Abstract
Context
Phosphate has gained recognition as a risk factor for adverse cardiovascular outcomes, potentially due to accelerated vascular calcification. Fibroblast growth factor-23 (FGF-23) is a counter-regulatory hormone that increases renal phosphate excretion to maintain normal levels.
Objective
The purpose of the study was to determine the association of phosphate and FGF-23 to atherosclerosis.
Design and Setting
A prospective cohort study (n = 204) of outpatients referred for coronary angiography over of a 1-year recruitment period at the Kingston General Hospital.
Intervention
Blood was collected, and a focused carotid ultrasound was performed.
Main Outcome Measure
Degree of angiographic coronary artery disease was scored. Carotid maximum plaque height, total area, grayscale median, and tissue pixel distribution were measured. Plasma phosphate was assessed by mineral assay and FGF-23 by ELISA.
Results
Carotid plaque burden [total plaque area (TPA)] was associated with higher levels of phosphate (TPA, r = 0.20, P < 0.01) and FGF-23 (r = 0.19, P < 0.01). FGF-23 was associated with increased plaque % calcium-like tissue. Participants with no coronary artery disease had significantly lower phosphate levels. Phosphate was associated with higher grayscale median (GSM) in male subjects but with lower GSM in female subjects. FGF-23 was associated with increased plaque % fat in male subjects but increased plaque % calcium in female subjects.
Conclusions
Phosphate was independently associated with the severity of atherosclerosis in terms of plaque burden and composition. FGF-23 was associated with plaque calcification. These findings suggest that abnormal phosphate homeostasis may play an under-recognized but potentially modifiable role in cardiovascular disease.
Keywords: phosphate, atherosclerosis, plaque, ultrasound, FGF-23, cardiovascular disease
Phosphate is an increasingly common additive to fast food, ready-to-eat food, canned or bottled beverages, enhanced meat products, and processed foods. Phosphate, in this inorganic form, is fully absorbed and metabolized by the normal kidney. However, chronic kidney impairment can lead to increased serum levels, which have been found to promote extraosseus calcification [1]. Specifically, it is thought that inorganic phosphate in this form is an important inducer of calcification. Vascular calcification has been linked to complications of chronic kidney disease and cardiovascular events [2, 3], suggesting that calcification is also regulated in the atherosclerotic process [4].
This link between phosphate metabolism and atherosclerosis is not well understood. Phosphate levels even within the normal range have been linked to cardiovascular disease in the general population [5], suggesting that a measurement of phosphate alone may not afford a good understanding of phosphate handling and atherosclerosis.
Less than 1% of total body phosphate is in extracellular fluid. The maintenance of serum phosphate within a normal range is complex and is regulated by three feedback loops involving phosphate absorption by the gut, exchange with bone storage between intracellular and intravascular compartments, and renal excretion [6]. It is unclear how human cells sense changes in extracellular phosphate; however, bone and the parathyroid gland are proposed as the key organs involved, and potential signaling pathways include the extracellular signal regulated kinase pathway and SLC20 sodium-phosphate cotransporters [7]. Evidence also points to the existence of an enteric-renal signaling mechanism for phosphate as exists for other ions [8]. In humans with normal kidney function, the maintenance of phosphate balance is dependent on urinary excretion of excess phosphate. Phosphate stimulates the synthesis and secretion of parathyroid hormone and fibroblast growth factor 23 (FGF-23) and blocks the synthesis of calcitriol [9]. At the kidney level, parathyroid hormone and FGF-23 reduce phosphate reabsorption by internalizing phosphate transporters and decreasing their expression, respectively [10].
Atherosclerotic plaque can be assessed by vascular ultrasound to determine components associated with the stability of a plaque. Plaques that are prone to rupture and cause acute clinical events have thin fibrous caps with large lipid cores and contain high levels of inflammatory cells and less extracellular matrix. In contrast, stable plaques are believed to be composed of more dense fibrous tissue [11]. Advances in ultrasound, such as grayscale median (GSM) and pixel distribution analysis (PDA), allow for characterization of the various tissue elements present in atherosclerotic plaque [12–14].
Our objective was to go beyond simply correlating serum phosphate level with atherosclerosis by examining the relationship between phosphate dysregulation and cardiovascular disease. Specifically, we examined the relationship between biomarkers of phosphate homeostasis and the presence of coronary and peripheral vascular atherosclerotic plaque. In addition to looking at the impact on atherosclerotic disease burden, we assessed associations to atherosclerotic plaque composition.
1. Materials and Methods
A. Study Population
Two-hundred four outpatients referred for a coronary angiogram between May and October 2013 participated in this study and agreed to have blood drawn and a carotid ultrasound performed. Participants meeting the following inclusion criteria were eligible: male or female subjects aged >18 years who were non-emergency outpatients referred for a clinically indicated angiogram for one of the following: nonspecific chest pain evaluation, stable or unstable angina pectoris, positive stress test, preoperative assessment, or old or recent myocardial infarction (>2 days). Demographic and medical data were collected from the participant’s medical chart, hospital information database, and participant interviews. The study was approved by Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board. All participants provided informed consent to participate in the study.
B. Measurement of Phosphate and FGF-23 Concentrations
Phosphate content was determined calorimetrically using the malachite green method as described by Heresztyn and Nicholson [15]. With the addition of ammonium molybdate, a green complex is formed between malachite green, molybdate, and free phosphate. The absorbance for this complex was measured in duplicate for both standards and plasma at 650 nm, and results were calculated directly from a linear standard curve (SynergyHT Microplate Reader; Bio-Tek Instruments, Winooski, VT). FGF-23 levels were determined using the Human FGF-23 (C-term) ELISA kit (Quidel Cat# 60-6100, RRID: AB_2722648; Immunotropics Inc., San Clemente, CA) [16]. To assess intra-assay variability, all samples were run in duplicate and had a coefficient of variation of <10%. To assess interassay variability, a control with a known concentration was run on each of the plates. The coefficient of variation was also <10% for the control across plates.
C. Coronary Atherosclerosis Detection
Coronary angiograms were scored as previously described [17]. In brief, an angiographic score of 0 indicated no or minimal disease (0% to 19% narrowing in any segment), 1 indicated mild disease (20% to 49% narrowing in any segment), 2 indicated moderate disease (luminal narrowing of at least one segment of 50% to 69%), and 3 indicated severe disease (≥70% narrowing within any segment of the main branches of the coronary artery or ≥50% in the left main coronary artery).
D. Peripheral Atherosclerosis: Carotid Arterial Plaque Detection
Carotid ultrasound was conducted using a Vivid E9 (GE Healthcare, Milwaukee, WI) vascular ultrasonography device equipped with a 9L-D transducer. All images were stored in Digital Imaging and Communications in Medicine format and analyzed offline using EchoPAC software for plaque quantification. Carotid intima media thickness (CIMT) was measured with the auto border detection function, and the mean of the right and left sides was used in the analysis. Plaque height was measured manually using calipers in the bulb/internal carotid artery region, and the maximum plaque height of either side was used in the analysis. Plaque area was traced manually, and the total areas of both sides were added to give a total plaque area (TPA) [17, 18].
E. Peripheral Atherosclerosis: Carotid Arterial Plaque Composition
E-1. GSM
New advances in ultrasound allow for the accurate characterization of the various elements present in atherosclerotic plaque. GSM analysis assesses the echolucency of an arterial plaque and is an indicator of plaque vulnerability when ≤25 (ICAROS study) [19]. Although GSM can assess plaque overall echogenicity, grayscale PDA can provide further information that reflects tissue types and degree of heterogeneity [12].
GSM was assessed in longitudinal sections of the bulb/internal carotid artery where carotid plaque was present. Digital Imaging and Communications in Medicine images were exported to uncompressed TIFF files and were opened in Adobe Photoshop CC (2015.0.1 Release; Adobe Inc., San Jose, CA). Images were then converted to grayscale to discard color information so that all pixel values fell between a range of 0 (black) and 255 (white). Images were normalized by linear scaling using the “curves” option so that a selected area within the lumen had a GSM of 0 and the brightest area of the adventitia had a GSM of 190 [20]. Plaque was outlined manually using the pen tool to create a region of interest. A histogram of gray values within the plaque was obtained to determine the GSM. If more than one plaque lesion was present in an image, all lesions were combined as one plaque to calculate the GSM, giving a single GSM value for both left and right carotid arteries. The average GSM of both sides was used in the analysis.
E-2. Grayscale PDA and tissue color mapping
Carotid plaque lesions were characterized based on the PDA method established by Lal et al. [12]. Normalized histogram data from each plaque region of interest were exported from Adobe Photoshop and used to determine the percentage of pixels within each plaque that fell within gray ranges associated with tissue types: 0 to 4 (blood), 8 to 26 (fat), 41 to 76 (muscle), 112 to 196 (fibrous), and 211 to 255 (calcium). For each participant, the % pixels that fell within each tissue range was represented by the average of all plaque lesions in both left and right carotid arteries.
F. Statistical Analysis
All data were analyzed using JMP® 12.0.1 software (SAS Institute Inc., Cary, NC). Fisher exact test was used to compare nominal variables, and the Wilcoxon-Mann-Whitney two-sample test (rank sums) was used for continuous variables. The Spearman correlation coefficient was used to evaluate the bivariate (unadjusted) associations of continuous variables. We used a backward selection criteria of P < 0.25 to select independent factors associated with increased phosphate or Log(FGF-23). The candidates included all atherosclerosis variables: CIMT, maximum plaque height, TPA, any coronary artery disease (angiographic score 1 to 3), GSM, plaque composition (% pixels within grayscale of ranges for blood, fat, muscle, fibrous, and calcium), and demographics such as age, sex, estimated glomerular filtration rate (eGFR), body mass index (BMI), and traditional cardiac risk factors (tobacco use, diabetes, hypertension, and dyslipidemia). After selection, multiple linear regression models were used to examine the independent associations between carotid plaque measures and phosphate or FGF-23 in all participants and when separated by sex. Because FGF-23 was not normally distributed, it was log transformed prior to multiple regression analysis. Statistical significance was accepted at α < 0.05.
2. Results
Table 1 describes the demographic, clinical, and laboratory variables of the participants. Of the 204 participants, 31% had diabetes, 39% had a BMI ≥30, and the majority had hypertension (75%) and dyslipidemia (82%). Only 36 participants had an eGFR <60 mL/min/1.73 m2, and there was no difference in eGFR between male and female subjects. Although the level of phosphate was the same in male and female subjects, the level of FGF-23 was significantly higher in female subjects compared with male subjects (median, 90.9 vs 61.7 RU/mL, respectively; P < 0.0001) (Table 1). As expected, FGF-23 and phosphate were significantly correlated to each other (r = 0.21, P < 0.01). There was an inverse association between FGF-23 and eGFR (r = −0.18, P < 0.05).
Table 1.
Demographic, Laboratory, and Atherosclerosis Variables in the Sample Population
| Variables | Overall (n = 204) | Male (n = 134) | Female (n = 70) |
|---|---|---|---|
| Demographic | |||
| Age, y (mean ± SD) | 65.5 ± 9.4 | 65.8 ± 9.8 | 65.0 ± 8.7 |
| Diabetes, n (%) | 63 (31) | 38 (28) | 25 (36) |
| Hypertension, n (%) | 154 (75) | 96 (72) | 58 (83) |
| Dyslipidemia, n (%) | 168 (82) | 110 (82) | 58 (83) |
| Tobacco use, n (%) | 32 (16) | 19 (14) | 13 (19) |
| BMI, (mean ± SD) | 30.1 ± 6.7 | 29.7 ± 6.1 | 30.8 ± 7.6 |
| BMI ≥30, n (%) | 79 (39) | 51 (38) | 28 (40) |
| WC, cm (mean ± SD) | 106.6 ± 14.1 | 107.9 ± 13.1 | 103.9 ± 15.9 |
| Laboratory variables | |||
| Creatinine, μmol/L (mean ± SD) | 85.1 ± 27.2 | 91.8 ± 28.6 | 72.4 ± 18.5a |
| eGFR, mL/min/m2 (mean ± SD) | 77.4 ± 21.8 | 78.5 ± 22.4 | 75.2 ± 20.5 |
| FGF-23, RU/m, median (IQR) | 69.1 (45.0–113.8) | 61.7 (43.4–95.7) | 90.9 (54.1–168.7)a |
| Phosphate, mmol/L (mean ± SD) | 1.13 ± 0.27 | 1.13 ± 0.27 | 1.13 ± 0.26 |
| Angiographic score (mean ± SD) | 2.1 ± 1.2 | 2.3 ± 1.2 | 1.8 ± 1.2b |
| 0 (normal, no CAD), n (%) | 32 (16) | 20 (15) | 12 (17) |
| 1 (mild CAD), n (%) | 38 (19) | 15 (11) | 23 (33) |
| 2 (moderate CAD), n (%) | 9 (4) | 5 (4) | 4 (6) |
| 3 (severe CAD), n (%) | 125 (61) | 94 (70) | 31 (44) |
| Carotid ultrasound measures (mean ± SD) | |||
| Mean CIMT, mm | 0.81 ± 0.15 | 0.82 ± 0.16 | 0.79 ± 0.14 |
| Maximum plaque height, mm | 2.97 ± 1.35 | 3.01 ± 1.33 | 2.88 ± 1.39 |
| Total plaque area, mm2 | 55.7 ± 40.6 | 59.4 ± 43.0 | 48.5 ± 34.8 |
| Mean GSM | 58.3 ± 13.6 | 58.1 ± 13.3 | 58.9 ± 14.2 |
Abbreviations: CAD, coronary artery disease; WC, waist circumference.
Two-tailed Fisher exact test was used to compare nominal variables, and the Wilcoxon test (rank sums) was used for continuous variables.
P < 0.0001.
P < 0.01.
A. Correlation of Phosphate and FGF-23 With Coronary Atherosclerosis
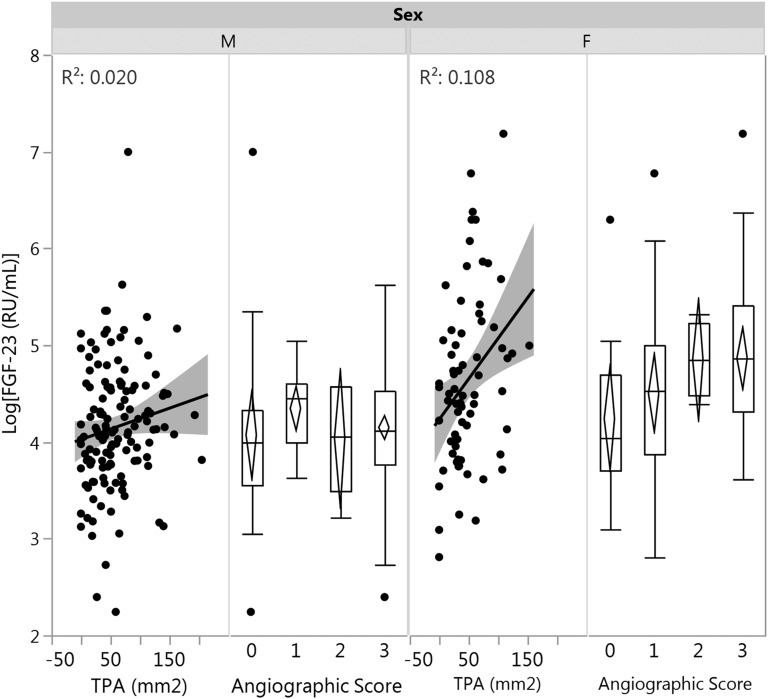
Phosphate, FGF-23, and atherosclerosis measures were compared between the presence and absence of cardiac risk factors (Table 2). Phosphate level was significantly higher in the presence of any coronary atherosclerosis (angiographic score 1 to 3), in smokers, and in participants with increased girth. FGF-23 levels were significantly higher in smokers and in participants with diabetes, hypertension, and obesity (elevated BMI and waist circumference), but no correlations were found with coronary atherosclerosis. Figure 1 demonstrates the association between FGF-23 and TPA values and angiographic score in male and female subjects.
Table 2.
Comparison Between Atherosclerosis Measures, Phosphate, and FGF-23 With the Presence of Cardiac Risk Factors
| Risk Factors |
Categorical Variables
|
||||
|---|---|---|---|---|---|
| CIMT (mm) | MPH (mm) | TPA (mm2) | Phosphate (mmol/L) | FGF-23 (RU/mL), Median (IQR) | |
| Diabetes | |||||
| Yes | 0.81 ± 0.16 | 3.41 ± 0.97a | 71.0 ± 43.7b | 1.11 ± 0.23 | 94.4 (45.0–166.7)a |
| No | 0.81 ± 0.15 | 2.77 ± 1.45 | 48.9 ± 37.3 | 1.14 ± 0.28 | 64.4 (45.2–95.8) |
| Hypertension | |||||
| Yes | 0.82 ± 0.15 | 3.07 ± 1.29 | 58.0 ± 40.4 | 1.13 ± 0.25 | 73.1 (47.6–129.7)a |
| No | 0.78 ± 0.16 | 2.65 ± 1.49 | 48.6 ± 40.7 | 1.11 ± 0.32 | 56.9 (39.3–92.2) |
| Dyslipidemia | |||||
| Yes | 0.82 ± 0.15a | 3.17 ± 1.21b | 61.1 ± 38.9c | 1.14 ± 0.26 | 71.3 (46.8–125.4) |
| No | 0.75 ± 0.16 | 2.02 ± 1.54 | 30.7 ± 39.7 | 1.06 ± 0.29 | 55.7 (44.6–91.7) |
| Tobacco use | |||||
| Yes | 0.82 ± 0.15 | 3.14 ± 1.38 | 63.8 ± 43.0 | 1.20 ± 0.20d | 95.1 (37.8–146.5)a |
| No | 0.81 ± 0.15 | 2.94 ± 1.34 | 54.2 ± 40.1 | 1.11 ± 0.28 | 64.2 (44.6–99.7) |
| BMI ≥30 | |||||
| Yes | 0.82 ± 0.15 | 2.96 ± 1.36 | 59.8 ± 43.1 | 1.12 ± 0.24 | 88.5 (53.1–143.6)a |
| No | 0.81 ± 0.15 | 2.97 ± 1.34 | 53.1 ± 38.9 | 1.13 ± 0.28 | 61.5 (42.8–93.6) |
| WC >88 cm (F) and >102 cm (M) | |||||
| Yes | 0.82 ± 0.16 | 3.04 ± 1.33 | 54.7 ± 37.3 | 1.14 ± 0.26d | 82.5 (47.7–133.9)a |
| No | 0.79 ± 0.15 | 2.74 ± 1.35 | 51.8 ± 42.0 | 1.07 ± 0.29 | 57.7 (41.7–77.5) |
| CAD | |||||
| Angio. score 1–3 | 0.82 ± 0.16d | 3.17 ± 1.21c | 60.9 ± 40.2c | 1.15 ± 0.27d | 72.1 (46.8–119.0) |
| Angio. score 0 | 0.75 ± 0.12 | 1.89 ± 1.52 | 27.8 ± 30.3 | 1.02 ± 0.23 | 55.3 (37.2, 95.1) |
Abbreviations: CAD, coronary artery disease; MPH, maximum plaque height; WC, waist circumference.
Values are mean ± SD unless noted otherwise. Significance determined with the Wilcoxon test (rank sums).
P < 0.01.
P < 0.001.
P < 0.0001.
P < 0.05.
Figure 1.
Correlation between FGF-23 and carotid TPA or angiographic coronary artery disease in male and female subjects. Total plaque area had a significant correlation with FGF-23 in female subjects (P = 0.006) but not in male subjects. As coronary disease severity increased in female subjects, levels of FGF-23 also increased. This was not observed in male subjects. F, female; M, male.
B. Correlation of Phosphate and FGF-23 With Peripheral Vascular Atherosclerosis
B-1. Plaque burden/quantity
Phosphate and FGF-23 were associated with higher carotid maximum plaque height (r = 0.15 and r = 0.18, respectively; P < 0.05) and TPA (r = 0.20 and r = 0.19, respectively; P < 0.05), whereas no association was found between eGFR and these plaque measures. After backward selection for independent predictors of phosphate or FGF-23, multivariable regression models were constructed. Included in the selection process were all measures of atherosclerosis (both quantitative and composition measures) as well as age, sex, BMI, eGFR, and traditional cardiac risk factors. The independent factors associated with serum phosphate are presented in Table 3. When all participants were included in the analysis, plaque height was significantly associated with the level of phosphate (β = 0.18, P = 0.01). In female subjects, plaque height was negatively correlated with FGF-23 (β = −0.41, P = 0.01), but plaque area was positively correlated (β = 0.52, P = 0.001), as was angiographic score.
Table 3.
Predictors of Phosphate After Stepwise Backward Regression Analysis
| Term | Estimate | SE | Lower 95% | Upper 95% | Std. β | P value |
|---|---|---|---|---|---|---|
| All | ||||||
| MPH, mm | 0.037 | 0.014 | 0.008 | 0.065 | 0.18 | 0.01 |
| Male | ||||||
| GSM | 0.010 | 0.004 | 0.002 | 0.018 | 0.50 | 0.01 |
| Female | ||||||
| Plaque % blood | −0.019 | 0.009 | −0.036 | −0.002 | −0.36 | 0.03 |
| GSM | −0.007 | 0.003 | −0.012 | −0.002 | −0.41 | 0.01 |
Abbreviations: MPH, maximum plaque height; Std., standardized.
Selection model included all atherosclerotic variables (quantity and composition), age, sex (if all participants), eGFR, BMI, and traditional cardiac risk factors (tobacco use, diabetes, hypertension, and dyslipidemia). Only contributors that remained in the model and were statistically significant are presented.
C. Plaque Type or Composition
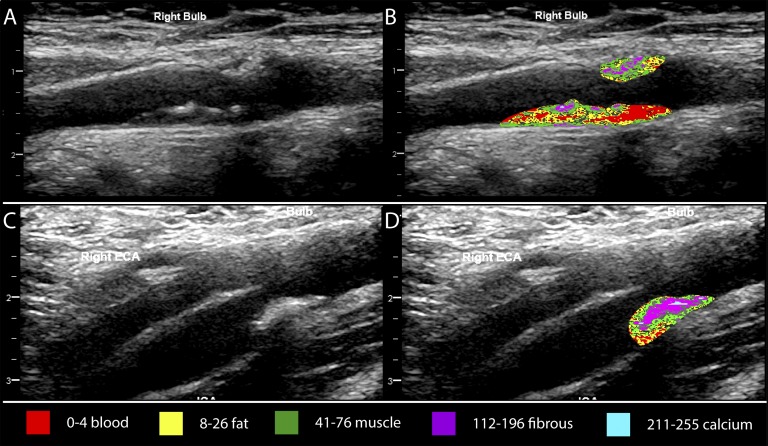
There was a significant correlation between FGF-23 and the percentage of pixels within carotid plaque in the range of calcification (r = 0.16, P < 0.05). Figure 2 provides examples of grayscale mapping ranges for different plaque tissue types.
Figure 2.
Ultrasound grayscale color mapping of carotid plaque. (A, B) Right carotid bulb longitudinal view of plaque from a 66-year-old man. Phosphate level was 1.65 mmol/L, and FGF-23 was 94.4 RU/mL. Grayscale color mapping indicates that the plaque had a higher percentage of pixels in the blood (red) and fat-like (yellow) tissue ranges. (C, D) Right carotid bulb longitudinal view of plaque from a 68-year-old man. Phosphate level was 1.25 mmol/L, and FGF-23 was 71.9 RU/mL. Grayscale color mapping indicates that the plaque had a higher percentage of pixels in the fibrous-like tissue range (purple) with some calcification (blue). ECA, external carotid artery.
When analyzed separately by sex, phosphate levels in male subjects were positively correlated to plaque GSM (indicative of increased echogenicity; i.e., brighter plaque). In female subjects, phosphate levels were negatively associated with GSM and plaque % blood (indicative of decreased echogenicity and decreased % blood).
Independent predictors of FGF-23 are presented in Table 4. When all participants were included in the analysis, eGFR and male sex were negatively associated with FGF-23. Tobacco use and plaque % pixels in the range of calcium were positively associated with FGF-23, consistent with calcification. When separated by sex, a higher level of FGF-23 was associated with increased GSM and plaque % pixels in the range of fat in male subjects (β = 0.56 and β = 0.50, respectively; P = 0.01). Plaque % pixels in the range of calcium was positively correlated to FGF-23 (β = 0.28, P = 0.01) in female subjects.
Table 4.
Predictors of Log(FGF-23) Levels After Stepwise Backward Regression Analysis
| Term | Estimate | SE | Lower 95% | Upper 95% | Std. β | P value |
|---|---|---|---|---|---|---|
| All | ||||||
| Sex (male) | −0.260 | 0.053 | −0.365 | −0.156 | −0.32 | <0.0001 |
| eGFR, mL/min/1.73 m2 | −0.007 | 0.002 | −0.011 | −0.002 | −0.19 | 0.01 |
| Tobacco use | 0.151 | 0.070 | 0.013 | 0.289 | 0.14 | 0.03 |
| Plaque % calcium | 0.178 | 0.064 | 0.053 | 0.304 | 0.18 | 0.01 |
| Male | ||||||
| GSM | 0.024 | 0.010 | 0.005 | 0.044 | 0.50 | 0.01 |
| Plaque % fat | 0.050 | 0.018 | 0.015 | 0.085 | 0.56 | 0.01 |
| Female | ||||||
| MPH, mm | −0.319 | 0.114 | −0.548 | −0.090 | −0.41 | 0.01 |
| TPA, mm2 | 0.014 | 0.004 | 0.006 | 0.022 | 0.52 | 0.001 |
| Plaque % calcium | 0.274 | 0.102 | 0.071 | 0.477 | 0.28 | 0.01 |
Abbreviations: MPH, maximum plaque height; Std., standardized.
Selection model included all atherosclerotic variables (quantity and composition), age, sex (if all participants), eGFR, BMI, traditional cardiac risk factors (tobacco use, diabetes, hypertension, and dyslipidemia). Only contributors that remained in the model and were statistically significant are presented.
3. Discussion
We found that both phosphate and FGF-23, an important biomarker of serum phosphate regulation, were significantly associated with atherosclerotic disease. In addition to finding an association between FGF-23 and the quantity of peripheral atherosclerosis, we found an association with the type or composition of plaque, which differed by sex. FGF-23 was correlated with increased calcification in the plaque lesions of female subjects, whereas in male subjects it was associated with fatty components of plaque. FGF-23 levels were significantly higher in female subjects despite equivalent levels of kidney function, suggesting the presence of underlying sex differences with respect to phosphate homeostasis that may affect cardiovascular disease risk.
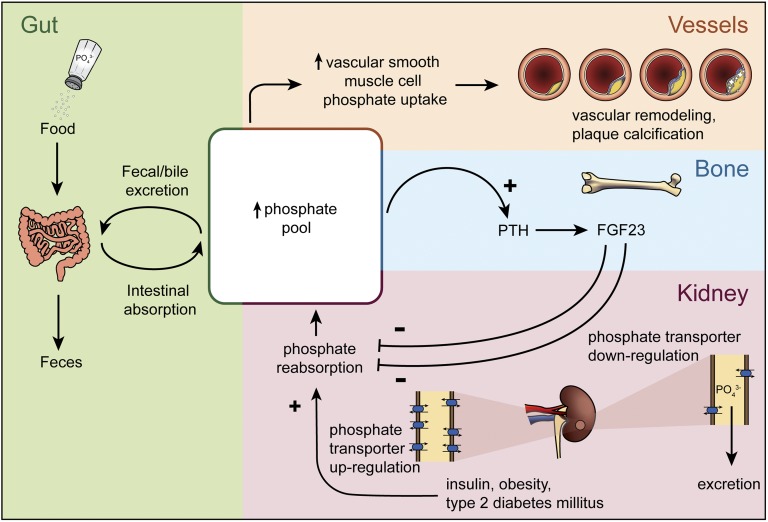
In people with normal phosphate levels, as largely reflected in this study, FGF-23 is a better biomarker to reflect the presence of dysregulated phosphate handling. That is, serum phosphate levels do not fully reflect phosphate homeostasis, and impaired phosphate excretion may only become evident once exposed to a dietary challenge [21–23]. For example, compared with healthy people, individuals with coronary artery disease with normal kidney function had significantly attenuated phosphate appearance in the urine 2 hours after an oral phosphate challenge (14% vs 62% increase) [23]. Dietary phosphate that is absorbed but not immediately excreted by the kidneys may increase the exposure of vascular smooth muscle cells to a procalcific milieu (Fig. 3). Phosphate transporters present on vascular smooth muscle cells mediate intracellular uptake of phosphate and conversion of vascular smooth muscle cells to an osteochondroblast-like phenotype that produces bone matrix proteins and accrues calcium and phosphate minerals in the form of hydroxyapatite.
Figure 3.
Association of phosphate intake and excretion with vascular remodeling. The phosphate pool is increased by the intake of phosphate-containing foods, including those fortified with phosphate additives. High systemic phosphate levels may lead to increased phosphate uptake by vascular smooth muscle cells, in turn accelerating vascular remodeling and calcification. In response to high phosphate, parathyroid hormone and FGF-23 promote the downregulation of phosphate transporters to increase urinary phosphate excretion (decreasing the phosphate pool). Conversely, high insulin levels observed in type 2 diabetes mellitus and obesity may decrease phosphate excretion (increasing the phosphate pool) by promoting the upregulation of phosphate transporters in urinary tubules.
Several studies have demonstrated a relationship between phosphate-related biomarkers and coronary artery calcification [24, 25], measures of arterial stiffness [26, 27], and degree of coronary stenosis at angiography [28]. In the current study, phosphate was independently associated with carotid plaque height. In our assessment of carotid plaque composition, phosphate was associated with increased echogenicity in male subjects but not in female subjects. In contrast, FGF-23 was associated with plaque calcification in female subjects and with increased plaque echogenicity in male subjects. Dysregulated phosphate metabolism may play a role in plaque remodeling, thereby contributing to progressive luminal narrowing and vascular stiffening.
Previous studies have indicated that FGF-23 may be associated with increased plaque burden. Shah et al. [20] reported an association between FGF-23 and the presence of carotid plaque and increased echogenicity in the Northern Manhattan study. This is the only previous study to consider plaque composition, which they assessed by a single GSM value as opposed to the pixel distribution method that we have used. Participants in the highest quintile of FGF-23 had significantly higher GSM after adjustment for age, sex, race, and eGFR. We have added to their findings by including both phosphate and FGF-23 in our assessments and have used a granular approach to characterizing plaque composition [29]. Our study is consistent with their findings, but we have also reported significant differences between sexes.
There is a paucity of studies to support the contention that dietary intake of phosphate is a modifiable risk factor for cardiovascular health in non-CKD populations [30, 31]. This may be due, in part, to the ability of current dietary assessment tools to accurately capture total phosphate intake. Inorganic phosphates are being increasingly added to the food supply without sufficient regulatory oversight because they are considered a Generally Recognized to be Safe ingredient. Most countries require that inorganic phosphate be listed on food packaging, but the amount of phosphate is not a required element. In a study of over 500 healthy middle-aged participants, Itkonin et al. [32] found that increased energy-adjusted total phosphate and food-additive inorganic phosphate intake was associated with increased CIMT. This is an emerging public health concern because phosphate salts are being increasingly used as an additive for food preservation with minimal consideration for its health risks [33].
A. Study Limitations
This study is limited due to the low number of participants without carotid plaque. Given that this is a cross-sectional study, the relationships we have found between phosphate homeostasis biomarkers and measures of atherosclerosis suggest that an association exists. Further studies are required to determine mechanisms and causality, especially in light of the observed sex differences.
4. Conclusion
In this study, phosphate and FGF-23 was associated with atherosclerotic disease. FGF-23 was significantly associated with increased plaque calcification. These findings suggest that abnormal phosphate homeostasis may play an under-recognized but potentially modifiable role in plaque development and in the progression of cardiovascular disease.
Acknowledgments
We thank the KGH catheterization laboratory for their support in this study.
Financial Support: This work was supported by Canada Foundation for Innovation Grant CFI IOF#29051 (to A.M.J.) and by funds from the Ontario Ministry of Research Innovation and Science, the South Eastern Ontario Academic Medical Organization, and the Heart and Stroke Foundation of Canada (to A.M.J.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- CIMT
carotid intima media thickness
- eGFR
estimated glomerular filtration rate
- FGF-23
fibroblast growth factor-23
- GSM
grayscale median
- PDA
pixel distribution analysis
- TPA
total plaque area
References and Notes
- 1. Stompór T. Coronary artery calcification in chronic kidney disease: an update. World J Cardiol. 2014;6(4):115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mizobuchi M, Towler D, Slatopolsky E. Vascular calcification: the killer of patients with chronic kidney disease. J Am Soc Nephrol. 2009;20(7):1453–1464. [DOI] [PubMed] [Google Scholar]
- 3. Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38(4):938–942. [DOI] [PubMed] [Google Scholar]
- 4. Wahlgren CM, Zheng W, Shaalan W, Tang J, Bassiouny HS. Human carotid plaque calcification and vulnerability: relationship between degree of plaque calcification, fibrous cap inflammatory gene expression and symptomatology. Cerebrovasc Dis. 2009;27(2):193–200. [DOI] [PubMed] [Google Scholar]
- 5. Onufrak SJ, Bellasi A, Shaw LJ, Herzog CA, Cardarelli F, Wilson PW, Vaccarino V, Raggi P. Phosphorus levels are associated with subclinical atherosclerosis in the general population. Atherosclerosis. 2008;199(2):424–431. [DOI] [PubMed] [Google Scholar]
- 6. Blaine J, Chonchol M, Levi M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol. 2015;10(7):1257–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khoshniat S, Bourgine A, Julien M, Weiss P, Guicheux J, Beck L. The emergence of phosphate as a specific signaling molecule in bone and other cell types in mammals. Cell Mol Life Sci. 2011;68(2):205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berndt T, Thomas LF, Craig TA, Sommer S, Li X, Bergstralh EJ, Kumar R. Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption [published correction appears in Proc Natl Acad Sci USA. 2007;104(52):21021]. Proc Natl Acad Sci USA. 2007;104(26):11085–11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chande S, Bergwitz C. Role of phosphate sensing in bone and mineral metabolism. Nat Rev Endocrinol. 2018;14(11):637–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Torres PA, De Brauwere DP. Three feedback loops precisely regulating serum phosphate concentration. Kidney Int. 2011;80(5):443–445. [DOI] [PubMed] [Google Scholar]
- 11. Naim C, Douziech M, Therasse E, Robillard P, Giroux MF, Arsenault F, Cloutier G, Soulez G. Vulnerable atherosclerotic carotid plaque evaluation by ultrasound, computed tomography angiography, and magnetic resonance imaging: an overview. Can Assoc Radiol J. 2014;65(3):275–286. [DOI] [PubMed] [Google Scholar]
- 12. Lal BK, Hobson RW II, Pappas PJ, Kubicka R, Hameed M, Chakhtoura EY, Jamil Z, Padberg FT Jr, Haser PB, Durán WN. Pixel distribution analysis of B-mode ultrasound scan images predicts histologic features of atherosclerotic carotid plaques. J Vasc Surg. 2002;35(6):1210–1217. [DOI] [PubMed] [Google Scholar]
- 13. Lal BK, Hobson RW II, Hameed M, Pappas PJ, Padberg FT Jr, Jamil Z, Durán WN. Noninvasive identification of the unstable carotid plaque. Ann Vasc Surg. 2006;20(2):167–174. [DOI] [PubMed] [Google Scholar]
- 14. Menezes FH, do Carmo Silveira T, Silveira SAF, Salles-Cunha SX, Metze K, de Menezes ASC. Preliminary comparisons between in vivo ultrasonographic virtual histology and histopathological findings of endarterectomized carotid plaque. J Vasc Bras. 2013;12(3):193–201. [Google Scholar]
- 15. Heresztyn T, Nicholson BC. A colorimetric protein phosphatase inhibition assay for the determination of cyanobacterial peptide hepatotoxins based on the dephosphorylation of phosvitin by recombinant protein phosphatase 1. Environ Toxicol. 2001;16(3):242–252. [DOI] [PubMed] [Google Scholar]
- 16. Reinert RB, Bixby D, Koenig RJ. Fibroblast growth factor 23-induced hypophosphatemia in acute leukemia. J Endocr Soc. 2018;2(5):437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johri AM, Behl P, Hétu MF, Haqqi M, Ewart P, Day AG, Parfrey B, Matangi MF. Carotid ultrasound maximum plaque height: a sensitive imaging biomarker for the assessment of significant coronary artery disease. Echocardiography. 2016;33(2):281–289. [DOI] [PubMed] [Google Scholar]
- 18. Johri AM, Calnan CM, Matangi MF, MacHaalany J, Hétu MF. Focused vascular ultrasound for the assessment of atherosclerosis: a proof-of-concept study. J Am Soc Echocardiogr. 2016;29(9):842–849. [DOI] [PubMed] [Google Scholar]
- 19. Biasi GM, Froio A, Diethrich EB, Deleo G, Galimberti S, Mingazzini P, Nicolaides AN, Griffin M, Raithel D, Reid DB, Valsecchi MG. Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. Circulation. 2004;110(6):756–762. [DOI] [PubMed] [Google Scholar]
- 20. Shah NH, Dong C, Elkind MS, Sacco RL, Mendez AJ, Hudson BI, Silverberg S, Wolf M, Rundek T, Wright CB. Fibroblast growth factor 23 is associated with carotid plaque presence and area: the Northern Manhattan Study. Arterioscler Thromb Vasc Biol. 2015;35(9):2048–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gutiérrez OM, Isakova T, Smith K, Epstein M, Patel N, Wolf M. Racial differences in postprandial mineral ion handling in health and in chronic kidney disease. Nephrol Dial Transplant. 2010;25(12):3970–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Isakova T, Gutierrez O, Shah A, Castaldo L, Holmes J, Lee H, Wolf M. Postprandial mineral metabolism and secondary hyperparathyroidism in early CKD. J Am Soc Nephrol. 2008;19(3):615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jozefacki A, White CA, Shobeiri NS, Hopman WM, Johri AM, Adams MA, Holden RM. Phosphate excretion is decreased in older cardiac patients with normal kidney function: an emerging dietary risk factor? Appl Physiol Nutr Metab. 2016;41(4):452–455. [DOI] [PubMed] [Google Scholar]
- 24. Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol. 2009;20(2):397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shin S, Kim KJ, Chang HJ, Cho I, Kim YJ, Choi BW, Rhee Y, Lim SK, Yang WI, Shim CY, Ha JW, Jang Y, Chung N. Impact of serum calcium and phosphate on coronary atherosclerosis detected by cardiac computed tomography. Eur Heart J. 2012;33(22):2873–2881. [DOI] [PubMed] [Google Scholar]
- 26. Ix JH, De Boer IH, Peralta CA, Adeney KL, Duprez DA, Jenny NS, Siscovick DS, Kestenbaum BR. Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin J Am Soc Nephrol. 2009;4(3):609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kendrick J, Ix JH, Targher G, Smits G, Chonchol M. Relation of serum phosphorus levels to ankle brachial pressure index (from the Third National Health and Nutrition Examination Survey). Am J Cardiol. 2010;106(4):564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu X, Ma X, Pan X, Hao Y, Luo Y, Lu Z, Bao Y, Jia W. Fibroblast growth factor 23 is associated with the presence of coronary artery disease and the number of stenotic vessels. Clin Exp Pharmacol Physiol. 2015;42(11):1152–1157. [DOI] [PubMed] [Google Scholar]
- 29. Halliday A, Harrison M, Hayter E, Kong X, Mansfield A, Marro J, Pan H, Peto R, Potter J, Rahimi K, Rau A, Robertson S, Streifler J, Thomas D; Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group . 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet. 2010;376(9746):1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis. 2009;53(3):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mataix J, Aranda P, López-Jurado M, Sánchez C, Planells E, Llopis J. Factors influencing the intake and plasma levels of calcium, phosphorus and magnesium in southern Spain. Eur J Nutr. 2006;45(6):349–354. [DOI] [PubMed] [Google Scholar]
- 32. Itkonen ST, Karp HJ, Kemi VE, Kokkonen EM, Saarnio EM, Pekkinen MH, Kärkkäinen MU, Laitinen EK, Turanlahti MI, Lamberg-Allardt CJ. Associations among total and food additive phosphorus intake and carotid intima-media thickness: a cross-sectional study in a middle-aged population in Southern Finland. Nutr J. 2013;12(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Calvo MS, Moshfegh AJ, Tucker KL. Assessing the health impact of phosphorus in the food supply: issues and considerations. Adv Nutr. 2014;5(1):104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]