Summary
Ensheathment of axons by myelin is a highly complex and multi-cellular process. Cytosolic calcium (Ca2+) changes in the myelin sheath have been implicated in myelin synthesis, but the source of this Ca2+ and the role of neuronal activity is not well understood. Using one-photon Ca2+ imaging, we investigated myelin sheath formation in the mouse somatosensory cortex and found a high rate of spontaneous microdomain Ca2+ transients and large-amplitude Ca2+ waves propagating along the internode. The frequency of Ca2+ transients and waves rapidly declines with maturation and reactivates during remyelination. Unexpectedly, myelin microdomain Ca2+ transients occur independent of neuronal action potential generation or network activity but are nearly completely abolished when the mitochondrial permeability transition pores are blocked. These findings are supported by the discovery of mitochondria organelles in non-compacted myelin. Together, the results suggest that myelin microdomain Ca2+ signals are cell-autonomously driven by high activity of mitochondria during myelin remodeling.
Keywords: axon, myelin, calcium, action potential, pyramidal neuron, mouse, remyelination, mitochondria, permeability transition pore, development
Graphical Abstract
Highlights
-
•
Developing myelin sheaths show high rates of calcium transients and calcium waves
-
•
Myelin calcium transients are independent from neuronal activity
-
•
Adaxonal and paranodal myelin contained mitochondria
-
•
Calcium transients require opening of mitochondrial permeability transition pores
Using epifluorescence imaging of oligodendrocytes during myelin development and remyelination, Battefeld et al. discovered that actively myelinating sheaths have high rates of local spontaneous calcium transients and propagating calcium waves. Myelin calcium transients are generated by the spontaneous activity of mitochondria in the non-compacted myelin, independently from neuronal activity.
Introduction
The multi-lamellar myelin sheath around vertebrate axons is paramount for rapid and efficient saltatory conduction of the action potential and formed by a complex multi-cellular process (Nave and Werner, 2014). Myelination of neuronal networks in the brain develops rapidly after birth and is a lifelong process, with newly generated myelin sheaths continually integrated in the mature cortex even in mice 2 years of age (Fard et al., 2017, Hill et al., 2018, Hughes et al., 2018, Vincze et al., 2008, Young et al., 2013). Myelin develops from oligodendrocyte precursor cells (OPCs) that undergo terminal differentiation through a short intermediate phase, termed newly formed (or pre-myelinating) oligodendrocytes, and subsequently turn into mature myelinating oligodendrocytes (OLs) (Barres and Raff, 1999, Nishiyama et al., 2009, Zhang et al., 2014). Both OPC differentiation and stabilization of axo-glial contacts depend on neuronal activity and vesicle release from axons (Hines et al., 2015, Koudelka et al., 2016, Mensch et al., 2015, Wake et al., 2011). In early phases of myelin formation, glutamate release from axons triggered by neuronal activity has been shown instructive for myelin induction (Kukley et al., 2007, Stevens et al., 2002, Sun et al., 2016, Wake et al., 2011). Furthermore, there is converging evidence that the signaling pathways to increase myelin basic protein (MBP) translation and synthesis require intracellular Ca2+ changes in OPCs (Friess et al., 2016, Wake et al., 2011).
Although the mechanisms of Ca2+ signaling have been studied in OPCs (Friess et al., 2016, Haak et al., 2000, Haak et al., 2002, Kirischuk et al., 1995), little is known about Ca2+ signaling in early developing or mature myelin sheaths. Recent in vivo Ca2+ imaging of newly formed OLs in zebrafish revealed spontaneous Ca2+ events in myelin sheaths lasting 15–20 s correlated with both growth and calpain-mediated retraction of the myelin sheath (Baraban et al., 2018, Krasnow et al., 2018). Whether Ca2+ transients occur in mammalian myelin sheaths and can integrate neuronal activity at the temporal resolution of single action potentials, typically lasting only milliseconds, remains to be examined. Here, we used an OL-specific reporter mouse line to combine visually guided patch-clamp recordings with Ca2+ imaging of early developing and adult OLs in the gray matter of the somatosensory neocortex. Using a combination of electrophysiology, one-photon Ca2+ imaging with high sensitivity, electron microscopy (EM), and immunohistochemistry, we investigated Ca2+ in single OLs in the first postnatal months and characterized myelin Ca2+ transients in connected pairs of neurons and OLs. Our experiments revealed a remarkably high rate of brief (∼1 s duration) microdomain Ca2+ transients and propagating Ca2+ waves in the cytoplasmic compartments of myelin. Microdomain Ca2+ transients required the spontaneous opening of the mitochondrial permeability transition pore (mPTP) but were independent of neuronal activity. These results suggest a model in which cell autonomous mitochondrial-driven Ca2+ homeostasis regulates myelin lipid biosynthesis and energy substrate production for radial and longitudinal refinement of the myelin sheath.
Results
Radial and Longitudinal Myelin Development in the Somatosensory Cortex in the First Postnatal Weeks
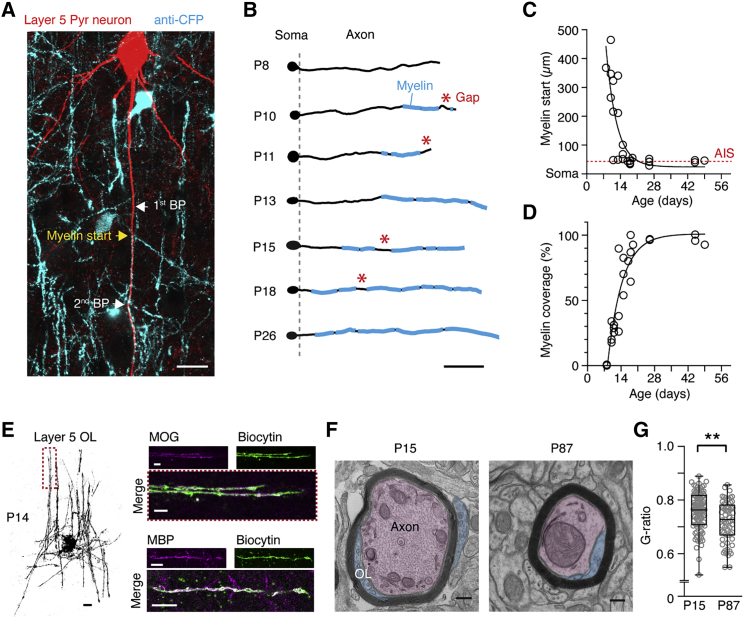
To characterize myelin development of the gray matter primary somatosensory layer 5 axons, we used a combination of single-axon tracing and myelin protein expression analysis by immunohistochemistry and EM (Figure 1). While the first myelin segments of layer 5 axons emerged near the corpus callosum at postnatal day 10 (P10), the first myelin internodes near the axon initial segment were formed approximately 1 week later (∼P17 to P18; Figures 1A–1C), consistent with previous light and electron microscopic work (Vincze et al., 2008). Although internode length was similar across all periods (Figure S1A), large gaps between myelinated segments indicated that myelination was not complete until P18 (Figures 1B and 1D). At P14, enhanced cyan fluorescent protein (ECFP)-positive OLs had ∼35 ± 8 internodes (n = 3 OLs) and expressed the myelin specific proteins myelin oligodendrocyte glycoprotein (MOG; n = 2) and MBP (n = 2; Figure 1E). At this age, axons had a significantly thinner myelin sheath (i.e., a larger g ratio of 0.76 ± 0.01) compared to those in the adult cortex (g ratio = 0.72 ± 0.01; p = 0.002, Mann-Whitney test) suggesting that radial myelin growth continues after P15 (Figures 1F and 1G). During early development, the OL resting membrane potential depolarized and input resistance decreased, reaching adult values around 4–5 weeks of age (Figures S1B–S1D). These data show that myelin wrapping around the layer 5 axon undergoes rapid longitudinal and radial refinement during the first postnatal weeks.
Figure 1.
Radial and Longitudinal Myelin Development in the Somatosensory Cortex in the First Postnatal Weeks
(A) Fluorescence image of a layer 5 neuron in the somatosensory cortex (P15) of a PLP-ECFP mouse. The neuron was filled with biocytin (red) and co-labeled for ECFP (cyan) to identify OLs and myelinated segments. Start of myelin and branchpoints (BPs) are indicated. Scale bar, 20 μm.
(B) Examples of reconstructed primary axon (black) overlaid with myelin segments (cyan). Scale bar, 100 μm. Asterisks indicate gaps larger than nodes of Ranvier.
(C) The start of the first myelinated segment moves closer to the axon initial segment (dotted line) within the third postnatal week. Data fit with a single exponential equation with a time constant of 4.4 days.
(D) Layer 5 axon myelination develops rapidly and reaches completion near P21. Data points represent individual axons and are corrected for an unmyelinated proximal length of 45 μm that includes the AIS. Data fit with a single exponential equation with a time constant of 6 days.
(E) Left: a P14 OL filled with biocytin. Right: post hoc staining of two OLs confirms that internodes are positive for MOG and MBP at P14. The dotted box (left) indicates the MOG-positive internode. Scale bars, 10 μm.
(F) Electron microscopy (EM) images of large axons in the somatosensory cortex during development (P15) and adulthood (P87). OL cytoplasmic ridges (cyan) and neuron (red) are false colored. Scale bars, 200 nm.
(G) G-ratio measurements (inner axon diameter/outer diameter including myelin) reveals an average of 0.76 ± 0.01 at P14 (n = 96 axons) and a smaller g ratio at P87 (n = 64 axons; p = 0.002, Mann-Whitney test). Horizontal bar in the 25th to 75th percentile boxplot indicates the median and the error bars the maximum and minimum.
See also Figures S1 and S3.
Myelin Ca2+ Transients and Ca2+ Waves during Early Development and Remyelination
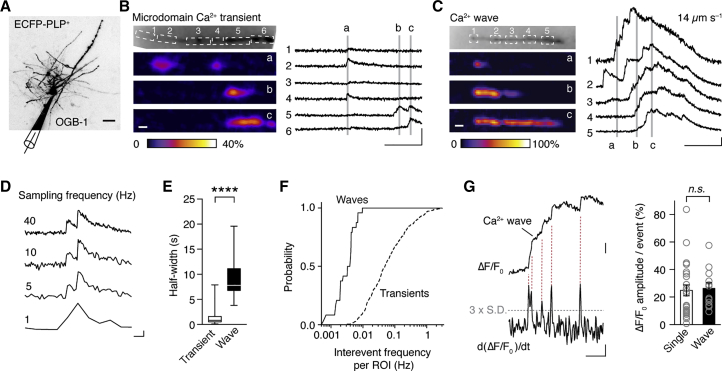
To investigate Ca2+ in myelin sheaths we made acute brain slices prepared from reporter mice expressing ECFP under the OL-specific proteolipid protein (PLP) promoter (Battefeld et al., 2016, Hirrlinger et al., 2005) and targeted single ECFP-positive OLs between P12 and P111 (Figure S2A). During whole-cell recordings of ECFP-positive OLs in layers 5 and 6, the OLs were filled with OGB-1 for ∼45 min before recording Ca2+ reporter fluorescence in internodes with a back-illuminated charge-coupled device (CCD) camera at 40 Hz (Figure 2A). Ca2+ changes appeared as transients restricted to small microdomains or as large-amplitude Ca2+ waves propagating longitudinally along the internode, clearly distinguishable with an average signal-to-noise ratio of 5.2 ± 0.6 (n = 51 events, N = 12 OLs) (Figures 2B, 2C, and S2B; Videos S1 and S2). Comparison of different sampling rates between 1 and 40 Hz revealed that higher rates were required to reliably identify the brief Ca2+ transients and analyze their properties and kinetics (Figure 2D). Although microdomain Ca2+ transients were of short half-width duration, lasting on average 1.3 ± 0.1 s, Ca2+ waves persisted significantly longer (on average 9.0 ± 0.8 s, n = 12 OLs; Figure 2E). Ca2+ transients were observed in the majority of imaged myelin sheaths (75% [160 of 216 sheaths], n = 53 OLs) and occurred ∼50 times more frequently than Ca2+ waves (10% [20 of 216 sheaths]; Figure 2F). Further analysis revealed that Ca2+ waves were composed of multiple smaller Ca2+ transients (12 of 31 waves; Figure 2G). Ca2+ waves were not observed to propagate between different myelin sheaths of the same OL, suggesting that Ca2+ activity is independently regulated within internodes. Most events decayed within 3 s, with a half maximum duration of 1.6 s and a Ca2+ increase <15% ΔF/F0 (Figures S2C–S2E). When corrected for the region of interest (ROI) size (events per micrometer), Ca2+ event probability was equally distributed along the internode (48 ± 5% at the end tips, 52 ± 5% in the rest of the internode, n = 365 events from 23 internodes of 7 OLs; p = 0.67, paired t test).
Figure 2.
High-Frequency Microdomain Ca2+ Transients and Ca2+ Waves during Early Myelin Remodeling
(A) Confocal z projection of a live-scanned OL filled with 100 μM OGB-1. Scale bar, 20 μm.
(B and C) Left: Ca2+ transients (B) and wave (C) from two different OLs (scale bar, 5 μm). Right: Ca2+ traces with corresponding ROI locations (numbers) and time points (letters) as indicated in epifluorescence images (scale bars, 5 s and 30% ΔF/F, B, and ΔF/F0, C).
(D) Comparison of different optical sampling frequencies. Scale bars, 1 s and 10% ΔF/F.
(E) Half-width duration of myelin Ca2+ events is longer in waves (n = 21) compared with transients (n = 421; p = 0.0001, Mann-Whitney test). Boxplot indicates 25th and 75th percentiles, the horizontal bar in the box indicates the median, and error bars show the maximum and minimum.
(F) Cumulative probability plot of inter-event frequencies reveals that Ca2+ transients occur 50 times more often than Ca2+ waves (transients: n = 418 events, n = 11 OLs; waves: n = 24, n = 14 OLs; p < 0.0001, Kolmogorov-Smirnov test).
(G) Left: Ca2+ wave with expanded timescale reveals a summation of brief Ca2+ transients. By using the first derivative of the fluorescence (lower trace − d[ΔF/F]/dt), peak detection could be performed when fluorescence change was fastest (peak). Several transients are indicated (vertical dotted lines) and the cut-off set as 3 × SD (horizontal dotted line). Scale bars, 10% ΔF/F0, 50% ΔF/F × s–1, and 1 s. Right: summary data comparing the predicted amplitude of Ca2+ events within a wave to individual Ca2+ transients (five first events of five control OLs; p = 0.42, Mann-Whitney test). Mean ± SEM.
See also Figure S2.
A microdomain Ca2+ transient is visible at the tip of the myelin sheath (arrow). Imaging data were overlaid with a confocal z-projected and cropped post-hoc scan at high spatial resolution (1024 × 1024 pixels; 0.207 μm/pixel). The raw imaging data were background subtracted and scaled to the maximum signal in the Video S2. Ca2+ signals were acquired at 40 Hz, played in real time for 6 s duration.
Example of a Ca2+ wave propagating along the myelin internode. In this internode, the wave initiates at the end and propagates toward the middle of the process. For display purposes, the Ca2+ imaging data were overlaid with a z-projected cropped high-resolution post-hoc confocal scan (1024 × 1024 pixels; 0.207 μm/pixel). Ca2+ signals were acquired at 40 Hz, background subtracted and scaled to the maximum intensity.
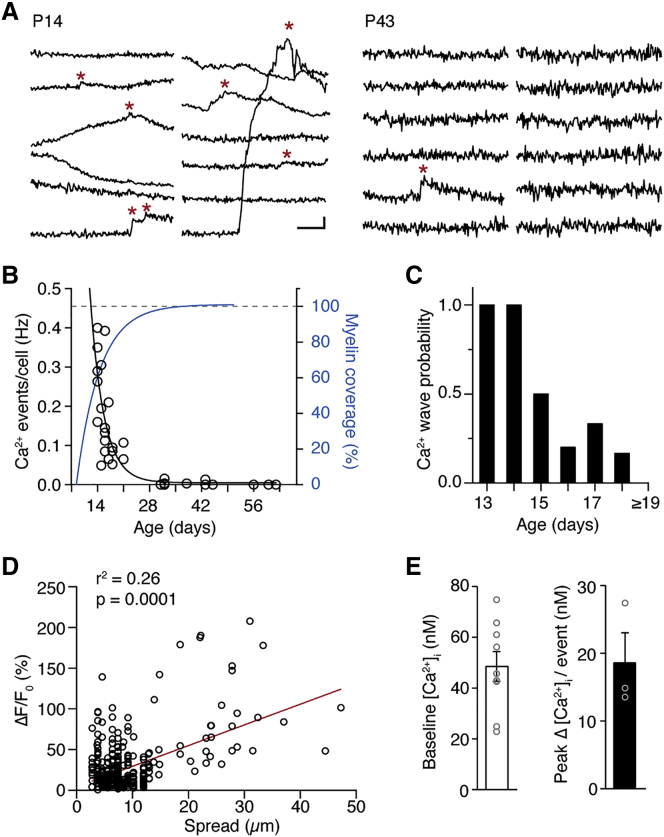
Ca2+ imaging experiments from mice between 2 weeks and 3 months of age revealed that Ca2+ event frequency was highest in the earliest detectable ECFP-positive OLs in layers 5 and 6 (P12). Ca2+ activity rapidly attenuated within the first 2 weeks, after which little to no activity could be detected within the time frame of whole-cell recording (Figures 3A and 3B). Interestingly, the rate of developmental decrease in Ca2+ events mirrored the change in myelin coverage of layer 5 pyramidal cell axons and the maturation of the intrinsic membrane properties of OLs (Figures 3B and S1). Indeed, the extent of myelin coverage was negatively correlated with the Ca2+ event frequency in the internodes (r = −0.89; Figure S3A) as well as the OL resting membrane potential, with Ca2+ event rate being highest in hyperpolarized OLs (r = −0.89; Figure S3B). Although microdomain Ca2+ transients were consistently observed between P13 and P43 (n = 32 OLs), large-amplitude Ca2+ waves occurred almost exclusively between P13 and P15 (93% [28 of 30 waves], n = 14 OLs), coinciding with a phase of rapid longitudinal remodeling of myelin (Figure 3C). Ca2+ spread along the internode was positively correlated with ΔF/F0 (Pearson r = 0.51, p = 0.0001, N = 22 OLs, n = 391 events; Figure 3D) but did not lead to membrane potential changes measured at the OL cell body (p = 0.97; Figure S3C).
Figure 3.
Ca2+ Events Attenuate during Myelin Maturation
(A) Example Ca2+ traces for two ages (P14 and P43). Note the large Ca2+ waves at P14. Scale bars, 10% ΔF/F and 1 s. Asterisks indicate Ca2+ events.
(B) Scatterplot of myelin Ca2+ events per OL (open black circles) revealing a rapid decrease until the third postnatal week. Dotted blue line represents the fit to myelin coverage of layer 5 axons (same fit as in Figure 1D).
(C) Probability of Ca2+ waves rapidly declines between P13 and P63. P13, 2 waves and 2 OLs; P14, 5 waves and 5 OLs; P15, 4 waves and 8 OLs; P16, 1 wave and 5 OLs; P17, 1 wave and 3 OLs; P18, 1 wave and 6 OLs; ≥P19, 0 waves and 13 OLs.
(D) Scatterplot of ΔF/F0 changes versus spatial spread shows a positive correlation (n = 391 events, N = 22 cells). Data fit with a linear function (red line).
(E) Left: bar plot for baseline intracellular internode Ca2+ concentration [Ca2+]i (n = 9 internodes). Right: bar plot for Ca2+ peak change during Ca2+ transients (n = 3 internodes from N = 3 animals) obtained with ratiometric Ca2+ imaging from internodes. Data are presented as mean ± SEM.
See also Figure S3.
To link the Ca2+ fluorescence changes to underlying signaling pathways, we measured the absolute baseline and spontaneous cytoplasmic Ca2+ concentration changes ([Ca2+]i) in myelin using the ratiometric Ca2+ indicator fura-2 (Figures S3D–S3F). These experiments revealed an internodal [Ca2+]i of 49 ± 6 nM at rest (nine internodes, three OLs), similar to estimates in OPCs (Friess et al., 2016), and spontaneous microdomain Ca2+ transients were producing a Δ[Ca2+]i of ∼19 nM (three internodes, two OLs; Figure 3E). On the basis of our finding that Ca2+ waves are composed of two to seven events, we estimate that Δ[Ca2+]i during Ca2+ waves rises from ∼90 to ∼200 nM, respectively, suggesting a large dynamic range in Ca2+ concentrations within the myelin sheath.
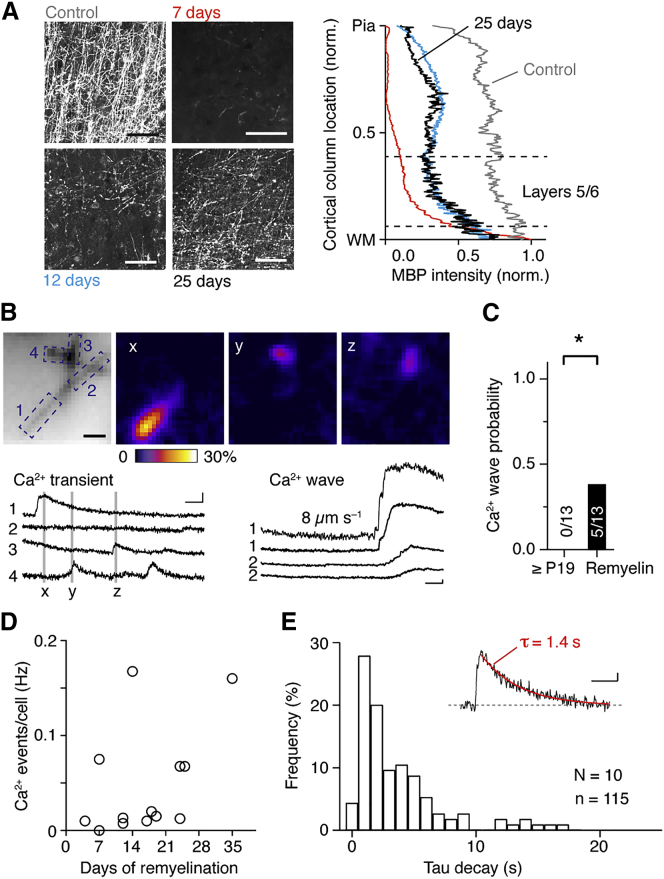
Because there is a strong age-dependent reduction in newly formed oligodendrocytes after myelin development (Fard et al., 2017), our low rate of Ca2+ events in myelin sheaths in adulthood may reflect a reduced probability to target newly formed OLs. We hypothesized that similar Ca2+ dynamics as observed during myelin maturation should occur during remyelination in adulthood. To test this, we used the cuprizone model, which effectively demyelinates hundreds of micrometers of the proximal part of the primary layer 5 axon after a 5 week cuprizone diet (Hamada and Kole, 2015), and then induced remyelination by terminating the cuprizone diet. Remyelination was variable across the cortical layers and still ongoing after 25 days of remyelination (Figure 4A), in line with previous data (Baxi et al., 2017, Clarner et al., 2012). We recorded from newly generated ECFP-expressing OLs in layers 5 and 6 and imaged internodal Ca2+ between 4 and 35 days of the remyelination phase (Figures 4B and S4A). These recordings showed that Ca2+ waves occurred in ∼40% of the OLs (Figure 4C), and Ca2+ transients in MBP-positive internodes from 13 OLs were significantly increased in frequency compared with adult controls (control data: P31–P62 from Figure 2J; p = 0.0004, Mann-Whitney test; n = 12 OLs; Figures 4D and S4B). The kinetics and amplitude of Ca2+ events were similar to those observed during normal early development (p = 0.18 and p = 0.66, Mann-Whitney test; Figures 4E, S4C, and S4D). One exception was the half-width being longer during remyelination (on average in control: 1.4 ± 0.07 s, n = 427 events; remyelination: 1.84 ± 0.16 s, n = 136 events; p = 0.008, Mann-Whitney test; Figure S4E). Similar to the OL maturation, Ca2+ transient frequency was reduced with more depolarized resting membrane potential (Figure S4F). In summary, these data suggest that microdomain Ca2+ transients and Ca2+ waves in the myelin sheath concur with the active phase of longitudinal and radial myelination.
Figure 4.
Remyelination Reactivates Ca2+ Microdomain Transients and Ca2+ Waves
(A) Left: representative MBP immunostainings of layer 5 in control and after 7, 12, and 25 days of remyelination. Scale bars, 50 μm. Right: normalized MBP intensity for the four stages plotted versus the normalized cortical column length. WM, white matter.
(B) Top: images of resting and frame subtracted fractional fluorescence change with time points indicated (x, y, z). Scale bar, 5 μm. Bottom: corresponding Ca2+ traces of the ROIs for transient Ca2+ events and Ca2+ waves from two different trials. The Ca2+ wave initiates at the end tip (ROI 1) and propagates toward the center of the sheath (ROI 2). Scale bars, 10% ΔF/F0 and 2 s.
(C) Ca2+ wave probability increases during remyelination (p = 0.039, Fisher exact test).
(D) Ca2+ event frequency is increased up to 35 days after remyelination onset.
(E) Histogram of the decay time constant for Ca2+ events during remyelination. Inset shows a typical Ca2+ event; scale bars, 5% ΔF/F0 and 1 s.
See also Figure S4.
Microdomain Ca2+ Transients in Myelin Are Independent of Neuronal Activity
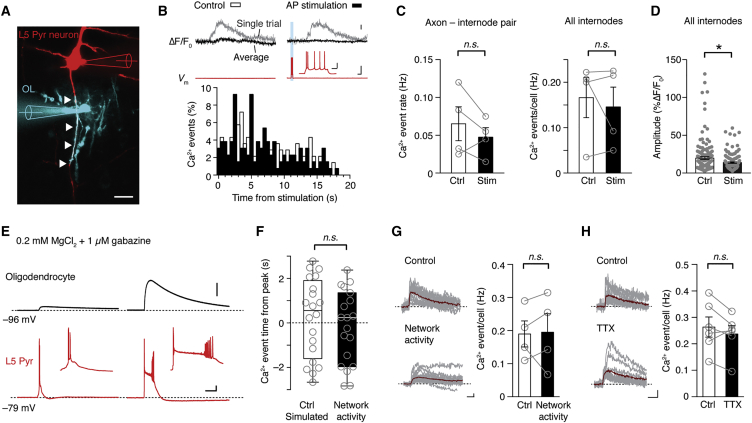
Are myelin Ca2+ events susceptible to neuromodulation? Neuronal activity has been shown to be important for recruitment of OPCs in de novo myelination and is linked to Ca2+ changes in OPC processes (McKenzie et al., 2014, Wake et al., 2011). We therefore hypothesized that action potential generation may alter the microdomain Ca2+ transient frequency or amplitude. To examine this, we simultaneously recorded from a layer 5 pyramidal neuron and the OL ensheathing its first internode (Figure 5A). Simultaneous whole-cell recordings were performed between P18 and P21, the age when myelin segments around the first internode were consistently detected (Figure 1). Myelin Ca2+ signals were recorded before and after a train of action potentials (on average ten action potentials at ∼50 Hz and more than ten trials). Surprisingly, the results indicated that the frequency of microdomain Ca2+ transients was independent of action potential generation in the corresponding axon (p = 0.73, Kolmogorov-Smirnov [KS] test; control, n = 138; stimulation, n = 130 events), and neither affected other internodes of the same OL (Figures 5B and 5C). In fact, the amplitude of microdomain Ca2+ transients significantly decreased by ∼30% following action potential stimulation (Figure 5D).
Figure 5.
Myelin Microdomain Ca2+ Transients Are Independent of Neuronal Activity
(A) Confocal image of a simultaneous OL-neuron whole-cell recording (white arrowheads indicate shared axon). Scale bar, 20 μm.
(B) Top: example traces of the neuronal membrane potential (Vm, red) and myelin Ca2+ signals (same ROI) for control and stimulation. Bottom: histogram of events relative to the action potential (AP) stimulus at 0 s. Ca2+ events in control were aligned to the AP onset. Scale bars, 5% ΔF/F, 30 mV, and 1 s. Inset: 30 mV and 0.1 s.
(C) Left: Ca2+ event frequency of single axon-internode pairs before and after stimulation (p = 0.38, Wilcoxon signed rank test; n = 4 paired recordings, N = 3 animals). Right: Ca2+ event frequency for all internodes (p = 0.88, Wilcoxon signed rank test; n = 4 paired recordings, n = 20 internodes, 2–6 internodes/OL).
(D) Ca2+ event amplitude (ΔF/F0) was reduced following AP stimulation (p = 0.018, Mann-Whitney test). Mean ± SEM.
(E) Network activity was evoked by reducing extracellular Mg2+ to 0.2 mM and blocking inhibition (GABAA receptor blocker gabazine). OL membrane potentials were temporally synchronized with network burst and exhibited peak depolarizations up to 20 mV. Insets show expanded timescales of ∼1 s. Scale bars, 10 mV and 1 s.
(F) Boxplot depicting the timing of myelin Ca2+ events 3 s before and after network activity compared with randomly generated event times (p = 0.99, Kolmogorov-Smirnov test; n = 4 OLs). The box indicates 25th and 75th percentiles, the horizontal bar indicates the median, and error bars maximum and minimum.
(G) Left: single-trial Ca2+ fluorescence traces overlaid with the average (red trace) before and after increase of network activity. Scale bars, 10% ΔF/F0 and 0.5 s. Right: summary plot showing Ca2+ event frequency before and after increased network activity (p = 0.88, Wilcoxon signed rank test; n = 4 OLs). Mean ± SEM.
(H) Single-trial Ca2+ fluorescence traces and summary data before and after bath application of TTX (p = 0.31, Wilcoxon signed rank test; n = 6 OLs). Scale bars, 1 s and 10% ΔF/F0. Mean ± SEM.
See also Figure S5.
Because neocortical OLs myelinate >30 axons, we hypothesized that modulation of myelin Ca2+ transients require synchronized network activity. To examine this hypothesis, we experimentally increased neuronal network activity by lowering extracellular Mg2+ and partially blocking inhibitory synaptic transmission with 1 μM gabazine (a selective GABAA antagonist). During the network burst, OLs showed highly synchronized depolarizations up to 20.2 ± 2.2 mV from the resting membrane potential (n = 17 events from four OLs; Figures 5E and S5A). These depolarizations likely reflect the extracellular K+ elevations (Battefeld et al., 2016). However, the microdomain Ca2+ transients were not temporally related to network bursts, and neither did we observe Ca2+ waves (Figure 5F). The Ca2+ event frequency, amplitude, and half-width remained similar (Figures 5G and S5B). Finally, blocking voltage-gated Na+ channels by bath application of TTX provided further evidence that myelin Ca2+ events are generated independent from neuronal activity (Figures 5H and S5C). Together, these data suggest that Ca2+ events in developing myelin sheaths are either intrinsically generated or require signaling from other glia cells such as astrocytes.
Myelin Ca2+ Transients Are Produced by Mitochondria Localized in the Non-compacted Myelin
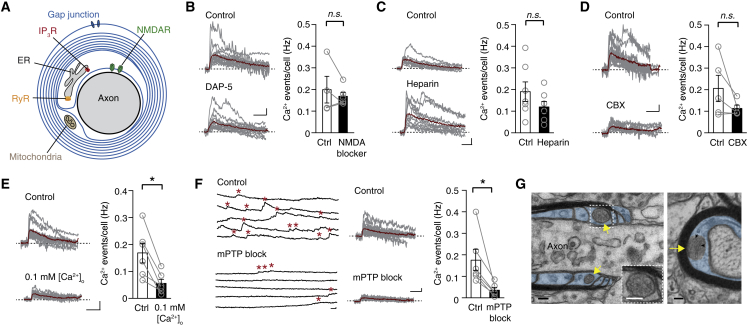
Which mechanisms produce the myelin Ca2+ transients? Previous studies showed that cultured OLs express voltage-gated Ca2+ channels and calcium-permeable NMDA receptors, which have been associated with glutamate mediated signaling between neurons and OL lineage cells (Káradóttir et al., 2005, Kirischuk et al., 1995, Micu et al., 2016). Furthermore, second messenger signaling via purinergic G protein-coupled receptors (P2YR) or activation of inositol trisphosphate receptor (IP3R) or ryanodine receptors (RyRs) is implicated in myelin calcium regulation (James and Butt, 2001, Kirischuk et al., 1995, Micu et al., 2016). Using pharmacological blockers, we tested multiple pathways that could be involved in the generation of myelin Ca2+ transients (Figure 6A). Blocking NMDA receptors (DAP-5/MK-801), IP3R (heparin), and gap junctions (carbenoxolone [CBX]) did not change the frequency, amplitude, and half-width of Ca2+ signals (Figures 6B–6D, S6A, and S6B), with the exception being an increased amplitude in the presence of heparin. This robust increase in Ca2+ amplitude (p = 0.026, Mann-Whitney test; Figure S6A) is potentially a consequence of endoplasmic reticulum (ER) IP3R block and a successive reduction in intracellular baseline Ca2+ level, leading to a larger ΔF/F amplitude. Moreover, simultaneously blocking IP3R and RyR with heparin, dantrolene, and ruthenium red did not change myelin Ca2+ transient frequency, amplitude, or half-width (Figure S6C). Reducing [Ca2+]o to 0.1 mM, however, led to a significant reduction in frequency and amplitude of Ca2+ transients in myelin without a change in half-width (Figures 6E, S6A, and S6B), suggesting an ion channel source or an indirect depletion of intracellular Ca2+ stores. Both in OPCs and in astrocytes, Ca2+ transients and waves are generated by the mitochondrial Ca2+ uptake and release from and into the cytosol (Agarwal et al., 2017, Haak et al., 2000, Haak et al., 2002). During periods of high oxidative phosphorylation, the mitochondria membrane depolarizes periodically, releasing Ca2+ into the cytosol mediated by the transient opening of the mitochondrial permeability pore (mPTP) (Wang et al., 2008, Ichas et al., 1997). To test the contribution of mitochondria in myelin Ca2+ transients, we blocked the mPTP by combining cyclosporin A (inhibiting the cyclophilin D component at the pore) and rotenone (a mitochondrial complex I blocker). Bath application of these mPTP-inhibiting drugs significantly and nearly completely abolished the frequency of Ca2+ events (80 ± 5% block) in 34 myelin sheaths of 6 oligodendrocytes, while leaving amplitude and event duration of the few remaining Ca2+ transients unchanged (Figures 6F, S6A, and S6B). The substantive role of mitochondria is surprising, as their presence within the myelin sheath cytoplasm has only recently been shown at the light and EM level (Rinholm et al., 2016). To determine whether mitochondria are present in myelin sheaths, we examined EM images of P15 and adult mice somatosensory cortex. Despite the small chance of detecting mitochondria in ultrathin EM cross sections of the axon (∼2%; Rinholm et al., 2016), in EM images from the P15 mice, we found mitochondria, characterized by at least a few cristae and double outer membrane layer, located within the non-compacted cytoplasmic ridges, including the inner cytoplasmic tongue (n = 5) and the paranodal loops (n = 2). Similarly, also in the EM from adult mice, mitochondria were observed within the inner cytoplasmic tongue (n = 8) and in the cytoplasm of paranodal loops (n = 2; Figure 6G). Taken together, these results reveal that a high rate of periodic myelin Ca2+ transients is correlated with phases of myelin remodeling and produced by the mitochondria localized to cytoplasmic domains of the myelin sheath.
Figure 6.
Myelin Microdomain Ca2+ Transients Are Generated by Mitochondria in Non-compacted Myelin
(A) Schematic overview of putative receptors and pathways implicated in myelin Ca2. Ca2+ entry could be triggered by activation of NMDA receptors (NMDAR), downstream activation of intracellular IP3R or ryanodine receptor (RyR), gap-junction coupling with astrocytes or mitochondria.
(B) Example traces and pooled summary data for paired DAP-5 wash-in experiments (connecting lines) and intracellular application of MK-801 (single data points). No effect of NMDA receptor block on event frequency was observed (p = 0.91, Mann-Whitney test; ctrl: n = 4 OLs, NMDA block = 6 OLs). Mean ± SEM.
(C) Example traces and summary plot for Ca2+ event frequency for control and intracellular IP3R block by heparin shows no change (p = 0.21, Mann-Whitney test; n = 7 OLs for each condition). Mean ± SEM.
(D) Examples traces and summary plots for the effect of gap-junction blocker carbenoxolone (p = 0.18, Wilcoxon-signed rank test; n = 5 OLs). Mean ± SEM.
(E) Reduction of extracellular [Ca2+] to 0.1 mM reduces Ca2+ event frequency in myelin (p = 0.03, Wilcoxon-signed rank test; n = 6 OLs). Mean ± SEM.
(F) Left: example traces showing 5 × 20 s of myelin Ca2+ imaging in control and after inhibition of the mitochondrial permeability transition pore (mPTP) with 20 μM cyclosporine A and 10 μM rotenone. Asterisks indicate Ca2+ transients. Middle and right: individual Ca2+ events (gray) and average Ca2+ event frequency (red) showing a significant reduction following mPTP block (p = 0.03, Wilcoxon-signed rank test; n = 34 internodes from 6 OLs). Scale bars for all imaging traces from (B) to (F), 1 s and 10% ΔF/F0. Mean ± SEM.
(G) Left: example EM image mitochondria (yellow arrows) in the cytoplasm of paranodal myelin (false blue colored) in the adult somatosensory cortex. Right: EM image of a mitochondrion located in the inner cytoplasmic loop, the adaxonal myelin. Note the presence of cristae within the mitochondrion (black arrowheads). Scale bars, 100 nm.
See also Figure S6.
Discussion
By imaging Ca2+ in the mammalian myelin sheath in the deeper layers of the somatosensory cortex at high sensitivity, we observed a highly active period of spontaneous Ca2+ events during the first postnatal weeks, characterized by Ca2+ signals in small microdomains as well as Ca2+ waves propagating along the myelin sheath (Figures 2 and 3). At this age, axonal growth, reorganization, and pruning as well as myelination rapidly develop (Romand et al., 2011, Vincze et al., 2008) (Figure 1). Our approach of filling individual OLs with OGB-1 resulted in high signal-to-noise ratios across experiments, allowing discrimination of Ca2+ changes ≥5% ΔF/F (Figures S2B and S2C) and the identification that large-amplitude propagating Ca2+ waves (Figure 2) are built up by a brief burst of multiple Ca2+ transients (∼0.8 Hz), suggesting that they share a common source. These findings are in good agreement with recent work linking myelin Ca2+ events with growth and disassembly of the myelin sheath in zebrafish during the early stages of myelination (Baraban et al., 2018, Krasnow et al., 2018). In contrast, we observed Ca2+ events at a remarkably high rate of ∼0.2 Hz (Figures 2F and 3B), two orders of magnitude higher than those in zebrafish (∼1–2 mHz in (Baraban et al., 2018, Krasnow et al., 2018). The discrepancy could be due to technical differences in acquiring Ca2+ signals (in vivo two-photon and confocal microscopy versus in vitro one-photon epifluorescence imaging in the present study) or the different kinetics of genetic versus synthetic Ca2+ reporters. It is equally possible that the Ca2+ events reported in zebrafish myelin (Baraban et al., 2018, Krasnow et al., 2018) imaged over longer time periods and low acquisition rates (∼0.4 Hz) are actually Ca2+ waves. Indeed, Ca2+ waves typically occur at a low rate of ∼0.2 events/min and have a relatively long half-width duration of ∼10 s (Figure 2).
Unexpectedly, we did not find evidence that neuronal activity drives oligodendroglial Ca2+ (Figure 5). Axonal vesicle release during neuronal activity in zebrafish initiates and stabilizes myelin segments during their transition from OPC to OL (Hines et al., 2015, Mensch et al., 2015, Wake et al., 2011), and extracellular axonal stimulation of the spinal cord doubles the rate of Ca2+ events in segments of developing OLs (Krasnow et al., 2018). In addition to the technical differences between the studies as mentioned above, we cannot exclude that the discrepancy stems from the distinct developmental stages of OLs (3–4 days post-fertilization in zebrafish versus 13–56 postnatal days in mice), interspecies differences, or OL cell-type differences between neuronal circuits. In support of the latter argument, myelination of commissural primary ascending axons in zebrafish spinal cord forms independently of vesicle release, whereas myelin ensheathment of reticulospinal axons is regulated by activity-dependent vesicle release (Koudelka et al., 2016). Mammalian OLs consist of six genetically distinct subpopulations of mature OLs, two types of which are found primarily in the somatosensory cortex and corpus callosum (Marques et al., 2016). Recent work shows that the auditory brain stem contains a large fraction of pre-myelinating OL types, of which some express Na+ channels, and in this brain region myelination is increased by neuronal activity (Berret et al., 2017). Whether myelin Ca2+ activity differs between OL populations and how these are linked with differential activity dependence of myelination remains to be established.
The high rate of microdomain Ca2+ transients permitted a controlled pharmacological characterization revealing that inhibition of the transient opening of the mitochondrial mPTP selectively and nearly completely abolished Ca2+ events in myelin sheaths (Figure 6). These findings are in good agreement with previous studies in OPCs and myelin-like processes in cultured oligodendrocytes, which identified a major role of mitochondria in producing Ca2+ transients and facilitating Ca2+ wave propagation (Haak et al., 2002, Haak et al., 2000, Simpson and Russell, 1996). In addition to the substantial impact of the mitochondrial inhibitors, we discovered that myelin in the neocortex does contain mitochondria organelles located in the cytoplasmic ridges of the sheath, including the adaxonal myelin and paranodal loops (Figure 6). These results are in support of the recently identified small and sparsely moving atypical mitochondria identified in cytoplasm of OLs (Rinholm et al., 2016). During periods of high respiration, mPTPs undergo periods of quantal low-conductance openings, leading to a resetting of the mitochondrial membrane potentials, transient efflux of Ca2+ from the mitochondria into the cytosol, and the continuation of ATP production (Ichas et al., 1997, Wang et al., 2008). Indeed, in OPCs and thin processes of astrocytes, a strong anatomical overlap was observed between mitochondrial locations and Ca2+ microdomains (Agarwal et al., 2017, Haak et al., 2000, Simpson and Russell, 1996). Interestingly, in myelin sheaths, we did not observe a contribution of ER Ca2+ release from IP3 or RyRs (Figures 6 and S6). Our findings resemble the spontaneous Ca2+ transients in astrocytic processes, which are generated by mPTP opening and persist in the absence of IP3 receptors (Agarwal et al., 2017, Srinivasan et al., 2015). One caveat of our approach is the rare occurrence of Ca2+ waves during typical patch-clamp recording experiments, which prohibited the investigation of the nature of the waves or their dependence on neuronal activity. To examine whether ER Ca2+ is critical for producing Ca2+ waves future experiments would require other means, such as, for example, the sparse expression of genetically encoded Ca2+ indicators, enabling in vivo and long-term imaging.
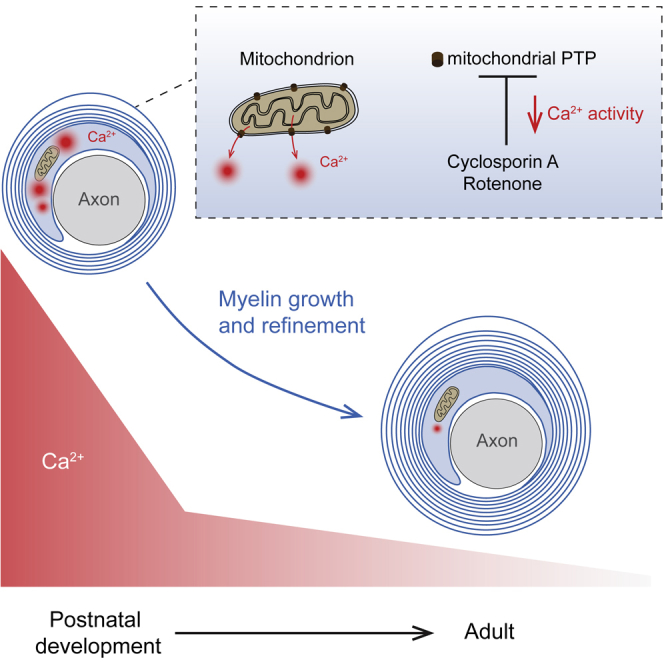
What could be the role of autonomous transient Ca2+ release by mitochondria in the myelin sheath? A downstream target of Ca2+ could be the recruitment of Ca2+/calmodulin-dependent kinase inducing MBP membrane binding via phosphatidylinositol 4,5-bisphosphate (PIP2), or actin disassembly to induce oligodendroglial membrane wrapping (Nawaz et al., 2009, Nawaz et al., 2015, Waggener et al., 2013, Zuchero et al., 2015). Although the biophysical axon-glia signaling pathways that match myelin sheath thickness to axon diameter remain to be identified (Chang et al., 2016), we speculate that in the deeper layers of the somatosensory cortex, axon diameter may suffice as an instructive signal for myelination. It has been estimated that rat oligodendrocytes may produce up to ∼5,000 μm2 of myelin membrane per day or 105 myelin proteins per minute (Pfeiffer et al., 1993). In this view, the high frequency of Ca2+ events ensures a high respiratory rate and mitochondrial ATP production (Ichas et al., 1997) for lipid trafficking and myelin biosynthesis (Voelker, 1984). Such a cell-autonomous signaling pathway is in good agreement with the notion that biosynthesis of the proteins and lipids for the myelin sheaths occurs largely in the absence of neurons (Nawaz et al., 2015, Rosenberg et al., 2008). Furthermore, cultured OLs ensheath synthetic nanofibers greater than ∼0.4 μm in diameter, suggesting that the molecular or biophysical mechanisms of axon selection and initiation of spiral myelin wrapping are intrinsic to the OL (Lee et al., 2012). In future studies, it will be interesting to address the interplay of molecular pathways regulating membrane growth and the signaling mechanisms regulating mitochondria respiration and myelin lipid production. Mitochondria-generated cytoplasmic Ca2+ may support coordination of myelin sheath remodeling at the inner tongue and paranodes, allowing the lateral movement of cytoplasmic loops and refinement of the organization of myelinated axons toward the nodes of Ranvier (Snaidero et al., 2014). One hypothesis emerging from the present data is that spatially distributed waves of high [Ca2+]i mediate actin disassembly, thereby providing the driving force for advancing the spiral wrapping of the lamellae (Baraban et al., 2018, Nawaz et al., 2015, Zuchero et al., 2015), while small [Ca2+]i changes evoked during microdomain Ca2+ transients stabilize the myelin sheath and mediate compaction and myelin sheath refinement. To further resolve the relationship between myelin Ca2+ activity and myelination patterns, longitudinal time-lapse in vivo studies simultaneously monitoring activity and cortical myelination would be required.
In summary, our study uncovered a remarkably high rate of mitochondria-dependent local Ca2+ transients and Ca2+ waves propagating along the mammalian myelin sheath during myelin remodeling, providing biochemical and cellular insights into the mechanism of de novo myelin formation and remyelination in neocortical circuits.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse anti myelin myelin oligodendrocyte glycoprotein (MOG, 1:1000) | Millipore | Cat#: MAB5680; RRID: AB_1587278 |
| Chicken anti proteo-lipid-protein (PLP, 1:250) | Millipore | Cat#: Ab15454; RRID: AB_805413 |
| Rabbit anti myelin basic protein (MBP, 1:250) | Millipore | Cat#: AB980; RRID: AB_92396 |
| Rabbit - anti-GFP (1:1500) | Abcam | Cat#: ab6556; RRID: AB_305564 |
| Alexa488 - Streptavidin (1:500) | Thermofisher | Cat#: S32354; RRID: AB_2336881 |
| Alexa555 - Streptavidin (1:500) | Thermofisher | Cate#: S21381; RRID: AB_2307336 |
| Alexa594 - Streptavidin (1:500) | Thermofisher | Cat#: S11227; RRID: AB_2313574 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Cuprizone | Sigma-Aldrich | Cat#: C9012 |
| Oregon Green BAPTA-1 hexapotassium salt | Thermo-Fisher | Cat#: O6806 |
| Fura-2 | Thermo-Fisher | Cat#: F1200 |
| Biocytin | Sigma | Cat#: B4261 |
| Tetrodotoxin (TTX) citrate | Tocris | Cat#: 1069/1 |
| D-AP5 | Tocris | Cat#: 0106/1 |
| Carbenoxolone | Sigma-Aldrich | Cat#: C4790 |
| Gabazine (SR-95531) | Sigma-Aldrich | Cat#: S106 |
| Heparin | Tocris | Cat#: 2812/100 |
| Ruthenium Red | Tocris | Cat#: 1439/100 |
| Dantrolene | Tocris | Cat#: 0507/100 |
| Rotenone | Tocris | Cat#: 3616/50 |
| Cyclosporin A | Tocris | Cat#: 1101/100 |
| Experimental Models: Organisms/Strains | ||
| Mouse: PLP-ECFP (line Q) | Frank Kirchhoff | (Battefeld et al., 2016, Hirrlinger et al., 2005) |
| Mouse: C57BL6/J | The Jackson Laboratory | Cat#: 000664 |
| Oligonucleotides | ||
| FW sequencing primers for PLP-PCFQ: 5′-ATGCGTACCTGACTTTCTCCTTCT-3′ | (Battefeld et al., 2016) | (Hirrlinger et al., 2005) |
| RV sequencing primers for PLP-PCFQ: 5′-ACTGGGTGCTCAG GTACTGGTTGT-3′ | (Battefeld et al., 2016) | (Hirrlinger et al., 2005) |
| Software and Algorithms | ||
| NeuroPlex | RedShirt Imaging | RRID: SCR_016193 |
| Axograph X | Axograph | RRID: SCR_014284 |
| Graph Pad Prism | GraphPad | RRID: SCR_002798 |
| FIJI | (Schindelin et al., 2012) | RRID: SCR_002285 |
| Neuromantic | (Myatt et al., 2012) | RRID: SCR_013597 |
| NEURON | (Hines and Carnevale, 2001) | RRID: SCR_005393 |
| Maxchelator | (Schoenmakers et al., 1992) | RRID: SCR_000459 |
| Frame splitter | This paper | https://github.com/Kolelab/Image-analysis |
| ΔF/F calculator | This paper | https://github.com/Kolelab/Image-analysis |
| Other | ||
| Vibratome | Leica | VT1200 |
| Upright microscope | Olympus | BX61WI |
| Blue LED 470 nm | Thorlabs | M470L3 |
| UV LED 340 nm | Thorlabs | M340L4 |
| Violet LED 420 nm | Thorlabs | M420L3 (discontinued) |
| NeuroCCD-SMQ camera | RedShirt | N/A |
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Maarten Kole (m.kole@nin.knaw.nl).
Experimental Model and Subject Details
Mice
All procedures involving experimental animals were in agreement with EU directive 2010/63/EU and Dutch law and were approved by the local animal ethics committee of the Royal Netherlands Academy of Arts and Sciences (NIN 12.67, NIN11.70). We used male and female mice of either the C57BL/6 strain or the transgenic PLP-ECFP strain (line Q, as previously described (Battefeld et al., 2016) – obtained from Frank Kirchhoff, University of Saarland, Germany) that were kept on a C57BL/6 background. All transgenic mice were bred in house in a SPF facility, kept on a 12/12 hour light/dark cycle and had access to ad libitum standard chow and water. Male mice were randomly assigned to experimentally induced demyelination. Demyelination was induced by feeding mice with 0.2% cuprizone (C9012, Sigma-Aldrich Chemie N.V., Zwijndrecht, the Netherlands) supplemented to the standard diet for 5 weeks (Hamada and Kole, 2015). The standard chow was ground before being mixed with fresh cuprizone and replaced every 2-3 days. Following the 5 weeks treatment, mice were put back on normal chow diet for 1 to 5 weeks to allow remyelination.
Method Details
Brain slice preparation
For all experiments acute slices were prepared from male (61 out of 93) or female (32 out of 93) mice between postnatal day 8 (P8) and P111 in the morning. Mice were anesthetized with 3% isoflurane inhalation, decapitated and the brain was quickly removed and placed in ice-cold ACSF (in mM: 125 NaCl, 25 NaHCO3, 1.25 NaH2PO4, 3 KCl, 25 glucose, 1 CaCl2, 6 MgCl2 and 1 kynurenic acid) saturated with 95% O2 and 5% CO2. Subsequently we prepared 300 - 400 μm thick parasagittal slices with a vibratome (VT1200S, Leica Microsystems, Germany) and incubated at 35°C for 35 min. Afterward slices were stored in the same solution at room temperature for the experimental day.
Electrophysiology
For physiology experiments slices were transferred to a submerged recording chamber mounted on an upright microscope (BX61WI, Olympus) perfused with ACSF (in mM: 125 NaCl, 25 NaHCO3, 1.25 NaH2PO4, 3 KCl, 25 glucose, 2 CaCl2, 1 MgCl2) heated to 33 ± 2°C. Cells were visualized with oblique contrast and OLs were identified by their ECFP fluorescence using a standard filter cube (U-MCFPHQ, Olympus). For whole-cell recordings the intracellular solution consisted of (in mM): 130 K-Gluconate, 10 KCl, 10 HEPES, 4 Mg-ATP, 0.3 Na2-GTP, 10 Na2-phosphocreatine and pH set to 7.25 (∼280 mOsm). This solution was supplemented with 100 μM of the fluorescent Ca2+ indicator Oregon Green BAPTA-1 hexapotassium (OGB-1, O6806, Thermo Fisher Scientific, Landsmeer, Netherlands) for Ca2+ imaging experiments (for details see epifluorescence calcium imaging). For some experiments, we included 5 mg/ml biocytin (Sigma) for post hoc morphology analysis. The intracellular solution for neurons in paired neuron-oligodendrocyte recordings was supplemented with 75-100 μM Alexa-594-hydrazide (Thermo Fisher Scientific) to identify myelin-axon assemblies. Voltage-clamp and current-clamp recordings were made with Axopatch 200B (Molecular Devices, Sunnyyvale, CA, USA) and BVC-700A amplifiers (Dagan Corporation, Minneapolis, MN, USA), respectively. Current or voltage signals were digitized with an AD board (ITC-18, HEKA Elektronik, Lambrecht, Germany) and Axograph X software (RRID: SCR_014284, v1.3.5, AxoGraph Scientific, Sydney, Australia). Recordings were analog filtered at 10 kHz (Bessel) before digitizing with a minimum of 20 kHz.
Epifluorescence calcium imaging of oligodendrocytes
Oligodendrocyte cell bodies were identified using the ECFP signal which also allows imaging [Ca2+]i with blue light, enabling the use of the high-affinity Ca2+ reporter OGB-1 with minimal fluorescence crosstalk. Epifluorescence Ca2+ imaging was performed on a BX61WI microscope (Olympus, Leiderdorp, the Netherlands) with a 60 × 1.0 NA water immersion objective. A blue LED (peak 470 nm, Thorlabs Inc, Newton NJ, USA) equipped with a band pass excitation filter (460-490 nm, Olympus) was used as fluorescence light source and coupled into the light path via a U-DP and U-DPXC1 adaptor (Olympus). Excitation light was reflected with a beam splitter (FF510-Di02, Semrock, Rochester, NY, USA) and emission was band pass (536/40-25, Semrock) or long pass (FGL515, Thorlabs) filtered before being projected to a NeuroCCD camera (RedShirtImaging LLC, Decatur, GA, USA). The camera has a low spatial resolution of 80 × 80 pixels, but low read noise and a high dynamic range. To fully illuminate the imaging chip, the camera was mounted on a 0.35 × or 0.38 × de-magnifier tube, resulting in a pixel size of 0.92 or 0.95 μm, respectively. Oligodendrocytes were loaded with OGB-1 for a minimum of 45 minutes before starting image acquisition that was performed with Neuroplex software (RRID: SCR_016193, v. 10.1.2, RedShirt Imaging) with a sampling frequency of 40 Hz and the camera gain set to 30. Using TTL synchronization, imaging and electrophysiological recording start times were matched. Usually, 10 to 20 trials with a duration of 10 or 20 s were collected for a given field of view. The imaged area was selected to obtain a focal image of a single or several internodes, located some distance from the cell body. Illumination of the field of view was restricted by a field stop with a variable iris that was mounted into the excitation light path. Using this approach the soma, pipette and other unwanted processes (including gap-junction coupled astrocytes) were not illuminated and the dynamic range was increased. The OL resting membrane potential was continuously monitored and depolarizations > 10 mV from the resting membrane potential led to abortion of the experiments and data were excluded from the analyses. In 95% of recordings the same internodes were imaged for control and drug condition. Drugs [1 μM TTX (5 ± 0.5 minutes, 50 μM DAP-5 (14 ± 5), 100 μM carbenoxolone (14 ± 2 minutes), and 1 μM gabazine (9 ± 2 minutes)] were washed in after control Ca2+ signals were collected with the exception of intracellular receptor blockers. For intracellular blockers, the incubation time was > 40 minutes during which OGB-1 was fully diffused into the cell. Intracellular block of endoplasmic reticulum IP3 receptors was accomplished with the broad-spectrum blocker heparin (5 mg/ml) and compared to a separate set of myelin Ca2+ imaging experiments from age-matched controls. Combined block of IP3 and RyRs was achieved with freshly adding heparin, 20 μM dantrolene and 10 μM ruthenium red (all Tocris) to the intracellular solution. The mitochondrial permeability pore (mPTP) was blocked by adding rotenone (10 μM) and cyclosporin A (20 μM) to the bath (19 ± 3 minutes). Stock solutions for these two blockers were prepared on the day of the experiment. To ensure sufficient Ca2+ transients pharmacology experiments were performed between postnatal day 12 and 21 (average 16 ± 2 days, n = 45 cells).
Ratiometric fura-2 Ca2+ imaging
As ECFP produces considerable crosstalk when excited at 420 nm we used wild-type littermates from the OL reporter mouseline. For calibration experiments we recorded from satellite OLs in layer 5 (Battefeld et al., 2016) and used 200 μM of the ratio metric Ca2+ reporter fura-2 (F1200, Thermo Fischer Scientific). The excitation light path was custom designed and fura-2 was excited with two LEDs with peaks at 340 nm (M340L4, Thorlabs) and 420 nm (M420L3, Thorlabs) fitted with bandpass excitation filters 340/22 nm (Semrock) and 420/10 nm (Semrock), respectively. LED collimation was achieved with an aspheric UV passing lens for 340 nm (#33-953, Edmund Optics, York, United Kingdom) and a 1” plano-convex lens (N-BK7, Thorlabs) for 420 nm. Both LEDs were combined into a single light path with a 405 nm dichroic mirror (Di02-R405, Semrock). A field stop was mounted into the excitation light path at a focal length of 17.5 cm from the tube lens. The standard tube lens of U-DPXC1 was replaced with a UV passing plano-convex lens (#48-289, Edmund Optics) and the length of the tube was adjusted accordingly. A dichroic mirror at 458 nm (FF458-Di02, Semrock) reflected excitation light to the sample and passed emission light that was then long passed through a colored glass filter of 455 nm (FGL455, Thorlabs) before being collected with the imaging camera (RedShirtImaging LLC, Decatur, GA, USA). Output current of the LEDs was kept identical for recordings and calibration. We minimized LED output changes by using LEDs equipped with proper cooling (passive heat sinks) and not driving LEDs continuously. The camera generates TTL output pulses at the beginning of each frame and each LED was triggered every other frame and all frames were collected and post hoc separated for the two wavelengths similarly as previously published (Miyazaki and Ross, 2015). Each camera frame was triggered for 12.5 ms and light was on for 10 ms to acquire images for both wavelengths at 40 Hz in a pseudo simultaneous manner. Light intensity was adjusted for each excitation wavelength.
Immunohistochemistry
For immunohistochemistry experiments we used brain slices from the electrophysiology experiments (∼300 μm thick). Tissue was fixed with 4% PFA for 20 minutes and washed with PBS. Subsequently slices were blocked in PBS supplemented with 2.5%–5% goat serum and 0.5% Triton X-100 for 1 hour at room temperature. The following primary antibodies were used and incubated at room temperature overnight: streptavidin conjugated Alexa488 (RRID: AB_2336881, S32354), Alexa555 (RRID: AB_2307336, S21381) and Alexa594 (RRID: AB_2313574, S11227, all 1:500, Thermofisher), rabbit anti-GFP (RRID: AB_305564, 1:1500, ab6556, Abcam), anti MOG (RRID: AB_1587278, 1:1000, MAB5680, Millipore), anti myelin basic protein (MBP, RRID: AB_92396, 1:250, AB980, Millipore), anti proteo-lipid-protein (PLP, RRID: AB_805413, 1:250, Ab15454, Millipore). Layer 5 neuron morphology was recovered by immunoreaction of biocytin with streptavidin and identification of myelinated axon segments was achieved by co-labeling with MBP, MOG or GFP as described above. Slices were mounted on glass slides and embedded in Vectashield with DAPI (H-1200, Vector Laboratories, Burlingame, CA, USA). Acquisition of z stacks was performed with image size set to 2048 × 2048 and a 40 × 1.3NA or 63 × 1.4NA lens using Leica SP5 or SP8 confocal microscopes equipped with Leica ASF software (RRID: SCR_013673, Leica, Germany).
EM
Tissue for EM from P15 neocortex was obtained from acutely prepared slices that were fixed for 30 min in freshly prepared 5% glutaraldehyde in Na-cacodylate (0.1 M, pH 7.4) and from a transcardially perfused P15 pup using the same fixation solution. No difference in the g-ratio between the two preparation methods was observed (p = 0.11, t test) and the data subsequently pooled. Slices were cryo-protected in 30% sucrose and once saturated, slices were placed in an aluminum boat, frozen on dry ice and recut at 40 μm with a freezing microtome (1205, Reichert-Jung). Adult tissue (12 weeks, ∼P87) was obtained from a transcardially-perfused mouse (5% glutaraldehyde in Na-cacodylate). For perfused samples the somatosensory cortex was carefully dissected after fixation and the tissue was subsequently washed in Na-cacodylate buffer. All samples were rinsed in Na-cacodylate and post fixed for 20 min in 1% OsO4 supplemented with 1% ferricyanide in Na-cacodylate buffer. Subsequently, the tissue was dehydrated using a gradient of ethanol and acetone and embedded in epoxy resin. Once fully hardened, ultrathin 60 nm sections (Ultracut UCT, Leica) were made and collected on EM grids. Contrasting of the tissue was performed with solutions of lead citrate and 0.5% uranyl acetate (Merck KGaA, Darmstadt, Germany). Sections were sampled with a transmission electron microscope (Tecnai 12, FEI Europe, Eindhoven, Netherlands) and digital images were acquired as 16 bit TIF files (Camera: Veleta, Emsis GmbH, Muenster, Germany; Software: Radius, Olympus).
Quantification and Statistical Analysis
Analysis of Ca2+ imaging data
Image analysis was performed with Neuroplex software (RedShirtImaging), Axograph X and Excel (RRID: SCR_016137, Microsoft Corp., USA). When images were acquired with a pulsed LED a script was executed to discard every other frame. Next, regions of interest (ROIs) were manually defined on myelinating processes and background areas. Raw Ca2+ fluorescence values were extracted from ROIs and an automated script subtracted the background for each ROI and calculated ΔF/F. For the total fluorescence (F) all data points of an ROI were averaged in one time series. During few experiments, acquisition of traces showed bleaching which could be described by a double exponential function. Bleaching is a non-linear process that not only bleaches cell processes, but also the background. A correction of this exponential bleach was not performed because it was negligible with used LED intensities. For an average peak signal of 10.1 ± 1.0% ΔF/F0 the difference with and without bleach correction was measured to be 4.7 ± 1.2% of the maximum peak (n = 34 events, N = 5 cells). Excitation light intensities were kept at the lowest possible level to prevent bleaching. Ca2+ event amplitude, half-width and kinetics were analyzed in Axograph X (Peak detection). For each event the peak (ΔF/F0) was calculated as an average of 3 data points relative to the baseline preceding the event (usually 100 to 200 ms). The onset of an event was detected at 25% of the peak. The half-width was determined at 50% of the peak. For presentation, individual events are displayed as (ΔF/F0) while imaging traces with longer duration are shown as ΔF/F. Kinetics of Ca2+ events were determined from single exponential fits for the decay. Calculation of propagation speed for Ca2+ events was determined from the Δ-time of the onset at 25% of the peak (starting to terminating ROI) over the distance in μm between the starting and terminating ROI. Random event times were generated in Excel using the RAND function (Figure 5F). Custom-written scripts are available on GitHub (see Key Resources Table).
Calculation of free [Ca2+]i
We calculated the free [Ca2+] of various dilutions of our pipette solution by using the online maxchelator tool (RRID: SCR_000459, http://maxchelator.stanford.edu/CaMgATPEGTA-TS.htm) that is based on the Chelator program (Schoenmakers et al., 1992). Our intracellular solution for patch-clamp experiments contained ATP, EGTA, Mg2+ and traces of Ca2+ thus we included a correction factor to obtain a constant concentration of 4 mM Mg2+ in all used dilutions. Intracellular binding or transport of Mg2+ was not factored into the calculation. Parameters for calculation were set to a recording temperature of 32°C, pH of 7.25 and an ionic strength of 0.29 based on the estimated total free ions in our solution. The free [Ca2+] has small increment steps in the nanomolar range to account for the low Kd of fura-2. For a standardization curve on our imaging system we prepared dilutions including 100 μM fura-2 and acquired fluorescence ratios (340/420) for all dilutions ex situ. The intracellular Ca2+ concentration ([Ca2+]i) was calculated as given by (Grynkiewicz et al., 1985).
Kd was set to 0.22 μM, determined from our calibration experiments, which is similar to previous published Kd for fura-2 (Helmchen et al., 1996, Yoo et al., 1999). R was the ratio (340/420) determined during experiments and Rmin and Rmax the ratio (340/420) measured with calibration solutions as described. Sf2 is the max and Sb2 the min fluorescence at 420 nm.
Analysis of myelin distribution and g-ratio
For identifying axonal segments and determining the length of co-localization with OL processes, confocal images were analyzed in FIJI (RRID: SCR_002285) (Schindelin et al., 2012) by measuring segment lengths along single axons using the segmented line tool. For g-ratio measurements EM images were analyzed in FIJI. Using the line tool the inner axon diameter and outer diameter including myelin were measured and subsequently divided.
Morphological reconstructions
For axonal reconstructions single channel z stacks were loaded into Neuromantic 1.7.5 (RRID: SCR_013597) (Myatt et al., 2012) and the axon proper was manually traced. Z stacks containing the myelin channel were subsequently loaded and traced axons were manually annotated by assigning unique identifiers for unmyelinated and myelinated segments. Reconstructions were saved in swc format and scaled according to the confocal metadata. Graphical display and pseudo coloring of reconstructed axons was performed using the NEURON environment (RRID: SCR_005393, Hines and Carnevale, 2001).
Statistical tests
Data was tested for normal distribution with Shapiro-Wilk test. When normal distributed and n ≥ 8 data was tested with paired or unpaired t test. Not normal distributed data was tested with non-parametric tests. Wilcoxon-signed rank test (WSR) was used for paired datasets and Mann-Whitney U tests (MW) for unpaired data. Distributions were tested with a Kolmogorov-Smirnov test (KS-test). Multiple comparisons were performed with ANOVA and post hoc Tukey-test. The respective tests are indicated in the results. All statistical analysis was performed in Prism 7.0a (RRID: SCR_002798, GraphPad Software Inc, La Jolla, CA, USA). No power analysis was performed to estimate the number of experiments.
Data and Software Availability
Datasets are available upon request to m.kole@nin.knaw.nl. Software scripts written for the imaging analyses are available at https://github.com/Kolelab/Image-analysis.
Acknowledgments
We thank the members of the Axonal Signaling Group (NIN) for helpful discussion and critical reading of the manuscript. This research was supported by the European Research Council (ERC StG 261114), Hersenstichting Nederland (grant 2013[1]-160), and the National Multiple Sclerosis Society (RG 4924A1/1).
Author Contributions
Conceptualization, A.B. and M.H.P.K.; Methodology, A.B., M.A.P., and M.H.P.K.; Investigation, A.B. and S.I.V.; Software, M.A.P.; Interpretation of Data, A.B., M.A.P., and M.H.P.K.; Writing – Original Draft, A.B.; Writing – Review & Editing, A.B. and M.H.P.K., with input from M.A.P. and S.I.V.; Funding Acquisition, M.H.P.K. All authors approved the final version of the manuscript.
Declaration of Interest
The authors declare no competing interests.
Published: January 2, 2019
Footnotes
Supplemental Information includes six figures and two videos and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.12.039.
Contributor Information
Arne Battefeld, Email: arne.battefeld@u-bordeaux.fr.
Maarten H.P. Kole, Email: m.kole@nin.knaw.nl.
Supplemental Information
References
- Agarwal A., Wu P.-H., Hughes E.G., Fukaya M., Tischfield M.A., Langseth A.J., Wirtz D., Bergles D.E. Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron. 2017;93:587–605.e7. doi: 10.1016/j.neuron.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban M., Koudelka S., Lyons D.A. Ca 2+ activity signatures of myelin sheath formation and growth in vivo. Nat. Neurosci. 2018;21:19–23. doi: 10.1038/s41593-017-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres B.A., Raff M.C. Axonal control of oligodendrocyte development. J. Cell Biol. 1999;147:1123–1128. doi: 10.1083/jcb.147.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battefeld A., Klooster J., Kole M.H.P. Myelinating satellite oligodendrocytes are integrated in a glial syncytium constraining neuronal high-frequency activity. Nat. Commun. 2016;7:11298. doi: 10.1038/ncomms11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxi E.G., DeBruin J., Jin J., Strasburger H.J., Smith M.D., Orthmann-Murphy J.L., Schott J.T., Fairchild A.N., Bergles D.E., Calabresi P.A. Lineage tracing reveals dynamic changes in oligodendrocyte precursor cells following cuprizone-induced demyelination. Glia. 2017;65:2087–2098. doi: 10.1002/glia.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berret E., Barron T., Xu J., Debner E., Kim E.J., Kim J.H. Oligodendroglial excitability mediated by glutamatergic inputs and Nav1.2 activation. Nat. Commun. 2017;8:557. doi: 10.1038/s41467-017-00688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K.-J., Redmond S.A., Chan J.R. Remodeling myelination: implications for mechanisms of neural plasticity. Nat. Neurosci. 2016;19:190–197. doi: 10.1038/nn.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarner T., Diederichs F., Berger K., Denecke B., Gan L., van der Valk P., Beyer C., Amor S., Kipp M. Myelin debris regulates inflammatory responses in an experimental demyelination animal model and multiple sclerosis lesions. Glia. 2012;60:1468–1480. doi: 10.1002/glia.22367. [DOI] [PubMed] [Google Scholar]
- Fard M.K., van der Meer F., Sánchez P., Cantuti-Castelvetri L., Mandad S., Jäkel S., Fornasiero E.F., Schmitt S., Ehrlich M., Starost L. BCAS1 expression defines a population of early myelinating oligodendrocytes in multiple sclerosis lesions. Sci. Transl. Med. 2017;9:eaam7816. doi: 10.1126/scitranslmed.aam7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friess M., Hammann J., Unichenko P., Luhmann H.J., White R., Kirischuk S. Intracellular ion signaling influences myelin basic protein synthesis in oligodendrocyte precursor cells. Cell Calcium. 2016;60:322–330. doi: 10.1016/j.ceca.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Haak L.L., Grimaldi M., Russell J.T. Mitochondria in myelinating cells: calcium signaling in oligodendrocyte precursor cells. Cell Calcium. 2000;28:297–306. doi: 10.1054/ceca.2000.0176. [DOI] [PubMed] [Google Scholar]
- Haak L.L., Grimaldi M., Smaili S.S., Russell J.T. Mitochondria regulate Ca2+ wave initiation and inositol trisphosphate signal transduction in oligodendrocyte progenitors. J. Neurochem. 2002;80:405–415. doi: 10.1046/j.0022-3042.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- Hamada M.S., Kole M.H.P. Myelin loss and axonal ion channel adaptations associated with gray matter neuronal hyperexcitability. J. Neurosci. 2015;35:7272–7286. doi: 10.1523/JNEUROSCI.4747-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen F., Imoto K., Sakmann B. Ca2+ buffering and action potential-evoked Ca2+ signaling in dendrites of pyramidal neurons. Biophys. J. 1996;70:1069–1081. doi: 10.1016/S0006-3495(96)79653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R.A., Li A.M., Grutzendler J. Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat. Neurosci. 2018;21:683–695. doi: 10.1038/s41593-018-0120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M.L., Carnevale N.T. NEURON: a tool for neuroscientists. Neuroscientist. 2001;7:123–135. doi: 10.1177/107385840100700207. [DOI] [PubMed] [Google Scholar]
- Hines J.H., Ravanelli A.M., Schwindt R., Scott E.K., Appel B. Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci. 2015;18:683–689. doi: 10.1038/nn.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirrlinger P.G., Scheller A., Braun C., Quintela-Schneider M., Fuss B., Hirrlinger J., Kirchhoff F. Expression of reef coral fluorescent proteins in the central nervous system of transgenic mice. Mol. Cell. Neurosci. 2005;30:291–303. doi: 10.1016/j.mcn.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Hughes E.G., Orthmann-Murphy J.L., Langseth A.J., Bergles D.E. Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci. 2018;21:696–706. doi: 10.1038/s41593-018-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichas F., Jouaville L.S., Mazat J.P. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 1997;89:1145–1153. doi: 10.1016/s0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- James G., Butt A.M. P2X and P2Y purinoreceptors mediate ATP-evoked calcium signalling in optic nerve glia in situ. Cell Calcium. 2001;30:251–259. doi: 10.1054/ceca.2001.0232. [DOI] [PubMed] [Google Scholar]
- Káradóttir R., Cavelier P., Bergersen L.H., Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirischuk S., Scherer J., Kettenmann H., Verkhratsky A. Activation of P2-purinoreceptors triggered Ca2+ release from InsP3-sensitive internal stores in mammalian oligodendrocytes. J. Physiol. 1995;483:41–57. doi: 10.1113/jphysiol.1995.sp020566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koudelka S., Voas M.G., Almeida R.G., Baraban M., Soetaert J., Meyer M.P., Talbot W.S., Lyons D.A. Individual neuronal subtypes exhibit diversity in CNS myelination mediated by synaptic vesicle release. Curr. Biol. 2016;26:1447–1455. doi: 10.1016/j.cub.2016.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnow A.M., Ford M.C., Valdivia L.E., Wilson S.W., Attwell D. Regulation of developing myelin sheath elongation by oligodendrocyte calcium transients in vivo. Nat. Neurosci. 2018;21:24–28. doi: 10.1038/s41593-017-0031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M., Capetillo-Zarate E., Dietrich D. Vesicular glutamate release from axons in white matter. Nat. Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- Lee S., Leach M.K., Redmond S.A., Chong S.Y.C., Mellon S.H., Tuck S.J., Feng Z.-Q., Corey J.M., Chan J.R. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat. Methods. 2012;9:917–922. doi: 10.1038/nmeth.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques S., Zeisel A., Codeluppi S., van Bruggen D., Mendanha Falcão A., Xiao L., Li H., Häring M., Hochgerner H., Romanov R.A. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science. 2016;352:1326–1329. doi: 10.1126/science.aaf6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie I.A., Ohayon D., Li H., de Faria J.P., Emery B., Tohyama K., Richardson W.D. Motor skill learning requires active central myelination. Science. 2014;346:318–322. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensch S., Baraban M., Almeida R., Czopka T., Ausborn J., El Manira A., Lyons D.A. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci. 2015;18:628–630. doi: 10.1038/nn.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micu I., Plemel J.R., Lachance C., Proft J., Jansen A.J., Cummins K., van Minnen J., Stys P.K. The molecular physiology of the axo-myelinic synapse. Exp. Neurol. 2016;276:41–50. doi: 10.1016/j.expneurol.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Miyazaki K., Ross W.N. Simultaneous sodium and calcium imaging from dendrites and axons. eNeuro. 2015;2 doi: 10.1523/ENEURO.0092-15.2015. ENEURO.0092-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myatt D.R., Hadlington T., Ascoli G.A., Nasuto S.J. Neuromantic - from semi-manual to semi-automatic reconstruction of neuron morphology. Front. Neuroinform. 2012;6:4. doi: 10.3389/fninf.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave K.-A., Werner H.B. Myelination of the nervous system: mechanisms and functions. Annu. Rev. Cell Dev. Biol. 2014;30:503–533. doi: 10.1146/annurev-cellbio-100913-013101. [DOI] [PubMed] [Google Scholar]
- Nawaz S., Kippert A., Saab A.S., Werner H.B., Lang T., Nave K.-A., Simons M. Phosphatidylinositol 4,5-bisphosphate-dependent interaction of myelin basic protein with the plasma membrane in oligodendroglial cells and its rapid perturbation by elevated calcium. J. Neurosci. 2009;29:4794–4807. doi: 10.1523/JNEUROSCI.3955-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz S., Sánchez P., Schmitt S., Snaidero N., Mitkovski M., Velte C., Brückner B.R., Alexopoulos I., Czopka T., Jung S.Y. Actin filament turnover drives leading edge growth during myelin sheath formation in the central nervous system. Dev. Cell. 2015;34:139–151. doi: 10.1016/j.devcel.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Komitova M., Suzuki R., Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S.E., Warrington A.E., Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–197. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- Rinholm J.E., Vervaeke K., Tadross M.R., Tkachuk A.N., Kopek B.G., Brown T.A., Bergersen L.H., Clayton D.A. Movement and structure of mitochondria in oligodendrocytes and their myelin sheaths. Glia. 2016;64:810–825. doi: 10.1002/glia.22965. [DOI] [PubMed] [Google Scholar]
- Romand S., Wang Y., Toledo-Rodriguez M., Markram H. Morphological development of thick-tufted layer V pyramidal cells in the rat somatosensory cortex. Front. Neuroanat. 2011;5:5. doi: 10.3389/fnana.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S.S., Kelland E.E., Tokar E., De la Torre A.R., Chan J.R. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc. Natl. Acad. Sc. U S A. 2008;105:14662–14667. doi: 10.1073/pnas.0805640105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers T.J., Visser G.J., Flik G., Theuvenet A.P. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques. 1992;12:870–879. [PubMed] [Google Scholar]
- Simpson P.B., Russell J.T. Mitochondria support inositol 1,4,5-trisphosphate-mediated Ca2+ waves in cultured oligodendrocytes. J. Biol. Chem. 1996;271:33493–33501. doi: 10.1074/jbc.271.52.33493. [DOI] [PubMed] [Google Scholar]
- Snaidero N., Möbius W., Czopka T., Hekking L.H.P., Mathisen C., Verkleij D., Goebbels S., Edgar J., Merkler D., Lyons D.A. Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell. 2014;156:277–290. doi: 10.1016/j.cell.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R., Huang B.S., Venugopal S., Johnston A.D., Chai H., Zeng H., Golshani P., Khakh B.S. Ca2+ signaling in astrocytes from Ip3r2-/- mice in brain slices and during startle responses in vivo. Nat. Neurosci. 2015;18:708–717. doi: 10.1038/nn.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B., Porta S., Haak L.L., Gallo V., Fields R.D. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;36:855–868. doi: 10.1016/s0896-6273(02)01067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Matthews E.A., Nicolas V., Schoch S., Dietrich D. NG2 glial cells integrate synaptic input in global and dendritic calcium signals. eLife. 2016;5:e16262. doi: 10.7554/eLife.16262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincze A., Mázló M., Seress L., Komoly S., Abrahám H. A correlative light and electron microscopic study of postnatal myelination in the murine corpus callosum. Int. J. Dev. Neurosci. 2008;26:575–584. doi: 10.1016/j.ijdevneu.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Voelker D.R. Phosphatidylserine functions as the major precursor of phosphatidylethanolamine in cultured BHK-21 cells. Proc. Natl. Acad. Sci. U S A. 1984;81:2669–2673. doi: 10.1073/pnas.81.9.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggener C.T., Dupree J.L., Elgersma Y., Fuss B. CaMKIIβ regulates oligodendrocyte maturation and CNS myelination. J. Neurosci. 2013;33:10453–10458. doi: 10.1523/JNEUROSCI.5875-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H., Lee P.R., Fields R.D. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333:1647–1651. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Fang H., Groom L., Cheng A., Zhang W., Liu J., Wang X., Li K., Han P., Zheng M. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo A.S., Krieger C., Kim S.U. Process extension and intracellular Ca2+ in cultured murine oligodendrocytes. Brain Res. 1999;827:19–27. doi: 10.1016/s0006-8993(99)01282-2. [DOI] [PubMed] [Google Scholar]
- Young K.M., Psachoulia K., Tripathi R.B., Dunn S.-J., Cossell L., Attwell D., Tohyama K., Richardson W.D. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77:873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen K., Sloan S.A., Bennett M.L., Scholze A.R., O’Keeffe S., Phatnani H.P., Guarnieri P., Caneda C., Ruderisch N. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchero J.B., Fu M.-M., Sloan S.A., Ibrahim A., Olson A., Zaremba A., Dugas J.C., Wienbar S., Caprariello A.V., Kantor C. CNS myelin wrapping is driven by actin disassembly. Dev. Cell. 2015;34:152–167. doi: 10.1016/j.devcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A microdomain Ca2+ transient is visible at the tip of the myelin sheath (arrow). Imaging data were overlaid with a confocal z-projected and cropped post-hoc scan at high spatial resolution (1024 × 1024 pixels; 0.207 μm/pixel). The raw imaging data were background subtracted and scaled to the maximum signal in the Video S2. Ca2+ signals were acquired at 40 Hz, played in real time for 6 s duration.
Example of a Ca2+ wave propagating along the myelin internode. In this internode, the wave initiates at the end and propagates toward the middle of the process. For display purposes, the Ca2+ imaging data were overlaid with a z-projected cropped high-resolution post-hoc confocal scan (1024 × 1024 pixels; 0.207 μm/pixel). Ca2+ signals were acquired at 40 Hz, background subtracted and scaled to the maximum intensity.