Abstract
Context
DAX1 (NR0B1) mutations cause X-linked adrenal hypoplasia congenita (AHC) and hypogonadotropic hypogonadism (HH) in affected male patients. Affected individuals typically present with early-onset adrenal insufficiency and develop HH during puberty. Rare cases can present with late-onset adrenal insufficiency or other unusual phenotypes.
Objectives
We sought to identify and functionally characterize DAX1 mutations in seven Thai male subjects in six families with X-linked AHC.
Patients and Methods
Six patients had classic phenotypes with early-onset adrenal failure. One patient presented with late-onset Addison disease at 17 years. In the early-onset group, one patient had GnRH-independent sexual precocity at 3 years of age, and another patient had growth hormone deficiency. The DAX1 gene was sequenced from all patients, and the transcriptional activities of the identified mutations were assessed in vitro using luciferase assays.
Results
DAX1 mutations were identified in all patients, including three novel mutations [c.363delG (p.Gly122Valfs*142), c.1062delC (p.Ala355Profs*17), and c.1156C>T (p.Leu386Phe)] and three known mutations [c.1148_1149delGG (p.Gly383Aspfs*5), c.501_502insG (p.Ala170Argfs*15), and c.805_807delGTC (p.Val269del)]. Functional studies showed that the DAX1 mutants had lower levels of repressor activity on the StAR gene promoter compared with the wild-type DAX-1 protein.
Conclusions
This study describes unusual phenotypes and three novel mutations, extending the phenotypic and mutational spectra of DAX1 mutations.
Keywords: X-linked adrenal hypoplasia congenital, DAX-1, novel, mutation, luciferase 23 assay
X-linked adrenal hypoplasia congenita (AHC) is a rare congenital disorder of primary adrenal insufficiency and hypogonadotropic hypogonadism (HH) in boys [1–4]. It is caused by loss-of-function mutations in dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1 (DAX1), which is a nuclear transcription factor that plays a critical role in human adrenal, pituitary, and gonadal development. DAX-1 is encoded by the NR0B1 gene located on the chromosome Xp21 [1, 2], which has two exons that encode a 470-amino-acid protein. The mechanism of DAX-1 action remains unclear, but most studies indicate that DAX-1 acts as a transcriptional repressor of genes involved in the steroidogenic pathway and at all levels of the hypothalamic–pituitary–gonadal axis [5–8]. Loss-of-function of DAX1 is associated with adrenal hypoplasia and reproductive dysfunction [1–3].
Most male subjects with DAX1 mutations present with primary adrenal failure or salt-losing in infancy or early childhood and develop HH and azoospermia in adolescence. Nonclassic phenotypes, including late-onset Addison disease or transient precocious puberty, have been described in rare cases [9–15]. Patients with X-linked AHC can present with different phenotypes, and a genotype–phenotype correlation is not well established [3, 4]. More than 200 DAX1 mutations have been described in different ethnic groups (http://www.hgmd.org) [16]. Most of these mutations are nonsense or frameshift mutations leading to premature truncation of the protein. Missense mutations are mainly clustered at the carboxyl terminus of the putative ligand-binding region of DAX-1 [4]. We describe seven patients with DAX1 mutations presenting with unusual phenotypes. Three novel DAX1 mutations and three known mutations were identified, and the activities of the mutants were characterized to explore genotype/phenotype correlations.
1. Patients and Methods
A. Patients
Five unrelated patients and two cousins with clinical and hormonal findings suggesting X-linked AHC were recruited from the Division of Pediatric Endocrinology, King Chulalongkorn Memorial Hospital from 2015 to 2017. This study was approved by the Institutional Review Board of Faculty of Medicine, Chulalongkorn University (COA no. 954/2016).
Patient I, the only child of nonconsanguineous parents, weighed 3.0 kg at birth and had normal male genitalia, with phallic length 3.5 cm and descended testes. The first symptoms were diarrhea and poor feeding at 1 month of age. At the local hospital, he was found to have salt-losing crisis and multiple seizure episodes due to hypoglycemia. Hydrocortisone was given, and the patient was referred to our hospital. The patient recovered slowly due to nosocomial pneumonia complication. He was discharged after 35 days. He was maintained with oral hydrocortisone 30 mg/m2/d and fludrocortisone 0.2 mg/d with the presumptive diagnosis of congenital adrenal hyperplasia. A 60-minute ACTH stimulation test was performed at age 3.5 months after the discontinuation of hydrocortisone for 36 h after weaning onto a lower daily dose (∼10 mg/m2/d) for 1 week. An ACTH test failed to increase serum levels of progesterone, 17-hydroxyprogesterone (17OHP), and cortisol [at 60 min: 0.1 ng/mL (0.32 nmol/L), 0.34 ng/mL (1.03 nmol/L), and <0.1 μg/dL (<3 nmol/L), respectively]. Hypogonadotropic hypogonadism was diagnosed at the age of 15 years, and testosterone therapy was started. The patient is now 20 years old with rather good physical health. He was diagnosed with well-controlled epilepsy and mild intellectual disability. He is on prednisolone 7.5 mg/d and fludrocortisone 0.15 mg/d. His penile length is 7 cm (Tanner stage V), and testicular volume is 4 mL bilaterally.
Patient II was the first cousin of patient I. He was born at term with a birth weight of 3.15 kg. His skin was noted to be severely dark at birth. At 2 days of age, he had drowsiness and was found to have hypoglycemia [blood sugar 9 mg/dL (0.5 mmol/L)]. At 6 days of age, electrolytes suggested salt-losing (Na 131, K 6.1, Cl 97, CO2 19 mmol/L). An ACTH stimulation test confirmed primary adrenal insufficiency [at 60 min: cortisol 1.1 μg/dL (30 nmol/L)]. The patient recovered under treatment with hydrocortisone. Ultrasound of the abdomen could not identify either adrenal gland.
Patient III was born at term with a birth weight of 3.17 kg to nonconsanguineous parents. He had moderate birth asphyxia and was intubated at birth. He was admitted to the neonatal intensive care unit of a local hospital for 1 week before being discharged. The initial diagnosis was Klebsiella pneumonia with hypovolemic shock. At age 24 days, he had intractable seizure and was admitted to the neonatal intensive care unit again. In the second admission, his laboratory results showed that he had salt-losing crisis and hypoglycemia. Physical examination revealed severe hyperpigmentation. Intravenous hydrocortisone and NaCl were given, and he was referred to our hospital. Critical blood samples obtained when the patient had clinical signs of shock showed ACTH levels of 185 pg/mL (41 pmol/L), cortisol levels of 1.4 μg/dL (38.6 nmol/L), and 17OHP levels of 1.9 ng/mL (5.7 nmol/L). Adrenal ultrasound demonstrated very small glands bilaterally without evidence of mass lesion. His pedigree was highly suggestive of X-linked recessive transmission because nine boys in previous generations had unexplained death during infancy.
Patient IV was a 17-year-old boy who presented with progressive hyperpigmentation over the past 2 years. At the time of admission, it was noted that he had not yet developed secondary sexual characteristics. Physical examination revealed an obese eunuchoid-appearance boy with generalized hyperpigmentation. His genitalia were normal prepubertal male (Tanner stage I), and his testes were small at 4 mL in volume. His baseline LH (1.15 IU/L), FSH (4.04 IU/L), and testosterone (14 ng/dL, 0.5 nmol/L) were all prepubertal. Electrolytes were normal, and the patient denied a serious past medical history. His morning basal ACTH was 2910 pg/mL (640 pmol/L), cortisol was 2.2 μg/dL (60.7 nmol/L), and 17OHP was 0.08 ng/mL (0.24 nmol/L). CT of the abdomen showed bilateral adrenal glands hypoplasia. MRI of the pituitary revealed a small pituitary gland for age.
Patient V was a 1-month-old term newborn from nonconsanguineous parents. Patient V presented with poor feeding, diarrhea, and lethargy for 8 days. Physical examination revealed poor weight gain, hypotension, severe dehydration, marked hyperpigmentation, and normal male genitalia (penile length 4 cm) with descended testes. Electrolytes documented salt-wasting (Na 109, K 9.9, Cl 82, CO2 13.6 mmol/L). Basal cortisol levels were normal (8.4 μg/dL, 232 nmol/L), but there was poor cortisol response to ACTH stimulation [10.9 μg/dL (301 nmol/L) at 60 min]. 17OHP was not hyper-responsive to ACTH [475 ng/dL (14.4 nmol/L) at 0 min, 716 ng/dL (21.7 nmol/L) at 60 min], whereas androstenedione (1.61 ng/mL, 5.6 nmol/L) and testosterone (243 ng/dL, 8.4 nmol/L) were normal. He was diagnosed with atypical congenital adrenal hyperplasia and was treated with oral hydrocortisone 12.5 mg/m2/d and remained well, with normal electrolytes and suppressed 17OHP levels. At the age of 10 months, he developed acne and rapid penile enlargement (penile length 8.5 cm, testes 3 mL bilaterally) without bone age advancement. Luteinizing hormone–releasing hormone stimulation test (Relisorm,® 100 µg IV, Serono, CA) demonstrated a GnRH-independent precocious puberty (basal LH 0.6, peak LH 7.5 IU/L by radioimmunoassay), with low baseline androstenedione (1.61 ng/mL, 5.6 nmol/L) and DHEAS (0.26 μg/mL, 706 nmol/L) but high testosterone levels (320 ng/dL, 11.1 nmol/L). MRI of the pituitary was normal, and MRI of the abdomen showed small adrenals. After increasing the hydrocortisone dose, the patient’s acne and genital development ceased, and testosterone decreased (30 ng/dL, 1.04 nmol/L). During follow-up, it appeared that acne, rapid growth, and elevated testosterone levels would recur when the hydrocortisone dose was inadequate. A repeated ACTH test (after 5 days hydrocortisone suspension) at 11 years of age showed very low stimulated cortisol and all steroidogenic precursors suggesting AHC.
Patient VI was first evaluated at 3.5 years of age due to progressive hyperpigmentation and symptoms of salt-losing (nausea, vomiting, and diarrhea). His electrolytes confirmed salt-losing. His baseline ACTH was 254 pg/mL (56 pmol/L), and he had undetectable cortisol levels. His aldosterone levels were 2 pg/mL (0.006 nmol/L) (normal, 10 to 160 pg/mL), and plasma renin activity was 9.6 ng/mL/h (2.7 ng/L/s) (normal, 0.2 to 2.8 ng/mL/h). An ACTH stimulation test failed to increase cortisol [0.04 μg/dL (1.1 nmol/L) at 60 minutes]. CT scan revealed no visible bilateral adrenal glands. He was given hydrocortisone and fludrocortisone replacement and remained well. At age 13 years, his pubertal development was assessed to be Tanner I (testicular volume 2 mL bilaterally). His laboratory results showed FSH of 1.5 IU/L, LH <0.1 IU/L, and testosterone <3 ng/dL (<0.1 nmol/L). A pituitary MRI was unremarkable. At age 14 years, he was commenced on hCG therapy for pubertal induction.
Patient VII was a 12-year-old boy referred to our endocrine service with a diagnosis of congenital adrenal insufficiency and severe short stature. The patient was born at term with a normal birth weight (2.7 kg). In the first month of life, he had salt-wasting syndrome and multiple seizure episodes due to hypoglycemia. He was diagnosed with adrenal insufficiency and received hydrocortisone replacement at a local community hospital. The mean daily glucocorticoid dose was ∼11 to 14 mg/m2/d. At age 12 years, he was referred for further evaluation of severe short stature (height, 112 cm; weight, 35 kg; bone age, 7 years). GH provocative tests were performed with estrogen priming, and complete GH deficiency was confirmed (peak GH 0.8 ng/mL, 0.8 μg/L). Pituitary MRI was normal. His free thyroxine was low normal (0.9 ng/dL; normal, 0.8 to 1.8 ng/dL; 11.6 pmol/L) with slightly high TSH (4.5 mU/L; normal, 0.3 to 4.1 mU/L), so l-thyroxine treatment was given at 50 μg/d. A thyrotropin-releasing hormone stimulation test was not performed due to unavailability of medication. An ACTH test proved primary adrenal insufficiency with baseline ACTH 1128 pg/mL (248 pmol/L), and peak cortisol at 60 minutes was undetectable at <1 μg/dL (<28 nmol/L). At age 13 years, he had no signs of puberty with small testes (1 mL bilaterally). His LH was <0.1 IU/L, FSH was 0.2 IU/L, and testosterone was <2.5 ng/dL (<0.1 nmol/L). Growth hormone therapy was started with a good clinical response, but sex steroid therapy was delayed to maximize final height outcome.
B. DNA Sequencing and Mutation Analysis
With informed consent, leukocyte genomic DNA was extracted using QIAamp® DNA Blood Mini Kit (Qiagen, Germantown, MD) according to the manufacturer’s instructions. The two exons of DAX1 (NR0B1) were amplified by PCR using specific oligonucleotide primer pairs designed by Primer3 program (http://bioinfo.ut.ee/primer3-0.4.0/). The PCR products were treated with Exonuclease I (ExoSAP-IT; USB Corporation, Cleveland, OH) and sent for direct sequencing at Macrogen Inc. (Seoul, Korea). The sequencing results were analyzed by Sequencher 4.2 (Gene Codes Corporation, Ann Arbor, MI) and compared with the published DAX1 sequence (accession no.: NM_000475) [1].
C. Functional Analysis of Novel DAX1 Mutations
C-1. Generation of expression vectors
Commercial human pcDNA3.1-DAX1, pcDNA3.1-SF1, and pGL4.10-StAR promoter cDNA clones were obtained (OriGene, Rockville, MD) [17]. StAR promoter sequences (+3 to −1222) from human genomic DNA were cloned into pGL4.10 (Promega, Madison, WI), encoding the luciferase reporter gene luc2 using pfu DNA polymerase (Stratagene, La Jolla, CA). All mutant DAX1 expression vectors were created using a QuickChange® Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA) according to the manufacturer’s instructions. The mutagenized and WT plasmids were separately transformed into bacterial cells by heat shock transformation. The transformed colonies were selected, and the plasmids were extracted by High-Speed Plasmid Mini Kit (Geneaid, Taiwan). The accuracy of the constructions was confirmed by direct sequencing.
C-2. Transient transfection and luciferase assay
Human embryonic kidney 293T cells (HEK293T) cells were cultured in DMEM containing 10% fetal bovine serum and antibiotics in T75 flasks at 37°C in 5% CO2 atmosphere. HEK293T cells were seeded into 12-well plates for transient transfection using X-tremeGENE9 DNA transfection reagent (Sigma-Aldrich, Singapore) according to the manufacturer’s instructions. HEK293T cells were transiently cotransfected with 100 ng WT or mutant DAX1 vector or empty vector with 100 ng SF1 vector, 100 ng StAR promoter reporter, and 100 ng Renilla luciferase reporter plasmids (Promega, Madison, WI). Gene expression was determined 48 hours later using the Dual-Luciferase Reporter Assay System (Promega) with a luminometer (SpectraMax Microplate Reader; Molecular Devices, San Jose, CA). The luciferase activities were reported as a percentage of the empty vector control. The results were presented as mean ± SEM from three independent experiments, each performed in triplicate.
3. Results
A. DAX1 Mutation Analysis
Direct DNA sequencing identified DAX1 mutations in all patients. The clinical features and mutational results of all seven patients are summarized in Table 1. Patients I and II (cousins) carried a previously described in-frame single codon deletion c.805_807delGTC [2]. Patient III had known frame-shift mutation c.1148_1149delGG, which was previously described in a Thai boy with adrenal failure at 4 years of age [18]. Patients V and VI harbored novel frame-shift mutations, including c.363delG, and c.1062delC in exon 1, respectively (NCBI c.DNA reference sequence: NM_000475.4). These deletion mutations produce premature stop codons and truncated proteins. Patient IV had the novel missense mutation c.1156C>T changing leucine 386 to phenylalanine (p.Leu386Phe). p.Leu386 is a highly conserved amino acid in the putative ligand binding domain. In silico analysis by SIFT (http://www.blocks.fhcrc.org/sift) and Polyphen (http://www.genetics.bwh.harvard.edu/) predicted p.Leu386Phe to be damaging and probably damaging, respectively. Patient VII had the insertion mutation c.501_502insG, which was previously described in a Spanish boy with complete adrenal failure in the neonatal period, hypogonadotropic hypogonadism, and severe short stature [19]. None of three novel mutations (c.363delG, c.1062delC, and c.1156C>T) were detected in our 1024 ethnic-matched in-house whole exome sequencing database.
Table 1.
Clinical Features and Molecular Data of Patients With AHC
| Patient No. | Sex | Age of Onset | Clinical Features |
Genotypes |
Unusual Phenotypes | ||||
|---|---|---|---|---|---|---|---|---|---|
| P | H | SW | HH | DNA Mutation | Protein Change | ||||
| I | M | 1 mo | X | X | X | X | c.805_807delGTC | p.Val269del | — |
| II | M | 2 d | X | X | X | N/A | c.805_807delGTC | p.Val269del | — |
| III | M | 24 d | X | X | X | N/A | c.1148_1149delGG | p.Gly383Aspfs*5 | — |
| IV | M | 17 y | X | - | — | X | c.1156C>T | p.Leu386Phe | Late-onset Addison |
| V | M | 1 mo | X | — | X | N/A | c.363delG | p.Gly122Valfs*142 | Precocious puberty |
| VI | M | 3.5 y | X | — | X | X | c.1062delC | p.Ala355Profs*17 | — |
| VII | M | 3 d | X | X | X | X | c.501_502insG | p.Ala170Argfs*15 | Growth hormone deficiency |
Novel mutations are indicated in bold.
Abbreviations: H, hypoglycemia; HH, hypogonadotropic hypogonadism; N/A, not applicable; P, hyperpigmentation; SW, salt-wasting.
B. Gene Expression Studies
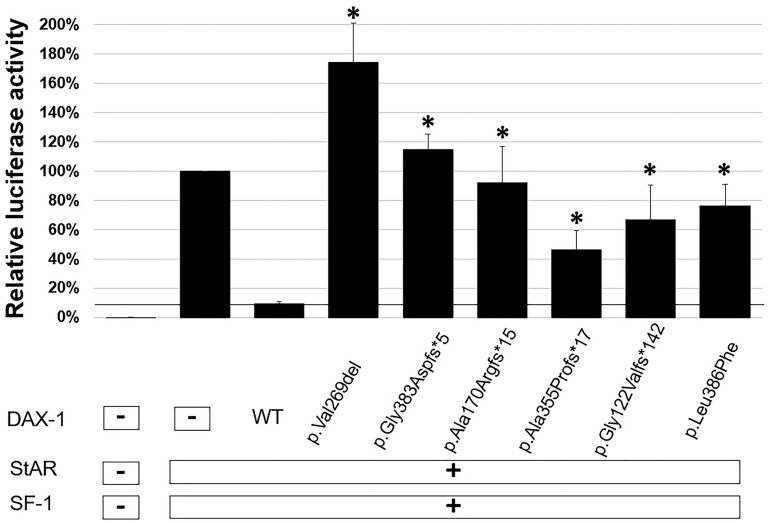
DAX-1 and SF-1 proteins interact on the promoter of the StAR gene [20]. DAX-1 acts as a powerful transcriptional repressor of StAR gene expression, whereas SF-1 acts as a transcriptional activator [7]. Therefore, we cotransfected HEK293T cells with a luciferase reporter construct containing the human StAR promoter region with expression constructs containing human full-length cDNAs for SF1 and DAX1 (WT or mutants) to investigate whether our patients’ mutations would impair the repressor function of DAX-1. WT DAX-1 suppressed basal transcription activity of the pcDNA3.1 vector down to 9%. This suppression was markedly reduced with each of the DAX-1 mutant vectors tested (p.Val269del, 174%; p.Gly383Aspfs*5, 115%; p.Ala170Argfs*15, 92%; p.Ala355Profs*17, 46%; p.Gly122Valfs*142, 67%; p.Leu386Phe, 76%) (Fig. 1). The respective repressor activities of p.Ala170Argfs*15, p.Ala355Profs*17, p.Gly122Valfs*142, and p.Leu386Phe were ∼9%, 59%, 36%, and 26% of WT activity, whereas p.Val269del and p.Gly383Aspfs*5 mutants led to increase StAR transcriptional activities by 74%, and 15% of the basal transcription activity of the empty vector. All of the mutant DAX-1 proteins exhibited significantly less repressive activity than WT (P < 0.05). These results confirm that the DAX-1 mutants displayed loss of trans-repression of StAR transcriptional activities.
Figure 1.
Functional effects of DAX-1 mutants. The effects of WT DAX-1 and its mutants on basal transcriptional activity were studied in dual-luciferase assays. WT DAX-1 greatly repressed on basal transcription activities of the pcDNA3.1 vector. Mutant DAX-1 expression vectors (p.Val269del, p.Gly383Aspfs*5, p.Leu386Phe, p.Gly122Valfs*142, p.Ala355Profs*17, p.Ala170Argfs*15) showed loss of repressor SF-1 activities on the promoter of the StAR gene. Relative luciferase activities are expressed as the mean ± SEM for three independent experiments, each performed in triplicate. *Significant differences of luciferase activities between the mutants and WT (P < 0.05).
4. Discussion
X-linked AHC is caused by mutations in DAX-1, encoded by the NR0B1 gene. DAX-1 is a nonliganded member of the nuclear hormone receptor superfamily that acts as a transcriptional repressor of genes involved in the steroidogenic pathway [21, 22]. We describe seven patients with X-linked AHC with four novel DAX1 mutations and two previously reported mutations. Our findings confirm a broad, heterogeneous spectrum of phenotypes associated with DAX1 mutations. Six patients had classic phenotypes with complete adrenal failure in infancy or early childhood. One patient (patient IV) presented with late-onset Addison disease during adolescence. Two patients had atypical clinical characteristics including GnRH-independent precocious puberty (patient V) and severe growth hormone deficiency (patient VII). Our results indicate that genotype–phenotype correlations within families (patients I and II) are likely. In addition, patient VII, who had a known frameshift mutation, had a clinical phenotype similar to a previously reported Spanish boy who presented with early-onset adrenal failure with complete growth hormone deficiency [19]. However, some DAX1 mutations do not have good genotype–phenotype correlations [23], possibly due to other modifying genes, epigenetic, and nongenetic factors [24].
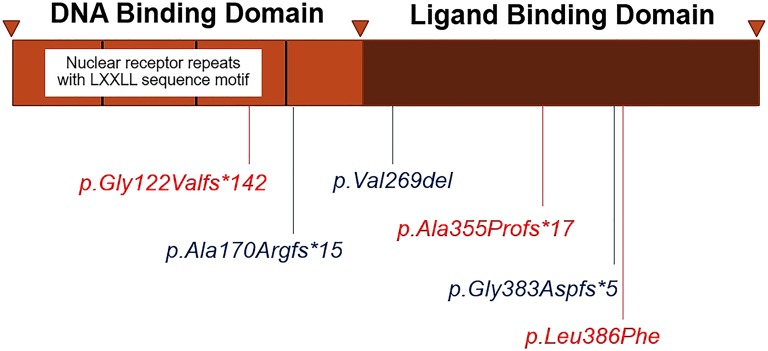
The DAX-1 protein consists of a DNA-binding domain and a putative ligand-binding domain (LBD). The DNA-binding domain, located at the amino terminus, contains repeating hydrophobic LXXLL motifs, which are essential for their interaction with nuclear receptors and protein–protein interactions. The putative LBD at the carboxyl terminus acts as a molecular switch by changing its conformation to convert the receptor from a transcriptional repressor to activator [5, 6].
At least 200 different mutations in DAX1 have been described, including nonsense, frameshifts, and point (missense) mutations. Nonsense and frameshift mutations are found throughout the gene/protein and are usually associated with a severe phenotype [4]. By contrast, missense mutations are clustered in the LBD with several hotspots, suggesting this domain is functionally essential [4]. Some specific changes in these regions have been associated with a late-onset phenotype [9, 14, 15]. Contiguous gene deletions account for approximately one-third of published cases [25].
We identified six DAX1 mutations, of which two are novel frame-shift mutations [c.363delG (p.Gly122Valfs*142), c.1062delC (p.Ala355Profs*17)], one is novel missense mutation (p.Leu386Phe), and three are the known mutations [c.1148_1149delGG (p.Gly383Aspfs*5), c.805_807delGTC (p.Val269del), and c.501_502insG (p.Ala170Argfs*15)] (Fig. 2). All these DAX1 mutations led to loss of repression of StAR transcriptional activities in vitro (Fig. 1). However, the manifestations and severity of disease differed substantially among our patients. Patients I and II, who had classic, severe phenotypes, had the in-frame single codon deletion [c.805_807delGTC (p.Val269del)], which severely impaired transcriptional repression of StAR in our in vitro assays. Patients III, V, and VII also had severe disease and carried frame-shift mutations that severely reduced activities. Patient IV had a milder phenotype presenting with progressive pigmentation and hypogonadotropic hypogonadism at age 17 years and carried the novel p.Leu386Phe mutation. To our knowledge, only 10 previously reported cases presented with late-onset Addison disease manifesting in adolescence or adulthood [9]. Most missense mutations in DAX1 are reported in the putative carboxyl terminal LBD, especially between codons 262 to 300 and 361 to 385 [4]. The mutations associated with late-onset phenotypes include p.Q37X, p.W39X, p.S259P, p.P279L, p.Gln305Hisfs*67 (c.915delG), p.Y380D, and p.I439S [9–15]. Some of these mutations were tested in vitro and retained partial function in different gene transcription assays [11, 14, 15, 26]. Our patient added to previously described cases of late-onset AHC and expands the genotypic spectrum of DAX1 mutations that can present with a milder or late-onset phenotype. p.Leu386Phe retained partial function in luciferase transcription assays. Leu386 is a highly conserved residue that is conserved among fish, chicken, frogs, and mammals. The crystal structure of DAX-1 bound to its target nuclear receptor LRH-1 [6] shows that Leu386 is in the hydrophobic core of the protein. We hypothesize that the bulkier phenylalanine residue interferes with a function of the conserved leucine. Thus, substitution of Phe for Leu may affect protein complex interactions or nuclear localization.
Figure 2.
Schematic representation of the position of all identified DAX1 mutations in this study.
The majority of DAX1 mutations with adult-onset AHC also had HH; therefore, late-onset Addison disease with HH or infertility should prompt genetic testing of DAX1. However, DAX1 mutations appear to be rare in men with only infertility or adult-onset Addison [25, 27]. Patient V, who carried p.Gly122Valfs*142 (c.363delG), presented with early-onset AHC but developed signs of puberty (acne, penile enlargement) at 10 months of age. The luteinizing hormone–releasing hormone stimulation test demonstrated GnRH-independent sexual precocity with high testosterone levels. After starting hydrocortisone treatment, acne and penile enlargement were reversed and testosterone levels were normalized, indicating ACTH-dependent sexual precocity. Sexual precocity in early childhood is a rare and atypical presentation of DAX1 mutations [28–31]. A Brazilian boy with X-linked AHC had pubic hair and enlarged penis at age 2 years [28], and a 9-month-old boy had classic AHC and early sexual development [29]. The GnRH test indicated gonadotropin-independent sexual precocity in both cases. In contrast, two previous studies [30, 31] described mild testicular enlargement in boys with DAX1 mutations who were stable on steroid replacement. In these cases, there was no evidence of true central precocious puberty with a prepubertal GnRH test. Although the exact mechanism of this condition is still unclear, the authors postulated that high ACTH might stimulate testicular steroidogenesis via melanocortin receptor type 1 and autonomous Leydig cell hyperplasia in testes, which could induce sexual precocity in boys with AHC [28–31]. However, further studies are needed to confirm these hypotheses.
Patient VII carried the frameshift mutation c.501_502insG (p.Ala170Argfs*15), which was described previously in a Spanish boy with complete neonatal adrenal failure, HH, and short stature without identified cause [19]. Patient VII had early-onset adrenal failure and very poor linear growth; hormonal tests identified complete growth hormone deficiency and possible central hypothyroidism. Short stature with growth hormone deficiency has been reported in a few children with DAX1 mutations [8, 32, 33]. This is a potentially new clinical finding of AHC. The mechanism of growth hormone deficiency in patients with AHC is unknown. DAX-1 plays a key role in the development of the adrenal gland and in pituitary development. DAX-1 is expressed in embryonic stem cells, steroidogenic tissues (gonads, adrenals), the ventromedial hypothalamus, and the pituitary early in development [5, 34]. Thus, it might affect differentiation of pituitary cells other than gonadotrophs. Our case and others highlight that defects in DAX-1 could result in rare pituitary hormone deficits, but the mechanisms by which pituitary somatotropes or thyrotropes respond to DAX-1 remain unknown. The identification of more patients with DAX1 mutations who have selective or combined hypopituitarism will be most informative.
Acknowledgments
We thank Professor Han-Wook Yoo (University of Ulsan College of Medicine, Seoul, South Korea) for the human DAX1, SF1 cDNA, and StAR promoter clones; Professor Walter L. Miller (University of California, San Francisco, CA) for critically reading and editing this manuscript; and the multiple specialists who participated in the care of these patients.
Financial Support: This study was supported by Ratchadaphiseksomphot Endowment Fund, Faculty of Medicine, the Chulalongkorn Academic Advancement into Its 2nd Century, Chulalongkorn University and the Thailand Research Fund Grants RSA5780054 (to T.S.) and DPG6180001 (to V.S.) and by the Science Achievement Scholarship of Thailand.
Author Contributions: C.S. carried out the molecular genetic studies and drafted the manuscript. T.S. and K.S. cared for the patients. T.S. conceived the idea of the report and drafted the manuscript. All authors participated in the study design, writing, review of the literature, and text editing. TS and VS were co-senior authors and finalized the manuscript. All authors read and approved the final manuscript.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 17OHP
17-hydroxyprogesterone
- AHC
adrenal hypoplasia congenita
- DAX1
dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1
- HH
hypogonadotropic hypogonadism
- LBD
ligand-binding domain
References and Notes
- 1. Zanaria E, Muscatelli F, Bardoni B, Strom TM, Guioli S, Guo W, Lalli E, Moser C, Walker AP, McCabe ER, Meitinger T, Monaco AP, Sassone-Corsi P, Camerino G. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature. 1994;372(6507):635–641. [DOI] [PubMed] [Google Scholar]
- 2. Muscatelli F, Strom TM, Walker AP, Zanaria E, Récan D, Meindl A, Bardoni B, Guioli S, Zehetner G, Rabl W, Peter SH, Kaplan JC, Camerino G, Meitinger T, Monaco AP. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature. 1994;372(6507):672–676. [DOI] [PubMed] [Google Scholar]
- 3. Landau Z, Hanukoglu A, Sack J, Goldstein N, Weintrob N, Eliakim A, Gillis D, Sagi M, Shomrat R, Kosinovsky EB, Anikster Y. Clinical and genetic heterogeneity of congenital adrenal hypoplasia due to NR0B1 gene mutations. Clin Endocrinol (Oxf). 2010;72(4):448–454. [DOI] [PubMed] [Google Scholar]
- 4. Suntharalingham JP, Buonocore F, Duncan AJ, Achermann JC. DAX-1 (NR0B1) and steroidogenic factor-1 (SF-1, NR5A1) in human disease. Best Pract Res Clin Endocrinol Metab. 2015;29(4):607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iyer AK, McCabe ER. Molecular mechanisms of DAX1 action. Mol Genet Metab. 2004;83(1-2):60–73. [DOI] [PubMed] [Google Scholar]
- 6. Sablin EP, Woods A, Krylova IN, Hwang P, Ingraham HA, Fletterick RJ. The structure of corepressor Dax-1 bound to its target nuclear receptor LRH-1. Proc Natl Acad Sci USA. 2008;105(47):18390–18395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ito M, Yu R, Jameson JL. DAX-1 inhibits SF-1-mediated transactivation via a carboxy-terminal domain that is deleted in adrenal hypoplasia congenita. Mol Cell Biol. 1997;17(3):1476–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reutens AT, Achermann JC, Ito M, Ito M, Gu WX, Habiby RL, Donohoue PA, Pang S, Hindmarsh PC, Jameson JL. Clinical and functional effects of mutations in the DAX-1 gene in patients with adrenal hypoplasia congenita. J Clin Endocrinol Metab. 1999;84(2):504–511. [DOI] [PubMed] [Google Scholar]
- 9. Kyriakakis N, Shonibare T, Kyaw-Tun J, Lynch J, Lagos CF, Achermann JC, Murray RD. Late-onset X-linked adrenal hypoplasia (DAX-1, NR0B1): two new adult-onset cases from a single center. Pituitary. 2017;20(5):585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sekiguchi Y, Hara Y, Matsuoka H, Hayashi Y, Katsumata N, Hirata Y. Sibling cases of Addison’s disease caused by DAX-1 gene mutations. Intern Med. 2007;46(1):35–39. [DOI] [PubMed] [Google Scholar]
- 11. Ozisik G, Mantovani G, Achermann JC, Persani L, Spada A, Weiss J, Beck-Peccoz P, Jameson JL. An alternate translation initiation site circumvents an amino-terminal DAX1 nonsense mutation leading to a mild form of X-linked adrenal hypoplasia congenita. J Clin Endocrinol Metab. 2003;88(1):417–423. [DOI] [PubMed] [Google Scholar]
- 12. Raffin-Sanson ML, Oudet B, Salenave S, Brailly-Tabard S, Pehuet M, Christin-Maitre S, Morel Y, Young J. A man with a DAX1/NR0B1 mutation, normal puberty, and an intact hypothalamic-pituitary-gonadal axis but deteriorating oligospermia during long-term follow-up. Eur J Endocrinol. 2013;168(4):K45–K50. [DOI] [PubMed] [Google Scholar]
- 13. Guclu M, Lin L, Erturk E, Achermann JC, Cangul H. Puberty, stress, and sudden death. Lancet. 2010;376(9751):1512. [DOI] [PubMed] [Google Scholar]
- 14. Mantovani G, Ozisik G, Achermann JC, Romoli R, Borretta G, Persani L, Spada A, Jameson JL, Beck-Peccoz P. Hypogonadotropic hypogonadism as a presenting feature of late-onset X-linked adrenal hypoplasia congenita. J Clin Endocrinol Metab. 2002;87(1):44–48. [DOI] [PubMed] [Google Scholar]
- 15. Tabarin A, Achermann JC, Recan D, Bex V, Bertagna X, Christin-Maitre S, Ito M, Jameson JL, Bouchard P. A novel mutation in DAX1 causes delayed-onset adrenal insufficiency and incomplete hypogonadotropic hypogonadism. J Clin Invest. 2000;105(3):321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stenson PD, Mort M, Ball EV, Shaw K, Phillips A, Cooper DN. The human gene mutation database: Building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet. 2014;133(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choi JH, Park JY, Kim GH, Jin HY, Lee BH, Kim JH, Shin CH, Yang SW, Yoo HW. Functional effects of DAX-1 mutations identified in patients with X-linked adrenal hypoplasia congenita. Metabolism. 2011;60(11):1545–1550. [DOI] [PubMed] [Google Scholar]
- 18. Boonyawat B, Phatarakijnirund V, Numbenjapon N, Chantrathammachart P. Novel DAX-1 (NROB1) mutation in a Thai boy with X-linked adrenal hypoplasia congenita (AHC): a first report. J Southeast Asian Med Res. 2017;2:38–41. [Google Scholar]
- 19. Pérez Rodríguez O, Ruibal Francisco JL, Loidi Fernández de Trocóniz L, Parajes Castro S, Martín Rojas-Marcos P. [Gene as a cause of adrenal hypoplasia, hypogonadism and short height novel mutation of DAX-1 gene (pGly168fsX17)]. An Pediatr (Barc). 2006;64(6):591–594. [DOI] [PubMed] [Google Scholar]
- 20. Lalli E, Bardoni B, Zazopoulos E, Wurtz JM, Strom TM, Moras D, Sassone-Corsi P. A transcriptional silencing domain in DAX-1 whose mutation causes adrenal hypoplasia congenita. Mol Endocrinol. 1997;11(13):1950–1960. [DOI] [PubMed] [Google Scholar]
- 21. Lehmann SG, Wurtz JM, Renaud JP, Sassone-Corsi P, Lalli E. Structure-function analysis reveals the molecular determinants of the impaired biological function of DAX-1 mutants in AHC patients. Hum Mol Genet. 2003;12(9):1063–1072. [DOI] [PubMed] [Google Scholar]
- 22. Clipsham R, McCabe ER. DAX1 and its network partners: exploring complexity in development. Mol Genet Metab. 2003;80(1-2):81–120. [DOI] [PubMed] [Google Scholar]
- 23. Li N, Liu R, Zhang H, Yang J, Sun S, Zhang M, Liu Y, Lu Y, Wang W, Mu Y, Ning G, Li X. Seven novel DAX1 mutations with loss of function identified in Chinese patients with congenital adrenal hypoplasia. J Clin Endocrinol Metab. 2010;95(9):E104–E111. [DOI] [PubMed] [Google Scholar]
- 24. Dipple KM, McCabe ER. Phenotypes of patients with “simple” Mendelian disorders are complex traits: thresholds, modifiers, and systems dynamics. Am J Hum Genet. 2000;66(6):1729–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin L, Gu WX, Ozisik G, To WS, Owen CJ, Jameson JL, Achermann JC. Analysis of DAX1 (NR0B1) and steroidogenic factor-1 (NR5A1) in children and adults with primary adrenal failure: ten years’ experience. J Clin Endocrinol Metab. 2006;91(8):3048–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oh CM, Chun S, Lee JE, Lee JS, Park S, Gee HY, Kim SW. A novel missense mutation in NR0B1 causes delayed-onset primary adrenal insufficiency in adults. Clin Genet. 2017;92(3):344–346. [DOI] [PubMed] [Google Scholar]
- 27. Mantovani G, Mancini M, Gazzano G, Spada A, Colpi GM, Beck-Peccoz P, Persani L. Somatic mutational analysis of DAX1 in testes from men with idiopathic azoospermia. Fertil Steril. 2005;84(5):1542–1544. [DOI] [PubMed] [Google Scholar]
- 28. Domenice S, Latronico AC, Brito VN, Arnhold IJP, Kok F, Mendonca BB. Adrenocorticotropin-dependent precocious puberty of testicular origin in a boy with X-linked adrenal hypoplasia congenita due to a novel mutation in the DAX1 gene. J Clin Endocrinol Metab. 2001;86(9):4068–4071. [DOI] [PubMed] [Google Scholar]
- 29. Yeste D, González-Niño C, Pérez de Nanclares G, Pérez-Nanclares G, Audi L, Castaño L, Carrascosa A. ACTH-dependent precocious pseudopuberty in an infant with DAX1 gene mutation. Eur J Pediatr. 2009;168(1):65–69. [DOI] [PubMed] [Google Scholar]
- 30. Argente J, Ozisik G, Pozo J, Teresa Muñoz M, Soriano-Guillén L, Larry Jameson J. A novel single base deletion at codon 434 (1301delT) of the DAX1 gene associated with prepubertal testis enlargement. Mol Genet Metab. 2003;78(1):79–81. [DOI] [PubMed] [Google Scholar]
- 31. Ahmad I, Paterson WF, Lin L, Adlard P, Duncan P, Tolmie J, Achermann JC, Donaldson MD. A novel missense mutation in DAX-1 with an unusual presentation of X-linked adrenal hypoplasia congenita. Horm Res. 2007;68(1):32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rojek A, Obara-Moszynska M, Malecka E, Slomko-Jozwiak M, Niedziela M. NR0B1 (DAX1) mutations in patients affected by congenital adrenal hypoplasia with growth hormone deficiency as a new finding. J Appl Genet. 2013;54(2):225–230. [DOI] [PubMed] [Google Scholar]
- 33. Chung ST, Chi CH, Haymond MW, Jeha GS Infantile growth hormone deficiency and X-linked adrenal hypoplasia congenita. Jacobs J Pediatr.2015;1(1):003. [PMC free article] [PubMed] [Google Scholar]
- 34. Ikeda Y, Takeda Y, Shikayama T, Mukai T, Hisano S, Morohashi KI. Comparative localization of Dax-1 and Ad4BP/SF-1 during development of the hypothalamic-pituitary-gonadal axis suggests their closely related and distinct functions. Dev Dyn. 2001;220(4):363–376. [DOI] [PubMed] [Google Scholar]