Abstract
BACKGROUND
Approximately 5% of patients with drug-susceptible tuberculosis have a relapse after 6 months of first-line therapy, as do approximately 20% of patients after 4 months of short-course therapy. We postulated that by analyzing pretreatment isolates of Mycobacterium tuberculosis obtained from patients who subsequently had a relapse or were cured, we could determine any correlations between the minimum inhibitory concentration (MIC) of a drug below the standard resistance breakpoint and the relapse risk after treatment.
METHODS
Using data from the Tuberculosis Trials Consortium Study 22 (development cohort), we assessed relapse and cure isolates to determine the MIC values of isoniazid and rifampin that were below the standard resistance breakpoint (0.1 μg per milliliter for isoniazid and 1.0 μg per milliliter for rifampin). We combined this analysis with clinical, radiologic, and laboratory data to generate predictive relapse models, which we validated by analyzing data from the DMID 01–009 study (validation cohort).
RESULTS
In the development cohort, the mean (±SD) MIC of isoniazid below the breakpoint was 0.0334±0.0085 μg per milliliter in the relapse group and 0.0286±0.0092 μg per milliliter in the cure group, which represented a higher value in the relapse group by a factor of 1.17 (P=0.02). The corresponding MIC values of rifampin were 0.0695±0.0276 and 0.0453±0.0223 μg per milliliter, respectively, which represented a higher value in the relapse group by a factor of 1.53 (P<0.001). Higher MIC values remained associated with relapse in a multivariable analysis that included other significant between-group differences. In an analysis of receiver-operating-characteristic curves of relapse based on these MIC values, the area under the curve (AUC) was 0.779. In the development cohort, the AUC in a multivariable model that included MIC values was 0.875. In the validation cohort, the MIC values either alone or combined with other patient characteristics were also predictive of relapse, with AUC values of 0.964 and 0.929, respectively. The use of a model score for the MIC values of isoniazid and rifampin to achieve 75.0% sensitivity in cross-validation analysis predicted relapse with a specificity of 76.5% in the development cohort and a sensitivity of 70.0% and a specificity of 100% in the validation cohort.
CONCLUSIONS
In pretreatment isolates of M. tuberculosis with decrements of MIC values of isoniazid or rifampin below standard resistance breakpoints, higher MIC values were associated with a greater risk of relapse than lower MIC values. (Funded by the National Institute of Allergy and Infectious Diseases.)
APPROXIMATELY 5% OF PATIENTS WITH drug-susceptible tuberculosis have a relapse after 6 months of first-line therapy,1 and approximately 20% of patients have a relapse after 4 months of short-course therapy, even when regimens include a fluoroquinolone.2,3 Lengthy tuberculosis treatment places burdens on public health systems4,5 and increases the risks of toxic effects, treatment nonadherence, and development of drug resistance.6,7 Thus, the standard 6-month, multidrug therapy that is recommended for the initial treatment of drug- susceptible tuberculosis8 represents a balance between the benefits and liabilities of longterm treatment and the risk of relapse associated with shortened treatments. No measures are available for reliably assigning patients with tuberculosis to different risk groups and treatment durations.9
We postulated that drug-susceptible Mycobacterium tuberculosis might have a graded spectrum of susceptibilities that could be used to determine the risk of relapse. (If the minimum inhibitory concentration [MIC] of a drug — the lowest concentration that prevents visible growth of a bacterium — is below the standard resistance breakpoint, the bacterium is considered to be susceptible; if the MIC is at or above the breakpoint, the bacterium is considered to be intermediate or resistant.) Isolates that have a MIC that is near the standard resistance breakpoint may be less susceptible than isolates with a MIC that is far below the breakpoint.
We assessed decrements of MIC values of isoniazid and rifampin that were below the breakpoint (0.1 μg per milliliter for isoniazid and 1.0 μg per milliliter for rifampin, according to World Health Organization criteria) in pretreatment M. tuberculosis isolates obtained from patients who had a relapse or who had been cured in the Tuberculosis Trials Consortium Study 22 (development cohort). We combined this analysis with clinical, radiologic, and laboratory data to generate predictive models for relapse and then validated the models using data from patients who had a relapse or were cured in the 4-month treatment group in the DMID 01–009 study (validation cohort).
METHODS
SAMPLE SELECTION
Development Cohort
From April 1995 through February 2001 in the United States and Canada, the Tuberculosis Trials Consortium of the Centers for Disease Control and Prevention conducted Study 22,10 which involved 1004 adult patients with drug-susceptible pulmonary tuberculosis who were seronegative for the human immunodeficiency virus (HIV). All the patients had completed at least 8 weeks of standard four-drug therapy containing rifampin, isoniazid, pyrazinamide, and ethambutol. The patients were then randomly assigned to receive either once-weekly rifapentine plus isoniazid or twice-weekly rifampin plus isoniazid for an additional 16 weeks. The patients were followed for 24 months after the completion of therapy to identify a bacteriologically confirmed relapse.
We obtained M. tuberculosis cultures from all 57 patients with a confirmed relapse for whom a frozen isolate of the pretreatment sample could be located and regrown by the Study 22 team (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). We randomly selected an equal number of control samples from pretreatment sputum samples obtained from patients who had been cured (i.e., those who did not meet the Study 22 definition of failure or relapse), plus 11 extra samples to account for possibly contaminated or failed cultures.
Validation Cohort
From April 2002 through December 2008 in Brazil, the Philippines, and Uganda, the Division of Microbiology and Infectious Diseases (DMID) of the National Institute of Allergy and Infectious Diseases conducted the DMID 01–009 study11 involving 394 adult patients with drug- susceptible, noncavitary pulmonary tuberculosis (Fig. S2 in the Supplementary Appendix). All the patients were treated for 2 months with isoniazid, rifampin, ethambutol, and pyrazinamide, which was followed by 2 months of isoniazid and rifampin. Patients who had two negative cultures at 2 months were randomly assigned either to stop therapy (4-month group) or to receive an additional 2 months of isoniazid and rifampin. The patients were followed for 24 months after the end of treatment.
We obtained M. tuberculosis cultures from all 13 patients in the 4-month group with a confirmed relapse for whom a frozen isolate of the pretreatment sample could be located and regrown by the DMID 01–009 study team. We excluded 2 of the 13 relapse isolates after retesting because of culture contamination, which left 11 relapse isolates for further analysis. We randomly selected an equal number of baseline control sputum samples provided by the DMID 01–009 study team from cured patients in the 4-month group, plus 7 extra samples to account for possibly contaminated or failed cultures. Six control isolates were later excluded at retesting because of culture contamination, leaving 14 control isolates for further analysis.
We measured MIC values of isoniazid and rifampin that were below the standard resistance breakpoint in the remaining baseline isolates, except for one relapse isolate that could be tested only for MIC values of isoniazid because of contamination of the culture stocks. Full details regarding the sample-selection methods for both Study 22 and the DMID 01–009 study are provided in the Supplementary Appendix.
CULTURE AND DRUG-SUSCEPTIBILITY TESTING
We performed drug-susceptibility testing using either TREK Sensititre 96-weII plates (Trek Diagnostic Systems), with customized quantities of IyophiIized drugs, or MycobacteriaI Growth Indicator Tubes (BD), according to the manufacturers’ recommendations.12,13 We measured fine gradations in the MIC vaIues that feII beIow standard resistance breakpoints. We performed a series of sequential dilutions of isoniazid by a factor of 1.26 (a value derived from the formula Iog2 X = 1/3) and of rifampin by a factor of 1.41 (a value derived from the formula Iog2 X=1/2) to ensure that the diIution series overIapped with the more conventionaI diIution by a factor of 2 at some points. The final drug concentrations tested (in μg per milliliter) were 0.013, 0.016, 0.020, 0.025, 0.031, 0.040, 0.050, and 0.063 for isoniazid and 0.016, 0.022, 0.031, 0.044, 0.063, 0.088, and 0.125 for rifampin. AdditionaI information regarding culture, susceptibility testing, and DNA sequencing is provided in the SuppIementary Appendix.
STATISTICAL ANALYSIS
We caIcuIated IikeIihood ratios and used Iogistic regression to evaIuate MIC vaIues as weII as other potentiaIIy infIuentiaI covariates (i.e., the characteristics of patients that were recorded as part of Study 22 that describe disease severity or may predict outcomes) as predictors of reIapse.10,14 We used muItivariabIe modeIs that were adjusted for the randomized treatment to evaluate predictors that were significant at the 0.2 IeveI in univariate anaIysis, with the exception of the presence of bilateral disease, because such a finding was coIIinear with cavitation on chest radiography. AII the avaiIabIe data were used for each anaIysis, with an impIicit assumption that within-group data were missing compIeteIy at random. We determined that this assumption was reasonabIe for the observed characteristics (Tables S2, S3, and S4 in the Supplementary Appendix).
Models were fit with the use of the Logistic Procedure (SAS Institute) on Study 22 data. The sampIe in the DMID 01–009 study was scored with the use of models that had been generated only with the use of Study 22 data. We used the pROC package15 to plot receiver-operating-characteristic (ROC) curves and to calculate the area under the ROC curve (AUC) and the PredictABEL package16 to caIcuIate the net recIassification index (NRI) P vaIues that compare ROC curves, both in R software, version 3.4.3 (R Foundation for StatisticaI Computing). We used the Bonferroni method to adjust for muItipIe comparisons in the anaIysis of the MIC vaIues of isoniazid and rifampin. AII other covariates are exploratory, and P vaIues have not been adjusted for muItipIe comparisons. CompIete detaiIs regarding the statisticaI anaIysis are provided in the SuppIementary Appendix.
RESULTS
DEVELOPMENT COHORT
Study Patients
Of the 1004 patients who were enroIIed in Study 22, a totaI of 803 compIeted foIIow-up or had an outcome event; 11 had cIinicaI or microbioIogic treatment faiIure, and 63 had a bacterioIogicaIIy confirmed reIapse.10 The Study 22 team couId not find or recuIture the baseIine M. tuberculosis isolates obtained from 6 patients who had a relapse. Additional isolates that had been obtained from 3 patients who had a relapse were excluded after retesting in the current substudy (2 because the MIC values of isoniazid were higher than the clinical resistance breakpoint of 0.1 μg per milliliter on repeat testing and 1 because of culture contamination), which left 54 relapse isolates for further analysis. The 11 isolates that had been obtained from patients who had treatment failure were also excluded. We randomly selected 68 of 201 isolates provided by the Study 22 team from patients who had been cured. Five control samples were later excluded after retesting (1 because of isoniazid resistance and 4 because of culture contamination), which left 63 control isolates for further analysis. On the remaining baseline isolates, we performed detailed MIC measurements below the breakpoint for isoniazid and rifampin, except in cases in which contamination of the culture stocks made it possible to test MIC values of only one of the two study drugs (Fig. S1 in the Supplementary Appendix).
The clinical and laboratory characteristics of the patients in Study 22 whom we examined in our substudy are shown in Table 1, with additional details shown in Table S1 in the Supplementary Appendix. Characteristics that were significantly associated with relapse were being underweight (defined as a weight that was ≥10% below the ideal body weight17) at diagnosis, the presence of cavitation and bilateral disease on chest radiography, white race, and sputum-culture positivity after 8 weeks of treatment. The Beijing strain of M. tuberculosis (which has been associated with increased virulence and multidrug resistance) was identified in 24% of the patients who had a relapse and in 20% of those who were cured, findings that were consistent with the overall results of Study 22 and of previous studies.
Table 1.
Characteristics ofthe Patients in the Development Cohort.*
| Characteristic | Relapse (N = 54) |
Cure (N = 63) |
P Value |
|---|---|---|---|
| Mean age ±SD — yr | 41±14 | 42±14 | 0.74 |
| Male sex — no. (%) | 45 (83) | 50 (79) | 0.64 |
| Race or ethnic group — no. (%)† | 0.31 | ||
| White non-Hispanic | 19 (35) | 12 (19) | |
| Black non-Hispanic | 20 (37) | 26 (41) | |
| Hispanic | 8 (15) | 13 (21) | |
| Asian or Pacific Islander | 5 (9) | 9 (14) | |
| American Indian or Alaskan Native | 2 (4) | 3 (5) | |
| Tuberculosis type — no. (%) | >0.99 | ||
| Pulmonary only | 52 (96) | 61 (97) | |
| Pulmonary and extrapulmonary | 2 (4) | 2 (3) | |
| Underweight by >10% — no. (%) | 32 (59) | 23 (37) | 0.02 |
| Diabetes — no. (%) | 6 (11) | 7 (11) | >0.99 |
| Results on radiography — no./total no. (%) | |||
| Chest cavitation | 40/52 (77) | 32/59 (54) | 0.02 |
| Bilateral disease | 41/53 (77) | 31/62 (50) | 0.002 |
| Results on 8-week sputum culture — no. (%) | <0.001 | ||
| No growth | 25 (46) | 48 (76) | |
| Mycobacterium tuberculosis | 29 (54) | 14 (22) | |
| Other mycobacteria | 0 | 1 (2) | |
| Randomized treatment — no. (%) | 0.13 | ||
| Rifapentine | 36 (67) | 32 (51) | |
| Rifampin | 18 (33) | 31 (49) | |
| Positive results on first acid-fast bacillus smear — no./total no. (%) | 47/54 (87) | 47/62 (76) | 0.25 |
| Median Karnofsky score (IQR)‡ | 90 (90–100) | 90 (90–100) | 0.45 |
| Beijing genotype — no./total no. (%)§ | 13/53 (25) | 10/51 (20) | 0.64 |
IQR denotes interquartile range.
Race or ethnic group was reported by the patients.
Scores on the Karnofsky performance scale range from 0 to 100, with higher scores indicating better performance.
The Beijing genotype has been associated with increased virulence and multidrug resistance.
Association between MIC Values and Relapse
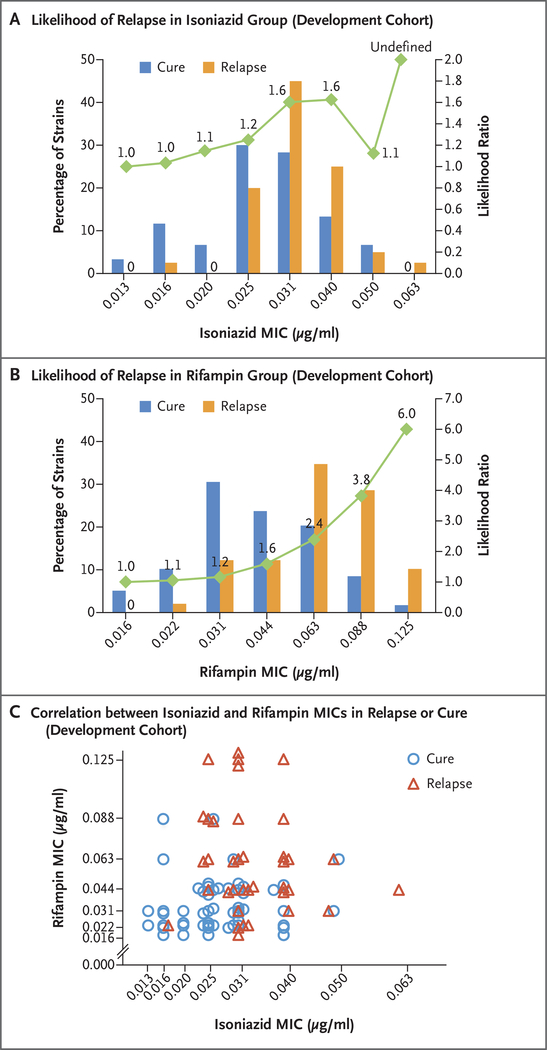
The baseline MIC values of isoniazid in the pretreatment isolates obtained from patients who had a relapse or were cured are shown in Figure 1A. The mean (±SD) MIC of isoniazid below the breakpoint was 0.0334±0.0085 μg per milliliter among the patients who had a relapse and 0.0286±0.0092 μg per milliliter among those who were cured, which represented a higher value in the relapse group by a factor of 1.17 (95% confidence interval [CI], 1.03 to 1.33; P = 0.02 after adjustment for multiple comparisons). As compared with the relapse risk at the lowest MIC of isoniazid, the likelihood ratio for relapse generally increased with increased MIC values.
Figure 1. MIC Values of Isoniazid and Rifampin and Treatment Outcomes in the Development Cohort.
Shown are the minimum inhibitory concentration (MIC) values of isoniazid (Panel A) and rifampin (Panel B) in Mycobacterium tuberculosis isolates obtained from patients in the development cohort, according to whether the patients were cured or had a relapse after the completion of treatment. The green curve indicates the likelihood ratios for a relapse for each MIC value shown. For MIC values at which no cure or relapse occurred, the absence is indicated by a zero. In Panel A, the likelihood ratio for the highest MIC value is undefined because there were no cures at this value. Panel C shows the MIC values of isoniazid and rifampin, according to cure or relapse status, in a subgroup of isolates in which the MIC values of both isoniazid and rifampin were known. Superimposed symbols have been slightly offset on the x and y axes for clarity.
The corresponding MIC values of rifampin are shown in Figure 1B. The mean MIC of rifampin below the breakpoint was 0.0695±0.0276 μg per milliliter among the patients who had a relapse and 0.0453±0.0223 μg per milliliter among those who were cured, which represented a higher value in the relapse group by a factor of 1.53 (95% CI, 1.27 to 1.86; adjusted P<0.001). The likelihood ratio for relapse increased with each increment of MIC, reaching observed likelihood ratios of 3.8 and 6.0 at the two highest rifampin concentrations tested.
For the isolates that had MIC values available for both isoniazid and rifampin, plotting each isolate with both MIC values again suggested a group of highly susceptible isolates that were predominately associated with cure (isolates with concordant low MIC values) and a second group of more resistant isolates that were predominantly associated with relapse (isolates with concordant higher MIC values) (Fig. 1C).
Models of Relapse Risk
In the univariate models, factors that were associated with an increased risk of relapse were a high MIC of isoniazid or rifampin, a positive 8-week sputum culture, being underweight, cavitation on chest radiography, white race, and bilateral disease. Although the odds ratios for relapse according to the MIC values of isoniazid and rifampin (1.83 and 1.47, respectively) were relatively small, these odds ratios were associated with each MIC increase of 0.01 μg per milliliter, whereas the total range of MIC values that were measured was approximately 10 times this increment. Thus, such calculations led to large odds ratios for large differences in MIC values. The differences in MIC values of both isoniazid and rifampin remained significant in a multivariable model that included only these MIC values as dependent variables (odds ratio for isoniazid, 1.38 [P = 0.002]; and odds ratio for rifampin, 2.14 [P=0.02]). In a model that included all the variables that were significant in the univariate analysis (all-variable model), the factors that showed an independent association with an increased risk of relapse were a high MIC of isoniazid or rifampin, a positive 8-week sputum culture, and being underweight.
The 8-week culture results are not measurable at or near the time of treatment initiation, which is an undesirable feature for a treatment biomarker. In the all-variable model that excluded the 8-week culture result, cavitation on chest radiography (a radiologic sign of an increased bacterial load) was significantly associated with an increased relapse risk, along with increased MIC values of isoniazid and rifampin and being underweight. Notably, there was no significant association between relapse and receipt of either rifampin or rifapentine in either the univariate model or the multivariable model, a finding that was consistent with the results from the parent study.10
ROC Curves for Relapse Risk
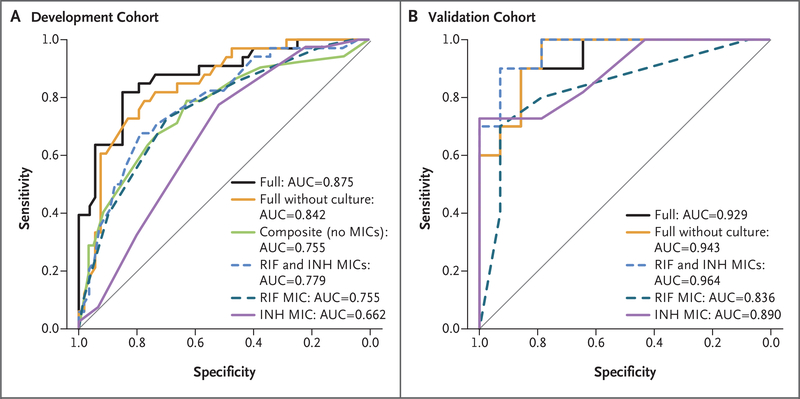
We examined the ROC curves for relapse to visualize the trade-off between sensitivity and specificity (Fig. 2A). The MIC values of isoniazid alone predicted relapse on ROC analysis (AUC, 0.662); the MIC values of rifampin alone or of isoniazid plus rifampin performed better (AUC, 0.755 [P = 0.07] and 0.779 [P<0.001], respectively). The ROC curves for either rifampin alone or isoniazid plus rifampin and their AUCs were similar to the ROC curves in a composite model that used clinical and laboratory measurements that were independently significant, with the exception of MIC values of isoniazid and rifampin (AUC, 0.755; P=0.81). Adding the MIC values of isoniazid and rifampin (full model) led to a better prediction of relapse (AUC, 0.875; P<0.001), and removing the 8-week culture result from the full model had a small but significant effect (AUC, 0.842; P<0.001). Estimated odds ratios and ROC AUCs are shown in Table 2, and coefficients for select models are shown in Table S5 in the Supplementary Appendix.
Figure 2. Receiver-Operating-Characteristic (ROC) Curves for Relapse after Tuberculosis Treatment.
Shown are ROC curves in the development cohort (Panel A) and the validation cohort (Panel B). Curves are displayed for MIC values of isoniazid (INH) and rifampin (RIF) alone, for MIC values of isoniazid plus rifampin, and for the other models discussed below, as indicated. ROC curves are graphical plots that illustrate the performance of a binary classifier system as its discrimination threshold is varied. The curves were created by plotting the true positive rate against the false positive rate at various threshold settings. The area under the curve (AUC) that is shown in each plot summarizes the overall biomarker performance in a single number, with 0.5 indicating no difference from chance and 1.0 indicating a perfect biomarker with sensitivity and specificity both equal to 100%. The full model includes the following factors: MIC values of isoniazid and rifampin, cavitary disease on radiography, being underweight, and a positive 8-week sputum culture. The full model without culture results includes the same covariates as the full model with the exclusion of a positive 8-week sputum culture. The composite model includes the same covariates as the full model with the exclusion of the MIC values of isoniazid and rifampin.
Table 2.
Logistic-Regression Models PredictingTuberculosis Relapse after Treatment.*
| Variable | Univariate Model | Rifampin and Isoniazid MIC Model |
All-Variable Model† | All-Variable Model without 8-Wk Sputum Culture |
Full Model‡ | Full Model without 8-Wk Sputum Culture |
Composite Model§ |
|---|---|---|---|---|---|---|---|
| Isoniazid MIC | NA | ||||||
| Odds ratio per increase of 0.01 μg/ml (95% CI) | 1.83 (1.08–3.28) |
2.14 (1.15–4.40) |
2.81 (1.29–7.34) |
2.43 (1.17–5.85) |
2.65 (1.36–5.84) |
2.41 (1.17–5.68) |
|
| P value | 0.02 | 0.02 | 0.01 | 0.02 | 0.008 | 0.02 | |
| Rifampin MIC | NA | ||||||
| Odds ratio per increase of 0.01 μg/ml (95% CI) | 1.47 (1.21–1.85) |
1.38 (1.12–1.75) |
1.44 (1.13–1.94) |
1.45 (1.15–1.90) |
1.43 (1.15–1.84) |
1.44 (1.15–1.89) |
|
| P value | <0.001 | 0.002 | 0.004 | 0.002 | 0.002 | 0.002 | |
| Underweight by >10% | NA | ||||||
| Odds ratio (95% CI) | 2.53 (1.21–5.41) |
4.06 (1.19–16.01) |
3.21 (1.06–10.55) |
3.99 (1.17–15.55) |
3.21 (1.06–10.56) |
3.03 (1.31–7.29) |
|
| P value | 0.01 | 0.03 | 0.04 | 0.03 | 0.04 | 0.01 | |
| Cavitation on chest radiography | NA | ||||||
| Odds ratio (95% CI) | 2.81 (1.26–6.58) |
2.75 (0.76–11.10) |
3.92 (1.20–14.85) |
2.71 (0.75–10.85) |
3.92 (1.20–14.80) |
1.88 (0.77–4.66) |
|
| P value | 0.01 | 0.13 | 0.03 | 0.14 | 0.03 | 0.17 | |
| Bilateral disease on chest radiography | NA | NA | NA | NA | NA | NA | |
| Odds ratio (95% CI) | 3.42 (1.54–7.93) |
||||||
| P value | 0.003 | ||||||
| Positive 8-wk sputum culture | NA | NA | NA | ||||
| Odds ratio (95% CI) | 4.06 (1.86–9.24) |
7.46 (2.18–29.48) |
6.85 (2.05–26.09) |
3.87 (1.60–9.84) |
|||
| P value | <0.001 | 0.002 | 0.003 | 0.003 | |||
| White race | NA | NA | NA | NA | |||
| Odds ratio (95% CI) | 2.31 (1.01–5.47) |
0.60 (0.13–2.41) |
0.93 (0.25–3.29) |
||||
| P value | 0.05 | 0.48 | 0.91 | ||||
| Treatment with rifapentine vs. rifampin |
NA | ||||||
| Odds ratio (95% CI) | 1.94 (0.92–4.16) |
1.14 (0.33–3.92) |
1.34 (0.44–4.16) |
1.09 (0.32–3.69) |
1.33 (0.44–4.13) |
1.66 (0.71–3.95) |
|
| P value | 0.08 | 0.83 | 0.61 | 0.89 | 0.61 | 0.24 | |
| ROC AUC (95% CI)¶ | NA | 0.779 (0.680–0.877) |
0.880 (0.806–0.954) |
0.841 (0.756–0.925) |
0.875 (0.798–0.952) |
0.842 (0.756–0.927) |
0.755 (0.663–0.847) |
Shown are the eight covariates that were included in models; there was no adjustment for additional covariates. Bilateral disease was included as a variable only in the univariate model, because it was collinear with cavitation on chest radiography. For the minimum inhibitory concentration (MIC) values of rifampin and isoniazid, the P values and 95% confidence intervals have been adjusted by the Bonferroni method; all other variables are exploratory, and the P values and 95% confidence intervals have not been adjusted for multiple comparisons.
NA denotes not applicable.
The all-variable model includes all the variables with the exception of bilateral disease.
The full model includes the MIC values of rifampin and isoniazid, being underweight by 10% or more, cavitation on chest radiography, positive 8-week sputum culture, and randomized study treatment but does not include white race.
The composite model includes being underweight, cavitation on chest radiography, positive 8-week sputum culture, and randomized study treatment.
Receiver-operating-characteristic (ROC) curves for relapse were examined to visualize the trade-off between sensitivity and specificity. The area under the curve (AUC) summarizes the overall biomarker performance in a single number, with 0.5 indicating no difference from chance and 1.0 indicating a perfect biomarker with sensitivity and specificity both equal to 100%.
Biologic Causes of Variation in MIC Values
To look for the biologic causes of variability in MIC values below the standard resistance breakpoint, we tested the DNA sequence of the 81-bp region of the M. tuberculosis gene encoding the beta subunit of RNA polymerase (rpoB), which is associated with approximately 95% of all rifampin resistance.18 We also performed whole-genome sequencing of eight isolates with a high MIC value and seven isolates with a low value. However, neither approach identified mutations that explained the observed variation in MIC values19,20 (see the Supplementary Appendix). Furthermore, the addition of pump inhibitors to bacterial cultures did not lower the MIC values in isolates with elevated values, as would be expected if the induction of efflux pumps were the cause of the elevated values (Fig. S3 in the Supplementary Appendix). On spoligotyping (i.e., the analysis of polymorphisms in certain repeat units in DNA), there was no significant association between the Beijing strain of M. tuberculosis and the MIC of isoniazid or rifampin (P = 0.21 and P = 0.70, respectively).
VALIDATION COHORT
Study Patients
Pertinent clinical and laboratory characteristics of the patients that we examined in our study are shown in Table S6 in the Supplementary Appendix. Factors that were significantly associated with relapse were an older age and an increased severity of disease on radiography, according to the criteria of the National Tuberculosis and Respiratory Disease Association. No patients in the DMID 01–009 study had cavitary disease or positive cultures after 8 weeks of therapy, since these factors were among the exclusion criteria.
Testing Predictive Models
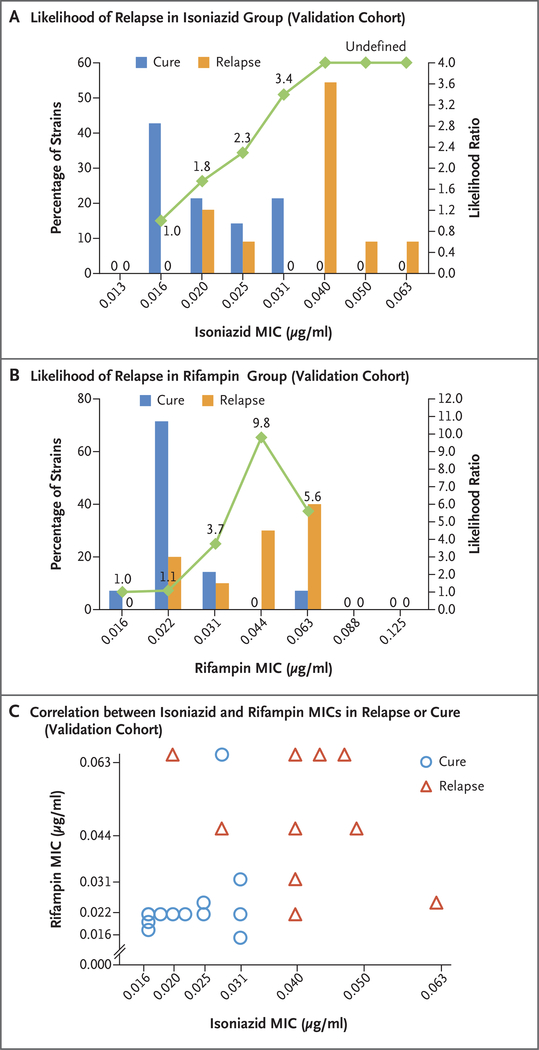
In the validation cohort, the mean MIC of isoniazid below the breakpoint was 0.0380±0.0127 μg per milliliter in patients who had a relapse and 0.0214±0.0061 μg per milliliter in patients who were cured, which represented a higher value in the relapse group by a factor of 1.78 (95% CI, 1.29 to 2.39; adjusted P = 0.002); the corresponding MIC value of rifampin was 0.0459±0.0168 μg per milliliter in the relapse isolate and 0.0258± 0.0114 μg per milliliter in the cure isolate, which represented a higher value in the relapse group by a factor of 1.78 (95% CI, 1.19 to 2.67; adjusted P=0.006). Furthermore, there was a correlation between the likelihood ratio for relapse and the MIC value (Fig. 3A and 3B). Plotting each isolate according to the MIC value of isoniazid and rifampin again suggested that several highly susceptible isolates were predominately associated with a cure and several other more resistant isolates were predominantly associated with a relapse (Fig. 3C).
Figure 3. MIC Values of Isoniazid and Rifampin and Treatment Outcomes in the Validation Cohort.
Shown are the MIC values of isoniazid (Panel A) and rifampin (Panel B), according to treatment outcome in the validation cohort, with corresponding likelihood ratios for relapse indicated. In Panel A, the likelihood ratios for the three highest MIC values are undefined because there were no cures at these values. For MIC values at which no cure or relapse occurred, such an absence is indicated by a zero. Panel C shows the MIC values of isoniazid and rifampin, according to cure or relapse status, in a subgroup of isolates for which the MIC values of both isoniazid and rifampin were known. Superimposed symbols have been slightly offset on the x and y axes for clarity.
We used models that had been generated with data from the development cohort to confirm the strong association between higher MIC values and relapse (Table S5 in the Supplementary Appendix). The model that was based only on MIC values of isoniazid and rifampin and the full model that was based on MIC values plus all significant clinical characteristics produced AUCs of 0.964 (95% CI, 0.903 to 1.00) and 0.929 (95% CI, 0.830 to 1.00), respectively (Fig. 2B). The use of the development cohort model that featured MIC values of both isoniazid and rifampin with breakpoint thresholds selected to correspond to 75% sensitivity in internal cross-validation provided a specificity of 76.5% (95% CI, 60.0 to 87.6) in the development cohort and a sensitivity of 70.0% (95% CI, 39.7 to 89.2) and a specificity of 100% (95% CI, 78.5 to 100) in the validation cohort for identifying relapse cases.
DISCUSSION
Our results suggest that measurable characteristics of the infecting M. tuberculosis strain are at least as important as clinical, radiologic, and microbiologic features that have been previously associated with relapse in patients with tuberculosis. The validation of our risk models in isolates obtained from patients who had been assigned to receive 4 months of treatment with antituberculosis drugs suggests that MIC measurements may be useful in selecting patients who can be safely treated with shorter-duration regimens. We selected MIC cutoffs for isoniazid and rifampin in the development cohort with the goal of detecting a sensitivity of 75% for relapse, which had a high specificity. However, lower cutoffs could be selected to enhance sensitivity with some loss of specificity if enhanced identification of relapse is desired. Further studies are needed to determine the ideal treatment duration for patients who have been classified as being at high risk for relapse. However, a treatment duration of more than 6 months may be warranted in this group.
Our results, combined with the findings of other recent studies showing an association between inadequate drug levels and a worse outcome in patients being treated for tuberculosis,21–25 suggest that regimens that include higher-potency drugs at higher doses could be beneficial. Differences in MIC values may also reflect more fundamental differences in the biologic characteristics of the infecting M. tuberculosis strain that were not detected on whole-genome sequencing because of their rarity and variability. With a more in-depth understanding of these factors, treatment may be improved by new antituberculosis agents.
Our study is limited by its relatively small size and retrospective design. However, we were able to confirm the predictive ability of our models in a validation cohort, an approach that is widely used for validating potential biomarkers.26–28 Another limitation of our study is that we could not test the MIC values of drugs in the primary M. tuberculosis culture made directly from the patient’s sputum isolate. Instead, isolates were subcultured at least three times before MIC testing. This period of in vitro culture might have allowed isolates to acquire new mutations that altered the MIC values. However, the isolates were not exposed to isoniazid or rifampin during any of these subcultures; thus, it is unlikely that repeated culturing of isolates affected the MIC results. Another potential limitation is that data for patients who were lost to follow-up (20% in Study 22 and 6% in the DMID 01–009 study) or who died were excluded from our analyses. These results could have been competing risks for the primary outcome.
In conclusion, we found that decrements of MIC values of isoniazid and rifampin that were below the standard resistance breakpoint in drugsusceptible M. tuberculosis strains had an influence on treatment outcomes, with the risk of relapse increasing together with the MIC value. In addition, we confirmed these findings in isolates obtained from patients with tuberculosis in a validation cohort. Additional studies that are performed in larger, well-defined prospective cohorts and that include MIC testing of pretreatment culture isolates will be useful to better validate these findings.
Supplementary Material
Acknowledgments
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or of the National Institutes of Health.
Supported by grants from the National Institute of Allergy and Infectious Diseases (U01AI065663, U19AI11276, NO1-AI95383, and HHSN266200700022C/N01-AI-70022).
We thank Lorna Bozeman, Beverly Metchock, and Kit Whitworth for making available the isolates and clinical data from patients in the Tuberculosis Trials Consortium Study 22; Andrey Vernon for providing insights and guidance in analyzing the data and in the preparation of an earlier version of the manuscript; and Nadine Sullivan and the staff of Trek Diagnostic Systems for providing custom Trek plates for drug-susceptibility testing.
APPENDIX
The authors’ full names and academic degrees are as follows: Roberto Colangeli, Ph.D., Hannah Jedrey, Ph.D., Soyeon Kim, Sc.D., Roy Connell, M.Ph., Shuyi Ma, Ph.D., Uma D. Chippada Venkata, M.S., Soumitesh Chakravorty, Ph.D., Aditi Gupta, Ph.D., Erin E. Sizemore, M.P.H., Lois Diem, B.S., David R. Sherman, Ph.D., Alphonse Okwera, M.B., Ch.B., Reynaldo Dietze, M.D., W. Henry Boom, M.D., John L. Johnson, M.D., William R. Mac Kenzie, M.D., and David Alland, M.D.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
R. Colangeli, Department of Medicine, Rutgers-New Jersey Medical School
H. Jedrey, Department of Medicine, Rutgers-New Jersey Medical School
U.D. Chippada Venkata, Department of Medicine, Rutgers-New Jersey Medical School.
S. Chakravorty, Department of Medicine, Rutgers-New Jersey Medical School
A. Gupta, Department of Medicine, Rutgers-New Jersey Medical School
D. Alland, Department of Medicine, Rutgers-New Jersey Medical School
S. Kim, Department of Biostatistics, Rutgers School of Public Health, Newark
R. Connell, Department of Biostatistics, Rutgers School of Public Health, Newark
S. Ma, Center for Infectious Disease Research, Seattle
D.R. Sherman, Center for Infectious Disease Research, Seattle
E.E. Sizemore, Centers for Disease Control and Prevention, Atlanta
L. Diem, Centers for Disease Control and Prevention, Atlanta
W.R. Mac Kenzie, Centers for Disease Control and Prevention, Atlanta.
A. Okwera, Uganda-Case Western Reserve University Research Collaboration, Kampala, Uganda
R. Dietze, Nucleo de Doencas Infecciosas, Centro de Ciencias da Saude, Universidade Federal do Espirito Santo, Vitoria, Brazil
W.H. Boom, Tuberculosis Research Unit, Department of Medicine, Case Western Reserve University School of Medicine and University Hospitals Cleveland Medical Center, Cleveland
J.L. Johnson, Tuberculosis Research Unit, Department of Medicine, Case Western Reserve University School of Medicine and University Hospitals Cleveland Medical Center, Cleveland
REFERENCES
- 1.Lambert ML, Hasker E, Van Deun A, Roberfroid D, Boelaert M, Van der Stuyft P. Recurrence in tuberculosis: relapse or reinfection? Lancet Infect Dis 2003;3:282–7. [DOI] [PubMed] [Google Scholar]
- 2.Merle CS, Fielding K, Sow OB, et al. A four-month gatifloxacin-containing regimen for treating tuberculosis. N Engl J Med 2014;371:1588–98. [DOI] [PubMed] [Google Scholar]
- 3.Gillespie SH, Crook AM, McHugh TD, et al. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 2014;371:1577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ukwaja KN, Alobu I, Lgwenyi C, Hopewell PC. The high cost of free tuberculosis services: patient and household costs associated with tuberculosis care in Ebonyi State, Nigeria. PLoS One 2013; 8(8):e73134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks SM, Hirsch-Moverman Y, Salcedo K, et al. Characteristics and costs of multidrug-resistant tuberculosis in-patient care in the United States, 2005–2007. Int J Tuberc Lung Dis 2016;20:435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Senousy BE, Belal SI, Draganov PV. Hepatotoxic effects of therapies for tuberculosis. Nat Rev Gastroenterol Hepatol 2010;7:543–56. [DOI] [PubMed] [Google Scholar]
- 7.de Steenwinkel JE, ten Kate MT, de Knegt GJ, et al. Consequences of noncompliance for therapy efficacy and emergence of resistance in murine tuberculosis caused by the Beijing genotype of Mycobacterium tuberculosis. Antimicrob Agents Chemother 2012;56:4937–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treatment for TB disease. Atlanta: Centers for Disease Control and Prevention, 2016. (http://www.cdc.gov/tb/topic/treatment/tbdisease.htm). [Google Scholar]
- 9.Horne DJ, Royce SE, Gooze L, et al. Sputum monitoring during tuberculosis treatment for predicting outcome: systematic review and meta-analysis. Lancet Infect Dis 2010;10:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benator D, Bhattacharya M, Bozeman L, et al. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet 2002;360:528–34. [DOI] [PubMed] [Google Scholar]
- 11.Johnson JL, Hadad DJ, Dietze R, et al. Shortening treatment in adults with noncavitary tuberculosis and 2-month culture conversion. Am J Respir Crit Care Med 2009;180:558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J, Armstrong DT, Ssengooba W, et al. Sensititre MYCOTB MIC plate for testing Mycobacterium tuberculosis susceptibility to first- and second-line drugs. Antimicrob Agents Chemother 2014;58: 11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Springer B, Lucke K, Calligaris-Maibach R, Ritter C, Böttger EC. Quantitative drug susceptibility testing of Mycobacterium tuberculosis by use of MGIT 960 and EpiCenter instrumentation. J Clin Microbiol 2009;47:1773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan A, Sterling TR, Reves R, Vernon A, Horsburgh CR. Lack of weight gain and relapse risk in a large tuberculosis treatment trial. Am J Respir Crit Care Med 2006;174:344–8. [DOI] [PubMed] [Google Scholar]
- 15.Robin X, Turck N, Hainard A, et al. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kundu S, Aulchenko YS, van Duijn CM, Janssens AC. PredictABEL: an R package for the assessment of risk prediction models. Eur J Epidemiol 2011;26:261–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.1983 Metropolitan height and weight tables. Stat Bull Metrop Life Found 1983; 64:3–9. [PubMed] [Google Scholar]
- 18.Ramaswamy S, Musser JM. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber Lung Dis 1998;79:3–29. [DOI] [PubMed] [Google Scholar]
- 19.Seifert M, Catanzaro D, Catanzaro A, Rodwell TC. Genetic mutations associated with isoniazid resistance in Mycobacterium tuberculosis: a systematic review. PLoS One 2015;10(3):e0119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jamieson FB, Guthrie JL, Neemuchwala A, Lastovetska O, Melano RG, Mehaffy C. Profiling of rpoB mutations and MICs for rifampin and rifabutin in Mycobacterium tuberculosis. J Clin Microbiol 2014;52: 2157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babalik A, Babalik A, Mannix S, Francis D, Menzies D. Therapeutic drug monitoring in the treatment of active tuberculosis. Can Respir J 2011;18:225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta JB, Shantaveerapa H, Byrd RP Jr, Morton SE, Fountain F, Roy TM. Utility of rifampin blood levels in the treatment and follow-up of active pulmonary tuberculosis in patients who were slow to respond to routine directly observed therapy. Chest 2001;120:1520–4. [DOI] [PubMed] [Google Scholar]
- 23.Totapally BR, Walsh WT. Pneumococcal bacteremia in childhood: a 6-year experience in a community hospital. Chest 1998;113:1207–14. [DOI] [PubMed] [Google Scholar]
- 24.Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 2013;208:1464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srivastava S, Pasipanodya JG, Ramachandran G, et al. A long-term co-perfused disseminated tuberculosis-3D liver hollow fiber model for both drug efficacy and hepatotoxicity in babies. EBioMedicine 2016;6:126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson ST, Kaforou M, Brent AJ, et al. Diagnosis of childhood tuberculosis and host RNA expression in Africa. N Engl J Med 2014;370:1712–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rassi A Jr, Rassi A, Little WC, et al. Development and validation of a risk score for predicting death in Chagas’ heart disease. N Engl J Med 2006;355:799–808. [DOI] [PubMed] [Google Scholar]
- 28.Taylor JM, Ankerst DP, Andridge RR. Validation of biomarker-based risk prediction models. Clin Cancer Res 2008;14: 5977–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.