Abstract
DNA replication is an essential biochemical reaction in dividing cells that frequently stalls at damaged sites. Homologous/homeologous recombination (HR)-mediated template switch and translesion DNA synthesis (TLS)-mediated bypass processes release arrested DNA replication forks. These mechanisms are pivotal for replication fork maintenance and play critical roles in DNA damage tolerance (DDT) and gap-filling. The avian DT40 B lymphocyte cell line provides an opportunity to examine HR-mediated template switch and TLS triggered by abasic sites by sequencing the constitutively diversifying immunoglobulin light-chain variable gene (IgV). During IgV diversification, activation-induced deaminase (AID) converts dC to dU, which in turn is excised by uracil DNA glycosylase and yields abasic sites within a defined window of around 500 base pairs. These abasic sites can induce gene conversion with a set of homeologous upstream pseudogenes via the HR-mediated template switch, resulting in templated mutagenesis, or can be bypassed directly by TLS, resulting in non-templated somatic hypermutation at dC/dG base pairs. In this review, we discuss recent works unveiling IgV diversification mechanisms in avian DT40 cells, which shed light on DDT mode usage in vertebrate cells and tolerance of abasic sites.
Keywords: homologous recombination, translesion DNA synthesis, replication, activation-induced deaminase, abasic site, DNA damage
1. Introduction
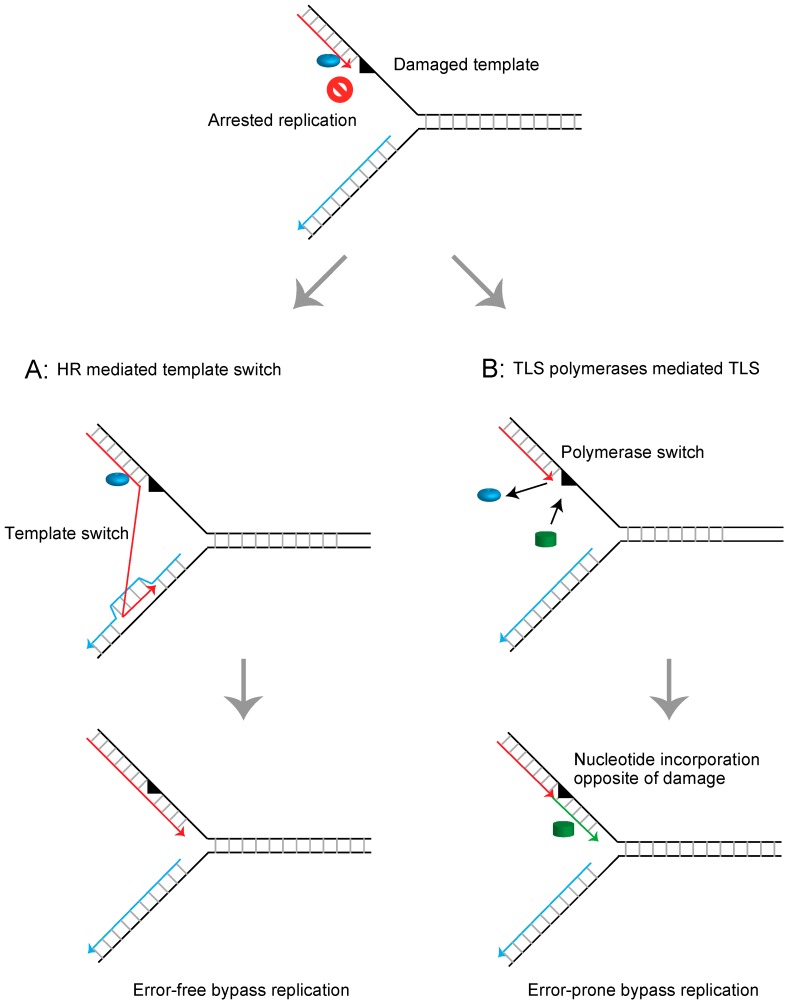
Cellular DNA is continuously damaged by chemical and physical agents from both endogenous metabolic processes and exogenous insults. Replicative DNA polymerases replicate genomic DNA with extraordinarily high accuracy, making only a single error per 106 nucleotides synthesized in vivo [1]. Due to this enzymatic property, replicative polymerases cannot accommodate nucleotides at damaged templates and thus arrest replication [2]. Cells employ several mechanisms for releasing arrested replication forks depending on the type of DNA lesions or the cell cycle phase [3]. One of the most common and formidable lesions is the abasic site. Abasic sites are estimated to occur at a frequency of over 10,000 sites per cell per day in mammalian cells [4,5]. These lesions can be repaired via accurate base excision repair [6,7,8,9]. However, when the replication fork encounters unrepaired abasic sites, they are dealt with by DNA damage tolerance (DDT) mechanisms that allow completion of replication beyond the damaged template and prevent formation of deleterious double strand breaks (DSBs) [10,11]. In general, there are two modes of DDT conserved in eukaryotes [10]. One mode is mediated through homologous recombination (HR), which mediates continuous replication using a newly synthesized sister strand [10,12,13] (Figure 1A). A second mode is translesion DNA synthesis (TLS), which employs specialized DNA polymerases, including polymerase η and polymerase ζ, to permit continuous replication beyond the damaged template [14,15,16,17,18] (Figure 1B).
Figure 1.
Schematic representation of mechanisms releasing the arrested replication fork at the damaged template. (A) Homologous recombination (HR)-mediated template switch releases the arrested replication fork using intact newly synthesized DNA as the template strand and promotes error-free bypass replication. (B) Translesion DNA synthesis (TLS) polymerases mediate direct bypass replication across the damaged template in an error-prone manner.
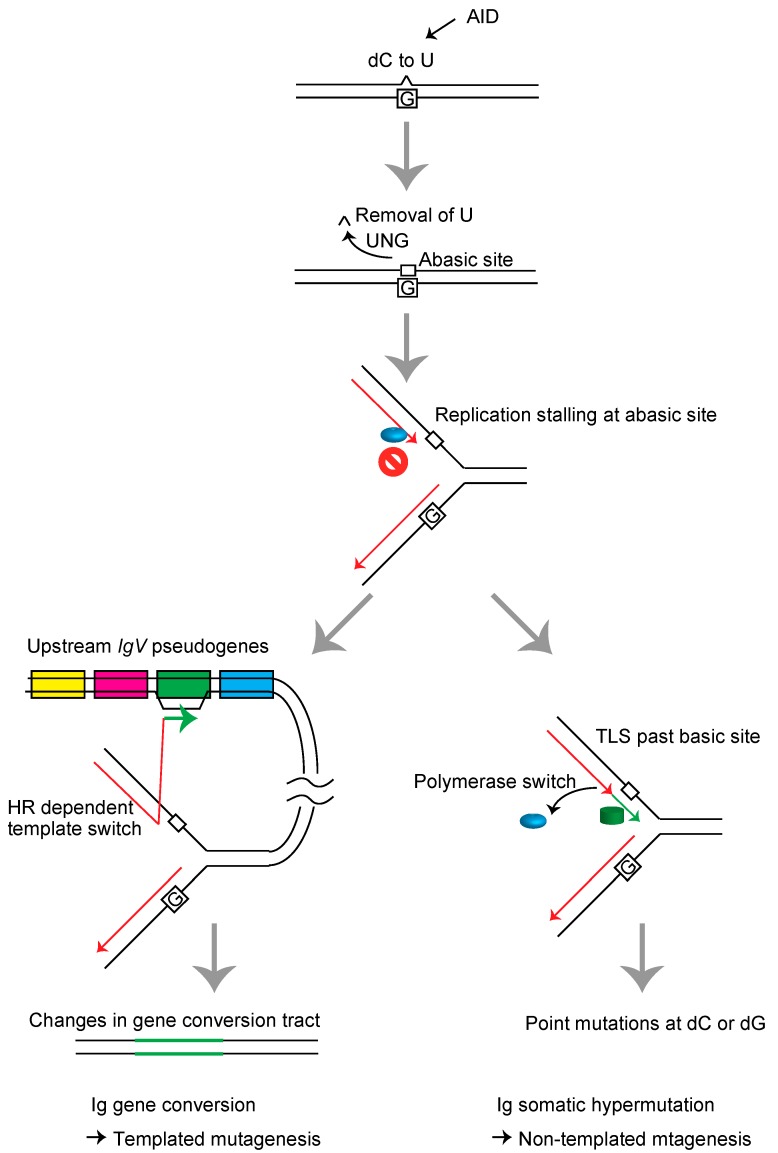
When the blocking lesions are abasic sites, DDT processes serve additional functions, such as generating immunoglobulin diversity in B cells [19,20]. Avian B cells diversify the immunoglobulin variable (IgV) gene through HR-mediated gene conversion and TLS-mediated somatic hypermutation [21]. In the initiation process of IgV diversification, activation-induced deaminase (AID) leads to conversion of dC to dU on regions of single-stranded DNA, the exposure of which is locally facilitated by the histone variant H3.3 [22], probably because nucleosome cores containing H3.3 are more unstable than those containing canonical H3 variants [23]. The uracil DNA glycosylase (UNG) efficiently excises the uracil residues, yielding a high frequency of abasic sites [24,25] within a defined window of around 500 base pairs. The restriction of AID-mediated abasic sites to the IgV gene is mediated by the cis-acting sequence referred to as DIVAC (diversification activator) [26]. The replication stalling at these abasic sites either induces template switch-mediated gene conversion with one of the 25 copies of upstream IgV pseudogenes carrying ~10% mismatch, resulting in HR-mediated diversification/mutagenesis of the immunoglobulin gene, or they induce TLS, resulting in somatic hypermutation at the dC/dG base pairs [14,15,24,27,28,29,30,31] (Figure 2). The induced hypermutations are most likely a consequence of replication bypass across abasic sites, rather than bypass of dU, as evidenced by the fact that most mutations are dC/dG to dG/dC transversions and loss of UNG instead leads to replication over the dU, resulting in dC/dG to dT/dA transitions [24].
Figure 2.
Schematic representation of the immunoglobulin variable (IgV) gene diversification mechanism in DT40 cells. The sequential actions of activation-induced deaminase (AID) and uracil DNA glycosylase (UNG) induce abasic sites in the IgV gene. The replication fork arrests at these lesions and induces template switch-mediated gene conversion with one of the 25 copies of upstream IgV pseudogenes carrying a ~10% mismatch rate, resulting in HR-mediated diversification/mutagenesis of the immunoglobulin gene (left), or TLS, resulting in somatic hypermutation at the dC/dG base pairs (right).
The avian DT40 cell line derived from bursal B cells continues to diversify its IgV gene by gene conversion and somatic hypermutation during in vitro passage [32]. This cell line exhibits extraordinarily high gene-targeting efficiency, as an exception among vertebrate cell lines, and it is thus used in genetic studies due to the relative ease of establishing mutants [33]. Using this cell line, a number of studies have assessed the contribution of genes of interest to HR-mediated template switch and TLS via analyzing IgV diversification mechanisms [19]. Herein, we discuss how the DT40 cell line provides an opportunity to examine HR-mediated template switch and TLS at abasic sites via sequencing the constitutively diversifying IgV gene.
2. Immunoglobulin variable (IgV) Gene Diversification in DT40 Cells
To examine IgV sequence diversification within the limited duration of in vitro passage (usually 2–5 weeks), the following methods increase the frequency of IgV sequence diversification and allow effective measurement of the IgV diversification rate.
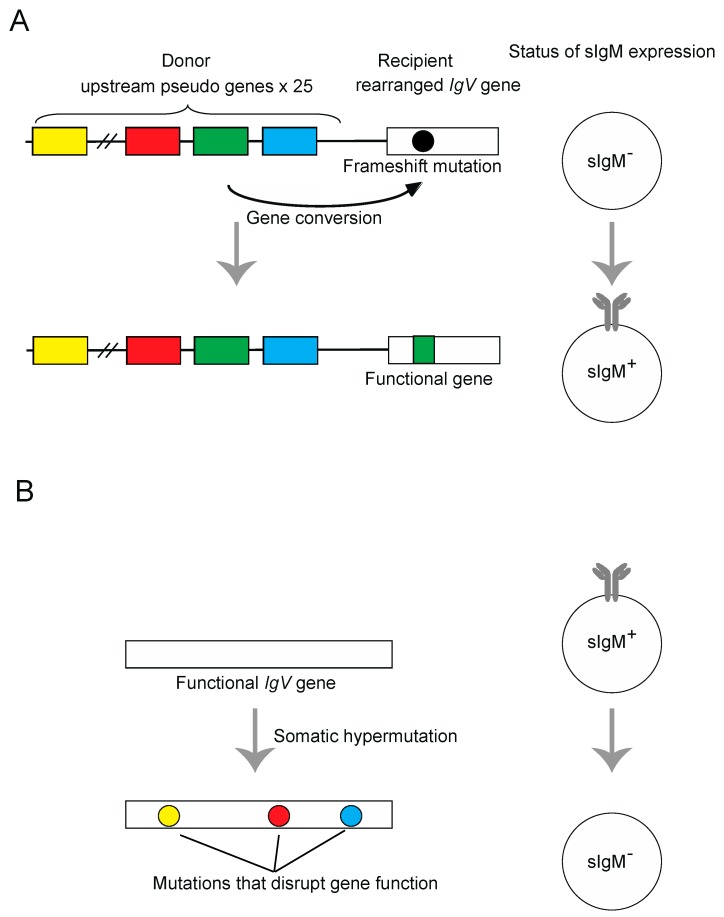
The first method is treating DT40 cells with a histone deacetylase inhibitor, trichostatin A (TSA). This method increases the frequency of HR-mediated gene conversion 50- to 100-fold, with limited effects on TLS-mediated somatic hypermutation [17,34]. Thus, for evaluating HR efficiency, TSA treatment during in vitro passage of DT40 cells allows for sensitive detection of gene conversion events. The mechanism underlying TSA-mediated activation of gene conversion has not been fully elucidated, but a likely scenario is that TSA mainly affects the chromatin in the upstream IgV pseudogenes, favoring their open configuration. Subsequently, the HR machinery can efficiently induce gene conversion using these pseudogenes as donor sequences. This view is supported by the observation that tethering of the heterochromatin protein HP1 to upstream IgV pseudogene regions diminishes histone acetylation within this region and alters the outcome of IgV diversification, causing somatic hypermutation to outweigh gene conversion [35]. The efficiency of gene conversion events can be estimated without conducting sequence analysis. Specifically, the status of surface immunoglobulin M (sIgM) expression in DT40 cl-18 cells, which carry a frameshift mutation in the IgVλ segment, alters from negative to positive (sIgM gain) by gene conversion-mediated replacement of the frameshift mutation. Thus, by measuring the rate of sIgM gain, the frequency of gene conversion can be estimated (Figure 3A). The percentage of sIgM-positive cells within expanding subclones can be analyzed by fluorescence activated cell sorting (FACS) [36].
Figure 3.
Schematic representations of the surface immunoglobulin M (sIgM) gain (A) and sIgM loss assays (B). (A) Principle of the Ig gene conversion assay. The sIgM-negative DT40 clone contains a frameshift in its rearranged V-Jλ segments, which can be repaired by pseudogene-templated conversion events. The rate of Ig gene conversion can be measured as a gain of sIgM expression in subclones by flow-cytometric analysis of sIgM staining. (B) Principle of the Ig hypermutation assay. The DT40 cells carrying wild type IgV gene, which can be disrupted by somatic hypermutation events. The rate of hypermutation events can be measured as a loss of sIgM expression in subclones by flow-cytometric analysis of sIgM staining. The sequence analysis of hypermutation event can be also carried out by selecting sIgM negative cells by cell sorter.
Another method is AID overexpression during in vitro passage of DT40 cells. In this approach, mouse AID (mAID) is overexpressed via infection with retrovirus containing the mAID gene, followed by an internal ribosomal entry site and the GFP gene, as described previously [37]. With this method, the expression level of the mouse AID protein is approximately 20 times higher than that of endogenous chicken AID [38]. With the same methodology, chicken AID cannot be efficiently overexpressed, presumably due to protein level control (author’s unpublished data). A variant of this method is to establish clones overexpressing an enzymatically hyperactive form of human AID, hAIDup 7.3 [39], which exhibits increased levels of deamination while generating an identical pattern of deamination to the wild-type enzyme [22,39].
The overexpression of AID increases gene conversion at a rate similar to that of gene conversion induced by TSA [38]. Moreover, somatic hypermutation is significantly increased by AID overexpression, and hypermutation events are observed in nearly all IgV sequences analyzed, while somatic hypermutation is barely detectable without AID overexpression. AID overexpression induces gene conversion and somatic hypermutation at a ratio of approximately 1:2. AID overexpression during in vitro passage of DT40 cells allows sensitive detection of gene conversion and somatic hypermutation.
An alternative approach to analyze hypermutation events without AID overexpression has also been used. Instead of increasing the frequency of diversification, cells with spontaneously diversified IgV sequences can be selected by the status of sIgM expression using the cell sorter. Since the hypermutation-mediated insertion of deleterious mutations results in the loss of sIgM expression, starting from sIgM positive cells and selecting for sIgM negative cells is proposed to be a good readout of the hypermutation events. In support of this hypermutation-mediated sIgM loss is the observation that complete deletion of upstream pseudogenes (ψV−) diminishes gene conversion while increasing hypermutation events and the frequency of sIgM loss [28]. However, the caveat with this approach is that sIgM loss can also derive in good part from gene conversion-associated frameshift in wild type (WT) cells [40]. Thus, perhaps a good compromise in this regard would be to use a cell line with complete deletion of upstream pseudogenes (ψV−) and to approximate the sIgM loss with the frequency of somatic hypermutation events (Figure 3B).
Importantly, the hypermutation analysis allows evaluation not only of TLS efficiency but also of the mutation spectrum. In wild type cells, dG to dC transversion predominantly occurs [24], while in certain cases when TLS polymerase(s) are inactivated, the mutation spectrum is altered, possibly due to a shift of used TLS polymerase(s) in the bypass of replication across abasic sites in the IgV gene [14,27,41].
Differentiating between hypermutation and gene conversion from the IgV nucleotide sequence should be carried out as previously described [40]. Briefly, all sequence changes are assigned to one of the following three categories: hypermutation, gene conversion or ambiguous mutation. A single base substitution can be categorized as hypermutation or ambiguous mutation. Using the genome database search, if one can find pseudogenes identical to the sequence containing the point mutation, such mutation is categorized as ambiguous mutation, while if no identical donor sequence can be identified, such point mutation should be categorized as hypermutation. An event with multiple mutations having an identical pseudogene is interpreted as a single gene conversion event. If a sequence containing multiple substitutions is not completely identical to one of the pseudogenes, but has few mismatches, such mutations can be interpreted as deriving from a single gene conversion event followed by several subsequent hypermutations.
3. Templated Mutagenesis by Gene Conversion
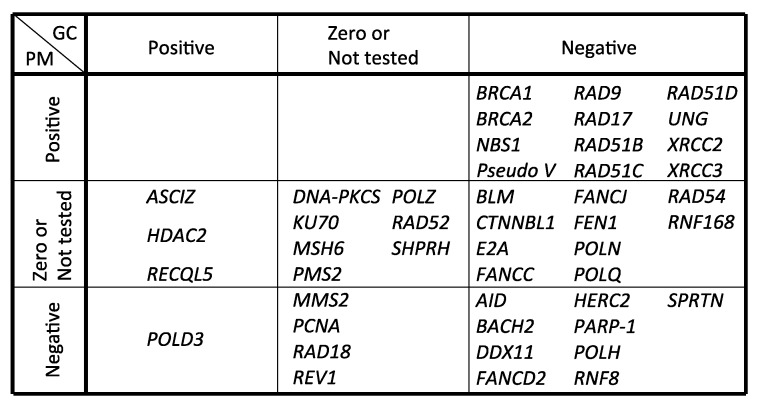
Ig gene conversion is mediated through HR as evidenced by the fact that deletion of upstream homeologous IgV pseudogenes completely abolishes gene conversion [28] (Figure 4, Table 1, Pseudo V). This observation also demonstrates that gene conversion and hypermutation are initiated from a common DNA lesion mediated by AID, since loss of upstream IgV pseudogenes activates AID-dependent hypermutation (Figure 4, Table 1). This idea is further strongly supported by the fact that in DT40 cells mutated in Rad51 paralog genes BRCA1 or BRCA2, in which recruitment of the Rad51 DNA-strand exchange protein to damaged DNA is strongly impaired [40,42,43], gene conversion is critically reduced and the IgV diversification is strongly shifted toward hypermutation (Figure 4, Table 1). Inactivation of RAD54 (a gene encoding a key component of homologous recombination) or FANCD2 (a gene responsible for Fanconi anemia, FA) reduces gene conversion without increasing hypermutation [44,45] (Figure 4, Table 1). These pathways might contribute to gene conversion after strand exchange, when the commitment towards Ig gene conversion has been made.
Figure 4.
Genes involved in Ig gene conversion and hypermutation. GC and PM represent gene conversion and hypermutation, respectively. Positive, zero or negative effects by gene deletion or mutation on gene conversion and hypermutation were categorized.
Table 1.
Genes involved in Ig gene conversion and hypermutation.
| Gene Name | GC | PM | Method | Reference |
|---|---|---|---|---|
| AID | − | − | IgM gain and sequencing | Arakawa et al., 2002 [105] |
| ASCIZ | + | 0 | IgM gain, mAID overexpression and sequencing | Oka et al., 2008 [106] |
| BACH2 | − | − | IgM gain and sequencing | Budzynska et al., 2017 [107] |
| BLM | − | NT | IgM gain | Kikuchi et al., 2009 [108] |
| BRCA1 | − | + | IgM loss and sequencing | Longerich et al., 2008 [42] |
| BRCA2 | − | + | IgM loss and sequencing | Hatanaka et al., 2005 [43] |
| CTNNBL1 | − | NT | IgM gain | Conticello et al., 2008 [109] |
| DDX11 | − | − | IgM gain, mAID overexpression and sequencing | Abe et al., 2018 [65] |
| DNA-PKCS | NT | 0 | IgM loss | Sale et al., 2001 [40] |
| E2A | − | 0 | IgM gain and sequencing | Kitao et al., 2008 [110] |
| FANCC | − | NT | IgM loss and sequencing | Pace et al., 2010 [111] |
| FANCD2 | − | − | IgM loss, gain and sequencing | Yamamoto et al., 2005 [45] |
| FANCJ | − | NT | IgM loss and sequencing | Kitao et al., 2011 [112] |
| FEN1 | − | NT | IgM gain and sequencing | Kikuchi et al., 2005 [113] |
| HDAC2 | + | 0 | IgM gain and sequencing | Lin et al., 2008 [114] |
| HERC2 | − | − | mAID overexpression and sequencing | Mohiuddin et al., 2016 [104] |
| KU70 | NT | 0 | IgM loss | Sale et al., 2001 [40] |
| MMS2 | 0 | − | IgM loss and sequencing | Simpson et al., 2005 [115] |
| MSH6 | 0 | 0 | IgM gain and sequencing | Campo et al., 2013 [116] |
| NBS1 (p70) | − | + | sIgM gain, mAID overexpression and sequencing | Nakahara et al., 2009 [117] |
| PARP-1 | − | − | IgM gain, mAID overexpression and sequencing | Paddock et al., 2010 [118] |
| PCNA (K164R) | NT | − | IgM loss and sequencing | Arakawa et al., 2006 [27] |
| PMS2 | 0 | 0 | IgM gain and sequencing | Campo et al., 2013 [116] |
| POLD3 | + | − | mAID overexpression and sequencing | Hirota et al., 2015 [16] |
| POLH | − | − | IgM gain and sequencing | Kawamoto et al., 2006 [46], Kohzaki et al., 2010 [17] |
| POLN | − | 0 | IgM gain, mAID overexpression and sequencing | Kohzaki et al., 2010 [17] |
| POLQ | − | 0 | IgM gain, mAID overexpression and sequencing | Kohzaki et al., 2010 [17] |
| POLH/POLN/POLQ | − | − | IgM gain, mAID overexpression and sequencing | Kohzaki et al., 2010 [17] |
| POLZ (REV3) | 0 | NT | IgM gain and sequencing | Okada et al., 2005 [83] |
| Pseudo V | − | + | IgM loss and sequencing | Arakawa et al., 2004 [28] |
| RAD9 | − | + | mAID overexpression and sequencing | Saberi et al., 2008 [38] |
| RAD17 | − | + | mAID overexpression and sequencing | Saberi et al., 2008 [38] |
| RAD18 | NT | − | IgM loss and sequencing | Arakawa et al., 2006 [27] |
| RAD51B | − | + | IgM loss and sequencing | Sale et al., 2001 [40] |
| RAD51C | − | + | IgM loss and sequencing | Hatanaka et al., 2005 [43] |
| RAD51D | − | + | IgM loss and sequencing | Hatanaka et al., 2005 [43] |
| RAD52 | NT | 0 | IgM loss | Sale et al., 2001 [40] |
| RAD54 | − | NT | IgM gain and sequencing | Bezzubova et al., 1997 [44] |
| RECQL5 | + | NT | IgM gain and sequencing | Hosono et al., 2014 [119] |
| RNF8 | − | − | mAID overexpression and sequencing | Mohiuddin et al., 2016 [104] |
| RNF168 | − | 0 | mAID overexpression and sequencing | Mohiuddin et al., 2016 [104] |
| SPRTN | − | − | IgM gain, mAID overexpression and sequencing | Nakazato et al., 2018 [78] |
| SHPRH | 0 | 0 | sIgM gain and sequencing | Tomi et al., 2014 [97] |
| REV1 | 0 | − | IgM loss and sequencing | Simpson et al., 2003 [120] |
| UNG | − | + | IgM gain, loss and sequencing | Saribasak et al., 2005 [25] |
| XRCC2 | − | + | IgM loss and sequencing | Sale et al., 2001 [40] |
| XRCC3 | − | + | IgM loss and sequencing | Sale et al., 2001 [40] |
GC and PM represent gene conversion and hypermutation, respectively. Positive, zero and negative effects by gene deletion or mutation are shown by +, 0 and −, respectively. NT: not tested.
Ig gene conversion is initiated at the AID-mediated lesion (Figure 4, Table 1). HR mediates a template switch to a homeologous upstream IgV pseudogene. HR is also accompanied by DNA synthesis, which is facilitated by TLS-polymerases, as evidenced by the following observations. Polη-deficient (POLη−/−) cells show a reduced gene conversion rate with increased length of gene conversion tract in comparison with wild type cells [46] (Table 1). A role for Polη in D-loop extensions during HR reactions in vitro has been reported [47,48,49,50]. These data indicate involvement of Polη in Ig gene conversion and further imply that some backup polymerase(s) carrying higher processivity might compensate for the absence of Polη in POLη−/− cells. Such backup polymerases might be Polν and Polθ, since Ig gene conversion is completely abolished in POLη−/−/POLν−/−/POLθ−/− cells [17] (Figure 4, Table 1). Thus, these TLS polymerases play roles not only in TLS but also in HR-mediated Ig gene conversion. The recruitment of TLS polymerases is promoted by ubiquitination of the proliferating cell nuclear antigen (PCNA) [51,52,53]. However, the role of PCNA ubiquitination in vertebrate HR and IgV diversification has not been fully elucidated.
The heterotrimeric checkpoint clamp, consisting of the Rad9, Hus1, and Rad1 subunits (hereafter referred to as 9-1-1), is structurally similar to PCNA and serves as a DNA damage sensor to mediate the ATR signal axis [54,55,56]. The 9-1-1 clamp is loaded onto DNA by Rad17-RFC (replication factor C) in vitro, which is analogous to the PCNA-RFC clamp loader system [57,58]. In vertebrates, Rad9 and Rad17 play significant roles in HR as previously described [59,60]. RAD9−/− and RAD17/− DT40 cells show strongly reduced Ig gene conversion and increased hypermutation [38] (Figure 4, Table 1). Considering limited effects on HR-mediated gene-targeting and DSB repair in comparison with severe defects in Ig gene conversion by the loss of Rad9 or Rad17, the role of these factors might be confined to a subset of the HR reaction initiated by replication fork stalling and associated with formation of single strand DNA (ssDNA) gaps, rather than induced by DSBs.
Recently, mutations in the XPD family helicase gene DDX11 were identified in the hereditary disorder Warsaw Breakage Syndrome (WABS) [61,62,63,64]. Cells from WABS patients have a shared feature with Fanconi anemia (FA) with respect to the hypersensitivity to interstrand-crosslink (ICL) inducing agents and increased chromosome aberrations, but to date DDX11 has not been formally classified as an FA gene [61,62,63,64]. A recent study revealed that DDX11 plays a critical backup role for the FA pathway regarding ICL repair in DT40 cells [65]. In addition to its role in ICL repair, DDX11 acts jointly with the 9-1-1 clamp to promote HR induced by various lesions. DDX11−/− DT40 cells are epistatic with RAD17/− with regards to DNA damage hypersensitivity and induction of sister-chromatid exchanges and chromosome aberrations [65]. Interestingly, DDX11−/− DT40 cells show reduced Ig gene conversion and hypermutation, with the effect of DDX11 in hypermutation being clearly observed in a rad17 defective background [65] (Figure 4, Table 1). Thus, DDX11 may be involved in the promotion of both HR and TLS for releasing stalled replication forks at lesions, with its function in these two modes possibly being regulated by 9-1-1 and its loader, Rad17. This dual role of DDX11 in affecting both gene conversion and hypermutation is reminiscent of other FA genes [66] known to integrate HR and TLS machineries to promote ICL repair. However, the interplay between the FA pathway as delineated so far, and DDX11 at abasic sites, remains to be elucidated by future studies.
SPARTAN, a ubiquitin-PCNA-interacting regulator, plays a complex role in regulating mutagenesis and in mediating repair of DNA-protein crosslink lesions. On one hand, SPARTAN promotes TLS by recruiting Polη to DNA lesions, which involves its association with ubiquitinated PCNA via its PCNA interacting peptide (PIP) domain and ubiquitin-binding zinc-finger 4 (UBZ4) domain [67,68,69,70,71]. On the other hand, SPARTAN recruits the p97 protein segregase and removes Polη from DNA damage sites, thereby preventing mutations [72,73]. In addition to its function as a ubiquitinated PCNA-binding TLS regulator, SPARTAN also mediates the repair of DNA-protein crosslink lesions through its DNA-dependent metalloprotease activity [74,75,76,77]. Thus, the role of SPARTAN at the stalled replication fork has been a matter of debate and remains controversial. Recent analysis using DT40 cells revealed that SPARTAN promotes not only TLS-mediated Ig hypermutation, but also Ig gene conversion [78] (Figure 4, Table 1). For promotion of Ig gene conversion, UBZ4 and not the PIP domain of SPARTAN is required, suggesting that SPARTAN recognizes ubiquitinated protein(s) other than PCNA to promote gene conversion [78]. Important questions for future studies concern the mechanisms underlying SPARTAN’s role in the promotion of Ig gene conversion.
Moreover, as a general remark applied to most of the studies using AID overexpression to study IgV gene diversification, the factors under study may affect AID activity itself, a possibility that has not been formally ruled out. Recent work from the Sale Lab (Cambridge, UK) has used an ingenuous strategy to address this issue, by co-overexpressing together with AID a bacterial uracil glycosylase inhibitor and looking at whether the mutation rates induced by dU are similar to the ones observed in control cells [22]. Likely, this strategy will be applied in the future to the study of other factors and this will increase the knowledge of the mechanisms mediating AID regulation and IgV diversification via bypass of abasic sites.
4. Nontemplated Somatic Hypermutation
Bypass replication by error-prone TLS polymerases causes hypermutation after the action of AID and UNG. Studies in budding yeast have revealed that mono-ubiquitination of PCNA at lysine 164 serves as a signal for the recruitment of error-prone TLS polymerases to sites of perturbed replication [79,80]. The role of PCNA ubiquitination was examined in ψV− DT40 cells, in which upstream IgV pseudogenes are completely eliminated and gene conversion events are diminished [27] (Figure 4, Table 1). The PCNA-K164R DT40 cells show impaired PCNA ubiquitination and exhibit hypersensitivity to a wide variety of DNA damaging agents. Moreover, Ig hypermutation is critically reduced in PCNA-K164R DT40 cells (Figure 4, Table 1), indicating that post-translational modification of PCNA at lysine 164 is required for efficient TLS events in IgV gene diversification. Rad18, an E3-ligase required for PCNA ubiquitination in yeast, is conserved in eukaryotic cells [79,81]. RAD18−/− DT40 cells exhibit a milder reduction in cellular tolerance to DNA damaging agents and Ig hypermutation in comparison to PCNA-K164R cells (Figure 4, Table 1), and this mutant strain showed residual PCNA ubiquitination [27,82]. Moreover, RAD18−/−/PCNA-K164R double mutant cells essentially show the same phenotype as PCNA-K164R mutant cells [27]. These observations suggest that Rad18-mediated PCNA ubiquitination is required for TLS events, but other ubiquitin ligases seem to function as a backup for Rad18 in vertebrate cells. The mutation spectrum in PCNA-K164R DT40 cells is changed, and this cell line shows biased reduction of dG:dC to dC:dG transversion [27]. Similarly, the loss of Rev1 results in nearly complete loss of the dG:dC to dC:dG transversion mutation [27] (Figure 4, Table 1). Ig hypermutation requires the deoxycytidyl transferase activity of Rev1 that is dispensable for cellular tolerance to DNA damaging agents, suggesting that the transferase activity of Rev1 incorporates dC opposite to the abasic site [41]. More importantly, other types of IgV hypermutations are also significantly reduced in PCNA-K164R cells, but not in REV1−/− cells, suggesting that PCNA ubiquitination activates other TLS polymerases apart from Rev1.
POLη−/−/POLζ−/− DT40 cells also show reduced dG:dC to dC:dG mutations and increased dG:dC to dA:dT mutations. Moreover, this cell line has a wild type level of Ig hypermutations [14]. These observations suggest that the absence of Polη and Polζ is largely compensated by the action of other polymerase(s) possessing activity to preferentially incorporate dA opposite the abasic site. Interestingly, POLζ−/− DT40 cells have pronounced defects in TLS across T-T (6-4) UV photoproducts, while loss of Polη critically restores this defect. Similarly, POLη−/−/POLζ−/− DT40 cells exhibit milder DNA damage sensitivity in comparison to POLζ−/− cells [14]. These observations suggest that actions of Polη in the absence of Polζ critically impair the functionality of TLS. The absence of both Polη and Polζ can be substituted by other polymerase(s), such as Pοlδ. Loss of PolD3, the third subunit of the Pοlδ holoenzyme, results in a five-fold reduction of Ig hypermutation efficiency [16], suggesting a possible role of Pοlδ in TLS across abasic sites (Figure 4, Table 1). This view is supported by the observation that a complete set of human Polδ holoenzyme, but not PolD3-deficient Polδ, efficiently replicates across abasic sites in vitro [16]. Moreover, inactivation of the proofreading exonuclease activity dramatically enhances TLS across abasic sites in vitro [15], suggesting that this activity counteracts bypass replication presumably due to excision of incorporated nucleotides at damaged templates. Strikingly, the expression of proofreading exonuclease activity-deficient Polδ by introducing a heteroallelic point mutation (POLD1+/exo−) rescues the mutant phenotype of POLD3−/− cells, and the Ig hypermutation in POLD3−/−/POLD1+/exo− returns to wild type levels [15]. These observations suggest that PolD3 counteracts Pοlδ’s proofreading exonuclease activity in order to promote TLS across abasic sites during IgV gene diversification, and support a pivotal role of PolD3 in Polδ-mediated TLS. The loss of PolD3 in POLη−/−/POLζ−/− DT40 cells results in synthetic lethality, and this lethality is partially suppressed by the expression of proofreading exonuclease activity-deficient Polδ [15]. Thus, TLS relying on Polη-Polζ and Polδ might participate in parallel pathways promoting cell survival.
Either loss of Rev1 (REV1−/−) or Polη-Polζ (POLη−/−/REV3−/−) reduces dG:dC to dC:dG transversions [14,27]. This might be attributable to the loss of Polζ activity in these mutant cells, as evidenced by the following observations. DT40 REV1−/− REV3−/− REV7−/− triple mutant cells show hypersensitivity to genotoxic agents as observed in each single mutant cell, indicating an epistatic relationship between these factors [83]. These observations further suggest that Rev1, Rev3 and Rev7 function cooperatively in the TLS pathway as Polζ.
Other TLS polymerases, including Polν and Polθ, might also be involved in Ig hypermutation, since inactivation of Polν and Polθ in POLη−/− cells significantly reduced Ig hypermutation [17] (Figure 4, Table 1). However, to fully understand the redundancy as well as the division of labor among polymerases in the TLS network further investigations are needed.
The spatiotemporal regulation of the repair of AID-induced abasic sites, that is, whether the repair/bypass of these sites happens at the level of the fork, or behind the fork, remains an issue of investigation. So far, indirect assays have been used to assess the involvement of a subset of the factors implicated in this process in regulating fork speed and gap-filling. Specifically, DNA fiber assays following UV or MMS (methylmethane sulfonate) damage can measure damage bypass events at the fork, whereas alkaline sucrose gradient sedimentation of pulse labeled cells show the efficiency of post-replicative processes [84]. For instance, POLη−/− POLζ−/− mutants show only defects in the post-replicative gap-filling assay, whereas POLD3−/− mutants show defects only in the fork speed assay [15,16]. Thus, although these assays do not probe for events at the IgV locus or in conditions with increased abasic sites, these data suggest that IgV diversification may happen both at the fork and in the G2 phase, and may be mediated by distinct factors.
5. Summary and Perspective
In this review, we summarized the current understanding of mechanisms promoting recovery of arrested replication forks at damaged DNA templates as unveiled by the analysis of IgV gene diversification in avian DT40 cells. This cell line exhibits extraordinarily high gene-targeting efficiency and has thus been extensively used for genetic studies [33]. Moreover, this cell line provides a great opportunity to analyze gene functions in replication fork recovery and/or gap-filling at damaged templates by analyzing IgV gene diversification. Thus, genetic studies using the DT40 cell line might continue to contribute to the understanding of DDT mechanisms in eukaryotic cells. The genes involved in IgV gene diversification (gene conversion and hypermutation) are summarized in Table 1.
Genetic studies of yeast have greatly contributed to the understanding of mechanisms regulating TLS and HR-mediated template switch to recover arrested forks at damaged DNA templates [79,85,86,87,88,89]. The mono-ubiquitination on PCNA at lysine 164 by Rad6 (E2 conjugating enzyme) and Rad18 (E3 ligase) serves as a signal for promoting TLS [87], while extension of this ubiquitin to a polyubiquitin chain by Ubc13 (E2 conjugating enzyme) and Rad5 (E3 ligase) facilitates template switch [85,89]. Studies in the mouse model using PCNA-K164R or RAD18−/− mutant cells demonstrated an important role of PCNA ubiquitination in the maintenance of hematopoeotic stem cells, avoidance of premature aging and DNA damage tolerance, highlighting the critical role of this pathway for genome maintenance in mammals [90,91]. Studies using DT40 cells also revealed conserved mechanisms of the Rad18-PCNA ubiquitination axis in TLS promotion and suggested the role of additional E3 ubiquitin ligases in this ubiquitination process [27,82]. In addition to promoting TLS, Rad18 functions in concert with another E3 ligase, Rnf8, and plays a significant role in HR promotion in DT40 cells [12], as it does in budding yeast [89]. The role of Rad18 in the promotion of HR in vertebrate cells occurs via a direct interaction with the RAD51 paralog Rad51C [92]. Thus, the roles of Rad18 and the mechanisms of PCNA mono-ubiquitination might be considerably more complex in higher eukaryotic cells in comparison to yeast cells. SHPRH and HLTF are identified human homologs of Rad5 [93,94,95,96], and depletion of these E3 ligases resulted in reduction of PCNA poly-ubiquitination and rendered cells hypersensitive to DNA damaging agents [93,94]. These observations highlighted the importance of PCNA poly-ubiquitination via Rad5 homologs in human cells. However, SHPRH−/− DT40 cells showed no detectable defects in the ubiquitination of PCNA or cellular tolerance to alkylating agents and Ig diversification (Figure 4, Table 1), but they did show higher sensitivity to the topoisomerase II inhibitor, etoposide, in comparison to wild type cells [97]. Moreover, HLTF is missing in the chicken genome [97]. The redundancy of E3 ligases in PCNA ubiquitination might be linked to different roles of this modification in genome maintenance and genetic plasticity. Moreover, in human cells, Ubc13 (an E2 conjugating enzyme) plays a role in DSB repair. Rnf8 (E3 ligase), in complex with Ubc13, ubiquitinates histone H2A surrounding DSB sites [98,99], and then the Rnf168 (E3 ligase)–Ubc13 complex further amplifies histone ubiquitination [100] to recruit downstream repair factors, including BRCA1, that are required for the recruitment of Rad51 to DSB sites [101,102]. Similarly, UBC13−/− DT40 cells show defective DSB repair due to impaired Rad51 recruitment [103]. Furthermore, Rnf8 (E3 ligase) also contributes to both Ig hypermutation and gene conversion in DT40 cells [104] (Figure 4, Table 1). Taken together, these observations suggest that the Ubc13-Rad5 axis unveiled in yeast studies cannot be directly extrapolated into higher eukaryotic cells, due to considerable redundancy in ubiquitination enzyme networks and differential functions of the key enzymes. Moreover, the role of PCNA in HR-mediated template switch has not been elucidated. Comprehensive analysis of the ubiquitination pathway is warranted to fully dissect the molecular mechanisms of the ubiquitination signal pathway in DNA damage responses.
Funding
This work was supported by the Network-type Joint Usage/Research Center for Radiation Disaster Medical Science of Hiroshima University, Nagasaki University, and Fukushima Medical University. This work was funded by JSPS KAKENHI (JP16K12598, JP 16H01314 and 16H02957), the Yamada Science Foundation, and the Takeda Science Foundation to K.H.; and the AIRC IG grant18976 to D.B.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.McCulloch S.D., Kunkel T.A. The fidelity of DNA synthesis by eukaryotic replicative and translesion synthesis polymerases. Cell Res. 2008;18:148–161. doi: 10.1038/cr.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitt M.W., Matsumoto Y., Loeb L.A. High fidelity and lesion bypass capability of human DNA polymerase delta. Biochimie. 2009;91:1163–1172. doi: 10.1016/j.biochi.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Branzei D., Foiani M. Regulation of DNA repair throughout the cell cycle. Nat. Rev. Mol. Cell Biol. 2008;9:297–308. doi: 10.1038/nrm2351. [DOI] [PubMed] [Google Scholar]
- 4.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 5.Friedberg E.C., Walker G.C., Siede W., Wood R.D., Schultz R.A., Ellenberger T. DNA Repair and Mutagenesis. ASM Press; Boston, MA, USA: 2006. [Google Scholar]
- 6.Barnes D.E., Lindahl T., Sedgwick B. DNA repair. Curr. Opin. Cell Biol. 1993;5:424–433. doi: 10.1016/0955-0674(93)90007-D. [DOI] [PubMed] [Google Scholar]
- 7.Klungland A., Rosewell I., Hollenbach S., Larsen E., Daly G., Epe B., Seeberg E., Lindahl T., Barnes D.E. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc. Natl. Acad. Sci. USA. 1999;96:13300–13305. doi: 10.1073/pnas.96.23.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loeb L.A. Apurinic sites as mutagenic intermediates. Cell. 1985;40:483–484. doi: 10.1016/0092-8674(85)90191-6. [DOI] [PubMed] [Google Scholar]
- 9.Prasad R., Horton J.K., Liu Y., Wilson S.H. Central steps in mammalian BER and regulation by PARP1. Base Excision Repair Pathw. 2017:253–280. doi: 10.1142/9789814719735_0007. [DOI] [Google Scholar]
- 10.Branzei D., Psakhye I. DNA damage tolerance. Curr. Opin. Cell Biol. 2016;40:137–144. doi: 10.1016/j.ceb.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y., Mutter-Rottmayer E., Zlatanou A., Vaziri C., Yang Y. Mechanisms of post-replication DNA repair. Genes. 2017;8:64. doi: 10.3390/genes8020064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi S., Kasaishi Y., Nakada S., Takagi T., Era S., Motegi A., Chiu R.K., Takeda S., Hirota K. Rad18 and Rnf8 facilitate homologous recombination by two distinct mechanisms, promoting Rad51 focus formation and suppressing the toxic effect of nonhomologous end joining. Oncogene. 2015;34:4403–4411. doi: 10.1038/onc.2014.371. [DOI] [PubMed] [Google Scholar]
- 13.Li X., Heyer W.D. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res. 2008;18:99–113. doi: 10.1038/cr.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirota K., Sonoda E., Kawamoto T., Motegi A., Masutani C., Hanaoka F., Szuts D., Iwai S., Sale J.E., Lehmann A., et al. Simultaneous disruption of two DNA polymerases, Poleta and Polzeta, in Avian DT40 cells unmasks the role of Poleta in cellular response to various DNA lesions. PLoS Genet. 2010;6:e1001151. doi: 10.1371/journal.pgen.1001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirota K., Tsuda M., Tsurimoto T., Cohen I.S., Livneh Z., Kobayashi K., Narita T., Nishihara K., Murai J., Iwai S., et al. In vivo evidence for translesion synthesis by the replicative DNA polymerase delta. Nucleic Acids Res. 2016;44:7242–7250. doi: 10.1093/nar/gkw439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirota K., Yoshikiyo K., Guilbaud G., Tsurimoto T., Murai J., Tsuda M., Phillips L.G., Narita T., Nishihara K., Kobayashi K., et al. The POLD3 subunit of DNA polymerase δ can promote translesion synthesis independently of DNA polymerase ζ. Nucleic Acids Res. 2015;43:1671–1683. doi: 10.1093/nar/gkv023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohzaki M., Nishihara K., Hirota K., Sonoda E., Yoshimura M., Ekino S., Butler J.E., Watanabe M., Halazonetis T.D., Takeda S. DNA polymerases ν and θ are required for efficient immunoglobulin V gene diversification in chicken. J. Cell Biol. 2010;189:1117–1127. doi: 10.1083/jcb.200912012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sale J.E. Competition, collaboration and coordination–determining how cells bypass DNA damage. J. Cell Sci. 2012;125:1633–1643. doi: 10.1242/jcs.094748. [DOI] [PubMed] [Google Scholar]
- 19.Arakawa H., Buerstedde J.M. Activation-induced cytidine deaminase-mediated hypermutation in the DT40 cell line. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:639–644. doi: 10.1098/rstb.2008.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sale J.E. Translesion DNA synthesis and mutagenesis in eukaryotes. Cold Spring Harb. Perspect. Biol. 2013;5:a012708. doi: 10.1101/cshperspect.a012708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sale J.E., Batters C., Edmunds C.E., Phillips L.G., Simpson L.J., Szuts D. Timing matters: Error-prone gap filling and translesion synthesis in immunoglobulin gene hypermutation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:595–603. doi: 10.1098/rstb.2008.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romanello M., Schiavone D., Frey A., Sale J.E. Histone H3.3 promotes IgV gene diversification by enhancing formation of AID-accessible single-stranded DNA. EMBO J. 2016;35:1452–1464. doi: 10.15252/embj.201693958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin C., Felsenfeld G. Nucleosome stability mediated by histone variants H3.3 and H2A.Z. Genes Dev. 2007;21:1519–1529. doi: 10.1101/gad.1547707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Noia J., Neuberger M.S. Altering the pathway of immunoglobulin hypermutation by inhibiting uracil-DNA glycosylase. Nature. 2002;419:43–48. doi: 10.1038/nature00981. [DOI] [PubMed] [Google Scholar]
- 25.Saribasak H., Saribasak N.N., Ipek F.M., Ellwart J.W., Arakawa H., Buerstedde J.M. Uracil DNA glycosylase disruption blocks Ig gene conversion and induces transition mutations. J. Immunol. 2006;176:365–371. doi: 10.4049/jimmunol.176.1.365. [DOI] [PubMed] [Google Scholar]
- 26.Blagodatski A., Batrak V., Schmidl S., Schoetz U., Caldwell R.B., Arakawa H., Buerstedde J.M. A cis-acting diversification activator both necessary and sufficient for AID-mediated hypermutation. PLoS Genet. 2009;5:e1000332. doi: 10.1371/journal.pgen.1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arakawa H., Moldovan G.L., Saribasak H., Saribasak N.N., Jentsch S., Buerstedde J.M. A role for PCNA ubiquitination in immunoglobulin hypermutation. PLoS Biol. 2006;4:e366. doi: 10.1371/journal.pbio.0040366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arakawa H., Saribasak H., Buerstedde J.M. Activation-induced cytidine deaminase initiates immunoglobulin gene conversion and hypermutation by a common intermediate. PLoS Biol. 2004;2:e179. doi: 10.1371/journal.pbio.0020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Noia J.M., Neuberger M.S. Molecular mechanisms of antibody somatic hypermutation. Annu. Rev. Biochem. 2007;76:1–22. doi: 10.1146/annurev.biochem.76.061705.090740. [DOI] [PubMed] [Google Scholar]
- 30.Sale J.E. Immunoglobulin diversification in DT40: A model for vertebrate DNA damage tolerance. DNA Repair. 2004;3:693–702. doi: 10.1016/j.dnarep.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 31.Sale J.E. Measurement of diversification in the immunoglobulin light chain gene of DT40 cells. Methods Mol. Biol. 2012;920:417–432. doi: 10.1007/978-1-61779-998-3_29. [DOI] [PubMed] [Google Scholar]
- 32.Buerstedde J.M., Reynaud C.A., Humphries E.H., Olson W., Ewert D.L., Weill J.C. Light chain gene conversion continues at high rate in an ALV-induced cell line. EMBO J. 1990;9:921–927. doi: 10.1002/j.1460-2075.1990.tb08190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buerstedde J.M., Takeda S. Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell. 1991;67:179–188. doi: 10.1016/0092-8674(91)90581-I. [DOI] [PubMed] [Google Scholar]
- 34.Seo H., Masuoka M., Murofushi H., Takeda S., Shibata T., Ohta K. Rapid generation of specific antibodies by enhanced homologous recombination. Nat. Biotechnol. 2005;23:731–735. doi: 10.1038/nbt1092. [DOI] [PubMed] [Google Scholar]
- 35.Cummings W.J., Yabuki M., Ordinario E.C., Bednarski D.W., Quay S., Maizels N. Chromatin structure regulates gene conversion. PLoS Biol. 2007;5:e246. doi: 10.1371/journal.pbio.0050246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arakawa H. Immunoglobulin gene conversion and hypermutation assay by FACs. Sub-Cell. Biochem. 2006;40:351–352. doi: 10.1007/978-1-4020-4896-8_23. [DOI] [PubMed] [Google Scholar]
- 37.Shinkura R., Ito S., Begum N.A., Nagaoka H., Muramatsu M., Kinoshita K., Sakakibara Y., Hijikata H., Honjo T. Separate domains of AID are required for somatic hypermutation and class-switch recombination. Nat. Immunol. 2004;5:707–712. doi: 10.1038/ni1086. [DOI] [PubMed] [Google Scholar]
- 38.Saberi A., Nakahara M., Sale J.E., Kikuchi K., Arakawa H., Buerstedde J.M., Yamamoto K., Takeda S., Sonoda E. The 9-1-1 DNA clamp is required for immunoglobulin gene conversion. Mol. Cell. Biol. 2008;28:6113–6122. doi: 10.1128/MCB.00156-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang M., Yang Z., Rada C., Neuberger M.S. AID upmutants isolated using a high-throughput screen highlight the immunity/cancer balance limiting DNA deaminase activity. Nat. Struct. Mol. Biol. 2009;16:769–776. doi: 10.1038/nsmb.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sale J.E., Calandrini D.M., Takata M., Takeda S., Neuberger M.S. Ablation of XRCC2/3 transforms immunoglobulin V gene conversion into somatic hypermutation. Nature. 2001;412:921–926. doi: 10.1038/35091100. [DOI] [PubMed] [Google Scholar]
- 41.Ross A.L., Sale J.E. The catalytic activity of REV1 is employed during immunoglobulin gene diversification in DT40. Mol. Immunol. 2006;43:1587–1594. doi: 10.1016/j.molimm.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Longerich S., Orelli B.J., Martin R.W., Bishop D.K., Storb U. Brca1 in immunoglobulin gene conversion and somatic hypermutation. DNA Repair. 2008;7:253–266. doi: 10.1016/j.dnarep.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hatanaka A., Yamazoe M., Sale J.E., Takata M., Yamamoto K., Kitao H., Sonoda E., Kikuchi K., Yonetani Y., Takeda S. Similar effects of Brca2 truncation and Rad51 paralog deficiency on immunoglobulin V gene diversification in DT40 cells support an early role for Rad51 paralogs in homologous recombination. Mol. Cell. Biol. 2005;25:1124–1134. doi: 10.1128/MCB.25.3.1124-1134.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bezzubova O., Silbergleit A., Yamaguchi-Iwai Y., Takeda S., Buerstedde J.M. Reduced X-ray resistance and homologous recombination frequencies in a RAD54−/− mutant of the chicken DT40 cell line. Cell. 1997;89:185–193. doi: 10.1016/S0092-8674(00)80198-1. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto K., Hirano S., Ishiai M., Morishima K., Kitao H., Namikoshi K., Kimura M., Matsushita N., Arakawa H., Buerstedde J.M., et al. Fanconi anemia protein FANCD2 promotes immunoglobulin gene conversion and DNA repair through a mechanism related to homologous recombination. Mol. Cell. Biol. 2005;25:34–43. doi: 10.1128/MCB.25.1.34-43.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawamoto T., Araki K., Sonoda E., Yamashita Y.M., Harada K., Kikuchi K., Masutani C., Hanaoka F., Nozaki K., Hashimoto N., et al. Dual roles for DNA polymerase eta in homologous DNA recombination and translesion DNA synthesis. Mol. Cell. 2005;20:793–799. doi: 10.1016/j.molcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 47.Li J., Holzschu D.L., Sugiyama T. PCNA is efficiently loaded on the DNA recombination intermediate to modulate polymerase δ, η, and ζ activities. Proc. Natl. Acad. Sci. USA. 2013;110:7672–7677. doi: 10.1073/pnas.1222241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McIlwraith M.J., Vaisman A., Liu Y., Fanning E., Woodgate R., West S.C. Human DNA polymerase η promotes DNA synthesis from strand invasion intermediates of homologous recombination. Mol. Cell. 2005;20:783–792. doi: 10.1016/j.molcel.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Sebesta M., Burkovics P., Haracska L., Krejci L. Reconstitution of DNA repair synthesis in vitro and the role of polymerase and helicase activities. DNA Repair. 2011;10:567–576. doi: 10.1016/j.dnarep.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sebesta M., Burkovics P., Juhasz S., Zhang S., Szabo J.E., Lee M.Y., Haracska L., Krejci L. Role of PCNA and TLS polymerases in D-loop extension during homologous recombination in humans. DNA Repair. 2013;12:691–698. doi: 10.1016/j.dnarep.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kannouche P.L., Wing J., Lehmann A.R. Interaction of human DNA polymerase η with monoubiquitinated PCNA: A possible mechanism for the polymerase switch in response to DNA damage. Mol. Cell. 2004;14:491–500. doi: 10.1016/S1097-2765(04)00259-X. [DOI] [PubMed] [Google Scholar]
- 52.Moldovan G.L., Pfander B., Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 53.Ulrich H.D. Deubiquitinating PCNA: A downside to DNA damage tolerance. Nat. Cell Biol. 2006;8:303–305. doi: 10.1038/ncb0406-303. [DOI] [PubMed] [Google Scholar]
- 54.Navadgi-Patil V.M., Burgers P.M. The unstructured C-Terminal tail of the 9-1-1 clamp subunit Ddc1 activates Mec1/ATR via two distinct mechanisms. Mol. Cell. 2009;36:743–753. doi: 10.1016/j.molcel.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Navadgi-Patil V.M., Burgers P.M. A tale of two tails: Activation of DNA damage checkpoint kinase Mec1/ATR by the 9-1-1 clamp and by Dpb11/TopBP1. DNA Repair. 2009;8:996–1003. doi: 10.1016/j.dnarep.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niida H., Nakanishi M. DNA damage checkpoints in mammals. Mutagenesis. 2006;21:3–9. doi: 10.1093/mutage/gei063. [DOI] [PubMed] [Google Scholar]
- 57.Bermudez V.P., Lindsey-Boltz L.A., Cesare A.J., Maniwa Y., Griffith J.D., Hurwitz J., Sancar A. Loading of the human 9-1-1 checkpoint complex onto DNA by the checkpoint clamp loader hRad17-replication factor C complex in vitro. Proc. Natl. Acad. Sci. USA. 2003;100:1633–1638. doi: 10.1073/pnas.0437927100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ellison V., Stillman B. Biochemical characterization of DNA damage checkpoint complexes: Clamp loader and clamp complexes with specificity for 5’ recessed DNA. PLoS Biol. 2003;1:E33. doi: 10.1371/journal.pbio.0000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Budzowska M., Jaspers I., Essers J., de Waard H., van Drunen E., Hanada K., Beverloo B., Hendriks R.W., de Klein A., Kanaar R., et al. Mutation of the mouse Rad17 gene leads to embryonic lethality and reveals a role in DNA damage-dependent recombination. EMBO J. 2004;23:3548–3558. doi: 10.1038/sj.emboj.7600353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pandita R.K., Sharma G.G., Laszlo A., Hopkins K.M., Davey S., Chakhparonian M., Gupta A., Wellinger R.J., Zhang J., Powell S.N., et al. Mammalian Rad9 plays a role in telomere stability, S- and G2-phase-specific cell survival, and homologous recombinational repair. Mol. Cell. Biol. 2006;26:1850–1864. doi: 10.1128/MCB.26.5.1850-1864.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bailey C., Fryer A.E., Greenslade M. Warsaw Breakage Syndrome—A further report, emphasising cutaneous findings. Eur. J. Med. Genet. 2015;58:235–237. doi: 10.1016/j.ejmg.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 62.Capo-Chichi J.M., Bharti S.K., Sommers J.A., Yammine T., Chouery E., Patry L., Rouleau G.A., Samuels M.E., Hamdan F.F., Michaud J.L., et al. Identification and biochemical characterization of a novel mutation in DDX11 causing Warsaw breakage syndrome. Hum. Mutat. 2013;34:103–107. doi: 10.1002/humu.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eppley S., Hopkin R.J., Mendelsohn B., Slavotinek A.M. Clinical Report: Warsaw Breakage Syndrome with small radii and fibulae. Am. J. Med. Genet. A. 2017;173:3075–3081. doi: 10.1002/ajmg.a.38382. [DOI] [PubMed] [Google Scholar]
- 64.Van der Lelij P., Chrzanowska K.H., Godthelp B.C., Rooimans M.A., Oostra A.B., Stumm M., Zdzienicka M.Z., Joenje H., de Winter J.P. Warsaw breakage syndrome, a cohesinopathy associated with mutations in the XPD helicase family member DDX11/ChlR1. Am. J. Hum. Genet. 2010;86:262–266. doi: 10.1016/j.ajhg.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abe T., Ooka M., Kawasumi R., Miyata K., Takata M., Hirota K., Branzei D. Warsaw breakage syndrome DDX11 helicase acts jointly with RAD17 in the repair of bulky lesions and replication through abasic sites. Proc. Natl. Acad. Sci. USA. 2018;115:8412–8417. doi: 10.1073/pnas.1803110115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Niedzwiedz W., Mosedale G., Johnson M., Ong C.Y., Pace P., Patel K.J. The Fanconi anaemia gene FANCC promotes homologous recombination and error-prone DNA repair. Mol. Cell. 2004;15:607–620. doi: 10.1016/j.molcel.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 67.Centore R.C., Yazinski S.A., Tse A., Zou L. Spartan/C1orf124, a reader of PCNA ubiquitylation and a regulator of UV-induced DNA damage response. Mol. Cell. 2012;46:625–635. doi: 10.1016/j.molcel.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghosal G., Leung J.W., Nair B.C., Fong K.W., Chen J. Proliferating cell nuclear antigen (PCNA)-binding protein C1orf124 is a regulator of translesion synthesis. J. Biol. Chem. 2012;287:34225–34233. doi: 10.1074/jbc.M112.400135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Juhasz S., Balogh D., Hajdu I., Burkovics P., Villamil M.A., Zhuang Z., Haracska L. Characterization of human Spartan/C1orf124, an ubiquitin-PCNA interacting regulator of DNA damage tolerance. Nucleic Acids Res. 2012;40:10795–10808. doi: 10.1093/nar/gks850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Machida Y., Kim M.S., Machida Y.J. Spartan/C1orf124 is important to prevent UV-induced mutagenesis. Cell Cycle. 2012;11:3395–3402. doi: 10.4161/cc.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toth A., Hegedus L., Juhasz S., Haracska L., Burkovics P. The DNA-binding box of human SPARTAN contributes to the targeting of Poleta to DNA damage sites. DNA Repair. 2017;49:33–42. doi: 10.1016/j.dnarep.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 72.Davis E.J., Lachaud C., Appleton P., Macartney T.J., Nathke I., Rouse J. DVC1 (C1orf124) recruits the p97 protein segregase to sites of DNA damage. Nat. Struct. Mol. Biol. 2012;19:1093–1100. doi: 10.1038/nsmb.2394. [DOI] [PubMed] [Google Scholar]
- 73.Mosbech A., Gibbs-Seymour I., Kagias K., Thorslund T., Beli P., Povlsen L., Nielsen S.V., Smedegaard S., Sedgwick G., Lukas C., et al. DVC1 (C1orf124) is a DNA damage-targeting p97 adaptor that promotes ubiquitin-dependent responses to replication blocks. Nat. Struct. Mol. Biol. 2012;19:1084–1092. doi: 10.1038/nsmb.2395. [DOI] [PubMed] [Google Scholar]
- 74.Maskey R.S., Flatten K.S., Sieben C.J., Peterson K.L., Baker D.J., Nam H.J., Kim M.S., Smyrk T.C., Kojima Y., Machida Y., et al. Spartan deficiency causes accumulation of Topoisomerase 1 cleavage complexes and tumorigenesis. Nucleic Acids Res. 2017;45:4564–4576. doi: 10.1093/nar/gkx107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morocz M., Zsigmond E., Toth R., Enyedi M.Z., Pinter L., Haracska L. DNA-dependent protease activity of human Spartan facilitates replication of DNA-protein crosslink-containing DNA. Nucleic Acids Res. 2017;45:3172–3188. doi: 10.1093/nar/gkw1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stingele J., Bellelli R., Alte F., Hewitt G., Sarek G., Maslen S.L., Tsutakawa S.E., Borg A., Kjaer S., Tainer J.A., et al. Mechanism and regulation of DNA-protein crosslink repair by the DNA-dependent metalloprotease SPRTN. Mol. Cell. 2016;64:688–703. doi: 10.1016/j.molcel.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vaz B., Popovic M., Newman J.A., Fielden J., Aitkenhead H., Halder S., Singh A.N., Vendrell I., Fischer R., Torrecilla I., et al. Metalloprotease SPRTN/DVC1 orchestrates replication-coupled DNA-protein crosslink repair. Mol. Cell. 2016;64:704–719. doi: 10.1016/j.molcel.2016.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakazato A., Kajita K., Ooka M., Akagawa R., Abe T., Takeda S., Branzei D., Hirota K. SPARTAN promotes genetic diversification of the immunoglobulin-variable gene locus in avian DT40 cells. DNA Repair. 2018;68:50–57. doi: 10.1016/j.dnarep.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 79.Lee K.Y., Myung K. PCNA modifications for regulation of post-replication repair pathways. Mol. Cells. 2008;26:5–11. [PMC free article] [PubMed] [Google Scholar]
- 80.McIntyre J., Woodgate R. Regulation of translesion DNA synthesis: Posttranslational modification of lysine residues in key proteins. DNA Repair. 2015;29:166–179. doi: 10.1016/j.dnarep.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kanao R., Masutani C. Regulation of DNA damage tolerance in mammalian cells by post-translational modifications of PCNA. Mutat. Res. 2017;803–805:82–88. doi: 10.1016/j.mrfmmm.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 82.Simpson L.J., Ross A.L., Szuts D., Alviani C.A., Oestergaard V.H., Patel K.J., Sale J.E. RAD18-independent ubiquitination of proliferating-cell nuclear antigen in the avian cell line DT40. EMBO Rep. 2006;7:927–932. doi: 10.1038/sj.embor.7400777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Okada T., Sonoda E., Yoshimura M., Kawano Y., Saya H., Kohzaki M., Takeda S. Multiple roles of vertebrate REV genes in DNA repair and recombination. Mol. Cell. Biol. 2005;25:6103–6111. doi: 10.1128/MCB.25.14.6103-6111.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edmunds C.E., Simpson L.J., Sale J.E. PCNA ubiquitination and REV1 define temporally distinct mechanisms for controlling translesion synthesis in the avian cell line DT40. Mol. Cell. 2008;30:519–529. doi: 10.1016/j.molcel.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 85.Branzei D., Seki M., Enomoto T. Rad18/Rad5/Mms2-mediated polyubiquitination of PCNA is implicated in replication completion during replication stress. Genes Cells. 2004;9:1031–1042. doi: 10.1111/j.1365-2443.2004.00787.x. [DOI] [PubMed] [Google Scholar]
- 86.Hishida T., Kubota Y., Carr A.M., Iwasaki H. RAD6-RAD18-RAD5-pathway-dependent tolerance to chronic low-dose ultraviolet light. Nature. 2009;457:612–615. doi: 10.1038/nature07580. [DOI] [PubMed] [Google Scholar]
- 87.Hoege C., Pfander B., Moldovan G.L., Pyrowolakis G., Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- 88.Torres-Ramos C.A., Prakash S., Prakash L. Requirement of RAD5 and MMS2 for postreplication repair of UV-damaged DNA in Saccharomyces cerevisiae. Mol. Cell. Biol. 2002;22:2419–2426. doi: 10.1128/MCB.22.7.2419-2426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Branzei D., Vanoli F., Foiani M. SUMOylation regulates Rad18-mediated template switch. Nature. 2008;456:915–920. doi: 10.1038/nature07587. [DOI] [PubMed] [Google Scholar]
- 90.Pilzecker B., Buoninfante O.A., van den Berk P., Lancini C., Song J.Y., Citterio E., Jacobs H. DNA damage tolerance in hematopoietic stem and progenitor cells in mice. Proc. Natl. Acad. Sci. USA. 2017;114:E6875–E6883. doi: 10.1073/pnas.1706508114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang Y., Poe J.C., Yang L., Fedoriw A., Desai S., Magnuson T., Li Z., Fedoriw Y., Araki K., Gao Y., et al. Rad18 confers hematopoietic progenitor cell DNA damage tolerance independently of the Fanconi Anemia pathway in vivo. Nucleic Acids Res. 2016;44:4174–4188. doi: 10.1093/nar/gkw072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang J., Huen M.S., Kim H., Leung C.C., Glover J.N., Yu X., Chen J. RAD18 transmits DNA damage signalling to elicit homologous recombination repair. Nat. Cell Biol. 2009;11:592–603. doi: 10.1038/ncb1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Motegi A., Liaw H.J., Lee K.Y., Roest H.P., Maas A., Wu X., Moinova H., Markowitz S.D., Ding H., Hoeijmakers J.H., et al. Polyubiquitination of proliferating cell nuclear antigen by HLTF and SHPRH prevents genomic instability from stalled replication forks. Proc. Natl. Acad. Sci. USA. 2008;105:12411–12416. doi: 10.1073/pnas.0805685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Motegi A., Sood R., Moinova H., Markowitz S.D., Liu P.P., Myung K. Human SHPRH suppresses genomic instability through proliferating cell nuclear antigen polyubiquitination. J. Cell Biol. 2006;175:703–708. doi: 10.1083/jcb.200606145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Unk I., Hajdu I., Blastyak A., Haracska L. Role of yeast Rad5 and its human orthologs, HLTF and SHPRH in DNA damage tolerance. DNA Repair. 2010;9:257–267. doi: 10.1016/j.dnarep.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 96.Unk I., Hajdu I., Fatyol K., Szakal B., Blastyak A., Bermudez V., Hurwitz J., Prakash L., Prakash S., Haracska L. Human SHPRH is a ubiquitin ligase for Mms2-Ubc13-dependent polyubiquitylation of proliferating cell nuclear antigen. Proc. Natl. Acad. Sci. USA. 2006;103:18107–18112. doi: 10.1073/pnas.0608595103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tomi N.S., Davari K., Grotzky D., Loos F., Bottcher K., Frankenberger S., Jungnickel B. Analysis of SHPRH functions in DNA repair and immunoglobulin diversification. DNA Repair. 2014;24:63–72. doi: 10.1016/j.dnarep.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 98.Kolas N.K., Chapman J.R., Nakada S., Ylanko J., Chahwan R., Sweeney F.D., Panier S., Mendez M., Wildenhain J., Thomson T.M., et al. Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science. 2007;318:1637–1640. doi: 10.1126/science.1150034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Panier S., Durocher D. Regulatory ubiquitylation in response to DNA double-strand breaks. DNA Repair. 2009;8:436–443. doi: 10.1016/j.dnarep.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 100.Stewart G.S., Panier S., Townsend K., Al-Hakim A.K., Kolas N.K., Miller E.S., Nakada S., Ylanko J., Olivarius S., Mendez M., et al. The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage. Cell. 2009;136:420–434. doi: 10.1016/j.cell.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 101.Moynahan M.E., Chiu J.W., Koller B.H., Jasin M. Brca1 controls homology-directed DNA repair. Mol. Cell. 1999;4:511–518. doi: 10.1016/S1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- 102.Scully R., Ganesan S., Vlasakova K., Chen J., Socolovsky M., Livingston D.M. Genetic analysis of BRCA1 function in a defined tumor cell line. Mol. Cell. 1999;4:1093–1099. doi: 10.1016/S1097-2765(00)80238-5. [DOI] [PubMed] [Google Scholar]
- 103.Zhao G.Y., Sonoda E., Barber L.J., Oka H., Murakawa Y., Yamada K., Ikura T., Wang X., Kobayashi M., Yamamoto K., et al. A critical role for the ubiquitin-conjugating enzyme Ubc13 in initiating homologous recombination. Mol. Cell. 2007;25:663–675. doi: 10.1016/j.molcel.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 104.Kobayashi S., Keka I.S., Guilbaud G., Sale J., Narita T., Abdel-Aziz H.I., Wang X., Ogawa S., Sasanuma H., Chiu R., et al. The role of HERC2 and RNF8 ubiquitin E3 ligases in the promotion of translesion DNA synthesis in the chicken DT40 cell line. DNA Repair. 2016;40:67–76. doi: 10.1016/j.dnarep.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arakawa H., Hauschild J., Buerstedde J.M. Requirement of the activation-induced deaminase (AID) gene for immunoglobulin gene conversion. Science. 2002;295:1301–1306. doi: 10.1126/science.1067308. [DOI] [PubMed] [Google Scholar]
- 106.Oka H., Sakai W., Sonoda E., Nakamura J., Asagoshi K., Wilson S.H., Kobayashi M., Yamamoto K., Heierhorst J., Takeda S., et al. DNA damage response protein ASCIZ links base excision repair with immunoglobulin gene conversion. Biochem. Biophys. Res. Commun. 2008;371:225–229. doi: 10.1016/j.bbrc.2008.04.052. [DOI] [PubMed] [Google Scholar]
- 107.Budzynska P.M., Kylaniemi M.K., Kallonen T., Soikkeli A.I., Nera K.P., Lassila O., Alinikula J. Bach2 regulates AID-mediated immunoglobulin gene conversion and somatic hypermutation in DT40 B cells. Eur. J. Immunol. 2017;47:993–1001. doi: 10.1002/eji.201646895. [DOI] [PubMed] [Google Scholar]
- 108.Kikuchi K., Abdel-Aziz H.I., Taniguchi Y., Yamazoe M., Takeda S., Hirota K. Bloom DNA helicase facilitates homologous recombination between diverged homologous sequences. J. Biol. Chem. 2009;284:26360–26367. doi: 10.1074/jbc.M109.029348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Conticello S.G., Ganesh K., Xue K., Lu M., Rada C., Neuberger M.S. Interaction between antibody-diversification enzyme AID and spliceosome-associated factor CTNNBL1. Mol. Cell. 2008;31:474–484. doi: 10.1016/j.molcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 110.Kitao H., Kimura M., Yamamoto K., Seo H., Namikoshi K., Agata Y., Ohta K., Takata M. Regulation of histone H4 acetylation by transcription factor E2A in Ig gene conversion. Int. Immunol. 2008;20:277–284. doi: 10.1093/intimm/dxm140. [DOI] [PubMed] [Google Scholar]
- 111.Pace P., Mosedale G., Hodskinson M.R., Rosado I.V., Sivasubramaniam M., Patel K.J. Ku70 corrupts DNA repair in the absence of the Fanconi anemia pathway. Science. 2010;329:219–223. doi: 10.1126/science.1192277. [DOI] [PubMed] [Google Scholar]
- 112.Kitao H., Nanda I., Sugino R.P., Kinomura A., Yamazoe M., Arakawa H., Schmid M., Innan H., Hiom K., Takata M. FancJ/Brip1 helicase protects against genomic losses and gains in vertebrate cells. Genes Cells. 2011;16:714–727. doi: 10.1111/j.1365-2443.2011.01523.x. [DOI] [PubMed] [Google Scholar]
- 113.Kikuchi K., Taniguchi Y., Hatanaka A., Sonoda E., Hochegger H., Adachi N., Matsuzaki Y., Koyama H., van Gent D.C., Jasin M., et al. Fen-1 facilitates homologous recombination by removing divergent sequences at DNA break ends. Mol. Cell. Biol. 2005;25:6948–6955. doi: 10.1128/MCB.25.16.6948-6955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin W., Hashimoto S., Seo H., Shibata T., Ohta K. Modulation of immunoglobulin gene conversion frequency and distribution by the histone deacetylase HDAC2 in chicken DT40. Genes Cells. 2008;13:255–268. doi: 10.1111/j.1365-2443.2008.01166.x. [DOI] [PubMed] [Google Scholar]
- 115.Simpson L.J., Sale J.E. UBE2V2 (MMS2) is not required for effective immunoglobulin gene conversion or DNA damage tolerance in DT40. DNA Repair. 2005;4:503–510. doi: 10.1016/j.dnarep.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 116.Campo V.A., Patenaude A.M., Kaden S., Horb L., Firka D., Jiricny J., Di Noia J.M. MSH6- or PMS2-deficiency causes re-replication in DT40 B cells, but it has little effect on immunoglobulin gene conversion or on repair of AID-generated uracils. Nucleic Acids Res. 2013;41:3032–3046. doi: 10.1093/nar/gks1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nakahara M., Sonoda E., Nojima K., Sale J.E., Takenaka K., Kikuchi K., Taniguchi Y., Nakamura K., Sumitomo Y., Bree R.T., et al. Genetic evidence for single-strand lesions initiating Nbs1-dependent homologous recombination in diversification of Ig V in chicken B lymphocytes. PLoS Genet. 2009;5:e1000356. doi: 10.1371/journal.pgen.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Paddock M.N., Buelow B.D., Takeda S., Scharenberg A.M. The BRCT domain of PARP-1 is required for immunoglobulin gene conversion. PLoS Biol. 2010;8:e1000428. doi: 10.1371/journal.pbio.1000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hosono Y., Abe T., Ishiai M., Islam M.N., Arakawa H., Wang W., Takeda S., Ishii Y., Takata M., Seki M., et al. Tumor suppressor RecQL5 controls recombination induced by DNA crosslinking agents. Biochim. Biophys. Acta. 2014;1843:1002–1012. doi: 10.1016/j.bbamcr.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Simpson L.J., Sale J.E. Rev1 is essential for DNA damage tolerance and non-templated immunoglobulin gene mutation in a vertebrate cell line. EMBO J. 2003;22:1654–1664. doi: 10.1093/emboj/cdg161. [DOI] [PMC free article] [PubMed] [Google Scholar]