Abstract
Despite its substantial clinical importance, specific genetic variants associated with depression have not yet been identified. We sought to identify genetic variants associated with depression by (a) focusing on a more homogenous subsample (vascular depression) and (b) applying a three-stage approach. First, we contacted 730 participants with a confirmed atherosclerotic disease (coronary artery disease) from a population-based study population (German Myocardial Infarction Family Study IV) for psychiatric assessment with the Mini International Neuropsychiatric Interview. Second, we genotyped these patients using genome-wide single nucleotide polymorphism (SNP) arrays. Third, we characterized the SNP via in-silico analysis. The final sample consisted of 342 patients (78.3% male, age = 63.2 ± 9.9 years), 22.8% with a severe depressive disorder. Variant rs528732638 on chromosome 18q11.2 was a genome-wide significant variant and was associated with 3.6-fold increase in the odds of lifetime depression. The locus belongs to a linkage disequilibrium block showing expression quantitative trait loci effects on three putative cis-regulated genes, including the aquaporin 4 (AQP4) locus. AQP4 is already known to mediate the formation of ischemic edema in the brain and heart, increasing the size and extent of resulting lesions. Our findings indicate that AQP4 may also play a role in the etiopathology of vascular depression.
Keywords: genome-wide association study, coronary artery disease, late-onset depression, vascular depression, aquaporin, AQP4, chromosome 18q11.2
1. Introduction
Unipolar depression is a common disorder, with 150 million men and women affected worldwide. With 65.5 million disability-adjusted life years (DALYs) worldwide, it is also one of the leading causes of loss of healthy life years [1]. Regarding the etiology, a bio-psycho-social model is assumed, with an estimated heritability of 35% [2]. However, no major gene locus has been consistently shown to be associated with unipolar depression [3,4,5,6], which may be attributable to a β error due to sample heterogeneity. Thus, research focusing on a more homogenous subsample holds promise, such as analyzing subjects with vascular depression. The vascular depression concept evolved at the end of the last century based on consistent differences between unipolar depression manifesting before or after the age of 50 (early onset depression (EOD) or late onset depression (LOD)) [7,8]. Regarding the clinical presentation, patients with LOD tend to exhibit more substantial psychomotor retardation and executive dysfunction but a less depressed affect than patients with EOD [9,10] and tend to respond poorly to treatment [11,12].
The term “vascular depression” places cerebrovascular lesions at the heart of the etiopathology of LOD. This assumption is based on morphological, functional, and epidemiological data: patients with LOD show greater carotid pathology [13] and more cerebrovascular lesions (such as white matter hyperintensities, silent brain infarctions, and microbleeds) [14,15] than patients with EOD. Additionally, cerebrovascular lesions are associated with a higher prevalence of depressive episodes [16]. The relevance of these cerebrovascular lesions is supported by functional imaging studies showing altered connectivity in patients with LOD, which led to the disconnectivity hypothesis of vascular depression [10,17,18,19]. Furthermore, epidemiological studies link unipolar depression with vascular risk factors [20] and with vascular diseases such as dementia, stroke, myocardial infarction and coronary artery disease (CAD) [21,22,23]. The prevalence of vascular depression in the elderly is estimated to range from 2.4 to 3.4% [24,25]. Regarding the genetics of vascular depression, the only study published to date failed to identify an association with the SLC6A4 locus of the serotonin transporter gene [26]. Therefore, we conducted a genome-wide association study (GWAS) of severe depressive disorders in patients with a confirmed atherosclerotic disorder, namely coronary artery disease.
2. Materials and Methods
2.1. Study Protocol and Study Subjects
The study protocol was approved by the Ethics Committee of the University of Lübeck (ID 04/041) and is depicted in Figure 1. Regarding our study population, we chose to study patients with CAD which correlates highly with cerebrovascular disease [27,28] and thus constitutes an acceptable surrogate marker. When admitted to the University Hospital of Lübeck, Germany, for cardiac catheterization between February 2004 and December 2012, 730 patients with coronary heart disease (154 females, 576 males; mean age = 59.5 years; standard deviation (SD) = 9.8 years; previously described as the German Myocardial Infarction Family Study IV [29]), provided fully informed consent to the use of their data and genetic material for research purposes and to be contacted for further research purposes, in accordance with the Declaration of Helsinki. Of these individuals, 342 patients agreed to participate in our telephone survey when contacted by phone several years later (median = 1.8 years), between March 2013 and January 2015. Exclusion criteria were a lack of informed consent, an inability to participate in a telephone survey (due to cognitive deficits, hearing impairment, aphasia, or insufficient language skills) and the exclusion of CAD during cardiac catheterization.
Figure 1.
Graphical depiction of the stepwise study design. GerMIFS: German Myocardial Infarction Study; CAD: coronary artery disease; M.I.N.I.: Mini International Neuropsychiatric Interview; severe depressive disorder: severe Major Depressive Episode and/or Dysthymia; GWA: genome-wide association; chr: chromosome; SNP: single nucleotide polymorphism.
2.2. Assessment of Depression
To further reduce the risk of a β error, we focused on severe depressive disorders (Major Depressive Episode and Dysthymia) which were diagnosed during the telephone survey using the Mini International Neuropsychiatric Interview (M.I.N.I. 5.0.0) [30]. The M.I.N.I. is a short, structured diagnostic interview for mental disorders according to Diagnostic and Statistical Manual of Mental Disorders (DSM) IV with good psychometric qualities. As the timing of the interview was arbitrary, current severe depressive disorders were not differentiated from previous, currently remitted disorders. A detailed report on the psychiatric morbidity found in our sample has been published elsewhere [31].
2.3. DNA Isolation and Genotyping
DNA was isolated at the Institute for Cardiogenetics at the University of Lübeck according to standard protocols. Genotyping was performed on a Affymetrix Genome-Wide Human SNP Array 6.0 (Thermo Fisher Scientific, Santa Clara, CA 95051, USA) in cooperation with the Helmholtz-Zentrum in Munich, Germany.
2.4. Pre/Post-Processing and Test of Genome-Wide Associations
In our analysis, we used variants that were previously imputed into the 1000 Genomes Phase 3 reference panel. Variants with poor imputation quality (i.e., info < 0.8), a minor allele frequency (MAF) < 0.05 and failing the Hardy–Weinberg-Equilibrium (p < 10−4) were filtered out. We defined cases as patients with CAD who were diagnosed with severe depressive disorder in the interview (regardless of remission status) and controls as patients with CAD for whom a lifetime diagnosis of severe depressive disorder had been excluded in the interview. Associations were tested with SNPTEST (Department of Statistics, University of Oxford, Oxford, UK) using the additive model [32]. Subsequently, variants with an association level of p < 10−5 were clustered into independent loci. Loci with at least two variants displaying p < 10−5 were retained.
2.5. Functional Annotation and Software
Statistical analyses of demographic and clinical characteristics were performed using the Software Package for the Social Sciences (SPSS) (IBM, Armonk, NY, USA). We annotated variants that passed our pre-selected filtering criteria using the Genehopper database (DB) [33]. The Genehopper DB is a relational database that integrates genetic and biomedical data from many public resources. Variants were annotated based on linkage disequilibrium (LD) information [34], expression quantitative trait loci (eQTL) mappings [35,36,37], and topologically associated domain boundaries (TADs) [38]. Figure 1 was created using Powerpoint (Microsoft, Redmond, WA, USA), Figure 2 using the programming language R and Figure 3 with Locuszoom (Department of Biostatistics, University of Michigan, Ann Arbor, MI, USA).
Figure 2.
The Manhattan plot shows association levels of severe depressive disorders in patients with coronary artery disease on the autosomal chromosomes.
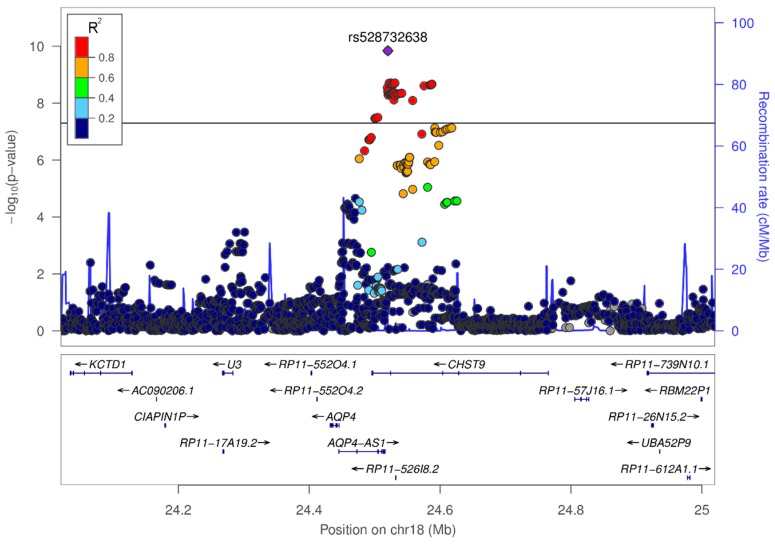
Figure 3.
Variant rs528732638 on chromosome 18q11.2 reached genome-wide significance.
3. Results
3.1. Demographic and Clinical Characteristics
Of the initial 730 patients, 112 patients were known to be deceased, 75 patients refused to participate, 10 patients were not able to participate due to cognitive limitations, 17 patients were not able to participate because of an inability to communicate (e.g., insufficient language skills, hearing impairment, or aphasia), 50 patients were not able to be reached within four attempts, 118 patients were not able to be reached due to change in contact details, and 6 patients could not be interviewed for other reasons. The final sample consisted of 342 participants (78.3% males; mean age = 63.2 years; SD = 9.9 years) with a lifetime prevalence of severe depressive disorders of 23.0%. The prevalence of severe depressive disorders was higher after manifestation of CAD than before (17.4% vs. 8.8%; McNemar’s χ2 (1) = 11.05; p = 0.001), although the corresponding time interval was shorter (median = 7 years vs. median = 54 years; Wilcoxon’s Z = −15.36; two-tailed p < 0.001). The initial manifestation of 81.0% of severe depressive disorders lay after the initial manifestation of CAD—which was on average at 53.6 years (SD = 9.9 years)—and thus the majority of severe depressive disorders in our sample fit the definition of LOD.
3.2. Results from GWAS
We tested the association of 6,341,066 variants using genotyping data from 78 cases and 264 controls. The genome-wide association levels on the autosomal chromosomes are shown as a Manhattan plot in Figure 2. Five loci passed our pre-defined selection criteria and contained lead variants with p < 10−5 (Table S1, Figures S2–S5). Among the lead variants, rs528732638 on chromosome 18q11.2 was a genome-wide significant variant (Beta/Se = 1.62; two-tailed p = 1.45 × 10−10; odds ratio (OR) = 3.62; 95% confidence interval (CI) = 2.38–5.50; Table 1 and Figure 3). Variant rs528732638 is located between the genes AQP4-AS1 (Aquaporin 4 antisense RNA 1) and CHST9 (Carbohydrate sulfotransferase 9).
Table 1.
The lead variant of a single locus, rs528732638, showed genome-wide significance.
| EAF | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variant | Locus | Nearest Genes | EA | NEA | Cas | Con | OR [95% CI] | P |
| rs528732638 | 18q11.2 | AQP4-AS1, CHST9 | GAA | G | 0.34 | 0.13 | 3.62 [2.38–5.50] | 1.45 × 10−10 |
EA: effect allele; NEA: non-effect allele; EAF: effect allele frequency; Cas: cases; Con: controls; OR: odds ratio; CI: confidence interval; p = p-value.
3.3. In-Silico Characterization of Variant rs528732638
We next assessed the putative effects of variant rs528732638 and its highly correlated variants (R2 > 0.8) on gene expression, according to LD information from 1000 Genomes Phase 3 individuals with a European background. Variant rs528732638 shares an LD block with 50 highly correlated variants (Table S2). This LD block showed eQTL effects on five genes in multiple tissues (Table 2 and Table S3) with the eQTL on AQP4 having the overall lowest p-value (p = 4.7 × 10−10 in blood). We used information about TAD boundaries to group genes into cis- and trans-located genes with respect to the LD block of rs528732638 (Table S4). Three of the five genes (AQP4, AQP4-AS1 and CHST9) are putative cis-regulated genes, i.e., these genes are located within a single spatial compartment in which increased physical interaction occurs, that is between regulatory DNA elements and gene promoters.
Table 2.
Variant rs528732638 and its high LD variants showed expression quantitative trait loci (eQTL) effects on multiple tissues.
| Tissue | Gene |
|---|---|
| Blood (4.7 × 10−11), skeletal muscle (4.7 × 10−11) | AQP4 |
| Average brain (1.2 × 10−10), white matter (6.6 × 10−9), parietal lobe (3 × 10−3) | AQP4-AS1 |
| liver (1 × 10−6) | CDH12 a |
| Peripheral blood monocytes (1.6 × 10−6) | CHST9 |
| Peripheral blood monocytes (1.6 × 10−6) | ZNF570 a |
atrans-regulated genes.
4. Discussion
4.1. Variant rs528732638 on Chromosome 18q11.2 Represents the First Vascular Depression Locus
Variant rs528732638 on chromosome 18q11.2 was associated with a 3.6-fold increase in the odds of lifetime depression in patients with CAD (95% CI = 2.38–5.50). It is the first locus meeting the formal threshold for genome-wide significance [39] of 7.2 × 10−8 for an association with depression in patients with coronary artery disease.
Variant rs528732638 is located between the genes AQP4-AS1 (Aquaporin 4 antisense RNA 1) and CHST9 (Carbohydrate sulfotransferase 9) and shares an LD block with 50 highly correlated variants. Prior to our study, the GWAS catalog for rs528732638 or one of its LD variants contained no entries [40]. In our study, however, this LD block showed eQTL effects on five genes, three of which (AQP4, AQP4-AS1 and CHST9) are putative cis-regulated genes.
AQP4 is the most abundant aquaporin isoform in the brain, where it is mainly expressed in perivascular astrocyte endfeet [41,42] and in neural stem cells [43,44]. It is a bidirectional water-specific channel that is essential for neurovascular coupling and glymphatic flow [45,46]. A reduced density of AQP4-positive endfeet in the orbitofrontal cortex is associated with major depressive disorder in humans [47] and the antidepressant fluoxetine enhances the endfeet density in an AQP4-dependent manner [48]. Additionally, AQP4 has been shown to play an important role in adult neural stem cell proliferation [49,50], which is downregulated in depression [51]. The antidepressant fluoxetine enhances neurogenesis [52,53,54] in an AQP4-dependent manner [50]. Regarding cerebrovascular disease, abundant evidence suggests a pivotal role for AQP4 in the formation of ischaemic brain edema [55,56,57,58,59,60,61], and AQP4 inhibition reduces infarct volume and improves clinical recovery in an animal model [62,63,64,65].
Outside of the central nervous system, AQP4 is expressed in myocytes in both cardiac and skeletal muscles [66]. Regarding coronary artery disease, AQP4 is involved in cell swelling and cardiac dysfunction in murine myocardial infarction models [67,68]. AQP4 knock-out in mice reduces the infarct size [69], and tigacelor-mediated cardioprotection relies on AQP4 downregulation [70].
In summary, AQP4 mediates the formation of ischaemic edema in the brain and heart, enhancing cytotoxicity and thus the number and extent of lesions. Based on our finding that variant rs528732638 which belongs to a LD block with eQTL effects on AQP4 is associated with depression in CAD patients, we speculate that AQP4 may play a role in the etiopathology of vascular depression via promotion of ischemic cytotoxicity.
Further studies that aim to replicate our findings in independent, possibly non-European samples, explore expression of AQP4 in atherosclerosis-relevant tissues as a function of genotype at variant rs528732638, and explore the expression of AQP4 in depressed patients with and without atherosclerosis, and functional studies (e.g., animal models) are the next potential steps to elucidating the role of AQP4 in vascular depression.
4.2. Limitations of the Study
Nevertheless, limitations of this study must be considered. First, our findings are solely based on a case-control study, not including unbiased prospective population-based cohorts. Second, our sample size is too small to draw definite conclusions. Third, due to the high morbidity in the sample and the length of the follow-up period, our study suffered from a high drop-out rate. As the prognosis of patients with CAD and depression is poorer than that of patients with CAD that are not depressed [71,72,73], selection bias may have occurred, leading to an underestimation of the prevalence of depression and reducing the power of our study. Thus, we may have missed other depression loci in CAD patients. Fourth, we did not diagnose cerebrovascular disease directly using cerebral imaging. Fifth, our finding has not yet been replicated in an independent sample. Sixth, the LD block of the locus we identified also exerts eQTL effects on the CHST9 gene. Although no previous data have implicated CHST9 in the etiopathology of vascular depression, we cannot yet exclude this possibility.
5. Conclusions
We conducted the first GWAS in a psychiatrically characterized cohort of patients with a confirmed atherosclerotic disorder, namely coronary artery disease. We thus identified a potential locus for vascular depression on chromosome 18q11.2. The lead variant rs528732638 belongs to a LD block with eQTL effects on putative cis-regulated genes (AQP4, AQP4-AS1, and CHST9). As AQP4 is already known to increase the size and extent of ischemic lesions in the heart and brain, we speculate that AQP4 may play a role in the etiopathology of vascular depression. Replication steps including non-European cohorts are needed to confirm this finding and establish the functional role of the AQP4 locus in the etiopathology of depression in CAD patients.
Acknowledgments
The authors would like to thank Marie Luise Bussmann and Sandra Köhne for assistance with data acquisition.
Supplementary Materials
The following are available online at http://www.mdpi.com/2218-273X/8/4/164/s1, Figure S1, Regional association plot of SNP rs11054833 ± 500 kb, Figure S2, Regional association plot of SNP rs11835646 ± 500 kb, Figure S3, Regional association plot of SNP rs7148335 ± 500 kb, Figure S4, Regional association plot of SNP rs60466238 ± 500 kb, Table S1, Lead Variants, Table S2, High LD variants of rs528732638, Table S3, Regulatory effects of rs528732638, Table S4, TAD of rs528732638.
Author Contributions
Conceptualization, H.S., U.S. and J.E.; methodology, M.M., C.W. and J.E.; software, M.M. and C.W.; validation, C.W., U.S. and J.E.; formal analysis, A.L.W., M.M. and L.M.M.V.; investigation, A.L.W., M.M., A.S., S.N., B.W.; resources, H.S., U.S. and J.E.; data curation, A.L.W., M.M. and C.W.; writing—original draft preparation, A.L.W. and M.M.; writing—review and editing, A.L.W., M.M., A.S., S.N., B.W., L.M.M.V., C.W., H.S., U.S. and J.E.; visualization, A.L.W. and M.M.; supervision, B.W., C.W., U.S. and J.E.; project administration, B.W., U.S. and J.E.; funding acquisition, A.L.W.
Funding
This research received no external funding. The APC was funded by Land Schleswig-Holstein within the funding program “Open Access Publikationsfonds”.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- 1.Mathers C., Fat D.M., Boerma J.T. The Global Burden of Disease: 2004 Update. World Health Organization; Geneva, Switzerland: 2008. [Google Scholar]
- 2.Geschwind D.H., Flint J. Genetics and genomics of psychiatric disease. Science. 2015;349:1489–1494. doi: 10.1126/science.aaa8954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosker F., Hartman C., Nolte I., Prins B., Terpstra P., Posthuma D., Van Veen T., Willemsen G., DeRijk R., De Geus E. Poor replication of candidate genes for major depressive disorder using genome-wide association data. Mol. Psychiatry. 2011;16:516–532. doi: 10.1038/mp.2010.38. [DOI] [PubMed] [Google Scholar]
- 4.Otte C., Gold S.M., Penninx B.W., Pariante C.M., Etkin A., Fava M., Mohr D.C., Schatzberg A.F. Major depressive disorder. Nat. Rev. Dis. Primers. 2016;2:160–165. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- 5.Ripke S., Wray N.R., Lewis C.M., Hamilton S.P., Weissman M.M., Breen G., Byrne E.M., Blackwood D.H., Boomsma D.I., Cichon S. A mega-analysis of genome-wide association studies for major depressive disorder. Mol. Psychiatry. 2013;18:497–511. doi: 10.1038/mp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flint J., Kendler K.S. The genetics of major depression. Neuron. 2014;81:484–503. doi: 10.1016/j.neuron.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexopoulos G.S., Meyers B.S., Young R.C., Campbell S., Silbersweig D., Charlson M. The ‘vascular depression’ hypothesis. Arch. Gen. Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 8.Krishnan K., Hays J.C., Blazer D.G. MRI-defined vascular depression. Am. J. Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- 9.Alexopoulos G.S., Kiosses D.N., Klimstra S., Kalayam B., Bruce M.L. Clinical presentation of the “depression–executive dysfunction syndrome” of late life. Am. J. Geriatr. Psychiatry. 2002;10:98–106. [PubMed] [Google Scholar]
- 10.Taylor W.D., Aizenstein H.J., Alexopoulos G.S. The vascular depression hypothesis: Mechanisms linking vascular disease with depression. Mol. Psychiatry. 2013;18:963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexopoulos G.S., Schultz S.K., Lebowitz B.D. Late-life depression: A model for medical classification. Biol. Psychiatry. 2005;58:283–289. doi: 10.1016/j.biopsych.2005.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Köhler S., Thomas A., Barnett N., O’Brien J. The pattern and course of cognitive impairment in late-life depression. Psychol. Med. 2010;40:591–602. doi: 10.1017/S0033291709990833. [DOI] [PubMed] [Google Scholar]
- 13.Paranthaman R., Burns A.S., Cruickshank J.K., Jackson A., Scott M.L., Baldwin R.C. Age at onset and vascular pathology in late-life depression. Am. J. Geriatr. Psychiatry. 2012;20:524–532. doi: 10.1097/JGP.0b013e318227f85c. [DOI] [PubMed] [Google Scholar]
- 14.Feng C., Fang M., Xu Y., Hua T., Liu X.-Y. Microbleeds in late-life depression: Comparison of early-and late-onset depression. Biomed. Res. Int. 2014;2014:682092. doi: 10.1155/2014/682092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrmann L.L., Le Masurier M., Ebmeier K.P. White matter hyperintensities in late life depression: A systematic review. J. Neurol. Neurosurg. Psychiatry. 2008;79:619–624. doi: 10.1136/jnnp.2007.124651. [DOI] [PubMed] [Google Scholar]
- 16.van Sloten T.T., Sigurdsson S., van Buchem M.A., Phillips C.L., Jonsson P.V., Ding J., Schram M.T., Harris T.B., Gudnason V., Launer L.J. Cerebral small vessel disease and association with higher incidence of depressive symptoms in a general elderly population: The AGES-Reykjavik Study. Am. J. Psychiatry. 2015;172:570–578. doi: 10.1176/appi.ajp.2014.14050578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sexton C.E., Le Masurier M., Allan C.L., Jenkinson M., McDermott L., Kalu U.G., Herrmann L.L., Bradley K.M., Mackay C.E., Ebmeier K.P. Magnetic resonance imaging in late-life depression: Vascular and glucocorticoid cascade hypotheses. Br. J. Psychiatry. 2012;201:46–51. doi: 10.1192/bjp.bp.111.105361. [DOI] [PubMed] [Google Scholar]
- 18.Andreescu C., Tudorascu D.L., Butters M.A., Tamburo E., Patel M., Price J., Karp J.F., Reynolds C.F., Aizenstein H. Resting state functional connectivity and treatment response in late-life depression. Psychiatry Res. Neuroimaging. 2013;214:313–321. doi: 10.1016/j.pscychresns.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin Y., Hou Z., Wang X., Sui Y., Yuan Y. Association between altered resting-state cortico-cerebellar functional connectivity networks and mood/cognition dysfunction in late-onset depression. J. Neural Transm. 2015;122:887–896. doi: 10.1007/s00702-014-1347-3. [DOI] [PubMed] [Google Scholar]
- 20.Valkanova V., Ebmeier K.P. Vascular risk factors and depression in later life: A systematic review and meta-analysis. Biol. Psychiatry. 2013;73:406–413. doi: 10.1016/j.biopsych.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 21.Saran R., Puri A., Agarwal M. Depression and the heart. Indian Heart J. 2012;64:397–401. doi: 10.1016/j.ihj.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson R.G., Spalletta G. Poststroke depression: A review. Can. J. Psychiatry. 2010;55:341–349. doi: 10.1177/070674371005500602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saczynski J.S., Beiser A., Seshadri S., Auerbach S., Wolf P., Au R. Depressive symptoms and risk of dementia The Framingham Heart Study. Neurology. 2010;75:35–41. doi: 10.1212/WNL.0b013e3181e62138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.González H.M., Tarraf W., Whitfield K., Gallo J.J. Vascular depression prevalence and epidemiology in the United States. J. Psychiatr. Res. 2012;46:456–461. doi: 10.1016/j.jpsychires.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park J.H., Lee S.B., Lee J.J., Yoon J.C., Han J.W., Kim T.H., Jeong H.-G., Newhouse P.A., Taylor W.D., Kim J.H. Epidemiology of MRI-defined vascular depression: A longitudinal, community-based study in Korean elders. J. Affect. Disord. 2015;180:200–206. doi: 10.1016/j.jad.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Seripa D., Panza F., D’Onofrio G., Paroni G., Bizzarro A., Fontana A., Paris F., Cascavilla L., Copetti M., Masullo C. The serotonin transporter gene locus in late-life major depressive disorder. Am. J. Geriatr. Psychiatry. 2013;21:67–77. doi: 10.1016/j.jagp.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Kim B.J., Lee S.-H., Kim C.K., Ryu W.-S., Kwon H.-M., Choi S.-Y., Yoon B.-W. Advanced coronary artery calcification and cerebral small vessel diseases in the healthy elderly. Circ. J. 2011;75:451–456. doi: 10.1253/circj.CJ-10-0762. [DOI] [PubMed] [Google Scholar]
- 28.Bos D., Ikram M.A., Elias-Smale S.E., Krestin G.P., Hofman A., Witteman J.C., van der Lugt A., Vernooij M.W. Calcification in major vessel beds relates to vascular brain disease. Arterioscler. Thromb. Vasc. Biol. 2011;31:2331–2337. doi: 10.1161/ATVBAHA.111.232728. [DOI] [PubMed] [Google Scholar]
- 29.Nikpay M., Goel A., Won H.-H., Hall L.M., Willenborg C., Kanoni S., Saleheen D., Kyriakou T., Nelson C.P., Hopewell J.C. A comprehensive 1000 Genomes–based genome-wide association meta-analysis of coronary artery disease. Nat. Genet. 2015;47:1121–1130. doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ackenheil M., Stotz-Ingenlath G., Dietz-Bauer R., Vossen A. MINI (Mini International Neuropsychiatric Interview), German Version 5.0.0 DSM IV. Psychiatric University Clinic Munich; Munich, Germany: 1999. [Google Scholar]
- 31.Schaich A., Westermair A.L., Munz M., Nitsche S., Willenborg B., Willenborg C., Schunkert H., Erdmann J., Schweiger U. Mental health and psychosocial functioning over the lifespan of German patients undergoing cardiac catheterization for coronary artery disease. Front. Psychiatry. 2018;9 doi: 10.3389/fpsyt.2018.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchini J., Howie B., Myers S., McVean G., Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat. Genet. 2007;39:906–913. doi: 10.1038/ng2088. [DOI] [PubMed] [Google Scholar]
- 33.Munz M., Tönnies S., Balke W.-T., Simon E. Multidimensional gene search with Genehopper. Nucleic Acids Res. 2015;43:W98–W103. doi: 10.1093/nar/gkv511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.1000 Genomes Project Consortium A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.GTEx Consortium The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science. 2015;348:648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lappalainen T., Sammeth M., Friedländer M.R., AC’t Hoen P., Monlong J., Rivas M.A., Gonzalez-Porta M., Kurbatova N., Griebel T., Ferreira P.G., et al. Transcriptome and genome sequencing uncovers functional variation in humans. Nature. 2013;501:506–511. doi: 10.1038/nature12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia K., Shabalin A.A., Huang S., Madar V., Zhou Y.-H., Wang W., Zou F., Sun W., Sullivan P.F., Wright F.A. seeQTL: A searchable database for human eQTLs. Bioinformatics. 2011;28:451–452. doi: 10.1093/bioinformatics/btr678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dixon J.R., Selvaraj S., Yue F., Kim A., Li Y., Shen Y., Hu M., Liu J.S., Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dudbridge F., Gusnanto A. Estimation of significance thresholds for genomewide association scans. Genet. Epidemiol. 2008;32:227–234. doi: 10.1002/gepi.20297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welter D., MacArthur J., Morales J., Burdett T., Hall P., Junkins H., Klemm A., Flicek P., Manolio T., Hindorff L. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2013;42:D1001–D1006. doi: 10.1093/nar/gkt1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iacovetta C., Rudloff E., Kirby R. The role of aquaporin 4 in the brain. Vet. Clin. Pathol. 2012;41:32–44. doi: 10.1111/j.1939-165X.2011.00390.x. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki Y., Nakamura Y., Yamada K., Huber V.J., Tsujita M., Nakada T. Aquaporin-4 Positron Emission Tomography Imaging of the Human Brain: First Report. J. Neuroimaging. 2013;23:219–223. doi: 10.1111/j.1552-6569.2012.00704.x. [DOI] [PubMed] [Google Scholar]
- 43.Venero J.L., Vizuete M.A.L., Machado A., Cano J. Aquaporins in the central nervous system. Prog. Neurobiol. 2001;63:321–336. doi: 10.1016/S0301-0082(00)00035-6. [DOI] [PubMed] [Google Scholar]
- 44.Cavazzin C., Ferrari D., Facchetti F., Russignan A., Vescovi A.L., La Porta C.A., Gritti A. Unique expression and localization of aquaporin-4 and aquaporin-9 in murine and human neural stem cells and in their glial progeny. Glia. 2006;53:167–181. doi: 10.1002/glia.20256. [DOI] [PubMed] [Google Scholar]
- 45.Nakada T., Kwee I.L., Igarashi H., Suzuki Y. Aquaporin-4 functionality and Virchow-Robin space water dynamics: Physiological model for neurovascular coupling and glymphatic flow. Int. J. Mol. Sci. 2017;18:1798. doi: 10.3390/ijms18081798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Previch L.E., Ma L., Wright J.C., Singh S., Geng X., Ding Y. Progress in AQP research and new developments in therapeutic approaches to ischemic and hemorrhagic stroke. Int. J. Mol. Sci. 2016;17:1146. doi: 10.3390/ijms17071146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajkowska G., Hughes J., Stockmeier C.A., Miguel-Hidalgo J.J., Maciag D. Coverage of Blood Vessels by Astrocytic Endfeet is Reduced in Major Depressive Disorder. Biol. Psychiatry. 2013;73:613–621. doi: 10.1016/j.biopsych.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Benedetto B., Malik V.A., Begum S., Jablonowski L., Gómez-González G.B., Neumann I.D., Rupprecht R. Fluoxetine requires the endfeet protein aquaporin-4 to enhance plasticity of astrocyte processes. Front. Cell. Neurosci. 2016;10:8. doi: 10.3389/fncel.2016.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kong H., Fan Y., Xie J., Ding J., Sha L., Shi X., Sun X., Hu G. AQP4 knockout impairs proliferation, migration and neuronal differentiation of adult neural stem cells. J. Cell Sci. 2008;121:4029–4036. doi: 10.1242/jcs.035758. [DOI] [PubMed] [Google Scholar]
- 50.Kong H., Sha L.-L., Fan Y., Xiao M., Ding J.-H., Wu J., Hu G. Requirement of AQP4 for antidepressive efficiency of fluoxetine: Implication in adult hippocampal neurogenesis. Neuropsychopharmacology. 2009;34:1263–1276. doi: 10.1038/npp.2008.185. [DOI] [PubMed] [Google Scholar]
- 51.Sheline Y.I., Gado M.H., Kraemer H.C. Untreated depression and hippocampal volume loss. Am. J. Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 52.Malberg J.E., Eisch A.J., Nestler E.J., Duman R.S. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manev H., Uz T., Smalheiser N.R., Manev R. Antidepressants alter cell proliferation in the adult brain in vivo and in neural cultures in vitro. Eur. J. Pharmacol. 2001;411:67–70. doi: 10.1016/S0014-2999(00)00904-3. [DOI] [PubMed] [Google Scholar]
- 54.Malberg J.E., Duman R.S. Cell proliferation in adult hippocampus is decreased by inescapable stress: Reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- 55.Manley G.T., Fujimura M., Ma T., Noshita N., Filiz F., Bollen A.W., Chan P., Verkman A. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 56.Thrane A.S., Rappold P.M., Fujita T., Torres A., Bekar L.K., Takano T., Peng W., Wang F., Thrane V.R., Enger R., et al. Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc. Natl. Acad. Sci. USA. 2011;108:846–851. doi: 10.1073/pnas.1015217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fazzina G., Amorini A.M., Marmarou C.R., Fukui S., Okuno K., Dunbar J.G., Glisson R., Marmarou A., Kleindienst A. The protein kinase C activator phorbol myristate acetate decreases brain edema by aquaporin 4 downregulation after middle cerebral artery occlusion in the rat. J. Neurotrauma. 2010;27:453–461. doi: 10.1089/neu.2008.0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li X., Bai R., Zhang J., Wang X. Effect of progesterone intervention on the dynamic changes of AQP-4 in hypoxic-ischaemic brain damage. Int. J. Clin. Exp. Med. 2015;8:18831–18836. [PMC free article] [PubMed] [Google Scholar]
- 59.Higashida T., Peng C., Li J., Dornbos D., Teng K., Li X., Kinni H., Guthikonda M., Ding Y. Hypoxia-inducible factor-1α contributes to brain edema after stroke by regulating aquaporins and glycerol distribution in brain. Curr. Neurovasc. Res. 2011;8:44–51. doi: 10.2174/156720211794520251. [DOI] [PubMed] [Google Scholar]
- 60.Yu L.S., Fan Y.Y., Ye G., Li J., Feng X.P., Lin K., Dong M., Wang Z. Curcumin alleviates brain edema by lowering AQP4 expression levels in a rat model of hypoxia-hypercapnia-induced brain damage. Exp. Ther. Med. 2016;11:709–716. doi: 10.3892/etm.2016.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X., An F., Wang S., An Z., Wang S. Orientin Attenuates Cerebral Ischemia/Reperfusion Injury in Rat Model through the AQP-4 and TLR4/NF-κB/TNF-α Signaling Pathway. J. Stroke Cerebrovasc. Dis. 2017;26:2199–2214. doi: 10.1016/j.jstrokecerebrovasdis.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Vella J., Zammit C., Di Giovanni G., Muscat R., Valentino M. The central role of aquaporins in the pathophysiology of ischemic stroke. Front. Cell. Neurosci. 2015;9:108. doi: 10.3389/fncel.2015.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Igarashi H., Huber V.J., Tsujita M., Nakada T. Pretreatment with a novel aquaporin 4 inhibitor, TGN-020, significantly reduces ischemic cerebral edema. Neurol. Sci. 2011;32:113–116. doi: 10.1007/s10072-010-0431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Popescu E.S., Pirici I., Ciurea R.N., Balseanu T.-A., Catalin B., Margaritescu C., Mogoanta L., Hostiuc S., Pirici D. Three-dimensional organ scanning reveals brain edema reduction in a rat model of stroke treated with an aquaporin 4 inhibitor. Rom. J. Morphol. Embryol. 2017;58:59–66. [PubMed] [Google Scholar]
- 65.Amiry-Moghaddam M., Otsuka T., Hurn P.D., Traystman R.J., Haug F.-M., Froehner S.C., Adams M.E., Neely J.D., Agre P., Ottersen O.P., et al. An α-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc. Natl. Acad. Sci. USA. 2003;100:2106–2111. doi: 10.1073/pnas.0437946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rutkovskiy A., Valen G., Vaage J. Cardiac aquaporins. Basic Res. Cardiol. 2013;108:393. doi: 10.1007/s00395-013-0393-6. [DOI] [PubMed] [Google Scholar]
- 67.Warth A., Eckle T., Köhler D., Faigle M., Zug S., Klingel K., Eltzschig H.K., Wolburg H. Upregulation of the water channel aquaporin-4 as a potential cause of postischemic cell swelling in a murine model of myocardial infarction. Cardiology. 2007;107:402–410. doi: 10.1159/000099060. [DOI] [PubMed] [Google Scholar]
- 68.Zhang H.Z., Kim M.H., Lim J.H., Bae H.-R. Time-dependent expression patterns of cardiac aquaporins following myocardial infarction. J. Korean Med. Sci. 2013;28:402–408. doi: 10.3346/jkms.2013.28.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rutkovskiy A., Stensløkken K.-O., Mariero L.H., Skrbic B., Amiry-Moghaddam M., Hillestad V., Valen G., Perreault M.-C., Ottersen O.P., Gullestad L., et al. Aquaporin-4 in the heart: Expression, regulation and functional role in ischemia. Basic Res. Cardiol. 2012;107:280. doi: 10.1007/s00395-012-0280-6. [DOI] [PubMed] [Google Scholar]
- 70.Vilahur G., Gutiérrez M., Casani L., Varela L., Capdevila A., Pons-Lladó G., Carreras F., Carlsson L., Hidalgo A., Badimon L. Protective effects of ticagrelor on myocardial injury after infarction. Circulation. 2016;134:1708–1719. doi: 10.1161/CIRCULATIONAHA.116.024014. [DOI] [PubMed] [Google Scholar]
- 71.Szpakowski N., Bennell M.C., Qiu F., Ko D.T., Tu J.V., Kurdyak P., Wijeysundera H.C. Clinical Impact of Subsequent Depression in Patients With a New Diagnosis of Stable Angina. Circ. Cardiovasc. Qual. Outcomes. 2016;9 doi: 10.1161/CIRCOUTCOMES.116.002904. [DOI] [PubMed] [Google Scholar]
- 72.Moussavi S., Chatterji S., Verdes E., Tandon A., Patel V., Ustun B. Depression, chronic diseases, and decrements in health: Results from the World Health Surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 73.de Jager T.A., Dulfer K., Pieters K., Utens E.M., Daemen J., Lenzen M.J., van Domburg R.T. The association between subjective health status and 14-year mortality in post-PCI patients. Int. J. Cardiol. 2017;229:108–112. doi: 10.1016/j.ijcard.2016.11.218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.