Abstract
MoS2 quantum dots (QDs) functionalized g-C3N4 nanosheets (MoS2@CNNS) were prepared through a protonation-assisted ion exchange method, which were developed as a highly efficient biomimetic catalyst. Structural analysis revealed that uniformly-dispersed MoS2 QDs with controllable size and different loading amount grew in-situ on the surface of CNNS, forming close-contact MoS2@CNNS nanostructures and exhibiting distinct surface properties. Compared to MoS2 QDs and CNNS, the MoS2@CNNS nanocomposites exhibited a more than four times stronger peroxidase-like catalytic activity, which could catalyze the oxidation of 3,3’,5,5’-tetramethylbenzidine (TMB) in the presence of H2O2 to generate a blue oxide. Among the MoS2@CNNS nanocomposites, MoS2@CNNS(30) was verified to present the best intrinsic peroxidase-like performance, which could be attributed to the more negative potential and larger specific surface area. A simple, rapid and ultrasensitive system for colorimetric detection of H2O2 was thus successfully established based on MoS2@CNNS, displaying nice selectivity, reusability, and stability. The detection limit of H2O2 could reach as low as 0.02 μM. Furthermore, the kinetic and active species trapping experiments indicated the peroxidase-like catalytic mechanism of MoS2@CNNS. This work develops a novel, rapid, and ultrasensitive approach for visual assay of H2O2, which has a potential application prospect on clinical diagnosis and biomedical analysis.
Keywords: MoS2, g-C3N4, peroxidase-like, colorimetric detection, H2O2
1. Introduction
Over past decades, enzyme mimetics have caused extensive concern due to their favorable superiorities against harsh conditions compared to natural enzymes, such as low cost, easy preparation and storage, better stability and reusability, and nice practicability [1,2,3]. Hence, it is interesting and challenging to develop novel and effective enzyme mimetics. Recently, various enzyme mimetics have been reported and widely used in clinical diagnosis and biomedical analysis, including nanomaterials [4,5,6,7], Schiff-base complexes [8], cyclodextrin [9], hemin [10], and DNA complexes [11]. Among them, nanomaterials are becoming a novel efficient mimic peroxidase in catalyzing H2O2-mediated reaction due to their intrinsic properties like natural enzymes in size, shape and surface charge [4,5,6]. Moreover, nanomaterials exhibit nice surface properties with larger specific surface areas, more surface activation centers, and controlled catalytic potentials, which can highly promote their peroxidase-like catalytic performances [5]. Thus, the field for seeking novel nanomaterials such as peroxidase mimetics has been rapidly developed since Fe3O4 magnetic nanoparticles were first found to present the intrinsic peroxidase-like activity like that of horseradish peroxidase (HRP) in 2007 [4]. Thereafter, many kinds of nanomaterials have been exploited as peroxidase mimetics, and have exhibited good peroxidase-like properties, such as magnetic nanomaterials (CoFe2O4 [12] and FeVO4 [13]), carbon nanomaterials (carbon nanotubes [14], carbon dots [15], graphene oxides [16], and C3N4 [17]), noble metal nanomaterials (gold, silver and platinum) [18] and their alloys (AgVO3 nanobelts [19], FeSe-Pt@SiO2 nanospheres [20], Fe3O4-Pt nanocomposites [21], and Fe3O4-Au nanohybrids [22]), and other nanomaterials (BiOI nanoflowers [23], CeVO4 nanorods [24] and MoS2 nanoflakes [25]). Despite this progress, there is still an urgent demand to pursue novel nanomaterials with highly-efficient and stable peroxidase-like activities to overcome their inherent disadvantages, including the loss of noble metals, environmental pollution, difficulty in separation, and recyclability.
Recently, special attention has been focused on graphene-like, two-dimensional (2D) nanomaterials owing to their 2D layer structure with high energy surfaces analogous to graphene [25,26,27]. As typical 2D nanomaterials, graphitic carbon nitride (g-C3N4) possesses a stacked 2D structure and appropriate band gap (2.7 eV) owing to the sp2 hybridization of carbon and nitrogen, resulting in the formation of a stable and extended π-conjugated system [25,26,27]. And the unique graphite-like structure and tunable electronic structure of g-C3N4 lead to its large specific surface area, high thermal and chemical stability, and rapid electron transfer, accompanied by the advantage of being metal-free, abundant in natural resources, and economical, endowing g-C3N4 with extensive potential for use in new energy, sensor, and catalysis applications [25,26,27]. Currently, g-C3N4 materials have been reported as peroxidase mimetics [17], showing nice peroxidase-like activities and further extending their application areas in biotechnology. However, in view of the high recombination rate of photoinduced electron-hole pairs, the catalytic efficiency of pure g-C3N4 is greatly restricted [27]. Hence, various of methods have been performed to further improve the catalytic activity. Among them, constructing a functionalized hybrid structure using g-C3N4 as the supporter by doping with other efficient catalysts is an especially effective way, which can apparently adjust the electronic structure and accelerate electron transport [28,29]. Up to now, lots of g-C3N4-based composites have been designed and exploited to explore the synergistic enhancement effect, such as Cu/g-C3N4 [28], MnSe-g-C3N4 nanosheets [29], Fe-g-C3N4 [30], Co-g-C3N4 [31], g-C3N4/BiFeO3 nanocomposites [32], and so on, all of which exhibited an improved peroxidase-like activity. Therefore, in-depth investigations are indeed of great demand for designing and fabricating novel g-C3N4-based nanocomposites to further facilitate the peroxidase-like activity.
As one of the typical 2D transition metal dichalcogenides, MoS2 materials show excellent catalytic activity and long-term stability, which has been widely used in the fields of electronic devices, battery materials, and catalysts [33,34]. In addition, when the size decreased to less than 10 nm, MoS2 QDs will exhibit unique extra electrical/optical properties owing to the stronger quantum confinement and edge effects [35,36], further improving its catalytic performance. Moreover, MoS2 with diverse nanostructures (nanoflakes [25] and nanoparticles [37]) and several MoS2-based hybrids [38,39,40] have been reported as enzyme mimetics recently. Thus, on account of the matching energy band structure and nice catalytic performance, MoS2 QDs is becoming an ideal candidate for coupling with g-C3N4 to facilitate the peroxidase-like ability by promoting the separation and transport of electron-hole pairs. However, to the best of our knowledge, the peroxidase-like activity of MoS2 QDs-g-C3N4 hybrids has not been investigated until now, which deserves further and deeper exploration.
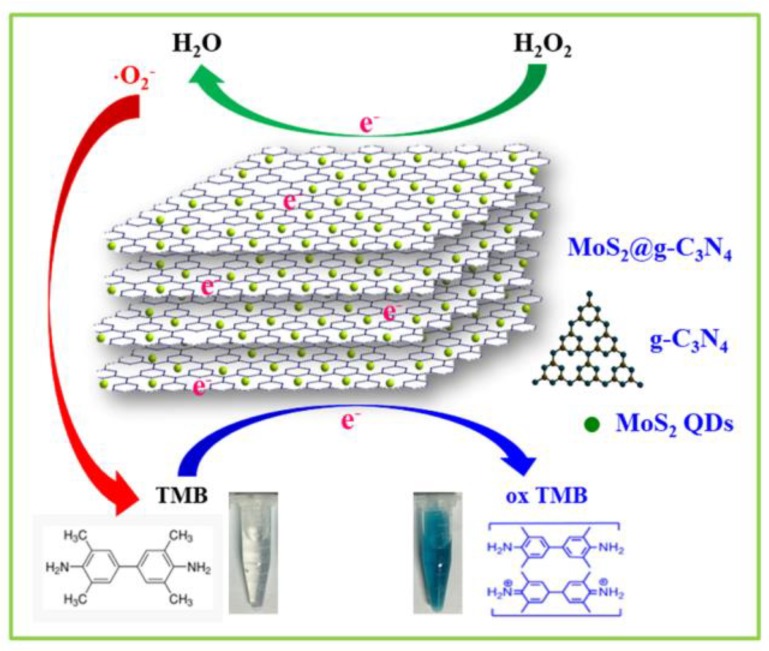
Herein, in view of the superiority of g-C3N4 and MoS2 QDs, the MoS2@CNNS nanocomposites with different morphologies and surface properties were successfully designed and prepared via a protonation-assisted ion exchange method, which were used as efficient artificial enzymes. With the assistance of H2O2, the MoS2@CNNS nanocomposites could catalyze the oxidation of the peroxidase substrate TMB to generate a blue colored reaction. Thus, a simple, rapid, ultrasensitive, selective, and stable system for the colorimetric detection of H2O2 was developed (Scheme 1). In addition, the morphology, crystal structure, surface properties, catalytic kinetics and mechanism, reusability and selectivity of the MoS2@CNNS nanocomposites were studied. The peroxidase-like catalytic activities of MoS2@CNNS nanocomposites had been greatly enhanced after the incorporation between CNNS and MoS2 QDs, which could be mainly attributed to the synergistic interaction, accompanied by the more negative charge and larger specific surface area. The MoS2@CNNS nanocomposites could have promising and broad applications in catalysis, biotechnology, and clinical diagnostics.
Scheme 1.
Schematic illustration of peroxidase-like catalytic reaction of MoS2@CNNS nanocomposites.
2. Materials and Methods
2.1. Materials and Reagents
MoS2 powder and 3,3’,5,5’-Tetramethylbenzidine (TMB) were purchased from Sigma-Aldrich (Shanghai, China). Melamine (C3H6N6), Na2MoO4∙2H2O, Tioacetamida (TAA, CH3CSNH2), 1-methyl-2-pyrrolidone (NMP, C5H9NO), HCl, H2O2 (3 wt %), ethanol, isopropanol alcohol (IPA), p-benzoquinone (BQ), FeCl3⋅6H2O, CuSO4⋅5H2O, KNO3, NaClO, glucose (Glu), lactose (Lac), l-valine (l-Val), glycine (Gly), and other chemicals were all of analytical grade and were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Lircon antiseptic liquid was obtained from Shandong Lierkang Disinfection Technology Co., Ltd. (Dezhou, China). All aqueous solutions were prepared with Milli-Q water (Millipore, Boston, MA, USA).
2.2. Preparation of the Catalysts
The MoS2@CNNS nanocomposites were prepared according to our previous report by an in-situ ion exchange method in a protonation process [36]. The bulk g-C3N4 was firstly prepared via a calcination process. Then g-C3N4 nanosheets (CNNS) were obtained by the ultrasonic exfoliation method using bulk g-C3N4 powder. After a protonation process in HCl (37%) and an ion exchange process under a hydrothermal condition, the MoS2@CNNS nanocomposites were finally obtained and denoted as MoS2@CNNS(30) (‘30’ stands for the molar ratio of Na2MoO4∙2H2O/CNNS was 30:1).
In addition, other MoS2@CNNS nanocomposites with different molar ratio were prepared as controls under the same conditions as those mentioned above, which were denoted as MoS2@CNNS(15) and MoS2@CNNS(45), respectively. Moreover, pure MoS2 QDs were prepared as control via an ultrasonic exfoliation method [41]. In brief, 100 mg of MoS2 powder was added into 10 mL of NMP and sonicated for 3.5 h. Then, the dispersion was kept stable overnight and centrifuged at 5500 r/min for 90 min. Finally, the MoS2 QDs were obtained by collecting the top part of the dispersion.
2.3. Characterization
The crystal phase and structure of the as-prepared samples were analyzed by powder X-ray diffraction (XRD) measurements on a Germany Bruker D8 Advanced powder diffractometer using Cu Kα radiation (λ = 0.15406 nm). The morphology and microstructure of the as-prepared samples were observed by transmission electron microscopy (TEM), high resolution transmission electron microscopy (HRTEM), and selected area electron diffraction (SAED) (JEOL JEM-2100, Tokyo, Japan). Contents of S and Mo in MoS2@CNNS nanocomposites were measured via an inductively coupled plasma emission spectrometer (ICP-AES, Varian725-ES, Palo Alto, CA, USA). The specific surface areas were determined by an automatic nitrogen adsorption specific surface and pore size distribution analyzer (NOVA 4000e, Quantachrome Instruments, Boynton Beach, FL, USA) at 77 K after a pretreatment at 473 K for 2 h. The zeta potential tests were determined on a zeta potential measuring instrument (Zetasizer Nano-ZS, Malvern Instruments Ltd., Malvern, UK), which was examined six times (each time being the average of 100 runs) at pH 4.0, and the mean values and standard deviations were calculated automatically based on Smoluchowski’s equation.
2.4. Peroxidase-Like Activities and Steady-State Kinetic Assay
The catalytic oxidation experiments (a peroxidase substrate TMB with H2O2) were carried out at room temperature to comparably investigate the peroxidase-like activities of the as-prepared catalysts, including MoS2 QDs, CNNS and MoS2@CNNS nanocomposites. Typically, the tests were performed by adding 200 μL of 600 μg/mL catalysts into the reaction systems containing 500 μL of 50.0 mM phosphate buffer solution (PBS, pH = 4.0), 100 μL of 8.0 mM TMB, and 200 μL of 10.0 mM H2O2. Then, the reaction systems were monitored in a time-scan mode at 652 nm by an UV-visible spectrophotometer (Shimadzu UV-2500, Kyoto, Japan) right after all of the components were added and mixed. The effect of MoS2@CNNS(30) concentration (0–200 μg/mL), H2O2 concentration (0–5.0 mM), pH (2.0–9.0), and temperature (10–50 °C) on the peroxidase-like activity of MoS2@CNNS(30) were also tested by the same procedures mentioned above to probe the optimal reaction conditions (Supplementary Information Figure S2 and Figure S3).
The steady-state kinetic tests were performed in a time course mode at 652 nm by an UV-visible spectrophotometer under the optimal experimental conditions (25.0 mM PBS, pH = 4.0, 25 °C, 120 μg/mL MoS2@CNNS(30)) by varying the concentration of TMB at a fixed concentration of H2O2 or vice versa [4,42]. The Michaelis-Menten constant was measured using the Lineweaver-Burk double reciprocal plot according to the equation 1/v = (Km/Vmax) × (1/[S]) + 1/Vmax [4,42], where v is the initial reaction velocity, Km is the Michaelis-Menten constant, Vmax is the maximum reaction velocity, and [S] is the concentration of substrate.
2.5. Analysis of Active Species
To explore the roles of active species played in the catalytic reaction, the active species trapping experiments were carried out. In brief, scavengers (10.0 mM IPA, a scavenger of ⋅OH; 10.0 mM BQ, a scavenger of ⋅O2−) were added into the reaction systems respectively to remove the active species under the optimal experimental conditions [23,24,43]. Then, the absorbance and color change of the reaction system was recorded at 652 nm by an UV-visible spectrophotometer.
2.6. H2O2 Detection
A colorimetric detection system of H2O2 was set up based on the nice peroxidase-like performances of MoS2@CNNS(30). The reaction system contained 100 μL of 8.0 mM TMB, 200 μL of 600 μg/mL MoS2@CNNS(30), 200 μL of H2O2 with different concentrations (0–0.1 mM), and 500 μL of 50.0 mM PBS (pH = 4.0). Then, the absorbance change of the mixture was recorded on time-mode at 652 nm with an UV-visible spectrophotometer to obtain a standard curve.
In addition, the specificity and selectivity of MoS2@CNNS(30)-based detection system were evaluated by adding some potential interfering substances into the reaction system under the same experimental conditions mentioned above, such as metal and non-metal ions (20.0 mM of Fe3+, Cu2+, NO3−, and ClO−) and common organic compounds (20.0 mM of glucose (Glu), lactose (Lac), L-valine (L-Val), and glycine (Gly)). The practical detection assay of H2O2 based on MoS2@CNNS(30) was performed by adding commercial antiseptic solution (containing about 0.79 M H2O2) into the reaction system instead of standard H2O2 with the other operations fixed. The antiseptic liquid was diluted 1000 times before the test.
2.7. Stability and Reusability of the Catalysts
The stability and reusability of MoS2@CNNS(30) were studied by recycling the H2O2 detection assays 10 times under optimal experimental conditions. The reaction system was monitored at 652 nm by a UV-visible spectrophotometer for 10 min. After each cycle, the MoS2@CNNS(30) samples were collected by centrifugation, washed with Milli-Q water and alcohol, dried at 60 °C for 30 min, and then reused in the next cycle. The crystal structure and morphology of MoS2@CNNS(30) samples after 10 cycles were characterized by XRD and TEM as described above.
3. Results
3.1. Characterization of the Catalysts
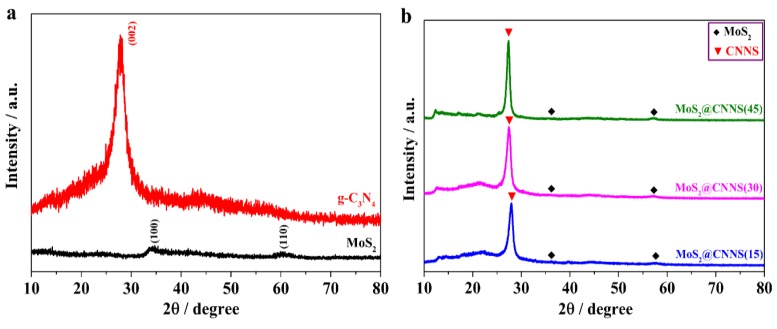
The crystal phase, structure, and crystallinity of the as-prepared samples were determined by XRD. It can be seen in Figure 1a that two tiny diffraction peaks can be barely seen in the XRD pattern of MoS2 QDs, which can be attributed to the (100) and (110) lattice planes of MoS2, respectively. However, the two diffraction peaks were extremely weak, which implied that the MoS2 QDs had poor crystallinity [36]. In addition, the CNNS samples show a strong peak at 27.29°, which can be attributed to the characteristic of the stacking peak of π-conjugated layers and indexed for the interlayer reflection of a graphite-like structure as the (002) peak [29,36,44], consistent with the literature value (JCPDS No. 87-1526). Moreover, the absence of a (100) peak and the presence of a sharp (002) peak further confirmed the layered structures of CNNS samples and the good crystallinity of CNNS. As for the MoS2@CNNS composites (Figure 1b), all the characteristic diffraction peaks can be well indexed to the graphite-like structure of CNNS (JCPDS No. 87-1526) and hexagonal phase MoS2 (JCPDS Card No. 37-1492), indicating that MoS2 QDs were successfully formed on the surface of CNNS through the aid of ion-exchange process. Furthermore, with increasing the loading amount of MoS2 QDs, the relative intensity of corresponding (100) and (110) diffraction peaks of MoS2 strengthened gradually, while the characteristic peaks of MoS2 were still relatively weak and broad in the composites owing to the size effect of quantum dots. There were no significant shifts of the characteristic diffraction peaks occurring in the MoS2@CNNS composites, implying that MoS2 QDs existed as a separate phase rather than being incorporated into the lattice of CNNS [36]. No impurity peaks were observed in the obtained samples, indicating the pure phase of the samples. Hence, these results indicated that the MoS2@CNNS nanocomposites were successfully obtained through the synergistic effect of anion-exchange and hydrothermal process.
Figure 1.
XRD patterns of MoS2 QDs and g-C3N4 (a) and MoS2@CNNS nanocomposites (b).
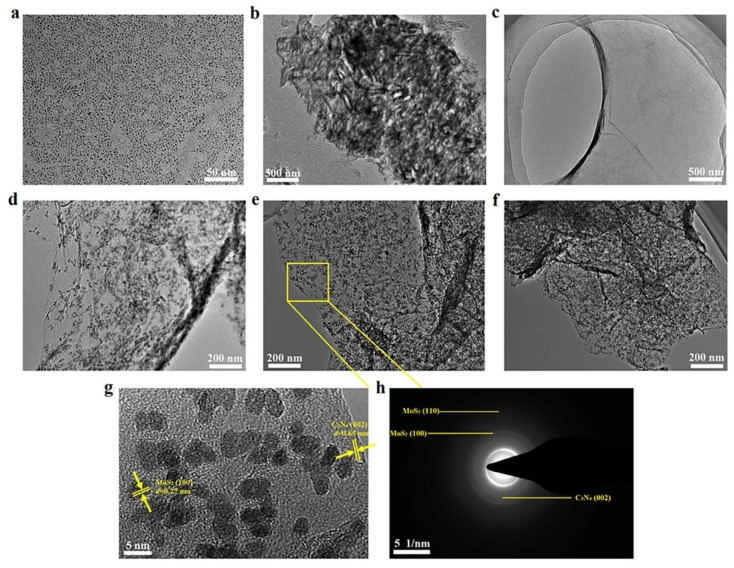
To obtain more detailed information about the morphologies and microstructures of the as-prepared catalysts, TEM and HRTEM characterizations were carried out. It can be seen in Figure 2a that the MoS2 QDs showed a highly homogeneously monodispersed QDs nanostructures with the diameter of about 2–3 nm, indicating the successful preparation of MoS2 QDs through the ultrasonic exfoliation method in NMP. Figure 2b presents a typical morphology of slabs with wrinkles of bulk g-C3N4, and after ultrasonic treatment for 16 h, the three-dimensional (3D) structure of bulk g-C3N4 was broken up into CNNS with 2D lamellar structure with the thickness of about 2.5 nm (Figure 2c). Thus, the as-prepared 2D CNNS nanosheets exhibited a thinner and explanate lamellar structure, which could provide a larger surface area and more reactive sites in the MoS2@CNNS nanocomposites. Figure 2d–f shows the typical TEM images of MoS2@CNNS nanocomposites. It can be seen that the MoS2 QDs with the diameter of about 2.5–5.5 nm were uniformly distributed on the surface of CNNS nanosheets, and with increasing the amount of Na2MoO4∙2H2O in the ion exchange process, more and larger MoS2 QDs were formed. No MoS2 QDs were observed except for the surface of CNNS, indicating that the elaborate protonation effect in the ion exchange process leaded to the in-situ nucleation and growth of MoS2 QDs on the surface of CNNS. And the large surface area of CNNS provided a nice reaction area, which effectively promoted the in-situ reaction process, resulting in the successful formation of MoS2@CNNS nanocomposites. Moreover, the HRTEM image was examined to give further insight into the crystal structure of the MoS2@CNNS(30) nanocomposites corresponding to the circled region in Figure 2e. It can be seen in Figure 2g that a lattice spacing of 0.65 nm was obviously shown in the CNNS, which is in accordance with the (002) lattice plane of tetragonal C3N4. In addition, the QDs displayed well-defined lattice fringes parallel to each other with the same interplanar spacing of 0.27 nm, which can be indexed to the (100) lattice plane of hexagonal-phase MoS2. Meanwhile, several apparent diffraction rings were observed in the selected area electron diffraction (SAED) pattern of MoS2@CNNS(30) (Figure 2h), which can be well indexed to the lattice planes of MoS2 and g-C3N4, respectively, coinciding well with the XRD results. Therefore, these results indicated that a close-contact and well-defined MoS2@CNNS nanostructure was formed via the planar in-situ growth of uniformly-dispersed MoS2 QDs on the surface of CNNS during the protonation-assisted ion exchange process, which is a facile and controllable approach to obtain uniform and dispersive MoS2 QDs on the surface of substrate.
Figure 2.
TEM images of MoS2 QDs (a), bulk g-C3N4 (b), CNNS (c), MoS2@CNNS(15) (d), MoS2@CNNS(30) (e), and MoS2@CNNS(45) (f); HRTEM image (g) and SAED pattern (h) of MoS2@CNNS(30).
In order to further probe the contents of S and Mo in MoS2@CNNS nanocomposites, ICP-AES was examined. Quantitative analysis results showed the loading amount of MoS2 QDs on the surface of CNNS is about 2.0, 5.7 and 5.7 wt % for MoS2@CNNS(15), MoS2@CNNS(30) and MoS2@CNNS(45), respectively (Table S1). These results indicated that with increasing the amount of Na2MoO4∙2H2O in the ion exchange process, the loading capacity and protonated reactive site of CNNS was reaching saturation, which greatly restricted the formation of more MoS2 QDs.
In addition, the Brunauer Emmett Teller (BET) specific surface area is a significant affecting factor for the catalytic abilities of catalysts, which was determined by the nitrogen adsorption method [23,24,45]. And the specific surface areas of MoS2@CNNS(15), MoS2@CNNS(30), MoS2@CNNS(45), CNNS, and MoS2 QDs were measured as 45.58, 75.93, 69.37, 29.85 and 16.13 m2/g, respectively. It can thus be seen that the MoS2@CNNS(30) nanocomposites presented a larger specific surface area among the as-prepared catalysts (Figure S1a), though MoS2@CNNS(45) loaded a little bit more MoS2 QDs, while the agglomeration of the QDs could decrease the specific surface area. As is well known, a larger specific surface area will lead to more active sites, better adsorption performance, and faster electron transfer for catalysts, and will further improve the catalytic activities. Hence, MoS2@CNNS(30) nanocomposites are expected to display enhanced peroxidase-like activity.
Furthermore, the zeta potentials of the MoS2@CNNS nanocomposites were tested to explore their adsorption properties to molecules with different charges, which is also a significant indicator of the catalytic activities for catalysts. Thus, the zeta potentials of MoS2@CNNS nanocomposites were determined as −30.48, −63.38, −53.66, −16.35 and −3.76 mV for MoS2@CNNS(15), MoS2@CNNS(30), MoS2@CNNS(45), CNNS, and MoS2 QDs, respectively (Figure S1b). The results indicated that different quantities of MoS2 QDs loaded onto the surface of CNNS contributed to different surface charges of MoS2@CNNS nanocomposites, which would further lead to different peroxidase-like activities. Therefore, based on the XRD, TEM, ICP-AES, BET, and zeta potential results mentioned above, it can be deduced that the MoS2@CNNS nanocomposites prepared via the protonation-assisted ion exchange process showed different morphologies and structures due to the molar ratio of Na2MoO4∙2H2O/CNNS, leading to their different surface/interface properties and further contributing to the distinct peroxidase-like performance.
3.2. Peroxidase-Like Activities of MoS2@CNNS(30) Nanocomposites
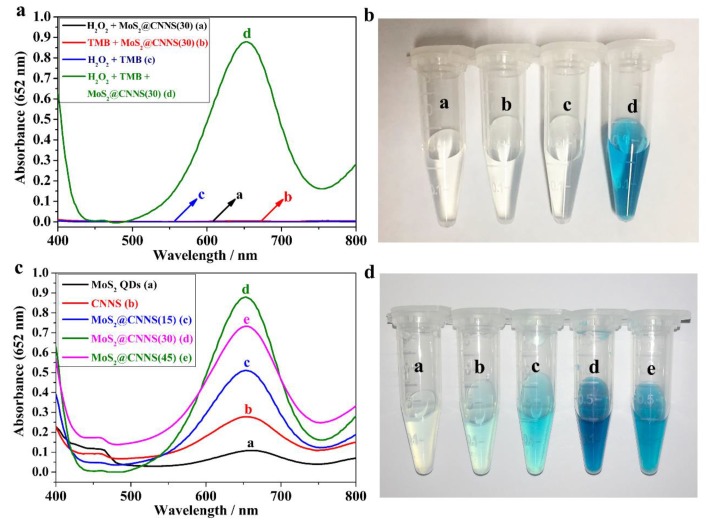
To investigate the peroxidase-like activity of MoS2@CNNS(30) nanocomposites, the TMB catalytic oxidation experiments were conducted with or without H2O2 by the UV-visible absorption spectra in the range of 400–800 nm. It can be seen in Figure 3a that low absorption presented in the TMB + MoS2@CNNS(30), the H2O2 + TMB system, and the H2O2 + MoS2@CNNS(30) system, while the H2O2 + TMB + MoS2@CNNS(30) system exhibited an evident absorption peak at 652 nm, indicating that the MoS2@CNNS(30) nanocomposites could play a key role in catalyzing the oxidation of TMB in the presence of H2O2. In addition, the significant color changes of different reaction systems were observed in Figure 3b, which coincided with the absorption spectra. It can be seen that the TMB + MoS2@CNNS(30) system, the H2O2 + TMB system, and the H2O2 + MoS2@CNNS(30) system were almost colorless. However, the H2O2 + TMB + MoS2@CNNS(30) system showed an apparent color variation, presenting a deep blue color, further demonstrating the excellent peroxidase-like properties of the MoS2@CNNS(30) nanocomposites.
Figure 3.
UV-visible absorption spectra (a) and color changes (b) of different reaction systems (a. H2O2 + MoS2@CNNS(30), b. TMB + MoS2@CNNS(30), c. H2O2 + TMB, and d. H2O2 + TMB + MoS2@CNNS(30); UV-visible absorption spectra (c) and color changes (d) in the presence of different MoS2@CNNS nanocomposites.
What’s more, the peroxidase-like activities of other MoS2@CNNS, pure MoS2 QDs, and CNNS samples were studied and compared via the catalytic oxidation of TMB in the presence of H2O2. It can be seen in Figure 3c that the MoS2@CNNS(30)-based assay system revealed the strongest absorption peak at 652 nm, followed by MoS2@CNNS(45), MoS2@CNNS(15), CNNS, and MoS2 QDs, confirming the best peroxidase-like catalytic activity of MoS2@CNNS(30). The color variations of different reaction systems also presented a similar result with the absorption spectra. As shown in Figure 3d, the MoS2@CNNS(30)-based assay system showed the deepest blue color compared to that of other materials. Therefore, these results further indicated the enhanced peroxidase-like activities of the MoS2@CNNS(30) nanocomposites compared to other MoS2@CNNS nanocomposites, pure CNNS, and MoS2 QDs. The more negative charge, larger specific surface area, and stable nanostructure of MoS2@CNNS(30) mainly facilitated its superior and enhanced peroxidase-like performance, which could increase the binding affinity and absorption to TMB molecules, improve the electron transfer, accelerate the reaction rate, and further improve the peroxidase-like catalytic performance [29]. On the other hand, one can find that both CNNS and MoS2 QDs showed a certain intrinsic peroxidase-like catalysis behavior toward TMB-H2O2 reaction, which could be attributed to the quantum effect and catalysis-active segments of MoS2 QDs and CNNS. Thus, when MoS2 QDs were loaded on CNNS by the in-situ growth process, the resulting MoS2@CNNS(30) nanocomposites could display much higher peroxidase-like activities than pure CNNS and MoS2 QDs on account of the synergistic effect of high conductivity and electron transfer capability for CNNS and highly-efficient intrinsic catalytic activity for MoS2 QDs [29,36]. The bonding carbon network in CNNS could donate electrons to reduce H2O2 by accelerating the electron transfer from microcosmic point of view [29]. Furthermore, as a good supporter, CNNS could apparently improve the aqueous dispersion and stability of the nanocomposites. Thus, all of the favorable factors effectively improved the peroxidase-like performances of MoS2@CNNS(30) nanocomposites. Hence, these results confirmed the enhanced peroxidase-like activities of the MoS2@CNNS(30) nanocomposites, making it a potential highly-efficient colorimetric sensor for H2O2 detection.
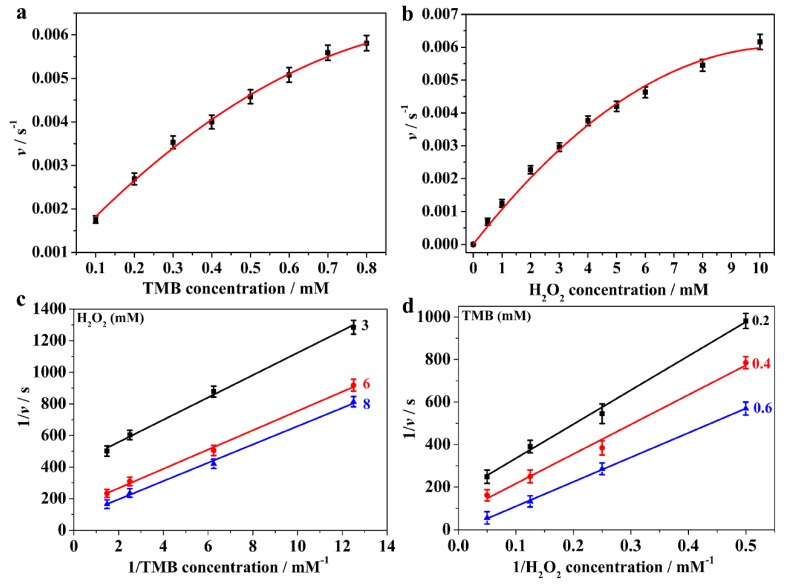
3.3. Steady-State Kinetics Assay
For further insight into the peroxidase-like catalytic behavior of the MoS2@CNNS(30) nanocomposites, the steady-state kinetic assays were carried out with H2O2 and TMB as substrates. The kinetic data were collected by changing the concentration of one substrate while keeping the other substrate concentration constant [4,42]. Thus, the typical Michaelis-Menten curves were recorded by varying the concentration of TMB or H2O2 while keeping the other one constant, as shown in Figure 4a,b. The steady-state kinetic reaction parameters were calculated on the basis of the Lineweaver-Burk double reciprocal plot (Figure 4c,d) according to the Michaelis-Menten equation: 1/v = (Km/Vmax) × (1/[S]) + 1/Vmax, where v is the initial velocity, Km is the Michaelis-Menten constant, Vmax represents the maximal reaction velocity, and [S] signifies the concentration of the substrate [4,29,38,42,46,47], which were listed in Table S2. It is well known that Km is an important indicator of the binding affinity of enzyme to the substrates, and that it affects the reaction rate; a smaller value of Km generally means a stronger affinity between the enzyme and the substrate [4,29,38,42,46,47]. As listed in Table S2, the Km value of MoS2@CNNS(30) with TMB was obviously lower than that of the natural enzyme HRP, implying that MoS2@CNNS(30) had a stronger binding affinity to TMB than HRP, which might be attributed to the CNNS carriers with strong adsorption to TMB [4,29,38,42]. In addition, the Vmax of MoS2@CNNS(30) with TMB was more than two times larger than that of HRP, indicating a favorable tendency of a higher reaction rate, which could the result of the presence of tiny MoS2 QDs loaded on the surface of CNNS, as well as the rapid electron transfer capability of CNNS itself [29,36,38]. The Km value of MoS2@CNNS(30) with H2O2 was higher than that of HRP, suggesting a lower binding affinity between MoS2@CNNS(30) and H2O2 than that of HRP. Furthermore, the typical Michaelis-Menten behavior towards TMB and H2O2 with various concentrations in the peroxidase-like catalytic reaction were measured, respectively. And the double-reciprocal plots (Figure 4c,d) of initial velocity against one substrate concentration showed the characteristic parallel lines, indicating a typical ping-pong mechanism of the peroxidase-like catalytic reaction [4,23,24,29,38,42,46,47]. Hence, these results inferred that MoS2@CNNS(30) bound and reacted with the first substrate and then released the first product before reacting with the second substrate, which is similar to that of HRP [1,2,3,4,42,48].
Figure 4.
Steady-state kinetic analysis. The reaction velocity (v) was measured using 120 μg/mL of MoS2@CNNS(30) in 25.0 mM PBS (pH = 4.0) at room temperature. The TMB concentration was varied while the concentration of H2O2 was 2.0 mM (a), the H2O2 concentration was varied while the concentration of TMB was 0.8 mM (b), and the double-reciprocal plots with a fixed concentration of one substrate relative to varying the concentration of the other substrate (c) and (d).
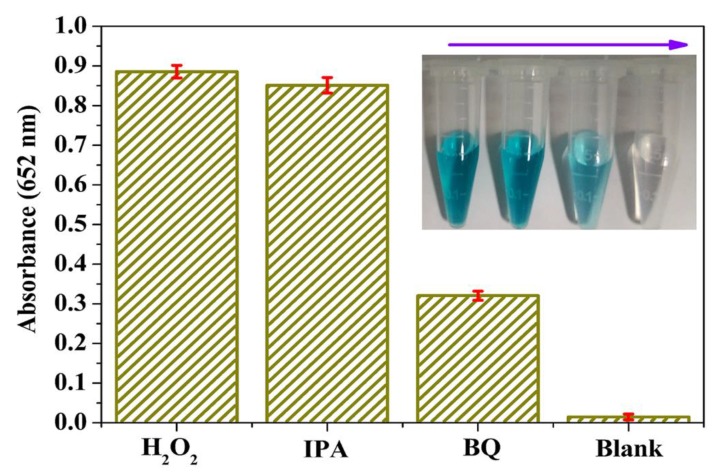
3.4. Active Species Analysis and Peroxidase-Like Catalytic Mechanism Study
It is reported that the reaction system contained H2O2, which would easily produce some active radicals such as ⋅OH and ⋅O2− that could play significant roles in catalytic reaction [23,24,49,50]. Hence, in order to explore the peroxidase-like catalytic mechanism of MoS2@CNNS(30) nanocomposites, actives species trapping experiments were carried out by adding different scavengers (IPA as ·OH scavengers and BQ as ⋅O2− scavengers) into the reaction systems under other fixed experimental conditions. It can be seen in Figure 5 that an evident decrease in absorption and color fading of the reaction system was seen with the addition of BQ, while little change in absorption and color was observed after adding IPA. These results indicated that the MoS2@CNNS(30) nanocomposites could catalytically activate H2O2 to generate ⋅O2− radicals in the peroxidase-like catalytic reaction, which subsequently play major roles in oxidizing TMB to produce a TMB oxide with a blue color.
Figure 5.
Time-dependent absorbance of reaction solutions at 652 nm in the absence or presence of scavengers (10 mM of IPA and BQ) containing 25.0 mM PBS (pH = 4.0), 2.0 mM H2O2, 0.8 mM TMB, and 120 μg/mL MoS2@CNNS(30) at room temperature. Inset: related color changes.
On the basis of the above experimental results and some previous literature [1,2,3,4,23,24,28,29,30,31,32,37,38,39,40], the peroxidase-like catalytic mechanism of MoS2@CNNS(30) nanocomposites was proposed, and is illustrated in Scheme 1. It can be seen that the negatively-charged MoS2@CNNS(30) nanocomposites could act as the peroxidase mimics, facilitating the electron transfer between TMB and H2O2 in the catalytic oxidation reaction. During the reaction process, many positively-charged TMB molecules would be absorbed onto the surface of MoS2@CNNS(30) nanocomposites owing to the large specific surface area, acting as the chromogenic electron donors. Then, the TMB molecules would donate the lone-pair electrons from the amino groups to the surface of MoS2@CNNS(30) nanocomposites, enhancing the density and mobility of electrons on the surface of MoS2@CNNS(30), then promoting the electron transfer from MoS2@CNNS(30) to H2O2, and further accelerating the TMB catalytic oxidation reaction rate [4,23,24,28,29,30,31,32,37,38,39,40]. Subsequently, the oxidized intermediate ·O2− radicals generated in the reaction between MoS2@CNNS(30) and H2O2 via one electron transfer would react with TMB molecules to generate a TMB oxide, leading to the color change of the system from colorless to blue [4,23,24,28,29,30,31,32,37,38,39,40]. The corresponding chemical equation was: . Hence, in light of the excellent peroxidase-like catalytic activity, MoS2@CNNS(30) nanocomposites exhibited a promising application prospect in medical diagnostics and environmental assay.
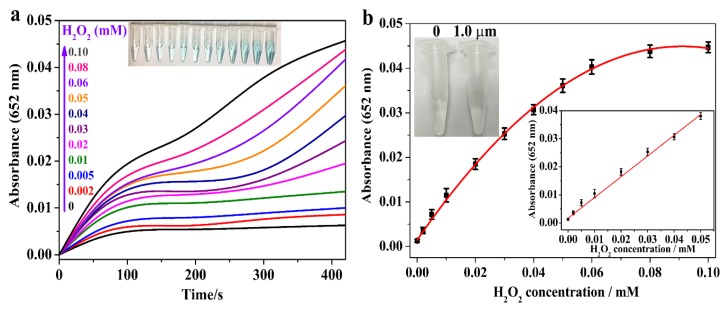
3.5. Detection of H2O2 by MoS2@CNNS(30)-Based Assay System
On the basis of the aforementioned intrinsic and enhanced peroxidase-like catalytic activity of MoS2@CNNS(30), a simple, rapid and ultrasensitive colorimetric method for the visual detection of H2O2 was developed. As the absorbance of oxidized TMB was in proportion to the H2O2 concentration, it was a simple approach to determine H2O2 at 652 nm only by the naked eye, or by using an UV-visible spectrometer. Figure 6a shows the time-course dependent absorbance changes at 652 nm of the oxidized TMB in the presence of H2O2 with different concentrations. It can be seen that the reaction rate increased by increasing the concentration of H2O2 from 0.002 mM to 0.10 mM. Moreover, it can be seen in Figure 6b that the typical H2O2 concentration-response curve displayed a linear response in the range of 2.0 μM to 50.0 μM, and the linear fitting equation is (mM), with a correlation coefficient of 0.9956. Hence, the detection limit of H2O2 was estimated to be 0.02 μM. In addition, as shown in the inset of Figure 6b, the color variation for H2O2 response as low as 1.0 μM was also apparently observed by the naked eye, which offered a convenient approach to detect H2O2 even at low concentrations. Furthermore, compared to some other previously-reported nanomaterials with peroxidase-like activities [17,25,29,30,37,39,42,46,51] (Table S3), MoS2@CNNS(30) nanocomposites revealed a reasonable linear range and a lower detection limit for H2O2 detection, further confirming their superior peroxidase-like catalytic activity and sensitivity. Therefore, these results indicated that the visual biosensing platform based on MoS2@CNNS(30) was a simple, rapid, and convenient method for H2O2 detection with ultrasensitive response.
Figure 6.
Time-dependent absorbance changes at 652 nm in the presence of different concentrations of H2O2 in 25.0 mM PBS (pH = 4.0) with 120 μg/mL MoS2@CNNS(30) and 0.8 mM TMB at room temperature (a) and a dose-response curve for H2O2 detection (b). Inset: related color changes and the linear calibration plot of H2O2.
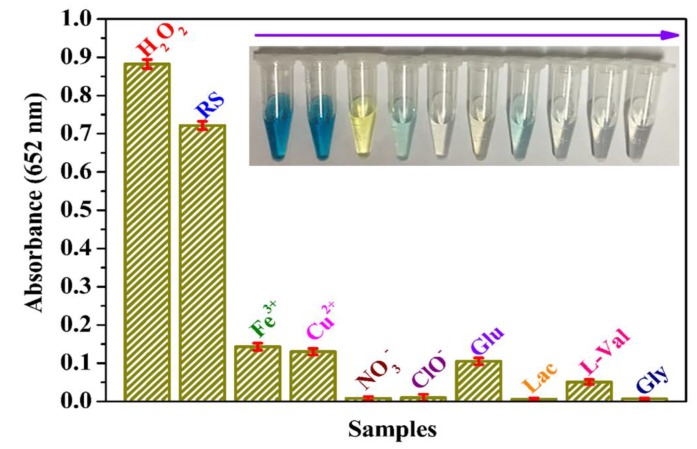
3.6. Selectivity and Applicability of MoS2@CNNS(30)-Based Assay System
To estimate the selectivity of MoS2@CNNS(30)-TMB-H2O2 detection system, some other substances, such as Fe3+, Cu2+, NO3−, ClO−, Glu, Lac, L-Val, and Gly, with the concentration of 20.0 mM were added into the reaction system respectively as the potential interferents instead of H2O2. In addition, a commercial antiseptic liquid (diluted 1000 times) was used to check the practical applicability and accuracy of the H2O2 detection system in a real sample (RS). It can be seen in Figure 7 that no evident absorbance at 652 nm and color change were observed, though the concentrations of these interferents were 10 times higher than that of standard H2O2 (2.0 mM), indicating a nice selectivity of the detection system. Moreover, the diluted commercial antiseptic liquid presented an obvious absorbance and light blue color, and the concentration of H2O2 in the commercial antiseptic liquid could be calculated to be about 0.77 M based on the calibration curve shown in Figure 6b, which was close to the actual concentration of the commercial antiseptic liquid (0.79 M). Therefore, these results exhibited the favorable applicability in complicated conditions of the MoS2@CNNS(30)-based H2O2 detection system, which favored the practical and rapid determination of H2O2 in various environments.
Figure 7.
Reveal sample (antiseptic liquid was diluted 1000 times) and selectivity analysis with interfering substances (20.0 mM of Fe3+, Cu2+, NO3−, ClO−, Glu, Lac, L-Val, and Gly) of MoS2@CNNS(30)-based assay system by monitoring the absorbance at 652 nm. Inset: related color changes.
3.7. Stability and Reusability of MoS2@CNNS(30)-Based Assay System
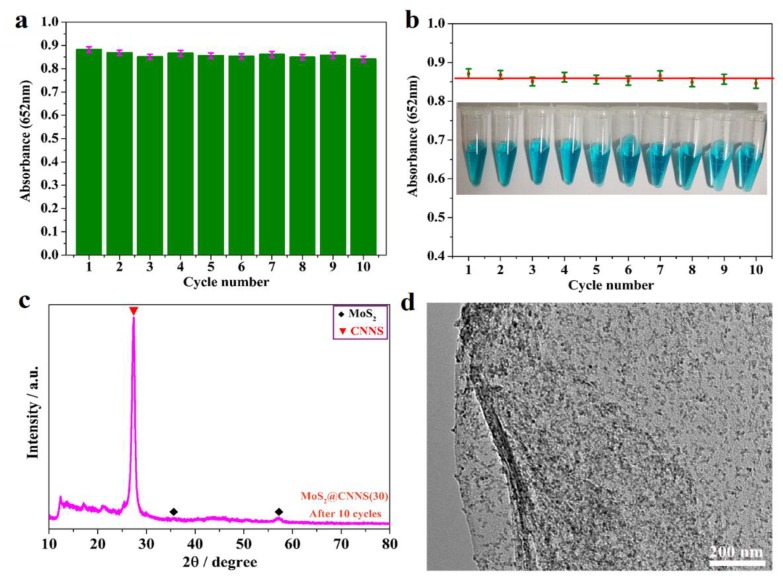
In order to study the stability and reusability of MoS2@CNNS(30)-based H2O2 detection system, the peroxidase-like experiments were conducted by repeating the reaction for ten successive cycles. After each cycle, the MoS2@CNNS(30) samples were collected, washed several times with Milli-Q water and ethanol, and dried, and then reused in the next cycle. Every cycle lasted for 10 min. It can be seen in Figure 8a,b that there was no significant change of the reaction system absorbance at 652 nm during the recycling tests, accompanied by no color variations of the reaction system observed in the recycling tests, showing the excellent reusability and stable peroxidase-like performance of the MoS2@CNNS(30)-based H2O2 detection system. The relative standard deviation (RSD) of the absorbance values was only 1.72%, which further confirmed the nice reproducibility, stability, and reusability of the MoS2@CNNS(30)-based assay system even for 10 cycles. Moreover, XRD and TEM were used to further analyze the crystal structure and morphology of MoS2@CNNS(30) after ten successive cycles. It can be seen in Figure 8c that the XRD pattern of MoS2@CNNS(30) after ten successive cycles displayed no apparent change in both peak intensity and position, implying the stable crystal structure of MoS2@CNNS(30) samples kept in the recycling tests. In addition, no significant morphology variation was observed in the TEM image of MoS2@CNNS(30) samples after ten successive cycles (Figure 8d), though a little impurity appeared on the surface of CNNS, exhibiting the good stability in crystal structure and morphology. Hence, good stability, reusability, reproducibility and precision of the MoS2@CNNS(30)-based assay system suggested that the peroxidase-like colorimetric method might be used to analyze H2O2 in real water samples, which also favored long-term use.
Figure 8.
Stability and reusability experiments of MoS2@CNNS(30)-based assay system containing 120 μg/mL MoS2@CNNS(30), 25.0 mM PBS (pH = 4.0), 2.0 mM H2O2, and 0.8 mM TMB (a), (b); XRD pattern (c) and TEM image (d) of MoS2@CNNS(30) after 10 cycles. Inset: related color changes in 10 cycles.
4. Conclusions
In conclusion, the MoS2@CNNS nanocomposites were successfully synthesized via a protonation-assisted ion exchange method, which were demonstrated to display an enhanced intrinsic peroxidase-like activity. The in-situ growth of MoS2 QDs on the surface of ultrathin CNNS formed a stable nanostructure, facilitating the synergetic effects of high conductivity and electron-transfer capability for CNNS and intrinsic catalytic activity for MoS2 QDs, and thus, greatly improving the peroxidase-like performance of the nanocomposites. In addition, the MoS2@CNNS nanocomposites revealed different morphologies, surface properties, and peroxidase-like activities, owing to the different amount of MoS2 QDs loading on the surface of CNNS. The MoS2@CNNS(30) nanocomposites showed the best peroxidase-like ability among the composites, which could be attributed to their more negative potential and larger specific surface area. In the presence of H2O2 and the peroxidase substrate TMB, MoS2@CNNS(30) could induce a typical blue color reaction, thus providing a colorimetric assay for H2O2. The catalytic activity was strongly dependent on the catalyst concentration, H2O2 concentration, pH, and temperature. Moreover, the kinetic and active species trapping experiments indicated that the catalytic reaction followed a ping-pong mechanism, and the ⋅O2− radicals played a pivotal role in the peroxidase-like catalytic reaction. Based on the excellent peroxidase-like catalytic performance of MoS2@CNNS(30) nanocomposites, a simple, rapid, and ultrasensitive platform for colorimetric detection of H2O2 was developed, which exhibited a nice selectivity, reusability, long-term stability, and practicability, making it a potential biosensing material for the practical applications in H2O2 detection and biomedical analysis. Furthermore, this work offers some new insights and targeted directions for novel enzymatic mimics with enhanced catalytic activity.
Supplementary Materials
The following are available online at http://www.mdpi.com/2079-4991/8/12/976/s1, Figure S1: Adsorption/desorption isotherms of MoS2@CNNS(30) (a) and zeta potentials of the MoS2@CNNS nanocomposites dispersed in ultrapure water (pH = 4.0) (b), Figure S2: Time-dependent absorbance at 652 nm and color changes of 0.8 mM TMB reaction solutions in the absence or presence of different concentrations of MoS2@CNNS(30) (a) and H2O2 (b) in 25.0 mM PBS (pH = 4.0) at room temperature. Inset: related color variations, Figure S3: Dependency of peroxidase-like activity of MoS2@CNNS(30) on pH (a) and temperature (b) and color changes. Experiments were conducted by using 120 μg/mL of MoS2@CNNS(30) in 25.0 mM PBS with 2.0 mM H2O2 and 0.8 mM TMB as substrates; Inset: related color variations, Table S1: MoS2 loading amount in MoS2/CNNS samples determined by ICP-AES, Table S2: Comparison of Km and Vmax between MoS2@CNNS(30) and HRP for H2O2 and TMB, Table S3: Comparison of peroxidase-like activity in the linear range and detection limit of H2O2 between MoS2@CNNS(30) and other peroxidase mimics.
Author Contributions
Conceptualization, P.J. and Y.H.H.; methodology, P.J. and Y.H.H.; software, X.X.H.; validation, P.J. and C.J.S.; formal analysis, Y.H.H. and M.W.; investigation, P.J., Y.H.H. and M.W.; resources, P.J. and C.J.S.; data curation, Y.H.H. and M.W.; writing—original draft preparation, P.J. and Y.H.H.; writing—review and editing, P.J.; visualization, F.H.J.; supervision, C.W. and C.J.S.; project administration, P.J. and C.J.S.; funding acquisition, P.J. and C.J.S.
Funding
This research was supported by National Natural Science Foundation of China (51702328), Key Lab of Marine Bioactive Substance and Modern Analytical Technique, SOA (MBSMAT-2016-05), Natural Science Foundation of Shandong Province, China (ZR2017BD002), China Postdoctoral Science Foundation Funded Project (2017M622179 and 2018T110681), CAS “Light of West China” Program, Open Fund of Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology (Qingdao) (LMEES201802), Open Foundation of Pilot National Laboratory for Marine Science and Technology (Qingdao) (QNLM2016ORP0410), and The Aoshan Scientific and Technological Innovation Project Financially Supported by Pilot National Laboratory for Marine Science and Technology (Qingdao) (2016ASKJ14). C.J. Sun would also like to thank support from Taishan Scholar and the Ministry of Human Resources and Social Security of China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Song Y.J., Wei W.L., Qu X.G. Colorimetric biosensing using smart materials. Adv. Mater. 2011;23:4215–4236. doi: 10.1002/adma.201101853. [DOI] [PubMed] [Google Scholar]
- 2.Wei H., Wang E.K. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013;42:6060–6093. doi: 10.1039/c3cs35486e. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q.Q., Wei H., Zhang Z.Q., Wang E.K., Dong S.J. Nanozyme: An emerging alternative to natural enzyme for biosensing and immunoassay. TrAC-Trend. Anal. Chem. 2018;105:218–224. doi: 10.1016/j.trac.2018.05.012. [DOI] [Google Scholar]
- 4.Gao L., Zhuang J., Nie L., Zhang J., Zhang Y., Gu N., Wang T., Feng J., Yang D., Perrett S., Yan X. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007;2:577–583. doi: 10.1038/nnano.2007.260. [DOI] [PubMed] [Google Scholar]
- 5.Tian R., Sun J.H., Qi Y.F., Zhang B.Y., Guo S.L., Zhao M.M. Influence of VO2 nanoparticle morphology on the colorimetric assay of H2O2 and glucose. Nanomaterials. 2017;7:347. doi: 10.3390/nano7110347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng H.Q., Liu C.Y., Zeng X.Y., Chen J., Lv J., Lin R.G., Cao R., Lin Z.J., Su J.W. MOF-808: A metal-organic framework with intrinsic peroxidase-like catalytic activity at neutral pH for colorimetric bciosensing. Inorg. Chem. 2018;57:9096–9104. doi: 10.1021/acs.inorgchem.8b01097. [DOI] [PubMed] [Google Scholar]
- 7.Zhou R., Guzman M.I. Photocatalytic reduction of fumarate to succinate on ZnS mineral surfaces. J. Phys. Chem. C. 2016;120:7349–7357. doi: 10.1021/acs.jpcc.5b12380. [DOI] [Google Scholar]
- 8.Vázquez-Fernández M.Á., Bermejo M.R., Fernández-García M.I., González-Riopedre G., Rodríguez-Doutón M.J., Maneiro M. Influence of the geometry around the manganese ion on the peroxidase and catalase activities of Mn(III)=Schiff base complexes. J. Inorg. Biochem. 2011;105:1538–1547. doi: 10.1016/j.jinorgbio.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Yang Z., Ji H. 2-Hydroxypropyl-β-cyclodextrin polymer as a mimetic enzyme for mediated synthesis of benzaldehyde in water. ACS Sustain. Chem. Eng. 2013;1:1172–1179. doi: 10.1021/sc4001059. [DOI] [Google Scholar]
- 10.Wang Q.G., Yang Z.M., Zhang X.Q., Xiao X.D., Chang C.K., Xu B. A supramolecular-hydrogel-encapsulated hemin as an artificial enzyme to mimic peroxidase. Angew. Chem. Int. Ed. 2007;46:4285–4289. doi: 10.1002/anie.200700404. [DOI] [PubMed] [Google Scholar]
- 11.Tan B., Zhao H.M., Wu W.H., Liu X., Zhang Y.B., Quan X. Fe3O4-AuNPs anchored 2D metal-organic framework nanosheets with DNA regulated switchable peroxidase-like activity. Nanoscale. 2017;9:18699–18710. doi: 10.1039/C7NR05541B. [DOI] [PubMed] [Google Scholar]
- 12.Wu L.H., Wan G.P., Hu N., He Z.Y., Shi S.H., Suo Y.R., Wang K., Xu X.F., Tang Y.L., Wang G.Z. Synthesis of porous CoFe2O4 and its application as a peroxidase mimetic for colorimetric detection of H2O2 and organic pollutant degradation. Nanomaterials. 2017;7:451. doi: 10.3390/nano8070451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Y.Z., Ju P., Zhang D., Han X.X., Yin X.F., Zheng L., Sun C.J. Peroxidase-like activity of FeVO4 nanobelts and its analytical application for optical detection of hydrogen peroxide. Sensor. Actuator. B Chem. 2016;233:162–172. doi: 10.1016/j.snb.2016.04.041. [DOI] [Google Scholar]
- 14.Wang H., Li P.H., Yu D.Q., Zhang Y., Wang Z.Z., Liu C.Q., Qiu H., Liu Z., Ren J.S., Qu X.G. Unraveling the enzymatic activity of oxygenated carbon nanotubes and their application in the treatment of bacterial infections. Nano Lett. 2018;6:3344–3351. doi: 10.1021/acs.nanolett.7b05095. [DOI] [PubMed] [Google Scholar]
- 15.Singh V.K., Yadav P.K., Chandra S., Bano D., Talat M., Hasan S.H. Peroxidase mimetic activity of fluorescent NS-carbon quantum dots and their application in colorimetric detection of H2O2 and glutathione in human blood serum. J. Mater. Chem. B. 2018;6:5256–5268. doi: 10.1039/C8TB01286E. [DOI] [PubMed] [Google Scholar]
- 16.Song Y., Qu K., Zhao C., Ren J., Qu X. Graphene oxide: intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 2010;22:2206–2210. doi: 10.1002/adma.200903783. [DOI] [PubMed] [Google Scholar]
- 17.Lin T.R., Zhong L.S., Wang J., Guo L.Q., Wu H.Y., Guo Q.Q., Fu F.F., Chen G.N. Graphite-like carbon nitrides as peroxidase mimetics and their applications to glucose detection. Biosens. Bioelectron. 2014;59:89–93. doi: 10.1016/j.bios.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Guo S.J., Wang E.K. Noble metal nanomaterials: Controllable synthesis and application in fuel cells and analytical sensors. Nano Today. 2011;6:240–264. doi: 10.1016/j.nantod.2011.04.007. [DOI] [Google Scholar]
- 19.Xiang Z.B., Wang Y., Ju P., Zhang D. Optical determination of hydrogen peroxide by exploiting the peroxidase-like activity of AgVO3 nanobelts. Microchim. Acta. 2016;183:457–463. doi: 10.1007/s00604-015-1670-x. [DOI] [Google Scholar]
- 20.Qiao F.M., Wang Z., Xu K., Ai S.Y. Double enzymatic cascade reactions within FeSe-Pt@SiO2 nanospheres: synthesis and application toward colorimetric biosensing of H2O2 and glucose. Analyst. 2015;140:6684–6691. doi: 10.1039/C5AN01268F. [DOI] [PubMed] [Google Scholar]
- 21.Kim M.S., Kweon S.H., Cho S.Y., An S.S.A., Kim M.I., Doh J.S., Lee J.W. Pt-decorated magnetic nanozymes for facile and sensitive point-of-care bioassay. ACS Appl. Mater. Interfaces. 2017;9:35133–35140. doi: 10.1021/acsami.7b12326. [DOI] [PubMed] [Google Scholar]
- 22.Cho S.Y., Shin H.Y., Kim M.I. Nanohybrids consisting of magnetic nanoparticles and gold nanoclusters as effective peroxidase mimics and their application for colorimetric detection of glucose. Biointerphases. 2017;12:01A401. doi: 10.1116/1.4974198. [DOI] [PubMed] [Google Scholar]
- 23.Ju P., Xiang Y.H., Xiang Z.B., Wang M., Zhao Y., Zhang D., Yu J.Q., Han X.X. BiOI hierarchical nanoflowers as novel robust peroxidase mimetics for colorimetric detection of H2O2. RSC Adv. 2016;6:17483–17493. doi: 10.1039/C6RA00368K. [DOI] [Google Scholar]
- 24.Ju P., Yu Y.Z., Wang M., Zhao Y., Zhang D., Sun C.J., Han X.X. Synthesis of EDTA-assisted CeVO4 nanorods as robust peroxidase mimics towards colorimetric detection of H2O2. J. Mater. Chem. B. 2016;4:6316–6325. doi: 10.1039/C6TB01881E. [DOI] [PubMed] [Google Scholar]
- 25.Yu J., Ma D.Q., Mei L.Q., Gao Q., Yin W.Y., Zhang X., Yan L., Gu Z.J., Ma X.Y., Zhao Y.L. Peroxidase-like activity of MoS2 nanoflakes with different modifications and their application for H2O2 and glucose detection. J. Mater. Chem. B. 2018;6:487–498. doi: 10.1039/C7TB02676E. [DOI] [PubMed] [Google Scholar]
- 26.Fan H., Wang N., Tian Y.J., Ai S.Y., Zhan J.H. Acetic acid induced synthesis of laminated activated carbon nitride nanostructures. Carbon. 2016;107:747–753. doi: 10.1016/j.carbon.2016.06.082. [DOI] [Google Scholar]
- 27.Li Y., Jin R., Xing Y., Li J., Song S., Liu X., Li M., Jin R. Macroscopic foam-like holey ultrathin g-C3N4 nanosheets for drastic improvement of visible-light photocatalytic activity. Adv. Energy Mater. 2016;24:1601273. doi: 10.1002/aenm.201601273. [DOI] [Google Scholar]
- 28.Wang N., Han Z.W., Fan H., Ai S.Y. Copper nanoparticles modified graphitic carbon nitride nanosheets as a peroxidase mimetic for glucose detection. RSC Adv. 2015;5:91302–91307. doi: 10.1039/C5RA18957H. [DOI] [Google Scholar]
- 29.Qiao F.M., Qi Q.Q., Wang Z.Z., Xu K., Ai S.Y. MnSe-loaded g-C3N4 nanocomposite with synergistic peroxidase-like catalysis: Synthesis and application toward colorimetric biosensing of H2O2 and glucose. Sensor. Actuator. B Chem. 2016;229:379–386. doi: 10.1016/j.snb.2015.12.109. [DOI] [Google Scholar]
- 30.Tian J.Q., Liu Q., Asiri A.M., Qusti A.H., Al-Youbi A.O., Sun X.P. Ultrathin graphitic carbon nitride nanosheets: a novel peroxidase mimetic, Fe doping-mediated catalytic performance enhancement and application to rapid, highly sensitive optical detection of glucose. Nanoscale. 2013;5:11604–11609. doi: 10.1039/c3nr03693f. [DOI] [PubMed] [Google Scholar]
- 31.Mu J.S., Li J., Zhao X., Yang E.C., Zhao X.J. Cobalt-doped graphitic carbon nitride with enhanced peroxidase-like activity for wastewater treatment. RSC Adv. 2016;6:35568–35576. doi: 10.1039/C6RA02911F. [DOI] [Google Scholar]
- 32.Ouyang H., Tu X.M., Fu Z.F., Wang W.W., Fu S.F., Zhu C.Z., Du D., Lin Y.H. Colorimetric and chemiluminescent dual-readout immunochromatographic assay for detection of pesticide residues utilizing g-C3N4/BiFeO3 nanocomposites. Biosens. Bioelectron. 2018;106:43–49. doi: 10.1016/j.bios.2018.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chhowalla M., Shin H.S., Eda G., Li L.J., Loh K.P., Zhang H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013;5:263–275. doi: 10.1038/nchem.1589. [DOI] [PubMed] [Google Scholar]
- 34.Huang Y.X., Guo J.H., Kang Y.J., Ai Y., Li C.M. Two dimensional atomically thin MoS2 nanosheets and their sensing applications. Nanoscale. 2015;7:19358–19376. doi: 10.1039/C5NR06144J. [DOI] [PubMed] [Google Scholar]
- 35.Wang X.J., Wu Q., Jiang K.L., Wang C.X., Zhang C. One-step synthesis of water-soluble and highly fluorescent MoS2 quantum dots for detection of hydrogen peroxide and glucose. Sensor. Actuator. B Chem. 2017;252:183–190. doi: 10.1016/j.snb.2017.05.177. [DOI] [Google Scholar]
- 36.Wang M., Ju P., Zhao Y., Li J.J., Han X.X., Hao Z.M. In situ ion exchange synthesis of MoS2/g-C3N4 heterojunction for highly efficient hydrogen production. New J. Chem. 2018;42:910–917. doi: 10.1039/C7NJ03483K. [DOI] [Google Scholar]
- 37.Zhao K., Gu W., Zheng S.S., Zhang C.L., Xian Y.Z. SDS-MoS2 nanoparticles as highly-efficient peroxidase mimetics for colorimetric detection of H2O2 and glucose. Talanta. 2015;141:47–52. doi: 10.1016/j.talanta.2015.03.055. [DOI] [PubMed] [Google Scholar]
- 38.Peng J., Weng J. Enhanced peroxidase-like activity of MoS2/graphene oxide hybrid with light irradiation for glucose detection. Biosens. Bioelectron. 2017;89:652–658. doi: 10.1016/j.bios.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y., Zhou Z.F., Wen F.F., Tan J., Peng T., Luo B.Q., Wang H.G., Yin S.X. A flower-like MoS2-decorated MgFe2O4 nanocomposite: Mimicking peroxidase and colorimetric detection of H2O2 and glucose. Sensor. Actuator. B Chem. 2018;275:155–162. doi: 10.1016/j.snb.2018.08.051. [DOI] [Google Scholar]
- 40.Vinita, Nirala N.R., Prakash R. One step synthesis of AuNPs@MoS2-QDs composite as a robust peroxidase-mimetic for instant unaided eye detection of glucose in serum, saliva and tear. Sensor. Actuator. B Chem. 2018;263:109–119. doi: 10.1016/j.snb.2018.02.085. [DOI] [Google Scholar]
- 41.Gopalakrishnan D., Damien D., Shaijumon M.M. MoS2 quantum dot-interspersed exfoliated MoS2 nanosheets. ACS Nano. 2014;8:5297–5303. doi: 10.1021/nn501479e. [DOI] [PubMed] [Google Scholar]
- 42.Wei H., Wang E.K. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal. Chem. 2008;80:2250–2254. doi: 10.1021/ac702203f. [DOI] [PubMed] [Google Scholar]
- 43.Ye L., Chen J., Tian L., Liu J., Peng T., Deng K., Zan L. BiOI thin film via chemical vapor transport: photocatalytic activity, durability, selectivity and mechanism. Appl. Catal. B Environ. 2013;130–131:1–7. doi: 10.1016/j.apcatb.2012.10.011. [DOI] [Google Scholar]
- 44.Zhang Y., Thomas A., Antonietti M., Wang X. Activation of carbon nitride solids by protonation: Morphology changes, enhanced ionic conductivity, and photoconduction experiments. J. Am. Chem. Soc. 2009;131:50–51. doi: 10.1021/ja808329f. [DOI] [PubMed] [Google Scholar]
- 45.Ju P., Wang Y., Sun Y., Zhang D. Controllable one-pot synthesis of a nest-like Bi2WO6/BiVO4 composite with enhanced photocatalytic antifouling performance under visible light irradiation. Dalton Trans. 2016;45:4588–4602. doi: 10.1039/C6DT00118A. [DOI] [PubMed] [Google Scholar]
- 46.Chen L.J., Sun B., Wang X., Qiao F.M., Ai S.Y. 2D ultrathin nanosheets of Co-Al layered double hydroxides prepared in L-asparagine solution enhanced peroxidase-like activity and colorimetric detection of glucose. J. Mater. Chem. B. 2013;1:2268–2274. doi: 10.1039/c3tb00044c. [DOI] [PubMed] [Google Scholar]
- 47.Qiao F.M., Chen L.J., Li X., Li L., Ai S.Y. Peroxidase-like activity of manganese selenide nanoparticles and its analytical application for visual detection of hydrogen peroxide and glucose. Sensor. Actuator. B Chem. 2014;193:255–262. doi: 10.1016/j.snb.2013.11.108. [DOI] [Google Scholar]
- 48.Porter D.J., Bright H.J. The mechanism of oxidation of nitroalkanes by horseradish peroxidase. J. Biol. Chem. 1983;258:9913–9924. [PubMed] [Google Scholar]
- 49.Li L., Ai L., Zhang C., Jiang J. Hierarchical {001}-faceted BiOBr microspheres as a novel biomimetic catalyst dark catalysis towards colorimetric biosensing and pollutant degradation. Nanoscale. 2014;6:4627–4634. doi: 10.1039/c3nr06533b. [DOI] [PubMed] [Google Scholar]
- 50.Wang B., Ju P., Zhang D., Han X.X., Zheng L., Yin X.F., Sun C.J. Colorimetric detection of H2O2 using flower-like Fe2(MoO4)3 microparticles as a peroxidase mimic. Microchim. Acta. 2016;183:3025–3033. doi: 10.1007/s00604-016-1955-8. [DOI] [Google Scholar]
- 51.Chen Q., Chen J., Gao C.J., Zhang M.L., Chen J.Y., Qiu H.D. Hemin-functionalized WS2 nanosheets as highly active peroxidase mimetics for label-free colorimetric detection of H2O2 and glucose. Analyst. 2015;140:2857–2863. doi: 10.1039/C5AN00031A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.