Abstract
Context
Many antihypertensive medications modulate the renin-angiotensin-aldosterone system, possibly skewing the diagnosis and subtyping of primary aldosteronism (PA). Particularly, mineralocorticoid receptor antagonists (MRA) might raise renin and stimulate aldosterone synthesis from nonautonomous areas, potentially obscuring lateralization on adrenal vein sampling (AVS). Withdrawal of MRA in severe PA, however, can precipitate hypokalemia and/or hypertension and therefore is not always practical.
Objective
To assess the effects of MRA on the interpretation of AVS data.
Design and Participants
A cohort study of all PA patients who underwent AVS at University of Michigan between January 2009 and January 2018 was conducted. Demographics, diagnostic, AVS, surgical pathology, and follow-up data were collected retrospectively.
Results
Of 191 patients who underwent AVS, 51 (27%) were exposed to MRA at the time of the procedure. Plasma aldosterone concentration and the daily defined dose of antihypertensives were higher in patients taking vs those not taking MRA. Unilateral PA was more frequent in the MRA group, both precosyntropin and postcosyntropin (P < 0.05). The MRA group included two patients with unsuppressed renin, who demonstrated unequivocal AVS lateralization. To date, 86 patients underwent unilateral adrenalectomy, including 30 patients taking MRA during AVS. The proportion of clinical and biochemical success was not statistically different between patients exposed to and those not exposed to MRA during AVS (P = 0.17 and 0.65, respectively).
Conclusion
Our data suggest that conclusive AVS lateralization is often achieved in patients with severe PA despite MRA use.
This retrospective study shows that conclusive adrenal vein sampling lateralization is often achieved in patients with severe primary aldosteronism despite mineralocorticoid receptor antagonists use.
Primary aldosteronism (PA) is the most common form of secondary hypertension (1, 2). Early diagnosis and appropriate treatment of PA are essential for preventing cardiovascular and renal complications (3–6). PA is subtyped into unilateral forms, most commonly aldosterone producing adenomas, and bilateral hyperaldosteronism. This distinction is clinically important because cases of unilateral PA can be effectively treated with adrenalectomy (7, 8). Diagnosis of PA relies on measurement of plasma (or serum) aldosterone concentration (PAC) and plasma renin activity (PRA) or direct renin concentration under basal and salt loading conditions (9, 10). Adrenal vein sampling (AVS) is considered the definitive procedure for PA subtyping (9, 11, 12). Many antihypertensive medications can interfere with the renin-angiotensin-aldosterone system (RAAS) and consequently with the interpretation of diagnostic procedures, including screening, confirmatory tests, and AVS (9, 12, 13).
Current expert guidelines recommend avoiding medications that influence the RAAS during both diagnosis and subtyping of PA, to prevent inaccurate clinical interpretation (9, 11). In particular, mineralocorticoid receptor antagonists (MRA) can increase renin and subsequently activate aldosterone secretion from the normal zona glomerulosa cells. Through this mechanism, MRA can lead to false-negative screening (14), and when used during AVS, MRA might mask lateralization, due to stimulation of aldosterone secretion from nonautonomous areas. Although guidelines recommend discontinuing MRA several weeks prior to diagnostic procedures, hypertension and/or hypokalemia might be difficult to control without MRA in some PA patients. Although the concern for using MRA during PA diagnosis and subtyping is theoretically well founded, clinical data on this subject have been minimal. In particular, evidence regarding AVS results in patients using MRA has been limited to a small case series (15). Herein we present a large experience of MRA use during PA subtyping with AVS from our institution.
Patients and Methods
Study participants
We conducted a retrospective cohort study of all patients with confirmed PA who underwent AVS at the University of Michigan between January 2009 and January 2018. All patients who underwent surgery based on AVS lateralization were also included. This study was approved by the University of Michigan Institutional Review Boards. A waiver of consent was granted for this retrospective study.
Clinical assessment
Patient demographics, blood pressure, PAC, PRA, CT imaging, AVS data, pathology reports, and postoperative follow-up data were retrospectively reviewed. The individual antihypertensive regimens were converted to a standardized daily defined dose (DDD) according to the World Health Organization Anatomical Therapeutic Chemical/DDD Index (16). PAC, PRA, and cortisol were measured in ambulatory setting, in seated position, by immunoassays, as previously reported (17). Diagnosis of PA was confirmed in accordance with the Endocrine Society Clinical Practice Guidelines (9). Postoperative evaluation was based on the Primary Aldosteronism Surgical Outcomes (PASO) criteria (18).
AVS
AVS was performed by experienced interventional radiologists. Samples were obtained simultaneously from the inferior vena cava (IVC) and both adrenal veins (AVs) before and 10 to 30 minutes after cosyntropin administration (0.125 mg bolus followed by continuous infusion at 0.075 mg/h or 0.125 mg/h prior to and after November 2014, respectively). AV catheterization was considered successful when the selectivity index, defined by the AV/IVC cortisol concentrations, was ≥2 before and ≥5 after cosyntropin administration, respectively. Lateralization of PA was assessed by the lateralization index (LI), defined as the aldosterone/cortisol ratio between the two AVs, and contralateral suppression index (CI), defined as (aldosterone/cortisol)nondominant AV / (aldosterone/cortisol)IVC. Unilateral PA was diagnosed if the LI was ≥ 4 before and/or after cosyntropin administration. Contralateral suppression was defined by a CI < 1.
Statistical analysis
Statistical differences in measured parameters between groups were evaluated using the Mann-Whitney U test. Differences in proportions were analyzed by the Fisher exact test. A P value < 0.05 was considered statistically significant. Analyses were performed using GraphPad Prism 7 (La Jolla, CA) and SAS 9.4 (Cary, NC).
Results
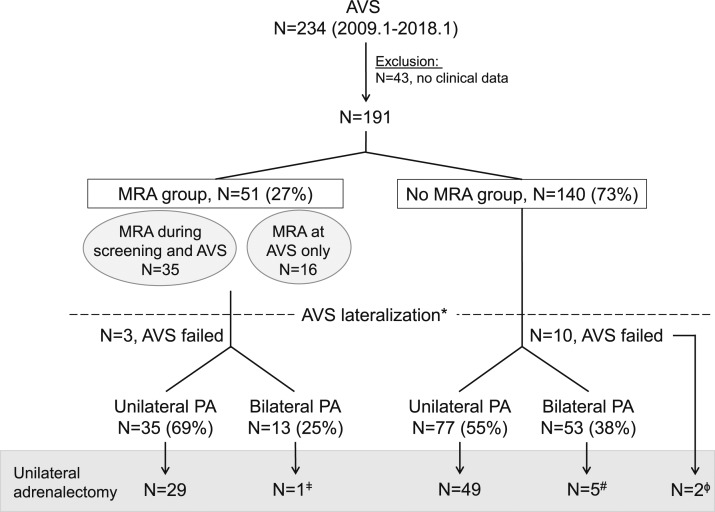
Between January 2009 and January 2018, 234 patients diagnosed with PA underwent AVS at the University of Michigan. Of these, 43 patients referred directly from outside for AVS who did not have sufficiently detailed clinical records available for our review were excluded. Of the remaining 191 patients, 51 (27%) were taking MRA at the time of AVS (Fig. 1). Of the latter group, eight patients were referred to AVS from outside providers, whereas the remaining 43 patients had difficult to control hypertension and/or hypokalemia (including two patients with unsuppressed renin). Sex, age, body mass index, blood pressure, PRA, and frequency of hypokalemia were similar between patients exposed to and those not exposed to MRA during AVS (Table 1). In contrast, the DDD index and PAC were significantly higher in patients taking MRA at the time of AVS (DDD index 6.7 vs 4.0, and PAC 38 ng/dL vs 26 ng/dL, P < 0.001 for both). Unilateral PA was determined by AVS in 35 (69%) patients taking MRA and in 77 (55%) patients not taking MRA at the time of the procedure based on postcosyntropin results (P = 0.03); and in 31 (61%) patients taking MRA vs 64 (46%) patients not taking MRA, based on unstimulated AVS data (P = 0.001). Patients exposed to MRA during AVS had similar LI and CI with those not taking MRA, regardless of lateralization (Table 2).
Figure 1.
Diagram of study flow. *Classification based on postcosyntropin AVS data. ǂAVS results showed modest right dominance (LI = 2.3). #Adrenalectomy was performed based on a precosyntropin LI > 2. ɸAdrenalectomy was performed based on CT findings.
Table 1.
Clinical Characteristics of Study Participants
| MRA, N = 51 | No MRA, N = 140 | P Value | |
|---|---|---|---|
| Sex, M (%) | 34 (67%) | 80 (57%) | 0.06 |
| Age, y | 55 (30–74) | 53 (32–79) | 0.6 |
| BMI, kg/m2 | 33 (29–39) | 33 (29–39) | 0.9 |
| SBP, mm Hg | 156 (140–172) | 151 (136–167) | 0.6 |
| DBP, mm Hg | 85 (74–94) | 85 (76–92) | 0.6 |
| DDD index | 6.7 (4.2–8.8) | 4.0 (2.5–6.3) | <0.0001 |
| Dose of MRA, mg/d | |||
| Spironolactone , 41 patients | 75 (25–100) | — | |
| Eplerenone , 10 patients | 50 (50–75) | — | |
| H/o hypokalemia, % | 76% | 86% | 0.06 |
| PAC, ng/dL | 38 (23–65) | 26 (21–36) | 0.0009 |
| PRA, ng/mL/h | 0.3 (0.1–0.6) | 0.3 (0.1–0.6) | 0.7 |
| ARR | 120 (50–327) | 111 (47–241) | 0.2 |
| Unilateral PA, N (%) | |||
| Precosyntropin | 31 (61%) | 64 (46%) | 0.001 |
| Postcosyntropin | 35 (69%) | 77 (55%) | 0.03 |
| Unilateral adrenalectomy, N (%) | 30 (59%) | 56 (40%) | 0.009 |
Data are expressed as medians (interquartile range) or numbers (N) and %. AVS was unsuccessful in three patients taking MRA and 10 patients not taking MRA.
Abbreviations: ARR, PAC/PRA ratio; BMI, body mass index; DBP, diastolic blood pressure; DDD index, a standardized DDD of antihypertensive medications; H/o, history of; SBP, systolic blood pressure.
Table 2.
Comparison of LI and CI Between Patients Exposed and Those Not Exposed to MRA at the Time of AVS
| MRA, N = 51 |
No MRA, N = 140 |
P Value MRA vs No MRA |
||||
|---|---|---|---|---|---|---|
| Unilateral,a N = 35 | Bilateral,a N = 13 | Unilateral,a N = 77 | Bilateral,a N = 53 | Unilaterala | Bilaterala | |
| LI | 22.2 (8.2–49.8) | 2.1 (1.5–2.5) | 14.1 (8.0–25.1) | 1.8 (1.1–2.6) | 0.4 | 0.4 |
| CI | 0.2 (0.1–0.4) | 0.7 (0.4–0.9) | 0.2 (0.1–0.5) | 1.1 (0.4–1.6) | 0.3 | 0.08 |
Data are expressed as medians (interquartile range). AVS was unsuccessful in three patients taking MRA and 10 patients not taking MRA.
Classification based on postcosyntropin AVS data.
Of the 51 patients taking MRA at the time of AVS, 35 patients had been exposed to MRA throughout PA testing, including screening (Table 3). AVS demonstrated unilateral PA in 26 (74%) of these patients: 24 patients with suppressed PRA and two patients in whom PRA was not suppressed. Both patients with unsuppressed PRA had substantial elevation of PAC, hypokalemia, and dramatic lateralization with contralateral suppression based on AVS (Tables 3 and 4).
Table 3.
Lateralization in 35 Patients Taking MRA Throughout PA Diagnostic Testing
|
|
Unilateral,a N = 26 |
Bilateral,a N = 8 |
P Value (Unilateral vs Bilateral) | ||
|---|---|---|---|---|---|
| Suppressed PRA, N = 24 |
Unsuppressed PRA, N = 2 |
Suppressed PRA, N = 8 |
|||
| Case 1 | Case 2 | ||||
| LI | 14.4 (5.5–57.6) | 46.0 | 88.2 | 1.9 (1.5–2.5) | <0.0001 |
| CI | 0.2 (0.1–0.5) | 0.1 | 0.1 | 0.7 (0.4–0.8) | 0.008 |
| PAC, ng/dL | 40 (21–69) | 31.2 | 98.3 | 35 (25–47) | >0.9 |
| PRA, ng/mL/h | 0.2 (0.1–0.3) | 1.4 | 6.4 | 0.5 (0.2–0.8) | 0.07 |
| H/o hypokalemia, % | 83% | + | + | 38% | 0.02 |
| DDD index | 6.7 (4.8–9.2) | 22.8 | 7.4 | 8.5 (4.9–12.1) | 0.6 |
| Dose of MRA, mg | |||||
| Spironolactone | 25–400 | 200 | 200 | 25–300 | |
| (N) | (19) | (6) | |||
| Eplerenone | 50–100 | — | — | 50, 300 | |
| (N) | (5) | (2) | |||
Data are expressed as medians (interquartile range). AVS was unsuccessful in one patient. PRA was suppressed in all patients with bilateral PA.
Abbreviations: CI, contralateral index after cosyntropin; DDD index, a standardized daily defined dose of antihypertensive medications; H/o, history of; LI, lateralized index after cosyntropin.
Classification based on postcosyntropin AVS data.
Table 4.
Characteristics of the Patients With Unsuppressed PRA While Taking MRA
|
|
Case 1, 41-y-old Male |
Case 2, 69-y-old Male |
||
|---|---|---|---|---|
| Preoperative | Postoperative | Preoperative | Postoperative | |
| PAC, ng/dL | 31.2 | 3.1 | 98.3 | 3.4 |
| PRA, ng/mL/h | 1.4 | 0.3 | 6.4 | 1.3 |
| ARR | 22.3 | 10.3 | 15.4 | 2.6 |
| H/o hypokalemia | + | — | + | — |
| DDD index | 22.8 | 7.67 | 7.4 | 0 |
| Medications | ||||
| MRA | + | — | + | — |
| ACE-I/ARB | + | + | + | — |
| K+ wasting diuretics | + | + | — | — |
Abbreviations: ACE-I, angiotensin converting enzyme inhibitors; ARB, angiotensin II receptor blockers; ARR, PAC/PRA ratio; DDD index, a standardized daily defined dose of antihypertensive medications; H/o, history of.
To date, 86 patients underwent unilateral adrenalectomy, including 30 patients who were exposed to MRA during AVS (Fig. 1). Postoperative hormonal data were available in 64/86 (74.4%) patients (Table 5). According to the PASO criteria (18), complete biochemical success was achieved in 22/23 (95.7%) patients exposed to MRA during AVS. One patient with AVS results consistent with bilateral PA underwent surgery to lessen disease severity (19) and had partial biochemical success. Complete or partial clinical success was achieved in five (16.7%) and 23 (76.7%) patients, respectively. Of the two patients with absent clinical success after adrenalectomy as proposed by the PASO criteria, one patient had complete biochemical success, whereas the other did not have hormonal data available. Taken together, complete or partial clinical (P = 0.17) and biochemical (P = 0.65) success was achieved in similar proportions of patients exposed to and not exposed to MRA at the time of AVS (Table 5). Both patients with unsuppressed PRA at the time of AVS while exposed to MRA experienced clinical and biochemical improvement of PA, with PAC < 10 ng/dL, normalized serum potassium, and a decline of the DDD index (Table 4).
Table 5.
Clinical Outcomes After Unilateral Adrenalectomy Based on PASO Criteria
| MRA, N = 30 | No MRA, N = 56 | |
|---|---|---|
| Complete clinical success | 5 (16.7%) | 17 (30.3%) |
| Partial clinical success | 23 (76.7%) | 31 (55.4%) |
| Absent clinical success | 2 (6.6%) | 8 (14.3%) |
| Complete biochemical success | 22 (95.7%) | 37 (90.2%) |
| Partial biochemical success | 1 (4.3%) | 3 (7.4%) |
| Absent biochemical success | 0 | 1 (2.4%) |
Biochemical data after surgery were available in 23/30 patients taking MRA and 41/56 patients not taking MRA. The proportion of clinical and biochemical success (complete or partial) was not statistically different between patients exposed to and those not exposed to MRA during AVS (P = 0.17 and 0.65, respectively).
Discussion
Our study provides data on AVS results in a relatively large number of PA patients exposed to MRA at the time of the procedure. Furthermore, a considerable proportion of these patients were treated with MRA throughout PA testing, including at the initiation of screening. These results suggest that in cases of severe PA, in which hypokalemia and/or hypertension cannot be safely controlled without MRA, these agents might not necessarily alter the interpretation of testing, particularly if renin remains suppressed, which indicates incomplete mineralocorticoid receptor blockade.
Remarkably, we have found that the proportion of cases with clear AVS lateralization was 69% in patients taking MRA as compared with 55% in patients not taking MRA at the time of AVS (P = 0.04). These findings might be attributed to the higher rate of severe PA, typical of aldosterone-producing adenomas, among patients treated with MRA. Overall, the proportion of unilateral PA in our cohort is similar to other reports (20, 21) and even higher in the group exposed to MRA. Previous studies have consistently shown that AVS lateralization is more likely in patients with severe PA (17, 21, 22); indeed, within our cohort, PAC and DDD were significantly higher in patients taking MRA than in their counterparts.
Several antihypertensive medications can affect the RAAS and confound results of PA testing and subtyping. In particular, MRA can lead to renin elevation and stimulation of aldosterone from normal zona glomerulosa cells. This effect can be prolonged, prompting the Endocrine Society and other experts’ consensus clinical practice guidelines to recommend stopping MRA for up to 6 weeks in patients undergoing testing for PA (9, 11). Although preferable, discontinuing all RAAS-interfering medications is often infeasible, especially in patients with resistant hypertension and/or hypokalemia. Furthermore, hypokalemia might impair aldosterone production, which attenuates lateralization in patients with unilateral PA. As an alternative, it has been proposed that MRA can be continued if renin is suppressed, which would indicate that the mineralocorticoid receptor blockade is incomplete (11, 15, 23). In PA patients, renin frequently remains low despite MRA doses sufficient to control blood pressure and potassium (24–26). A previous study showed AVS lateralization in four patients with severe PA taking MRA at the time of the procedure, all having a suppressed renin; and all four patients experienced favorable surgical outcomes after adrenalectomy (15).
In our institution, 51 patients were exposed to MRA during AVS over the duration of this study. Although MRA can stimulate aldosterone secretion from nonautonomous zona glomerulosa areas and possibly mask AVS lateralization, LI and CI were comparable between patients taking and those not taking MRA. Notably, our report includes two patients with unsuppressed renin while taking 200 mg/d of spironolactone. Other medications with tendency to raise renin were also used in both patients, including potassium-wasting diuretics and angiotensin-converting-enzyme inhibitors/angiotensin II receptor blockers, thus, making it impossible to assess the sole effect of MRA on RAAS. Despite this, AVS demonstrated dramatic LI in both of these patients, and both experienced biochemical and clinical improvement of PA after adrenalectomy. Overall, the postoperative clinical and biochemical outcomes were not only comparable to those previously reported (18), but also similar between patients exposed and those not exposed to MRA during AVS.
The limitations to our study include referral and treatment bias. An important caveat of our cohort is that the proportion of historic hypokalemia was overall very high, suggesting a referral bias and underscoring that many PA cases might never be referred to AVS. As such, our results might only apply to patients with severe PA and cannot be extrapolated to milder cases. In addition, treatment with MRA was not randomized, and AVS was not performed with and without MRA in the same patients. Although the proportion of unilateral PA was considerably higher in patients exposed to MRA during AVS than in their counterparts, our study cannot exclude potential masking of lateralization in the 13 patients (25%) with bilateral AVS results while exposed to MRA. Our data are insufficient to support unrestricted use of MRA or other RAAS-interfering medications during PA testing and subtyping. Furthermore, our study was not designed to objectively measure the MRA impact on RAAS in patients with and without PA. Carefully designed prospective studies are needed to further elucidate these aspects.
In summary, our findings suggest that the majority of patients with severe PA treated with MRA still demonstrate conclusive lateralization by AVS, particularly if renin remains suppressed. Clinical judgment is implicit to determine if MRA should be permitted during PA testing on case-by-case basis, to ensure patient safety and accurate subtyping.
Acknowledgments
We thank Donald A. Giacherio, Clinical Pathology, University of Michigan, for invaluable support with clinical laboratory data, and Drs. James Findling and Tracy Wang from the Medical College of Wisconsin for sharing their clinical experience with similar cases from their institution. We thank the University of Michigan adrenal team members for their contributions to the care of these patients.
Financial Support: A.F.T. was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 1K08DK109116. J.B.B. was supported by National Heart, Lung, and Blood Institute Grant K23HL128909.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AV
adrenal vein
- AVS
adrenal vein sampling
- CI
contralateral index
- DDD
daily defined dose
- IVC
inferior vena cava
- LI
lateralization index
- MRA
mineralocorticoid receptor antagonists
- PA
primary aldosteronism
- PAC
plasma aldosterone concentration
- PASO
Primary Aldosteronism Surgical Outcomes
- PRA
plasma renin activity
- RAAS
renin-angiotensin-aldosterone system
References
- 1. Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Mannelli M, Mattarello MJ, Moretti A, Palumbo G, Parenti G, Porteri E, Semplicini A, Rizzoni D, Rossi E, Boscaro M, Pessina AC, Mantero F; PAPY Study Investigators . A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48(11):2293–2300. [DOI] [PubMed] [Google Scholar]
- 2. Douma S, Petidis K, Doumas M, Papaefthimiou P, Triantafyllou A, Kartali N, Papadopoulos N, Vogiatzis K, Zamboulis C. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet. 2008;371(9628):1921–1926. [DOI] [PubMed] [Google Scholar]
- 3. Catena C, Colussi G, Lapenna R, Nadalini E, Chiuch A, Gianfagna P, Sechi LA. Long-term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension. 2007;50(5):911–918. [DOI] [PubMed] [Google Scholar]
- 4. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(1):51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fourkiotis V, Vonend O, Diederich S, Fischer E, Lang K, Endres S, Beuschlein F, Willenberg HS, Rump LC, Allolio B, Reincke M, Quinkler M; Mephisto Study Group . Effectiveness of eplerenone or spironolactone treatment in preserving renal function in primary aldosteronism. Eur J Endocrinol. 2012;168(1):75–81. [DOI] [PubMed] [Google Scholar]
- 6. Iwakura Y, Morimoto R, Kudo M, Ono Y, Takase K, Seiji K, Arai Y, Nakamura Y, Sasano H, Ito S, Satoh F. Predictors of decreasing glomerular filtration rate and prevalence of chronic kidney disease after treatment of primary aldosteronism: renal outcome of 213 cases. J Clin Endocrinol Metab. 2014;99(5):1593–1598. [DOI] [PubMed] [Google Scholar]
- 7. Chao CT, Wu VC, Kuo CC, Lin YH, Chang CC, Chueh SJ, Wu KD, Pimenta E, Stowasser M. Diagnosis and management of primary aldosteronism: an updated review. Ann Med. 2013;45(4):375–383. [DOI] [PubMed] [Google Scholar]
- 8. Mulatero P, Bertello C, Verhovez A, Rossato D, Giraudo G, Mengozzi G, Limerutti G, Avenatti E, Tizzani D, Veglio F. Differential diagnosis of primary aldosteronism subtypes. Curr Hypertens Rep. 2009;11(3):217–223. [DOI] [PubMed] [Google Scholar]
- 9. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. [DOI] [PubMed] [Google Scholar]
- 10. Rossi GP, Pessina AC, Heagerty AM. Primary aldosteronism: an update on screening, diagnosis and treatment. J Hypertens. 2008;26(4):613–621. [DOI] [PubMed] [Google Scholar]
- 11. Rossi GP, Auchus RJ, Brown M, Lenders JW, Naruse M, Plouin PF, Satoh F, Young WF Jr. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014;63(1):151–160. [DOI] [PubMed] [Google Scholar]
- 12. Wolley MJ, Stowasser M. New advances in the diagnostic workup of primary aldosteronism. J Endocr Soc. 2017;1(3):149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stowasser M, Ahmed A, Guo Z, Wolley M, Ungerer J, McWhinney B, Poglitsch M, Gordon R. Can screening and confirmatory testing in the management of patients with primary aldosteronism be improved? Horm Metab Res. 2017;49(12):915–921. [DOI] [PubMed] [Google Scholar]
- 14. Stowasser M, Ahmed AH, Pimenta E, Taylor PJ, Gordon RD. Factors affecting the aldosterone/renin ratio. Horm Metab Res. 2012;44(3):170–176. [DOI] [PubMed] [Google Scholar]
- 15. Haase M, Riester A, Kröpil P, Hahner S, Degenhart C, Willenberg HS, Reincke M. Outcome of adrenal vein sampling performed during concurrent mineralocorticoid receptor antagonist therapy. J Clin Endocrinol Metab. 2014;99(12):4397–4402. [DOI] [PubMed] [Google Scholar]
- 16. Guidelines for ATC Classification and DDD Assignment. Oslo, Norway: Who Collaborating Centre for Drug Statistics Methodology; 2002. [Google Scholar]
- 17. Nanba AT, Nanba K, Byrd JB, Shields JJ, Giordano TJ, Miller BS, Rainey WE, Auchus RJ, Turcu AF. Discordance between imaging and immunohistochemistry in unilateral primary aldosteronism. Clin Endocrinol (Oxf). 2017;87(6):665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, Satoh F, Amar L, Quinkler M, Deinum J, Beuschlein F, Kitamoto KK, Pham U, Morimoto R, Umakoshi H, Prejbisz A, Kocjan T, Naruse M, Stowasser M, Nishikawa T, Young WF Jr, Gomez-Sanchez CE, Funder JW, Reincke M. Primary Aldosteronism Surgery Outcome (PASO) investigators . Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5(9):689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sukor N, Gordon RD, Ku YK, Jones M, Stowasser M. Role of unilateral adrenalectomy in bilateral primary aldosteronism: a 22-year single center experience. J Clin Endocrinol Metab. 2009;94(7):2437–2445. [DOI] [PubMed] [Google Scholar]
- 20. Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007;66(5):607–618. [DOI] [PubMed] [Google Scholar]
- 21. Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, Gabetti L, Mengozzi G, Williams TA, Rabbia F, Veglio F, Mulatero P. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69(14):1811–1820. [DOI] [PubMed] [Google Scholar]
- 22. Kline GA, Pasieka JL, Harvey A, So B, Dias VC. High-probability features of primary aldosteronism may obviate the need for confirmatory testing without increasing false-positive diagnoses. J Clin Hypertens (Greenwich). 2014;16(7):488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Monticone S, Viola A, Rossato D, Veglio F, Reincke M, Gomez-Sanchez C, Mulatero P. Adrenal vein sampling in primary aldosteronism: towards a standardised protocol. Lancet Diabetes Endocrinol. 2015;3(4):296–303. [DOI] [PubMed] [Google Scholar]
- 24. Ghose RP, Hall PM, Bravo EL. Medical management of aldosterone-producing adenomas. Ann Intern Med. 1999;131(2):105–108. [DOI] [PubMed] [Google Scholar]
- 25. Karashima S, Yoneda T, Kometani M, Ohe M, Mori S, Sawamura T, Furukawa K, Seta T, Yamagishi M, Takeda Y. Comparison of eplerenone and spironolactone for the treatment of primary aldosteronism. Hypertens Res. 2016;39(3):133–137. [DOI] [PubMed] [Google Scholar]
- 26. Matsuda Y, Kawate H, Matsuzaki C, Sakamoto R, Shibue K, Ohnaka K, Anzai K, Nomura M, Takayanagi R. Eplerenone improves carotid intima-media thickness (IMT) in patients with primary aldosteronism. Endocr J. 2016;63(3):249–255. [DOI] [PubMed] [Google Scholar]