Abstract
The role of the concomitant intake of zinc, potassium, calcium, and magnesium in the glycemic control of individuals with type 2 diabetes mellitus (T2DM) has not been extensively discussed. We evaluated the relationship between the dietary intake of these micronutrients and glycemic markers in 95 individuals with T2DM (mean age 48.6 ± 8.4 years). Hierarchical grouping analysis was used to divide the individuals into two clusters according to their micronutrient intake, and differences between clusters were statistically assessed. Effects of individual and combination intake of micronutrients on glycated hemoglobin percentage (%HbA1c) were assessed using multiple linear regression and binary logistic regression analysis. We observed a high likelihood of inadequate intake of the four micronutrients. The group with lower micronutrient intake (cluster 1) displayed higher %HbA1c (p = 0.006) and triglyceride (p = 0.010) levels. High %HbA1c showed an association with cluster 1 (odds ratio (OR) = 3.041, 95% confidence interval (CI) = 1.131; 8.175) and time of T2DM diagnosis (OR = 1.155, 95% CI = 1.043; 1.278). Potassium (β = −0.001, p = 0.017) and magnesium (β = −0.007, p = 0.015) intakes were inversely associated with %HbA1c. Reduced concomitant intake of the four micronutrients studied proved to be associated with risk of increased %HbA1c in individuals with T2DM, which was particularly predicted by magnesium and potassium intakes.
Keywords: micronutrients, trace elements, food, glycated hemoglobin A, hyperglycemia
1. Introduction
Type 2 diabetes mellitus (T2DM) is characterized by failures in blood glucose homeostasis and is considered a global public health problem [1]. Chronic hyperglycemia leads to increased oxidative stress and production of proinflammatory cytokines, disrupting insulin signaling pathways, lipid metabolism, protein synthesis, and cell differentiation, and may alter body concentrations of micronutrients [2,3,4,5]. The maintenance of the glycemic control in individuals with T2DM involves strategies for lifestyle changes, drug therapy, and adoption of healthy eating habits [1,2]. A balanced diet with adequate nutrient content can reduce the glycated hemoglobin percentage (%HbA1c) in subjects with T2DM by 0.3–2% [6]. Over the last few years, studies have verified the participation of minerals in the synthesis, secretion, and action of insulin. Among them, the minerals zinc, potassium, calcium, and magnesium are considered essential for the homeostasis of glucose metabolism [5,7,8].
Zinc plays a key role in the insulin biosynthesis as part of the hexameric structure of this hormone, and in the sensitivity to insulin in target tissues through stimulation of insulin receptors [9,10]. Calcium and potassium regulate voltage-dependent channels in pancreatic β-cells, which are essential for insulin exocytosis [11,12]. Magnesium is important for β-cell functioning and acts as a cofactor of many enzymes involved in the glucose metabolism, like tyrosine kinase enzymes, which phosphorylate insulin receptors and trigger the signaling cascade [4,12,13].
Thus, an inadequate intake of these micronutrients may impair the insulin synthesis, secretion, and signaling pathways [4,11,14]. It is possible to observe in the literature studies that associate the low intake and serum concentration of these minerals with risk of T2DM development [2,13,15,16,17,18]. Nevertheless, studies that evaluate the relationship between the dietary intake of these minerals in glycemic control in subjects with established disease are not widely found. In addition, no studies have assessed the relationship of the concomitant intake of these four micronutrients with the glycemic control, insulin biosynthesis, and insulin sensitivity of individuals with T2DM. In this scenario, the present study aimed to evaluate the relationship of the dietary intake of zinc, potassium, calcium, and magnesium with glycemic markers in individuals with T2DM in order to expand the understanding of these relationships in glycemic control.
2. Materials and Methods
2.1. Study Design and Participants
In this cross-sectional study, to be eligible for enrollment, individuals had to be 19–59 years old and have diagnosis of T2DM. Thus, 102 individuals with T2DM who visited the Family Health Units in the state of Sergipe, Brazil, were evaluated. Exclusion criteria adopted were the use of vitamin–mineral supplements, pregnancy, status as a current smoker, and the presence of the following diseases: rheumatoid arthritis, cancer, chronic renal failure, thyroid dysfunction, and acute infections or inflammatory processes such as influenza and urinary tract infection. The study followed the guidelines of the Declaration of Helsinki and all participants provided written informed consent. The study was approved by the Ethical Committee of the Federal University of Sergipe (Approval number 1.370.831).
Venous blood samples were obtained, after an overnight fast for at least 10 hours, in ethylenediamine tetra-acetic acid (EDTA) tubes for estimation of concentration of glycated hemoglobin (HbA1c) in whole blood, and in gel tubes for the determination of fasting glucose, lipid profile, insulin, and C-peptide in serum. Measurements of anthropometric parameters, body fat percentage, and blood pressure were performed. All the measurements were realized by trained technicians, and the individuals were instructed to avoid physical activity or effort one day before the tests. In combination, dietary intake assessments of macro and micronutrients was performed. Information about socioeconomic conditions, medical history, and lifestyle were obtained using a questionnaire.
2.2. Anthropometric Parameters, Bioelectric Impedance and Blood Pressure Measurements
Weight and height measurements were performed to calculate the body mass index (BMI). The BMI was calculated by dividing the body weight value (in kilograms) by the square value of the body height (in meters). The participants were classified as malnourished (BMI < 18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2) or overweight/obese (BMI ≥ 25 kg/m2), assessed according to the cut-off values proposed by the World Health Organization [19]. Waist circumference was measured with a non-extendable tape positioned at the midpoint between the last floating rib and the top of the iliac crest. The cut-off point used for waist circumference was ≥80 cm for women and ≥94 cm for men [20].
Measurement of body fat percentage was performed using the BIA 310 Bioimpedance Analyzer (Biodynamics Corporation, Shoreline, WA, USA), following the manufacturer’s instructions. Blood pressure was measured using aneroid sphygmomanometers and a stethoscope according to the recommendations of the Brazilian Society of Cardiology [21] at rest in a sitting position.
2.3. Biochemical Analyses
Serum concentrations of glucose, triglycerides, total cholesterol, and high-density lipoprotein cholesterol (HDL-c) were measured by an enzymatic colorimetric method using commercial kits (Labtest®, Lagoa Santa/MG, Brazil). The HbA1c was measured by immunoturbidimetric inhibition. All biochemical tests were performed using an automatic biochemical analyzer CMD 800i (Wiener Lab Group®, Rosario, Argentina). Serum insulin and C-peptide concentrations were measured by chemiluminescence using commercial kits (Abbott®, Abbott Park, IL, USA) and the automatic immunoassay analyzer Architect i1000SR (Abbott®, Abbott Park, IL, USA). The homeostasis model assessment (HOMA) was used to assess insulin sensitivity, based on C-peptide and glucose levels. Calculations were made using the HOMA calculator (University of Oxford, United Kingdom).
2.4. Food Intake
The 24-h dietary recalls over three days (including two weekdays and one weekend day) were obtained by Multiple-Pass method [22], and analyzed using the NutWin software (Department of Informatics, Paulista School of Medicine/UNIFESP, São Paulo/SP, Brazil), which includes the updated versions of the Brazilian Table of Food Composition (TACO), the Table of Nutritional Composition of Food Consumed in Brazil, the United States Department of Agriculture (USDA) Food Composition Databases, and food labels. The adequacy of usual dietary intake of nutrients by each group was evaluated according to the estimated average requirement (EAR) and adequate intake (AI) for potassium values proposed by the dietary reference intakes [23,24]. The probability of inadequate food intake by group was evaluated by z-score, which was calculated from the difference between the EAR corresponding to each nutrient and the mean ingestion of the group divided by the standard deviation of the intake. Potassium intake values were compared with AI values [24].
2.5. Statistical Analyses
The Kolmogorov–Smirnov test was used to evaluate data homogeneity. Non-homogeneous data were subjected to logarithmic transformation. The identification of individuals with inaccurate report of energy intake was performed using the methodology proposed by Mccrory et al. [25], based on the ratio between the reported energy intake and the total energy expenditure predicted, calculated by the equation of Vinken et al. [26] and considering the cut-off value of ± one standard deviation. The usual dietary intake was calculated using the Multiple Sources Method (MSM) (https://msm.dife.de/) [27]. Micronutrient intake values were adjusted for total energy intake according to residual method proposed by Willett et al. [28].
To verify associations between response variables, and to characterize individuals according to dietary intake of zinc, magnesium, calcium, and potassium, a hierarchical cluster analysis was used. From this analysis, the mean values corresponding to the energy-adjusted dietary intake of the four minerals were auto-scaled, and the similarities between the individuals calculated according to the Euclidean squared distance, while the Ward’s method was used for the formation of clusters. Differences between the two clusters formed was assessed using the Student’s t-test (for normal data distribution) or the Mann–Whitney test (for non-normal data distribution).
Regression models were used to assess the effect of the micronutrient intake on the glycemic control. Independent variables with p ≤ 0.20 in the bivariate analyses were included in the models using the enter method. p ≤ 0.20 was adopted according to the criterion of Wang et al. [29], established for the testing of a greater number of predictor variables in the model. Thus, a binary logistic regression model was applied using %HbA1c values above 7.0 [30] and cluster 1 (lower micronutrient intake) as risk variables. In addition, multiple regression models were applied to assess the effect of each micronutrient intake on %HbA1c separately. Multicollinearity was assessed by variance inflation factors (VIF) and no correlation was identified between the independent variables (VIF <10). Both regression models used sex, age, and time of T2DM diagnosis as adjustment variables.
The results were expressed as means and standard deviations, and as absolute and relative frequencies. A significance level of 5% was adopted for all tests. All statistical analyses were performed using SPSS for Windows Version 17.0 (SPSS Inc., Chicago, IL, USA).
3. Results
After exclusion of participants with missing dietary information, 95 individuals with T2DM were finally evaluated; 69.5% of them were female and mean age was 48.6 ± 8.4 years. Most individuals were overweight or obese (81.0%), and mean BMI and body fat percentage values were 30.22 ± 6.78 kg/m2 and 34.98 ± 7.94%, respectively. Waist circumference measurement showed 85.3% of the participants at increased risk of obesity-associated metabolic diseases (Table 1).
Table 1.
Clinical, biochemical and nutrient intake variables of individuals with type 2 diabetes mellitus (T2DM).
| Variables | T2DM (n = 95) |
|---|---|
| Age, years | 48.6 ± 8.4 |
| Sex | |
| Men, n (%) | 29 (30.5) |
| Women, n (%) | 66 (69.5) |
| Time of T2DM diagnosis 1, years | 7.3 ± 6.2 |
| Insulin therapy 2, n (%) | 21 (22.1) |
| Oral antidiabetic agents 2, n (%) | 70 (73.7) |
| Lipid-lowering agents 2, n (%) | 24 (25.3) |
| Antihypertensive agents 2, n (%) | 46 (48.4) |
| Weight, kg | 78.9 ± 19.3 |
| BMI, kg/m2 | 30.2 ± 6.8 |
| Fat mass 2, % | 35.0 ± 7.9 |
| Waist circumference, cm | 99.8 ± 14.3 |
| Men, n (%) | |
| <94 cm | 8 (27.6) |
| ≥94 cm | 21 (72.4) |
| Women, n (%) | |
| <80 cm | 6 (9.1) |
| ≥80 cm | 60 (90.9) |
| SBP 3, mmHg | 129.2 ± 17.0 |
| DBP 3, mmHg | 83.5 ± 15.3 |
| Fasting glucose, mg/dL | 180.1 ± 84.1 |
| %Hb1Ac | 8.1 ± 2.1 |
| Insulin, µU/mL | 13.8 ± 13.5 |
| C-peptide, ng/mL | 2.7 ± 0.8 |
| HOMA2-%B | 77.0 ± 56.2 |
| HOMA2-%S | 42.8 ± 20.9 |
| HOMA2-IR | 3.3 ± 2.9 |
| Total cholesterol, mg/dL | 193.4 ± 47.3 |
| HDL-c, mg/dL | 41.1 ± 10.4 |
| LDL-c, mg/dL | 117.4 ± 39.8 |
| Triglycerides, mg/dL | 174.3 ± 117.1 |
| Energy intake, kcal/day | 1469.4 ± 478.5 |
| Protein intake, g/day | 80.0 ± 20.2 |
| Carbohydrate intake, g/day | 210.1 ± 73.6 |
| Lipid intake, g/day | 37.1 ± 15.6 |
| Zinc intake, mg/day | 5.2 ± 1.5 |
| Potassium intake, mg/day | 1848.5 ± 543.4 |
| Calcium intake, mg/day | 469.0 ± 195.3 |
| Magnesium intake mg/day | 218.4 ± 68.1 |
Results presented in mean ± standard deviation and absolute frequency; 1 n = 91; 2 n = 93; 3 n = 89. %HbA1c: glycated hemoglobin percentage; BMI: body mass index; DBP: diastolic blood pressure; HDL-c: high-density lipoprotein cholesterol; HOMA: Homeostasis Assessment Model; LDL-c: low-density lipoprotein cholesterol; T2DM: type 2 diabetes mellitus; SBP: systolic blood pressure. Micronutrients adjusted for total energy intake using the residual method [28].
Deficient glycemic control was observed, as indicated by values of fasting glucose and %HbA1c above the cut-off point for disease control (fasting glucose < 154 mg/dL; %HbA1c < 7.0) [30]. Regarding lipid profile, 73.7% of the participants presented low HDL-c levels, and 37.9%, 10.5%, and 3.1% presented hypertriglyceridemia, mixed hyperlipidemia, and hypercholesterolemia, respectively.
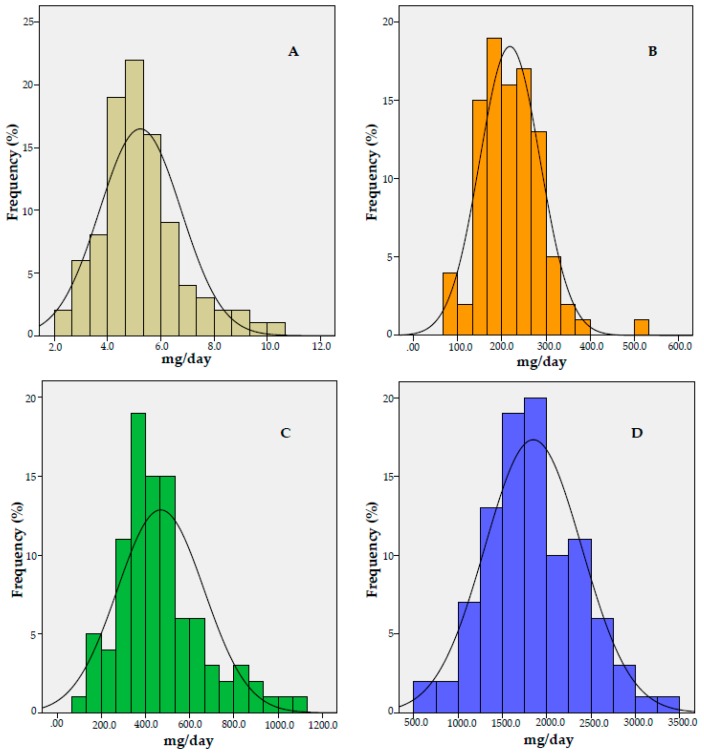
Energy intake was found to be underreported by 84.2% of the individuals. A prevalence of inadequacy in energy-adjusted zinc intake of 99.9% in males and 82.6% in females was observed. The prevalence of inadequate energy-adjusted magnesium intake for males and females was 96.4% and 74.9%, respectively. The energy-adjusted calcium intake showed a prevalence of inadequacy of 95.5%. All the individuals evaluated had energy-adjusted potassium intake below the AI values (Figure 1).
Figure 1.
Histogram of energy-adjusted intake of zinc (A), magnesium (B), calcium (C), and potassium (D), and prevalence of inadequate intake in individuals with type 2 diabetes mellitus (T2DM) (n = 95). (A) Group intake (mean ± SD): 5.23 ± 1.53 mg/day; Z-score: 3.06 (men) and 0.94 (women). (B) Group intake (mean ± SD): 218.36 ± 68.13 mg/day; Z-score: 1.80 (men) and 0.67 (women). (C) Group intake (mean ± SD): 468.95 ± 195.3 mg/day; Z-score: 1.96 (group). (D) Group intake (mean ± SD): 1848.45 ± 543.43 mg/day. SD: Standard deviation.
When energy-adjusted micronutrient (zinc, potassium, calcium, and magnesium) intake was compared between clusters, the group with lower micronutrient intake (cluster 1) showed significantly higher %HbA1c (p = 0.006) and serum triglyceride concentration (p = 0.01) in comparison with the group with higher intake (cluster 2). No statistical differences were seen with regard to the other variables studied (Table 2).
Table 2.
Clinical, biochemical and nutrient intake variables of individuals with type 2 diabetes mellitus (T2DM).
| Variables | Cluster 1 (n = 65) | Cluster 2 (n = 30) | p-value |
|---|---|---|---|
| Age, years | 48.6 ± 8.6 | 48.9 ± 7.9 | 0.866 ¥ |
| Time of T2DM diagnosis, years | 7.2 ± 6.4 | 7.4 ± 6.0 | 0.909 |
| BMI, kg/m2 | 30.4 ± 6.9 | 29.9 ± 6.7 | 0.762 ¥ |
| Waist circumference, cm | 100.8 ± 13.7 | 97.7 ± 15.5 | 0.332 |
| Fat mass, % | 34.6 ± 8.0 | 35.8 ± 7.8 | 0.483 |
| Total cholesterol, mg/dL | 198.8 ± 49.7 | 181.5 ± 40.0 | 0.097 |
| HDL-c, mg/dL | 41.9 ± 10.5 | 39.2 ± 10.3 | 0.247 |
| LDL-c, mg/dL | 117.9 ± 42.2 | 116.4 ± 34.5 | 0.867 |
| Triglycerides, mg/dL | 194.9 ± 131.1 | 129.6 ± 58.5 | 0.010 |
| %Hb1Ac | 8.3 ± 2.1 | 7.2 ± 1.7 | 0.006 |
| C-peptide, ng/mL | 2.7 ± 0.9 | 2.7 ± 0.7 | 0.963 |
| Fasting glucose, mg/dL | 190.3 ± 88.2 | 158.1 ± 70.7 | 0.082 ¥ |
| Insulin, µU/mL | 13.2 ± 11.7 | 15.1 ± 16.8 | 0.525 |
| HOMA2-%B | 73.7 ± 59.4 | 84.0 ± 48.8 | 0.407 ¥ |
| Energy intake, kcal/day | 1452.3 ± 471.4 | 1506.3 ± 499.7 | 0.611 |
| Lipid intake, g/day | 37.4 ± 15.1 | 36.5 ± 16.8 | 0.796 |
| Protein intake, g/day | 80.2 ± 19.7 | 79.4 ± 21.4 | 0.856 |
| Carbohydrate intake, g/day | 203.6 ± 74.1 | 224.2 ± 71.7 | 0.207 |
| Zinc intake, mg/day | 4.8 ± 1.4 | 6.1 ± 1.5 | <0.001 |
| Potassium intake, mg/day | 1556.5 ± 344.3 | 2480.9 ± 301.4 | <0.001 |
| Calcium intake, mg/day | 400.6 ± 136.4 | 616.9 ± 222.4 | <0.001 |
| Magnesium intake mg/day | 191.5 ± 54.2 | 276.6 ± 58.5 | <0.001 |
Results presented in mean ± standard deviation. p-value <0.05 was considered statistically significant. Student’s t-test for independent samples and ¥ Mann–Whitney test. %HbA1c: glycated hemoglobin percentage; BMI: body mass index; HDL-c: high-density lipoprotein cholesterol; HOMA: Homeostasis Assessment Model; LDL-c: low-density lipoprotein cholesterol; T2DM: type 2 diabetes mellitus. Micronutrients adjusted for total energy intake using the residual method [28].
Considering the significant difference in %HbA1c between clusters, a binary logistic regression model was applied in order to investigate the risk factors for increased %HbA1c (Table 3). We found that alterations in the %HbA1c were dependent of the time (in years) taken to diagnose T2DM (p = 0.005) and lower micronutrient intake (cluster 1) (p = 0.028).
Table 3.
Binary logistic regression model of glycated hemoglobin percentage (%HbA1c) and clusters formed from energy-adjusted mineral intake by individuals with type 2 diabetes mellitus (T2DM).
| Dependent Variable | Covariables | OR (95% CI) | p-value |
|---|---|---|---|
| %Hb1Ac § | Age (years) £ | 0.987 (0.931; 1.046) | 0.661 |
| Sex § | 1.598 (0.580; 4.405) | 0.365 | |
| Time of T2DM diagnosis (years) £ | 1.155 (1.043; 1.278) | 0.005 | |
| Cluster 1 § | 3.041 (1.131; 8.175) | 0.028 |
p-value <0.05 was considered significant. p-value of model: 0.003 and r2: 0.221. Hosmer and Lemeshow test: 0.591. § Variables included in the model in the dichotomous format. £ Variables included in the model in continuous format. Model adjusted by sex, age, and time of T2DM diagnosis. %HbA1c: glycated hemoglobin percentage; CI: confidence interval; OR: odds ratio; T2DM: type 2 diabetes mellitus. Risk classification used %HbA1c >7% [30] and cluster 1 with lower combined intake of zinc, potassium, calcium, and magnesium.
Multiple regression analyses showed that, for every 1 g of potassium and 100 mg of magnesium ingested, there was 1% and 0.7% reduction in %HbA1c, respectively. These results were influenced by sex and time taken to diagnose T2DM (Table 4).
Table 4.
Multiple linear regression models of glycated hemoglobin percentage (%HbA1c; dependent variable) and energy-adjusted mineral intake by individuals with type 2 diabetes mellitus (T2DM).
| Independent Variables | β (95%CI) | p-value | r2 Adjusted | |
|---|---|---|---|---|
| Model 1 1 | Age (years) | −0.032 (−0.082; 0.019) | 0.216 | 0.143 |
| Sex § | 1.069 (0.209; 1.930) | 0.015 | ||
| Time of T2DM diagnosis (years) | 0.117 (0.051; 0.183) | 0.001 | ||
| Zinc intake (mg/day) | −0.017 (−0.283; 0.250) | 0.902 | ||
| Model 2 2 | Age (years) | −0.028 (−0.077; 0.021) | 0.259 | 0.198 |
| Sex § | 0.925 (0.086; 1.765) | 0.031 | ||
| Time of T2DM diagnosis (years) | 1.118 (0.054; 0.182) | <0.001 | ||
| Potassium intake (mg/day) | −0.001 (−0.002; 0.000) | 0.017 | ||
| Model 3 2 | Age (years) | −0.029 (−0.079; 0.021) | 0.253 | 0.151 |
| Sex § | 1.025 (0.163; 1.887) | 0.020 | ||
| Time of T2DM diagnosis (years) | 0.119 (0.053; 0.184) | 0.001 | ||
| Calcium intake (mg/day) | −0.001 (−0.003; 0.001) | 0.377 | ||
| Model 4 2 | Age (years) | −0.031 (0.080; 0.017) | 0.206 | 0.201 |
| Sex § | 1.009 (0.177; 1.840) | 0.018 | ||
| Time of T2DM diagnosis (years) | 0.117 (0.053; 0.181) | <0.001 | ||
| Magnesium intake (mg/day) | −0.007 (−0.012; −0.001) | 0.015 |
1 p-value of model: 0.002. 2 p-value of model: <0.001. β non-standardized. Variables included in the models in the continuous format and in the § dichotomous format. All models adjusted by sex, age and time of T2DM diagnosis. %HbA1c: glycated hemoglobin percentage; CI: confidence interval; T2DM: type 2 diabetes mellitus.
4. Discussion
After assessing the effect of the energy-adjusted dietary intake of zinc, potassium, calcium, and magnesium on the glycemic control of individuals with T2DM, we found a three-fold increased risk of high %HbA1c when dietary intake was reduced. In addition, potassium and magnesium intakes were predictive of %HbA1c decrease. It is noteworthy that most evaluated individuals were likely to have inadequate ingestion of these micronutrients.
The association between low micronutrient intake and deficient glycemic control is well known and poor intake of these micronutrients is widely documented in T2DM [2,3,12,31]. However, most studies looked at the intake of each micronutrient individually, and studies evaluating the concomitant intake thereof are scarce.
Epidemiological studies have shown a significant association between low daily zinc intake and risk of T2DM [10,18,32], and the risk can be reduced by 20% upon daily intake of more than 13 mg of zinc [33]. Kanoni et al. [34] found a significant correlation between zinc intake and fasting glucose values, where the daily intake of every 1 mg of zinc was able to reduce the glucose concentration by 0.02 mg/dL. The same study was included in a recent systematic review, which reported that the serum zinc concentration has been negatively correlated with %HbA1c and fasting glucose values in T2DM [16].
The role of zinc in the functioning and maintenance of pancreatic β-cell mass, insulin biosynthesis, and maturation of secretory granules is well documented [9,10]. In addition, zinc inhibits tyrosine phosphatases by stimulating auto phosphorylation of insulin receptors [14,16]. Moreover, it is essential in the redox mechanisms as a component of the superoxide dismutase enzyme, thus being helpful against the oxidative stress generated by hyperglycemia [10,14].
The role of magnesium in the glycemic control has been shown in experimental studies on rats with induced T2DM. Magnesium supplementation improved insulin secretion and sensitivity, lipid profile, and inflammatory status [35,36]. Morakinyo et al. [36] also showed that rats supplemented with magnesium improved GLUT4 translocation and, consequently, the metabolic control.
Previous evidence also points to an inverse association between magnesium intake and T2DM risk [13,37]. Hypomagnesemia and low magnesium intake are very prevalent in individuals diagnosed with T2DM, especially in those with poor glycemic control [12,31,38]. In addition, reduced plasma concentration of magnesium may lead to changes in the glycemic control, since the body distribution of this micronutrient has an impact on insulin secretion and action [12].
However, studies addressing the effect of magnesium supplementation on glycemic control are controversial. Some studies have shown positive effects of magnesium supplementation on blood glucose and %HbA1c lowering, and on the increased insulin sensitivity [8,39,40,41]. However, other studies did not observe such effects, which may be explained by the presence of normomagnesemia, time of supplementation, and reduced number of individuals assessed [42,43,44,45,46,47]. Two meta-analyses studies showed reduction of 0.56 mmol/L (95% confidence interval (CI) = −1.10; −0.01) [48] and 0.37 mmol/L (95% CI = −0.74; −0.00) [4], respectively, in the fasting glucose levels upon magnesium supplementation.
Magnesium is an important cofactor of enzymes involved in the glucose metabolism, in which it binds to an ATP molecule, yielding the Mg–ATP complex that acts in phosphate transfer reactions [4,12,37]. Thus, magnesium participates in the autophosphorylation of the β-subunit of insulin receptors, proliferation and maintenance of pancreatic β-cells, activity of tyrosine kinases, and stimulation of proteins and substrates of the insulin signaling cascade [12,13].
Enhanced intakes of calcium and potassium are also associated with reduced risk of T2DM [5,15], due to the combined action of these minerals in the process of insulin release [7]. Within the pancreatic β-cells, the intracellular ATP/ADP ratio that follows the glucose metabolism leads to the closure of ATP-sensitive potassium channels, and subsequent depolarization of the plasma membrane. Calcium channels are then opened, allowing the calcium influx to the cells and subsequent activation of exocytosis of the insulin granules [7,11].
The fundamental role of calcium in body weight control and, consequently, in the maintenance of insulin sensitivity in the adipose tissue, is observed in animal models. Dietary calcium induces suppression of calcitriol, thus reducing lipogenesis and increasing lipolysis. In addition, it increases the uncoupling protein-2 (UCP2) expression in the adipose tissue. UCP2 is responsible for the transport and oxidation of mitochondrial fatty acids, which reduces lipid storage and adiposity [49].
Few studies have assessed the effect of calcium intake on the glycemic control. In an eight-week study, calcium supplementation (1500 mg per day) improved the insulin sensitivity in individuals with T2DM [50]. However, insulin sensitivity was not changed after non-diabetic obese individuals underwent 24 weeks of supplementation with 1000 mg of calcium per day [49].
Furthermore, magnesium intake is likely to be a confounding factor as it is highly correlated with calcium metabolism as observed in studies addressing the association between intake of both minerals and the risk of T2DM [51,52]. A relationship between calcium and vitamin D intakes was also observed as the interaction of these nutrients leads to reduced fasting insulin and %HbA1c levels in individuals with T2DM [53].
The relation between potassium intake and variables of glycemic control in individuals with T2DM is not widely addressed in the literature. Both low potassium intake and reduced potassium concentration in serum were shown to be significantly associated with reduced insulin sensitivity and with a compensatory increase in insulin secretion [5], as well as with risk of T2DM [17,54].
Considering that the adoption of healthy dietary and body weight control patterns is fundamental for the T2DM management [1,6], a high rate of underreported energy intake is observed among individuals with T2DM in this study, and this can be the main reason for the high prevalence of overweight and obesity here documented. Underreporting is a commonly observed practice among obese individuals and can affect the estimation of nutrient intake, consequently impairing nutritional and disease assessments [55,56]. In addition, the analysis of food intake that relies on 24-hour recalls may have limitations, since this method is susceptible to inaccurate reports about both the food consumed and the size of its portions [25]. However, these limitations were mitigated after calculation of intra and interindividual variability, in combination with the adjustment for energy intake of nutrients.
Furthermore, food evaluation involves data of tables of food composition, and such tables can be different depending on where the food is produced. Therefore, similar to the present study, the use of region or country-specific food composition tables may help to correct this confounding factor.
5. Conclusions
Overall, studies evaluating the effect of the concomitant intake of micronutrients on variables of glycemic metabolism in individuals with T2DM are scarce, especially when it comes to zinc, potassium, calcium, and magnesium. This highlights the importance of our results for a better evaluation of usual food intake in this population, which is a simple and low-cost procedure, as %HbA1c is routinely measured in individuals with T2DM and it is the gold standard for glycemic control evaluation.
Therefore, inadequate concomitant intake of zinc, potassium, calcium, and magnesium is related to poor glycemic control in individuals with T2DM, and the intakes of magnesium and potassium are predictors of %HbA1c reduction. Further studies on dietary intake of individuals with T2DM and their relation with glucose metabolism are needed.
Acknowledgments
The authors would like to thank the research volunteers, Alan Santos Oliveira for the support in data collection, the National Council for Scientific and Technological Development (CNPq) for the financial support, the Foundation for Research and Technological Innovation Support of the State of Sergipe (Fapitec/SE), and the Coordination of Improvement of Higher Education Personnel (CAPES).
Author Contributions
P.N.B.-L. and L.V.P. designed the study; All authors performed the analysis and contributed to the writing of the manuscript. All authors approved the final version of the manuscript.
Funding
This research was funded by the National Council for Scientific and Technological Development (CNPq), grant number 455117/2014-4.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.International Diabetes Federation . IDF Diabetes Atlas. 8th ed. IDF; Brussels, Belgium: 2017. [Google Scholar]
- 2.Shah M., Vasandani C., Adams-Huet B., Garg A. Comparison of nutrient intakes in South Asians with type 2 diabetes mellitus and controls living in the United States. Diabetes Res. Clin. Pract. 2018;138:47–56. doi: 10.1016/j.diabres.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Perez A., Rojas P., Carrasco F., Basfi-fer K., Perez-Bravo F., Codoceo J., Inostroza J., Galgani J.E., Gilmore L.A., Ruz M. Association between zinc nutritional status and glycemic control in individuals with well-controlled type-2 diabetes. J. Trace Elem. Med. Biol. 2018;50:560–565. doi: 10.1016/j.jtemb.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Veronese N., Watutantrige-Fernando S., Luchini C., Solmi M., Sartore G., Sergi G., Manzato E., Barbagallo M., Maggi S., Stubbs B. Effect of magnesium supplementation on glucose metabolism in people with or at risk of diabetes: A systematic review and meta-analysis of double-blind randomized controlled trials. Eur. J. Clin. Nutr. 2016;70:1354–1359. doi: 10.1038/ejcn.2016.154. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee R., Biggs M.L., de Boer I.H., Brancati F.L., Svetkey L.P., Barzilay J., Djoussé L., Ix J.H., Kizer J.R., Siscovick D.S., et al. Potassium and Glucose Measures in Older Adults: The Cardiovascular Health Study. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70:255–261. doi: 10.1093/gerona/glu071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association Lifestyle Management: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S38–S50. doi: 10.2337/dc18-S004. [DOI] [PubMed] [Google Scholar]
- 7.Fu Z., Gilbert E.R., Liu D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013;9:25–53. doi: 10.2174/157339913804143225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solati M., Ouspid E., Hosseini S., Soltani N., Keshavarz M., Dehghani M. Oral magnesium supplementation in type II diabetic patients. Med. J. Islam. Repub. Iran. 2014;28:67. [PMC free article] [PubMed] [Google Scholar]
- 9.Wijesekara N., Chimienti F., Wheeler M.B. Zinc, a regulator of islet function and glucose homeostasis. Diabetes Obes. Metab. 2009;11:202–214. doi: 10.1111/j.1463-1326.2009.01110.x. [DOI] [PubMed] [Google Scholar]
- 10.Sun Q., Van Dam R.M., Willett W.C., Hu F.B. Prospective Study of Zinc Intake and Risk of Type 2 Diabetes in Women. Diabetes Care. 2009;32:629–634. doi: 10.2337/dc08-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonfanti D.H., Alcazar L.P., Arakaki P.A., Martins L.T., Agustini B.C., de Moraes Rego F.G., Frigeri H.R. ATP-dependent potassium channels and type 2 diabetes mellitus. Clin. Biochem. 2015;48:476–482. doi: 10.1016/j.clinbiochem.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Sales C.H., Pedrosa L.F., Lima J.G., Lemos T.M., Colli C. Influence of magnesium status and magnesium intake on the blood glucose control in patients with type 2 diabetes. Clin. Nutr. 2011;30:359–364. doi: 10.1016/j.clnu.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Hruby A., Meigs J.B., O’Donnell C.J., Jacques P.F., McKeown N.M. Higher magnesium intake reduces risk of impaired glucose and insulin metabolism and progression from prediabetes to diabetes in middle-aged americans. Diabetes Care. 2014;37:419–427. doi: 10.2337/dc13-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chabosseau P., Rutter G.A. Zinc and Diabetes. Arch. Biochem. Biophys. 2016;611:79–85. doi: 10.1016/j.abb.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Villegas R., Gao Y.T., Dai Q., Yang G., Cai H., Li H., Zheng W., Shu X.O. Dietary calcium and magnesium intakes and the risk of type 2 diabetes: The Shanghai Women’s Health Study. Am. J. Clin. Nutr. 2009;89:1059–1067. doi: 10.3945/ajcn.2008.27182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Carvalho G.B., Brandão-Lima P.N., Maia C.S., Barbosa K.B.F., Pires L.V. Zinc’s role in the glycemic control of patients with type 2 diabetes: A systematic review. BioMetals. 2017;30:151–162. doi: 10.1007/s10534-017-9996-y. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee R., Colangelo L.A., Yeh H.C., Anderson C.A., Daviglus M.L., Liu K., Brancati F.L. Potassium intake and risk of incident type 2 diabetes mellitus: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetologia. 2012;55:1295–1303. doi: 10.1007/s00125-012-2487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drake I., Hindy G., Ericson U., Orho-Melander M. A prospective study of dietary and supplemental zinc intake and risk of type 2 diabetes depending on genetic variation in SLC30A8. Genes Nutr. 2017;12:30. doi: 10.1186/s12263-017-0586-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization . Obesity: Preventing and Managing the Global Epidemic. WHO; Geneva, Switzerland: 2000. (WHO Technical Report Series 894). [PubMed] [Google Scholar]
- 20.World Health Organization . Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. World Health Organization; Geneva, Switzerland: 2011. [Google Scholar]
- 21.Brazilian Society of Cardiology . 7ª Diretriz Brasileira de Hipertensão Arterial. Arq Bras Cardiol; Rio de janeiro, Brazil: 2016. [Google Scholar]
- 22.Guenther P.M., DeMaio T.J., Ingwersen L.A., Berlin M. The multiple-pass approach for the 24 hour recall in the Continuing Survey of food intakes by individuals (CSFII) 1994–1996; Proceedings of the 2nd International Conference on Dietary Assessment Methods; Boston, MA, USA. 22–24 January 1995. [Google Scholar]
- 23.Institute of Medicine . Dietary Reference Intakes for Calcium and Vitamin D. National Academy of Sciences; Washington, DC, USA: 2011. [Google Scholar]
- 24.Institute of Medicine . Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. National Academy of Sciences; Washington, DC, USA: 2006. [Google Scholar]
- 25.Mccrory M.A., Hajduk C.L., Roberts S.B. Procedures for screening out inaccurate reports of dietary energy intake. Public Health Nutr. 2002;5:873–882. doi: 10.1079/PHN2002387. [DOI] [PubMed] [Google Scholar]
- 26.Vinken A.G., Bathalon G.P., Sawaya A.L., Dallal G.E., Tucker K.L., Roberts S.B. Equations for predicting the energy requirements of healthy adults aged 18–81 y. Am. J. Clin. Nutr. 1999;69:920–926. doi: 10.1093/ajcn/69.5.920. [DOI] [PubMed] [Google Scholar]
- 27.German Institute of Human Nutrition Potsdam-Rehbrücke (DIfE) The Multiple Source Method (MSM) DIfE; Nuthetal, Germany: 2012. [(accessed on 25 April 2018)]. Available online: https://msm.dife.de. [Google Scholar]
- 28.Willett W.C., Howe G.R., Kushi L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997;65:1220S–1231S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q., Koval J.J., Mills C.A., Lee K.-I.D. Determination of the Selection Statistics and Best Significance Level in Backward Stepwise Logistic Regression. Commun. Stat. Simul. C. 2007;37:62–72. doi: 10.1080/03610910701723625. [DOI] [Google Scholar]
- 30.American Diabetes Association Glycemic Targets: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S55–S64. doi: 10.2337/dc18-S006. [DOI] [PubMed] [Google Scholar]
- 31.Sampaio F.A., Feitosa M.M., Sales C.H., Silva D.M.C., Cruz K.J.C., Oliveira F.E., Colli C., Marreiro D.N. Influence of magnesium on biochemical parameters of iron and oxidative stress in patients with type 2 diabetes. Nutr. Hosp. 2014;30:570–576. doi: 10.3305/nh.2014.30.3.7333. [DOI] [PubMed] [Google Scholar]
- 32.Eshak E.S., Iso H., Maruyama K., Muraki I., Tamakoshi A. Associations between dietary intakes of iron, copper and zinc with risk of type 2 diabetes mellitus: A large population-based prospective cohort study. Clin. Nutr. 2018;37:667–674. doi: 10.1016/j.clnu.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Vashum K.P., McEvoy M., Shi Z., Milton A.H., Islam M.R., Sibbritt D., Patterson A., Byles J., Loxton D., Attia J. Is dietary zinc protective for type 2 diabetes? Results from the Australian longitudinal study on women’s health. BMC Endocr. Disord. 2013;13:40. doi: 10.1186/1472-6823-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanoni S., Nettleton J.A., Hivert M.F., Ye Z., van Rooij F.J., Shungin D., Sonestedt E., Ngwa J.S., Wojczynski M.K., Lemaitre R.N., et al. Total Zinc Intake May Modify the Glucose-Raising Effect of a Zinc Transporter (SLC30A8) Variant: A 14-Cohort Meta-analysis. Diabetes. 2011;60:2407–2416. doi: 10.2337/db11-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ige A.O., Ajayi O.A., Adewoye E.O. Anti-inflammatory and insulin secretory activity in experimental type-2 diabetic rats treated orally with magnesium. J. Basic Clin. Physiol. Pharmacol. 2018;29:507–514. doi: 10.1515/jbcpp-2017-0104. [DOI] [PubMed] [Google Scholar]
- 36.Morakinyo A.O., Samuel T.A., Adekunbi D.A. Magnesium upregulates insulin receptor and glucose transporter-4 in streptozotocin-nicotinamide-induced type-2 diabetic rats. Endocr. Regul. 2018;52:6–16. doi: 10.2478/enr-2018-0002. [DOI] [PubMed] [Google Scholar]
- 37.Dong J.Y., Xun P., He K., Qin L.Q. Magnesium Intake and Risk of Type 2 Diabetes: Meta-analysis of prospective cohort studies. Diabetes Care. 2011;34:2116–2122. doi: 10.2337/dc11-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbagallo M., Dominguez L.J. Magnesium and type 2 diabetes. World J. Diabetes. 2015;6:1152–1157. doi: 10.4239/wjd.v6.i10.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodríguez-Morán M., Guerrero-Romero F. Oral magnesium supplementation improves insulin sensitivity and metabolic control in type 2 diabetic subjects: A randomized double-blind controlled trial. Diabetes Care. 2003;26:1147–1152. doi: 10.2337/diacare.26.4.1147. [DOI] [PubMed] [Google Scholar]
- 40.Paolisso G., Sgambato S., Pizza G., Passariello N., Varricchio M., D’Onofrio F. Improved insulin response and action by chronic magnesium administration in aged NIDDM subjects. Diabetes Care. 1989;12:265–269. doi: 10.2337/diacare.12.4.265. [DOI] [PubMed] [Google Scholar]
- 41.Paolisso G., Scheen A., Cozzolino D., Di Maro G., Varricchio M., D’Onofrio F., Lefebvre P.J. Changes in glucose turnover parameters and improvement of glucose oxidation after 4-week magnesium administration in elderly noninsulin-dependent (type II) diabetic patients. J. Clin. Endocrinol. Metab. 1994;78:1510–1514. doi: 10.1210/jcem.78.6.8200955. [DOI] [PubMed] [Google Scholar]
- 42.Navarrete-Cortes A., Ble-Castillo J.L., Guerrero-Romero F., Cordova-Uscanga R., Juárez-Rojop I.E., Aguilar-Mariscal H., Tovilla-Zarate C.A., Lopez-Guevara Mdel R. No effect of magnesium supplementation on metabolic control and insulin sensitivity in type 2 diabetic patients with normomagnesemia. Magnes. Res. 2014;27:48–56. doi: 10.1684/mrh.2014.0361. [DOI] [PubMed] [Google Scholar]
- 43.Lima M.L., Cruz T., Pousada J.C., Rodrigues L.E., Barbosa K., Cangucu V. The Effect of Magnesium Supplementation in Increasing Doses on the Control of Type 2 Diabetes. Diabetes Care. 1998;21:682–686. doi: 10.2337/diacare.21.5.682. [DOI] [PubMed] [Google Scholar]
- 44.de Valk H.W., Verkaaik R., van Rijn H.J., Geerdink R., Struyvenberg A. Oral magnesium supplementation in insulin-requiring Type 2 diabetic patients. Diabet. Med. 1998;15:503–507. doi: 10.1002/(SICI)1096-9136(199806)15:6<503::AID-DIA596>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 45.Gullestad L., Jacobsen T., Dolva L. Effect of Magnesium Treatment on Glycemic Control and Metabolic Parameters in NIDDM Patients. Diabetes Care. 1994;17:460–461. doi: 10.2337/diacare.17.5.460. [DOI] [PubMed] [Google Scholar]
- 46.Purvis J.R., Cummings D.M., Landsman P., Carroll R., Barakat H., Bray J., Whitley C., Horner R.D. Effect of oral magnesium supplementation on selected cardiovascular risk factors in non-insulin-dependent diabetics. Arch. Fam. Med. 1994;3:503–508. doi: 10.1001/archfami.3.6.503. [DOI] [PubMed] [Google Scholar]
- 47.Eibl N.L., Kopp H.P., Nowak H.R., Schnack C.J., Hopmeier P.G., Schernthaner G. Hypomagnesemia in Type II Diabetes: Effect of a 3-month replacement therapy. Diabetes Care. 1995;18:188–192. doi: 10.2337/diacare.18.2.188. [DOI] [PubMed] [Google Scholar]
- 48.Song Y., He K., Levitan E.B., Manson J.E., Liu S. Effects of oral magnesium supplementation on glycaemic control in Type 2 diabetes: A meta-analysis of randomized double-blind controlled trials. Diabet. Med. 2006;23:1050–1056. doi: 10.1111/j.1464-5491.2006.01852.x. [DOI] [PubMed] [Google Scholar]
- 49.Shalileh M., Shidfar F., Haghani H., Eghtesadi S., Heydari I. The influence of calcium supplement on body composition, weight loss and insulin resistance in obese adults receiving low calorie diet. J. Res. Med. Sci. 2010;15:191–201. [PMC free article] [PubMed] [Google Scholar]
- 50.Pikilidou M.I., Lasaridis A.N., Sarafidis P.A., Befani C.D., Koliakos G.G., Tziolas I.M., Kazakos K.A., Yovos J.G., Nilsson P.M. Insulin sensitivity increase after calcium supplementation and change in intraplatelet calcium and sodium-hydrogen exchange in hypertensive patients with Type 2 diabetes. Diabet. Med. 2009;26:211–219. doi: 10.1111/j.1464-5491.2009.02673.x. [DOI] [PubMed] [Google Scholar]
- 51.Dong J.Y., Qin L.Q. Dietary calcium intake and risk of type 2 diabetes: Possible confounding by magnesium. Eur. J. Clin. Nutr. 2012;66:408–410. doi: 10.1038/ejcn.2012.5. [DOI] [PubMed] [Google Scholar]
- 52.Van Dam R.M., Hu F.B., Rosenberg L., Krishnan S., Palmer J.R. Dietary Calcium and Magnesium, Major Food Sources, and Risk of Type 2 Diabetes in U.S. Black Women. Diabetes Care. 2006;29:2238–2243. doi: 10.2337/dc06-1014. [DOI] [PubMed] [Google Scholar]
- 53.Santos R.K.F., Brandão-Lima P.N., Tete R.M.D.D., Freire A.R.S., Pires L.V. Vitamin D ratio and glycaemic control in individuals with type 2 diabetes mellitus: A systematic review. Diabetes Metab. Res. Rev. 2018;34:1–11. doi: 10.1002/dmrr.2969. [DOI] [PubMed] [Google Scholar]
- 54.Chatterjee R., Yeh H.C., Shafi T., Selvin E., Anderson C., Pankow J.S., Miller E., Brancati F. Serum and dietary potassium and risk of incident type 2 diabetes mellitus: The Atherosclerosis Risk in Communities (ARIC) study. Arch. Intern. Med. 2010;170:1745–1751. doi: 10.1001/archinternmed.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Becker W., Welten D. Under-reporting in dietary surveys—Implications for development of food-based dietary guidelines. Public Health Nutr. 2001;4:683–687. doi: 10.1079/PHN2001154. [DOI] [PubMed] [Google Scholar]
- 56.Mirmiran P., Esmaillzadeh A., Azizi F. Under-reporting of energy intake affects estimates of nutrient intakes. Asia Pac. J. Clin. Nutr. 2006;15:459–464. [PubMed] [Google Scholar]