Abstract
To explore novel methods for the analysis of metabolomics data, we compared the ability of Partial Least Squares Discriminant Analysis (PLS-DA) and Bayesian networks (BN) to build predictive plasma metabolite models of age three asthma status in 411 three year olds (n = 59 cases and 352 controls) from the Vitamin D Antenatal Asthma Reduction Trial (VDAART) study. The standard PLS-DA approach had impressive accuracy for the prediction of age three asthma with an Area Under the Curve Convex Hull (AUCCH) of 81%. However, a permutation test indicated the possibility of overfitting. In contrast, a predictive Bayesian network including 42 metabolites had a significantly higher AUCCH of 92.1% (p for difference < 0.001), with no evidence that this accuracy was due to overfitting. Both models provided biologically informative insights into asthma; in particular, a role for dysregulated arginine metabolism and several exogenous metabolites that deserve further investigation as potential causative agents. As the BN model outperformed the PLS-DA model in both accuracy and decreased risk of overfitting, it may therefore represent a viable alternative to typical analytical approaches for the investigation of metabolomics data.
Keywords: Partial Least-Squares Discriminant analysis, Bayesian networks, asthma, arginine metabolism, overfitting
1. Introduction
Asthma is a complex chronic disease, estimated to affect more than 300 million people worldwide, with the majority of cases originating in early life [1]. While many genetic and environmental influences have been characterized [2], the exact etiology of asthma and its trajectory throughout the life-course is still not perfectly understood [3]. Identifying asthma and associated wheeze phenotypes early in life could help to initiate appropriate treatment as early as possible and mitigate the effects on lung function and growth associated with progression of disease.
Advances in high-throughput technologies provide novel tools for the identification of biomarkers of disease, while simultaneously providing new insights into underlying pathogenesis. Metabolomics, which aims to identify and quantify the small molecules (<10 kDa) in a biological sample and is the “ome” most closely related to phenotype, offers a particularly promising approach. Metabolites are involved in all the biochemical reactions regulating the functions of a cell, and represent the downstream products of both the genome and its interactions with the environment [4]. Metabolomics has previously been utilized to identify novel biomarkers of common chronic complex diseases [5]. Furthermore, existing metabolomic studies of asthma have shown promising results. However, accurate prediction or diagnosis of asthma using metabolomic profiles is not yet a reality [6].
Partial Least Squares Discriminant Analysis (PLS-DA) [7] is one of the most commonly used methods in the metabolomics literature, primarily for the assessment of the discriminatory and predictive ability of metabolites and metabolite profiles for diseases and phenotypes [8]. Despite their common use, there are several limitations to PLS-DA models, most notably the issue of overfitting [9,10]; i.e., the generation of models that too closely fit the existing data. Therefore, in addition to identifying real disease discriminators, the PLS-DA models may also explain random variability in the dataset that has no biological relevance to the disease of interest. Unsurprisingly, such overfit models tend to demonstrate limited generalizability, and are difficult to replicate in independent studies.
Consequently, investigators are increasingly looking to alternative approaches [11]. Here we consider one such alternative approach, Bayesian networks (BN). A Bayesian network is a graphical model of the statistical interactions between random variables in a data set, based on Bayesian statistics [12]. These “random variables” are typically metabolites, demographic and clinical covariates, and some phenotype(s) of interest, although in principle any data can be used. The BN then is a directed acyclic graph where nodes represent random variables, and directed edges between nodes represent a statistical dependence of the sink node on the source node. This dependence is determined by a Bayesian likelihood ratio test, called a Bayes Factor [13]. The process of building a BN relies on heuristic algorithms for identifying desirable models, as well as techniques more common in metabolomic analysis including cross-validation and permutation testing. BNs have several desirable properties for metabolomic analysis. (1) BNs have the potential to better capture the relationships between metabolites and a phenotype of interest, through the explicit modeling of nonlinear interactions; (2) BNs include mechanisms which mitigate overfitting, including Bayesian prior probability distributions used in the Bayes Factor tests which bias toward no association; (3) A BN model can be used to identify important individual metabolites and to predict the disease outcome from a subset of available metabolites [14]. We have previously demonstrated the power of Bayesian networks when applied to metabolomic data [15]. In this current study, we assessed and compared the ability of PLS-DA and a metabolomic Bayesian network to predict asthma status at age three in children from the Vitamin D Antenatal Asthma Reduction Trial (VDAART) [16].
2. Results
The study schematic is outlined in Figure 1. To maximize the value of our limited data, we first used cross-validation to identify optimal parameters for the classifiers. We then used those parameters to build a model using all of the data. This allows us to utilize the greatest amount of available data to obtain the most accurate classifier possible. To check for overfitting, we then used a permutation test.
Figure 1.
Study Schematic.
2.1. Study Population
A total of 411 children from the Vitamin D Antenatal Asthma Reduction Trial [16] (VDAART) with mass-spectrometry-based metabolomic profiling on blood samples extracted at age three were included in these analyses. By the age three visit, 59 of the children had been defined by a physician as asthma cases; 352 did not have a physician-diagnosis of asthma during the same period and were considered as controls.
Children with asthma were statistically more likely to be Black (p = 0.003), but there were no other significant differences between cases and controls (Table 1). Due to its cohort design, VDAART did not contain an equal number of asthma cases and asthma-free controls. Since strongly unbalanced datasets are a problem for most machine learning classification algorithms [17], leading to algorithms that are biased toward the majority class, we used bootstrap resampling to obtain a balanced dataset [18]. By randomly resampling from the asthma cases with replacement, we obtained a final bootstrap-balanced dataset of 704 subjects with 352 cases and 352 controls (see Section 4). Measurements of 481 known metabolites were available in blood plasma samples collected at age three years for these subjects. Of these, 433 metabolites passed the QC and data processing pipeline and were included in the analyses. The majority of metabolites were lipids (n = 142) and amino acids (n = 141). Nucleotides, peptides, cofactors and vitamins, energy metabolites, carbohydrates and 57 xenobiotics were also among the measured metabolites (Table S1).
Table 1.
Characteristics of the asthma cases and controls at age three.
| Variable | Controls (n = 352) | Cases (n = 59) | p-Value | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Gender | Male | 183 | 52.0% | 36 | 61.0% | 0.208 |
| Female | 169 | 48.0% | 23 | 39.0% | ||
| Race | White | 119 | 33.8% | 15 | 25.4% | 0.003 |
| Black | 159 | 45.2% | 40 | 67.8% | ||
| Other | 74 | 21.0% | 4 | 6.8% | ||
| Treatment Group | Placebo | 182 | 51.7% | 28 | 47.5% | 0.576 |
| Intervention | 170 | 48.3% | 31 | 52.5% | ||
| BMI | Mean (SD) | 16.6 (2.1) | 17.1 (1.9) | 0.063 | ||
2.2. Partial Least Squares Discriminant Analysis
We constructed PLS-DA prediction models using two sample-splitting approaches commonly employed in metabolomics studies: (1) splitting the data into training (67%) and testing (33%) sets, and (2) using five-fold cross-validation (CV). First, using the 2:1 training/testing split and all 433 metabolites, we constructed several models by varying the number of PLS components included for prediction from 1 to 70 (Figure S1). This test suggested that PLS-DA analysis with a model including one component would have the best performance (as assessed by the Area under the Curve Convex Hull (AUCCH), which was 75% on the test set) on potential replication data. Because using a single training-validation partition might be sensitive to random anomalies specific to any particular split, we next implemented PLS-DA with five-fold cross-validation, and again observed model performance with varying numbers of components (see Section 4). The cross-validated AUCCHs also suggested that a model with 1 component would have the strongest performance on replication data (Figure S2). Accordingly, we proceeded by building a PLS-DA model of the full dataset with a single component. The one-component PLS-DA model achieved an AUCCH of 81% (95% confidence interval: [77.7–84.4%]) (Figure 2) on the full data, indicating high accuracy for the prediction of asthma at age three. However, a permutation test indicated a possibility of such accuracy being due to chance (p = 0.057: Figure S3), and that the empirical null distribution of PLS-DA accuracy had a mean 76.2% AUCCH ± 2.6%.
Figure 2.
Discrimination of asthma at age three based on plasma metabolomic profiles by PLS-DA (AUCCH = 0.810) and by Bayesian network (AUCCH = 0.921) on the full dataset. AUCCH–Area under the Convex Hull of the Receiver Operator Characteristic curve; BN–Bayesian Network; ROC–Receiver Operator Characteristic curve; ROCCH–ROC Convex Hull.
2.3. Bayesian Network Analysis
Next, we explored the predictive ability of conditional Gaussian Bayesian networks (CGBN). Typically, predictive modeling with a Bayesian network proceeds as follows. A phenotype is selected; this and the potential predictor variables are arranged in a table. These might include metabolite concentrations, demographics, clinical data, possibly or genomic variants for example. A CGBN is a type of BN that cleanly handles a mix of ordinal (typically binary) data and continuous data (typically normally distributed) within a Bayesian mathematical framework. Then a Bayesian network must be found, or discovered. Because the number of possible networks is super exponential to the number of variables, all networks cannot be considered. Heuristic algorithms are employed to identify networks likely to fit the data. Among all networks considered, the “best” network is the one that best fits the available data, as measured by the posterior probability of that data. The posterior probability is computed using Bayesian statistics and is described in more detail in Sebastiani et al. [19]. This network can then be used to identify the most likely value of a variable given the values of the other variables nearby, and in this way can be used to predict age three asthma status given metabolite information. More details are given in McGeachie et al. [20].
Following the same methodological strategy as used for PLS-DA, we used two sample-splitting approaches of (1) a 2:1 training/testing split, and (2) 5-fold cross-validation. The CGBN has one major parameter to determine: an edge-inclusion threshold for the Bayes Factor tests of statistical association. If the BF is above the threshold, an edge between two variables is added in the CGBN model. A high threshold results in a sparse model, typically with a lower risk of overfitting, but may lead to unnecessarily conservative prediction performance. As with PLS-DA, we formed a training/testing split of the dataset, and varied the BN model threshold for edge inclusion. This identified no clear edge-inclusion threshold that produced a better testing performance than the others (Figure S1), so we repeated the random process of splitting the data into training and testing portions. This ultimately produced inconclusive results. As before, we then used five-fold cross-validation to identify the best threshold for edge inclusion; with the highest cross-validated AUCCH achieved using a log BF threshold of eight (Figure S2). We then used this threshold to build a CGBN model on the full dataset. This identified a BN with 42 metabolites within the Markov Neighborhood of childhood asthma—the predictive portion of the network required to predict childhood asthma in VDAART at age three—and obtained an accuracy of 92.1% AUCCH (95% CI [90–94.1%]), indicating strong prediction (Figure 2) on the full dataset. Permutation testing indicated this level of accuracy was unlikely to be due to chance (p < 0.001) (Figure S4), and that the empirical null distribution of BN accuracy had mean 75.1% AUCCH ± 6.7%.
2.4. Comparison of Models
Both methods integrate the measurements of many metabolites into a single number that can be used to classify a subject; assigning a probability between 0.0 and 1.0 of an asthma diagnosis at age 3. When comparing the performance of the models, the classification accuracy of the Bayesian network exceeded that of the PLS-DA model (with one component). The AUCCH for the BN model was 92% on the full dataset, with a corresponding sensitivity of 82% and specificity of 84%. The one-component PLS-DA model had an AUCCH of 81% on the full dataset, with a corresponding sensitivity of 73% and specificity of 72%. The difference in AUCCHs between the PLS-DA and the BN models was statistically significant (p < 0.001) (Figure 2). Combined with the observations from the permutation tests, this suggests that BN model performs better at predicting age-three asthma status from the metabolites than the PLS-DA model, and this is unlikely to be due to random fluctuations in the statistical properties of the cohort or to the fitting of noise in the data.
2.5. Biological Interpretation of Findings
2.5.1. PLS-DA
Metabolites with influential loadings (defined as ≤−2 or ≥2) in the first component were selected for further analysis. Fifty-one metabolites had a loading in the first principal component ≤−2 and three had a loading ≥2 (Table 2).
Table 2.
Metabolites with an influential loading in the first PLSDA component.
| Metabolite | Super Pathway | Sub Pathway | HMDB ID | Loading |
|---|---|---|---|---|
| glycochenodeoxycholate sulfate | Lipid | Primary Bile Acid Metabolism | −9.12 | |
| stachydrine | Xenobiotics | Food Component/Plant | HMDB04827 | −6.41 |
| N-methylproline | Amino Acid | Urea cycle; Arginine and Proline Metabolism | −6.31 | |
| glycolithocholate sulfate | Lipid | Secondary Bile Acid Metabolism | HMDB02639 | −4.87 |
| methyl glucopyranoside (alpha + beta) | Xenobiotics | Food Component/Plant | −4.54 | |
| theobromine | Xenobiotics | Xanthine Metabolism | HMDB02825 | −4.44 |
| cysteine s-sulfate | Amino Acid | Methionine, Cysteine, SAM and Taurine Metabolism | HMDB00731 | −4.23 |
| 4-vinylguaiacol sulfate | Xenobiotics | Food Component/Plant | −4.14 | |
| taurolithocholate 3-sulfate | Lipid | Secondary Bile Acid Metabolism | HMDB02580 | −4.12 |
| 3-hydroxyhippurate | Xenobiotics | Benzoate Metabolism | HMDB06116 | −3.72 |
| 2,3-dihydroxyisovalerate | Xenobiotics | Food Component/Plant | HMDB12141 | −3.34 |
| 4-methylcatechol sulfate | Xenobiotics | Benzoate Metabolism | −3.33 | |
| vanillic alcohol sulfate | Amino Acid | Tyrosine Metabolism | −3.25 | |
| 3-(3-hydroxyphenyl)propionate | Xenobiotics | Benzoate Metabolism | HMDB00375 | −3.21 |
| p-cresol-glucuronide | Amino Acid | Tyrosine Metabolism | HMDB11686 | −3.2 |
| CMP | Nucleotide | Pyrimidine Metabolism, Cytidine containing | HMDB00095 | −3.16 |
| indolepropionate | Amino Acid | Tryptophan Metabolism | HMDB02302 | −3.11 |
| beta-cryptoxanthin | Xenobiotics | Food Component/Plant | HMDB33844 | −3.08 |
| xylose | Carbohydrate | Pentose Metabolism | HMDB00098 | −3.05 |
| tauro-beta-muricholate | Lipid | Primary Bile Acid Metabolism | HMDB00932 | −3.04 |
| 5-hydroxyindoleacetate | Amino Acid | Tryptophan Metabolism | HMDB00763 | −2.93 |
| gamma-glutamylglutamate | Peptide | Gamma-glutamyl Amino Acid | HMDB11737 | −2.87 |
| ferulic acid 4-sulfate | Xenobiotics | Food Component/Plant | HMDB29200 | −2.8 |
| cinnamoylglycine | Xenobiotics | Food Component/Plant | HMDB11621 | −2.71 |
| tryptophan betaine | Amino Acid | Tryptophan Metabolism | HMDB61115 | −2.7 |
| 1,2,3-benzenetriol sulfate (2) | Xenobiotics | Chemical | −2.66 | |
| catechol sulfate | Xenobiotics | Benzoate Metabolism | HMDB59724 | −2.65 |
| quinate | Xenobiotics | Food Component/Plant | HMDB03072 | −2.62 |
| inosine 5’-monophosphate (IMP) | Nucleotide | Purine Metabolism, (Hypo)Xanthine/Inosine containing | HMDB00175 | −2.59 |
| gamma-glutamylvaline | Peptide | Gamma-glutamyl Amino Acid | HMDB11172 | −2.58 |
| ergothioneine | Xenobiotics | Food Component/Plant | HMDB03045 | −2.49 |
| ribitol | Carbohydrate | Pentose Metabolism | HMDB00508 | −2.49 |
| glycerophosphoinositol | Lipid | Phospholipid Metabolism | −2.49 | |
| umbelliferone sulfate | Xenobiotics | Food Component/Plant | −2.43 | |
| pyrraline | Xenobiotics | Food Component/Plant | HMDB33143 | −2.4 |
| 4-acetylphenyl sulfate | Xenobiotics | Drug | −2.37 | |
| gamma-glutamylisoleucine | Peptide | Gamma-glutamyl Amino Acid | HMDB11170 | −2.36 |
| N-acetylproline | Amino Acid | Urea cycle; Arginine and Proline Metabolism | −2.34 | |
| 3-methoxycatechol sulfate (1) | Xenobiotics | Benzoate Metabolism | −2.32 | |
| 4-vinylphenol sulfate | Xenobiotics | Benzoate Metabolism | HMDB04072 | −2.29 |
| sphinganine | Lipid | Sphingolipid Metabolism | HMDB00269 | −2.29 |
| hydantoin-5-propionic acid | Amino Acid | Histidine Metabolism | HMDB01212 | −2.23 |
| trigonelline (N’-methylnicotinate) | Cofactors and Vitamins | Nicotinate and Nicotinamide Metabolism | HMDB00875 | −2.19 |
| O-methylcatechol sulfate | Xenobiotics | Benzoate Metabolism | HMDB60013 | −2.18 |
| 4-allylphenol sulfate | Xenobiotics | Food Component/Plant | −2.16 | |
| 2-aminophenol sulfate | Xenobiotics | Chemical | HMDB61116 | −2.14 |
| citrulline | Amino Acid | Urea cycle; Arginine and Proline Metabolism | HMDB00904 | −2.13 |
| uridine 3’-monophosphate (3’-UMP) | Nucleotide | Pyrimidine Metabolism, Uracil containing | −2.09 | |
| 3-methyl catechol sulfate (1) | Xenobiotics | Benzoate Metabolism | −2.09 | |
| 3-hydroxypyridine sulfate | Xenobiotics | Chemical | −2.06 | |
| isoursodeoxycholate | Lipid | Secondary Bile Acid Metabolism | HMDB00686 | −2.05 |
| propyl 4-hydroxybenzoate sulfate | Xenobiotics | Benzoate Metabolism | 2.18 | |
| 2’-O-methylcytidine | Nucleotide | Pyrimidine Metabolism, Cytidine containing | 2.33 | |
| methyl-4-hydroxybenzoate sulfate | Xenobiotics | Benzoate Metabolism | 2.37 |
The most strongly negative loading metabolites were glycodeoxycholate sulfate (loading: −9.1), stachydrine (−6.4), and N-methylproline (−6.3); the positive loading metabolites were 2-O-methylcytidine (2.3), and two metabolites involved in benzoate metabolism: methyl-4-hydrozybenzoate sulfate (2.4) and propyl 4-hydroxybenzoate sulfate (2.2). Half (n = 27) of the influential metabolites were xenobiotics; the next most represented subgroup was amino acids (n = 10).
2.5.2. Bayesian Network Model
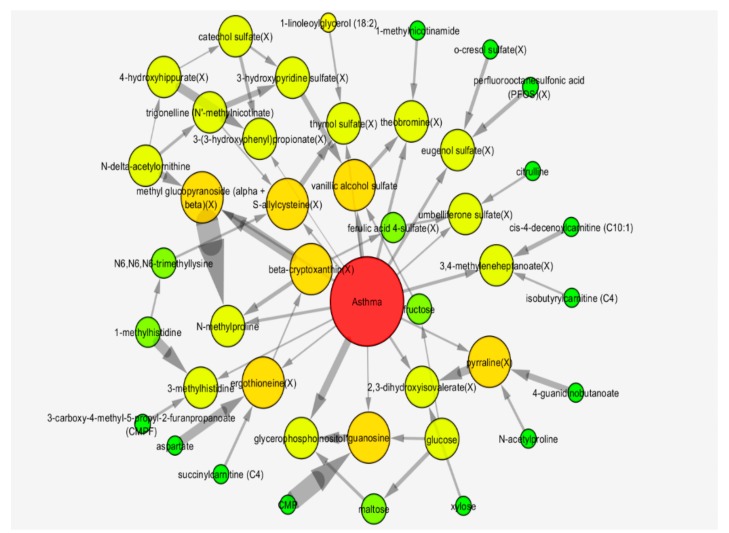
Forty-two metabolites were included in the Markov neighborhood of childhood asthma in the Bayesian network (Table 3, Figure 3).
Table 3.
Metabolites included in the Bayesian network.
| Metabolite | Super Pathway | Sub Pathway | HMDB ID |
|---|---|---|---|
| 1-linoleoylglycerol (18:2) | Lipid | Monoacylglycerol | |
| 1-methylhistidine | Amino Acid | Histidine Metabolism | HMDB00001 |
| 1-methylnicotinamide | Cofactors and Vitamins | Nicotinate and Nicotinamide Metabolism | HMDB00699 |
| 2,3-dihydroxyisovalerate (X) | Xenobiotics | Food Component/Plant | HMDB12141 |
| 3-(3-hydroxyphenyl)propionate (X) | Xenobiotics | Benzoate Metabolism | HMDB00375 |
| 3-carboxy-4-methyl-5-propyl-2-furanpropanoate (CMPF) | Lipid | Fatty Acid, Dicarboxylate | HMDB61112 |
| 3-hydroxypyridine sulfate (X) | Xenobiotics | Chemical | |
| 3-methylhistidine | Amino Acid | Histidine Metabolism | HMDB00479 |
| 3,4-methyleneheptanoate (X) | Xenobiotics | Food Component/Plant | |
| 4-guanidinobutanoate | Amino Acid | Guanidino and Acetamido Metabolism | HMDB03464 |
| 4-hydroxyhippurate (X) | Xenobiotics | Benzoate Metabolism | HMDB13678 |
| aspartate | Amino Acid | Alanine and Aspartate Metabolism | HMDB00191 |
| beta-cryptoxanthin (X) | Xenobiotics | Food Component/Plant | HMDB33844 |
| catechol sulfate (X) | Xenobiotics | Benzoate Metabolism | HMDB59724 |
| cis-4-decenoylcarnitine (C10:1) | Lipid | Fatty Acid Metabolism (Acyl Carnitine) | |
| citrulline | Amino Acid | Urea cycle; Arginine and Proline Metabolism | HMDB00904 |
| CMP | Nucleotide | Pyrimidine Metabolism, Cytidine containing | HMDB00095 |
| Ergothioneine (X) | Xenobiotics | Food Component/Plant | HMDB03045 |
| eugenol sulfate (X) | Xenobiotics | Food Component/Plant | |
| ferulic acid 4-sulfate (X) | Xenobiotics | Food Component/Plant | HMDB29200 |
| fructose | Carbohydrate | Fructose, Mannose and Galactose Metabolism | HMDB00660 |
| glucose | Carbohydrate | Glycolysis, Gluconeogenesis, and Pyruvate Metabolism | HMDB00122 |
| glycerophosphoinositol | Lipid | Phospholipid Metabolism | |
| guanosine | Nucleotide | Purine Metabolism, Guanine containing | HMDB00133 |
| isobutyrylcarnitine (C4) | Amino Acid | Leucine, Isoleucine and Valine Metabolism | HMDB00736 |
| maltose | Carbohydrate | Glycogen Metabolism | HMDB00163 |
| methyl glucopyranoside (alpha + beta) (X) | Xenobiotics | Food Component/Plant | |
| N-acetylproline | Amino Acid | Urea cycle; Arginine and Proline Metabolism | |
| N-delta-acetylornithine | Amino Acid | Urea cycle; Arginine and Proline Metabolism | |
| N-methylproline | Amino Acid | Urea cycle; Arginine and Proline Metabolism | |
| N6,N6,N6-trimethyllysine | Amino Acid | Lysine Metabolism | HMDB01325 |
| o-cresol sulfate (X) | Xenobiotics | Benzoate Metabolism | |
| perfluorooctanesulfonic acid (PFOS) (X) | Xenobiotics | Chemical | HMDB59586 |
| Pyrraline (X) | Xenobiotics | Food Component/Plant | HMDB33143 |
| S-allylcysteine (X) | Xenobiotics | Food Component/Plant | HMDB34323 |
| succinylcarnitine (C4) | Energy | TCA Cycle | HMDB61717 |
| Theobromine (X) | Xenobiotics | Xanthine Metabolism | HMDB02825 |
| thymol sulfate (X) | Xenobiotics | Food Component/Plant | HMDB01878 |
| trigonelline (N’-methylnicotinate) | Cofactors and Vitamins | Nicotinate and Nicotinamide Metabolism | HMDB00875 |
| umbelliferone sulfate (X) | Xenobiotics | Food Component/Plant | |
| vanillic alcohol sulfate | Amino Acid | Tyrosine Metabolism | |
| xylose | Carbohydrate | Pentose Metabolism | HMDB00098 |
Figure 3.
Markov neighborhood of metabolic Bayesian network for the identification of asthma at age three. Bayesian network of Year-3 asthma in VDAART. Pictured is the Markov neighborhood of the CGBN network predictive of asthma. Node size and color is proportional to degree. Directed edges represent statistical conditional dependence of the target node on the source node. Edge thickness is proportional to the statistical evidence for the edge (log Bayes Factor). Metabolite names are appended with “(X)” for those metabolites indicated as xenobiotic by Metabolon.
Again, xenobiotics (n = 18) and amino acids (n = 11) represented the majority of the included metabolites. In particular, these were exogenous food/plant components and metabolites of the urea cycle.
Figure 3 shows edges between metabolites with thickness proportional to the statistical evidence of that edge (in log Bayes factors; a Bayesian likelihood ratio test for presence of an edge compared with its absence). We found that the strongest edge was between methyl glucopyranoside (alpha + beta) and N-methylproline. Since this edge is much stronger than either of the edges between these metabolites and asthma, this indicates that these two metabolites together had a much stronger effect on asthma status than either separately. Other metabolites with strong direct relationships with asthma were glycerophosphoinositol, vanillic alcohol sulfate, theobromine and eugonal sulfate. There were an additional 12 metabolites with direct edges linking them to asthma status, which were comprised mainly of xenobiotic food or plant components. (Table S2).
Nineteen metabolites in the Bayesian network were also identified as being influential in the first PLS-DA component. These cross-over metabolites included nine xenobiotics, four amino acids, xylose, trigonelline, glycerophosphoinositol and cytidine monophosphate (CMP) (Table 4, Figure 4).
Table 4.
Metabolites that were identified as influential in the PLS-DA first component and were included in the Bayesian Network.
| Metabolite | Super Pathway | Sub Pathway | HMDB ID |
|---|---|---|---|
| vanillic alcohol sulfate | Amino Acid | Tyrosine Metabolism | |
| citrulline | Amino Acid | Urea cycle; Arginine and Proline Metabolism | HMDB00904 |
| N-acetylproline | Amino Acid | Urea cycle; Arginine and Proline Metabolism | |
| N-methylproline | Amino Acid | Urea cycle; Arginine and Proline Metabolism | |
| xylose | Carbohydrate | Pentose Metabolism | HMDB00098 |
| trigonelline (N’-methylnicotinate) | Cofactors and Vitamins | Nicotinate and Nicotinamide Metabolism | HMDB00875 |
| Glycerophosphoinositol | Lipid | Phospholipid Metabolism | |
| CMP | Nucleotide | Pyrimidine Metabolism, Cytidine containing | HMDB00095 |
| 3-(3-hydroxyphenyl)propionate | Xenobiotics | Benzoate Metabolism | HMDB00375 |
| catechol sulfate | Xenobiotics | Benzoate Metabolism | HMDB59724 |
| 2,3-dihydroxyisovalerate | Xenobiotics | Food Component/Plant | HMDB12141 |
| beta-cryptoxanthin | Xenobiotics | Food Component/Plant | HMDB33844 |
| ergothioneine | Xenobiotics | Food Component/Plant | HMDB03045 |
| ferulic acid 4-sulfate | Xenobiotics | Food Component/Plant | HMDB29200 |
| methyl glucopyranoside (alpha + beta) | Xenobiotics | Food Component/Plant | |
| pyrraline | Xenobiotics | Food Component/Plant | HMDB33143 |
| umbelliferone sulfate | Xenobiotics | Food Component/Plant | |
| theobromine | Xenobiotics | Xanthine Metabolism | HMDB02825 |
| 3-hydroxypyridine sulfate | Xenobiotics | Chemical |
Figure 4.
Super pathways of metabolites identified as constituent of the Bayesian network and as influential in the 1 component of the PLS-DA model, and those which are common to both methods.
2.6. Sensitivity Analyses
To explore the potential impact of race, which was significantly associated with asthma status in this population, we conducted several sensitivity analyses. First, the PLS-DA and the BN models were reconstructed while including race as a potential explanatory variable together with the 433 metabolites. In this analysis, race was determined to be important in both the PLS-DA model and the CGBN model. In the PLS-DA model, race had a higher loading even than the highest-loading metabolite, and in the BN it had a direct edge with asthma. Although race was important in both models, it had little impact on the predictive performance of either; when including race, the one-component PLS-DA model had an AUCCH of 81% on the full dataset, while for the BN the AUCCH was 93% on the full dataset (Figure S5). The models also identified very similar sets of metabolites, and were consistent in terms of loadings and edge weights, with glycodeoxycholate sulfate the highest loading metabolite (PLS-DA) and the relationship between methyl glucopyranoside (alpha + beta) and N-methylproline demonstrating the biggest influence on asthma risk (BN).
We next investigated the power of race as an explanatory variable for asthma status by building PLS-DA and CGBN models using only race, with no metabolites included. The PLS-DA model using 1 PLS component and only race had 67% AUCCH (95% CI: 64.7–69.3%), and the BN model with only race had 65.5% AUCCH (95% CI: 63.2–67.8%). These results show that race is a strong indicator of age-3 asthma status in VDAART, but far from the only important indicator, and does not display as strong predictive indices as the metabolite models.
As a third investigation of the role of race, we tested the main PLS-DA and CGBN models’ ability to predict race, rather than asthma status. To accomplish this, we used a binary race attribute (White vs. non-White), and found that our CGBN (without race included) predicted the binarized race attribute with reasonable accuracy: 70.2% AUCCH (95% CI: 66.2–74.1%). Our PLS-DA model (without race) had stronger accuracy on the binarized race attribute: 76.8% AUCCH (CI: 73.2–80.3%), indicating significant performance, but statistically worse (p < 0.05) than predicting actual age three asthma status. These results show that both the PLS-DA and CGBN models have identified more than just the non-White VDAART participants with asthma, and that race is not driving our findings.
3. Discussion
To date, no definitive metabolomic profile has been identified for asthma [6]. Although many studies, particularly those utilizing PLS-DA, report impressive diagnostic and predictive indices with AUCCHs, classification rates, sensitivities, specificities and R2 values in excess of 90%, very few if any of these findings have been replicated in truly independent populations, and none have been translated into clinical practice [6]. One of the underlying reasons for the lack of validation of predictive models is the issue of overfitting, whereby the model fits the data so well, that in addition to identifying real disease discriminators, the model explains random variability in the dataset that has no biological meaning. This is particularly common when the number of predictors far exceeds the number of subjects, as is often the case in metabolomics. While overfitting is a problem with any machine learning methodology, in this current study, we have tried to both minimize and assess the impact of overfitting on PLS-DA and CGBN models through permutation testing. Despite these efforts, our results show that both methods are powerful enough to overfit data, since the permutation test distributions are significantly greater than 50% AUCCH. These tests showed that the PLS-DA model was on the borderline of what might be possible purely through overfitting, and this is a danger that has been noted in existing literature [8,9,10,11]. The alternative approach using Bayesian network methodology appears to be more robust, significantly outperforming the empirical null distribution. With both models, the high-powered methods and capacity for overfitting mean that the observed accuracies in the present cohort likely overestimate what would be seen on an independent replication cohort.
In this study, we compared the ability of a PLS-DA-derived plasma metabolomic signature and a novel Bayesian network derived plasma metabolomic signature to identify asthma in children aged three. Both models demonstrated extremely high accuracy, with AUCCHs in excess of 0.8 on the full dataset; however, the Bayesian network significantly out-performed the one-component PLS-DA model both in terms of accuracy, and in the likelihood of overfitting. The Bayesian network’s ability to accommodate nonlinear interactions between metabolites and relate these to the phenotype could be responsible for the increased performance, while the ability to define Bayesian priors and the Bayes Factor threshold lead to relatively parsimonious models which may explain why Bayesian networks are less susceptible to overfitting than PLS-DA.
These models reflect one of the greatest strengths of metabolomic profiling: the potential to identify biomarkers that inform on underlying etiology and pathological mechanisms. Both the PLS-DA and the BN models were enriched for metabolites and metabolic pathways with biologically interesting relationships with asthma, airway function, or other respiratory disease. Nineteen metabolites were common to the two models. This provides strong evidence for their role in asthma pathogenesis, although it must be noted, by definition, the BN identifies networks of metabolites working together which may not have a linear relationship with asthma. The fact that not all the BN metabolites are identified by the PLS-DA model should not be interpreted to mean their biological associations are not real. Common metabolites included citrulline, which has been shown to be produced at significantly higher levels from the neutrophils of asthmatics compared to non-asthmatics, with a dose-response relationship with disease severity [21]; catechol sulfate, reported to be higher in the plasma of asthmatic cases [22]; and beta-cryptoxanthin, which is inversely associated with the risk of asthma when measured in whole blood [23]. Similarly, metabolites of the gut microbiota, which have been increasingly shown to play an important role in the pathogenesis of asthma, such as 3-(3-hydroxyphenyl)propionate and 2,3-dihydroxyisovalerate ferulic acid 4-sulfate [24,25], were also identified as both influential for the PLS-DA one-component model, and as constituents of the BN. Furthermore, several of the metabolites from both the PLS-DA and BN models are involved in histidine metabolism. Histamine, a downstream product of this biological pathway, is crucial for the processes of inflammation, and has been shown to be elevated in the serum of asthmatics [26]; likely due to its role in bronchospasm and edema [27].
Both models were dominated by xenobiotics, which may not be surprising given that asthma is known to arise from complex gene-environment interactions and to be exacerbated by exogenous factors. There were also a large number of exogenous metabolites acquired through the diet. These include theobromine, a xanthine alkaloid that is known to have bronchodilator effects [28], ferulic acid 4-sulfate, a coffee metabolite that ameliorates airway inflammation in mice [29], and eugenol sulfate, which is commonly used in flavorings, but which has also been shown to have anti-asthmatic effects in mouse models [30].
In addition to its increased performance, the BN has the advantage of describing visually whether the constituent metabolites have direct or joint influences on asthma, and whether they may be mediated by or interacting with other metabolites within the network. This is because of BN’s ability to identify non-linear interactions; consequently, it can identify groups of metabolites working in combination to influence asthma risk that PLS-DA cannot. For example, two of the BN metabolites were aspartate and citrulline, which react to form argininosuccinate in the urea cycle. Aspartate is also involved in the nicotinate and nicotinamide metabolism pathway which is essential for the generation of coenzymes that act as precursors of redox reactions. Metabolites of this pathway, including nicotinamide are markers of inflammation and have been shown to be at higher levels in the plasma of asthmatics [22]. This is thought to be related to increased production of NAD, which would also likely lead to the altered levels of methylnicotinamide and trigonelline observed within the BN. Similarly, arginine is produced from citrulline, and it has been shown that when arginine bioavailability is low, nitric oxide (NO) production by nitric oxide synthase (for which arginine is the only substrate) is reduced. NO affects both airway tone and inflammation and has been used as a biomarker of asthma [31]. The synthesis of NO additionally produces more citrulline which can be used for recycling back to arginine. Arginine uptake can be inhibited by l-ornithine and l-lysine, and metabolites involved in the pathways that regulate levels of these metabolites, N-delta-acetylornithine and N6,N6,N6-trimethyllysine, were also identified within the BN.
In their excellent review on multivariate classification techniques in metabolomics data, Trainor et al. [11] discuss the trade-off between model interpretability versus model accuracy. However, we counter that by its nature, and as the “ome” closest to phenotype, metabolomics has the potential to do both. A growing number of classification techniques are now being applied to metabolomic data [8,11,32,33]. These include, but are not limited to, Support Vector Machine (SVM) learning models and Artificial Neural Networks (ANN), which both allow the modelling of non-linear interactions; however, these are both notoriously difficult models to interpret [33]. Similarly, Random Forests, a decision tree-based method has demonstrated high accuracy, but with somewhat limited biological interpretability [8]. Clustering approaches such as K-Nearest Neighbor, hierarchical clustering and self-organizing maps have all also been applied [32]. Furthermore, extensions to PLS-DA have been proposed, including Orthogonal PLS (OPLS), which removes the variation in the dataset unrelated to the outcome (the orthogonal variance) [34], as well as multilevel partial least squares discriminant analysis and multiblock partial least squares, which aim to improve the interpretability of the models [8,9]. On balance, Trainor et al. conclude when applied to real metabolomics data, if prediction is the goal then SVM and Random Forests perform best [11]. However, we contend that BN also represent a compelling alternative allowing for strong prediction while enabling biological interpretation and visualization of the predictors and their relationships with both each other and the outcome.
However, there were limitations to this study. In common with the majority of metabolomics studies to date, we were limited in our ability to fully explore the biology underlying these metabolomic profiles. Pathway enrichment analyses are increasing in popularity among metabolomics studies, yet metabolomics is currently less amenable to such analyses than other omic technologies such as genetics. Several excellent reviews have discussed the reasons for this in detail, and compared the currently available tools, concluding that the incompleteness of the current underlying databases and the lack of available tissue specific pathway information render their utility limited [35,36,37]. The full biological meaning of our results can only be explored with further development of metabolomics databases and enrichment tools. Given that some of the children may have been on medication for their asthma, it is possible these profiles may be driven by therapeutic regime, although we identified only one drug metabolite within our models, 4-acetylphenyl sulfate (PLS-DA model), which is not associated with asthma management. We also explored the possibility that these profiles were being driven by race. The similar results obtained when race was included as a covariate in the model suggest this is not the case, as does the greater difference in the CGBN model’s prediction of race (70.2% AUCCH) vs. the model’s prediction of asthma status (92.1% AUCCH). Finally, although we use cross-validation to identify parameters least likely to lead to over-fit models, and we show with permutation testing that the Bayesian network, and to a lesser extent the PLS-DA model, are accurate in excess of what is due to overfitting, true validation can only be achieved by replication in an independent population. In future work, it would be of interest to investigate alternative regularization methods that may provide a more complex PLS model with a smaller, but greater than one, number of components. These represent the next steps for this study.
4. Materials and Methods
4.1. The VDAART Clinical Trial: Study Participants
VDAART is a randomized double-blinded, placebo-controlled trial with centers in Boston, San Diego and St Louis, that aimed to investigate the effect of vitamin D supplementation in pregnant women on the incidence of asthma in their offspring [16]. VDAART recruited non-smoking pregnant women between 10 and 18 weeks of gestation who reported a history of asthma, eczema, or allergic rhinitis, or who had conceived the child with a man with a history of such diseases. For the remainder of their pregnancy, women were randomized 1:1 to a daily dose of 4000 IU vitamin D3 plus a multivitamin containing 400 IU vitamin D3, or a matching placebo tablet plus a multivitamin containing 400 IU vitamin D3. The women were followed monthly throughout their pregnancy; their offspring were then followed via telephone interviews every three months and by in-person clinic visits at age 1, 2 and 3 years. A child with a physician-diagnosis of asthma at any time during the three years of follow up was considered an asthma case. VDAART was approved by the Institutional Review Boards (IRB) of the participating Clinical Centers and the Data Coordinating Center, with pregnant women signing informed consent at the enrollment visit covering both primary and secondary analyses of data.
4.2. Plasma Metabolomic Profiling
Metabolomic profiling was performed on blood plasma samples extracted during the age-three visit for all children defined by a physician as asthma cases (n = 59), and a subset of children from the same population who did not have a physician-diagnosis of asthma during follow-up (n = 352). All 411 samples were stored at −80 °C until processing. Non-targeted global metabolomic profiling was conducted at Metabolon, Inc. (Durham, NC, USA), as described previously [38,39], using four ultra-performance liquid chromatography-tandem mass spectroscopy (UPLC-MS/MS) profiling platforms: (1) UPLC-MS/MS under positive ionization; (2) UPLC-MS/MS under negative ionization; (3) UPLC-MS/MS, polar platform (negative ionization); and (4) GC-MS. Metabolites were identified by their mass-to-charge ratio (m/z), retention time (rt), and through a comparison to a library of purified known standards. Data were processed in two batches sent six months apart (batch one n = 245; batch two n = 166) then scaled and merged together based on equivalence of the control groups, as follows. The second batch’s controls were scaled to the first using scaling factors making the median of the control groups in each batch equivalent. If a metabolite had a missingness of 50% or greater in either batch it was excluded from further analysis. Metabolite intensities were log transformed and those metabolites with an interquartile range of zero or with a skewness >2 or <−2 were excluded from further analyses to remove metabolites with extreme or uninformative distributions, which may bias the results.
4.3. Statistical Analysis
Data were bootstrap-balanced by randomly resampling the cases to obtain an equal number of cases and controls.
We used the balanced data in subsequent analyses to assure learning of an unbiased classifier. Two different methods were then utilized to build predictive models that could distinguish asthma cases from controls based on their plasma metabolomic profiles at age three: PLS-DA and Bayesian Networks. Area under the Convex Hull of the Receiver Operator Characteristic Curves (AUCCH) was used to assess predictive performance of all models, since all points on the convex hull represent realizable classification performance [40]. The difference between the AUCCHs from the PLS-DA model and the BN model were compared using the method of DeLong et al [41], following suggestions by Lasko et al. [42]. Details of each analysis are presented below.
To identify the best parameter settings for each of the classification algorithms (PLS-DA and Bayesian Networks), two strategies were employed: (1) a training (67%) and testing (33%) split of the data, and (2) five-fold cross-validation. The first was used to identify parameters of PLS-DA and Bayesian Networks that might lead to greater performance on an entirely new (replication) dataset. The second was used as a principled way to make multiple training/testing splits of the data, to limit reliance on any one particular random split of the data while still identifying parameters leading to stronger performance. Cross-validation was performed on the original 411 subjects, randomly apportioning the cases and controls separately into 5 groups, and then performing bootstrap-balancing on each fold. The parameters resulting in the best cross-validation AUCCH metrics for each strategy were then used to build PLS-DA and Bayesian network models, respectively, on the complete bootstrap-balanced dataset. The models were assessed for possible overfitting by a permutation testing, using label-shuffling with 1000 realizations. Permutation of labels was performed with the original 411 subjects, which then included new bootstrap-balancing with each permutation.
4.3.1. Partial Least Squares Discriminant Analysis
PLS-DA is a supervised approach that aims to differentiate between classes (Y) in highly complex data sets, despite within-class variability in the observed variables (X). It determines the relationship between the two matrices (X and Y), by modelling their covariance structure. It finds the multidimensional direction in the X space (i.e., the metabolomic data) that explains the maximum multidimensional variance direction in the Y space (asthma status). The new subspace in X is-based upon a reduced number of factors (i.e., latent factors or components) [10,43]. The loading score provides a measure of the importance of an individual metabolite to these components. For each component of the PLS-DA models, we used a loading threshold of >2 or <−2 to identify the important contributors to that component. This is a threshold that has been shown to be a robust method of variable selection [44]. Metabolites with a loading for a component of >2 or <−2 were considered to be influential and were taken forward for further analysis. PLS-DA was performed using MATLAB version R2016a (Natick, MA, USA), with the plsregress function, which implements the SIMPLS algorithm [45]. Default parameter settings were used in all cases.
4.3.2. Bayesian Network Analysis
To precisely model interactions between continuous-valued metabolites, a type of Bayesian network known as a conditional Gaussian Bayesian network (CGBN) (see Section 2.3) [12] was learned using the CGBayesNets [20] package in MATLAB version R2016a (MATLAB, The Mathworks Inc.; CGBayesNets, www.cgbayesnets.com). We used default parameters and algorithms to build a metabolite network predictive of asthma status at age three years in VDAART. We used the default K2 learning algorithm [46] in the CGBayesNet package throughout, which prioritizes Bayesian network (BN) edges by likelihood of association to the phenotype, and then limits potential edges to those from nodes with higher priority to those with lower priority [46]. We used the default Bayesian prior parameters (nu = 10, alpha = 10, sigma2 = 1), which control the strength of the Bayesian prior probability in each Bayesian likelihood test and default network parameter (max parents = 2), which sets a limit on the number of incoming edges to each node in the final Bayesian network Following our approach to the PLS-DA modeling, we used both (1) a hold-out set and (2) five-fold cross-validation to identify the optimal threshold for inclusion of edges in the network, measured in Bayes Factors (BF) [13]. Results from the hold-out data set were inconclusive, and therefore we focused on the cross-validation step for the Bayesian network analysis. Five-fold cross-validation was performed keeping parameter settings fixed except for varying the BF threshold from 2 to 30 by increments of 1. The cross-validation AUCCH was measured at each BF threshold. This identified the optimal BF threshold to build a CGBN on the whole dataset. The Markov Property of Bayesian networks states that only the metabolites in the Markov Neighborhood of age-three asthma are necessary to predict asthma status; these are those nodes that are parents, children, or other parents of the children of the age three asthma node. All metabolites in the Markov neighborhood, i.e., the parents, children, and other parents of those children of age-three asthma, were taken forward for further analysis.
4.3.3. Permutation Tests to Assess Likelihood of Overfitting
The parameters resulting in the best cross-validation AUCCH metrics for each strategy were then used to build PLS-DA and Bayesian Network models, respectively, on the bootstrap-balanced dataset. The models were assessed for possible overfitting by permutation testing, using label-shuffling with 1000 realizations. Permutation of labels was performed with the original 411 subjects, which then included new bootstrap-balancing with each permutation.
5. Conclusions
In conclusion, at a time when we are still determining the optimal methodologies with which to explore metabolomic datasets, this study demonstrates the potential for Bayesian network approaches to produce robust and biologically meaningful results. This method demonstrated superior performance to PLS-DA; an approach that is commonly used in metabolomics, but has rarely identified validated profiles. This suggests BN approaches may be beneficial in the study of metabolomic datasets of other complex and chronic diseases. We present a discriminatory network for asthma in children that is characterized by several exogenous metabolites, particularly those originating from the diet, as well as dysregulation of arginine metabolism. The primacy of exogenous metabolites in our models indicates that altered metabolism of common food components is a potentially distinguishing feature of asthma.
Acknowledgments
We wish to thank all mothers and children who were involved in the VDAART study, and all our VDAART collaborators.
Supplementary Materials
The following are available online at http://www.mdpi.com/2218-1989/8/4/68/s1, Table S1: Super Pathway and Sub Pathway of 433 Metabolites included in the analyses, Table S2: Edge Weights between a pair of nodes in the BN, Table S3: Metabolites that were identified as influential in the PLS-DA first component and were included in the Bayesian Network, Figure S1: Hold-out testing of PLS-DA and BN models, Figure S2: Permutation test of 1 PLS-DA Component, Figure S3: Cross-validation of Bayesian Network and PLS-DA for Asthma in VDAART, Figure S4: Permutation test of Bayesian Network, Figure S5 VDAART Metabolite Asthma Prediction by BN (AUCCH = 0.926) and PLS-DA (AUCCH = 0.803) including race on the full dataset.
Author Contributions
Conceptualization, M.J.M., R.S.K., J.L.-S.; Methodology, M.J.M., R.S.K., J.L.-S., S.T.W., A.A.L.; Validation, S.H.C.; Formal Analysis, M.J.M., R.S.K.; Resources, J.L.-S., S.T.W., A.A.L.; Data Curation, R.S.K., K.A.L.-S.; Writing-Original Draft Preparation M.J.M., R.S.K. and J.L.-S. Writing-Review & Editing, R.S.K., M.J.M., K.A.L.-S., P.K., S.H.C., Y.V.V., M.H.; Funding Acquisition, J.L.-S., S.T.W., A.A.L.
Funding
VDAART was supported by U01HL091528, 1R01HL123915-01 and 1R01HL123546-01A1 from the National Heart, Lung, and Blood Institute (NHLBI); and U54TR001012 from the National Centers for Advancing Translational Sciences. Metabolomic profiling and additional analyses were supported by 5R01HL123915-05, W81XWH-17-1-0533 and 1R01HL141826-0. M.J.M. is funded by R01 HL139634. K.A.L.-S. is funded by 5T32AI007306-30.
Conflicts of Interest
J.L.-S. is a consultant for Metabolon. The other authors have no conflicts of interest to declare.
References
- 1.Eder W., Ege M.J., von Mutius E. The Asthma Epidemic. N. Engl. J. Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 2.Subbarao P., Mandhane P.J., Sears M.R. Asthma: Epidemiology, etiology and risk factors. Can. Med. Assoc. J. 2009;181:181–190. doi: 10.1503/cmaj.080612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mims J.W. Asthma: Definitions and pathophysiology. Int. Forum Allergy Rhinol. 2015;5:2–6. doi: 10.1002/alr.21609. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson J.K., Wilson I.D. Opinion: Understanding ‘global’ systems biology: Metabonomics and the continuum of metabolism. Nat. Rev. Drug Discov. 2003;2:668–676. doi: 10.1038/nrd1157. [DOI] [PubMed] [Google Scholar]
- 5.Johnson C.H., Ivanisevic J., Siuzdak G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016;17:451–459. doi: 10.1038/nrm.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly R.S., Dahlin A., McGeachie M.J., Qiu W., Sordillo J., Wan E.S., Wu A.C., Lasky-Su J. Asthma metabolomics and the potential for integrative omics in research and the clinic. Chest. 2017;151:262–277. doi: 10.1016/j.chest.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garthwaite P.H. An Interpretation of Partial Least Squares. J. Am. Stat. Assoc. 1994;89:122–127. doi: 10.1080/01621459.1994.10476452. [DOI] [Google Scholar]
- 8.Gromski P.S., Muhamadali H., Ellis D.I., Xu Y., Correa E., Turner M.L., Goodacre R. A tutorial review: Metabolomics and partial least squares-discriminant analysis—A marriage of convenience or a shotgun wedding. Anal. Chim. Acta. 2015;879:10–23. doi: 10.1016/j.aca.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Westerhuis J.A., Hoefsloot H.C.J., Smit S., Vis D.J., Smilde A.K., van Velzen E.J.J., van Duijnhoven J.P.M., van Dorsten F.A. Assessment of PLSDA cross validation. Metabolomics. 2008;4:81–89. doi: 10.1007/s11306-007-0099-6. [DOI] [Google Scholar]
- 10.Worley B., Powers R. Multivariate Analysis in Metabolomics. Curr. Metabolomics. 2013;1:92–107. doi: 10.2174/2213235X11301010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trainor P.J., DeFilippis A.P., Rai S.N. Evaluation of Classifier Performance for Multiclass Phenotype Discrimination in Untargeted Metabolomics. Metabolites. 2017;7:e30. doi: 10.3390/metabo7020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heckerman D., Gieger D. Uncertainty in Artificial Intelligence. Morgan Kaufmann; Burlington, MA, USA: 1995. Learning Bayesian Networks: A unification for discrete and Gaussian domains. [Google Scholar]
- 13.Kass R.E., Raftery A.E. Bayes Factors. J. Am. Stat. Assoc. 1995;90:773–795. doi: 10.1080/01621459.1995.10476572. [DOI] [Google Scholar]
- 14.McGeachie M.J., Dahlin A., Qiu W., Croteau-Chonka D.C., Savage J., Wu A.C., Wan E.S., Sordillo J.E., Al-Garawi A., Martinez F.D., et al. The metabolomics of asthma control: A promising link between genetics and disease. Immun. Inflamm. Dis. 2015;3:224–238. doi: 10.1002/iid3.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers A.J., McGeachie M., Baron R.M., Gazourian L., Haspel J.A., Nakahira K., Fredenburgh L.E., Hunninghake G.M., Raby B.A., Matthay M.A., et al. Metabolomic derangements are associated with mortality in critically ill adult patients. PLoS ONE. 2014;9:e87538. doi: 10.1371/journal.pone.0087538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Litonjua A.A., Lange N.E., Carey V.J., Brown S., Laranjo N., O’Connor G.T., Sandel M., Strunk R.C., Bacharier L.B., Zeiger R.S., et al. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): Rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemp. Clin. Trials. 2014;38:37–50. doi: 10.1016/j.cct.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Y., Wong A.K.C., Kamel M.S. Classification of imbalanced data: A review. Int. J. Pattern Recognit. Artif. Intell. 2009;23:687–719. doi: 10.1142/S0218001409007326. [DOI] [Google Scholar]
- 18.Branco P., Torgo L., Ribeiro R.P. A Survey of Predictive Modeling on Imbalanced Domains. ACM Comput. Surv. 2016;49:1–50. doi: 10.1145/2907070. [DOI] [Google Scholar]
- 19.Sebastiani P., Abad M., Ramoni M.F. Bayesian Networks for Genomic Analysis. In: Dougherty E.R., Shmulevich I., Chen J., Wang Z.J., editors. Genomic Signal Processing and Statistics. Volume 2. Hindawi Publishing Corporation; New York, NY, USA: 2005. pp. 281–320. [Google Scholar]
- 20.McGeachie M.J., Chang H.H., Weiss S.T. CGBayesNets: Conditional Gaussian Bayesian network learning and inference with mixed discrete and continuous data. PLoS Comput. Biol. 2014;10:e1003676. doi: 10.1371/journal.pcbi.1003676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramesh G., Jindal S.K., Ganguly N.K., Dhawan V. Increased nitric oxide production by neutrophils in bronchial asthma. Eur. Respir. J. 2001;17:868–871. doi: 10.1183/09031936.01.17508680. [DOI] [PubMed] [Google Scholar]
- 22.Comhair S.A.A., McDunn J., Bennett C., Fettig J., Erzurum S.C., Kalhan S.C. Metabolomic Endotype of Asthma. J. Immunol. 2015;195:643–650. doi: 10.4049/jimmunol.1500736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood L.G., Garg M.L., Blake R.J., Garcia-Caraballo S., Gibson P.G. Airway and circulating levels of carotenoids in asthma and healthy controls. J. Am. Coll. Nutr. 2005;24:448–455. doi: 10.1080/07315724.2005.10719490. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y.J., Boushey H.A. The Microbiome in Asthma. J. Allergy Clin. Immunol. 2015;135:25–30. doi: 10.1016/j.jaci.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Rymenant E., Abrankó L., Tumova S., Grootaert C., van Camp J., Williamson G., Kerimi A. Chronic exposure to short-chain fatty acids modulates transport and metabolism of microbiome-derived phenolics in human intestinal cells. J. Nutr. Biochem. 2017;39:156–168. doi: 10.1016/j.jnutbio.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung J., Kim S.H., Lee H.S., Choi G.S., Jung Y.S., Ryu D.H., Park H.S., Hwang G.S. Serum metabolomics reveals pathways and biomarkers associated with asthma pathogenesis. Clin. Exp. Allergy. 2013;43:425–433. doi: 10.1111/cea.12089. [DOI] [PubMed] [Google Scholar]
- 27.White M.V. The role of histamine in allergic diseases. J. Allergy Clin. Immunol. 1990;86:599–605. doi: 10.1016/S0091-6749(05)80223-4. [DOI] [PubMed] [Google Scholar]
- 28.Simons F.E., Becker A.B., Simons K.J., Gillespie C.A. The bronchodilator effect and pharmacokinetics of theobromine in young patients with asthma. J. Allergy Clin. Immunol. 1985;76:703–707. doi: 10.1016/0091-6749(85)90674-8. [DOI] [PubMed] [Google Scholar]
- 29.Lee C.C., Wang C.C., Huang H.M., Lin C.L., Leu S.J., Lee Y.L. Ferulic Acid Induces Th1 Responses by Modulating the Function of Dendritic Cells and Ameliorates Th2-Mediated Allergic Airway Inflammation in Mice. Evid.-Based Complement. Altern. Med. 2015;2015:678487. doi: 10.1155/2015/678487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan C., Dong Z. Antiasthmatic Effects of Eugenol in a Mouse Model of Allergic Asthma by Regulation of Vitamin D3 Upregulated Protein 1/NF-κB Pathway. Inflammation. 2015;38:1385–1393. doi: 10.1007/s10753-015-0110-8. [DOI] [PubMed] [Google Scholar]
- 31.Ricciardolo F.L., Sorbello V., Ciprandi G. FeNO as biomarker for asthma phenotyping and management. Allergy Asthma. Proc. 2015;36:e1–8. doi: 10.2500/aap.2015.36.3805. [DOI] [PubMed] [Google Scholar]
- 32.Bartel J., Krumsiek J., Theis F.J. Statistical methods for the analysis of high-throughput metabolomics data. Comput. Struct. Biotechnol. J. 2013;4:e201301009. doi: 10.5936/csbj.201301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riekeberg E., Powers R. New frontiers in metabolomics: From measurement to insight. F1000Res. 2017;6:1148. doi: 10.12688/f1000research.11495.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wold J.T.S. Orthogonal projections to latent structures (O-PLS) J. Chemometr. 2002;16:119–128. [Google Scholar]
- 35.Marco-Ramell A., Palau-Rodriguez M., Alay A., Tulipani S., Urpi-Sarda M., Sanchez-Pla A., Andres-Lacueva C. Evaluation and comparison of bioinformatic tools for the enrichment analysis of metabolomics data. BMC Bioinformatics. 2018;19:1. doi: 10.1186/s12859-017-2006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosato A., Tenori L., Cascante M., de Atauri Carulla P.R., Martins dos Santos V.A.P., Saccenti E. From correlation to causation: Analysis of metabolomics data using systems biology approaches. Metabolomics. 2018;14:37. doi: 10.1007/s11306-018-1335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barupal D.K., Fan S., Fiehn O. Integrating bioinformatics approaches for a comprehensive interpretation of metabolomics datasets. Curr. Opin. Biotechnol. 2018;54:1–9. doi: 10.1016/j.copbio.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blighe K., Chawes B.L., Kelly R.S., Mirzakhani H., McGeachie M., Litonjua A.A., Weiss S.T., Lasky-Su J.A. Vitamin D prenatal programming of childhood metabolomics profiles at age 3 y. Am. J. Clin. Nutr. 2017;106:1092–1099. doi: 10.3945/ajcn.117.158220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly R.S., Sordillo J.E., Lasky-Su J., Dahlin A., Perng W., Rifas-Shiman S.L., Weiss S.T., Gold D.R., Litonjua A.A., Hivert M.F., et al. Plasma metabolite profiles in children with current asthma. Clin. Exp. Allergy. 2018;48:1297–1304. doi: 10.1111/cea.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Provost F., Fawcett T. Robust Classification for imprecise environments. Mach. Learn. 2001;44:203–231. doi: 10.1023/A:1007601015854. [DOI] [Google Scholar]
- 41.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 42.Lasko T.A., Bhagwat J.G., Zou K.H., Ohno-Machado L. The use of receiver operating characteristic curves in biomedical informatics. J. Biomed. Inform. 2005;38:404–415. doi: 10.1016/j.jbi.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 43.Barker M., Rayens W. Partial least squares for discrimination. J. Chemometr. 2003;17:166–173. doi: 10.1002/cem.785. [DOI] [Google Scholar]
- 44.Mehmood T., Martens H., Sæbø S., Warringer J., Snipen L. A Partial Least Squares based algorithm for parsimonious variable selection. Algorithms Mol. Biol. 2011;6:27. doi: 10.1186/1748-7188-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Jong S. SIMPLS: An Alternative Approach to Partial Least Squares Regression. Chemometr. Intell. Lab. 1993;18:251–263. doi: 10.1016/0169-7439(93)85002-X. [DOI] [Google Scholar]
- 46.Cooper G.F., Herskovits E. A Bayesian method for the induction of probabilistic networks from data. Mach. Learn. 1992;9:309–347. doi: 10.1007/BF00994110. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.