Abstract
AlphoidtetO-type human artificial chromosome (HAC) has been recently synthetized as a novel class of gene delivery vectors for induced pluripotent stem cell (iPSC)-based tissue replacement therapeutic approach. This HAC vector was designed to deliver copies of genes into patients with genetic diseases caused by the loss of a particular gene function. The alphoidtetO-HAC vector has been successfully transferred into murine embryonic stem cells (ESCs) and maintained stably as an independent chromosome during the proliferation and differentiation of these cells. Human ESCs and iPSCs have significant differences in culturing conditions and pluripotency state in comparison with the murine naïve-type ESCs and iPSCs. To date, transferring alphoidtetO-HAC vector into human iPSCs (hiPSCs) remains a challenging task. In this study, we performed the microcell-mediated chromosome transfer (MMCT) of alphoidtetO-HAC expressing the green fluorescent protein into newly generated hiPSCs. We used a recently modified MMCT method that employs an envelope protein of amphotropic murine leukemia virus as a targeting cell fusion agent. Our data provide evidence that a totally artificial vector, alphoidtetO-HAC, can be transferred and maintained in human iPSCs as an independent autonomous chromosome without affecting pluripotent properties of the cells. These data also open new perspectives for implementing alphoidtetO-HAC as a gene therapy tool in future biomedical applications.
Keywords: human artificial chromosome (HAC), alphoidtetO-HAC, induced pluripotent stem cells (iPSCs), microcell-mediated chromosome transfer (MMCT), cell reprogramming
1. Introduction
Gene therapy includes approaches to either correct gene function or provide a wild-type copy of a mutated gene. Traditional gene delivery and therapy techniques using viruses, plasmids, bacterial and yeast artificial chromosomes can cause random DNA insertions into the host genome, often leading to unpredicted transgene expression and cancer development in humans [1,2,3,4]. Included among the several disadvantages of commonly used virus-based delivery systems are low cloning capacity, unstable episomal maintenance, and the lack of long-term gene expression. Human artificial chromosomes (HACs) avoid these disadvantages and also provide the physiological expression of genes of interests as analogous to the native chromosome [5].
Originally and commonly used HACs have been built by a top-down approach by means of the truncation of various human chromosomes [6,7,8], referred to as “mini-chromosomes”. The presence of a functional kinetochore in HACs allows them to be maintained as additional functional chromosomes in mammalian cells over multiple cell divisions [9,10]. Such HACs were used as high capacity gene delivery vectors in mouse models of muscular dystrophies [11,12,13]. HACs carrying megabase-size DNA inserts were also employed for gene therapy in CYP-humanized and human antibody-producing mice [6,11,14,15,16].
Another type of HAC is synthesized based on the bottom-up approach. A novel synthetic HAC has recently been assembled from a synthetic α-satellite (alphoid) DNA array, in which the tetracycline operator (tetO) sequences were embedded allowing the binding of Tet repressor fusion proteins. This feature provides the opportunity to conditionally inhibit a kinetochore function, resulting in the loss of the HAC in dividing cells [17,18,19]. In addition to this feature, the alphoidtetO-HAC vector has several other advantages, such as a fully defined megabase-size synthetic alphoid DNA array lacking any cryptic transcripts [20,21]. The structural integrity of this HAC has been demonstrated during gene loading and its transfer into different host cells, along with the high mitotic and transcriptional stability of the transgenes over multiple rounds of cell division in culture [18,22]. AlphoidtetO-HAC shows several characteristics required for an ideal gene delivery vector and can be stably maintained in murine embryonic stem cells and their derivatives throughout mouse ontogeny [23]. In human cancer cell lines, like HeLa, the alphoidtetO-HAC has been reported to be rather unstable, however, tethering histone acetyl transferase (HAT) to the centromere can significantly stabilize the HACs [24]. The behavior of the alphoidtetO-HAC in pluripotent stem cells and human tissues remains uncharacterized.
Microcell-mediated chromosome transfer (MMCT) is the main technique to transfer HACs from donor to recipient cells [25,26]. Chinese hamster ovary (CHO) cells have traditionally been used as the most efficient chromosome donor cells because unlike most cell lines, they undergo repetitive hyperploidization in the presence of colcemid, leading to micronucleation and the formation of micronuclei. These are subsequently ripped off the donor cells, along with fragments of cytoplasm and cell membrane, by centrifugation in the presence of actin inhibitors (cytochalasin B or latrunculin B) acting as cytoskeleton disruptors [26,27]. The resulting cell fragments, referred to as microcells, are then fused with the target cells using different cell-fusion agents. Traditionally, polyethylenglicol has commonly been used as a cell fusion agent. However, several new commercially available transfection reagents and the modified cell fusion micronucleated technique have also been developed [23,28,29,30]. Due to low efficiency and an increased risk of cell aneuploidy induced by polyethylenglicol, a new modified MMCT method applicable to human cells that utilizes an envelope protein of murine leukemia retroviruses (MLVs), was introduced [31]. Amphotropic MLV infects mammalian cells via binding to the Pit-2 phosphate transporter, which is a highly conserved and ubiquitously expressed membrane protein in mammals. The modified MLV-envelop protein was successfully utilized as a one-directional fusion agent for donor CHO-derived microcells, showing an increased efficiency of the MMCT method (retro-MMCT) [31].
In this study, we took advantage of the retro-MMCT method to transfer an alphoidtetO-HAC expressing GFP into de novo derived human iPSCs (hiPSCs) for the first time. We analyzed mitotic stability of the alphoidtetO-HAC and showed that it can be maintained in these cells for multiple passages without affecting their pluripotent properties.
2. Materials and Methods
2.1. Lentivirus Preparations
Lentiviruses encoding pluripotency factors OCT4, SOX2, cMYC, and KLF4 (within polycistronic cassette) and rtTA were prepared. Namely, 293T cells were transfected with envelope-encoding pMD2.G (2.5 μg), packaging psPAX2 (7.5 μg), and either pHAGE2-tetO-miniCMV-hOct4-F2A-hKlf4-IRES-hSox2-E2A-hcMyc-W-loxP (OKSM), FUW-M2rtTA (hereafter rtTA), or EnvΔR-IRES-tdTomato plasmids (10 μg) by polyethylenimine hydrochloride (PEI 40 kDa, 40 μg) transfection method [32,33]. Lentiviruses in cell culture supernatant were collected and processed, as described elsewhere [33,34,35,36].
2.2. Reprogramming Human Mesenchymal Stem Cells with OKSM/rt-TA
Human mesenchymal stem cells (hMSC) were grown in a DMEM medium (Biolot, St-Petersburg, Russia) supplemented with 10% fetal bovine serum, FBS (HyClone, Thermo Fisher Scientific, Waltham, MA, USA), 100 U/mL penicillin, 100 mg/mL streptomycin, and 2 mM l-Glutamine (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) [37,38]. The hMSCs were treated for mycoplasma by culturing them in media with 10 μg/mL ciprofloxacin (Myco-3, AppliChem, Darmstadt, EU) or 10 μg/mL Plasmocin (Invivogen, Toulouse, EU) for 1 passage (7 days). The cells were seeded, 6.5 × 104 cells per well of 0.1% gelatin-pretreated 12 well-plate in the above media. Next day, the media was replaced with Opti-MEM media containing the packaged lentiviruses rtTA [39] and pHAGE2 [40], adjusting the multiplicity of infection (MOI) to 10–12 for each virus. Following 3–4 h of incubation, 500 μL Opti-MEM were added, and incubation was continued overnight. The next day, the media was changed to the above serum-containing media supplemented with 2 μg/mL Doxycycline (Dox). The media was changed every second day and after 6 days, the cultured cells were trypsinized, seeded onto 6-well plates pre-coated with L7 hPSC matrix, and cultured (37 °C, 5% CO2) in L7 hPSC BulletKit media (Lonza Group, Basel, Switzerland) containing 2 μg/mL Dox. The media was changed every third day. The hiPSC colonies were picked on day 28, expanded in the same medium, and frozen in liquid nitrogen.
The derivation of human mesenchymal stem cells (hMSC) was performed according to the Helsinki declaration, and approval was obtained from the local Ethics Committee of the Almazov National Medical Research Centre. Written informed consent was obtained from all subjects prior to tissue biopsy. The specific clinical research protocol was approved by the local Ethics Committee of the Almazov National Medical Research Center (Ethical permit number 12.26/2014).
2.3. MMCT into hiPSCs
MMCT was performed as previously described [15,23,27,31,41], with modifications. CHO cells carrying alphoidtetO-HAC-GFP [23] were transduced with the lentivirus-bearing EnvΔR-IRES-TdTomato transgene (MOI = 4, virus titer = 6 × 106 tU/mL). The expanded CHO cells were cultured in T-25 flasks (Greiner) covered with 50 μg/mL collagen-I solution (Santa Cruz Biotechnology, Dallas, TX, USA) in a standard DMEM/F12 media (Biolot, St-Petersburg, Russia) supplemented with 10% FBS (HyClone, USA), 100 U/mL penicillin, 100 mg/mL streptomycin, 2 mM L-glutamine (Gibco, Thermo Fisher Scientific, Waltham, MA, USA), and 100 ng/mL colcemid (Wako Pure Chemical, Osaka, Japan), at 37 °C for 72 h with daily media change. The T-25 flask with micronucleated CHO cells was treated with DMEM containing 2 μM latrunculin B (Santa Cruz Biotechnology) and centrifuged at 8000× rpm at 34 °C for 1 h using an Avanti HP-26XP, JLA-10.500 rotor (Beckman Coulter Life Sciences, Indianapolis, IN, USA). The microcells fraction was collected as the total microcells pellets were consequently filtered through 8-, 5-, and 3-μm Whatman™ Nuclepore filters (Whatman, Piscataway, NJ, USA) and spun down by centrifugation at 3000 rpm for 5 min. The prepared microcell fractions were frozen with Cellbanker freezing media (Zenoaq, Tokyo, Japan) at −80 °C [41]. The hiPSCs were grown in mTeSR-1 media (Stemcell Technologies, Vancouver, BC, Canada) in 12-well plates up to 80–90% confluence. The cells were treated with Dispase (Stemcell Technologies, Vancouver, BC, Canada) for seven minutes and dissociated by fine micropipette tip scratching to produce small detached cell clusters. The hiPSCs were collected by centrifugation and softly suspended in 250 μL of pre-warmed mTeSR-1 media. Simultaneously, ¼ portion of the prepared microcells containing alphoidtetO-HAC-GFP were defrosted, washed in 10 mL of cold DMEM media, and suspended in 250 μL of pre-warmed mTeSR-1. Each 250 μL of hiPSC suspension and alphoidtetO-HAC-GFP microcell aliquots were mixed and carefully resuspended, spun down, and transferred into one well of 12-well plates covered with Matrigel (Corning, New York, NY, USA). The plates with the cell mixture were centrifuged (500 rpm, RT, 2 min), and incubated for 3–5 h at 37 °C in the standard CO2 incubator. The detached microcells were transferred into a separate Matrigel-covered well, and fresh mTeSR-1 media (1.5 mL) was added to the adhered hiPSCs. Next, the cells were grown for 24–48 h and passaged to 3 wells of 12-well plates covered with Matrigel. After separate colonies were grown, the GFP-positive ones were picked manually and transferred to separate wells of 12-well plates. The isolated GFP+ clones were grown to 25% confluency, then partly passed to fresh wells and partly frozen in 0.4 mL FreSR™-S media (Stemcell Technologies, Vancouver, BC, Canada). The hiPSCs were grown in various human embryonic stem cell media, including mTeSR-1, TeSR-E8 (Stemcell Technologies, Vancouver, BC, Canada), L7 hPSC BulletKit media (Lonza Group, Basel, Switzerland), StemFlex medium (Thermo Fisher Scientific, Waltham, MA, USA), cell matrices based on Matrigel (Corning, New York, NY, USA), and L7 hPSC Matrix (Lonza Group, Basel, Switzerland).
2.4. Preparation of Metaphase Spreads
The preparation of the metaphase nuclei was performed as previously described [23,27]. In brief, the exponentially growing HAC-carrying hiPSCs were incubated with 100 ng/mL colcemid (Wako Pure Chemical, Japan) for 4 h or overnight at 37 °C in 5% CO2 atmosphere. The cells were treated with hypotonic 0.56% KCl solution for 20 min and fixed by methanol/acetic acid solution (3:1, v/v). The prepared cell suspensions were placed dropwise on glass slides (Superfrost; Thermo Scientific, Darmstadt, Germany) and air-dried.
2.5. Fluorescence In Situ Hybridization with the PNA Probes
The slides with the metaphase spreads were treated with PBS for 15 min at RT, fixed in 4% paraformaldehyde (PFA), and washed four times for 7 min with PBS. The slides were consequently dehydrated with 70%, 90%, and 100% ethanol for five minutes each. A hybridization solution (20 μL) containing 10 M Tris–HCl pH 7.4, 70% formamide (Sigma-Aldrich, St. Louis, MO, USA), 5% dextran sulfate, 10 ng tetO PNA-FITC (Panagen Company, Bethel, PA, USA), and 10 ng telomere PNA-TRITS (Panagen Company, Bethel, PA, USA), was applied onto each slide and covered with cover glass. The slides were heated at 80 °C for 3 min and incubated for two to six hours at RT in darkness. The slides were then washed two times for 15 min with 70% formamide, 10 mM Tris–HCl (pH 7.4), 0.1% BSA, then washed three times for 5 min with 20 mM Tris–HCl (pH 7.4), 136 mM NaCl, 0.08% Tween-20, and finally rinsed in PBS. The slides were dehydrated, as indicated above, and mounted in Vectashield media containing 4′,6-diamidino-2-phenylindole (DAPI) (Santa Cruz Biotechnology, Dallas, TX, USA). Images were captured using the EVOS Cell Imaging Systems (Thermo Fisher Scientific, Waltham, MA, USA).
2.6. FACS Sorting of hiPSCs
The hiPSCs were grown in six-well plates in standard StemFlex medium/Matrigel conditions until 90% confluency, treated with L13 hPSC passaging solution (Lonza Group, Basel, Switzerland), washed with DMEM/F12, and resuspended in 1 mL StemFlex medium. Cell sorting of the GFP-positive and GFP-negative hiPSCs was done using flow cytofluorimeter EPIX XL (Beckman Coulter, Brea, CA, USA). The sorted cells were collected in PBS, centrifuged, and seeded on single wells of 12-well plates pre-covered with Matrigel.
2.7. Karyotype Analysis
The logarithmically grown hiPSCs (confluence 50–60%) were treated with 400 ng/mL colcemide for 16 h and trypsinized. The metaphase spreads were prepared as described above. The karyotype of the hiPSCs was defined using G-banding metaphase chromosomes analysis at a resolution of 400 bands, with twenty metaphase plates being analyzed.
2.8. Cell Immunostaining
Immunostaining of the cells was done as described previously [33]. The cells grown attached to the culture surface were fixed in 4% PFA in PBS, washed with PBS, and treated for 30 min with a blocking PBS solution of 1% BSA, 2% nonimmune sheep serum, and 0.1% Tween-20. The cells were next incubated with mouse antibodies to OCT4 (Santa Cruz Biotech, Dallas, TX, USA), SOX2 (NBC, Astana, Kazakhstan), NANOG (NBC, Astana, Kazakhstan), and KLF4 (Santa Cruz Biotech, Dallas, TX, USA), washed several times in 0.1% Tween20-PBS, and incubated with secondary antibodies conjugated with Cy-3 (Jackson ImmunoResearch, West Grove, PA, USA). Subsequently, the cells were washed in 0.1% Tween20-PBS, counterstained with DAPI, and embedded under coverslips into an anti-fading media.
2.9. Southern-Blot Hybridization Analysis
Southern-blot hybridization was performed with a 32P-labelled DNA probe [27]. The genomic DNA from 5 × 105 cells was digested by SpeI in an agarose plug. The digested CHEF DNA (CHEF Mapper, Bio-Rad Laboratories, Hercules, CA, USA) was gel-separated (5–250 kb range, 16 h run), transferred onto membrane (Amersham Hybond-N+), and hybridized with a 201-bp YAC/BAC DNA probe specific for alphoidtetO-HAC. The DNA probe was PCR-amplified from the genomic DNA in the presence of 32P-labeled dNTPs, using the 5′-GGGCAATTTGTCACAGGG-3′ and 5′-ATCCACTTATCCACGGGGAT-3′ primers. The blot was pre-hybridized for two hours at 65 °C in Church’s buffer containing 7% SDS and 0.5 M Na-phosphate buffer supplemented with 100 µg/mL salmon sperm DNA, then hybridized overnight at 65 °C with heat denatured 201-bp YAC/BAC DNA probe. The blot was washed twice in 0.05% SDS, 2× SSC for 10 min at RT, then twice in 0.05% SDS, 2× SSC for five minutes at 60 °C, twice in 0.05% SDS, 0.5× SSC for 5 min at 60 °C and twice in 0.05% SDS, 0.25× SSC for 5 min at 60 °C, developed for 24–72 h at −80°C.
2.10. Treatment of Cells with Inhibitors of Chromatin Modifiers
The AlphoidtetO-HAC-GFP hiPSCs were cultured for 24 h in mTeSR-1 media in the presence of DNA methyltransferase inhibitor 5-Aza-2’-deoxycytidine (AZA) (Sigma-Aldrich, St. Louis, MO, USA) or histone deacetylase inhibitor trichostatin A (TSA) (Sigma-Aldrich, St. Louis, MO, USA) at concentrations of 5–10 μM and 0.5 μM, respectively. The AlphoidtetO-HAC-GFP hiPSCs were cultured for 72 h in the presence of 100–400 nM AZA or 19–38 nM TSA. The GFP-expression in the living cells was monitored by fluorescent and phase contrast light microscopy (EVOS FL Auto Imaging System, Thermo Fisher Scientific, Waltham, MA, USA).
The AlphoidtetO-HAC-GFP hiPSCs were cultured for 24 h in mTeSR-1 media in the presence of DNA methyltransferase inhibitor 5-Aza-2’-deoxycytidine (AZA) (Sigma-Aldrich, St. Louis, MO, USA) or histone deacetylase inhibitor trichostatin A (TSA) (Sigma-Aldrich, St. Louis, MO, USA) at concentrations of 5–10 μM and 0.5 μM, respectively. The AlphoidtetO-HAC-GFP hiPSCs were cultured for 72 h in the presence of 100–400 nM AZA or 19–38 nM TSA. The GFP-expression in the living cells was monitored by fluorescent and phase contrast light microscopy (EVOS FL Auto Imaging System, Thermo Fisher Scientific, Waltham, MA, USA).
3. Results and Discussion
3.1. Reprogramming Human Endometrial MSCs in Lonza’s cGMP Culture Conditions
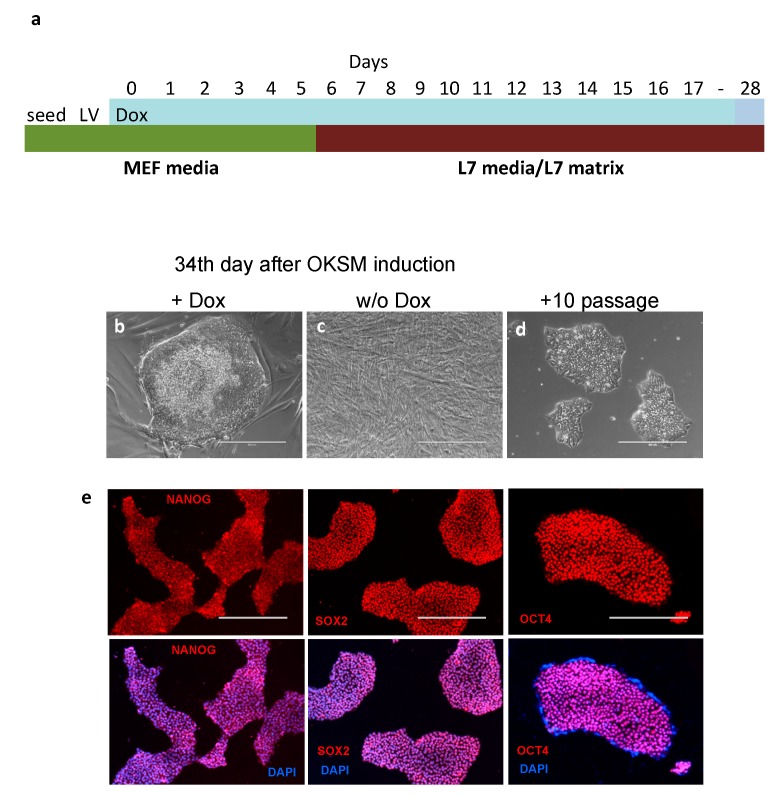
To generate hiPSCs in conditions compliant with current Good Medical Practice (cGMP), we used the culture reagents from Lonza [42,43]. These reagents, originally developed for the generation of hiPSCs from human peripheral blood mononuclear cells, were applied in this paper for the reprogramming of human mesenchymal stem cells (hMSCs). The hMSCs were taken from healthy women and grown in a standard serum-containing media (see Materials and Methods). The cells were simultaneously infected with lentiviruses containing tetO-driven Oct4, Klf4, Sox2, cMyc (OKSM) polycistronic cassette, and reverse tetracycline-controlled transactivator (rtTA) constructs [40]. Following the addition of Doxycycline (Dox), the cells were trypsinized, transferred onto fresh wells pre-covered with L7 matrix, and cultured in Lonza L7 hPSC medium (Figure 1a). On day 28 of the reprogramming, four iPSC clones were picked and expanded (Figure 1b). Although we found that the Matrigel/mTeSR-1 medium was superior for hiPSCs maintenance, L7 matrix/L7 medium conditions were preferred because they were compliant with cGMP standards. Importantly, the use of the OKSM polycistronic construct is very suitable for hiPSCs generation from hMSCs. Antibody staining confirmed that newly derived hiPSCs express the pluripotency markers NANOG, SOX2, and OCT4 (Figure 1e). Thus, the feeder cell- and serum-free L7 matrix/L7 media is both compliant with cGMP and very suitable for hMSCs reprogramming to pluripotent state. Notably, a case of successful reprogramming of hMSCs to iPSCs using a cell-feeder based conventional method has been previously reported [44]. At the same time, the reprogramming of human dermal fibroblast, using the same Lonza’s culture conditions and OKSM construct, has not been successful, as the fibroblasts showed a dramatic overgrowth. This culture media system was originally designed to reprogram human peripheral blood mononuclear cells under hypoxic conditions. Because we used the normoxic conditions and hMSCs at rather advanced passages, this probably yielded a relatively low number of hiPSC clones. We believe that the method could be significantly improved by applying hypoxic conditions (3–5% O2) and by using hMSCs at early passages. Additionally, we have found that the obtained hiPSCs can be maintained for multiple passages in TeSR-E8 and StemFlex human embryonic stem cell media with the use of both Matrigel and L7 matrixes. In summary, we applied in this study, for the first time, feeder-/serum-free cGMP-compliant conditions to reprogram hMSCs into hiPSCs.
Figure 1.
Generation of human pluripotent stem cells (hiPSCs) from human mesenchymal stem cells (hMSCs). (a) OKSM cassette expression was induced by Dox (2 μg/mL) on day 0, and on day 6 cells medium was changed to the L7. Distinctive hiPSC clones were observed on day 28. Representative images of (b) hiPSC clone on day 34 after OKSM induction, (c) uninfected hMSCs cultured for the same period of time, and (d) hiPSCs after 10 passages in culture. (e) hiPSCs cultured for 7 passages retain pluripotent characteristics, such as expression of NANOG, SOX2, and OCT4 marker genes (red). Scale bar represents 400 μm.
3.2. MLV Envelope Protein-Mediated Transfer of AlphoidtetO-HAC to Human iPSCs.
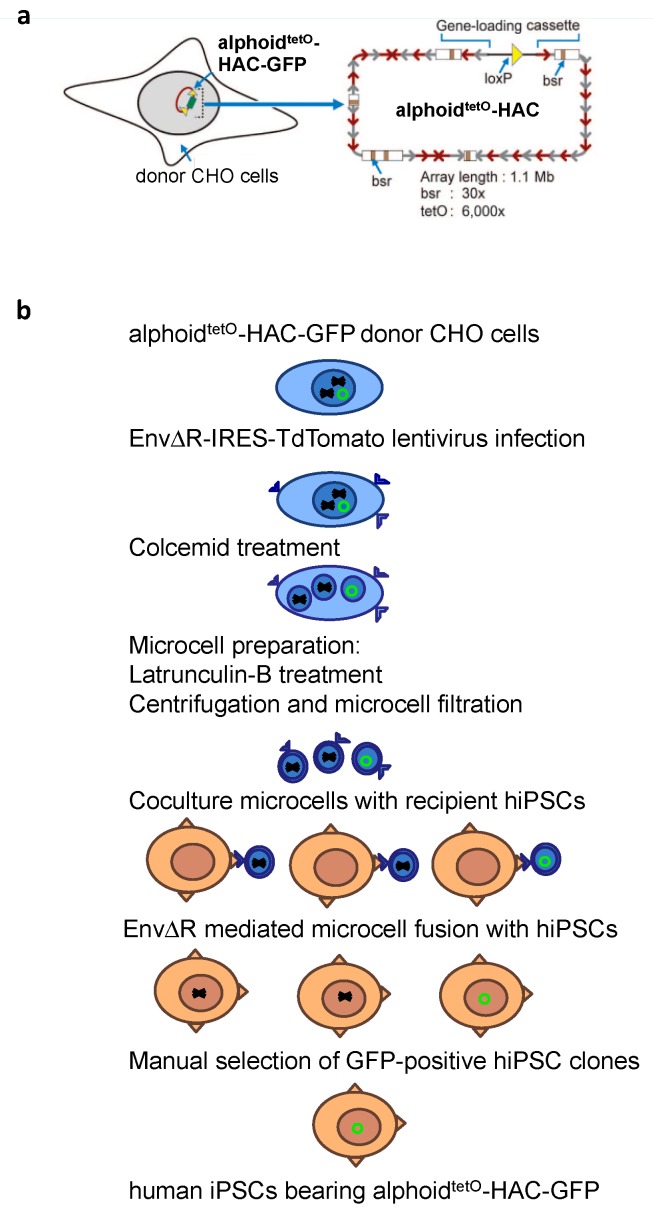
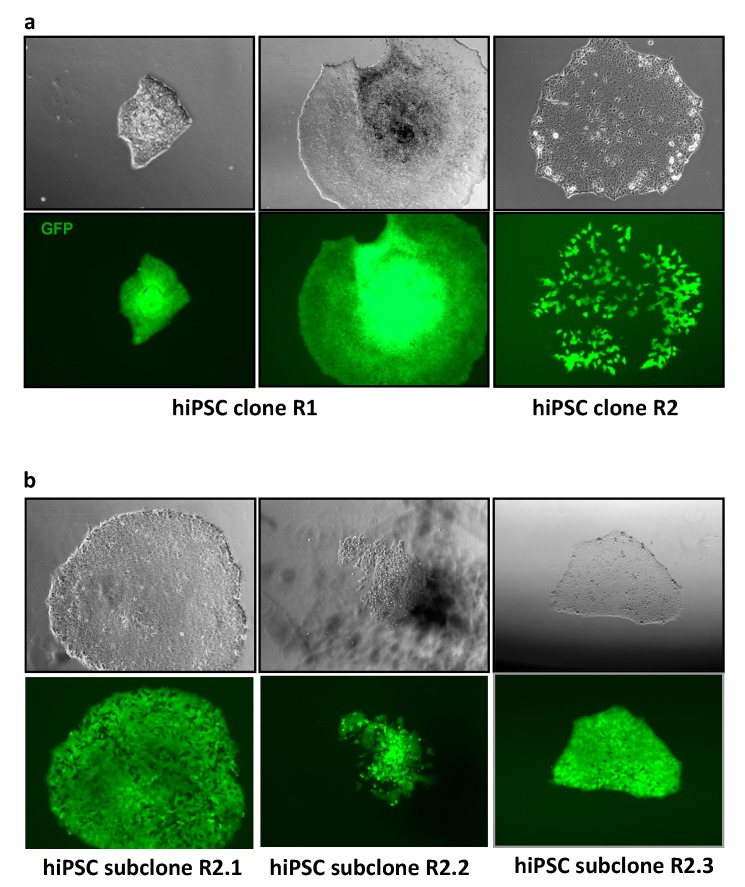
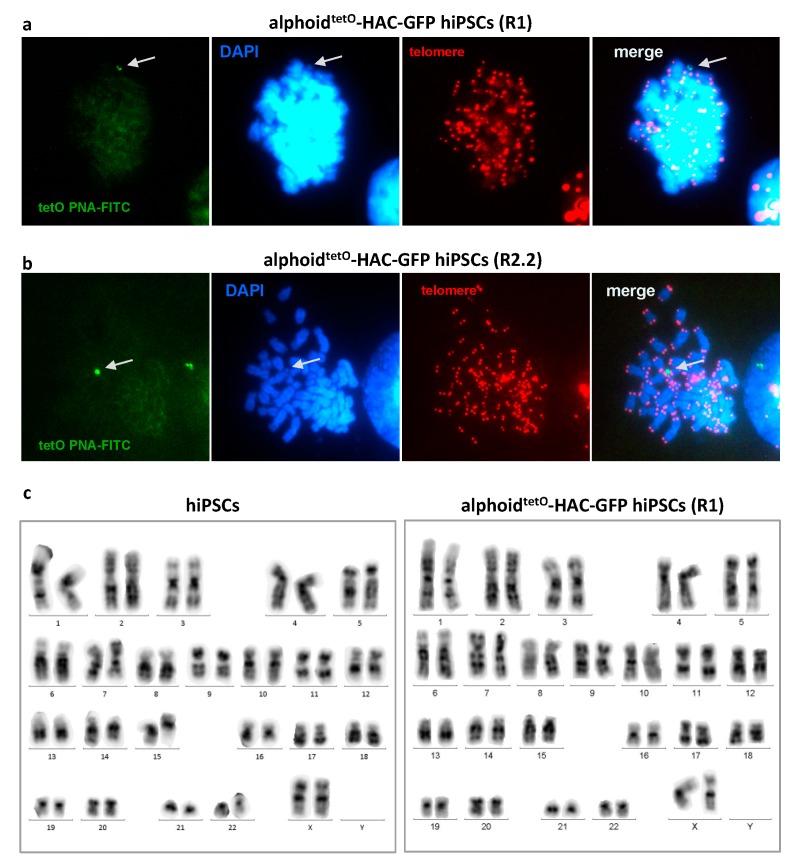
Conventional polyethylenglycol- and hemagglutinating virus of Japan (HVJ) envelope-based MMCT methods were highly inefficient for the transfer of the HACs into embryonic stem cells and iPSCs of the human origin. The efficiency of these methods was significantly improved by several recent modifications [27,31,45]. In this study, we applied the novel method developed by Suzuki et al [31] to transfer GFP-expressing alphoidtetO-HAC to hiPSCs (Figure 2). In addition, latrunculin B was used as a more efficient actin inhibitor for cytoskeleton disruption during microcells preparation [27]. Hamster donor CHO cells bearing alphoidtetO-HAC-GFP were infected with the EnvΔR protein-expressing lentivirus, resulting in >90% of the cells being positive for the EnvΔR protein expression. The prepared microcells were frozen and then fused with the hiPSCs. Two hiPSC clones bearing alphoidtetO-HAC-GFP were selected based on GFP expression, and subsequently expanded (referred to as R1 and R2 clones, Figure 3a). One of these clones (R2) was found to be a mixture of GFP-positive and GFP-negative cells. Therefore, it was further sub-cloned, resulting in three independent subclones homogeneously expressing GFP (R2.1, R2.2, and R2.3) (Figure 3b). Based on fluorescence in situ hybridization (FISH) assay, the alphoidtetO-HAC-GFP was maintained as an independent chromosome in the analyzed hiPSC clones (Figure 4). To analyze whether or not the HAC has undergone structural rearrangements during the course of the MMCT from hamster CHO to hiPSCs, Southern blot hybridization was carried out with genomic DNA possessing the alphoidtetO-HAC-GFP (clone R1), digested by SpeI endonuclease. This nuclease cuts the RCA/SAT43 vector sequence once but does not have a recognition site in the 1.1 Mb alphoid DNA array of alphoidtetO-HAC [20]. The original alphoidtetO-HAC carries 47 copies of the RCA/SAT43 vector used for the assembly and propagation of the synthetic alphoid DNA array [19]. SpeI-digested genomic DNA was separated by CHEF and hybridized with the probe specific to the tetO-alphoid sequence (see Materials and Methods for details). As seen in Figure S1, multiple identical bands of different sizes were observed on the Southern blot after SpeI digestion of CHO and hiPS cells. Thus, no detectable changes in the HAC structure were detected by Southern blot following the MMCT transfer of the HAC from CHO to hiPSCs.
Figure 2.
Scheme of microcell mediated chromosome transfer (MMCT) method used in this paper. (a) Schematic representation of alphoidtetO-HAC-GFP vector. (b) MMCT of the alphoidtetO-HAC-GFP into human pluripotent stem cells (iPSCs) with the use of mouse leukemia virus envelop protein (EnvΔR) as a cell fusion agent and latrunculin B as a cytoskeleton disruptor.
Figure 3.
Evaluation of GFP expression stability in primary alphoidtetO-HAC-GFP human pluripotent stem cells (hiPSC) colonies. (a) Following the retro-MMCT procedure, GFP-positive colonies became visible on day 5–7; they were picked up and expanded as R1 and R2 alphoidtetO-HAC-GFP hiPSC clones. (b) The mixed clone R2 was further sub-cloned, giving rise to the homogeneous GFP-positive subclones R2.1, R2.2, and R2.3.
Figure 4.
AlphoidtetO-HAC-GFP is maintained as an independently replicating chromosome in human pluripotent stem cells (hiPSCs), as revealed by fluorescence in situ hybridization (FISH) analysis using the tetO-PNA-FITC as a probe. The human artificial chromosome (HAC) (arrow) within the alphoidtetO-HAC-GFP carrying iPSC clone R1 (a) and sub-clone R2.2 (b) colocalizes with diamidino-2-phenylindole (DAPI) (blue) but not with the telomere labeling PNA-TRITS probe (red) specific exclusively for host chromosomes. (c) Representative results of karyotype analysis of the initial hiPSCs and the alphoidtetO-HAC-GFP hiPSCs (clone R1), showing normal 46XX karyotype.
Unlike mouse ESCs and iPSCs, their human counterparts are maintained in primed pluripotency state culture conditions which require more complex culture media and an extra-cellular matrix to sustain multiple cell passages. We have found that, contrary to all cell lines tested so far, hiPSCs are not susceptible to blasticidin (Bsd) selection. More specifically, hiPSCs bearing alphoidtetO-HAC-GFP, which contains multiple copies of the Bsd resistance gene [20], died within 48 h in the presence of 5 μg/mL Bsd. At the same time, both the parental iPSCs and the alphoidtetO-HAC containing hiPSCs could survive for at least 120 h in media containing 3.5 μg/mL Bsd. Thus, the sorting approach based on the living marker (GFP) is the method of choice for the selection of alphoidtetO-HAC in hiPSCs cells.
3.3. AlphoidtetO-HAC Maintenance in Human Induced-Pluripotent Stem Cells
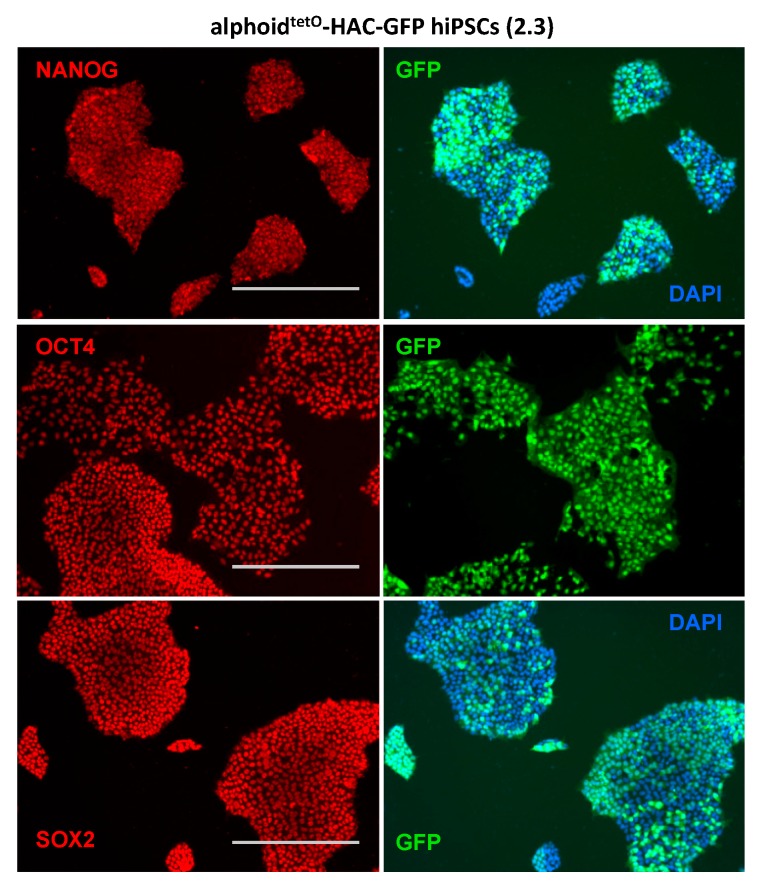
The hiPSCs carrying alphoidtetO-HAC-GFP remained similar to the parental wild-type hiPSCs with regard to growth and pluripotent stem cell characteristics for at least 15 passages. Importantly, even after such a prolonged time in culture, the hiPSCs showed stable karyotype, colony morphology, and expression of stem cell marker genes SOX2, OCT4, and NANOG (Figure 5 and Figure S2). G-banding analysis of the alphoidtetO-HAC-GFP bearing hiPSC clones revealed a normal 46XX karyotype with no apparent chromosomal abnormalities (Figure 4C). Interestingly, we noticed that after the first passage of alphoidtetO-HAC-GFP hiPSCs both GFP-positive and GFP-negative cells could be found (see Figure S3). After several passages, however, the number of GFP-negative cells of initially homogenous GFP-positive population of alphoidtetO-HAC bearing hiPSCs is significantly increased (up to 50% cells become GFP negative). This might be the result of either HAC loss or the silencing of GFP expression in some cells, or both. To address this, we separated the GFP-positive and GFP-negative cells by FACS and followed GFP expression in these sub-populations. Importantly, both sub-populations behaved similarly in standard culture conditions. However, even after one passage, GFP-negative cells emerged in population enriched for GFP-positive cells (Figure S3). On the other side, GFP-positive cells also emerged in GFP-negative population (Figure S3), suggesting that the former cells contain the HAC with silenced GFP whose expression is re-initiated after 1–2 passages. To further clarify the reason for the loss of GFP expression, we performed FISH analysis of the GFP-sorted cells, using the HAC-specific labeled probes (see Materials and Methods). More than 85% of the cells (from 25 metaphase spreads) exhibited a positive FISH signal in the population of GFP-positive cells (Figure S4). These results indicate that silencing of the GFP-expressing cassette within the HAC may occur. It was shown that epigenetic chromatin modifiers, such as inhibitors of DNA methyltransferase (5-Aza-2’-deoxycytidine or AZA) and histone deacetylase (Trichostatin A or TSA), can reactivate GFP-expression from alphoidtetO-HAC [46]. Treatment of GFP-positive cells by AZA or TSA resulted in a significant increase of GFP expression. However, neither of these inhibitors promoted an increase of GFP expression in the hiPSCs population sorted for GFP-negative cells (Figure S5). These data confirm that the alphoidtetO-HAC-GFP was lost in these cells and that HAC is mitotically unstable in hiPSCs.
Figure 5.
Human pluripotent stem cells (hiPSC) bearing alphoidtetO-HAC-GFP cells express pluripotency markers. AlphoidtetO-HAC-GFP hiPSCs maintained for over 5 passages remain pluripotent as they express OCT4, NANOG, and SOX2 markers (red), indicated on the panels. Scale bar, 400 μm.
4. Conclusions
De novo assembled alphoidtetO-HAC represents a promising new generation of high-capacity episomal vectors for biomedical applications [17,20,47]. In this study, we took advantage of the recent progress made in the MMCT methodology and succeeded in delivering the alphoidtetO-HAC into the hiPSCs which are currently considered to be the most clinically promising cell types in regenerative medicine. However, there are several limitations of the HAC-based technology that need to be resolved before it can be implemented in hiPSC-based clinical practice. These limitations include the low efficiency of HAC formation, the complex repeated DNA structure of the HACs, significant challenges in the amplification of a large amount of the HAC vector outside of eukaryotic cells, poor efficiency of the HAC delivery into target tissues or organs [6,7,48,49,50] and, as shown in this paper, insufficient mitotic stability of the HAC in hiPSCs. However, understanding the mechanism of HAC propagation in hiPSCs is critical for its application in gene therapy. Addressing the latter problem is a highly relevant pursuit in our future research.
Acknowledgments
We thank Nikolai D. Aksenov for assistance with FACS cell sorting. We thank Teruhiko Suzuki for EnvΔR-IRES-TdTomato construct, and Darrell N. Kotton and Gustavo Mostoslavsky for pHAGE2-OKSM construct. Part of experimental work was performed in the St-Petersburg State University equipment resource center “The development of molecular and cellular technologies”.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4409/7/12/261/s1. Figure S1: Southern blot analysis of integrity of the alphoidtetO-HAC-GFP in hamster CHO cells (clone 38-18) and after MMCT into hiPSCs (clone R1). Figure S2: hiPSCs bearing the alphoidtetO-HAC-GFP express pluripotency markers. Figure S3: Silencing of GFP expression in a fraction of alphoidtetO-HAC-GFP hiPSCs. Figure S4: FISH analysis of FACSed alphoidtetO-HAC-GFP hiPSCs revealed loss of the HAC in the population of GFP-negative cells. Figure S5: AZA and TSA treatment of alphoidtetO-HAC-GFP hiPSCs does not increase number of GFP-positive cells.
Author Contributions
S.A.S. and A.N.T. developed the project; S.A.S. designed the experiments, and performed the generation of hiPSCs, and MMCT experiments; E.V.S. performed FISH assays, lentiviruses generation, and assisted with MMCT experiments; M.A.L., S.V.P., A.A.K. (Andrey A. Kuzmin), A.A.K. (Aleksandr A. Khudiakov) performed the molecular biology and karyotype analysis experiments; A.B.M., N.A., V.L., N.K., and A.N.T. provided the funding; V.L. and N.K. provided critical feedback and suggestions; and S.A.S. and A.N.T. discussed the results and wrote the manuscript.
Funding
This work was supported essentially by the grant from the fundamental research program of the Presidium of the Russian Academy of Sciences “Basic research for biomedical technologies” and by the grant from the Russian Science Foundation (RSF) No. 17-14-01407. DNA plasmid construction was supported by Russian Foundation for Basic Research (RFBR) grant No. 18-04-01199. N.A. was supported by a joint tri-lateral Volkswagen Germany-Russia-Ukraine grant. M.A.L., V.L. and N.K. were supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, USA.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.O’Connor T.P., Crystal R.G. Genetic medicines: Treatment strategies for hereditary disorders. Nat. Rev. Genet. 2006;7:261–276. doi: 10.1038/nrg1829. [DOI] [PubMed] [Google Scholar]
- 2.Costantini F., Radice G., Lee J.L., Chada K.K., Perry W., Son H.J. Insertional mutations in transgenic mice. Prog. Nucleic Acid Res. Mol. Biol. 1989;36:159–169. doi: 10.1016/s0079-6603(08)60169-5. [DOI] [PubMed] [Google Scholar]
- 3.Soriano P., Gridley T., Jaenisch R. Retroviruses and insertional mutagenesis in mice: Proviral integration at the Mov 34 locus leads to early embryonic death. Genes Dev. 1987;1:366–375. doi: 10.1101/gad.1.4.366. [DOI] [PubMed] [Google Scholar]
- 4.Hotta A., Yamanaka S. From genomics to gene therapy: Induced pluripotent stem cells meet genome editing. Annu. Rev. Genet. 2015;49:47–70. doi: 10.1146/annurev-genet-112414-054926. [DOI] [PubMed] [Google Scholar]
- 5.Oshimura M., Katoh M. Transfer of human artificial chromosome vectors into stem cells. Reprod. Biomed. Online. 2008;16:57–69. doi: 10.1016/S1472-6483(10)60557-3. [DOI] [PubMed] [Google Scholar]
- 6.Kazuki Y., Hiratsuka M., Takiguchi M., Osaki M., Kajitani N., Hoshiya H., Hiramatsu K., Yoshino T., Kazuki K., Ishihara C., et al. Complete genetic correction of ips cells from Duchenne muscular dystrophy. Mol. Ther. 2010;18:386–393. doi: 10.1038/mt.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kouprina N., Tomilin A.N., Masumoto H., Earnshaw W.C., Larionov V. Human artificial chromosome-based gene delivery vectors for biomedicine and biotechnology. Expert Opin. Drug Delivery. 2014;11:517–535. doi: 10.1517/17425247.2014.882314. [DOI] [PubMed] [Google Scholar]
- 8.Oshimura M., Uno N., Kazuki Y., Katoh M., Inoue T. A pathway from chromosome transfer to engineering resulting in human and mouse artificial chromosomes for a variety of applications to bio-medical challenges. Chromosome Res. 2015;23:111–133. doi: 10.1007/s10577-014-9459-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu P.D., Lander E.S., Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi S., Kazuki Y., Nakayama Y., Nanba E., Oshimura M., Ohbayashi T. A method for producing transgenic cells using a multi-integrase system on a human artificial chromosome vector. PLoS ONE. 2011;6:e17267. doi: 10.1371/journal.pone.0017267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoshiya H., Kazuki Y., Abe S., Takiguchi M., Kajitani N., Watanabe Y., Yoshino T., Shirayoshi Y., Higaki K., Messina G., et al. A highly stable and nonintegrated human artificial chromosome (HAC) containing the 2.4 Mb entire human dystrophin gene. Mol. Ther. 2009;17:309–317. doi: 10.1038/mt.2008.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tedesco F.S., Hoshiya H., D’Antona G., Gerli M.F., Messina G., Antonini S., Tonlorenzi R., Benedetti S., Berghella L., Torrente Y., et al. Stem cell-mediated transfer of a human artificial chromosome ameliorates muscular dystrophy. Sci. Transl. Med. 2011;3:96ra78. doi: 10.1126/scitranslmed.3002342. [DOI] [PubMed] [Google Scholar]
- 13.Tedesco F.S., Gerli M.F., Perani L., Benedetti S., Ungaro F., Cassano M., Antonini S., Tagliafico E., Artusi V., Longa E., et al. Transplantation of genetically corrected human iPSC-derived progenitors in mice with limb-girdle muscular dystrophy. Sci. Transl. Med. 2012;4:140ra189. doi: 10.1126/scitranslmed.3003541. [DOI] [PubMed] [Google Scholar]
- 14.Kazuki Y., Kobayashi K., Aueviriyavit S., Oshima T., Kuroiwa Y., Tsukazaki Y., Senda N., Kawakami H., Ohtsuki S., Abe S., et al. Trans-chromosomic mice containing a human CYP3A cluster for prediction of xenobiotic metabolism in humans. Hum. Mol. Genet. 2013;22:578–592. doi: 10.1093/hmg/dds468. [DOI] [PubMed] [Google Scholar]
- 15.Tomizuka K., Yoshida H., Uejima H., Kugoh H., Sato K., Ohguma A., Hayasaka M., Hanaoka K., Oshimura M., Ishida I. Functional expression and germLine transmission of a human chromosome fragment in chimaeric mice. Nat. Genet. 1997;16:133–143. doi: 10.1038/ng0697-133. [DOI] [PubMed] [Google Scholar]
- 16.Kuroiwa Y., Kasinathan P., Choi Y.J., Naeem R., Tomizuka K., Sullivan E.J., Knott J.G., Duteau A., Goldsby R.A., Osborne B.A., et al. Cloned transchromosomic calves producing human immunoglobulin. Nat. Biotechnol. 2002;20:889–894. doi: 10.1038/nbt727. [DOI] [PubMed] [Google Scholar]
- 17.Ebersole T., Okamoto Y., Noskov V.N., Kouprina N., Kim J.H., Leem S.H., Barrett J.C., Masumoto H., Larionov V. Rapid generation of long synthetic tandem repeats and its application for analysis in human artificial chromosome formation. Nucleic Acids Res. 2005;33:e130. doi: 10.1093/nar/gni129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J.H., Kononenko A., Erliandri I., Kim T.A., Nakano M., Iida Y., Barrett J.C., Oshimura M., Masumoto H., Earnshaw W.C., et al. Human artificial chromosome (HAC) vector with a conditional centromere for correction of genetic deficiencies in human cells. Proc. Natl. Acad. Sci. USA. 2011;108:20048–20053. doi: 10.1073/pnas.1114483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakano M., Cardinale S., Noskov V.N., Gassmann R., Vagnarelli P., Kandels-Lewis S., Larionov V., Earnshaw W.C., Masumoto H. Inactivation of a human kinetochore by specific targeting of chromatin modifiers. Dev. Cell. 2008;14:507–522. doi: 10.1016/j.devcel.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kouprina N., Samoshkin A., Erliandri I., Nakano M., Lee H.S., Fu H., Iida Y., Aladjem M., Oshimura M., Masumoto H., et al. Organization of synthetic alphoid DNA array in human artificial chromosome (HAC) with a conditional centromere. ACS Synth. Biol. 2012;1:590–601. doi: 10.1021/sb3000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kouprina N., Earnshaw W.C., Masumoto H., Larionov V. A new generation of human artificial chromosomes for functional genomics and gene therapy. Cell. Mol. Life Sci. 2013;70:1135–1148. doi: 10.1007/s00018-012-1113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee N.C., Kononenko A.V., Lee H.S., Tolkunova E.N., Liskovykh M.A., Masumoto H., Earnshaw W.C., Tomilin A.N., Larionov V., Kouprina N. Protecting a transgene expression from the HAC-based vector by different chromatin insulators. Cell. Mol. Life Sci. 2013;70:3723–3737. doi: 10.1007/s00018-013-1362-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liskovykh M., Ponomartsev S., Popova E., Bader M., Kouprina N., Larionov V., Alenina N., Tomilin A. Stable maintenance of de novo assembled human artificial chromosomes in embryonic stem cells and their differentiated progeny in mice. Cell Cycle. 2015;14:1268–1273. doi: 10.1080/15384101.2015.1014151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohzeki J., Bergmann J.H., Kouprina N., Noskov V.N., Nakano M., Kimura H., Earnshaw W.C., Larionov V., Masumoto H. Breaking the HAC Barrier: Histone H3K9 acetyl/methyl balance regulates CENP-A assembly. EMBO J. 2012;31:2391–2402. doi: 10.1038/emboj.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doherty A.M., Fisher E.M. Microcell-mediated chromosome transfer (MMCT): Small cells with huge potential. Mammalian Genome. 2003;14:583–592. doi: 10.1007/s00335-003-4002-0. [DOI] [PubMed] [Google Scholar]
- 26.Fournier R.E., Ruddle F.H. Microcell-mediated transfer of murine chromosomes into mouse, Chinese hamster, and human somatic cells. Proc. Natl. Acad. Sci. USA. 1977;74:319–323. doi: 10.1073/pnas.74.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liskovykh M., Lee N.C., Larionov V., Kouprina N. Moving toward a higher efficiency of microcell-mediated chromosome transfer. Mol. Ther. 2016;3:16043. doi: 10.1038/mtm.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulis M., Bensi M., Orioli D., Mondello C., Mazzini G., D’Incalci M., Falcioni C., Radaelli E., Erba E., Raimondi E., et al. Transfer of a human chromosomal vector from a hamster cell line to a mouse embryonic stem cell line. Stem Cells. 2007;25:2543–2550. doi: 10.1634/stemcells.2007-0052. [DOI] [PubMed] [Google Scholar]
- 29.Paulis M. Chromosome transfer via cell fusion. Methods Mol. Biol. 2011;738:57–67. doi: 10.1007/978-1-61779-099-7_4. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki N., Itou T., Hasegawa Y., Okazaki T., Ikeno M. Cell to cell transfer of the chromatin-packaged human β-globin gene cluster. Nucleic Acids Res. 2010;38:e33. doi: 10.1093/nar/gkp1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki T., Kazuki Y., Oshimura M., Hara T. Highly efficient transfer of chromosomes to a broad range of target cells using Chinese hamster ovary cells expressing murine leukemia virus-derived envelope proteins. PLoS ONE. 2016;11:e0157187. doi: 10.1371/journal.pone.0157187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang Y., Garson K., Li L., Vanderhyden B.C. Optimization of lentiviral vector production using polyethylenimine-mediated transfection. Oncol. Lett. 2015;9:55–62. doi: 10.3892/ol.2014.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skvortsova E.V., Sinenko S.A., Tomilin A. Immortalized murine fibroblast cell lines are refractory to reprogramming to pluripotent state. Oncotarget. 2018;9:35241–35250. doi: 10.18632/oncotarget.26235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liskovykh M., Chuykin I., Ranjan A., Safina D., Popova E., Tolkunova E., Mosienko V., Minina J.M., Zhdanova N.S., Mullins J.J., et al. Derivation, characterization, and stable transfection of induced pluripotent stem cells from Fischer344 rats. PLoS ONE. 2011;6:e27345. doi: 10.1371/journal.pone.0027345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kostina A., Bjork H., Ignatieva E., Irtyuga O., Uspensky V., Semenova D., Maleki S., Tomilin A., Moiseeva O., Franco-Cereceda A., et al. Notch, BMP and WNT/β-catenin network is impaired in endothelial cells of the patients with thoracic aortic aneurysm. Atheroscler. Suppl. 2018;35:e6–e13. doi: 10.1016/j.atherosclerosissup.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Malashicheva A., Kanzler B., Tolkunova E., Trono D., Tomilin A. Lentivirus as a tool for lineage-specific gene manipulations. Genesis. 2007;45:456–459. doi: 10.1002/dvg.20313. [DOI] [PubMed] [Google Scholar]
- 37.Shilina M.A., Grinchuk T.M., Anatskaya O.V., Vinogradov A.E., Alekseenko L.L., Elmuratov A.U., Nikolsky N.N. Cytogenetic and transcriptomic analysis of human endometrial MSC retaining proliferative activity after sublethal heat shock. Cells. 2018;7 doi: 10.3390/cells7110184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malashicheva A., Bogdanova M., Zabirnyk A., Smolina N., Ignatieva E., Freilikhman O., Fedorov A., Dmitrieva R., Sjoberg G., Sejersen T., et al. Various lamin A/C mutations alter expression profile of mesenchymal stem cells in mutation specific manner. Mol. Genet. Metab. 2015;115:118–127. doi: 10.1016/j.ymgme.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Carey B.W., Markoulaki S., Hanna J., Saha K., Gao Q., Mitalipova M., Jaenisch R. Reprogramming of murine and human somatic cells using a single polycistronic vector. Proc. Natl. Acad. Sci. USA. 2009;106:157–162. doi: 10.1073/pnas.0811426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Somers A., Jean J.C., Sommer C.A., Omari A., Ford C.C., Mills J.A., Ying L., Sommer A.G., Jean J.M., Smith B.W., et al. Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette. Stem Cells. 2010;28:1728–1740. doi: 10.1002/stem.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uno N., Uno K., Zatti S., Ueda K., Hiratsuka M., Katoh M., Oshimura M. The transfer of human artificial chromosomes via cryopreserved microcells. Cytotechnology. 2013;65:803–809. doi: 10.1007/s10616-013-9548-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baghbaderani B.A., Syama A., Sivapatham R., Pei Y., Mukherjee O., Fellner T., Zeng X., Rao M.S. Detailed characterization of human induced pluripotent stem cells manufactured for therapeutic applications. Stem Cell Rev. 2016;12:394–420. doi: 10.1007/s12015-016-9662-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baghbaderani B.A., Tian X., Neo B.H., Burkall A., Dimezzo T., Sierra G., Zeng X., Warren K., Kovarcik D.P., Fellner T., et al. cGMP-manufactured human induced pluripotent stem cells are available for pre-clinical and clinical applications. Stem Cell Rep. 2015;5:647–659. doi: 10.1016/j.stemcr.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park I.H., Zhao R., West J.A., Yabuuchi A., Huo H., Ince T.A., Lerou P.H., Lensch M.W., Daley G.Q. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 45.Dance A. Core concept: Human artificial chromosomes offer insights, therapeutic possibilities, and challenges. Proc. Natl. Acad. Sci. USA. 2017;114:9752–9754. doi: 10.1073/pnas.1713319114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kononenko A.V., Lee N.C., Earnshaw W.C., Kouprina N., Larionov V. Re-engineering an alphoid(tetO)-HAC-based vector to enable high-throughput analyses of gene function. Nucleic Acids Res. 2013;41:e107. doi: 10.1093/nar/gkt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kouprina N., Larionov V. Transformation-associated recombination (TAR) cloning for genomics studies and synthetic biology. Chromosoma. 2016;125:621–632. doi: 10.1007/s00412-016-0588-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hiratsuka M., Uno N., Ueda K., Kurosaki H., Imaoka N., Kazuki K., Ueno E., Akakura Y., Katoh M., Osaki M., et al. Integration-free iPS cells engineered using human artificial chromosome vectors. PLoS ONE. 2011;6:e25961. doi: 10.1371/journal.pone.0025961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park I.H. DYS-HAC-iPS cells: The combination of gene and cell therapy to treat duchenne muscular dystrophy. Mol. Ther. 2010;18:238–240. doi: 10.1038/mt.2009.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yakura Y., Ishihara C., Kurosaki H., Kazuki Y., Komatsu N., Okada Y., Doi T., Takeya H., Oshimura M. An induced pluripotent stem cell-mediated and integration-free factor VIII expression system. Biochem. Biophys. Res. Commun. 2013;431:336–341. doi: 10.1016/j.bbrc.2012.12.096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.