Abstract
Abnormal mitochondrial morphology, especially fragmented mitochondria, and mitochondrial dysfunction are hallmarks of a variety of human diseases including heart failure (HF). Although emerging evidence suggests a link between mitochondrial fragmentation and cardiac dysfunction, it is still not well described which cardiac signaling pathway regulates mitochondrial morphology and function under pathophysiological conditions such as HF. Mitochondria change their shape and location via the activity of mitochondrial fission and fusion proteins. This mechanism is suggested as an important modulator for mitochondrial and cellular functions including bioenergetics, reactive oxygen species (ROS) generation, spatiotemporal dynamics of Ca2+ signaling, cell growth, and death in the mammalian cell- and tissue-specific manners. Recent reports show that a mitochondrial fission protein, dynamin-like/related protein 1 (DLP1/Drp1), is post-translationally modified via cell signaling pathways, which control its subcellular localization, stability, and activity in cardiomyocytes/heart. In this review, we summarize the possible molecular mechanisms for causing post-translational modifications (PTMs) of DLP1/Drp1 in cardiomyocytes, and further discuss how these PTMs of DLP1/Drp1 mediate abnormal mitochondrial morphology and mitochondrial dysfunction under adrenergic signaling activation that contributes to the development and progression of HF.
Keywords: adrenoceptor, Ca2+/calmodulin-dependent protein kinase II (CaMKII), protein kinase A (PKA), protein kinase D (PKD), calcineurin, GTPase, mitochondrial permeability transition pore, apoptosis, phosphorylation
1. Introduction
Mitochondria are essential eukaryotic organelles that generate the energy necessary for a myriad of cellular processes (see reviews [1,2,3,4,5]). Cellular distribution, organization, and the shape of mitochondria vary greatly in different mammalian cells/tissues, which is presumed to be a result of adaptation for specific functions of highly differentiated organ systems in multi-cellular organisms [1,6,7]. It is widely reported using cell culture systems that mitochondria can change their shape and location frequently, collectively termed “mitochondrial dynamics”, which is suggested as one of the most important modulators for bioenergetics, reactive oxygen species (ROS) generation, spatiotemporal dynamics of Ca2+ signaling, cell growth, and death in cell- and tissue-specific manners [8,9,10,11]. The most well-characterized processes for changes in mitochondrial shape are fission and fusion, which are regulated by the members of the dynamin family of large GTPases ubiquitously expressed in the various cells/tissues; Dynamin-like/related protein 1 (DLP1/Drp1) drive mitochondrial fission, whereas mitofusin 1 and 2 (Mfn1 and Mfn2), and optic atrophy 1 (OPA1) mediate fusion of the outer and inner mitochondrial membranes, respectively [12,13,14].
The mitochondrial dynamics of excitable cells such as cardiomyocytes (CMs) have attracted attention among researchers due to their cellular energetic demands as well as their abundant expression of mitochondrial fission/fusion proteins [15,16]. In adult CMs, the globular-shaped mitochondria are densely packed into the intermyofibrillar spaces with one or more mitochondria for each sarcomere, which likely restricts mitochondrial dynamics [15,16,17,18]. Therefore, mitochondrial dynamics in striated muscles (i.e., cardiac and skeletal muscles) as well as their physiological relevance to mitochondrial and cellular function have long been under debate. By using a photoactivable green fluorescent protein (GFP) in the mitochondrial matrix both in isolated cells and in vivo, it has been reported that mitochondria form local networks and undergo fusion events to share mitochondrial matrix content to neighboring mitochondria in both adult skeletal and cardiac muscles [19,20,21]. However, Hajnoczky’s group further demonstrated that these fusion events (or mitochondrial networks/interactions) were significantly slower (≅3–5 min), less frequent, and more stable compared to non-differentiated myoblasts or neonatal cells [19,20]. They also found that fusion events are dependent on the activity of mitochondrial fusion proteins, OPA1 and Mfn1 [20]. Indeed, Balaban’s group also used three-dimensional (3D) electron microscopy (EM) to show that mitochondria in both skeletal and cardiac muscles form highly connected networks and communicate through junctions, although they did not precisely demonstrate whether there is continuity in the matrix all along the conducting elements [22,23,24]. Thus, this emerging evidence suggests that the muscle mitochondria possess at least slow fusion machinery activity under physiological conditions, which maintains limited mitochondrial networks inside the myofibrils. However, since the experimental techniques for detecting mitochondrial fission events in live myofibrils are still being developed [25], the frequency and speed of fission events in striated muscles including adult CMs remain unclear. Moreover, it is largely unknown whether mitochondrial fission/fusion events influence the beat-to-beat-based regulation of excitation–contraction (E–C) coupling in CMs.
In the heart (especially in the ventricles), cardiac mitochondria occupy over 30% of the cell volume [6,26] and are usually classified into three groups according to their location: intermyofibrillar mitochondria (IFM), subsarcolemmal mitochondria (SSM) and perinuclear mitochondria (PNM) [7,15]. Importantly, abnormal mitochondrial morphologies concomitant with mitochondrial dysfunction in the heart are frequently observed in both human patients and animal models of cardiac diseases. For instance, transmitted EM (TEM) images from human ventricle samples of cardiomyopathy and heart failure (HF) frequently show the misalignment of IFM lying along the sarcomeres with smaller, rounder mitochondria compared to non-failing hearts [27,28,29,30]. In line with these observations in human hearts, animal models of HF through hypertrophy and ischemia exhibit similar changes in IFM morphology [31,32,33,34,35,36,37]. On the other hand, in animal hearts after acute ischemia, cardiac mitochondria tend to maintain their morphology and integrity, but the matrix density of IFM and SSM are significantly decreased, as seen in EM images [38,39]. This is mainly caused by opening of the mitochondrial permeability transition pore (mPTP) [40]. These observations indicate that mitochondrial fragmentation frequently occurs under chronic pathological conditions such as HF in the heart, possibly due to increased mitochondrial fission activity [41] and/or the inhibition of the fusion activity [20]. Notably, fragmented mitochondria are frequently observed in a variety of human diseases including neurodegenerative diseases, diabetes, and cancer in addition to cardiac diseases [42,43]. Although the evidence clearly suggests a link between mitochondrial fragmentation and cardiac pathology, several important questions remain:
-
(1)
Which cardiac signaling pathways regulate mitochondrial morphology and function under pathophysiological conditions?
-
(2)
How do the cardiac signaling pathways modulate the activities of mitochondrial fission and fusion proteins?
-
(3)
Do abnormal morphologies of cardiac mitochondria contribute to the development of cardiac pathology or are they just an adaptation/maladaptation to pathological changes in the heart?
Since the early 1990s, sympathetic nervous system activation and its downstream signaling in CMs (i.e., adrenoceptor (AR) signaling) has been well recognized as a main factor causing or exacerbating cardiac pathology, especially HF [44,45,46,47,48,49], in addition to its role as the primary regulator of E–C coupling under physiological conditions [50,51] (Figure 1). Moreover, growing evidence indicates that post-translational modifications (PTMs) of mitochondrial fission and fusion proteins play a role in regulating mitochondrial dynamics in various cell types/tissues [52,53,54,55]. Recently, new studies have reported novel signaling mechanisms located downstream of AR (e.g., kinases and phosphatases), which lead to PTMs of a mitochondrial fission protein DLP1 and regulate mitochondrial and cellular function in CMs [56,57,58,59,60]. Therefore, in this review, we summarize molecular mechanisms underlying PTMs of DLP1 in regulating mitochondrial fission under AR signaling, and further discuss whether PTMs of DLP1 by AR signaling pathways mediate mitochondrial and cellular injury in HF.
Figure 1.
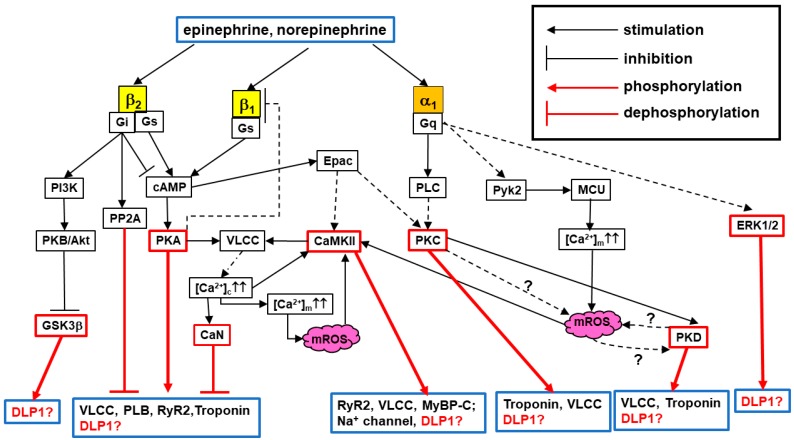
Overview of adrenergic signaling pathways in cardiomyocytes: Schematic diagram of cardiac AR subtypes and their downstream signaling. α1-AR consists of three highly homologous subtypes, including α1A-, α1B-, and α1D-AR, but we only show the downstream pathways from the main subtype in CMs, α1A-AR, in this diagram. Major Ca2+ handling proteins are also shown as the substrates for the downstream kinases of ARs. Dot lines indicate the pathways with multiple steps that are abbreviated in this scheme. Dot lines with “?” indicate the pathways with less evidence from publications, but highly promising pathways. PI3K, phosphoinositide 3-kinase; PLC, phospholipase C; PKB, protein kinase B; Pyk2, Protein tyrosine kinase 2; Epac, exchange factor directly activated by cAMP; MyBP-C, myosin-binding protein C; PP2A, Protein phosphatase 2; mROS, mitochondrial ROS; GSK3β, glycogen synthase kinase 3β; CaN, calcineurin.
2. Overview: Adrenergic Regulation of Mitochondrial Functions in Cardiomyocytes
In adult CMs (in this review, we refer mostly to adult ventricular myocytes), several subtypes of ARs including β1-, β2-, β3-, and α1-ARs [45,61,62,63,64] are expressed with β1-AR being the most abundant [49]. As mentioned above, AR signaling is an important regulator for physiological E–C coupling on a beat-to-beat basis. Catecholamines (i.e., epinephrine and norepinephrine) act on these ARs in CMs to regulate heart inotropy, chronotropy, and growth [45,49,50,61,62,63,64,65,66] via AR subtype-specific downstream pathways [45,61,65,67]. For instance, β-AR signaling increases the transient rise of cytosolic Ca2+ concentration ([Ca2+]c) (i.e., Ca2+ transient: CaT) about 1.5–2-fold at each beat, which triggers increased cardiac contraction [50,51]. This mechanism has been well-described in terms of the acute PTMs (mainly phosphorylation) of the major Ca2+ handling proteins such as voltage-gated L-type Ca2+ channel (VLCC), ryanodine receptor type 2 (RyR2), and phospholamban. The stimulation of α1-AR also increases CaT, although much less than that of β-AR stimulation (≅10–15%), but also contributes to positive inotropic effects in the heart. This difference in the contribution of β-ARs and α1-AR to E–C coupling is mainly due to the difference in downstream signaling activated under each AR. ARs are part of the large family of G protein-coupled receptors (GPCRs) and AR subtypes couple to different G proteins (e.g., β1- and α1-AR interact mainly with Gs and Gq, respectively), thus activating distinct signaling pathways [45,46] (Figure 1), though cross-talk signaling between α1- or β-ARs may also exist [45,68,69,70]. Under acute AR stimulation (e.g., “fight-or-flight” responses), heart rate and contractile force generated by CMs increase, indicating that cardiac mitochondria increase their ATP production temporarily for this additional energy demand. Though further investigations are required, possible mechanisms for matching the energy demand to supply under acute AR stimulation include:
-
(1)
β-AR signaling dramatically increases CaT, promoting the acceleration of Ca2+ uptake from the cytosol to the mitochondrial matrix via mitochondrial Ca2+ uniporter (MCU), thus increasing mitochondrial matrix Ca2+ ([Ca2+]m) and consequently activating the tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS) to enhance ATP production [71,72].
-
(2)
α1-AR stimulation increases the phosphorylation of MCU, which additionally enhances Ca2+ uptake, maintaining the higher [Ca2+]m [73,74] (see Figure 1).
Importantly, [Ca2+]m elevation by prolonged β-AR or α1-AR stimulation (e.g., ≥15 min) also increases mitochondrial ROS (mROS) generation, which triggers mPTP opening or arrhythmogenic [Ca2+]c oscillation [74,75]. Sheu and colleagues showed that increased [Ca2+]m evoked by [Ca2+]c elevation using an inhibitor of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) thapsigargin can promote DLP1 translocation to the mitochondria, followed by mitochondria fragmentation and increased mROS in cultured CMs [41]. However, there are several remaining questions to be resolved:
-
(1)
Does DLP1 translocation occur under physiological [Ca2+]c elevation, such as increased CaT under acute β-AR stimulation?
-
(2)
Does DLP1 translocation occur via increased [Ca2+]m and/or [Ca2+]c?
-
(3)
Are mitochondrial dynamics, especially fission, involved in the process for energy matching and/or mROS production under AR stimulation (see also Section 4)?
In addition to acute and subacute effects of AR signaling, it is well-established that chronic AR stimulation promotes pathological remodeling, leading to cardiac hypertrophy and/or HF [49,76]. Since α1-AR density in the heart is considerably lower than that of β-ARs (≅20% in human myocardium), the contribution of α1-ARs to AR-mediated cardiac hypertrophy may be less prominent than that of β-ARs in vivo [61]. However, another important observation is that β-AR signaling is downregulated via β-AR internalization under pathophysiological conditions including HF (see also Figure 1), whereas α1-AR expression at the plasma membrane has been shown to be unchanged [62,67,77]. Therefore, as the relative proportion of α1-AR increases, α1-AR stimulation has a more important role exacerbating pathological conditions of myocardium such as HF. Indeed, in addition to β-AR pathways, signaling pathways of cardiac Gq-protein-coupled receptors (GqPCRs)—including α1-ARs, angiotensin II receptors, and endothelin I receptors—are critical for the development and progression of HF [78,79]. It should be noted that experimentally-induced chronic AR stimulation causes mitochondrial fragmentation in isolated CMs and in vivo animal hearts [56,60,80,81,82,83,84]. Though it is still unclear whether the alteration in the stoichiometric ratio of fission/fusion proteins creates an imbalance between fusion and fission in HF ([34,35,85]), these observations have introduced the idea that PTMs of mitochondrial fission/fusion proteins by AR signaling may be at least part of the mechanism for promoting mitochondrial fragmentation in the CMs during HF. Indeed, there are few publications showing the PTMs of mitochondrial fusion protein and their functional consequences (i.e., inhibition of fusion) in the CMs [86,87,88]. In this review, we specifically focus on the PTMs of mitochondrial fission protein DLP1 and discuss the possible molecular mechanisms of enhanced mitochondrial fission under chronic AR stimulation (see following Section 3 and Section 4).
3. DLP1-Mediated Mitochondrial Fission in Non-Cardiac and Cardiac Cells
The major proteins involved in mammalian mitochondrial fission are a soluble cytosolic protein DLP1 (also called Drp1) and its receptor mitochondrial fission factor (Mff) which is anchored at the outer mitochondrial membrane (OMM) (see reviews [89,90,91,92]). In addition, DLP1 is known to associate with various membrane structures in the cell including peroxisomes [93], lysosomes, late endosomes and the plasma membrane [94]. DLP1 possesses GTPase activity that provides DLP1 with membrane remodeling activity [95,96] after associating with the membrane structures such as OMM or the peroxisomal membrane, thus promoting fission of mitochondria or peroxisomes, respectively [93,97,98]. The domain structure of DLP1 includes the N-terminal GTPase domain, middle domain, variable domain (VD, also known as B-insert), and the C-terminal GTPase effector domain (GED) [99,100,101] (Figure 2).
Figure 2.
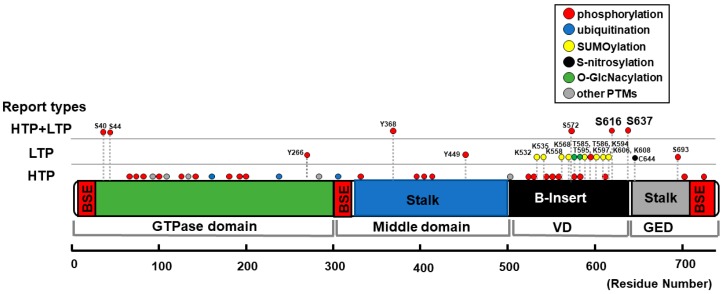
Schematic diagram of DLP1 structure and distribution of PTMs. Structure-based domain architecture of human DLP1 isoform 1 is depicted by the modified information of DLP1 isoform 2 from Fröhlich et al. [100]. If the publication record(s) is (are) only from proteomics and mass spectrometry data (“high-throughput papers” [HTP]), these sites are shown as HTP. The HTP information was collected from PhosphoSitePlus [102]. If the function of the specific site is investigated by methods other than high-throughput mass spectrometry (e.g., mutagenesis), then these records are categorized as “low-throughput papers” (LTP). If both low- and high-throughput records exist, then these records are shown as HTP + LTP. All the reports from LTP are summarized in Table 1. “Other PTMs” include acetylation, methylation, and proteolytic cleavage. If the information is from rat and mouse DLP1, site numbers are shown as the orthologous sites in human. BSE, bundle signaling element; GED, GTPase effector domain; VD, variable domain.
Based on the reports of crystal structure of DLP1, this protein has a globular head and stalk structures, similar to conventional dynamin [100]; the GTPase domain forms the head, and the stalk is composed of a middle domain and GED connected by a loop formed by B-insert; hence, the B-insert is located at the tip of the stalk, at the opposite end of the GTPase head domain. Importantly, the B-insert loop contains the two well-known serine (Ser or S) phosphorylation sites (S616 and S637) that determine mitochondrial translocation of DLP1 (Figure 2), suggesting that phosphorylation-mediated conformational changes may regulate DLP1 recruitment to the OMM [100,103,104].
A DLP receptor, Mff is a C-terminally anchored protein located at the OMM [105,106] and the peroxisomal membrane [107], whereas mitochondrial dynamics proteins of 49 and 51kDa, termed MiD49 and MiD51, respectively, are N-terminally anchored proteins at the OMM [108,109]. While both Mff and MiD49/51 are DLP1 receptors, the relative contribution of each receptor for the overall fission activity in various cell types/tissues is still controversial [110,111]. In the heart, Mff is likely the primary receptor at the OMM, since the loss of Mff in vivo causes a significant decrease in mitochondrial fission and more severe cardiomyopathy [112]. Based on recent publications [104,113,114,115], a model of how DLP1-Mff interaction at OMM contributes to fission is proposed as follows:
-
(1)
B-insert structure of DLP1 normally blocks the Mff-binding sites in DLP1.
-
(2)
B-insert binds to cardiolipin at the OMM, which allows the exposure of the Mff-binding site of DLP1 for DLP1-Mff interaction.
-
(3)
Binding of DLP1 dimers to Mff allows DLP1 assemblies to form functionally active DLP1 oligomers on the OMM in the form of a helical ring. GTP-induced constriction of the DLP1 helical ring plays a crucial role in mitochondrial membrane fission.
Since B-insert contains sites for phosphorylation, O-GlcNAcylation, and SUMOylation [56,58], PTMs of DLP1 may induce a conformational change of B-insert that regulate interactions between DLP1 and Mff on the membrane undergoing fission. However, further studies are required to delineate the detailed molecular mechanism of how the interactions between DLP1 and Mff are regulated at the OMM to provide the force for fission. Moreover, in addition to mitochondrial fission, DLP1-Mff interactions are also critical for peroxisomal fission. Considering that fatty acid metabolism is the primary energy source of the heart, the mitochondria and peroxisomes are the two main sites of β-oxidation of fatty acids in CMs [116], and peroxisome proliferator-activated receptors (PPARs) has been shown to be important pathways for mitochondrial protection and biogenesis in the heart [117,118,119], it is reasonable to surmise that the regulation of peroxisome morphology by DLP/Mff may have important roles for modulating cardiac energy metabolism and/or ROS production. However, there are currently only a few studies assessing peroxisome morphology in adult CMs. Further studies are required for precisely understanding the role of DLP/Mff on peroxisome function, morphology and the consequent role in regulating energy metabolism in the heart.
Multiple PTMs of DLP1 have been detected by mass spectrometry-based proteomics as shown in the online open data base (e.g., PhosphoSitePlus [102]) (Figure 2). Some of the PTM sites in DLP1 have been further investigated, and their physiological and pathological relevance to mitochondrial and cellular functions have been assessed in various cell types including CMs (see also reviews from others [52,55,89]). These include (1) phosphorylation, (2) ubiquitination, (3) SUMOylation, (4) S-nitrosylation, and (5) O-linked-N-acetyl-glucosamine glycosylation (O-GlcNAcylation), which can change functional properties of DLP1 such as subcellular localization, protein–protein interactions (e.g., DLP1-Mff binding), protein stability, and GTPase activity. Figure 2 and Table 1 summarize the PTM sites within the DLP1 structure, the mediating molecules (e.g., kinases and phosphatases), and their effects reported in non-cardiac cells and CMs.
Table 1.
Post-translational modifications of DLP1 and their effects on mitochondrial morphology.
| Position | Type of Modification | Type of Reports | Upstream Molecules | Effect on Mitochondrial Morphology | Detected/Tested in CMs? |
|---|---|---|---|---|---|
| S40 S44 |
phosphorylation | HTP + LTP | GSK3β [120] | phosphorylation → fragmentation [120] |
No (primary cultured hippocampus neurons [120]) |
| Y266 Y368, Y449 |
phosphorylation | LTP only or HTP + LTP | c-Abl [121] | phosphorylation → fragmentation [121] |
No (neuron-specific c-Abl knockout mice [121]) |
| K532 K535 K558 K568 K594 K597 K606 K608 |
SUMOylation | LTP | Ubc9 [122] MAPL [123,124] SENP5 [125,126,127] |
SUMOylation → fragmentation [122,123,124] deSUMOylation → elongation [125] → enlarged and swollen mitochondria [127] → fragmentation [126] |
Yes [127] |
| S572 | phosphorylation | HTP + LTP | CDK5 [128] | phosphorylation → fragmentation [128] dephosphorylation → elongation [128] |
No (cerebellar granule neurons [128]) |
| T585 T586 |
O-GlcNAcylation | LTP | N.D. (O-GlcNAc-transferase?) |
O-GlcNAcylation → fragmentation [129] |
Yes [129] |
| T595 | phosphorylation | LTP | LRRK2-G2019S [130] | phosphorylation → fragmentation [130] dephosphorylation → elongation [130] |
No (HeLa cells, HEK293 cells, human fibroblasts [130]) |
| S616 | phosphorylation | HTP + LTP | CDK1/cyclin B [131,132,133,134] CDK5 [135] ERK1/2 [103,136,137,138]. PKCδ [133,139] CaMKII [57,140] ROCK [141] PP2A [142] Rip1 [143] |
phosphorylation → fragmentation [103,133,136,137,138,142] → elongation [135] dephosphorylation → elongation [133,139] → fragmentation [135] S616D → increased fission event rate [140] → no significant changes [144] → elongation [135] S616A → elongation [131] → fragmentation [56,135] → no significant changes [132,137,139,141,144] |
Yes [56,57,141,143] (See also foot notes for 1 [57], 2 [103], 3 [133], and 4 [56]) |
| S637 | phosphorylation | HTP + LTP | PKA [58,145,146,147] CaMKIα [148] ROCK1 [149] PKD [56] Pim-1 [150] CaN [58,60,133,144,151,152] PP2A [153,154] |
phosphorylation → fragmentation [56,148,149] → elongation [58,145,146,147,152,153,154] dephosphorylation → fragmentation [60,133,151,153,154] S637D → elongation [58,144,145,152] → fragmentation [148] S637A → fragmentation [58,144,152] → elongation [148] → no significant changes [56] |
Yes [56,58,60,150] (see also foot notes for 3 [133], and 5 [150]) |
| C644 | S-Nitrosylation |
LTP | NO [155,156,157] | S-Nitrosylation → fragmentation [156,157,158] → no significant changes [155] |
No (Neurons [156,157,158], human brain tissue [155]) |
| S693 | phosphorylation | HTP + LTP | GSK3β [159] | phosphorylation → elongation [159] dephosphorylation → fragmentation [159] |
No (HeLa cells, HEK293 cells [159]) |
| N.D. | ubiquitination | HTP + LTP | MARCH5 [160,161,162,163] Parkin [164,165] |
ubiquitination → degradation of DLP1, followed by elongation [160,161,164,165] → recruitment of DLP1 to the OMM followed by fragmentation [162,163] |
No (HeLa cells [160,162,164], COS7 cells [161], RGC5 cells [163], SH-SY5Y cells [164,165]. (See also foot note for 6 [166]) |
HTP, high-throughput papers (see also Figure 2); LTP, Low-throughput papers (see also Figure 2); LRRK2, Leucine-rich repeat kinase 2; c-Abl, abelson murine leukemia viral oncogene homolog 1; CDK, Cyclin-dependent kinase; N.D., not determined; Pim-1, Proto-oncogene serine/threonine-protein kinase; GSK3β, glycogen synthase kinase 3β; CaN, calcineurin; Rho-associated protein kinase (ROCK). 1 Xu et al. detected DLP1 phosphorylation at S616 and S637 in CMs, but the effect of CaMKII on mitochondrial morphology was analyzed in H9C2 cardiac myoblasts [57]. 2 Yu et al. detected phosphorylation of DLP1 at S616 using an in vitro kinase assay and mitochondrial morphological changes were analyzed in H9c2 cells [103]. 3 Zaja et al. used the HL-1 CM line derived from the AT-1 mouse atrial myocyte tumor lineage for detecting DLP1 phosphorylation at S616 and S637 [133]. 4 Jhun et al. detected DLP1 phosphorylation at S616 in H9c2 cells, and heart trusses, but the effect of S616 phosphorylation on mitochondrial morphology was analyzed in H9C2 cells [56]. 5 Din et al. showed that Pim-1 decreases DLP1 expression and increases S637 phosphorylation, which results in mitochondrial elongation [150]. 6 Song et al. showed that cardiac-specific parkin ablation moderated hyper-mitophagy in DLP1-deficient hearts in vivo, but the phenotype of single deletion of Parkin in the heart was not documented [166].
Among these phosphorylation sites on DLP1, S616 and S637 are well reported (Figure 2). Amino acid sequences surrounding S616 matches the consensus motif for Cyclin-dependent kinase (CDK1/cyclin B) [131,132,133,134] and extracellular signal–regulated kinase 1/2 (ERK1/2) [103,136,137,138], and this site was shown to be phosphorylated by these kinases. In addition, protein kinase Cδ (PKCδ) and Ca2+/calmodulin-dependent protein kinase II (CaMKII) have been shown to be upstream kinases for DLP1 phosphorylation at S616, although the sequences around this site do not match the consensus motifs of these kinases [57,133,139,140]. Another Ser phosphorylation site S637 is reported as a site for protein kinase A (PKA) [58,145,146,147], Ca2+/calmodulin-dependent protein kinase Iα (CaMKIα) [148], protein kinase D (PKD) [56], Ca2+-dependent phosphatase calcineurin (CaN) [60,144,151], and protein phosphatase 2 (PP2A) [153,154]. Importantly, S637 is located within a consensus motif for Rho-associated protein kinase (ROCK), and its phosphorylation was detected in endothelial cells [149]. However, Brand and his colleagues showed that in neonatal CMs, ROCK cannot phosphorylate S637, but can phosphorylate S616 [141].
S616 phosphorylation consistently has resulted in enhanced mitochondrial fission in various cell types tested by multiple labs. However, the functional consequences of S637 phosphorylation for regulating mitochondrial morphology is still highly controversial [167]. For instance, various reports on the overexpression of phospho- or dephospho-mimetic mutants of DLP1 (S637D and S637A, respectively) have resulted in conflicting outcomes for mitochondrial morphology: S637D introduction promotes mitochondrial elongation [58,144,145], or fragmentation [148]; and S637A promotes fragmentation [58,144], elongation [148], or no significant changes in mitochondrial morphology [56,145] (Table 1). Moreover, another PTM of DLP1, O-GlcNAcylation at threonine (Thr or T) 585 and 586, has been shown to lead to dephosphorylation of DLP1 at S637, followed by DLP1 translocation to the OMM and mitochondrial fragmentation under hyperglycemic conditions in neonatal CMs [129] (Table 1). However, we recently reported that PKD-dependent phosphorylation of S637 promotes DLP1 translocation to the OMM and mitochondrial fragmentation under α1-AR stimulation in neonatal CMs [56]. The differing and conflicting outcomes of DLP1-S637 phosphorylation observed by different groups shown above may be partly due to:
-
(1)
Different cell types used for the overexpression of DLP1 mutants which possess distinct mitochondrial shapes/networks in resting conditions (i.e., highly elongated and interconnected, or mixture of small and tubular mitochondria).
-
(2)
Involvement of different upstream molecules (i.e., kinases and phosphatases) listed above.
-
(3)
Co-existence of additional PTMs within DLP1 (or in other fission/fusion proteins) caused by the different upstream molecules in addition to S637 phosphorylation.
These discrepancies will be discussed further in Section 4, specifically in the case of CMs. DLP1 has also been shown to be ubiquitinated by E3 ubiquitin ligases including MARCH5 (also known as MITOL) [160,161,162,163] and Parkin [164,165] (Table 1). Initial studies for MARCH5-DLP1 interactions showed DLP1 ubiquitination and consequent degradation [160,161], which may be a direction towards decreased fission activity. However, later studies suggest that DLP1 ubiquitination by MARCH5 instead promotes mitochondrial fission, possibly by mediating the recruitment of DLP1 at specific fission sites [162,163]. On the other hand, the consensus understanding for Parkin-mediated DLP1 ubiquitination is that this signal induces DLP1 degradation in a proteasome-dependent pathway [164,165].
SUMOylation is a PTM that promotes the addition of small ubiquitin-like modifier [SUMO] proteins to multiple lysine (Lys or K) residues of various proteins including DLP1 [122,168] (Figure 2 and Table 1). The SUMOylation of DLP1 is driven by a mitochondrial-anchored SUMO E3 ligase, mitochondrial-anchored protein ligase (MAPL), that stabilizes the oligomeric form of DLP1 on the OMM to increase mitochondrial fission during apoptosis [123,124]. On the other hand, Sentrin/SUMO-specific protease 5 (SENP5) participates in the deSUMOylation of DLP1, which reduces mitochondrial fission [125].
S-nitrosylation of DLP1 has been reported in neuronal cells but may need further investigation to confirm its functional relevance due to the limited number of publications from only a few groups to date (Figure 2 and Table 1). For instance, nitric oxide (NO)-mediated S-nitrosylation of DLP1 at cysteine (Cys or C) 644 was proposed to increase mitochondrial fission in neurons, and contribute to the pathologies of neurodegenerative diseases including Alzheimer’s and Huntington’s diseases especially from Lipton’s group [156,157] (see also their reviews [169,170]). However, other groups’ studies indicate that S-nitrosylation of DLP1 itself does not change DLP1 activity [155], but rather NO or S-nitrosylation of DLP1 mediates increased S616 phosphorylation to promote fission [155,158].
As listed above, in addition to phosphorylation and O-GlcNAcylation, several other PTMs of DLP1 (ubiquitination, SUMOylation, and S-nitrosylation) were reported in cultured cell lines or non-cardiac primary cells. However, these PTMs have not yet been established as having physiological and/or pathological roles in the CMs. Therefore, in the following section, we will briefly discuss the possibility that these PTMs participate in signaling pathways that promote or exacerbate HF.
Finally, it should be mentioned that several reports show the PTMs of DLP1 receptors (adenosine monophosphate-activated protein kinase [AMPK]-dependent phosphorylation of Mff [171,172] and MARCH5-dependent ubiquitination of MiD49 [173]) by cellular signaling that may modulate mitochondrial fission activity. Though these findings are also crucial in understanding the whole picture of regulation of mitochondrial fission activity by cardiac signaling pathways, we mainly focus on PTMs of DLP1 in this review due to the limited number of reports related to PTMs of DLP1 receptors compared to those of DLP1.
4. Molecular Mechanisms Underlying the Modulation of DLP1 Function by Adrenergic Signaling in Cardiomyocytes
4.1. Suitable Models for Investigating Post-Translational Modifications of DLP1 and Their Roles in Cardiomyocytes
Historically, DLP1 research has thrived off knowledge obtained from cultured cell lines and/or non-excitable cells (Table 1). Moreover, the physiological and pathological importance of DLP1 function in vivo has emerged from recent clinical reports showing that individuals who possess a DLP1 mutation exhibit neurological disorders [174,175,176,177,178]. Although a DLP1 mutation (DLP1-C452F) causing cardiomyopathy was reported in mice [179,180], cardiac disorders in patients who possess DLP1 mutations have not been yet documented. These prior studies lead us to the idea that other types of DLP1 modifications in CMs are likely to have more pivotal roles for driving cardiac dysfunctions in vivo compared to its mutations. In this context, many laboratories have started investigating the physiological and pathological roles of DLP1 and its PTMs, specifically in the adult CMs (especially, adult ventricular myocytes)/hearts. However, there are multiple technical challenges for using adult CMs in DLP1 research that needs to be overcome. These include:
-
(1)
Difficulty in observing mitochondrial morphology/dynamics (especially fission events) in adult CMs under light/confocal microscopy [19,20] (See also Section 1)
-
(2)
Difficulty in maintaining the specific cellular membrane integrity (i.e., T-tubules) in cultured adult CMs [11,57,74]
-
(3)
Limitation for gene manipulations in isolated CMs without culturing certain periods (e.g., 24–48 h) [11,57,74]
For point (1), a combination of the conventional TEM and state-of-the-art 3D EM [22,23,24] provides us with some clues (see also Introduction), although live cell imaging is still a challenge. Mainly due to the points of (2) and (3), it is still a reasonable idea to extract information of cardiac-specific DLP1 function from various “culture cell models for adult CMs” that possess a similar structure of signaling pathways, even though their mitochondrial morphologies/dynamics at resting conditions differ from those of adult CMs [181]. For instance, the following cell types are commonly used in addition to cultured adult CMs: HL-1 mouse atrial CM cell line [133], H9c2 cardiac myoblasts [56,57,182], primary neonatal CMs [56,141,150], and human induced pluripotent stem cells (iPSCs)-derived CMs [183] (see also the notes for Table 1). Therefore, we introduce the data from culture cell models mentioned above in addition to adult CMs. The use of putative DLP1 inhibitors, including Mdivi-1, has been widely applied to reduce the GTPase activity of DLP1 in cells and in vivo, which frequently shows protective effects from mitochondrial injury and cell death (see review [184]). However, the effects of these beneficial effects are likely via non-specific inhibition of complex I by these drugs [185], and thus the data obtained by the use of Mdivi-1 may not be suitable for understanding the role of DLP1 function as well as PTMs of DLP1. Therefore, to precisely understand the role of PTMs of DLP1 under AR stimulation in CMs, we develop our discussion here based on the accumulating data using the mutants of DLP1 that mimic or diminish PTMs of DLP1 as well as the experimental results from the genetic modification of upstream signaling pathways, rather than the reports using the pharmacological inhibition of DLP1 activity and upstream signaling.
4.2. Overview of DLP1 Phosphorylation by Adrenergic Signaling in Cardiomyocytes
It is widely accepted that chronic AR stimulation (either the stimulation of β-AR alone [81,82,83,84], α1-AR alone [56,80], or both [60], which commonly occurs under HF, contributes to mitochondrial fragmentation and dysfunctions in CMs and/or in vivo animal hearts. In addition, emerging evidence shows that chronic GqPCR stimulation including α1-AR also causes mitochondrial fragmentation and dysfunctions [186,187,188]. These observations lead to the idea that prolonged stimulation of Gs/Gq-protein signaling promotes mitochondrial fission in CMs. Several signaling molecules under the cascades of AR subtypes and Gs/Gq proteins (see Figure 1) were found as a possible candidate to modulate DLP1 via its PTMs in CMs (Figure 3A) (see also Section 3). As shown in Figure 3B and Table 1, most accumulated records of publications from adult CMs, cultured CM-model cells (see Section 4.1), and cardiac tissues concern phosphorylation at S616 and S637. Therefore, next we will summarize the possible molecular mechanisms for phosphorylation at S616 and S637 in DLP1 by each AR-subtype signaling pathway and discuss the physiological and pathological relevance of each phosphorylation to mitochondrial morphology and function.
Figure 3.
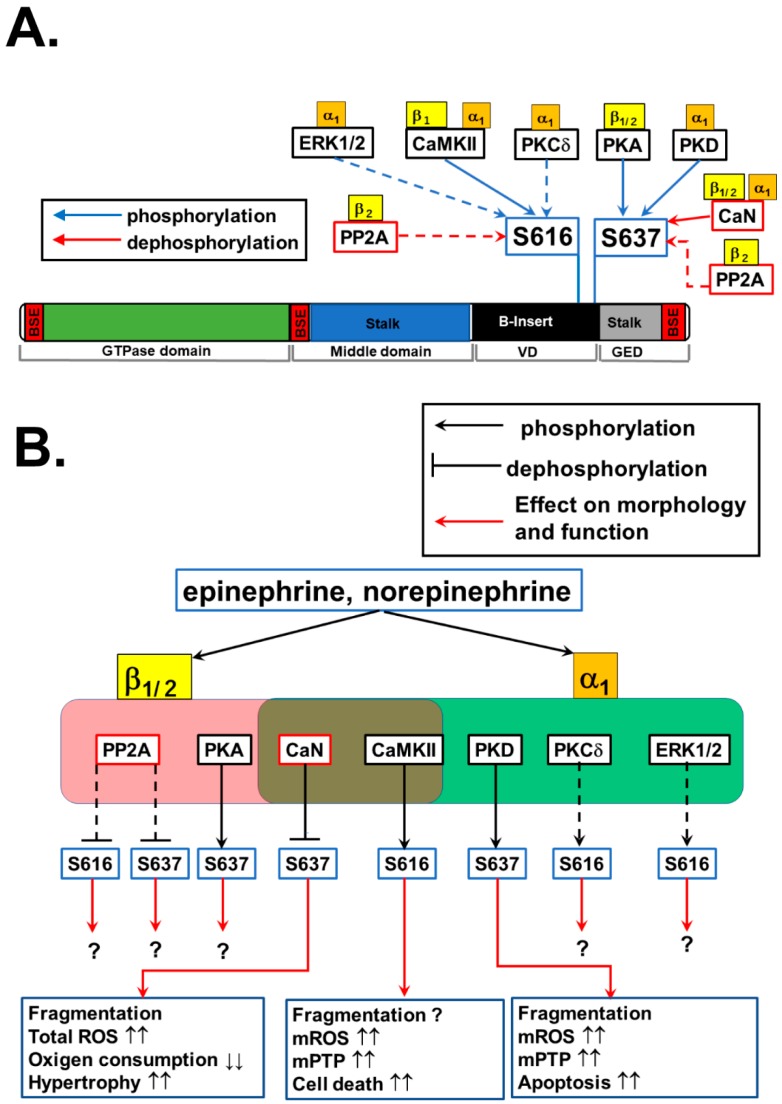
Phosphorylation of DLP1 and their effects on mitochondrial morphology and mitochondrial and cellular functions in cardiomyocytes: (A) DLP1 phosphorylation at S616 and S637 and their potential upstream signaling under AR stimulation. The upstream AR subtype(s) of each enzyme is(are) also shown. Dotted lines indicate the pathways reported in non-cardiac cells, but not yet tested in CMs. PP2A, Protein phosphatase 2; CaN, calcineurin. (B) Schematic diagram of DLP1 phosphorylation at S616 and S637 by AR signaling and their effects on mitochondrial morphology as well as mitochondrial and cellular functions in CMs. Dotted lines indicate the pathways reported in non-cardiac cells. If the functional effect of the signaling pathway has not yet been tested in CMs, question mark “?” is shown. The effects of CaN, CaMKII, and PKD are extracted from the following publications [56,57,60].
4.3. Phosphorylation of DLP1 at S616 by β-Adrenoceptor Signaling
Wang’s group showed that S616 is phosphorylated in isolated adult CMs by chronic treatment (over 12 h) of a β-AR agonist isoproterenol (Iso) [57]. Moreover, 2-week continuous infusion of Iso in vivo also promotes significant S616 phosphorylation in the heart. They also confirmed that (1) β-AR-mediated S616 phosphorylation is via CaMKII in CMs, and (2) S616 phosphorylation promotes DLP1 translocation to the OMM and increases mitochondrial flashes (i.e., the rate of transient mPTP opening [189]) (Figure 3B), although the sequences around this site do not match the consensus motif of CaMKII [57].
4.4. S616 Phosphorylation of DLP1 by α1-Adrenoceptor Signaling
Several upstream kinases have been reported both in non-cardiac and cardiac cells that may be activated in CMs under α1-AR stimulation, which include CaMKII, PKCδ, and ERK1/2 (See Figure 1, Figure 3A, and Table 1). RhoA/ROCK [141] can also be activated under α1-AR-Gq by an unknown mechanism, but this signaling is not efficacious compared to G12/13 protein-coupled receptor stimulation such as sphingosine 1-phosphatase (S1P) receptors [190]. All three potential candidate kinases listed above are known to be acutely activated by α1-AR stimulation (Figure 1 and Figure 3), but we have shown that 30-min treatment of an α1-AR agonist phenylephrine (Phe) did not promote S616 phosphorylation in DLP1 in H9c2 cells [56]. On the other hand, we also found that significant increases in both S616 and S637 phosphorylation in ventricular tissues from transgenic mice with the overexpression of a constitutively active Gq-protein under HF [56], suggesting that S616 may occur under chronic α1-AR stimulation, but the time course for this phosphorylation is different from that for S637 (see also Section 4.5 and Section 4.7). Therefore, further investigations are required to identify the upstream kinases for S616 phosphorylation under acute α1-AR stimulation and the onset of the activation of this signaling cascades under chronic α1-AR stimulation.
4.5. Role of DLP1 Phosphorylation at S616 in Mitochondrial Morphology and Function during Adrenergic Stimulation
The next question is whether S616 phosphorylation of DLP1 by chronic β-AR and/or α1-AR modulates mitochondrial morphology and function in CMs. Recently, Wang and Sheu’s group proposed that DLP1 has both canonical (i.e., regulation of mitochondrial fission) and non-canonical roles (e.g., regulation of transient mPTP opening and OXPHOS) in adult CMs [25]. Therefore, it is still unclear whether this phosphorylation promotes canonical functions of DLP1 (i.e., increased mitochondrial fission via S616 phosphorylation), and/or increases non-canonical functions of DLP1 (i.e., acceleration of transient mPTP opening independently of fission activity) (Figure 3B) [57]. Further studies may be needed to quantitatively assess whether the canonical and non-canonical functions of DLP1 are linked or occur independently of each other and how S616 phosphorylation regulates both functions in CMs under chronic β-AR stimulation. Lastly, since the upstream kinases for S616 phosphorylation under α1-AR stimulation have not yet been determined (see Section 4.4) and the changes in the level of S637 phosphorylation is likely more prominent than S616 phosphorylation under chronic α1-AR stimulation (see the following Section 4.7), further study will be necessary to investigate the relative contribution of the two phosphorylation sites to modulate mitochondrial fission under α1-AR stimulation in CMs (see also Section 4.8).
4.6. Phosphorylation of DLP1 at S637 by β-Adrenoceptor Signaling
Another phosphorylation site detectable under AR stimulation in CMs is S637. The sequences around the S637 match the consensus motif of PKA. Cribbs and Strack first showed that acute β-AR stimulation by an injection of Iso to the animals and exercise (forced swimming) promotes S637 phosphorylation in DLP1 in the heart in vivo, assuming that this phosphorylation is via PKA based on the observation from non-cardiac cells (Figure 3B) [58]. Next, Wang’s group confirmed using isolated adult CMs that 12-hr incubation with Iso exhibits increased S637 phosphorylation by PKA (but not via CaMKII) [57]. Moreover, they also found that this increased S637 phosphorylation disappears after 2-week Iso infusion in vivo [57]. These observations can be partly attributed to (1) the activation of Ca2+-dependent phosphatase CaN in the later phase of chronic β-AR stimulation (Figure 1 and Figure 3B) [191] and/or (2) the activation of PP2A via β2-AR-Gi pathway [192] (Figure 1 and Figure 3B), which may dephosphorylate DLP1 at S637 [58,60,153,154]. Thus, the phosphorylation levels of S637 may vary time-dependently during β-AR stimulation and HF development in vivo.
4.7. Phosphorylation of DLP1 at S637 by α1-Adrenoceptor Signaling
In addition to PKA, the sequences around the S637 also match to the consensus motif of PKD. Indeed, our group recently showed in neonatal CMs that 30-min treatment of an α1-AR agonist Phe in combination with a β-AR antagonist propranolol promotes S637 phosphorylation in DLP1 via PKD leading to increased association of DLP1 to the OMM [56]. Moreover, we also found a significant increase in S637 phosphorylation in ventricular tissues from mice with the overexpression of the constitutively active Gq-protein concomitant with chronic PKD activation under HF [56], suggesting that S637 phosphorylation occurs in vivo via PKD activation during the course of acute and chronic α1-AR stimulation. However, Pennanen and his colleagues showed that long-term stimulation (24–48 h) with high concentrations of norepinephrine (which stimulates simultaneously both α1-AR and β-AR) causes dephosphorylation of S637 in DLP1 via a CaN-dependent mechanism [60], implying that AR subtypes may have cross-talk signaling that regulates the phosphorylation levels of DLP1 at S637.
4.8. Role of DLP1 Phosphorylation at S637 on Mitochondrial Morphology and Function during Adrenergic Stimulation: Interplay of S637 and S616 Phosphorylation
As briefly mentioned in Section 3, the functional consequences of S637 phosphorylation for the regulation of mitochondrial morphology are still highly controversial in both non-cardiac and cardiac cells. Our recent publication shows that PKD-mediated phosphorylation at S637 under GqPCR stimulation including α1-AR promoted mitochondrial fragmentation, confirmed by the overexpression of a S637A mutant (Figure 3B). Our results are consistent with reports of CaMKIα- and ROCK1-dependent DLP1 phosphorylation in non-cardiac cells [148,149], but these results show the opposite effect of what the majority of groups have reported [58,145,146,147,152,153,154]. This discrepancy may be partly due to the involvement of different upstream molecules in different cell types or in different AR-subtype stimulations in CMs, that may regulate not only the phosphorylation levels of S637 in DLP1, but also impact the PTMs of other site(s) within DLP1 or other mitochondrial fission/fusion machinery proteins. For instance, we assessed β-AR- and PKA-mediated changes in DLP1 phosphorylation, its translocation to the OMM, and mitochondrial morphology in our previous study [56]. We found that β-AR stimulation activates PKA and increases DLP1 phosphorylation at S637, which promotes DLP1 translocation to mitochondria in H9c2 cells, similar to the case of PKD-dependent DLP1 phosphorylation at S637 [56]. However, β-AR stimulation did not show any significant changes in mitochondrial morphology. To understand the molecular mechanisms producing this discrepancy between the effect of PKA and PKD on mitochondrial fission in H9c2 cells, we further assessed the changes in the phosphorylation levels of DLP1 at S616 before and after α1-AR stimulation using a dephospho-mimetic mutant of DLP1, DLP1-S637A. We found that α1-AR stimulation significantly decreases S616 phosphorylation in cells overexpressing a DLP1-S637A mutant. These data sets indicate that phosphorylation at one site within DLP1 may prevent or initiate the phosphorylation or dephosphorylation at the other sites by changing the access of other kinases or phosphatases, since S616 and S637 may be proximal within the 3D structure of DLP1 [100]. Indeed, in neurological diseases, the DLP1 S616/S637 phosphorylation ratio dictates the severity of pathogenesis [193,194]. Another speculation is that the basal S637 phosphorylation state is critical for maintaining S616 basal phosphorylation, indicating that a priming phosphorylation at S637 is required for S616 phosphorylation. The use of DLP1 mutants with double mutations at S616 and S637 [144] in CMs may provide us with more detailed mechanistic insights for the relative roles of these two sites in mitochondrial fission. In summary, it is likely that S637 phosphorylation by AR signaling participates in the mechanism for mitochondrial fragmentation under the chronic AR stimulation in the CMs. Future studies using DLP1 double mutants in CMs are needed to delineate whether the mechanisms for the S637 and S616 phosphorylation of DLP1 are linked to (or independent of) each other under AR stimulation, and if so, how the phosphorylation levels of these two sites cooperatively regulate the overall activity of DLP1 in CMs. These further studies would also provide us with mechanistic insights of the critical difference in the role of S637 phosphorylation on mitochondrial morphology/dynamics between non-cardiac and cardiac cells.
4.9. Role of Adrenergic Signaling-Mediated DLP1 Phosphorylation in the Development of Heart Failure
The remaining questions are (1) how does the status of S616 and/or S637 phosphorylation influence mitochondrial function in CMs under AR stimulation and (2) whether AR-mediated PTMs of DLP1 participate in the process of HF development. As discussed above, short-term stimulation of both β-AR and α1-AR mediates S637 phosphorylation [56,57] (Figure 3A) and this phosphorylation (at least by α1-AR-PKD signaling) is likely to enhance mitochondrial fission in CMs (Figure 3B). Both β- and α1-AR stimulations can sub-acutely increase respiration to match ATP production for this acute additional energy demand [56,71,195] and this effect is possible by the following three separated mechanisms:
-
(1)
Increased [Ca2+]m stimulates the TCA cycle (see also Section 2) [40].
-
(2)
Accumulation of DLP1 to the OMM and the subsequent enhancement of mitochondrial fission by PTMs of DLP1 increase the efficiency of the electron transport chain (ETC) by affecting ultrastructural and spatial organization of the respiratory chain and ATP synthase, which stimulates forward electron flow through the ETC and ATP production [196].
-
(3)
Fragmented mitochondria by PTMs of DLP1 can sustain higher [Ca2+]m levels without propagation of Ca2+ to the neighboring mitochondria [197], which may also activate the TCA cycle to increase ATP generation.
This increased respiration by mitochondrial fission in CMs may be initially beneficial for the failing heart to boost its ATP production and maintain the ability to pump blood. Under well-coupled conditions, stimulation of forward flow by itself preferentially oxidizes the ETC and thereby lowers ROS levels [198]. However, increased electron flow at the ETC may oxidize the reduced forms of nicotinamide adenine dinucleotide and nicotinamide adenine dinucleotide phosphate (NADH and NADPH, respectively), thereby gradually depleting the anti-oxidative capacity [199]. Indeed, 24–48-h treatment of high-dose norepinephrine decreases oxygen consumption concomitant with lowering cellular ATP levels and increasing mROS levels in cultured neonatal CMs [60]. One possibility is that mitochondrial fragmentation via S637 phosphorylation of DLP1 under AR stimulation itself may structurally limit the size of the matrix cavity, which decreases the amount of anti-oxidative enzymes in the matrix. Though S637 phosphorylation may start to decrease due to the increased activity of phosphatases [60] during chronic AR stimulation, CaMKII-dependent S616 phosphorylation increases time-dependently, which increases the probability of mPTP opening [57]. Indeed, it is frequently observed that chronic AR stimulation not only causes mitochondrial fragmentation, but also increases the number of swollen mitochondria with structurally damaged mitochondrial cristae in isolated CMs and/or in vivo animal hearts, indicating enhanced mPTP opening and or OMM permeability under chronic AR stimulations [56,84,200]. Thus, DLP1 phosphorylation may be involved in upstream signaling for mPTP opening and excessive mROS production under chronic AR stimulation, which increases CM apoptosis followed by cardiac fibrosis, ROS-dependent HF signaling activation, and ROS-dependent arrhythmogenic events during HF. Finally, since DLP1 stimulates tBid-induced Bax oligomerization at OMM and promotes cytochrome c release in non-cardiac cells [201], further studies are also required for investigating whether DLP1 can promote OMM permeability via mPTP-independent pathway in CMs under chronic AR stimulations.
4.10. Other Post-Translational Modifications of DLP1 by Adrenergic Signaling
In addition to S616 and S637 phosphorylation, several phosphorylation candidate sites for glycogen synthase kinase 3β (GSK3β) within the structure of DLP1 have been reported [120,159], but the functional relevance of these sites have not yet been tested in CMs (Figure 2 and Table 1). GSK3β activity may be modulated under chronic AR stimulation (Figure 1), but the importance of this kinase activity for regulating mitochondrial function is well described in ischemic/reperfusion (I/R) injury [202] rather than non-ischemic HF induced by pressure overload or neurohumoral injury where chronic AR stimulation exists. In addition to phosphorylation, SUMOylation [127] and O-GlcNAcylation of DLP1 [129] are reported in CMs. SUMOylation of DLP1 promotes its association with the OMM followed by increased CM apoptosis [127], but it is still unknown whether this pathway is activated under AR stimulation during HF. The O-GlcNAcylation of DLP1 associated with hyperglycemia may be involved in diabetes-induced mitochondrial dysfunction such as diabetic cardiomyopathy [129], rather than non-ischemic HF. Currently, there are no reports regarding the role of DLP1 ubiquitination and S-nitrosylation in CMs (see Table 1), though emerging evidence has revealed that these PTM mechanisms are important for cardiac remodeling under chronic β-AR stimulation in the heart [203,204]. Specifically, DLP1 ubiquitination-mediated DLP1 degradation may have an important role for the development of HF since CM-specific DLP1-knockout mice exhibit a detrimental cardiac phenotype by the disturbances of mitochondrial autophagy (i.e., mitophagy) group [205,206]. Sadoshima’s group proposed using the CM-specific DLP1-knockout mice that endogenous expression of DLP1, translocation of DLP1 to the mitochondria, and DLP1-dependent mitophagy are required for protecting the heart against pressure overload-induced mitochondrial dysfunction and HF [205,206]. DLP1 expression in the heart indeed decreases under pressure overload [205], but the detailed mechanism for causing decreased DLP1 is not still clear in this model. Furthermore, this group also reported that mitophagy during myocardial ischemia is mediated via Rab9-associated autophagosomes and S616 phosphorylation of Drp1 by Rip1 [143]. Further studies are needed to investigate whether cardiac AR signaling has a potential to modulate DLP1-mediated mitophagy under pathological conditions.
5. Summary and Concluding Remarks
As summarized in this review, there has been substantial progress in understanding the detailed molecular mechanisms of how PTMs of DLP1, especially phosphorylation of DLP1 at S616 and S637, modulate important mitochondrial functions such as mitochondrial fission, bioenergetics, ROS generation, mPTP activity, and mitophagy using non-cardiac cells. Moreover, thanks to the recent studies using cultured CM-model cells, adult CMs, and cardiac-specific DLP1 knockout mouse lines, the biological importance of DLP1 in cardiac physiology and its contribution to cardiac pathologies have emerged. Phosphorylation of DLP1 by the downstream kinases of AR signaling likely contributes to the development of cardiac dysfunction such as non-ischemic HF. However, it is still up for debate whether altered levels of PTMs in DLP1 are a reflection of overall impairment of cellular function at the end-stage of the disease, or DLP1 PTM-mediated mitochondrial dysfunction serves as one of the pathogeneses of cardiac disease. Furthermore, though this review does not focus on other types of HF such as I/R injury, myocardial infarction, and diabetic cardiomyopathy (see also Section 4.10), it is also possible that other upstream signaling (e.g., ROCK [141], GSK3β [120,159] and CaN [151] under I/R injury and myocardial infarction)-mediated phosphorylation and other types of PTMs may have significant roles for the development of HF. Approaches such as quantitative measurements of the mitochondrial morphology/dynamics, bioenergetics, mROS production, and mPTP activity during the time course of AR stimulation in cultured CM-model cells, adult CMs, and in vivo animal models may provide further information regarding the cardiac-specific regulation of DLP1 function by AR signaling. Lastly, in addition to cardiac-specific DLP1 knockout animals, the development of knock-in mouse lines carrying DLP1 mutations that prevent or enhance its PTM effects (e.g., dephospho- or phospho-mimetic mutant of DLP1) will help resolve the remaining questions in the future.
Author Contributions
Writing and Original Draft Preparation, B.S.J., J.O.-U., and Y.Y.; Writing, Review and Editing, B.S.J., J.O.-U., S.M.A., M.W.C. and Y.Y.; Funding Acquisition, B.S.J., J.O.-U., and Y.Y.
Funding
This work was supported by American Heart Association (AHA) 18CDA34110091 (to B.S.J.), NIH/NHLBI R01HL136757 (to J.O.-U.), NIH/NIGMS P30GM1114750 (to J.O.-U.), AHA 16SDG27260248 (to J.O.-U.), Rhode Island Foundation Medical Research Grant No. 20164376 (to J.O.-U.), American Physiological Society (APS) 2017 Shih-Chun Wang Young Investigator Award (to J.O.-U.), Brown University Karen T. Romer Undergraduate Teaching and Research Award (to S.M.A.), and AHA 16GRNT31170032 (to Y.Y.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Vafai S.B., Mootha V.K. Mitochondrial Disorders as Windows into an Ancient Organelle. Nature. 2012;491:374–383. doi: 10.1038/nature11707. [DOI] [PubMed] [Google Scholar]
- 2.Nunnari J., Suomalainen A. Mitochondria: In Sickness and in Health. Cell. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huttemann M., Lee I., Pecinova A., Pecina P., Przyklenk K., Doan J.W. Regulation of Oxidative Phosphorylation, the Mitochondrial Membrane Potential, and their Role in Human Disease. J. Bioenerg. Biomembr. 2008;40:445–456. doi: 10.1007/s10863-008-9169-3. [DOI] [PubMed] [Google Scholar]
- 4.Formosa L.E., Ryan M.T. Mitochondrial OXPHOS Complex Assembly Lines. Nat. Cell Biol. 2018;20:511–513. doi: 10.1038/s41556-018-0098-z. [DOI] [PubMed] [Google Scholar]
- 5.Cogliati S., Enriquez J.A., Scorrano L. Mitochondrial Cristae: Where Beauty Meets Functionality. Trends Biochem. Sci. 2016;41:261–273. doi: 10.1016/j.tibs.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Yu S.B., Pekkurnaz G. Mechanisms Orchestrating Mitochondrial Dynamics for Energy Homeostasis. J. Mol. Biol. 2018;430:3922–3941. doi: 10.1016/j.jmb.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuznetsov A.V., Margreiter R. Heterogeneity of Mitochondria and Mitochondrial Function within Cells as another Level of Mitochondrial Complexity. Int. J. Mol. Sci. 2009;10:1911–1929. doi: 10.3390/ijms10041911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liesa M., Palacin M., Zorzano A. Mitochondrial Dynamics in Mammalian Health and Disease. Physiol. Rev. 2009;89:799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 9.Westermann B. Bioenergetic Role of Mitochondrial Fusion and Fission. Biochim. Biophys. Acta. 2012;1817:1833–1838. doi: 10.1016/j.bbabio.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 10.Youle R.J., van der Bliek A.M. Mitochondrial Fission, Fusion, and Stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisner V., Picard M., Hajnoczky G. Mitochondrial Dynamics in Adaptive and Maladaptive Cellular Stress Responses. Nat. Cell Biol. 2018;20:755–765. doi: 10.1038/s41556-018-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wai T., Langer T. Mitochondrial Dynamics and Metabolic Regulation. Trends Endocrinol. Metab. 2016;27:105–117. doi: 10.1016/j.tem.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Pernas L., Scorrano L. Mito-Morphosis: Mitochondrial Fusion, Fission, and Cristae Remodeling as Key Mediators of Cellular Function. Annu. Rev. Physiol. 2016;78:505–531. doi: 10.1146/annurev-physiol-021115-105011. [DOI] [PubMed] [Google Scholar]
- 14.Chan D.C. Fusion and Fission: Interlinked Processes Critical for Mitochondrial Health. Annu. Rev. Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- 15.Piquereau J., Caffin F., Novotova M., Lemaire C., Veksler V., Garnier A., Ventura-Clapier R., Joubert F. Mitochondrial Dynamics in the Adult Cardiomyocytes: Which Roles for a Highly Specialized Cell? Front. Physiol. 2013;4:102. doi: 10.3389/fphys.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong S.B., Hall A.R., Hausenloy D.J. Mitochondrial Dynamics in Cardiovascular Health and Disease. Antioxid. Redox Signal. 2013;19:400–414. doi: 10.1089/ars.2012.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vendelin M., Beraud N., Guerrero K., Andrienko T., Kuznetsov A.V., Olivares J., Kay L., Saks V.A. Mitochondrial Regular Arrangement in Muscle Cells: A “Crystal-Like” Pattern. Am. J. Physiol. Cell. Physiol. 2005;288:C757–C767. doi: 10.1152/ajpcell.00281.2004. [DOI] [PubMed] [Google Scholar]
- 18.Beraud N., Pelloux S., Usson Y., Kuznetsov A.V., Ronot X., Tourneur Y., Saks V. Mitochondrial Dynamics in Heart Cells: Very Low Amplitude High Frequency Fluctuations in Adult Cardiomyocytes and Flow Motion in Non Beating Hl-1 Cells. J. Bioenerg. Biomembr. 2009;41:195–214. doi: 10.1007/s10863-009-9214-x. [DOI] [PubMed] [Google Scholar]
- 19.Eisner V., Lenaers G., Hajnoczky G. Mitochondrial Fusion is Frequent in Skeletal Muscle and Supports Excitation-Contraction Coupling. J. Cell Biol. 2014;205:179–195. doi: 10.1083/jcb.201312066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisner V., Cupo R.R., Gao E., Csordas G., Slovinsky W.S., Paillard M., Cheng L., Ibetti J., Chen S.R., Chuprun J.K., et al. Mitochondrial Fusion Dynamics is Robust in the Heart and Depends on Calcium Oscillations and Contractile Activity. Proc. Natl. Acad. Sci. USA. 2017;114:E859–E868. doi: 10.1073/pnas.1617288114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pham A.H., McCaffery J.M., Chan D.C. Mouse Lines with Photo-Activatable Mitochondria to Study Mitochondrial Dynamics. Genesis. 2012;50:833–843. doi: 10.1002/dvg.22050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel K.D., Glancy B., Balaban R.S. The Electrochemical Transmission in I-Band Segments of the Mitochondrial Reticulum. Biochim. Biophys. Acta. 2016;1857:1284–1289. doi: 10.1016/j.bbabio.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glancy B., Hartnell L.M., Malide D., Yu Z.X., Combs C.A., Connelly P.S., Subramaniam S., Balaban R.S. Mitochondrial Reticulum for Cellular Energy Distribution in Muscle. Nature. 2015;523:617–620. doi: 10.1038/nature14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glancy B., Hartnell L.M., Combs C.A., Femnou A., Sun J., Murphy E., Subramaniam S., Balaban R.S. Power Grid Protection of the Muscle Mitochondrial Reticulum. Cell. Rep. 2017;19:487–496. doi: 10.1016/j.celrep.2017.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H., Wang P., Bisetto S., Yoon Y., Chen Q., Sheu S.S., Wang W. A Novel Fission-Independent Role of Dynamin-Related Protein 1 in Cardiac Mitochondrial Respiration. Cardiovasc. Res. 2017;113:160–170. doi: 10.1093/cvr/cvw212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bers D.M. Excitation-Contraction Coupling and Cardiac Contractile Force. 2nd ed. Springer; Berlin/Heidelberg, Germany: 2001. [Google Scholar]
- 27.Pisano A., Cerbelli B., Perli E., Pelullo M., Bargelli V., Preziuso C., Mancini M., He L., Bates M.G., Lucena J.R., et al. Impaired Mitochondrial Biogenesis is a Common Feature to Myocardial Hypertrophy and End-Stage Ischemic Heart Failure. Cardiovasc. Pathol. 2016;25:103–112. doi: 10.1016/j.carpath.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diakos N.A., Navankasattusas S., Abel E.D., Rutter J., McCreath L., Ferrin P., McKellar S.H., Miller D.V., Park S.Y., Richardson R.S., et al. Evidence of Glycolysis Up-Regulation and Pyruvate Mitochondrial Oxidation Mismatch during Mechanical Unloading of the Failing Human Heart: Implications for Cardiac Reloading and Conditioning. JACC Basic Transl. Sci. 2016;1:432–444. doi: 10.1016/j.jacbts.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karamanlidis G., Bautista-Hernandez V., Fynn-Thompson F., Del Nido P., Tian R. Impaired Mitochondrial Biogenesis Precedes Heart Failure in Right Ventricular Hypertrophy in Congenital Heart Disease. Circ. Heart Fail. 2011;4:707–713. doi: 10.1161/CIRCHEARTFAILURE.111.961474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaper J., Froede R., Hein S., Buck A., Hashizume H., Speiser B., Friedl A., Bleese N. Impairment of the Myocardial Ultrastructure and Changes of the Cytoskeleton in Dilated Cardiomyopathy. Circulation. 1991;83:504–514. doi: 10.1161/01.CIR.83.2.504. [DOI] [PubMed] [Google Scholar]
- 31.Santulli G., Xie W., Reiken S.R., Marks A.R. Mitochondrial Calcium Overload is a Key Determinant in Heart Failure. Proc. Natl. Acad. Sci. USA. 2015;112:11389–11394. doi: 10.1073/pnas.1513047112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wust R.C., de Vries H.J., Wintjes L.T., Rodenburg R.J., Niessen H.W., Stienen G.J. Mitochondrial Complex I Dysfunction and Altered NAD(P)H Kinetics in Rat Myocardium in Cardiac Right Ventricular Hypertrophy and Failure. Cardiovasc. Res. 2016;111:362–372. doi: 10.1093/cvr/cvw176. [DOI] [PubMed] [Google Scholar]
- 33.Bugger H., Schwarzer M., Chen D., Schrepper A., Amorim P.A., Schoepe M., Nguyen T.D., Mohr F.W., Khalimonchuk O., Weimer B.C., et al. Proteomic Remodelling of Mitochondrial Oxidative Pathways in Pressure Overload-Induced Heart Failure. Cardiovasc. Res. 2010;85:376–384. doi: 10.1093/cvr/cvp344. [DOI] [PubMed] [Google Scholar]
- 34.Boudina S., Sena S., Theobald H., Sheng X., Wright J.J., Hu X.X., Aziz S., Johnson J.I., Bugger H., Zaha V.G., et al. Mitochondrial Energetics in the Heart in Obesity-Related Diabetes: Direct Evidence for Increased Uncoupled Respiration and Activation of Uncoupling Proteins. Diabetes. 2007;56:2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 35.Chen L., Gong Q., Stice J.P., Knowlton A.A. Mitochondrial OPA1, Apoptosis, and Heart Failure. Cardiovasc. Res. 2009;84:91–99. doi: 10.1093/cvr/cvp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai D.F., Hsieh E.J., Chen T., Menendez L.G., Basisty N.B., Tsai L., Beyer R.P., Crispin D.A., Shulman N.J., Szeto H.H., et al. Global Proteomics and Pathway Analysis of Pressure-Overload-Induced Heart Failure and its Attenuation by Mitochondrial-Targeted Peptides. Circ. Heart Fail. 2013;6:1067–1076. doi: 10.1161/CIRCHEARTFAILURE.113.000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Javadov S., Karmazyn M., Escobales N. Mitochondrial Permeability Transition Pore Opening as a Promising Therapeutic Target in Cardiac Diseases. J. Pharmacol. Exp. Ther. 2009;330:670–678. doi: 10.1124/jpet.109.153213. [DOI] [PubMed] [Google Scholar]
- 38.Lesnefsky E.J., Tandler B., Ye J., Slabe T.J., Turkaly J., Hoppel C.L. Myocardial Ischemia Decreases Oxidative Phosphorylation through Cytochrome Oxidase in Subsarcolemmal Mitochondria. Am. J. Physiol. 1997;273:H1544–H1554. doi: 10.1152/ajpheart.1997.273.3.H1544. [DOI] [PubMed] [Google Scholar]
- 39.Lesnefsky E.J., Chen Q., Slabe T.J., Stoll M.S., Minkler P.E., Hassan M.O., Tandler B., Hoppel C.L. Ischemia, rather than Reperfusion, Inhibits Respiration through Cytochrome Oxidase in the Isolated, Perfused Rabbit Heart: Role of Cardiolipin. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H258–H267. doi: 10.1152/ajpheart.00348.2003. [DOI] [PubMed] [Google Scholar]
- 40.Kwong J.Q., Molkentin J.D. Physiological and Pathological Roles of the Mitochondrial Permeability Transition Pore in the Heart. Cell. Metab. 2015;21:206–214. doi: 10.1016/j.cmet.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hom J., Yu T., Yoon Y., Porter G., Sheu S.S. Regulation of Mitochondrial Fission by Intracellular Ca2+ in Rat Ventricular Myocytes. Biochim. Biophys. Acta. 2010;1797:913–921. doi: 10.1016/j.bbabio.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Archer S.L. Mitochondrial Fission and Fusion in Human Diseases. N. Engl. J. Med. 2014;370:1074. doi: 10.1056/NEJMc1316254. [DOI] [PubMed] [Google Scholar]
- 43.Chen L., Knowlton A.A. Mitochondria and Heart Failure: New Insights into an Energetic Problem. Minerva Cardioangiol. 2010;58:213–229. [PMC free article] [PubMed] [Google Scholar]
- 44.Bernstein D., Fajardo G., Zhao M. The role of beta-adrenergic receptors in heart failure: differential regulation of cardiotoxicity and cardioprotection. Prog. Pediatr. Cardiol. 2011;31:35–38. doi: 10.1016/j.ppedcard.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O-Uchi J., Lopes C.M. Combined Blockade of Beta- and Alpha(1)-Adrenoceptors in Left Ventricular Remodeling Induced by Hypertension: Beneficial or Not? Hypertens. Res. 2010;33:984–985. doi: 10.1038/hr.2010.157. [DOI] [PubMed] [Google Scholar]
- 46.Woo A.Y., Xiao R.P. Beta-Adrenergic Receptor Subtype Signaling in Heart: From Bench to Bedside. Acta Pharmacol. Sin. 2012;33:335–341. doi: 10.1038/aps.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shannon R., Chaudhry M. Effect of Alpha1-Adrenergic Receptors in Cardiac Pathophysiology. Am. Heart J. 2006;152:842–850. doi: 10.1016/j.ahj.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 48.De Lucia C., Eguchi A., Koch W.J. New Insights in Cardiac Beta-Adrenergic Signaling during Heart Failure and Aging. Front. Pharmacol. 2018;9:904. doi: 10.3389/fphar.2018.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Triposkiadis F., Karayannis G., Giamouzis G., Skoularigis J., Louridas G., Butler J. The Sympathetic Nervous System in Heart Failure Physiology, Pathophysiology, and Clinical Implications. J. Am. Coll. Cardiol. 2009;54:1747–1762. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 50.Bers D.M. Cardiac Excitation-Contraction Coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 51.Bers D.M., Guo T. Calcium Signaling in Cardiac Ventricular Myocytes. Ann. N. Y. Acad. Sci. 2005;1047:86–98. doi: 10.1196/annals.1341.008. [DOI] [PubMed] [Google Scholar]
- 52.Nan J., Zhu W., Rahman M.S., Liu M., Li D., Su S., Zhang N., Hu X., Yu H., Gupta M.P., et al. Molecular Regulation of Mitochondrial Dynamics in Cardiac Disease. Biochim. Biophys. Acta. 2017;1864:1260–1273. doi: 10.1016/j.bbamcr.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Otera H., Ishihara N., Mihara K. New Insights into the Function and Regulation of Mitochondrial Fission. Biochim. Biophys. Acta. 2013;1833:1256–1268. doi: 10.1016/j.bbamcr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 54.Elgass K., Pakay J., Ryan M.T., Palmer C.S. Recent Advances into the Understanding of Mitochondrial Fission. Biochim. Biophys. Acta. 2013;1833:150–161. doi: 10.1016/j.bbamcr.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 55.Chang C.R., Blackstone C. Dynamic Regulation of Mitochondrial Fission through Modification of the Dynamin-Related Protein Drp1. Ann. N. Y. Acad. Sci. 2010;1201:34–39. doi: 10.1111/j.1749-6632.2010.05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jhun B.S., O-Uchi J., Adaniya S.M., Mancini T.J., Cao J.L., King M.E., Landi A.K., Ma H., Shin M., Yang D., et al. Protein Kinase D Activation Induces Mitochondrial Fragmentation and Dysfunction in Cardiomyocytes. J. Physiol. 2018;596:827–855. doi: 10.1113/JP275418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu S., Wang P., Zhang H., Gong G., Gutierrez Cortes N., Zhu W., Yoon Y., Tian R., Wang W. CaMKII Induces Permeability Transition through Drp1 Phosphorylation during Chronic Beta-AR Stimulation. Nat. Commun. 2016;7:13189. doi: 10.1038/ncomms13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cribbs J.T., Strack S. Reversible Phosphorylation of Drp1 by Cyclic AMP-Dependent Protein Kinase and Calcineurin Regulates Mitochondrial Fission and Cell Death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monterisi S., Lobo M.J., Livie C., Castle J.C., Weinberger M., Baillie G., Surdo N.C., Musheshe N., Stangherlin A., Gottlieb E., et al. PDE2A2 Regulates Mitochondria Morphology and Apoptotic Cell Death Via Local Modulation of cAMP/PKA Signalling. Elife. 2017;6 doi: 10.7554/eLife.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pennanen C., Parra V., Lopez-Crisosto C., Morales P.E., Del Campo A., Gutierrez T., Rivera-Mejias P., Kuzmicic J., Chiong M., Zorzano A., et al. Mitochondrial Fission is Required for Cardiomyocyte Hypertrophy Mediated by a Ca2+-Calcineurin Signaling Pathway. J. Cell. Sci. 2014;127:2659–2671. doi: 10.1242/jcs.139394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brodde O.E., Michel M.C. Adrenergic and Muscarinic Receptors in the Human Heart. Pharmacol. Rev. 1999;51:651–690. [PubMed] [Google Scholar]
- 62.Woodcock E.A., Du X.J., Reichelt M.E., Graham R.M. Cardiac Alpha 1-Adrenergic Drive in Pathological Remodelling. Cardiovasc. Res. 2008;77:452–462. doi: 10.1093/cvr/cvm078. [DOI] [PubMed] [Google Scholar]
- 63.Xiao R.P. Beta-Adrenergic Signaling in the Heart: Dual Coupling of the Beta2-Adrenergic Receptor to G(s) and G(i) Proteins. Science. 2001;2001:re15. doi: 10.1126/stke.2001.104.re15. [DOI] [PubMed] [Google Scholar]
- 64.O’Connell T.D., Jensen B.C., Baker A.J., Simpson P.C. Cardiac Alpha1-Adrenergic Receptors: Novel Aspects of Expression, Signaling Mechanisms, Physiologic Function, and Clinical Importance. Pharmacol. Rev. 2013;66:308–333. doi: 10.1124/pr.112.007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Endoh M., Blinks J.R. Actions of Sympathomimetic Amines on the Ca2+ Transients and Contractions of Rabbit Myocardium: Reciprocal Changes in Myofibrillar Responsiveness to Ca2+ Mediated through Alpha- and Beta-Adrenoceptors. Circ. Res. 1988;62:247–265. doi: 10.1161/01.RES.62.2.247. [DOI] [PubMed] [Google Scholar]
- 66.Bers D.M. Calcium Cycling and Signaling in Cardiac Myocytes. Annu. Rev. Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 67.Jensen B.C., O’Connell T.D., Simpson P.C. Alpha-1-Adrenergic Receptors: Targets for Agonist Drugs to Treat Heart Failure. J. Mol. Cell. Cardiol. 2011;51:518–528. doi: 10.1016/j.yjmcc.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blinks J.R. The Nature of the Antagonism by Methoxamine of the Chronotropic and Inotropic Effects of Catecholamines. Naunyn Schmiedebergs. Arch. Exp. Pathol. Pharmakol. 1964;248:73–84. doi: 10.1007/BF00247060. [DOI] [PubMed] [Google Scholar]
- 69.Ruiz-Hurtado G., Morel E., Dominguez-Rodriguez A., Llach A., Lezoualc’h F., Benitah J.P., Gomez A.M. Epac in Cardiac Calcium Signaling. J. Mol. Cell. Cardiol. 2013;58:162–171. doi: 10.1016/j.yjmcc.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 70.Xiao R.P., Zhu W., Zheng M., Cao C., Zhang Y., Lakatta E.G., Han Q. Subtype-Specific Alpha1- and Beta-Adrenoceptor Signaling in the Heart. Trends Pharmacol. Sci. 2006;27:330–337. doi: 10.1016/j.tips.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 71.Kwong J.Q., Lu X., Correll R.N., Schwanekamp J.A., Vagnozzi R.J., Sargent M.A., York A.J., Zhang J., Bers D.M., Molkentin J.D. The Mitochondrial Calcium Uniporter Selectively Matches Metabolic Output to Acute Contractile Stress in the Heart. Cell. Rep. 2015;12:15–22. doi: 10.1016/j.celrep.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luongo T.S., Lambert J.P., Yuan A., Zhang X., Gross P., Song J., Shanmughapriya S., Gao E., Jain M., Houser S.R., et al. The Mitochondrial Calcium Uniporter Matches Energetic Supply with Cardiac Workload during Stress and Modulates Permeability Transition. Cell. Rep. 2015;12:23–34. doi: 10.1016/j.celrep.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie A., Song Z., Liu H., Zhou A., Shi G., Wang Q., Gu L., Liu M., Xie L.H., Qu Z., et al. Mitochondrial Ca2+ Influx Contributes to Arrhythmic Risk in Nonischemic Cardiomyopathy. J. Am. Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O-Uchi J., Jhun B.S., Xu S., Hurst S., Raffaello A., Liu X., Yi B., Zhang H., Gross P., Mishra J., et al. Adrenergic Signaling Regulates Mitochondrial Ca2+ Uptake through Pyk2-Dependent Tyrosine Phosphorylation of the Mitochondrial Ca2+ Uniporter. Antioxid. Redox Signal. 2014;21:863–879. doi: 10.1089/ars.2013.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bovo E., Lipsius S.L., Zima A.V. Reactive Oxygen Species Contribute to the Development of Arrhythmogenic Ca2+ Waves during Beta-Adrenergic Receptor Stimulation in Rabbit Cardiomyocytes. J. Physiol. 2012;590:3291–3304. doi: 10.1113/jphysiol.2012.230748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Osadchii O.E. Cardiac Hypertrophy Induced by Sustained Beta-Adrenoreceptor Activation: Pathophysiological Aspects. Heart Fail. Rev. 2007;12:66–86. doi: 10.1007/s10741-007-9007-4. [DOI] [PubMed] [Google Scholar]
- 77.Jensen B.C., O’Connell T.D., Simpson P.C. Alpha-1-Adrenergic Receptors in Heart Failure: The Adaptive Arm of the Cardiac Response to Chronic Catecholamine Stimulation. J. Cardiovasc. Pharmacol. 2014;63:291–301. doi: 10.1097/FJC.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adams J.W., Sakata Y., Davis M.G., Sah V.P., Wang Y., Liggett S.B., Chien K.R., Brown J.H., Dorn G.W., 2nd Enhanced Galphaq Signaling: A Common Pathway Mediates Cardiac Hypertrophy and Apoptotic Heart Failure. Proc. Natl. Acad. Sci. USA. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mende U., Kagen A., Cohen A., Aramburu J., Schoen F.J., Neer E.J. Transient Cardiac Expression of Constitutively Active Galphaq Leads to Hypertrophy and Dilated Cardiomyopathy by Calcineurin-Dependent and Independent Pathways. Proc. Natl. Acad. Sci. USA. 1998;95:13893–13898. doi: 10.1073/pnas.95.23.13893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yin W., Li R., Feng X., James Kang Y. The Involvement of Cytochrome C Oxidase in Mitochondrial Fusion in Primary Cultures of Neonatal Rat Cardiomyocytes. Cardiovasc. Toxicol. 2018;18:365–373. doi: 10.1007/s12012-018-9447-1. [DOI] [PubMed] [Google Scholar]
- 81.Acin-Perez R., Lechuga-Vieco A.V., Del Mar Munoz M., Nieto-Arellano R., Torroja C., Sanchez-Cabo F., Jimenez C., Gonzalez-Guerra A., Carrascoso I., Beninca C., et al. Ablation of the Stress Protease OMA1 Protects Against Heart Failure in Mice. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aan4935. [DOI] [PubMed] [Google Scholar]
- 82.Yates J.C., Taam G.M., Singal P.K., Beamish R.E., Dhalla N.S. Protection Against Adrenochrome-Induced Myocardial Damage by various Pharmacological Interventions. Br. J. Exp. Pathol. 1980;61:242–255. [PMC free article] [PubMed] [Google Scholar]
- 83.Li Y., Fang J., Hua Y., Wang C., Mu D., Zhou K. The Study of Fetal Rat Model of Intra-Amniotic Isoproterenol Injection Induced Heart Dysfunction and Phenotypic Switch of Contractile Proteins. Biomed. Res. Int. 2014;2014:360687. doi: 10.1155/2014/360687. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Mikusova A., Kralova E., Tylkova L., Novotova M., Stankovicova T. Myocardial Remodelling Induced by Repeated Low Doses of Isoproterenol. Can. J. Physiol. Pharmacol. 2009;87:641–651. doi: 10.1139/Y09-053. [DOI] [PubMed] [Google Scholar]
- 85.Javadov S., Rajapurohitam V., Kilic A., Hunter J.C., Zeidan A., Said Faruq N., Escobales N., Karmazyn M. Expression of Mitochondrial Fusion-Fission Proteins during Post-Infarction Remodeling: The Effect of NHE-1 Inhibition. Basic Res. Cardiol. 2011;106:99–109. doi: 10.1007/s00395-010-0122-3. [DOI] [PubMed] [Google Scholar]
- 86.Chen Y., Dorn G.W., 2nd PINK1-Phosphorylated Mitofusin 2 is a Parkin Receptor for Culling Damaged Mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsushima K., Bugger H., Wende A.R., Soto J., Jenson G.A., Tor A.R., McGlauflin R., Kenny H.C., Zhang Y., Souvenir R., et al. Mitochondrial Reactive Oxygen Species in Lipotoxic Hearts Induce Post-Translational Modifications of AKAP121, DRP1, and OPA1 that Promote Mitochondrial Fission. Circ. Res. 2018;122:58–73. doi: 10.1161/CIRCRESAHA.117.311307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Samant S.A., Zhang H.J., Hong Z., Pillai V.B., Sundaresan N.R., Wolfgeher D., Archer S.L., Chan D.C., Gupta M.P. SIRT3 Deacetylates and Activates OPA1 to Regulate Mitochondrial Dynamics during Stress. Mol. Cell. Biol. 2014;34:807–819. doi: 10.1128/MCB.01483-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Otera H., Mihara K. Mitochondrial Dynamics: Functional Link with Apoptosis. Int. J. Cell. Biol. 2012;2012:821676. doi: 10.1155/2012/821676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Al-Husseini A., Wijesinghe D.S., Farkas L., Kraskauskas D., Drake J.I., Van Tassel B., Abbate A., Chalfant C.E., Voelkel N.F. Increased Eicosanoid Levels in the Sugen/Chronic Hypoxia Model of Severe Pulmonary Hypertension. PLoS ONE. 2015;10:e0120157. doi: 10.1371/journal.pone.0120157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Serasinghe M.N., Chipuk J.E. Mitochondrial Fission in Human Diseases. Handb. Exp. Pharmacol. 2017;240:159–188. doi: 10.1007/164_2016_38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kraus F., Ryan M.T. The Constriction and Scission Machineries Involved in Mitochondrial Fission. J. Cell. Sci. 2017;130:2953–2960. doi: 10.1242/jcs.199562. [DOI] [PubMed] [Google Scholar]
- 93.Tanaka A., Kobayashi S., Fujiki Y. Peroxisome Division is Impaired in a CHO Cell Mutant with an Inactivating Point-Mutation in Dynamin-Like Protein 1 Gene. Exp. Cell Res. 2006;312:1671–1684. doi: 10.1016/j.yexcr.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 94.Itoh K., Adachi Y., Yamada T., Suzuki T.L., Otomo T., McBride H.M., Yoshimori T., Iijima M., Sesaki H. A Brain-Enriched Drp1 Isoform Associates with Lysosomes, Late Endosomes, and the Plasma Membrane. J. Biol. Chem. 2018;293:11809–11822. doi: 10.1074/jbc.RA117.001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yoon Y., Pitts K.R., McNiven M.A. Mammalian Dynamin-Like Protein DLP1 Tubulates Membranes. Mol. Biol. Cell. 2001;12:2894–2905. doi: 10.1091/mbc.12.9.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoon Y., Pitts K.R., Dahan S., McNiven M.A. A Novel Dynamin-Like Protein Associates with Cytoplasmic Vesicles and Tubules of the Endoplasmic Reticulum in Mammalian Cells. J. Cell Biol. 1998;140:779–793. doi: 10.1083/jcb.140.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smirnova E., Griparic L., Shurland D.L., van der Bliek A.M. Dynamin-Related Protein Drp1 is Required for Mitochondrial Division in Mammalian Cells. Mol. Biol. Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pitts K.R., Yoon Y., Krueger E.W., McNiven M.A. The Dynamin-Like Protein DLP1 is Essential for Normal Distribution and Morphology of the Endoplasmic Reticulum and Mitochondria in Mammalian Cells. Mol. Biol. Cell. 1999;10:4403–4417. doi: 10.1091/mbc.10.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Strack S., Cribbs J.T. Allosteric Modulation of Drp1 Mechanoenzyme Assembly and Mitochondrial Fission by the Variable Domain. J. Biol. Chem. 2012;287:10990–11001. doi: 10.1074/jbc.M112.342105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frohlich C., Grabiger S., Schwefel D., Faelber K., Rosenbaum E., Mears J., Rocks O., Daumke O. Structural Insights into Oligomerization and Mitochondrial Remodelling of Dynamin 1-Like Protein. EMBO J. 2013;32:1280–1292. doi: 10.1038/emboj.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wenger J., Klinglmayr E., Frohlich C., Eibl C., Gimeno A., Hessenberger M., Puehringer S., Daumke O., Goettig P. Functional Mapping of Human Dynamin-1-Like GTPase Domain Based on X-Ray Structure Analyses. PLoS ONE. 2013;8:e71835. doi: 10.1371/journal.pone.0071835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hornbeck P.V., Zhang B., Murray B., Kornhauser J.M., Latham V., Skrzypek E. PhosphoSitePlus, 2014: Mutations, PTMs and Recalibrations. Nucleic Acids Res. 2015;43:D512–D520. doi: 10.1093/nar/gku1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yu T., Jhun B.S., Yoon Y. High-Glucose Stimulation Increases Reactive Oxygen Species Production through the Calcium and Mitogen-Activated Protein Kinase-Mediated Activation of Mitochondrial Fission. Antioxid. Redox Signal. 2011;14:425–437. doi: 10.1089/ars.2010.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Clinton R.W., Francy C.A., Ramachandran R., Qi X., Mears J.A. Dynamin-Related Protein 1 Oligomerization in Solution Impairs Functional Interactions with Membrane-Anchored Mitochondrial Fission Factor. J. Biol. Chem. 2016;291:478–492. doi: 10.1074/jbc.M115.680025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gandre-Babbe S., van der Bliek A.M. The Novel Tail-Anchored Membrane Protein Mff Controls Mitochondrial and Peroxisomal Fission in Mammalian Cells. Mol. Biol. Cell. 2008;19:2402–2412. doi: 10.1091/mbc.e07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Otera H., Wang C., Cleland M.M., Setoguchi K., Yokota S., Youle R.J., Mihara K. Mff is an Essential Factor for Mitochondrial Recruitment of Drp1 during Mitochondrial Fission in Mammalian Cells. J. Cell Biol. 2010;191:1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Itoyama A., Michiyuki S., Honsho M., Yamamoto T., Moser A., Yoshida Y., Fujiki Y. Mff Functions with Pex11pbeta and DLP1 in Peroxisomal Fission. Biol. Open. 2013;2:998–1006. doi: 10.1242/bio.20135298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Palmer C.S., Osellame L.D., Laine D., Koutsopoulos O.S., Frazier A.E., Ryan M.T. MiD49 and MiD51, New Components of the Mitochondrial Fission Machinery. EMBO Rep. 2011;12:565–573. doi: 10.1038/embor.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhao J., Liu T., Jin S., Wang X., Qu M., Uhlen P., Tomilin N., Shupliakov O., Lendahl U., Nister M. Human MIEF1 Recruits Drp1 to Mitochondrial Outer Membranes and Promotes Mitochondrial Fusion rather than Fission. EMBO J. 2011;30:2762–2778. doi: 10.1038/emboj.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Palmer C.S., Elgass K.D., Parton R.G., Osellame L.D., Stojanovski D., Ryan M.T. Adaptor Proteins MiD49 and MiD51 can Act Independently of Mff and Fis1 in Drp1 Recruitment and are Specific for Mitochondrial Fission. J. Biol. Chem. 2013;288:27584–27593. doi: 10.1074/jbc.M113.479873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Loson O.C., Song Z., Chen H., Chan D.C. Fis1, Mff, MiD49, and MiD51 Mediate Drp1 Recruitment in Mitochondrial Fission. Mol. Biol. Cell. 2013;24:659–667. doi: 10.1091/mbc.e12-10-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen H., Ren S., Clish C., Jain M., Mootha V., McCaffery J.M., Chan D.C. Titration of Mitochondrial Fusion Rescues Mff-Deficient Cardiomyopathy. J. Cell Biol. 2015;211:795–805. doi: 10.1083/jcb.201507035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu R., Chan D.C. The Mitochondrial Fission Receptor Mff Selectively Recruits Oligomerized Drp1. Mol. Biol. Cell. 2015;26:4466–4477. doi: 10.1091/mbc.E15-08-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Osellame L.D., Singh A.P., Stroud D.A., Palmer C.S., Stojanovski D., Ramachandran R., Ryan M.T. Cooperative and Independent Roles of the Drp1 Adaptors Mff, MiD49 and MiD51 in Mitochondrial Fission. J. Cell. Sci. 2016;129:2170–2181. doi: 10.1242/jcs.185165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rosenbloom A.B., Lee S.H., To M., Lee A., Shin J.Y., Bustamante C. Optimized Two-Color Super Resolution Imaging of Drp1 during Mitochondrial Fission with a Slow-Switching Dronpa Variant. Proc. Natl. Acad. Sci. USA. 2014;111:13093–13098. doi: 10.1073/pnas.1320044111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Colasante C., Chen J., Ahlemeyer B., Baumgart-Vogt E. Peroxisomes in Cardiomyocytes and the Peroxisome/Peroxisome Proliferator-Activated Receptor-Loop. Thromb. Haemost. 2015;113:452–463. doi: 10.1160/TH14-06-0497. [DOI] [PubMed] [Google Scholar]
- 117.Wang P., Liu J., Li Y., Wu S., Luo J., Yang H., Subbiah R., Chatham J., Zhelyabovska O., Yang Q. Peroxisome Proliferator-Activated Receptor {Delta} is an Essential Transcriptional Regulator for Mitochondrial Protection and Biogenesis in Adult Heart. Circ. Res. 2010;106:911–919. doi: 10.1161/CIRCRESAHA.109.206185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu J., Wang P., He L., Li Y., Luo J., Cheng L., Qin Q., Brako L.A., Lo W.K., Lewis W., et al. Cardiomyocyte-Restricted Deletion of PPARbeta/Delta in PPARalpha-Null Mice Causes Impaired Mitochondrial Biogenesis and Defense, but no further Depression of Myocardial Fatty Acid Oxidation. PPAR Res. 2011;2011:372854. doi: 10.1155/2011/372854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chuang Y.C., Lin T.K., Yang D.I., Yang J.L., Liou C.W., Chen S.D. Peroxisome Proliferator-Activated Receptor-Gamma Dependent Pathway Reduces the Phosphorylation of Dynamin-Related Protein 1 and Ameliorates Hippocampal Injury Induced by Global Ischemia in Rats. J. Biomed. Sci. 2016;23:44. doi: 10.1186/s12929-016-0262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yan J., Liu X.H., Han M.Z., Wang Y.M., Sun X.L., Yu N., Li T., Su B., Chen Z.Y. Blockage of GSK3beta-Mediated Drp1 Phosphorylation Provides Neuroprotection in Neuronal and Mouse Models of Alzheimer’s Disease. Neurobiol. Aging. 2015;36:211–227. doi: 10.1016/j.neurobiolaging.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 121.Zhou L., Zhang Q., Zhang P., Sun L., Peng C., Yuan Z., Cheng J. C-Abl-Mediated Drp1 Phosphorylation Promotes Oxidative Stress-Induced Mitochondrial Fragmentation and Neuronal Cell Death. Cell. Death Dis. 2017;8:e3117. doi: 10.1038/cddis.2017.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Figueroa-Romero C., Iniguez-Lluhi J.A., Stadler J., Chang C.R., Arnoult D., Keller P.J., Hong Y., Blackstone C., Feldman E.L. SUMOylation of the Mitochondrial Fission Protein Drp1 Occurs at Multiple Nonconsensus Sites within the B Domain and is Linked to its Activity Cycle. FASEB J. 2009;23:3917–3927. doi: 10.1096/fj.09-136630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wasiak S., Zunino R., McBride H.M. Bax/Bak Promote Sumoylation of DRP1 and its Stable Association with Mitochondria during Apoptotic Cell Death. J. Cell Biol. 2007;177:439–450. doi: 10.1083/jcb.200610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Prudent J., Zunino R., Sugiura A., Mattie S., Shore G.C., McBride H.M. MAPL SUMOylation of Drp1 Stabilizes an ER/Mitochondrial Platform Required for Cell Death. Mol. Cell. 2015;59:941–955. doi: 10.1016/j.molcel.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 125.Zunino R., Schauss A., Rippstein P., Andrade-Navarro M., McBride H.M. The SUMO Protease SENP5 is Required to Maintain Mitochondrial Morphology and Function. J. Cell. Sci. 2007;120:1178–1188. doi: 10.1242/jcs.03418. [DOI] [PubMed] [Google Scholar]
- 126.Guo C., Hildick K.L., Luo J., Dearden L., Wilkinson K.A., Henley J.M. SENP3-Mediated deSUMOylation of Dynamin-Related Protein 1 Promotes Cell Death Following Ischaemia. EMBO J. 2013;32:1514–1528. doi: 10.1038/emboj.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim E.Y., Zhang Y., Beketaev I., Segura A.M., Yu W., Xi Y., Chang J., Wang J. SENP5, a SUMO Isopeptidase, Induces Apoptosis and Cardiomyopathy. J. Mol. Cell. Cardiol. 2015;78:154–164. doi: 10.1016/j.yjmcc.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 128.Jahani-Asl A., Huang E., Irrcher I., Rashidian J., Ishihara N., Lagace D.C., Slack R.S., Park D.S. CDK5 Phosphorylates DRP1 and Drives Mitochondrial Defects in NMDA-Induced Neuronal Death. Hum. Mol. Genet. 2015;24:4573–4583. doi: 10.1093/hmg/ddv188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gawlowski T., Suarez J., Scott B., Torres-Gonzalez M., Wang H., Schwappacher R., Han X., Yates J.R., 3rd, Hoshijima M., Dillmann W. Modulation of Dynamin-Related Protein 1 (DRP1) Function by Increased O-Linked-Beta-N-Acetylglucosamine Modification (O-GlcNAc) in Cardiac Myocytes. J. Biol. Chem. 2012;287:30024–30034. doi: 10.1074/jbc.M112.390682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Su Y.C., Qi X. Inhibition of Excessive Mitochondrial Fission Reduced Aberrant Autophagy and Neuronal Damage Caused by LRRK2 G2019S Mutation. Hum. Mol. Genet. 2013;22:4545–4561. doi: 10.1093/hmg/ddt301. [DOI] [PubMed] [Google Scholar]
- 131.Taguchi N., Ishihara N., Jofuku A., Oka T., Mihara K. Mitotic Phosphorylation of Dynamin-Related GTPase Drp1 Participates in Mitochondrial Fission. J. Biol. Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 132.Marsboom G., Toth P.T., Ryan J.J., Hong Z., Wu X., Fang Y.H., Thenappan T., Piao L., Zhang H.J., Pogoriler J., et al. Dynamin-Related Protein 1-Mediated Mitochondrial Mitotic Fission Permits Hyperproliferation of Vascular Smooth Muscle Cells and Offers a Novel Therapeutic Target in Pulmonary Hypertension. Circ. Res. 2012;110:1484–1497. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zaja I., Bai X., Liu Y., Kikuchi C., Dosenovic S., Yan Y., Canfield S.G., Bosnjak Z.J. Cdk1, PKCdelta and Calcineurin-Mediated Drp1 Pathway Contributes to Mitochondrial Fission-Induced Cardiomyocyte Death. Biochem. Biophys. Res. Commun. 2014;453:710–721. doi: 10.1016/j.bbrc.2014.09.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Strack S., Wilson T.J., Cribbs J.T. Cyclin-Dependent Kinases Regulate Splice-Specific Targeting of Dynamin-Related Protein 1 to Microtubules. J. Cell Biol. 2013;201:1037–1051. doi: 10.1083/jcb.201210045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cho B., Cho H.M., Kim H.J., Jeong J., Park S.K., Hwang E.M., Park J.Y., Kim W.R., Kim H., Sun W. CDK5-Dependent Inhibitory Phosphorylation of Drp1 during Neuronal Maturation. Exp. Mol. Med. 2014;46:e105. doi: 10.1038/emm.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kashatus J.A., Nascimento A., Myers L.J., Sher A., Byrne F.L., Hoehn K.L., Counter C.M., Kashatus D.F. Erk2 Phosphorylation of Drp1 Promotes Mitochondrial Fission and MAPK-Driven Tumor Growth. Mol. Cell. 2015;57:537–551. doi: 10.1016/j.molcel.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Serasinghe M.N., Wieder S.Y., Renault T.T., Elkholi R., Asciolla J.J., Yao J.L., Jabado O., Hoehn K., Kageyama Y., Sesaki H., et al. Mitochondrial Division is Requisite to RAS-Induced Transformation and Targeted by Oncogenic MAPK Pathway Inhibitors. Mol. Cell. 2015;57:521–536. doi: 10.1016/j.molcel.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Roe A.J., Qi X. Drp1 Phosphorylation by MAPK1 Causes Mitochondrial Dysfunction in Cell Culture Model of Huntington’s Disease. Biochem. Biophys. Res. Commun. 2018;496:706–711. doi: 10.1016/j.bbrc.2018.01.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Qi X., Disatnik M.H., Shen N., Sobel R.A., Mochly-Rosen D. Aberrant Mitochondrial Fission in Neurons Induced by Protein Kinase C{Delta} Under Oxidative Stress Conditions in Vivo. Mol. Biol. Cell. 2011;22:256–265. doi: 10.1091/mbc.e10-06-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Divakaruni S.S., Van Dyke A.M., Chandra R., LeGates T.A., Contreras M., Dharmasri P.A., Higgs H.N., Lobo M.K., Thompson S.M., Blanpied T.A. Long-Term Potentiation Requires a Rapid Burst of Dendritic Mitochondrial Fission during Induction. Neuron. 2018 doi: 10.1016/j.neuron.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brand C.S., Tan V.P., Brown J.H., Miyamoto S. RhoA Regulates Drp1 Mediated Mitochondrial Fission through ROCK to Protect Cardiomyocytes. Cell. Signal. 2018;50:48–57. doi: 10.1016/j.cellsig.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cho M.H., Kim D.H., Choi J.E., Chang E.J., Seung -Y. Increased Phosphorylation of Dynamin-Related Protein 1 and Mitochondrial Fission in Okadaic Acid-Treated Neurons. Brain Res. 2012;1454:100–110. doi: 10.1016/j.brainres.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 143.Saito T., Nah J., Oka S.I., Mukai R., Monden Y., Maejima Y., Ikeda Y., Sciarretta S., Liu T., Li H., et al. An Alternative Mitophagy Pathway Mediated by Rab9 Protects the Heart Against Ischemia. J. Clin. Investig. 2018 doi: 10.1172/JCI122035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cereghetti G.M., Stangherlin A., Martins de Brito O., Chang C.R., Blackstone C., Bernardi P., Scorrano L. Dephosphorylation by Calcineurin Regulates Translocation of Drp1 to Mitochondria. Proc. Natl. Acad. Sci. USA. 2008;105:15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chang C.R., Blackstone C. Cyclic AMP-Dependent Protein Kinase Phosphorylation of Drp1 Regulates its GTPase Activity and Mitochondrial Morphology. J. Biol. Chem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- 146.Kim H., Scimia M.C., Wilkinson D., Trelles R.D., Wood M.R., Bowtell D., Dillin A., Mercola M., Ronai Z.A. Fine-Tuning of Drp1/Fis1 Availability by AKAP121/Siah2 Regulates Mitochondrial Adaptation to Hypoxia. Mol. Cell. 2011;44:532–544. doi: 10.1016/j.molcel.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gomes L.C., Di Benedetto G., Scorrano L. During Autophagy Mitochondria Elongate, are Spared from Degradation and Sustain Cell Viability. Nat. Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Han X.J., Lu Y.F., Li S.A., Kaitsuka T., Sato Y., Tomizawa K., Nairn A.C., Takei K., Matsui H., Matsushita M. CaM Kinase I Alpha-Induced Phosphorylation of Drp1 Regulates Mitochondrial Morphology. J. Cell Biol. 2008;182:573–585. doi: 10.1083/jcb.200802164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang W., Wang Y., Long J., Wang J., Haudek S.B., Overbeek P., Chang B.H., Schumacker P.T., Danesh F.R. Mitochondrial Fission Triggered by Hyperglycemia is Mediated by ROCK1 Activation in Podocytes and Endothelial Cells. Cell. Metab. 2012;15:186–200. doi: 10.1016/j.cmet.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Din S., Mason M., Volkers M., Johnson B., Cottage C.T., Wang Z., Joyo A.Y., Quijada P., Erhardt P., Magnuson N.S., et al. Pim-1 Preserves Mitochondrial Morphology by Inhibiting Dynamin-Related Protein 1 Translocation. Proc. Natl. Acad. Sci. USA. 2013;110:5969–5974. doi: 10.1073/pnas.1213294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sharp W.W., Fang Y.H., Han M., Zhang H.J., Hong Z., Banathy A., Morrow E., Ryan J.J., Archer S.L. Dynamin-Related Protein 1 (Drp1)-Mediated Diastolic Dysfunction in Myocardial Ischemia-Reperfusion Injury: Therapeutic Benefits of Drp1 Inhibition to Reduce Mitochondrial Fission. FASEB J. 2014;28:316–326. doi: 10.1096/fj.12-226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Slupe A.M., Merrill R.A., Flippo K.H., Lobas M.A., Houtman J.C., Strack S. A Calcineurin Docking Motif (LXVP) in Dynamin-Related Protein 1 Contributes to Mitochondrial Fragmentation and Ischemic Neuronal Injury. J. Biol. Chem. 2013;288:12353–12365. doi: 10.1074/jbc.M113.459677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dickey A.S., Strack S. PKA/AKAP1 and PP2A/Bbeta2 Regulate Neuronal Morphogenesis Via Drp1 Phosphorylation and Mitochondrial Bioenergetics. J. Neurosci. 2011;31:15716–15726. doi: 10.1523/JNEUROSCI.3159-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Merrill R.A., Slupe A.M., Strack S. N-Terminal Phosphorylation of Protein Phosphatase 2A/Bbeta2 Regulates Translocation to Mitochondria, Dynamin-Related Protein 1 Dephosphorylation, and Neuronal Survival. FEBS J. 2013;280:662–673. doi: 10.1111/j.1742-4658.2012.08631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bossy B., Petrilli A., Klinglmayr E., Chen J., Lutz-Meindl U., Knott A.B., Masliah E., Schwarzenbacher R., Bossy-Wetzel E. S-Nitrosylation of DRP1 does Not Affect Enzymatic Activity and is Not Specific to Alzheimer’s Disease. J. Alzheimers Dis. 2010;20(Suppl. 2):S513–S526. doi: 10.3233/JAD-2010-100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cho D.H., Nakamura T., Fang J., Cieplak P., Godzik A., Gu Z., Lipton S.A. S-Nitrosylation of Drp1 Mediates Beta-Amyloid-Related Mitochondrial Fission and Neuronal Injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Haun F., Nakamura T., Shiu A.D., Cho D.H., Tsunemi T., Holland E.A., La Spada A.R., Lipton S.A. S-Nitrosylation of Dynamin-Related Protein 1 Mediates Mutant Huntingtin-Induced Mitochondrial Fragmentation and Neuronal Injury in Huntington’s Disease. Antioxid. Redox Signal. 2013;19:1173–1184. doi: 10.1089/ars.2012.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee D.S., Kim J.E. PDI-Mediated S-Nitrosylation of DRP1 Facilitates DRP1-S616 Phosphorylation and Mitochondrial Fission in CA1 Neurons. Cell. Death Dis. 2018;9:869. doi: 10.1038/s41419-018-0910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chou C.H., Lin C.C., Yang M.C., Wei C.C., Liao H.D., Lin R.C., Tu W.Y., Kao T.C., Hsu C.M., Cheng J.T., et al. GSK3beta-Mediated Drp1 Phosphorylation Induced Elongated Mitochondrial Morphology Against Oxidative Stress. PLoS ONE. 2012;7:e49112. doi: 10.1371/journal.pone.0049112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yonashiro R., Ishido S., Kyo S., Fukuda T., Goto E., Matsuki Y., Ohmura-Hoshino M., Sada K., Hotta H., Yamamura H., et al. A Novel Mitochondrial Ubiquitin Ligase Plays a Critical Role in Mitochondrial Dynamics. EMBO J. 2006;25:3618–3626. doi: 10.1038/sj.emboj.7601249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nakamura N., Kimura Y., Tokuda M., Honda S., Hirose S. MARCH-V is a Novel Mitofusin 2- and Drp1-Binding Protein Able to Change Mitochondrial Morphology. EMBO Rep. 2006;7:1019–1022. doi: 10.1038/sj.embor.7400790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Karbowski M., Neutzner A., Youle R.J. The Mitochondrial E3 Ubiquitin Ligase MARCH5 is Required for Drp1 Dependent Mitochondrial Division. J. Cell Biol. 2007;178:71–84. doi: 10.1083/jcb.200611064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fang L., Hemion C., Goldblum D., Meyer P., Orgul S., Frank S., Flammer J., Neutzner A. Inactivation of MARCH5 Prevents Mitochondrial Fragmentation and Interferes with Cell Death in a Neuronal Cell Model. PLoS ONE. 2012;7:e52637. doi: 10.1371/journal.pone.0052637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang H., Song P., Du L., Tian W., Yue W., Liu M., Li D., Wang B., Zhu Y., Cao C., et al. Parkin Ubiquitinates Drp1 for Proteasome-Dependent Degradation: Implication of Dysregulated Mitochondrial Dynamics in Parkinson Disease. J. Biol. Chem. 2011;286:11649–11658. doi: 10.1074/jbc.M110.144238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lutz A.K., Exner N., Fett M.E., Schlehe J.S., Kloos K., Lammermann K., Brunner B., Kurz-Drexler A., Vogel F., Reichert A.S., et al. Loss of Parkin Or PINK1 Function Increases Drp1-Dependent Mitochondrial Fragmentation. J. Biol. Chem. 2009;284:22938–22951. doi: 10.1074/jbc.M109.035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Song M., Gong G., Burelle Y., Gustafsson A.B., Kitsis R.N., Matkovich S.J., Dorn G.W., 2nd Interdependence of Parkin-Mediated Mitophagy and Mitochondrial Fission in Adult Mouse Hearts. Circ. Res. 2015;117:346–351. doi: 10.1161/CIRCRESAHA.117.306859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hu C., Huang Y., Li L. Drp1-Dependent Mitochondrial Fission Plays Critical Roles in Physiological and Pathological Progresses in Mammals. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Harder Z., Zunino R., McBride H. Sumo1 Conjugates Mitochondrial Substrates and Participates in Mitochondrial Fission. Curr. Biol. 2004;14:340–345. doi: 10.1016/j.cub.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 169.Nakamura T., Cieplak P., Cho D.H., Godzik A., Lipton S.A. S-Nitrosylation of Drp1 Links Excessive Mitochondrial Fission to Neuronal Injury in Neurodegeneration. Mitochondrion. 2010;10:573–578. doi: 10.1016/j.mito.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nakamura T., Prikhodko O.A., Pirie E., Nagar S., Akhtar M.W., Oh C.K., McKercher S.R., Ambasudhan R., Okamoto S., Lipton S.A. Aberrant Protein S-Nitrosylation Contributes to the Pathophysiology of Neurodegenerative Diseases. Neurobiol. Dis. 2015;84:99–108. doi: 10.1016/j.nbd.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ducommun S., Deak M., Sumpton D., Ford R.J., Nunez Galindo A., Kussmann M., Viollet B., Steinberg G.R., Foretz M., Dayon L., et al. Motif Affinity and Mass Spectrometry Proteomic Approach for the Discovery of Cellular AMPK Targets: Identification of Mitochondrial Fission Factor as a New AMPK Substrate. Cell. Signal. 2015;27:978–988. doi: 10.1016/j.cellsig.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 172.Toyama E.Q., Herzig S., Courchet J., Lewis T.L., Jr., Loson O.C., Hellberg K., Young N.P., Chen H., Polleux F., Chan D.C., et al. Metabolism. AMP-Activated Protein Kinase Mediates Mitochondrial Fission in Response to Energy Stress. Science. 2016;351:275–281. doi: 10.1126/science.aab4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xu S., Cherok E., Das S., Li S., Roelofs B.A., Ge S.X., Polster B.M., Boyman L., Lederer W.J., Wang C., et al. Mitochondrial E3 Ubiquitin Ligase MARCH5 Controls Mitochondrial Fission and Cell Sensitivity to Stress-Induced Apoptosis through Regulation of MiD49 Protein. Mol. Biol. Cell. 2016;27:349–359. doi: 10.1091/mbc.e15-09-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fahrner J.A., Liu R., Perry M.S., Klein J., Chan D.C. A Novel De Novo Dominant Negative Mutation in DNM1L Impairs Mitochondrial Fission and Presents as Childhood Epileptic Encephalopathy. Am. J. Med. Genet. A. 2016;170:2002–2011. doi: 10.1002/ajmg.a.37721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chao Y.H., Robak L.A., Xia F., Koenig M.K., Adesina A., Bacino C.A., Scaglia F., Bellen H.J., Wangler M.F. Missense Variants in the Middle Domain of DNM1L in Cases of Infantile Encephalopathy Alter Peroxisomes and Mitochondria when Assayed in Drosophila. Hum. Mol. Genet. 2016;25:1846–1856. doi: 10.1093/hmg/ddw059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nasca A., Legati A., Baruffini E., Nolli C., Moroni I., Ardissone A., Goffrini P., Ghezzi D. Biallelic Mutations in DNM1L are Associated with a Slowly Progressive Infantile Encephalopathy. Hum. Mutat. 2016;37:898–903. doi: 10.1002/humu.23033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vanstone J.R., Smith A.M., McBride S., Naas T., Holcik M., Antoun G., Harper M.E., Michaud J., Sell E., Chakraborty P., et al. DNM1L-Related Mitochondrial Fission Defect Presenting as Refractory Epilepsy. Eur. J. Hum. Genet. 2016;24:1084–1088. doi: 10.1038/ejhg.2015.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sheffer R., Douiev L., Edvardson S., Shaag A., Tamimi K., Soiferman D., Meiner V., Saada A. Postnatal Microcephaly and Pain Insensitivity due to a De Novo Heterozygous DNM1L Mutation Causing Impaired Mitochondrial Fission and Function. Am. J. Med. Genet. A. 2016;170:1603–1607. doi: 10.1002/ajmg.a.37624. [DOI] [PubMed] [Google Scholar]
- 179.Cahill T.J., Leo V., Kelly M., Stockenhuber A., Kennedy N.W., Bao L., Cereghetti G.M., Harper A.R., Czibik G., Liao C., et al. Resistance of Dynamin-Related Protein 1 Oligomers to Disassembly Impairs Mitophagy, Resulting in Myocardial Inflammation and Heart Failure. J. Biol. Chem. 2015;290:25907–25919. doi: 10.1074/jbc.M115.665695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ashrafian H., Docherty L., Leo V., Towlson C., Neilan M., Steeples V., Lygate C.A., Hough T., Townsend S., Williams D., et al. A Mutation in the Mitochondrial Fission Gene Dnm1l Leads to Cardiomyopathy. PLoS Genet. 2010;6:e1001000. doi: 10.1371/journal.pgen.1001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang W., Fernandez-Sanz C., Sheu S.S. Regulation of Mitochondrial Bioenergetics by the Non-Canonical Roles of Mitochondrial Dynamics Proteins in the Heart. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:1991–2001. doi: 10.1016/j.bbadis.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yu T., Robotham J.L., Yoon Y. Increased Production of Reactive Oxygen Species in Hyperglycemic Conditions Requires Dynamic Change of Mitochondrial Morphology. Proc. Natl. Acad. Sci. USA. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hoque A., Sivakumaran P., Bond S.T., Ling N.X.Y., Kong A.M., Scott J.W., Bandara N., Hernandez D., Liu G.S., Wong R.C.B., et al. Mitochondrial Fission Protein Drp1 Inhibition Promotes Cardiac Mesodermal Differentiation of Human Pluripotent Stem Cells. Cell. Death Discov. 2018;4:39. doi: 10.1038/s41420-018-0042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rosdah A.A., K Holien J., Delbridge L.M., Dusting G.J., Lim S.Y. Mitochondrial Fission—A Drug Target for Cytoprotection Or Cytodestruction? Pharmacol. Res. Perspect. 2016;4:e00235. doi: 10.1002/prp2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bordt E.A., Clerc P., Roelofs B.A., Saladino A.J., Tretter L., Adam-Vizi V., Cherok E., Khalil A., Yadava N., Ge S.X., et al. The Putative Drp1 Inhibitor Mdivi-1 is a Reversible Mitochondrial Complex I Inhibitor that Modulates Reactive Oxygen Species. Dev. Cell. 2017;40:583–594. doi: 10.1016/j.devcel.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Czarnowska E., Bierla J.B., Toczek M., Tyrankiewicz U., Pajak B., Domal-Kwiatkowska D., Ratajska A., Smolenski R.T., Mende U., Chlopicki S. Narrow Time Window of Metabolic Changes Associated with Transition to Overt Heart Failure in Tgaq*44 Mice. Pharmacol. Rep. 2016;68:707–714. doi: 10.1016/j.pharep.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 187.Dai D.F., Johnson S.C., Villarin J.J., Chin M.T., Nieves-Cintron M., Chen T., Marcinek D.J., Dorn G.W., 2nd, Kang Y.J., Prolla T.A., et al. Mitochondrial Oxidative Stress Mediates Angiotensin II-Induced Cardiac Hypertrophy and Galphaq Overexpression-Induced Heart Failure. Circ. Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Qi J., Wang F., Yang P., Wang X., Xu R., Chen J., Yuan Y., Lu Z., Duan J. Mitochondrial Fission is Required for Angiotensin II-Induced Cardiomyocyte Apoptosis Mediated by a Sirt1-p53 Signaling Pathway. Front. Pharmacol. 2018;9:176. doi: 10.3389/fphar.2018.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang W., Gong G., Wang X., Wei-LaPierre L., Cheng H., Dirksen R., Sheu S.S. Mitochondrial Flash: Integrative Reactive Oxygen Species and pH Signals in Cell and Organelle Biology. Antioxid. Redox Signal. 2016;25:534–549. doi: 10.1089/ars.2016.6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Miyamoto S., Del Re D.P., Xiang S.Y., Zhao X., Florholmen G., Brown J.H. Revisited and Revised: Is RhoA always a Villain in Cardiac Pathophysiology? J. Cardiovasc. Transl. Res. 2010;3:330–343. doi: 10.1007/s12265-010-9192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Parra V., Rothermel B.A. Calcineurin Signaling in the Heart: The Importance of Time and Place. J. Mol. Cell. Cardiol. 2017;103:121–136. doi: 10.1016/j.yjmcc.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lei M., Wang X., Ke Y., Solaro R.J. Regulation of Ca2+ Transient by PP2A in Normal and Failing Heart. Front. Physiol. 2015;6:13. doi: 10.3389/fphys.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.DuBoff B., Gotz J., Feany M.B. Tau Promotes Neurodegeneration Via DRP1 Mislocalization in Vivo. Neuron. 2012;75:618–632. doi: 10.1016/j.neuron.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kim J.E., Ryu H.J., Kim M.J., Kang T.C. LIM Kinase-2 Induces Programmed Necrotic Neuronal Death Via Dysfunction of DRP1-Mediated Mitochondrial Fission. Cell Death Differ. 2014;21:1036–1049. doi: 10.1038/cdd.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Williams G.S., Boyman L., Lederer W.J. Mitochondrial Calcium and the Regulation of Metabolism in the Heart. J. Mol. Cell. Cardiol. 2015;78:35–45. doi: 10.1016/j.yjmcc.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cogliati S., Frezza C., Soriano M.E., Varanita T., Quintana-Cabrera R., Corrado M., Cipolat S., Costa V., Casarin A., Gomes L.C., et al. Mitochondrial Cristae Shape Determines Respiratory Chain Supercomplexes Assembly and Respiratory Efficiency. Cell. 2013;155:160–171. doi: 10.1016/j.cell.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.O-Uchi J., Jhun B.S., Hurst S., Bisetto S., Gross P., Chen M., Kettlewell S., Park J., Oyamada H., Smith G.L., et al. Overexpression of Ryanodine Receptor Type 1 Enhances Mitochondrial Fragmentation and Ca2+-Induced ATP Production in Cardiac H9c2 Myoblasts. Am. J. Physiol. Heart Circ. Physiol. 2013;305:H1736. doi: 10.1152/ajpheart.00094.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nicholls D.G. Mitochondrial Membrane Potential and Aging. Aging Cell. 2004;3:35–40. doi: 10.1111/j.1474-9728.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 199.Aon M.A., Cortassa S., O’Rourke B. Redox-Optimized ROS Balance: A Unifying Hypothesis. Biochim. Biophys. Acta. 2010;1797:865–877. doi: 10.1016/j.bbabio.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hassan M.Q., Akhtar M.S., Akhtar M., Ansari S.H., Ali J., Haque S.E., Najmi A.K. Benidipine Prevents Oxidative Stress, Inflammatory Changes and Apoptosis Related Myofibril Damage in Isoproterenol-Induced Myocardial Infarction in Rats. Toxicol. Mech. Methods. 2015;25:26–33. doi: 10.3109/15376516.2014.972531. [DOI] [PubMed] [Google Scholar]
- 201.Montessuit S., Somasekharan S.P., Terrones O., Lucken-Ardjomande S., Herzig S., Schwarzenbacher R., Manstein D.J., Bossy-Wetzel E., Basanez G., Meda P., et al. Membrane Remodeling Induced by the Dynamin-Related Protein Drp1 Stimulates Bax Oligomerization. Cell. 2010;142:889–901. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Juhaszova M., Zorov D.B., Yaniv Y., Nuss H.B., Wang S., Sollott S.J. Role of Glycogen Synthase Kinase-3beta in Cardioprotection. Circ. Res. 2009;104:1240–1252. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Irie T., Sips P.Y., Kai S., Kida K., Ikeda K., Hirai S., Moazzami K., Jiramongkolchai P., Bloch D.B., Doulias P.T., et al. S-Nitrosylation of Calcium-Handling Proteins in Cardiac Adrenergic Signaling and Hypertrophy. Circ. Res. 2015;117:793–803. doi: 10.1161/CIRCRESAHA.115.307157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Daniels C.R., Foster C.R., Yakoob S., Dalal S., Joyner W.L., Singh M., Singh K. Exogenous Ubiquitin Modulates Chronic Beta-Adrenergic Receptor-Stimulated Myocardial Remodeling: Role in Akt Activity and Matrix Metalloproteinase Expression. Am. J. Physiol. Heart Circ. Physiol. 2012;303:H1459–H1468. doi: 10.1152/ajpheart.00401.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shirakabe A., Zhai P., Ikeda Y., Saito T., Maejima Y., Hsu C.P., Nomura M., Egashira K., Levine B., Sadoshima J. Drp1-Dependent Mitochondrial Autophagy Plays a Protective Role Against Pressure Overload-Induced Mitochondrial Dysfunction and Heart Failure. Circulation. 2016;133:1249–1263. doi: 10.1161/CIRCULATIONAHA.115.020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ikeda Y., Shirakabe A., Maejima Y., Zhai P., Sciarretta S., Toli J., Nomura M., Mihara K., Egashira K., Ohishi M., et al. Endogenous Drp1 Mediates Mitochondrial Autophagy and Protects the Heart Against Energy Stress. Circ. Res. 2015;116:264–278. doi: 10.1161/CIRCRESAHA.116.303356. [DOI] [PubMed] [Google Scholar]