Summary
The role of stress-induced increases in SUMO2/3 conjugation during the heat shock response (HSR) has remained enigmatic. We investigated SUMO signal transduction at the proteomic and functional level during the HSR in cells depleted of proteostasis network components via chronic heat shock factor 1 inhibition. In the recovery phase post heat shock, high SUMO2/3 conjugation was prolonged in cells lacking sufficient chaperones. Similar results were obtained upon inhibiting HSP90, indicating that increased chaperone activity during the HSR is critical for recovery to normal SUMO2/3 levels post-heat shock. Proteasome inhibition likewise prolonged SUMO2/3 conjugation, indicating that stress-induced SUMO2/3 targets are subsequently degraded by the ubiquitin-proteasome system. Functionally, we suggest that SUMOylation can enhance the solubility of target proteins upon heat shock, a phenomenon that we experimentally observed in vitro. Collectively, our results implicate SUMO2/3 as a rapid response factor that coordinates proteome degradation and assists the maintenance of proteostasis upon proteotoxic stress.
Keywords: small ubiquitin-like modifier, SUMO2/3, ubiquitin, proteasome, heat shock factor 1, heat shock response, proteotoxic stress, chaperone, HSP90, proteostasis
Graphical Abstract
Highlights
-
•
Chaperone depletion delays recovery of SUMOylation in response to heat shock
-
•
SUMOylated proteins are targeted to the proteasome during heat shock recovery
-
•
Chaperone depletion impairs clearance of Ub/SUMO co-modified proteins after stress
-
•
SUMO is a rapid response factor likely increasing protein solubility under heat shock
Functional consequences of SUMO2/3 conjugation during proteotoxic stress remain unclear. Liebelt et al. show that SUMO2/3 acts synergistically with the proteostasis network to reduce aggregation during acute heat shock and facilitate target degradation by the proteasome during stress recovery. SUMO2/3, therefore, acts as a first protective response to proteotoxic stress.
Introduction
The composition of the cellular proteome is dynamically matched to functional requirements via transcription, splicing, new protein synthesis, and protein turnover. Throughout this process, protein misfolding and aggregation are minimized by an extensive proteostasis network comprising chaperones, quality control mechanisms, and degradation pathways. The proteostasis network both facilitates productive folding and removes terminally misfolded or aggregated proteins (Kim et al., 2013, Labbadia and Morimoto, 2015).
Upon proteotoxic stress, the proteostasis network can become overloaded by excess misfolded proteins. Cellular responses such as the heat shock response (HSR) are induced to resolve the misfolding and prevent the formation of toxic and/or insoluble protein aggregates. The HSR is coordinated by HS factors (HSFs) that induce the transcription of chaperones and quality control factors to resolve excessive protein misfolding (Åkerfelt et al., 2010). HSF1 is the master regulator of the HSR. HSF1 is continuously expressed and displays low basal activity sufficient only to maintain normal levels of cytosolic and nuclear proteostasis network components in the absence of proteotoxic stress. The majority of HSF1 under these conditions is maintained inactive in the cytoplasm in a monomeric form, apparently via binding to chaperones (Shi et al., 1998, Zou et al., 1998). Increasing levels of misfolded proteins owing to either chronic proteotoxic stress (e.g., constitutive expression of a mutant or misfolding protein) or acute proteotoxic stress (e.g., HS or heavy metal oxidants) lead to chaperones being titrated off of HSF1 by interactions with the accumulating misfolding proteins, inducing HSF1 homotrimerization and translocation to the nucleus to initiate the extensive HSR transcriptional program (Zhong et al., 1998). Once the proteotoxic stress has been resolved, HSF1 is inactivated again via post-translational modifications or via binding to excess chaperones (Westerheide et al., 2009).
In addition to transcriptional remodeling, proteotoxic stress is known to rapidly but transiently induce high levels of specific protein post-translational modifications, including substantial increases in conjugation to ubiquitin (Ub), O-linked glycans, and small Ub-like modifiers (SUMOs) (Golebiowski et al., 2009, Sohn et al., 2004, Tatham et al., 2011, Tempé et al., 2008). It is well appreciated that Ub modification can enhance proteostasis in response to proteotoxic stress via promoting proteasomal degradation of misfolded proteins and that O-glycosylation can inhibit the aggregation of at least certain intrinsically disordered proteins (Hastings et al., 2017, Marotta et al., 2015). However, the role of dramatic increases in SUMO2/3 modification levels upon proteotoxic stress has remained enigmatic.
The SUMO family is a group of highly conserved proteins with a similar three-dimensional fold to Ub (Flotho and Melchior, 2013, Geiss-Friedlander and Melchior, 2007). Similar to Ub, SUMO dynamically modifies lysine side chains on substrate proteins (van der Veen and Ploegh, 2012). Three major isoforms (SUMO1, 2, and 3) are ubiquitously expressed in mammalian cells (Geiss-Friedlander and Melchior, 2007, Saitoh and Hinchey, 2000). SUMO1 only shares 48% sequence identity with SUMO2 and SUMO3. Mature SUMO2 and SUMO3 share the vast majority of their amino acid sequences (95% sequence identity) and cannot be distinguished by extant antibodies. As such, they are often referred to collectively as SUMO2/3.
Shortly after the discovery of the SUMO2/3 family members, it was observed that these SUMO modifications rapidly accumulate upon HS, in contrast to SUMO1 (Saitoh and Hinchey, 2000, Tempé et al., 2008). Proteomic analysis revealed increases in SUMO2/3 conjugation for a wide range of substrates (Golebiowski et al., 2009, Hendriks et al., 2014, Tatham et al., 2011). Notably, SUMO2 is recruited to open chromatin during HS (Seifert et al., 2015) and appears to be indispensable for full activation of the inducible HSPA1A (HSP70) gene (Martin et al., 2009). Modification of HSF1 by both SUMO1 and SUMO2/3 is also induced during stress and may modulate the transcription of HS proteins during later stages of stress (Brunet Simioni et al., 2009, Hietakangas et al., 2003). Although stress-induced SUMOylation is widespread, the potential proteostatic functions and regulation of this modification, which typically recover to normal levels in a matter of 2–4 hr after HS, are poorly understood.
We present evidence that the composition and activities of the cellular proteostasis network regulate SUMO2/3 dynamics during HS and are critical determinants in the degradation of SUMOylated substrates by the Ub-proteasome system. We further identify a unique subset of SUMOylated proteins that preferentially maintain SUMOylation for prolonged time periods during chronic proteostasis impairment. Finally, we present evidence that SUMOylation reduces the aggregation of substrate proteins in vitro. Our results implicate SUMOylation as a rapid response mechanism that is integrated within the chaperone and quality control networks, is essential for coordinating substrate degradation in response to proteotoxic stress, and may prevent substrate aggregation at least in certain cases. Considering the number of SUMOylated substrates associated with neurodegeneration and cancer (Krumova and Weishaupt, 2013, Seeler and Dejean, 2017), these findings lay the foundation for investigating the intersection of SUMO2/3 modifications with protein quality control systems in disease progression and severity.
Results
Depletion of Proteostasis Factors by Chronic HSF1 Inhibition Delays Recovery of SUMO2/3 Conjugation Levels after HS
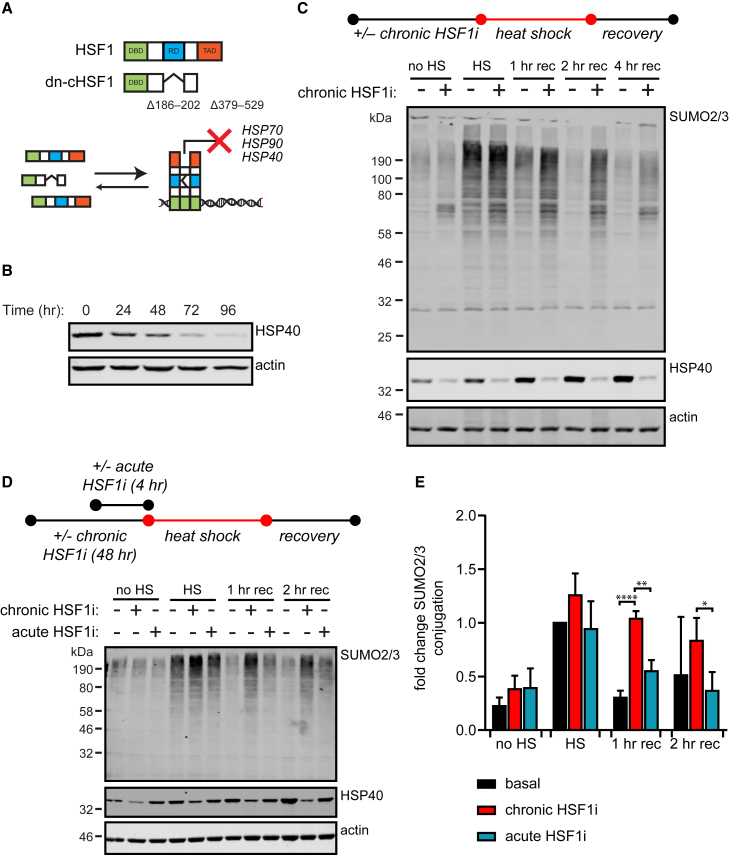
As SUMO2/3 conjugation is stimulated by a variety of stressors associated with protein misfolding, including heat and oxidative stress, we were first interested in determining whether an impaired protein-folding environment sensitizing cells to proteotoxic stress would modulate stress-induced SUMOylation. To create an impaired proteostasis network, we globally depleted cytosolic and nuclear proteostasis factors by using chemical biology tools to regulate the activity of the master cytosolic proteostasis transcription factor HSF1. We previously showed that a doxycycline (Dox)-inducible, constitutively active dominant-negative variant of HSF1, termed dn-cHSF1 (Moore et al., 2016), can inhibit the activity of endogenous HSF1 with high selectivity and potency (Figure 1A). Chronic expression of dn-cHSF1 results in reduced steady-state levels of key proteostasis network components, especially HSP40, HSP70, and HSP90 (Figure 1B; see also Moore et al., 2016).
Figure 1.
Depletion of Proteostasis Factors by Chronic HSF1 Inhibition Delays Recovery to Normal SUMO2/3 Conjugation Levels after Heat Shock
(A) Use of a dominant-negative, constitutively active HSF1 construct (dn-cHSF1), created via disruption of the regulatory domain (RD) by removing amino acids 186–202 combined with deletion of the transcription activation domain (TAD), for inhibition of endogenous HSF1.
(B) Western blot analysis of HSP40 protein levels in response to different time periods of Dox-induced dn-cHSF1 induction in HEK293T-REx cells.
(C) HEK293T-REx cells expressing Dox-inducible dn-cHSF1 were treated with Dox for 48 hr (chronic HSF1i) prior to heat shock (HS). Cells were exposed to a HS at 43°C for 75 min before returning to 37°C for a recovery period (rec) and lysed as indicated. Total amounts of SUMOylated proteins were analyzed by immunoblotting. HSP40 levels were used to confirm the induction of the heat shock response and functional HSF1 inhibition.
(D) HEK293T-REx cells constitutively expressing DHFR.dn-cHSF1 were treated with trimethoprim (TMP) for 48 hr (chronic HSF1 inhibition) or 4 hr (acute HSF1 inhibition) prior to HS. Cells were subsequently exposed to HS and recovery as described in (A). Total amounts of SUMOylated proteins were determined by immunoblotting.
(E) Quantification of total SUMO2/3 conjugation from (D) as compared to SUMOylation post-heat shock in basal proteostasis conditions. n = 3, error bars indicate SD. Significance was determined using ANOVA analysis followed by post hoc Tukey analysis, ∗p < 0.05; ∗∗p < 0.005, ∗∗∗∗p < 0.0001.
See also Figure S1.
We examined the effects of this chaperone depletion on the dynamics of SUMO2/3 conjugation both during and after HS in HEK293T-REx cells. As expected, immediately post-HS, a strong increase in SUMO2/3 conjugation was observed (Figure 1C). When HSF1 was not inhibited prior to HS, the rate of return to normal SUMO2/3 conjugation levels was rapid, with complete return to basal SUMOylation achieved within 2 hr of recovery at 37°C. In sharp contrast, the chronic inhibition of HSF1 prior to HS delayed the recovery of SUMO2/3 conjugation levels beyond 4 hr during recovery at 37°C (Figures 1C and S1A). Importantly, control experiments in HEK293T-REx cells expressing a Dox-inducible GFP construct did not show any Dox-mediated changes in SUMOylation dynamics (Figure S1B), confirming that the delayed recovery was due to the induction of dn-cHSF1. Metabolic activity after HS was not significantly reduced following chronic HSF1 inhibition (Figure S1C), indicating that cell growth was not influenced and that sustained SUMOylation could not be attributed to an overall impairment in cell health during HS recovery.
The difference in the recovery rates could not be attributed to either increases in basal SUMO2/3 conjugation, or to an increase in initial SUMOylation levels immediately following HS (Figures 1C and S1A). Instead, it was specifically the recovery to normal SUMO2/3 levels that was delayed by chronic HSF1 inhibition. Notably, these observations were not limited to HEK293T-REx cells, as we observed a similar delay in recovery when HSF1 was chronically inhibited prior to HS in LX2 hepatic stellate cells (Figure S1D).
Chronic inhibition of HSF1 via Dox-induced expression of dn-cHSF1 not only basally depletes chaperones but also prevents HSR induction upon HS. To distinguish between SUMO2/3 retention resulting from inhibition of the HS-induced HSR versus reduced basal proteostasis capacity prior to HS, we next sought to uncouple the HSR inhibition from basal depletion of chaperones owing to chronic HSF1 inhibition by transiently inhibiting the HSR without significantly reducing basal expression of chaperones. Briefly, we expressed a genetic fusion of dn-cHSF1 conjugated to a destabilized variant of Escherichia coli dihydrofolate reductase (DHFR), which is rapidly degraded by the proteasome unless a stabilizing ligand (trimethoprim [TMP]) is added to the cell culture medium (Moore et al., 2016), to rapidly increase protein levels of dn-cHSF1 only 4 hr before the HS. Using this system, we found that acute TMP treatment (4 hr) did not substantially impact basal chaperone expression (Figure 1D). However, HS-induced transcription of HSF1-mediated genes was substantially impaired (Figure S1E).
Using cells expressing the DHFR.dn-cHSF1 construct, we examined the dynamics of stress-responsive SUMO2/3 conjugation following acute (4 hr TMP) HS inhibition versus chronic (48 hr TMP) chaperone depletion prior to HS. Acute TMP treatment did not substantially alter either the accumulation of SUMO2/3 conjugates during HS or the rate of recovery (Figures 1D and 1E). In contrast, chronic inhibition of HSF1 by using this TMP-regulated HSF1 construct fully recapitulated the consequences of Dox-inducible dn-cHSF1 expression (Figures 1D and 1E). Thus, alterations in stress-responsive SUMOylation dynamics are attributable to chronic HSF1 inhibition that engenders the depletion of critical components within the proteostasis network and sensitizes the system to proteotoxic stress.
Proteomic Identification of SUMOylated Proteins Whose Recovery to Normal SUMO-Conjugation Levels Post Heat Shock Is Delayed by Chronic HSF1 Inhibition
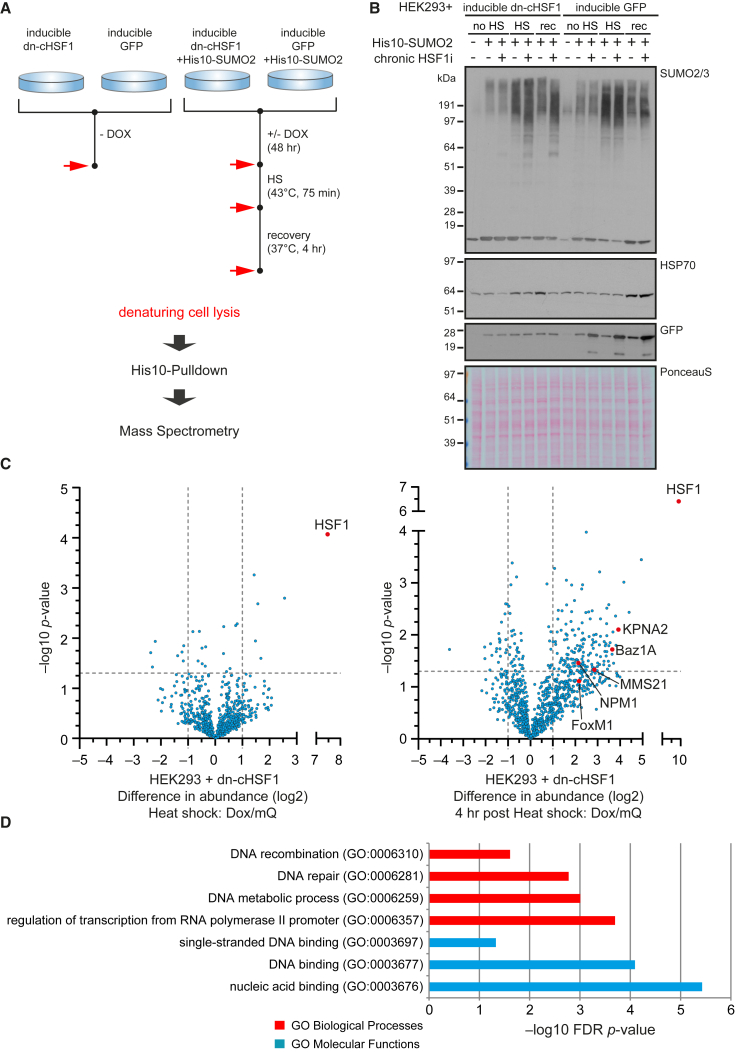
We next sought to identify the specific SUMOylation targets that preferentially retain SUMO2/3 when proteostasis capacity is reduced. To address this question, we used nickel-nitrilotriacetic acid (Ni-NTA) beads to purify SUMOylated proteins from HEK293T-REx cells co-expressing a His10-tagged SUMO2 along with Dox-inducible dn-cHSF1. Cells co-expressing a Dox-inducible GFP and His10-SUMO2 were used as a control for any effects of Dox treatment. Cells lacking the His10-SUMO2 construct were used as a control for non-specific binding to Ni-NTA beads.
We then used quantitative proteomics to study SUMO2 target-protein dynamics before, during, and after HS in basal and chronic HSF1 inhibition conditions (Figure 2A). Immunoblot analysis of the input samples prior to mass spectrometry analysis fully recapitulated our findings from Figure 1 (Figure 2B). In the proteomics, with a low stringency requiring only an average fold change of ≥2, we identified 450 proteins that consistently showed increased SUMOylation immediately following HS. The extent of SUMO2 conjugation on 89% (n = 399) of these proteins returned to normal levels during the 4-hr recovery period in untreated cells. In contrast, recovery to normal SUMO2 levels was delayed for 77% (n = 306) of the identified proteins when HSF1 was chronically inhibited (Table S1). We also observed striking enrichment of SUMOylated HSF1 immediately after HS and during recovery following Dox treatment, which can be attributed to a large extent to overexpression of dn-cHSF1 (Figure 2C). These observations demonstrate the vast influence of the proteostasis network on SUMOylated protein dynamics during HS recovery. Notably, we did not observe a global effect on the extent of SUMOylation immediately post-HS owing to chronic HSF1 inhibition. We also did not observe global changes in SUMOylation or SUMOylation dynamics as a result of Dox treatment in the Dox-inducible GFP control cells (Figures 2C and S2A; Table S1).
Figure 2.
Proteomic Identification of SUMOylated Proteins Whose Recovery to Normal SUMO-Conjugation Levels Post Heat Shock Is Delayed by Chronic HSF1 Inhibition
(A) Workflow for Figure 2. HEK293T-REx cells stably co-expressing His10-SUMO2 and either Dox-inducible dn-cHSF1 or Dox-inducible GFP were treated with Dox for 48 hr (chronic HSF1i) prior to heat shock (HS). Cells were exposed to HS at 43°C for 75 min before returning to 37°C for a recovery period (rec). Red arrows indicate time points at which cells were lysed. SUMOylated proteins were purified by means of His10 pull-down, proteins were trypsinized, and peptides were analyzed by mass spectrometry.
(B) Immunoblotting of cell lysates from (A) using antibodies against SUMO2/3, HSP70, and GFP. Total amounts of proteins in each lane were visualized by Ponceau S staining.
(C) Volcano plots depicting statistical differences in abundance between proteins identified by mass spectrometry. Dashed lines indicate a cut-off at a p value ≤ 0.05 (–log10 p value ≤ 1.3) and a fold change of 2 (log2 = 1). The left panel shows SUMOylated proteins identified immediately post-HS in the Dox-inducible dn-cHSF1 cell line, specifically in the Dox-treated sample compared to the untreated sample. The right panel shows SUMOylated proteins identified 4-hr post-HS in the Dox-inducible dn-cHSF1 cell line compared to the untreated sample. Proteins marked in red are either HSF1 or proteins that were further validated.
(D) Selection of enriched gene ontology terms of biological processes and molecular functions for selected proteins that significantly retained SUMOylation 4-hr post-HS when HSF1 activity was chronically inhibited.
We next set out to identify high-confidence proteins for validation and follow-up studies. We, therefore, adjusted our selection criteria to proteins having a ≥2-fold change as well as a p value < 0.05 and identified 344 high-confidence SUMO2 target proteins that were significantly enriched immediately following HS, regardless of treatment condition (Tables S1 and S2). The extent of SUMO2 conjugation on 49% (n = 170) of these proteins returned to normal levels during the 4-hr recovery period in untreated cells (Table S3). Of this subset, recovery to normal SUMOylation levels was delayed for 42% (n = 72) of the identified proteins when HSF1 was chronically inhibited (Figure 2C; Table S4), as compared to vehicle treatment.
High-confidence SUMO2 conjugates whose recovery to basal SUMOylation levels was delayed by chronic HSF1 inhibition were enriched for DNA-associated factors, including proteins involved in transcription, recombination, and DNA repair (Figures 2D and S2B), consistent with the predominantly nuclear localization of SUMO. We further validated delayed SUMO2/3 recovery under conditions of chronic HSF1 inhibition for a number of the proteins identified in our mass spectrometry screen, engendering high confidence in our mass spectrometry results (Figure S3). Interestingly, we could also confirm a HSF1 inhibition-dependent effect on the SUMOylation dynamics of FoxM1 post-HS. FoxM1 is a protein that did not quite meet our strict filtering conditions for Figure 2C, indicating that such a stringent selection criteria can lead to false negatives. In combination with our low-stringency data analysis, these results further support the thesis that the proteostasis network is required to resolve a significant fraction of HS-induced SUMO2 targets.
Chronic HSF1 Inhibition Interferes with Degradation of SUMOylated and Ubiquitinated Proteins during Heat Shock Recovery
The clearance of SUMOylated proteins observed during HS recovery may occur either by the removal of SUMO2/3 by the action of SUMO-specific proteases (SENPs) or by degradation of the SUMO2/3-conjugated proteins (Schimmel et al., 2008). Using a recently developed set of activity-based probes (Mulder et al., 2018), we performed activity profiling of a set of endogenously expressed SENPs. The results indicated that proteostasis modulation by chronic HSF1 inhibition did not affect the activity of SENPs during HS or recovery (Figure S4).
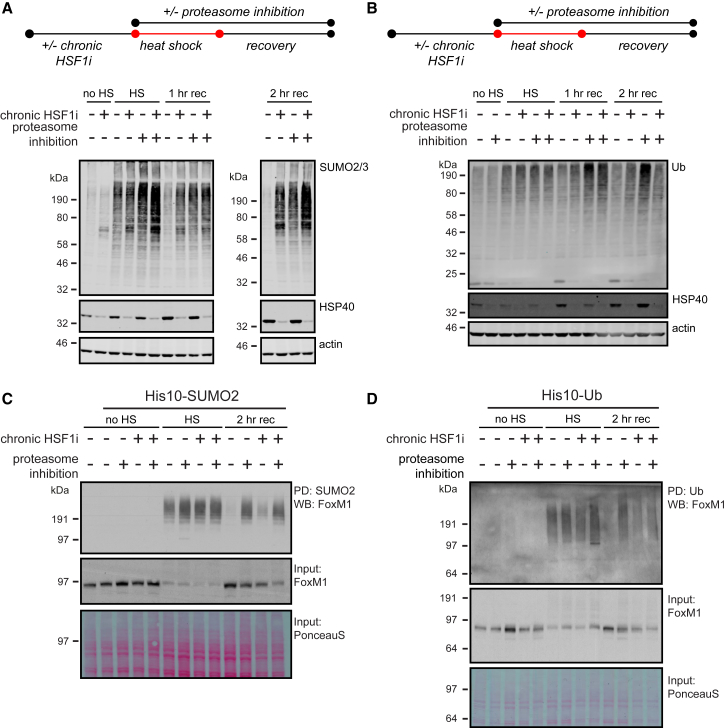
We next sought to define if SUMOylated proteins were subjected to degradation during HS recovery. We first examined the effects of proteasome inhibition on SUMO2/3 conjugation during HS and recovery by using two commercially available proteasome inhibitors, MG-132 and bortezomib. We observed that bortezomib did not induce basal SUMOylation at concentrations with comparable proteasome inhibitory activity as MG-132 (Figure S5A; note that bortezomib does not inhibit the β2 catalytic subunit of the proteasome, whereas MG-132 does inhibit this subunit [Berkers et al., 2005]). We, therefore, chose to proceed with bortezomib to isolate HS and proteostasis-dependent impacts on degradation of SUMOylated proteins.
We found that proteasome inhibition by using bortezomib delayed the recovery of global SUMO2/3 conjugates post-HS in a manner similar to the consequences of chronic HSF1 inhibition prior to HS. Indeed, either proteasome inhibition or chronic HSF1 inhibition resulted in SUMOylation remaining at >80% of the maximal SUMOylation levels in vehicle-treated samples following a 2-hr recovery period. Notably, the effects of proteasome inhibition and chronic HSF1 inhibition on delaying SUMO2/3 recovery were partially additive, with SUMO2/3 conjugation remaining near 100% throughout the recovery period when the treatments were applied in combination (Figures 3A, S5B, and S5C). Collectively, these observations suggest that a large fraction of SUMO2/3 conjugates are degraded by the proteasome in the recovery phase post-HS, indicating impaired degradation and, therefore, a stabilization of SUMO2/3 conjugates as a consequence of chronic HSF1 inhibition.
Figure 3.
Chronic HSF1 Inhibition Interferes with Degradation of SUMOylated and Ubiquitinated Proteins during Heat Shock Recovery
(A) HEK293T-REx cells expressing Dox-inducible dn-cHSF1 were treated with Dox for 48 hr (chronic HSF1i), with 100 nM bortezomib (proteasome inhibition) immediately prior to heat shock (HS), or with a combination of both. Cells were exposed to HS at 43°C for 75 min before returning to 37°C for a recovery period (rec) and lysed as indicated. Total amounts of SUMOylated proteins were analyzed by immunoblotting. HSP40 levels were used to confirm induction of the heat shock response and functional HSF1 inhibition.
(B) HEK293T-REx cells expressing Dox-inducible dn-cHSF1 were treated as in (A). Total amounts of ubiquitinated proteins were analyzed by immunoblotting.
(C) HEK293T-REx cells stably co-expressing His10-SUMO2 and Dox-inducible dn-cHSF1 were treated as in (A). SUMOylated proteins were purified by means of His10 purification. Elutions and inputs were analyzed by immunoblotting for FoxM1. Ponceau S stain was used as a loading control for inputs.
(D) HEK293T-REx cells stably co-expressing His10-Ub and Dox-inducible dn-cHSF1 were treated as in (A). Ubiquitinated proteins were purified by means of His10-purification. Elutions and inputs were analyzed by immunoblotting for FoxM1. Ponceau S stain was used as a loading control for inputs.
See also Figures S4 and S5.
Because proteasomal degradation of proteins is regulated by ubiquitination, we next investigated Ub dynamics during HS recovery. Similar to SUMOylation dynamics, we observed an increase in Ub conjugates during HS that was rapidly reduced to pre-HS levels during the recovery period (Figure 3B). Chronic HSF1 inhibition prior to HS delayed the recovery of Ub conjugation post-HS, although to a substantially lesser extent than for proteasome inhibition (Figures 3B and S5D). These results suggest that proteostasis factors, transcribed by HSF1, facilitate proteasomal degradation of ubiquitinated as well as SUMOylated substrates during HS recovery. As the simultaneous inhibition of the proteasome and HSF1 resulted in a slight additive stabilization of SUMOylated proteins, but not of ubiquitinated proteins, we would not exclude an additional proteasome-independent pathway by which chaperones and other proteostasis factors stimulate a decrease in SUMOylation during recovery post-HS.
We next investigated if the identities of the ubiquitinated and SUMOylated substrates affected by chronic HSF1 inhibition coincided. Here, we examined Ub- and SUMO2-purified fractions for the presence and dynamics of specific proteins that we previously identified as showing delayed recovery to normal SUMO2 levels in Figure 2. The SUMOylation of the cell cycle regulator Forkhead box transcription factor FoxM1 was induced upon HS and decreased to pre-HS levels during recovery but was stabilized by either proteasome inhibition or chronic HSF1 inhibition (Figure 3C). FoxM1 ubiquitination was also induced by HS and recovered post-HS in a proteasome and HSF1-dependent manner (Figure 3D). Additional SUMO2 substrates, identified by proteomics, showed similar SUMOylation and ubiquitination dynamics, indicating that both modifications were affected by chronic HSF1 inhibition on the same substrate proteins (Figures S5E and S5F).
Collectively, these data suggest that proteasomal degradation is a major, but not the exclusive, fate of SUMO2/3 and Ub conjugates during HS recovery. Chronic HSF1 inhibition interferes with this proteasomal degradation and therefore stabilizes ubiquitinated as well as SUMOylated subsets of the same target protein.
Chronic HSF1 Inhibition Selectively Impairs Degradation of SUMO2/3 and Ub Co-modified Proteins during Heat Shock Recovery
SUMO and Ub both covalently attach to lysine residues in target proteins. The modifications are mutually exclusive at a specific modification site. However, SUMOylation and ubiquitination frequently co-exist at different sites on the same protein (Cuijpers et al., 2017, Lamoliatte et al., 2017). We observed in Figures 3C and 3D that FoxM1 was modified by both SUMO2/3 and Ub and that the dynamics of both modifications were influenced by chronic HSF1 inhibition. We, therefore, wondered if depletion of the proteostasis network components by chronic HSF1 inhibition would preferentially impair degradation of the co-modified fraction of ubiquitinated or SUMOylated proteins.
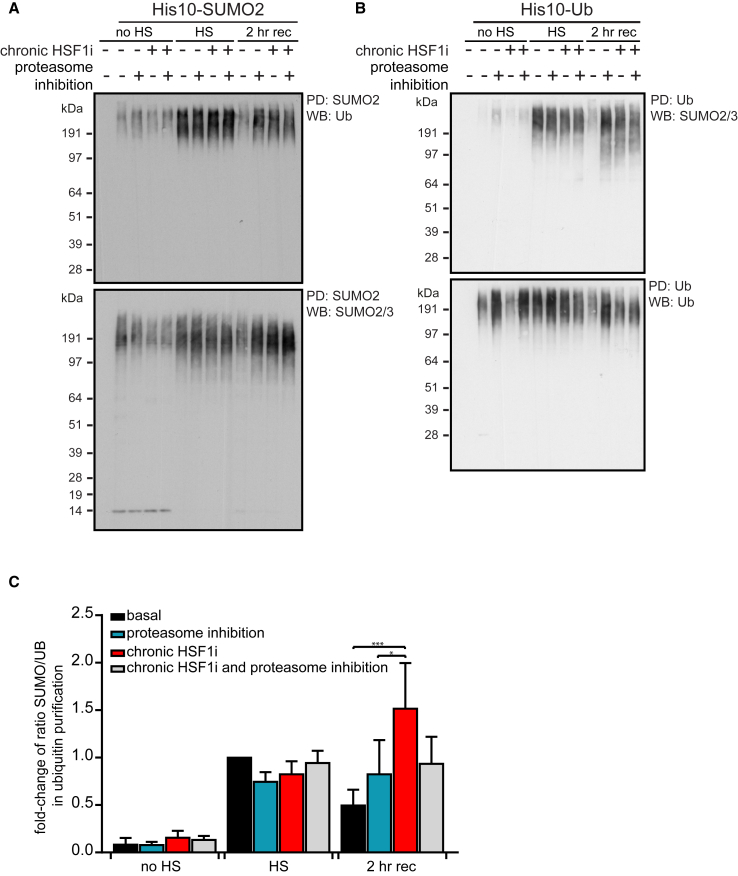
We first examined the dynamics of ubiquitinated proteins in SUMO2 pull-downs and vice versa during either proteasome inhibition or chronic HSF1 inhibition. Within the SUMOylated fraction, Ub was co-enriched upon HS, indicating an enrichment of co-modified proteins. The extent of Ub co-enrichment fell during HS recovery but was enhanced by either chronic HSF1 inhibition or proteasome inhibition (Figure 4A). We observed the same dynamics for SUMO2/3 substrates within the Ub-purified proteins (Figure 4B). Collectively, these results support the conclusion that chaperone depletion interferes with the degradation of co-modified proteins during HS recovery.
Figure 4.
Chronic HSF1 Inhibition Selectively Impairs Degradation of SUMO2/3 and Ub Co-modified Proteins during Heat Shock Recovery
(A) HEK293T-REx cells stably co-expressing His10-SUMO2 and Dox-inducible dn-cHSF1 were treated with Dox for 48 hr (chronic HSF1i) prior to heat shock (HS), with 100 nM bortezomib (proteasome inhibition) immediately prior to HS, or with a combination of both. Cells were exposed to a HS at 43°C for 75 min before returning to 37°C for a recovery period (rec) and lysed as indicated. Elutions were analyzed by immunoblotting for Ub and SUMO2/3.
(B) HEK293T-REx cells stably co-expressing His10-Ub and Dox-inducible dn-cHSF1 were treated as in (A). Elutions were analyzed by immunoblotting for SUMO2/3 and Ub.
(C) Quantification of (B). Graph shows fold change of the ratio of SUMOylated proteins over ubiquitinated proteins in His10-Ub purified elutions as compared to the ratio after HS under basal proteostasis conditions. n = 3, error bars represent SD. Significance was determined using ANOVA followed by post hoc Tukey’s multiple comparison test, ∗p < 0.05; ∗∗∗p < 0.001.
We next wondered if the effect of chronic HSF1 inhibition was restricted to co-modified proteins or if the stabilization of SUMO2/3 was merely a consequence of the stabilization of all ubiquitinated proteins. To answer this question, we investigated the ratio of SUMO2/3 in the pool of Ub-modified proteins. By inhibiting the proteasome, we expected to inhibit the degradation of all Ub-modified substrates, including co-modified proteins. If chronic HSF1 inhibition interfered mainly with the degradation of co-modified proteins, we would expect the ratio of SUMO2/3-modified to total Ub-modified proteins within the Ub-purified substrates to increase, as co-modified proteins would be stabilized, whereas substrates modified by Ub alone would continue to be degraded. As shown in Figure 4C, we indeed observed a significant difference in the SUMO/Ub ratio in the Ub-purified pool of proteins between proteasome inhibition and chronic HSF1 inhibition during the recovery post-HS, indicating a mechanism by which HSF1-induced factors selectively influence the degradation of co-modified proteins rather than all ubiquitinated proteins.
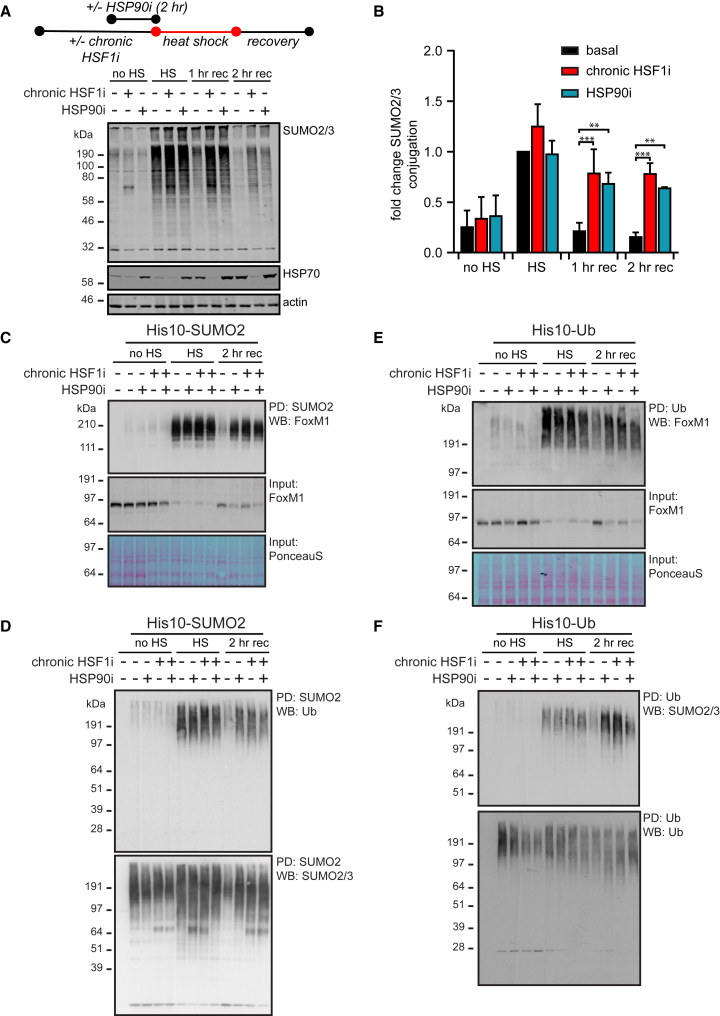
HSP90 Plays a Key Role in the Recovery to Normal SUMO2/3 Modification Levels following Heat Shock
As chronic HSF1 inhibition results in a reduction in proteostasis network capacity (Figure 1B), our data suggest that maintaining sufficient proteostasis capacity is critical for regulating the dynamics of SUMO2/3 and Ub substrates during HS recovery. We, therefore, sought to identify whether the inhibition of specific proteostasis components would be sufficient to recapitulate the delayed recovery of SUMO2/3- and Ub-modified targets we initially observed with chronic HSF1 inhibition.
Using STA-9090 to inhibit HSP90, we examined the dynamics of SUMO2/3 and Ub conjugates following HS. We found that HSP90 inhibition, similar to chronic HSF1 inhibition, resulted in delayed recovery of >50% of SUMO2/3 conjugates following a 2-hr recovery period (Figures 5A, 5B, and S6A). Similarly, we observed a delayed recovery post-HS of total ubiquitination upon HSP90 inhibition (Figures S6B and S6C). To investigate if HSP90 inhibition affects the same SUMOylated substrates we previously identified using proteomics (Figure 2), we purified SUMO2 conjugates and Ub conjugates and examined the dynamics of target proteins upon HS following either chaperone depletion or HSP90 inhibition. Indeed, we observed a HSF1- and HSP90-dependent decrease in FoxM1 SUMOylation and ubiquitination during the recovery period (Figures 5C and 5E). Similar effects were observed for other proteins (Figures S6D and S6E). HSP90 inhibition also prolonged the retention of co-modified proteins, as observed for SUMO2/3 co-modified proteins in the Ub pull-downs and vice versa (Figures 5D, 5F, and S6F), and led to an increase in SUMO2/3 relative to Ub within Ub-purified proteins (Figure S6F).
Figure 5.
HSP90 Plays a Key Role in the Recovery of SUMO2/3 Modification following Heat Shock
(A) HEK293T-REx cells expressing Dox-inducible dn-cHSF1 were either treated with Dox for 48 hr (chronic HSF1i) prior to heat shock (HS), with 500 nM STA-9090 (HSP90i) 2 hr prior to HS, or with a combination of both. Cells were exposed to a HS at 43°C for 75 min before returning to 37°C for a recovery period (rec) and lysed as indicated. Total amounts of SUMOylated proteins were analyzed by immunoblotting. HSP70 levels were used to confirm induction of the heat shock response and functional HSF1 inhibition.
(B) Quantification of fold change of SUMOylation from (A) as compared to SUMOylation after HS under the basal proteostasis conditions. n = 3, error bars indicate SD. Significance was determined using ANOVA analysis followed by post hoc Tukey analysis, ∗p < 0.05; ∗∗∗p < 0.0005.
(C) HEK293T-REx cells stably co-expressing His10-SUMO2 and Dox-inducible dn-cHSF1 were treated as in (A) with a 2-hr recovery at 37°C. SUMOylated proteins were purified by means of His10-purification. Elutions and inputs were analyzed by immunoblotting for FoxM1. Ponceau S stain was used as a loading control for inputs.
(D) As for (C). Elutions were analyzed by immunoblotting for Ub and SUMO2/3.
(E) HEK293T-REx cells stably co-expressing His10-Ub and Dox-inducible dn-cHSF1 were treated as in (A) with a 2-hr recovery at 37°C. Ubiquitinated proteins were purified by means of His10-purification. Elutions and inputs were analyzed by immunoblotting for FoxM1. Ponceau S stain was used as a loading control for inputs.
(F) As for (E). Elutions were analyzed by immunoblotting for SUMO2/3 and Ub.
See also Figure S6.
Collectively, these data indicate that HSP90 inhibition alone can at least partially recapitulate the effects of chronic HSF1 inhibition, delaying the recovery of SUMOylated and Ub co-modified substrates post-HS. These observations suggest that HSP90 is a key chaperone effector that promotes the degradation of co-modified proteins.
SUMOylation of Substrate Proteins Enhances Solubility
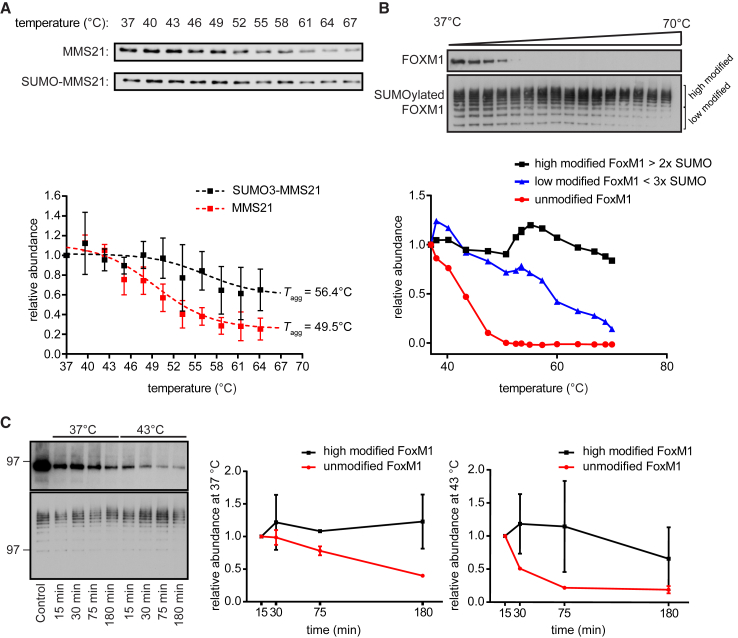
A key role of Ub on the co-modified proteins appears to be efficient proteasome delivery and processing of the target protein. In contrast, the role of SUMO is less clear. We noted that SUMO has been used extensively as a solubility tag to prevent inclusion body formation during protein expression in Escherichia coli (Butt et al., 2005, Malakhov et al., 2004) and is also proposed to modulate the aggregation of proteins associated with neurodegenerative disorders (Henley et al., 2014, Krumova and Weishaupt, 2013). Therefore, we next used an in vitro assay to explore the possibility that rapid SUMO2/3 conjugation could act as a solubility tag on target proteins identified from our proteomic studies. Solubility enhancement mediated by SUMOylation could prevent aggregation of terminally misfolded proteins prior to their clearance by proteasomal degradation, a process that could be facilitated by chaperones during HS recovery.
To address this possibility, we investigated the aggregation behavior of selected SUMO-tagged proteins identified from our proteomics screen in Figure 2 by using in vitro thermal shift assays. MMS21 was expressed in E. coli with or without an N-terminal SUMO3 tag, and the isolated protein was analyzed by a thermal shift assay to determine the aggregation temperature (Tagg) (Vedadi et al., 2006). We observed that the Tagg of N-SUMO3-MMS21 was ∼7°C higher than that of untagged MMS21 (Figure 6A).
Figure 6.
SUMOylation of Substrate Proteins Enhances Solubility
(A) Recombinant MMS21 fused to SUMO3 was exposed to increasing temperatures (37–67°C). Soluble fractions were isolated from temperature-induced aggregates by centrifugation and analyzed by immunoblotting with a specific antibody against MMS21. The plot shows relative fold change of soluble MMS21 normalized to the soluble MMS21 at 37°C, n = 3, error bars represent SD. Data were fit to a sigmoidal curve in Prism to determine aggregation temperature (Tagg).
(B) Recombinant FoxM1 was expressed and in vitro SUMOylated in E. coli and in vitro thermal shift performed as in (A). The soluble fraction of non-modified and SUMOylated FoxM1 was then analyzed by immunoblotting for FoxM1. The plots show relative fold change of FoxM1 in the soluble fraction, comparing the highly SUMOylated FoxM1 species (>FoxM1-3x SUMO), modestly SUMOylated FoxM1 species (<FoxM1-3x SUMO), and unmodified FoxM1.
(C) Recombinant FoxM1 was expressed and in vitro SUMOylated in E. coli, subsequently purified, and incubated at either 37°C or 43°C for 15, 30, 75 min, or 180 min. The soluble fraction of non-modified and SUMOylated FoxM1 was then analyzed by immunoblotting for FoxM1. The plots show the relative fold change of FoxM1 in the soluble fraction comparing the highly SUMOylated FoxM1 species (>FoxM1-3x SUMO) to unmodified FoxM1 in two biological replicates. Error bars indicate SD.
This intriguing result, obtained using a non-natural N-terminal fusion of a single SUMO3 moiety to MMS21, prompted us to then further investigate a more biologically realistic situation. Given that proteins from the proteomics screen were often conjugated to multiple SUMO2/3 moieties, we explored the impact of multiple SUMO2/3 modifications on FoxM1 using in vitro SUMOylation. Similar to MMS21, conjugation of SUMO2/3 protected FoxM1 against heat-induced aggregation (Figure 6B). Furthermore, increasing SUMOylation appeared to stabilize FoxM1 additively, with higher order SUMO2/3 conjugates showing almost no aggregation at temperatures up to 70°C. The presence of unmodified FoxM1 was also prolonged in the soluble fraction when SUMOylated proteins were present, indicating that the presence of SUMOylated proteins could even have a beneficial effect on the solubility of non-SUMOylated proteins in the vicinity (Figure 6B). We further investigated the impact of SUMOylation on FoxM1 during extended HS at moderate temperatures identical to the temperatures used in cellular studies. Although unmodified FoxM1 demonstrated progressive aggregation at 43°C, highly SUMOylated FoxM1 remained soluble throughout the duration of HS (Figure 6C). These results suggest that SUMO alters the biophysical properties of the target protein and, thereby, decreases aggregation propensity. In cells, a likely consequence would be the promotion of proteasomal clearance during HS recovery by preventing the formation of otherwise insoluble and potentially irreversible aggregates.
Discussion
The dynamic nature of protein post-translational modifications makes them particularly well suited to regulate cellular processes during stress responses, as they can act rapidly in response to stimuli and their reversible nature makes them suitable to act in a transient manner. Here, we have explored the enigmatic role of SUMO signaling in the HSR and identified a unique subset of HS-induced SUMO2/3 targets preferentially regulated by the proteostasis network.
The precise mechanism for enhanced SUMOylation upon heat stress remains to be investigated in detail. Pinto and co-workers have shown that heat stress inactivates SUMO proteases (Pinto et al., 2012). Our data recapitulate this observation, as we observed the inactivation of SENP1 and SENP3 in response to HS (Figure S4B). Whether prolonged inactivation of SUMO proteases is responsible for prolonged increases of SUMOylation upon heat stress combined with chronic HSF1 inhibition is less clear, as our data did not convincingly show an HSF1-dependent inactivation of SENPs (Figure S4B). Alternatively, prolonged increases in the activity of the SUMO conjugation machinery could explain prolonged increases of SUMOylation upon heat stress combined with chronic HSF1 inhibition. If this is the case, then we would expect higher auto-SUMOylation activity of SUMO E3 ligases (Zhou et al., 2004). SUMOylation of PIAS family members PIAS4 and MMS21 did increase in response to chronic HSF1 inhibition, as shown by mass spectrometry. We confirmed this result for MMS21 by immunoblotting (Figure S3A). We, therefore, expect, that PIAS4 and MMS21 could mechanistically contribute to enhanced SUMOylation upon chronic HSF1 inhibition.
Conjugation of SUMO2/3 May Act as an Early Responder to Misfolded Proteins during Heat Shock
The key function of the transcription factor HSF1 is to activate genes encoding chaperones and other proteostasis factors required to address protein misfolding induced by increased temperature or other stressors (Anckar and Sistonen, 2011). The transcriptional nature of this response results in a time gap between the rapid onset of proteotoxic stress and the availability of sufficient amounts of new chaperones to address the misfolded proteins (Khalil et al., 2006). Given that SUMOylation accumulates rapidly after onset of HS and the extensive use of SUMO as a linear fusion to recombinant proteins to enhance solubility (Butt et al., 2005, Marblestone et al., 2006), we hypothesized that SUMOylation could potentially be functioning to deter aggregation during early stages of HS.
We explored the effect of SUMOylation on the solubility of target proteins upon HS in vitro and established that SUMOylation can have a profoundly solubilizing effect on its target proteins. Given the low substrate occupancy of SUMO2/3 on target proteins, it is unlikely that SUMOylation in vivo globally shifts the aggregation propensity of the total pool for its targets. Rather, these data suggest that SUMOylation may function to survey and tag unfolded proteins at particular risk for aggregation and facilitate their degradation through subsequent ubiquitination and proteasomal degradation. N-Linked glycosylation in the endoplasmic reticulum is also known to enhance solubility and facilitate interactions with protein-folding machinery (Helenius and Aebi, 2004), suggesting that cells commonly use post-translational modifications to support proteostasis during stress conditions. These results, therefore, provide a possible method for cells to address the time-gap between stress onset and increased chaperone levels by acting as an early-response factor that can act rapidly to keep unfolded proteins soluble and prevent their accumulation into insoluble aggregates.
Mechanistically, it has been shown previously that the SUMO conjugation machinery recognizes disordered regions in target proteins (Hendriks et al., 2017). As proteins that are unfolded will display increased levels of disordered regions, direct recognition of these regions by the SUMO conjugation machinery may provide a mechanism for this machinery to focus on unfolded target proteins. Under normal proteostasis conditions, SUMO mostly targets lysines, which are located in a SUMOylation consensus motif (ψKxE, where ψ is a large hydrophobic amino acid). SUMOylation was reported to become more promiscuous during HS, targeting lysines that are not located in the consensus motif and demonstrating the possibility of a more general protective purpose for SUMOylation during HS (Hendriks et al., 2014).
Our results indicate that both SUMO2/3 and Ub are involved in the HSR. Whereas Ub has been named after its “ubiquitous” cellular levels (Cook and Chock, 1988), SUMO has previously been considered to be a low-abundance protein (Wilson and Heaton, 2008). Our data thus pose the challenge as to why a low-abundance protein modifier would be used to deal with extensive protein misfolding. Proteomics methods have been developed that can determine the copy numbers of proteins in cells (Nagaraj et al., 2011). The authors have shown that Ub is indeed one of the most abundant cellular proteins, with over 14 million copies per cell, ranking as the 10th most abundant cellular protein. Intriguingly, they found nearly 9 million copies of SUMO2 per cell, ranking SUMO2 as the 20st most abundant cellular protein. Thus, the difference in overall copy number per cell between Ub and SUMO2 is small. Additionally, given that Ub is present throughout the cell, whereas SUMO2 is predominantly located in the nucleus, nuclear SUMO2 and Ub levels are expected to be rather similar. Therefore, SUMO2 should also be considered to be an abundant protein, making it a suitable early-response factor.
Despite the high abundance of SUMO and global SUMO conjugation within the nucleus, especially after HS, only a small fraction of a specific protein pool is targeted for SUMOylation. Whereas the SUMO- and Ub-modified versions of target proteins are degraded by the proteasome, we did not observe that proteasomal inhibition affected the total pool of these targets, due to the substoichiometric nature of the modifications. We did, however, observe a clear stabilization of the SUMOylated and ubiquitinated version of selected targets in our bortezomib experiments (Figures 3 and S5). This finding is in line with earlier observations in the Ub field indicating that the ubiquitinated portion of many proteins increased in response to proteasomal inhibition without noticeable changes in total levels of these proteins (Kim et al., 2011).
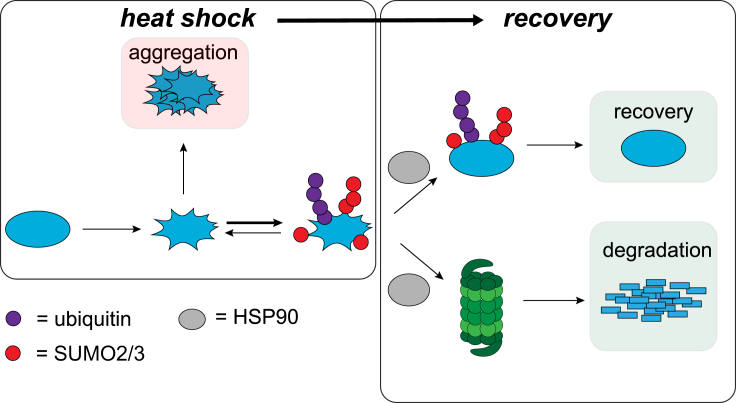
Because SUMO modifications are not known to directly target proteins to the proteasome for degradation, we consider it likely that subsequent ubiquitination is required for target protein degradation. Therefore, early conjugation of SUMO2/3 and subsequent ubiquitination work in concert to likely minimize aggregation of misfolded proteins by ensuring solubility and also by promoting degradation, respectively (Figure 7).
Figure 7.
SUMO2/3 Plays a Key Role as an Integrated Component of the Proteotoxic Stress Response
Upon HS, a transient increase in SUMO2/3 conjugation to target proteins is observed, which may function in part to prevent irreversible aggregation of target proteins (reduced aggregation was demonstrated in vitro). SUMO2/3-modified proteins are frequently co-modified by Ub, and a large fraction of these co-modified proteins are subsequently degraded by the proteasome during HS recovery in a chaperone-dependent manner.
Proteostasis Factors Facilitate Proteasomal Degradation of Co-modified Proteins
In the absence of HSF1, prolonged retention of SUMO2/3 during HS recovery indicates that proteostasis factors are required to resolve irreversible folding problems. Although SUMO2/3 might be able to act as an early-response factor to prevent the aggregation of misfolded proteins, this action is apparently insufficient for refolding of the majority of proteins involved. Dissolution of misfolded proteins can either be carried out via refolding of the misfolded proteins or via degradation by the Ub-proteasome system. The pronounced effects of proteasome inhibition on overall SUMO2/3 conjugate levels during HS recovery indicates that a majority of SUMO2/3 targets are degraded. Refolding of SUMOylated misfolded proteins by the chaperones, thus, appears to not be very successful. Nevertheless, chaperones, especially HSP90, appear to be needed to facilitate the proteasomal degradation of misfolded proteins. Whether this degradation is mediated by HSP90 directly via either shuttling co-modified proteins to the proteasome or an effect of HSP90 on the activity of the proteasome itself remains to be resolved. Both scenarios seem possible, as chaperones have been identified as proteasome-interacting factors (Guerrero et al., 2006). Of note, chaperones are predominantly located in the cytoplasm, indicating the limited availability of chaperones for the regulation of nuclear proteins. In contrast, SUMO2/3 are predominantly located in the nucleus and could, therefore, potentially compensate for the limited availability of chaperones in the nucleus at the early phase of the HSR.
Chaperone-dependent degradation or refolding both prevent the formation of protein aggregates that are linked to many diseases, including aging-related neurodegeneration (Balchin et al., 2016, Kopito, 2000). Once aggregates have formed, they appear to be resistant to protein degradation and possess a high propensity to self-propagate and accrue in inclusion bodies (Jucker and Walker, 2013). Interestingly, SUMO has been found in disease-associated protein aggregates (Liebelt and Vertegaal, 2016), suggesting a possible role in responding to or modifying aggregation-prone proteins. Our findings demonstrate clear connections between a functional proteostasis network and the post-translational modifications of Ub and SUMO2/3. Further improving our understanding of these connections might aid the development of treatment strategies for diseases characterized by the accumulation of toxic protein aggregates.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| SUMO2/3 mouse monoclonal | Abcam | Cat# ab81371; RRID:AB_1658424 |
| SUMO2/3 mouse monoclonal | University of Iowa | 8A2: RRID: AB_2198421 |
| HSP40 rabbit polyclonal | Enzo | Cat# ADI-SPA-400; RRID:AB_10631418 |
| HSP70 rabbit polyclonal | Enzo | Cat# ADI-SPA-811D; RRID:AB_2621431 |
| β-Actin mouse monoclonal | Sigma | Cat# A1978; RRID:AB_476692 |
| ACF1/Baz1A rabbit polyclonal | Bethyl Laboratories | Cat# A301-318A-T; RRID:AB_937730 |
| RCH1/KPNA2 rabbit polyclonal | Bethyl Laboratories | Cat# A300-483A-T; RRID:AB_451018 |
| MMS21 rabbit polyclonal | Bethyl Laboratories | Cat# A300-129A; RRID:AB_2142840 |
| NPM 1 rabbit polyclonal | Bethyl Laboratories | Cat# A302-402A; RRID:AB_1907285 |
| SENP1 rabbit monoclonal | Abcam | Cat# ab108981; RRID:AB_10862449 |
| SENP3 rabbit monoclonal | Cell Signaling Techology | Cat# 5591P; RRID:AB_10695540 |
| SETDB1 rabbit monoclonal | Cell Signaling Techology | Cat# 2196S; RRID:AB_823637 |
| SENP6 mouse monoclonal | Sigma | Cat # WHP0026054M1; RRID:AB_1843525 |
| FoxM1 | Santa Cruz | Cat# sc-502; RRID:AB_631523 |
| Ubiquitin Mouse monoclonal | Millipore | Cat# 04-263; RRID:AB_612093 |
| Ubiquitin | Santa Cruz | Cat# sc-8017; RRID:AB_628423 |
| Bacterial and Virus Strains | ||
| BL21(DE3) | New England BioLabs | Cat# C2527I |
| Chemicals, Peptides, and Recombinant Proteins | ||
| STA-9090 | MedChem Express | Cat# HY-15205 |
| Doxycyline | Alfa Aesar | Cat# J63805 |
| Trimethoprim | Matrix | Cat# 058373 |
| Bortezomib | ChemGood | Cat# C-1279 |
| MG-132 | Fischer Scientific | Cat# NC9326288 |
| Resazurin Sodium Salt | Sigma | Cat# R7017-5G |
| Rho-SUMO2-PA | (Mulder et al., 2018) | N/A |
| K11 di-SUMO VA ABP | (Mulder et al., 2018) | N/A |
| Critical Commercial Assays | ||
| 4–12% Bolt™ Bis–Tris | ThermoFisher | NW04125BOX |
| NuPage™ 3–8% Tris-acetate | ThermoFisher | EA03755BOX |
| EZNA Total RNA Kit I | Omega | Cat# R6834-02 |
| High-Capacity cDNA Reverse Transcription Kit | Applied Biosystems | Cat# 4368814 |
| Fast Start Universal SYBR Green Master Mix | Roche | Cat# 4913850001 |
| Deposited Data | ||
| ProteomeXchange (via PRIDE database) | This paper | PXD010231 |
| Experimental Models: Cell Lines | ||
| Human: HEK293T-REx | Life Technologies | Cat# R71007 |
| Human: LX2 | EMD Millipore | Cat# SCC064; RRID:CVCL_5792 |
| Recombinant DNA | ||
| pET-15b | Novagen | Cat# 69661 |
| pET11a-SUMO3 | (Flotho et al., 2012) | Addgene Plasmid #53143 |
| pDEST17 | Invitrogen | Cat# 11803012 |
| pT-E1E2S2 | (Uchimura et al., 2004) | N/A |
| Lentiviral His10-SUMO2 IRES GFP | (Hendriks and Vertegaal, 2016) | N/A |
| Lentiviral His10-Ubiquitin IRES Puro | (Cuijpers et al., 2017) | N/A |
| pLenti CMV/TO Zeo dn-cHSF1 | (Moore et al., 2016) | N/A |
| pLenti CMV/TO Zeo GFP | (Moore et al., 2016) | N/A |
| pLenti CMV Puro DHFR.dn-cHSF1 | (Moore et al., 2016) | N/A |
| Software and Algorithms | ||
| MaxQuant ver 1.5.6.0 | Max Planck Institute | http://www.biochem.mpg.de/5111795/maxquant |
| Perseus Software ver. 1.5.6 | Max Planck Institute | http://www.biochem.mpg.de/5111810/perseus |
| PANTHER | Gene Ontology Consortium | http://pantherdb.org/ |
| Image Studio Lite ver 5.2 | Li-COR | https://www.licor.com/bio/products/software/ |
| GraphPad Prism 7 | GraphPad | https://www.graphpad.com |
| ImageJ | Fiji | https://imagej.net/Welcome |
| Other | ||
| Triple-disc C18 reverse phase stage-tips | (Rappsilber et al., 2007) | N/A |
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Matthew D. Shoulders (mshoulde@mit.edu).
Experimental Model and Subject Details
Human cell lines
HEK293T-REx cells (human embryonic kidney, female) and LX2 cells (human hepatic stellate, male) were cultured at 37°C in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin/glutamine in a 5% CO2 atmosphere.
Environmental Stress Conditions
All heat shock experiments were performed at 43°C and 5% CO2 for 75 min. For the recovery post-heat shock, cells were kept at 37°C and 5% CO2 for the indicated time periods.
Method Details
Lentivirus
Stable HEK293T-REx cells containing the Dox-inducible dn-cHSF1 and constitutively expressing FKBP.cHSF1 were prepared as previously described (Moore et al., 2016). Lentiviruses encoding His10-SUMO2-WT-IRES-GFP (Hendriks and Vertegaal, 2016) or His10-ubiquitin-WT-IRES-puro were transduced with an MOI (multiplicity of infection) of 3 using the third- generation lentiviral system into HEK293T-REx cells stably expressing both FKBP.cHSF1 and Dox-inducible dn-cHSF1 (Moore et al., 2016). His10-SUMO2-WT-IRES-GFP expressing cells were sorted by fluorescence-aided cell sorting (FACS) on the BD FACSAriaIII™ cell sorter for low GFP expression to limit His10-SUMO2 expression. His10-ubiquitin-WT-IRES-PURO expressing cells were selected by supplementing the cell medium with 1μg/ml puromycin.
Immunoblotting Analysis
Cells were lysed in SNTBS buffer consisting of 2% SDS, 1% N-P40, 50 mM TRIS at pH 7.5, and 150 mM NaCl for whole lysate analysis of HEK293T-REx and LX2 cells. Proteins were separated by a 4%–10% SDS-PAGE gel then transferred to a nitrocellulose membrane. Pulldown elutions and inputs were separated on either 4%–12% Bolt™ Bis-Tris or NuPage™ 3%–8% Tris-acetate (anti-Baz1A) gradient gels. Following a 30 min blocking step in 5% milk, blots were incubated overnight with the appropriate primary antibodies and for 1 hr with the appropriate 680 or 800 nm fluorophore labeled secondary antibodies from Li-COR Biosciences or HRP-conjugated goat anti-mouse IgG, HRP-conjugated donkey anti-rabbit IgG from Pierce. Detection was performed on a Li-COR Biosciences Odyssey Imager for fluorophore-coupled secondary antibodies and with Pierce™ ECL Plus Western Blotting substrate for HRP-coupled secondary antibodies. Band quantification was performed in Image Studio Lite or ImageJ (Fiji).
Quantitative RT-PCR
The relative mRNA expression levels of selected heat shock response genes were measured using quantitative RT-PCR. HEK293T-REx cells expressing DHFR.dn-cHSF1 were treated with 10 μM TMP for 0, 2, 4, or 8 hr prior to challenge with a heat shock at 43°C for 75 min. RNA was extracted using the EZNA Total RNA Kit I (Omega). qRT-PCR reactions were performed on cDNA prepared from 1000 ng of total cellular RNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The Fast Start Universal SYBR Green Master Mix (Roche) and appropriate primers purchased Sigma were used for amplifications (6 min at 95°C then 45 cycles of 10 s at 95°C, 30 s at 60°C) in a Light Cycler 480 II Real-Time PCR machine. The primers used for DNAJB1 were 5′-TGTGTGGCTGCACAGTGAAC-3′ (forward) and 5′-ACGTTTCTCGGGTGTTTTGG-3′ (reverse), primers for HSPA1A were 5′-GGAGGCGGAGAAGTACA-3′ (forward) and 5′- GCTGATGATGGGGTTACA-3′ (reverse), primers for HSP90AA1 were 5′-GATAAACCCTGACCATTCC −3′ (forward) and 5′-AAGACAGGAGCGCAGTTTCATAAA-3′ (reverse) and primers for RPLP2 5′-CCATTCAGCTCACTGATAACCTTG-3′ (forward) and 5′-CGTCGCCTCCTACCTGCT-3′ (reverse). Transcripts were normalized to the housekeeping genes RPLP2 and all measurements were performed in technical triplicate. Data were analyzed using the LightCycler® 480 Software, Version 1.5 (Roche) and data are reported as the mean ± 95% confidence intervals.
Resazurin Assay
HEK293T-REx cells expressing dn-cHSF1 were plated at 5,000 cells/well in a 96-well black bottom plate. Cells were subsequently treated with either Dox or DMSO for 48 hr prior to HS. Cells were exposed to HS at 43°C for 75 min before returning to 37°C for recovery. At the indicated time points post recovery, 15.00 μL of a 0.10 mg/mL solution of resazurin (sodium salt) was added to wells. Reduction of resazurin was monitored by fluorescence on a Gen5 plate reader (λex: 530, λem: 590) following a 2-hr incubation at 37°C. Fold change in metabolic activity was determined from the non-heat shocked controls within each treatment condition.
Isolation of His10-SUMO2 and His10-Ub substrates
Purification of SUMOylated and ubiquitinated proteins was performed as described previously (Hendriks and Vertegaal, 2016). In short, cells expressing His10-SUMO2 or His10-Ub were washed three time in ice-cold PBS, and then a small fraction was used as an input control lysed in SNTBS lysis buffer (2% SDS, 1% NP-40, 50 mM Tris pH 7.5, 150 mM NaCl) while the remaining cells were lysed in denaturing lysis buffer containing 6 M guanidine-HCl, 100 mM Na2HPO4/NaH2PO4 and 10 mM TRIS, buffered at pH 8. Lysates were normalized for protein content, supplemented with 10 mM imidazole pH 8 and 5 mM β-mercaptoethanol and subsequently incubated with 20 μl dry volume Ni-NTA agarose beads/ml lysate overnight at 4°C. Beads were subsequently washed with wash buffers 1 to 4. Wash buffer 1 contained 6M guanidine-HCL, 100 mM Na2HPO4/NaH2PO4, 10 mM Tris pH 8, 10 mM imidazole pH 8, 0.2% Triton X-100 and 5 mM β-mercaptoethanol. Wash buffer 2 contained 8 M urea, 100 mM Na2HPO4/NaH2PO4, 10 mM Tris pH 8, 10 mM imidazole pH 8, 0.2% Triton X-100 and 5 mM β-mercaptoethanol. Wash buffer 3 contained 8 M urea, 100 mM Na2HPO4/NaH2PO4, 10 mM Tris pH 6.3, 10 mM imidazole pH 7, 0.2% Triton X-100 and 5 mM β-mercaptoethanol. Wash buffer 4 contained 8 M urea, 100 mM Na2HPO4/NaH2PO4, 10 mM Tris pH 6.3, 0.1% Triton X-100 and 5 mM β-mercaptoethanol. For purifications which were subsequently analyzed by mass spectrometry, wash buffer 2 contained only 0.1% Triton X-100 and Triton X-100 was left out of wash buffers 3 and 4. Washes were performed for 15 min at room temperature and wash 4 was performed twice. His10-tagged proteins were eluted twice with 7 M urea, 100 mM Na2HPO4/NaH2PO4, 10 mM Tris pH 7 and 500 mM imidazole. For mass spectrometry, elutions were concentrated by using a 100k cut-off filter and diluted with ammonium bicarbonate (ABC) to a concentration of 50 mM. Samples were reduced with dithiothreitol (DTT) and alkylated with cloroacetamide (CAA). Eluted fractions were further diluted with 50 mM ABC to attain a final urea concentration of 2 M. Proteins were digested with sequencing grade trypsin in a 1:50 enzyme-to-protein ratio overnight at 25°C. Peptides were acidified with 2% trifluoroacedic acid (TFA). The peptide samples were subsequently desalted and concentrated using homemade reversed phase StageTips. For the StageTips, three layers of C18 matrix was gently pushed into filter-less 200 μl pipet tips. The matrix was activated by HPLC-grade methanol, subsequently washed twice with 80% acetonitrile (ACN) in 0.1% formic acid (FA) and equilibrated with 0.1% FA. Sample was passed through the StageTip and was subsequently washed twice with 0.1% FA and centrifuged to complete dryness.(Rappsilber et al., 2007). Peptides were eluted twice from StageTips using 40% acetonitrile (ACN) in 1% formic acid (FA) and 60% ACN in 1% FA, respectively.
Mass Spectrometry Data Collection and Analysis
Four biological replicates were measured in technical duplicates by nanoflow liquid chromatography-tandem mass spectrometry (nanoLC-MS/MS) on a Q-Exactive Orbitrap (ThermoFisher). Raw data were processed using MaxQuant (1.5.6.0) software. MaxQuant analysis was carried out as previously described (Hendriks and Vertegaal, 2016). Default settings of MaxQuant software were employed with the following changes: “Label-free quantification (LFQ)” and “Match between runs” were enabled. All settings of MaxQuant parameters can be found via the PRIDE repository and dataset identifier PXD010231. Identified proteins were statistically analyzed using Perseus Software (version 1.5.6). The same experimental conditions of four biological replicates were grouped and LFQ intensities were log2-transformed. Protein groups that were identified in at least three biological replicates in at least one group were selected for further analysis. Missing values were imputed by the software using normally distributed values with a 1.8 (log2) downshift and a 0.3 (log2) width based on total matrix values. Differences in LFQ intensities between groups were analyzed by a two-sample t test. Proteins were selected based on their fold change and p value. Protein groups identified in the His10-SUMO2 dn-cHSF1 or GFP cell lines, which did not show a significant change in LFQ intensities (p value ≤ 0.05) in at least one of the experimental conditions compared to the appropriate parental cell lines, were excluded. Heat shock-responsive SUMOylation of proteins was defined by a ≥ 2-fold increase in abundance post-heat shock compared to no heat shock controls with a p value ≤ 0.05, in both vehicle and Dox-treated conditions. Proteins that recovered to normal SUMOylation levels post-heat shock were selected based on a ≥ 2-fold decrease 4 hr post-heat shock compared to immediately post-heat shock with a p value ≤ 0.05. Proteins that retained SUMOylation during heat shock recovery owing to chronic HSF1 inhibition were selected if they showed ≥ 2-fold enrichment in the Dox-treated condition 4 hr post-heat shock condition compared to the vehicle-treated control condition 4 hr post-heat shock with a p value ≤ 0.05 in addition to no significant difference under the same condition in the inducible GFP cell line. Gene Ontology enrichment analysis was performed on 125 proteins retained during heat shock recovery after chronic HSF1 inhibition using the Gene Ontology Consortium PANTHER Overrepresentation test (release date 2017-12-05) with the annotation version that was released on 2017-11-12.
Cloning, expression and purification of SUMO-MMS21
PCR amplifications of the cDNA coding region of human MMS21 (Dharmacon OHS1770-202324443) and SUMO3 (Addgene #53143) were cloned into pET-15b vectors containing a 6 × -His tag for purification. BL21(DE3) E.coli were transformed with these plasmids, and expression was induced overnight at 16°C by the addition of isopropyl β-D-1-thiogalactopyranoside (IPTG). Bacteria were lysed by sonication in lysis buffer containing 50 mM Tris, 300 mM NaCl, 10 mM imidazole, 5 mM DTT, 1 mM PMSF, 1 mg/mL lysozyme, 10% glycerol, at pH 7.8. Recombinant His6-SUMO3-MMS21 and His6-MMS21 were purified by Ni-NTA beads and eluted using 250 mM Imidazole (pH 7.8).
SUMOylation and purification of recombinant FOXM1
Plasmids containing His6-FoxM1 (pDEST17) and SUMO2+SUMOylation machinery (pT-E1E2S2) (Uchimura et al., 2004) were transfected or co-transfected into BL21 E.coli. Expression of proteins was induced overnight at 25°C by the addition of isopropyl β-D-1-thiogalactopyranoside (IPTG). Bacteria were lysed in 50mM HEPES (pH 7.6), 0.5 M NaCl. 25 mM MgCl2, 50% glycerol, 10% NP-40, 20 mM Imidazole (pH 8), 1 mM PMSF, 1/10ml protease inhibitor cocktail tablets, EDTA-free (SIGMA). Recombinant His6-FoxM1 was purified using TALON metal affinity resin and eluted from beads with 400 mM imidazole (pH 8).
Thermal Shift Analyses
Recombinant MMS21 or FoxM1 was aliquoted into 11 or 16 samples of 50 μl each and incubated at increasing temperatures from 30–70°C for 5 min using gradient settings on a Bio-Rad C1000™ Thermal Cycler or a Veriti ™ 96-Well Thermal Cycler. For the experiments described in Figure 6C, FoxM1 was aliquoted into samples of 50 μl and incubated for the indicated time periods at the indicated temperatures using a thermomixer (Eppendorf). Samples were left to cool down at RT for 3 min then 4°C for 3 min. 40 μl aliquots were transferred to a micro-centrifuge tube and centrifuged at 20000 x g for 20 min. 25 μl of the soluble fractions were used for immunoblotting analysis.
SENP labeling with activity-based probes (ABPs)
HEK293T-REx cells stably expressing Dox-inducible dn-cHSF1 were treated with Dox for 48 hr prior to heat shock. Cell were exposed to heat shock at 43°C for 75 min before returning to 37°C for a 2 hr recovery period. Cells were lysed prior to heat shock, immediately after heat shock, or after 2 hr of recovery. After washing with PBS, cell pellet was split into two. Cell lysates were prepared by resuspending cell pellets in two pellet volumes of lysis buffer (containing 0.25 M sucrose, 20 mM Mops-KOH (pH = 7.4), 1 mM EDTA-NaOH, 1 tablet/10 mL complete mini protease inhibitor cocktail (Sigma), 1 mM DTT) or lysis buffer containing 20 mM N-ethylmaleimide (NEM) and lysed by sonication. After centrifugation (20,000 × g, 4°C, 5 min), total protein concentration in the supernatant was determined by Bradford. 100 μl of supernatant (∼200 μg total protein) was labeled with either 8 μg of Rho-SUMO2-PA or K11 di-SUMO VA ABP for 30 min at 37°C. The reaction was terminated by the addition of NuPage LDS sample buffer (Invitrogen) (Mulder et al., 2018).
Quantification and Statistical Analysis
Unless otherwise indicated, experiments were performed in biological triplicate. Results are presented as mean ± standard deviation. Significance for immunobloting quantification was determined using ANOVA analysis followed by post hoc Tukey analysis (∗ = p < 0.05; ∗∗∗ = p < 0.0005). For analysis of data from the mass spectrometry experiments, differences in LFQ intensities between treatment groups were analyzed by a two-sample t test. Information on biological replicates and statistical significance is included in the figure legends.
Data and Software Availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PRIDE: PXD010231.
Acknowledgments
We thank Dr. R. González-Prieto for assistance with mass spectrometry. The laboratory of A.C.O.V. is supported by the European Research Council (ERC; grant 310913) and the Netherlands Organisation for Scientific Research (NWO; grant 724.016.003). This work was also supported by the National Institute on Aging (NIH grant 5R21AG054961 to M.D.S.) and the National Institute of Environmental Health Sciences (NIH award P30-ES002109). C.L.M. was supported by an NSF Graduate Research Fellowship.
Author Contributions
A.C.O.V. and M.D.S. conceived the project; F.L., R.M.S., C.L.M., A.C.O.V., and M.D.S. designed experiments; F.L., R.M.S., and C.L.M. conducted experiments; M.P.C.M. and H.O. provided key reagents; F.L., R.M.S., A.C.O.V., and M.D.S. drafted the manuscript. All authors analyzed data and edited the manuscript.
Declaration of Interests
H.O. is a co-founder and shareholder of UbiQ Bio B.V., a company that markets research reagents. All other authors declare no competing interests.
Published: January 2, 2019
Footnotes
Supplemental Information includes six figures and four tables and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.12.027.
Contributor Information
Matthew D. Shoulders, Email: mshoulde@mit.edu.
Alfred C.O. Vertegaal, Email: vertegaal@lumc.nl.
Supplemental Information
References
- Åkerfelt M., Morimoto R.I., Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anckar J., Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu. Rev. Biochem. 2011;80:1089–1115. doi: 10.1146/annurev-biochem-060809-095203. [DOI] [PubMed] [Google Scholar]
- Balchin D., Hayer-Hartl M., Hartl F.U. In vivo aspects of protein folding and quality control. Science. 2016;353:aac4354. doi: 10.1126/science.aac4354. [DOI] [PubMed] [Google Scholar]
- Berkers C.R., Verdoes M., Lichtman E., Fiebiger E., Kessler B.M., Anderson K.C., Ploegh H.L., Ovaa H., Galardy P.J. Activity probe for in vivo profiling of the specificity of proteasome inhibitor bortezomib. Nat. Methods. 2005;2:357–362. doi: 10.1038/nmeth759. [DOI] [PubMed] [Google Scholar]
- Brunet Simioni M., De Thonel A., Hammann A., Joly A.L., Bossis G., Fourmaux E., Bouchot A., Landry J., Piechaczyk M., Garrido C. Heat shock protein 27 is involved in SUMO-2/3 modification of heat shock factor 1 and thereby modulates the transcription factor activity. Oncogene. 2009;28:3332–3344. doi: 10.1038/onc.2009.188. [DOI] [PubMed] [Google Scholar]
- Butt T.R., Edavettal S.C., Hall J.P., Mattern M.R. SUMO fusion technology for difficult-to-express proteins. Protein Expr. Purif. 2005;43:1–9. doi: 10.1016/j.pep.2005.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J., Chock P.B. Ubiquitin: a review on a ubiquitous biofactor in eukaryotic cells. Biofactors. 1988;1:133–146. [PubMed] [Google Scholar]
- Cuijpers S.A.G., Willemstein E., Vertegaal A.C.O. Converging small ubiquitin-like modifier (SUMO) and ubiquitin signaling: improved methodology identifies co-modified target proteins. Mol. Cell. Proteomics. 2017;16:2281–2295. doi: 10.1074/mcp.TIR117.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotho A., Melchior F. Sumoylation: a regulatory protein modification in health and disease. Annu. Rev. Biochem. 2013;82:357–385. doi: 10.1146/annurev-biochem-061909-093311. [DOI] [PubMed] [Google Scholar]
- Flotho A., Werner A., Winter T., Frank A.S., Ehret H., Melchior F. Recombinant reconstitution of sumoylation reactions in vitro. Methods Mol. Biol. 2012;832:93–110. doi: 10.1007/978-1-61779-474-2_5. [DOI] [PubMed] [Google Scholar]
- Geiss-Friedlander R., Melchior F. Concepts in sumoylation: a decade on. Nat. Rev. Mol. Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- Golebiowski F., Matic I., Tatham M.H., Cole C., Yin Y., Nakamura A., Cox J., Barton G.J., Mann M., Hay R.T. System-wide changes to SUMO modifications in response to heat shock. Sci. Signal. 2009;2:ra24. doi: 10.1126/scisignal.2000282. [DOI] [PubMed] [Google Scholar]
- Guerrero C., Tagwerker C., Kaiser P., Huang L. An integrated mass spectrometry-based proteomic approach: quantitative analysis of tandem affinity-purified in vivo cross-linked protein complexes (QTAX) to decipher the 26 S proteasome-interacting network. Mol. Cell. Proteomics. 2006;5:366–378. doi: 10.1074/mcp.M500303-MCP200. [DOI] [PubMed] [Google Scholar]
- Hastings N.B., Wang X., Song L., Butts B.D., Grotz D., Hargreaves R., Fred Hess J., Hong K.K., Huang C.R., Hyde L. Inhibition of O-GlcNAcase leads to elevation of O-GlcNAc tau and reduction of tauopathy and cerebrospinal fluid tau in rTg4510 mice. Mol. Neurodegener. 2017;12:39. doi: 10.1186/s13024-017-0181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- Hendriks I.A., Vertegaal A.C.O. Label-free identification and quantification of SUMO target proteins. Methods Mol. Biol. 2016;1475:171–193. doi: 10.1007/978-1-4939-6358-4_13. [DOI] [PubMed] [Google Scholar]
- Hendriks I.A., D’Souza R.C.J., Yang B., Verlaan-de Vries M., Mann M., Vertegaal A.C.O. Uncovering global SUMOylation signaling networks in a site-specific manner. Nat. Struct. Mol. Biol. 2014;21:927–936. doi: 10.1038/nsmb.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks I.A., Lyon D., Young C., Jensen L.J., Vertegaal A.C.O., Nielsen M.L. Site-specific mapping of the human SUMO proteome reveals co-modification with phosphorylation. Nat. Struct. Mol. Biol. 2017;24:325–336. doi: 10.1038/nsmb.3366. [DOI] [PubMed] [Google Scholar]
- Henley J.M., Craig T.J., Wilkinson K.A. Neuronal SUMOylation: mechanisms, physiology, and roles in neuronal dysfunction. Physiol. Rev. 2014;94:1249–1285. doi: 10.1152/physrev.00008.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietakangas V., Ahlskog J.K., Jakobsson A.M., Hellesuo M., Sahlberg N.M., Holmberg C.I., Mikhailov A., Palvimo J.J., Pirkkala L., Sistonen L. Phosphorylation of serine 303 is a prerequisite for the stress-inducible SUMO modification of heat shock factor 1. Mol. Cell. Biol. 2003;23:2953–2968. doi: 10.1128/MCB.23.8.2953-2968.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M., Walker L.C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil S., Luciano J., Chen W., Liu A.Y.-C. Dynamic regulation and involvement of the heat shock transcriptional response in arsenic carcinogenesis. J. Cell. Physiol. 2006;207:562–569. doi: 10.1002/jcp.20599. [DOI] [PubMed] [Google Scholar]
- Kim W., Bennett E.J., Huttlin E.L., Guo A., Li J., Possemato A., Sowa M.E., Rad R., Rush J., Comb M.J. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol. Cell. 2011;44:325–340. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.E., Hipp M.S., Bracher A., Hayer-Hartl M., Hartl F.U. Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 2013;82:323–355. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- Kopito R.R. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–530. doi: 10.1016/s0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- Krumova P., Weishaupt J.H. Sumoylation in neurodegenerative diseases. Cell. Mol. Life Sci. 2013;70:2123–2138. doi: 10.1007/s00018-012-1158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbadia J., Morimoto R.I. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 2015;84:435–464. doi: 10.1146/annurev-biochem-060614-033955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoliatte F., McManus F.P., Maarifi G., Chelbi-Alix M.K., Thibault P. Uncovering the SUMOylation and ubiquitylation crosstalk in human cells using sequential peptide immunopurification. Nat. Commun. 2017;8:14109. doi: 10.1038/ncomms14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebelt F., Vertegaal A.C.O. Ubiquitin-dependent and independent roles of SUMO in proteostasis. Am. J. Physiol. Cell Physiol. 2016;311:C284–C296. doi: 10.1152/ajpcell.00091.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malakhov M.P., Mattern M.R., Malakhova O.A., Drinker M., Weeks S.D., Butt T.R. SUMO fusions and SUMO-specific protease for efficient expression and purification of proteins. J. Struct. Funct. Genomics. 2004;5:75–86. doi: 10.1023/B:JSFG.0000029237.70316.52. [DOI] [PubMed] [Google Scholar]
- Marblestone J.G., Edavettal S.C., Lim Y., Lim P., Zuo X., Butt T.R. Comparison of SUMO fusion technology with traditional gene fusion systems: enhanced expression and solubility with SUMO. Protein Sci. 2006;15:182–189. doi: 10.1110/ps.051812706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotta N.P., Lin Y.H., Lewis Y.E., Ambroso M.R., Zaro B.W., Roth M.T., Arnold D.B., Langen R., Pratt M.R. O-GlcNAc modification blocks the aggregation and toxicity of the protein α-synuclein associated with Parkinson’s disease. Nat. Chem. 2015;7:913–920. doi: 10.1038/nchem.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N., Schwamborn K., Schreiber V., Werner A., Guillier C., Zhang X.D., Bischof O., Seeler J.S., Dejean A. PARP-1 transcriptional activity is regulated by sumoylation upon heat shock. EMBO J. 2009;28:3534–3548. doi: 10.1038/emboj.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C.L., Dewal M.B., Nekongo E.E., Santiago S., Lu N.B., Levine S.S., Shoulders M.D. Transportable, chemical genetic methodology for the small molecule-mediated inhibition of heat shock factor 1. ACS Chem. Biol. 2016;11:200–210. doi: 10.1021/acschembio.5b00740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder M.P.C., Merkx R., Witting K.F., Hameed D.S., El Atmioui D., Lelieveld L., Liebelt F., Neefjes J., Berlin I., Vertegaal A.C.O., Ovaa H. Total chemical synthesis of SUMO and SUMO-based probes for profiling the activity of SUMO-specific proteases. Angew. Chem. Int. Ed. Engl. 2018;57:8958–8962. doi: 10.1002/anie.201803483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj N., Wisniewski J.R., Geiger T., Cox J., Kircher M., Kelso J., Pääbo S., Mann M. Deep proteome and transcriptome mapping of a human cancer cell line. Mol. Syst. Biol. 2011;7:548. doi: 10.1038/msb.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto M.P., Carvalho A.F., Grou C.P., Rodríguez-Borges J.E., Sá-Miranda C., Azevedo J.E. Heat shock induces a massive but differential inactivation of SUMO-specific proteases. Biochim. Biophys. Acta. 2012;1823:1958–1966. doi: 10.1016/j.bbamcr.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Rappsilber J., Mann M., Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007;2:1896–1906. doi: 10.1038/nprot.2007.261. [DOI] [PubMed] [Google Scholar]
- Saitoh H., Hinchey J. Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J. Biol. Chem. 2000;275:6252–6258. doi: 10.1074/jbc.275.9.6252. [DOI] [PubMed] [Google Scholar]
- Schimmel J., Larsen K.M., Matic I., van Hagen M., Cox J., Mann M., Andersen J.S., Vertegaal A.C.O. The ubiquitin-proteasome system is a key component of the SUMO-2/3 cycle. Mol. Cell. Proteomics. 2008;7:2107–2122. doi: 10.1074/mcp.M800025-MCP200. [DOI] [PubMed] [Google Scholar]
- Seeler J.S., Dejean A. SUMO and the robustness of cancer. Nat. Rev. Cancer. 2017;17:184–197. doi: 10.1038/nrc.2016.143. [DOI] [PubMed] [Google Scholar]
- Seifert A., Schofield P., Barton G.J., Hay R.T. Proteotoxic stress reprograms the chromatin landscape of SUMO modification. Sci. Signal. 2015;8:rs7. doi: 10.1126/scisignal.aaa2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Mosser D.D., Morimoto R.I. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 1998;12:654–666. doi: 10.1101/gad.12.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn K.C., Lee K.Y., Park J.E., Do S.I. OGT functions as a catalytic chaperone under heat stress response: a unique defense role of OGT in hyperthermia. Biochem. Biophys. Res. Commun. 2004;322:1045–1051. doi: 10.1016/j.bbrc.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Tatham M.H., Matic I., Mann M., Hay R.T. Comparative proteomic analysis identifies a role for SUMO in protein quality control. Sci. Signal. 2011;4:rs4. doi: 10.1126/scisignal.2001484. [DOI] [PubMed] [Google Scholar]
- Tempé D., Piechaczyk M., Bossis G. SUMO under stress. Biochem. Soc. Trans. 2008;36:874–878. doi: 10.1042/BST0360874. [DOI] [PubMed] [Google Scholar]
- Uchimura Y., Nakamura M., Sugasawa K., Nakao M., Saitoh H. Overproduction of eukaryotic SUMO-1- and SUMO-2-conjugated proteins in Escherichia coli. Anal. Biochem. 2004;331:204–206. doi: 10.1016/j.ab.2004.04.034. [DOI] [PubMed] [Google Scholar]
- van der Veen A.G., Ploegh H.L. Ubiquitin-like proteins. Annu. Rev. Biochem. 2012;81:323–357. doi: 10.1146/annurev-biochem-093010-153308. [DOI] [PubMed] [Google Scholar]
- Vedadi M., Niesen F.H., Allali-Hassani A., Fedorov O.Y., Finerty P.J., Jr., Wasney G.A., Yeung R., Arrowsmith C., Ball L.J., Berglund H. Chemical screening methods to identify ligands that promote protein stability, protein crystallization, and structure determination. Proc. Natl. Acad. Sci. USA. 2006;103:15835–15840. doi: 10.1073/pnas.0605224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerheide S.D., Anckar J., Stevens S.M., Jr., Sistonen L., Morimoto R.I. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323:1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V.G., Heaton P.R. Ubiquitin proteolytic system: focus on SUMO. Expert Rev. Proteomics. 2008;5:121–135. doi: 10.1586/14789450.5.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M., Orosz A., Wu C. Direct sensing of heat and oxidation by Drosophila heat shock transcription factor. Mol. Cell. 1998;2:101–108. doi: 10.1016/s1097-2765(00)80118-5. [DOI] [PubMed] [Google Scholar]
- Zhou W., Ryan J.J., Zhou H. Global analyses of sumoylated proteins in Saccharomyces cerevisiae. Induction of protein sumoylation by cellular stresses. J. Biol. Chem. 2004;279:32262–32268. doi: 10.1074/jbc.M404173200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J., Guo Y., Guettouche T., Smith D.F., Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.