possible and it could be used as a low cost defluoridation technique to minimize the wastage of bone char.
Keywords: adsorption capacity, defluoridation, repeated heat regeneration, hydroxyapatite, ion exchange, chemical precipitation
1. Introduction
The contamination of groundwater by fluoride is one of the major global issues. An excess amount of fluoride intake causes dental fluorosis and skeletal fluorosis in humans [1]. Further, fluoride is supposed to be a suspicious cause for the multifactorial, deadly disease [2]; chronic kidney disease of unknown etiology (CKDu) in Sri Lanka [3]. The world health organization (WHO) guideline for fluoride in drinking water is 1.5 mg/L [4]. A high concentration of fluoride up to 30 mg/L was found in the groundwater of many countries in the world including Sri Lanka [3], India, Pakistan, China, West Indies, Spain, Holland, Poland, Italy, Iraq, Iran, Sudan, Uganda, Kenya, United Republic of Tanzania, Mexico, Thailand, Serbia, Ethiopia, Eritrea (North East Africa), West Africa, Southern Africa and North and South American countries [5]. Therefore, Defluoridation of drinking water is essential to protect the human life from fluoride contaminated water [6].
Adsorption is one of the techniques widely used as a low cost method in defluoridation [6]. Several adsorbents were reported in the literature such as activated carbon, activated alumina [7], clay, zeolites, fly ash, specific ion exchange resins [8], brick powder, bentonite, montmorillonite, laterite [9], rare earth oxides, red mud, hydroxyapatite, fluorspar, calcite, quartz, bauxite and gypsum [10]. Among the adsorbents, bone char was found to be a low cost, efficient [6] and environmentally friendly adsorbent.
Continuous usage of bone char in fluoride removal causes exhaustion of the bone char. Regeneration of exhausted bone char by heating is a new approach in fluoride removal [11].
In this study our attention was focused on the repeated regeneration of exhausted bone char in relation to its adsorption capacity, in order to reuse bone char in fluoride removal from contaminated water.
2. Materials and Methods
2.1. Preparation of Chicken Bone Char
The raw chicken bones were placed in closed metal containers and carbonized by an electrical muffle furnace (Koyo thermo system Co. Ltd. Tokyo, Japan, KBF 794N1) for 1.5 h at 873 K under an anaerobic condition. The carbonized CBC was washed several times with clean water and dried at 378 K in an electrical oven for one night [12].
2.2. Preparation of Fluoride Exhausted Bone Char
The CBC with a diameter of 1–2 cm was used to prepare the exhausted bone char. Figure 1 shows the schematic diagram of the adsorption setup used to prepare exhausted bone char. Four setups (A, B, C and D) were used separately to prepare fluoride exhausted CBC for use in the same setup in the same manner for fluoride adsorption after the regeneration of CBC.
Figure 1.
Schematic diagram of the adsorption setup used to prepare exhausted CBC.
Four liters (4 L) of synthesized drinking water with an initial fluoride concentration of 50 mg/L was prepared by dissolving sodium fluoride (NaF) in tap water. 1 L of the prepared solution was put into each 1 L beaker in each setup. Four tea bags each filled with ten grams of CBC were dipped in the solutions in the four setups. The four setups were allowed to adsorb fluoride for 25 days on a magnetic stirrer with a 270 rpm. The fluoride concentrations of the solutions were measured regularly using an ion selective electrode (ORION STAR A324 pH/ISE Meter and ORION 9609BNWP Ionplus Sure-Flow Fluoride Electrode). The Total Ionic Strength Adjustment Buffer (TISAB III) was added to each sample before analysis to avoid interference during the measurements.
2.3. Regeneration of Chicken Bone Char
Heat regeneration was selected as the regeneration method due to its easy operation and cost effectiveness. Since heat regeneration of CBC is not a chemical treatment method, the release of chemical components into treated water during a chemical regeneration may not occur. Therefore, the treated water may be safer for human consumption than from a chemical treatment.
The exhausted CBC in setups B, C and D was taken out from the tea bags after 25 days and dried at 338 K in an electrical oven for 24 h. Thereafter the CBC in the three setups (B, C and D) was reactivated by heat at 673 K, 773 K and 873 K under an anaerobic condition in closed metal containers in an electrical muffle furnace for 2 h [12,13]. The same amount of CBC used to prepare exhausted CBC in setups B, C and D was used for the regeneration (1st regeneration). The setup A was continued as the control setup without regeneration. In Figure 1, the A, B, C and D setups show the control, 673 K regenerated, 773 K regenerated and 873 K regenerated setups, respectively after regeneration.
The CBC in setup B only (673 K regenerated CBC) was heat regenerated at 673 K under an anaerobic condition in a closed metal container in an electrical muffle furnace for 2 h 5 times repeatedly as the 1st, 2nd, 3rd, 4th and 5th regenerations. The setup A was continued as the control setup without regeneration, until the 5th regeneration was completed for setup B.
2.4. Fluoride Removal by Regenerated Bone Char
Regenerated CBC in setups B, C and D was returned to the same setups to continue the experiment after regeneration. The same amount of CBC used to prepare exhausted CBC in setups B, C and D was used in the adsorption setups after regeneration.
Regenerated CBC in setup B was returned to the same setup to continue the experiment in the same manner after the 2nd, 3rd, 4th and 5th regenerations. The same amount of CBC used in the adsorption setup B in the 1st regeneration was used in the adsorption setup B after the 2nd, 3rd, 4th and 5th regenerations. The setup A was continued as the control setup without regeneration until the 5th regeneration.
Fluoride concentrations of the solutions were measured regularly by the ion selective electrode. The pH of the solutions was measured using a pH meter (ORION STAR A324 pH/ISE Meter and Beckman Electrode 511070).
The F− content in CBC before (raw CBC) and after the adsorption (control, 673 K regenerated CBC, 773 K regenerated CBC and 873 K regenerated CBC) was determined by digesting 0.1 g of CBC with 1 mL of concentrated nitric acid [12] [13]. F− content in digested CBC was measured using the ion selective electrode. The X-ray diffraction (XRD) of hydroxyapatite (HAP) and CBC before and after the adsorption were analyzed using MiniFlex (Rigaku Co., Tokyo, Japan); X-ray 30 kV/15 mA, radiation CuK alpha line (Ni filter), scintillation detector.
Anion and cation concentrations of the solutions were measured using ion chromatography (for anions: Dionex ICS-2000, separation column IonPac AS18, eluent KOH 23–40 mmol/L (gradient), suppressor ASRS 300 4 mm; for cations: Dionex ICS-1500, separation column IonPac CS12, eluent methanesulfonic acid 30 mmol/L (isocratic), suppressor CSRS 500 4 mm).
3. Results and Discussion
3.1. Preparation of Fluoride Exhausted Bone Char
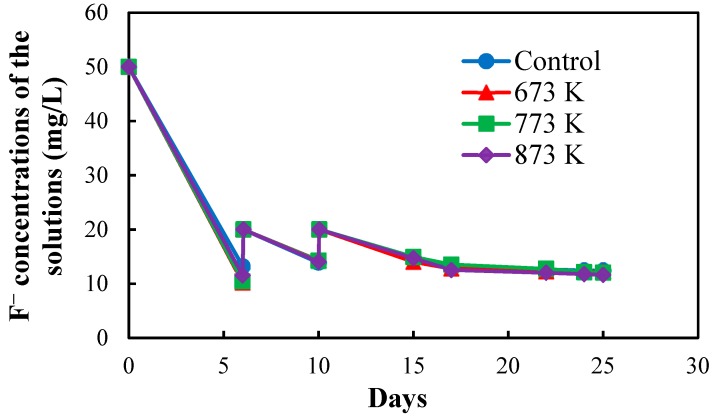
Figure 2 shows the fluoride concentrations of the solutions in the four setups used to prepare exhausted CBC throughout the operation period of 25 days. The fluoride concentrations of the solutions in the four setups were increased to 20 mg/L at days 6 and 10 to allow them to adsorb more amount of fluoride in order to obtain a quick equilibrium with the F− in the solutions. NaF solution with a concentration of 1000 mg/L was used to increase the fluoride concentrations of the solutions in the four setups.
Figure 2.
Fluoride concentrations of the solutions in the four setups used to prepare exhausted CBC.
3.2. Fluoride Removal by Regenerated Bone Char
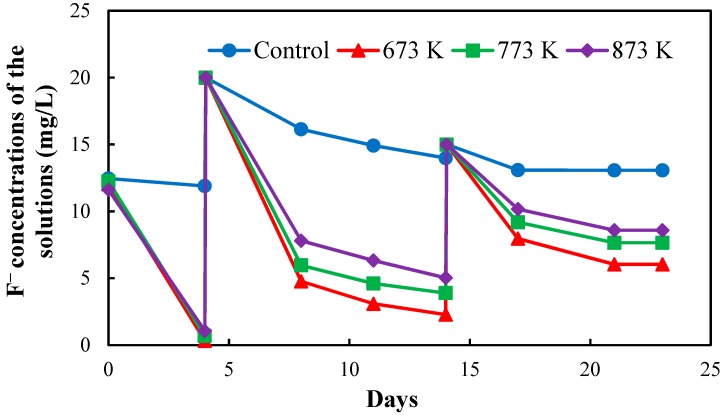
Figure 3 shows the fluoride concentrations of the solutions in the four setups after the 1st regeneration of CBC throughout the operation period of 23 days. The fluoride concentrations of the solutions in the four setups were increased to 20 mg/L at day 4 and 15 mg/L at day 14 to obtain a quick equilibrium with the fluoride in the solutions. NaF solution with the concentration of 1000 mg/L was used to increase the fluoride concentrations of the solutions in the four setups.
Figure 3.
Fluoride concentrations of the solutions in the four setups after 1st regeneration of CBC.
The equilibrium fluoride concentrations of the solutions in the four setups were used to determine the fluoride adsorption capacity of regenerated CBC.
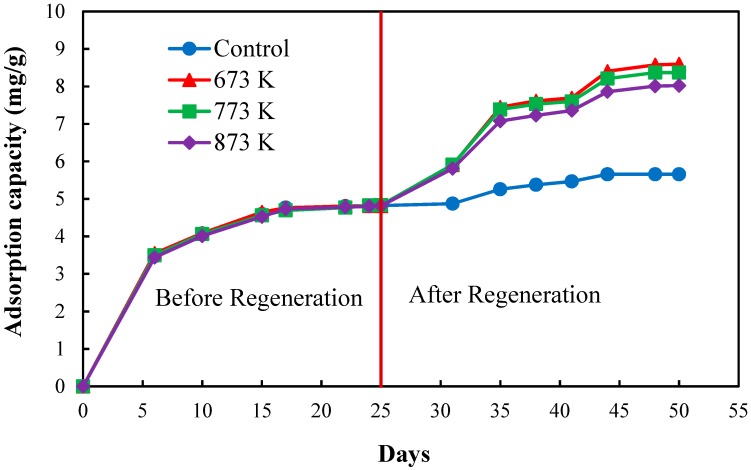
Figure 4 shows the effect of the 1st regeneration on the adsorption capacities of CBC obtained from the mass balance calculation for the solutions throughout the operation period of 50 days. The total adsorption capacities both before and after the regeneration were considered in order to evaluate the adsorption capacities after each regeneration cycle of CBC [12].
Figure 4.
Adsorption capacities of CBC used for the 1st regeneration obtained from the mass balance calculation for the solutions throughout the operation period.
The CBC regenerated at 673 K showed the highest total adsorption capacity; hence, 673 K was the best regenerating temperature. The total adsorption capacity of CBC regenerated at 773 K was slightly lower than that of the CBC regenerated at 673 K but was slightly higher than that of the CBC regenerated at 873 K. The adsorption capacity of regenerated CBC decreased with increasing temperature. When comparing the total adsorption capacities of regenerated CBC and control CBC, the total adsorption capacities of regenerated CBC were higher than that of the control CBC confirming that the heat regeneration of CBC was an effective method in defluoridation of drinking water.
Adsorption of fluoride onto heat regenerated bone char has been reported in the literature. Kaseva studied heat regenerated cattle bone char in fluoride removal at different temperatures and determined that the best regenerating temperature was 773 K [11]. Nigri et al. studied heat regenerated bovine bone char in fluoride removal at different temperatures and determined that the best regenerating temperature was 673 K [14], as we obtained in our study.
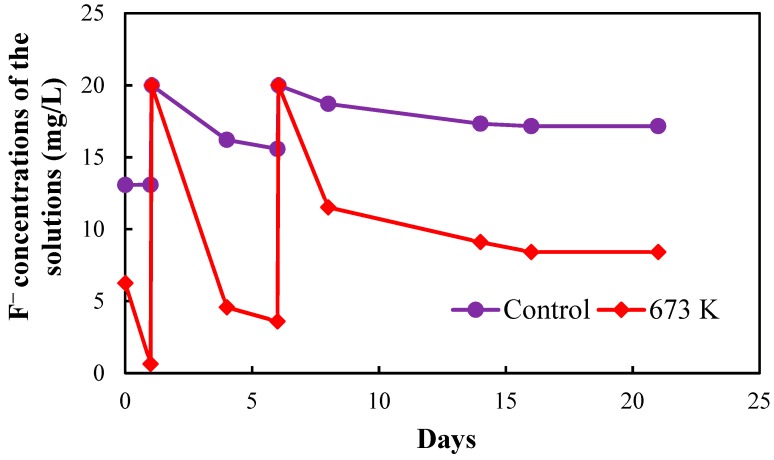
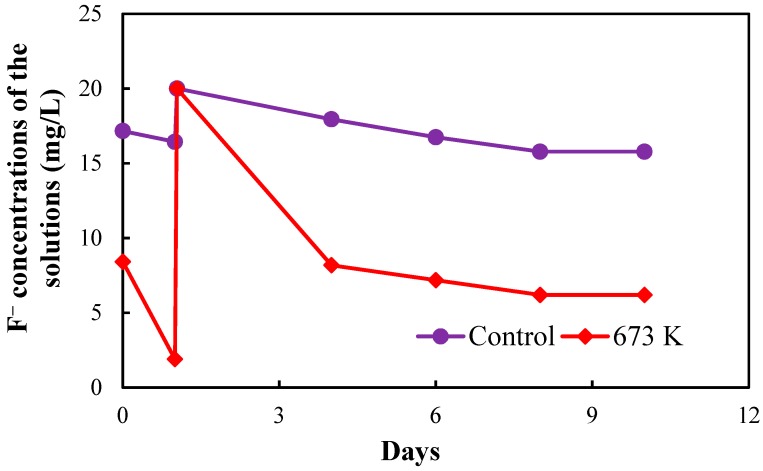
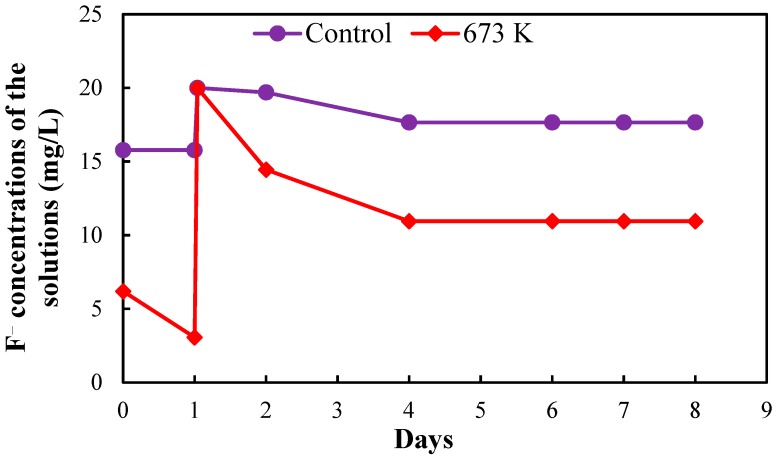
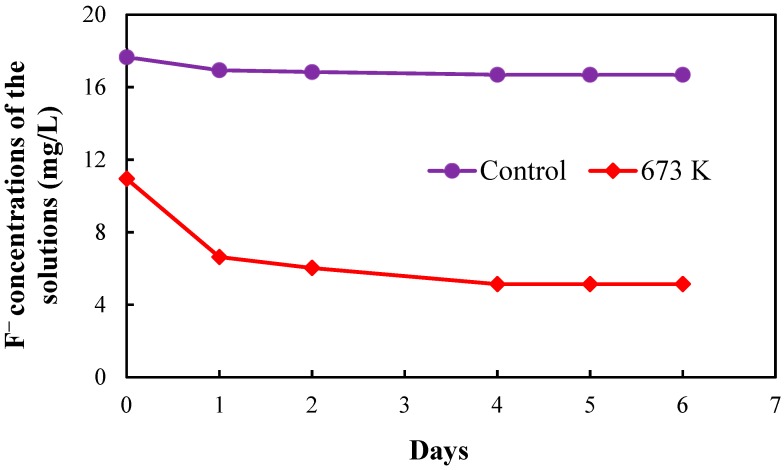
Figure 5, Figure 6, Figure 7 and Figure 8, respectively, show the fluoride concentrations of the solutions; control and 673 K regenerated setups after the 2nd, 3rd, 4th and 5th regenerations of CBC throughout the operation periods of 21, 10, 8 and 6 days, respectively.
Figure 5.
Fluoride concentrations of the solutions in the two setups after 2nd regeneration of CBC.
Figure 6.
Fluoride concentrations of the solutions in the two setups after 3rd regeneration of CBC.
Figure 7.
Fluoride concentrations of the solutions in the two setups after 4th regeneration of CBC.
Figure 8.
Fluoride concentrations of the solutions in the two setups after 5th regeneration of CBC.
The operation period of the 2nd regeneration was 21 days. The fluoride concentrations of the solutions in the two setups were increased to 20 mg/L at day 1 and day 6 in the 2nd regeneration. The operation period of the 3rd regeneration was 10 days. The fluoride concentrations of the solutions in the two setups were increased to 20 mg/L at day 1 in the 3rd regeneration. The operation period of the 4th regeneration was 8 days. The fluoride concentrations of the solutions in the two setups were increased to 20 mg/L at day 1 in the 4th regeneration. The operation periods of the 5th regeneration was 6 days. The fluoride concentrations of the solutions in the two setups were not increased in the 5th regeneration.
NaF solution with the concentration of 1000 mg/L was used to increase the fluoride concentrations of the solutions in the two setups. The fluoride concentrations of the two setups were increased in the 2nd, 3rd and 4th regenerations to obtain a quick equilibrium with the fluoride in the solutions.
The equilibrium fluoride concentrations of the solutions in the two setups were used to determine the fluoride adsorption capacity of CBC after the 2nd, 3rd, 4th and 5th regenerations.
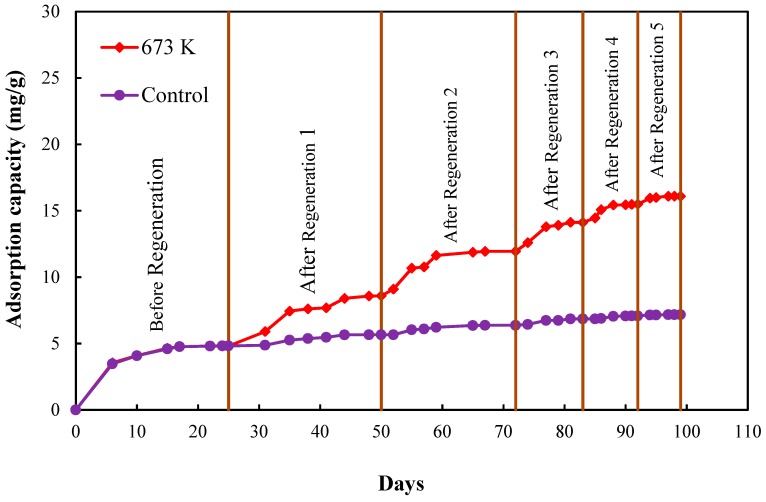
Figure 9 shows the F− adsorbed by CBC before and after the 1st, 2nd, 3rd, 4th and 5th regenerations, obtained from the mass balance calculation for the solutions throughout the operation period of 99 days for the control and the 673 K regenerated setups. Table 1 shows the total amount of F− adsorbed by CBC before and after regeneration, obtained from the mass balance calculation for the solutions; control, 673 K regenerated, 773 K regenerated and 873 K regenerated setups. Table 2 shows the adsorption capacities of CBC before and after regeneration obtained by the acid digestion of CBC; control, 673 K regenerated, 773 K regenerated and 873 K regenerated setups. F− content in CBC before the adsorption (raw CBC) was considered when calculating the adsorption capacity by acid digestion of CBC.
Figure 9.
Adsorption of F− by CBC (control and 673 K regenerated CBC) before and after 1st, 2nd, 3rd, 4th and 5th regenerations obtained from the mass balance calculation for the solutions throughout the operation period.
Table 1.
Adsorption capacities of CBC used for the regeneration obtained from the mass balance calculation for the solutions.
| Setups | State | Weight of CBC (g) | Adsorbed F− (mg) | Adsorption Capacity (mg/g) | Total Adsorption Capacity (mg/g) |
|---|---|---|---|---|---|
| Control | Before regeneration | 10.07 | 48.57 | 4.82 | 7.17 |
| After regeneration 1 | 8.41 | 0.84 | |||
| After regeneration 2 | 7.22 | 0.72 | |||
| After regeneration 3 | 4.84 | 0.48 | |||
| After regeneration 4 | 2.31 | 0.23 | |||
| After regeneration 5 | 0.89 | 0.09 | |||
| 673 K regenerated | Before regeneration | 10.66 | 51.25 | 4.81 | 16.11 |
| After regeneration 1 | 9.87 | 37.39 | 3.79 | ||
| After regeneration 2 | 9.81 | 32.88 | 3.35 | ||
| After regeneration 3 | 8.99 | 19.62 | 2.18 | ||
| After regeneration 4 | 8.75 | 12.00 | 1.37 | ||
| After regeneration 5 | 8.60 | 5.22 | 0.61 | ||
| 773 K regenerated | Before regeneration | 10.21 | 49.35 | 4.83 | 8.37 |
| After regeneration | 9.52 | 33.66 | 3.54 | ||
| 873 K regenerated | Before regeneration | 10.36 | 49.90 | 4.81 | 8.02 |
| After regeneration | 9.62 | 30.86 | 3.21 |
Table 2.
Adsorption capacities of CBC according to the HNO3 digestion.
| Setups | F− Adsorption Capacity (mg/g) | ||
|---|---|---|---|
| F− Content in CBC before Adsorption (raw CBC) | F− Content in CBC after Adsorption | Net Adsorption | |
| Control | 0.20 | 6.84 | 6.64 |
| 673 K regenerated | 15.77 | 15.57 | |
| 773 K regenerated | 7.93 | 7.73 | |
| 873 K regenerated | 7.63 | 7.43 | |
Adsorption capacities of CBC obtained from the mass balance calculation for the solutions were coincided with the adsorption capacities obtained from the acid digestion of CBC. However, there is a slight difference between the adsorption capacities obtained from the mass balance calculation for the solutions and from the acid digestion. This may be due to an error in the volume measurement when sampling the solutions for the regular measurement of F− concentration of the solution in each setup.
Table 3 shows the recovery rate of 673 K regenerated CBC during the five regeneration cycles. The recovery rate of CBC gradually decreased after each regeneration cycle. The recovery rate decreased approximately by half with each regeneration cycle. It is possible to reuse CBC five times repeatedly by continuous heat regeneration. This phenomenon is environmentally friendly, since it minimizes the wastage of CBC. Repeated heat regeneration of CBC is a new approach in defluoridation techniques. No study has used bone char continuously for five heat regeneration cycles in fluoride removal.
Table 3.
Recovery rate of 673 K regenerated CBC.
| State | Adsorption Capacity (mg/g) | Recovery Rate (%) |
|---|---|---|
| Before regeneration | 4.81 | - |
| After regeneration 1 | 8.60 | 79 |
| After regeneration 2 | 11.95 | 39 |
| After regeneration 3 | 14.13 | 18 |
| After regeneration 4 | 15.50 | 10 |
| After regeneration 5 | 16.11 | 4 |
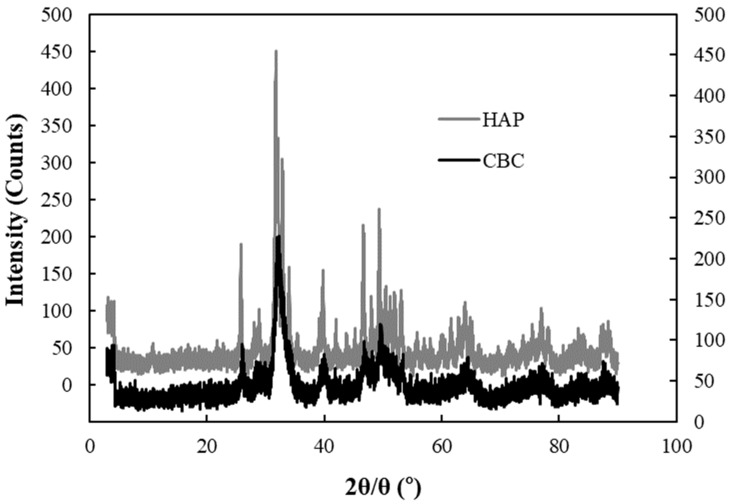
The major component of chicken bone char (CBC) is Hydroxyapatite (HAP) [6,12]. Figure 10 shows the XRD patterns of HAP and CBC. The XRD patterns overlap, indicating that the major component of CBC is HAP.
Figure 10.
XRD patterns of CBC and HAP. HAP (left Y axis), CBC (right Y axis).
Fluoride removal by chicken bone char (CBC) is associated with the mechanism of ion exchange.
The ion exchange mechanism is shown in Equation (1):
| (1) |
The hydroxyl ion in HAP in bone char (CBC) is replaced by fluoride ion, in the presence of fluoride ions. Insoluble fluorapatite (FAP) is formed from HAP [15] and the hydroxyl ion is released into the solution, as represented in Equation (1) [16].
The hydroxyl ion can replace fluoride ion in mineral structures because of the same charge and similar radius of F− and OH− [17].
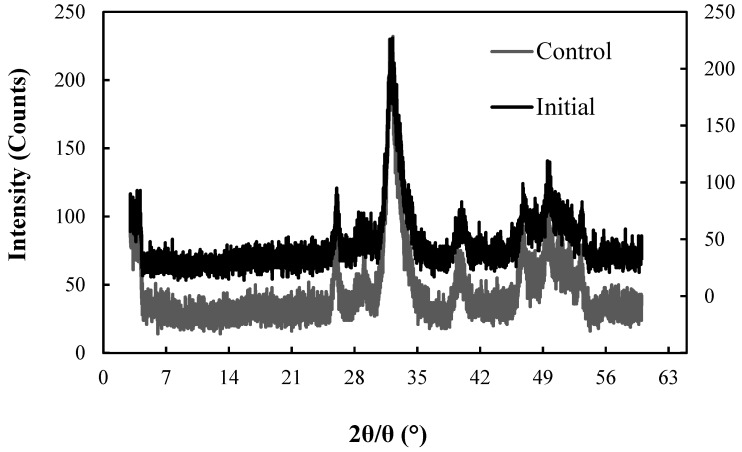
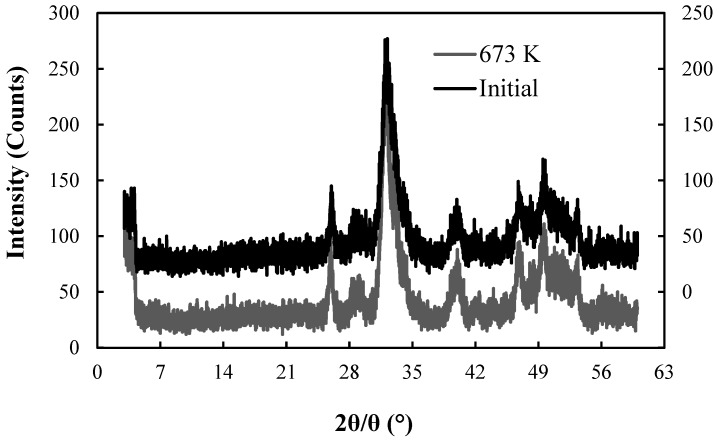
Figure 11 and Figure 12 show the XRD patterns of CBC before (initial) and after the fluoride adsorption for the control and the 673 K regenerated CBC, respectively.
Figure 11.
XRD patterns for the initial CBC and control setup after F− adsorption. Initial (right Y axis), After 99 days’ adsorption for control setup (left Y axis).
Figure 12.
XRD patterns for the initial CBC and CBC after F− adsorption. Initial (right Y axis), After 99 days’ adsorption for 673 K regenerated setup (left Y axis).
It was obvious that the XRD patterns of CBC before and after adsorption overlapped reveling that the structure of CBC has not changed during the fluoride adsorption by heat regenerated CBC, even after five regeneration cycles. The HAP structure of chicken bone char (CBC) was not changed by repeated heat regenerations. The fluoride incorporated to HAP (FAP) was detected by the acid digestion of CBC (Table 2) after 1st, 2nd, 3rd, 4th and 5th heat regenerations. The Equation (1) took place during the adsorption [12].
Nigri et al., showed that the XRD patterns of bone char were not changed during the fluoride adsorption by heat regenerated bone char by studying bovine bone char for a single heat regeneration cycle. They also reported the formation of FAP during the fluoride adsorption by heat regenerated bovine bone char [14].
Kaseva reported that the fluoride adsorbed on exhausted bone char (CBC) evaporated as HF gas during the heat regeneration, as shown in Equation (2) [11]:
| (2) |
The same amount of adsorbed fluoride onto CBC after the 1st, 2nd, 3rd, 4th and 5th regenerations (Table 1) was detected both by the acid digestion of CBC (Table 2) and by the mass balance calculation. It was concluded that the adsorbed fluoride during the formation of exhausted CBC was not evaporated as HF gas during the heat regeneration as mentioned in Equation (2) [12].
Nigri et al., reported that the diffusion of adsorbed fluoride inside to the bone char pores was observed during the heat regeneration [14]. This phenomenon might have occurred during our study as well.
Table 4 shows the solution pH. There was an increase in pH in the final solutions after regeneration when compared to the initial solution. The pH value of the solution should increase due to the release of OH− into the solution with the formation of FAP as shown in Equation (1).
Table 4.
Solution pH.
| Solutions | State | pH |
|---|---|---|
| Initial solutions | - | 7.39 |
| Final solutions before regeneration | Control | 8.23 |
| 673 K | 8.31 | |
| 773 K | 8.28 | |
| 873 K | 8.29 | |
| Final solutions after regeneration 1 | Control | 8.28 |
| 673 K | 8.21 | |
| 773 K | 8.27 | |
| 873 K | 8.47 | |
| Final solution after regeneration 2 | Control | 8.31 |
| 673 K | 8.23 | |
| Final solution after regeneration 3 | Control | 8.27 |
| 673 K | 8.23 | |
| Final solution after regeneration 4 | Control | 8.27 |
| 673 K | 8.31 | |
| Final solution after regeneration 5 | Control | 8.26 |
| 673 K | 8.35 |
Table 5 shows the changes in anion and cation concentrations of the solutions used for the regeneration before and after adsorption. The Na+ in the final solutions increased mainly due to the addition of NaF to the solutions. A considerable increase of Na+ was detected in the regenerated solutions; 673 K, 773 K and 873 K when compared to the control. PO43− and K+ were dissolved from CBC [6] and increased their concentration in the final solutions. SO42− was also detected in the final solutions. A considerable increase of SO42− was detected in the regenerated solutions; 673 K, 773 K and 873 K when compared to the control. Mg2+ and Ca2+ ions were adsorbed by CBC and were not detected in the final solutions.
Table 5.
Anion and cation concentrations of solutions.
| Solutions | Concentrations (mg/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | State | F− | Cl− | SO42− | NO3− | PO43− | Na+ | NH4+ | K+ | Mg2+ | Ca2+ |
| Initial solutions | - | 50 | 10 | 6 | 1 | 0 | 68 | 0 | 1 | 1 | 7 |
| Final solutions before regeneration | Control | 12 | 10 | 10 | 1 | 46 | 86 | 1 | 13 | 0 | 0 |
| 673 K | 12 | 11 | 12 | 1 | 49 | 89 | 1 | 15 | 0 | 0 | |
| 773 K | 12 | 10 | 10 | 1 | 49 | 88 | 1 | 13 | 0 | 0 | |
| 873 K | 12 | 13 | 12 | 1 | 45 | 87 | 1 | 14 | 0 | 0 | |
| Final solutions after regeneration 1 | Control | 13 | 9 | 9 | 1 | 43 | 85 | 1 | 12 | 0 | 0 |
| 673 K | 6 | 12 | 126 | 1 | 32 | 126 | 2 | 15 | 0 | 0 | |
| 773 K | 8 | 11 | 90 | 1 | 45 | 116 | 2 | 13 | 0 | 0 | |
| 873 K | 9 | 15 | 39 | 1 | 46 | 121 | 2 | 16 | 0 | 0 | |
| Final solution after regeneration 2 | Control | 17 | 8 | 8 | 1 | 42 | 88 | 1 | 11 | 0 | 0 |
| 673 K | 8 | 11 | 166 | 1 | 40 | 149 | 2 | 13 | 0 | 0 | |
| Final solution after regeneration 3 | Control | 16 | 7 | 7 | 1 | 40 | 83 | 1 | 10 | 0 | 0 |
| 673 K | 6 | 11 | 182 | 1 | 44 | 158 | 3 | 13 | 0 | 0 | |
| Final solution after regeneration 4 | Control | 18 | 7 | 7 | 1 | 38 | 82 | 1 | 9 | 0 | 0 |
| 673 K | 11 | 11 | 173 | 3 | 52 | 174 | 3 | 13 | 0 | 0 | |
| Final solution after regeneration 5 | Control | 17 | 7 | 7 | 1 | 38 | 82 | 1 | 9 | 0 | 0 |
| 673 K | 5 | 11 | 181 | 5 | 56 | 176 | 3 | 13 | 0 | 0 | |
Fluoride removal by bone char (CBC) is also associated with the precipitation of calcium fluoride (CaF2).
The CaF2 precipitation mechanism is shown in Equation (3):
| (3) |
HAP in bone char (CBC) precipitates into calcium fluoride (CaF2), in the presence of excess fluoride ions. Phosphate (PO43−) in HAP is released into the solution [12] as shown in Equation (3) [17].
The Equation (3) also took place during the adsorption, since phosphate was detected in the final solutions before and after the 1st, 2nd, 3rd, 4th and 5th heat regenerations, as well as in the control setup [12].
According to the Equation (3), the molar ratio of F− to PO43− in our study was 0.0938 mol/L:0.0011 mol/L (F− = ((163.58 − 74.49) mg/L/1000/19 g/mol) × 20; based on adsorbed F− for control (74.49 mg/L) and 673 K regenerated CBC after 5th regeneration (163.58 mg/L)), PO43− = ((56 − 38) mg/L/1000/94.97 g/mol) × 6; based on the PO43− data for final solutions for control and 673 K regenerated CBC after the 5th regeneration reported in Table 5).
The molar ratio of F− to PO43− obtained in our study did not coincide with the molar ratio of F− to PO43− in Equation (3). The amount of F− was much higher than the appropriate molar ratio representing by Equation (3). The molar ratio obtained by our study for F− and PO43− was evidence that the adsorption of F− was not only due to CaF2 precipitation. Therefore, we can confirm that the ion exchange incorporated with CaF2 precipitation occurred during the adsorption [12].
Nigri et al., detected phosphate in fluoride adsorption experiments by heat regenerated bovine bone char for a single regeneration cycle and showed that the chemical precipitation occurred during the fluoride adsorption by heat regenerated bone char [14].
Further, it is possible that the unopened pores in chicken bone char (CBC) may reactivate the fluoride adsorption [12] after each regeneration cycle, instead of the above mentioned phenomenon.
4. Conclusions
Fluoride removal from contaminated water is essential in order to protect human health. CBC was selected as a low cost adsorbent for fluoride removal. Regeneration of CBC was investigated and heat regeneration was selected as the regeneration method. It was found that 673 K was the best regenerating temperature. Repeated heat regeneration of CBC was studied by five heat regeneration cycles at 673 K. The recovery percentage of CBC decreased gradually after each regeneration cycle; however, the total F− adsorbed on to CBC was 16.1 mg/g, which was almost two times higher than that without regeneration.
The XRD patterns of CBC before and after the five regeneration cycles revealed that the structure of CBC did not change even after five continuous regeneration cycles. The results revealed that the ion exchange incorporated with the CaF2 precipitation occurred during the adsorption.
The repeated heat regeneration of CBC is possible and it is a new approach in fluoride removal techniques. CBC can be reused to minimize the wastage of CBC by using this technique, hence, it is a sustainable, environmentally friendly and efficient technique in fluoride removal. Therefore, this technique is highly recommended for developing countries.
Author Contributions
Data curation, H.M.A.S.H., T.K. and M.T.; Formal analysis, H.M.A.S.H., T.K. and M.T.; Funding acquisition, T.K.; Investigation, H.M.A.S.H., T.K. and M.T.; Methodology, H.M.A.S.H., T.K. and M.T.; Resources, H.M.A.S.H., T.K. and M.T.; Supervision, T.K.; Writing—original draft, H.M.A.S.H.; Writing—review & editing, H.M.A.S.H. and T.K.
Funding
This research was funded by the Japanese government’s (Monbukagakusho) Ministry of Education, Culture, Sports, Science and Technology (MEXT) and partly supported by JSPS KAKENHI Grant Numbers 23404003, 15H05120 and 25257306.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Yadav A.K., Kaushik C.P., Haritash A.K., Kansal A., Rani N. defluoridation of groundwater using brick powder as an adsorbent. J. Hazard. Mater. 2006;128:289–293. doi: 10.1016/j.jhazmat.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Herath H.M.A.S., Kawakami T., Nagasawa S., Serikawa Y., Motoyama A., Chaminda G.G.T., Weragoda S.K., Yatigammana S.K., Amarasooriya A.A.G.D. Arsenic, cadmium, lead and chromium in well water, rice and human urine in sri lanka relation to chronic kidney disease of unknown etiology. J. Water Health. 2018;16:212–222. doi: 10.2166/wh.2018.070. [DOI] [PubMed] [Google Scholar]
- 3.Herath H.M.A.S., Kubota K., Kawakami T., Nagasawa S., Motoyama A., Weragoda S.K., Chaminda G.G.T., Yatigammana S. Potential risk of drinking water to human health in Sri Lanka. J. Environ. Forensics. 2017;18:241–250. doi: 10.1080/15275922.2017.1340364. [DOI] [Google Scholar]
- 4.World Health Organization . Guidelines for drinking—Water Quality. 4th ed. WHO; Geneva, Switzerland: 2011. [Google Scholar]
- 5.Tomar V., Prasad S., Kumar D. Adsorptive removal of fluoride from aqueous media using Citrus limonum (lemon) leaf. Microchem. J. 2014;112:97–103. doi: 10.1016/j.microc.2013.09.010. [DOI] [Google Scholar]
- 6.Herath H.M.A.S., Kawakami T., Tafu M. The extremely high adsorption capacity of fluoride by chicken bone char (CBC) in defluoridation of drinking water in relation to its finer particle size for better human health. Healthcare. 2018;6:123. doi: 10.3390/healthcare6040123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang N., Yang X., Yu X., Jia Y., Wang J., Kong L., Jin Z., Sun B., Luo T., Liu J. Al-1,3,5-benzenetricarboxylic of water with pH insensitivity and low aluminum residual. Chem. Eng. J. 2014;252:220–229. doi: 10.1016/j.cej.2014.04.090. [DOI] [Google Scholar]
- 8.Chauhan V.S., Dwivedi P.D., Iyengar L. Investigation on activated alumina based domestic defluoridation units. J. Hazard. Mater. 2007;B139:103–107. doi: 10.1016/j.jhazmat.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Thakre D., Rayalu S., Kawade R., Meshram S., Subrt J., Labhsetwar N. Magnesium incorporated bentonite clay for defluoridation of drinking water. J. Hazard. Mater. 2010;180:122–130. doi: 10.1016/j.jhazmat.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Lunge S., Biniwale R., Labhsetwar N., Rayalu S.S. User perception study for performance evaluation of domestic defluoridation techniques for its application in rural areas. J. Hazard. Mater. 2011;191:325–332. doi: 10.1016/j.jhazmat.2011.04.088. [DOI] [PubMed] [Google Scholar]
- 11.Kaseva M.E. Optimization of regenerated bone char for fluoride removal in drinking water: A case study in Tanzania. J. Water Health. 2006;4:139–147. doi: 10.2166/wh.2006.0011. [DOI] [PubMed] [Google Scholar]
- 12.Herath H.M.A.S., Kawakami T., Tafu M. Regeneration of exhausted chicken bone char (CBC) to optimize its usage in the defluoridation of drinking water. J. Ecotechnol. Res. 2018;18:39–46. [Google Scholar]
- 13.Herath H.M.A.S. Ph.D. Thesis. Toyama Prefectural University; Imizu, Toyama Prefecture, Japan: 2018. Public health and groundwater quality in sri lanka and defluoridation of drinking water in relation to chronic kidney disease of unknown etiology. [Google Scholar]
- 14.Nigri E.M., Bhatnagar A., Rocha S.D.F. Thermal regeneration process of bone char used in the fluoride removal from aqueous solution. J. Clean. Prod. 2017;142:3558–3570. doi: 10.1016/j.jclepro.2016.10.112. [DOI] [Google Scholar]
- 15.Ismail Z.Z., Abdelkareem H.N. Sustainable approach for recycling waste lamb and chicken bones for fluoride removal from water followed by reusing fluoride-bearing waste in concrete. Waste Manag. 2015;45:66–75. doi: 10.1016/j.wasman.2015.06.039. [DOI] [PubMed] [Google Scholar]
- 16.Fawell J., Bailey K., Chilton J., Dahi E., Fewtrell L., Magara Y. 2006 Fluoride in Drinking Water. World Health Organization; Geneva, Switzerland: IWA Publishing; 2006. [Google Scholar]
- 17.Brunson L.R., Sabatini D.A. An Evaluation of Fish Bone Char as an Appropriate Arsenic and Fluoride Removal Technology for Emerging Regions. Environ. Eng. Sci. 2009;26:1777–1784. doi: 10.1089/ees.2009.0222. [DOI] [Google Scholar]