Abstract
Ethnopharmacological Relevance
Mental and neurological disorders are a serious public health challenge globally, particularly in developing countries where cultural factors and limited access to standard healthcare have led to a reliance on traditional medicines. However, ethnopharmacological characterization of traditional medicines used to treat these diseases is lacking. In this study, an ethnobotanical description of plant species used in treating mental and neurological disorders in Ghana and an update of their experimentally validated pharmacological relevance are provided.
Materials and Methods
Two hundred herbalists agreed to participate but sixty-six specialized in treating mental and neurological disorders were interviewed on their traditional medical practice. Literature review was conducted to verify the experimentally validated pharmacological importance of the reported plants.
Results
Thirty-two plant species belonging to twenty-eight families were identified. Most plant species had either analgesic (50%), anxiolytic (18.8%), or anticonvulsant (15.6%) properties. Others had reported sedative, anti-Alzheimer's disease, motor coordination, antipsychotic, antidepressant, cognitive enhancement, and neuroprotective properties. While Ageratum conyzoides L. (Asteraceae) and Ocimum gratissimum L. (Lamiaceae) were the most commonly mentioned species with analgesic properties, Lantana camara L. (Verbenaceae) was the most-reported anxiolytic product, with Cymbopogon citratus DC. (Gramineae), Mangifera indica L., Tetrapleura tetraptera Schum Taub. (Fabaceae), and Persea Americana Mill (Lauraceae) being the most studied anticonvulsants.
Conclusions
This study provides the first report specifically on medicinal plants used in treating mental and neurological disorders in Ghana. Most of the identified plants have been scientifically confirmed to possess neuro- and psychopharmacological properties and may serve as templates for drug development.
1. Introduction
The World Health Organization (WHO) estimates that more than one billion people suffer from central and peripheral nervous system (CNS/PNS) disorders globally [1, 2]. These diseases include Parkinson's disease, epilepsy, schizophrenia, bipolar disorder, Alzheimer's disease and other dementias, neuroinfections, brain tumors, traumatic disorders, and cerebrovascular diseases such as stroke and migraine. More than 6 million people reportedly die each year due to stroke, with over 80% of these deaths occurring in low- and middle-income countries [2]. Moreover, although little research attention has been paid to diseases such as schizophrenia, bipolar disorder, and other psychotic disorders in Africa, some studies have shown that schizophrenia is a major psychiatric diagnosis leading to in-patient admissions on these continents [3–6]. In addition, the CNS/PNS disease burden in Africa is exacerbated by the numerous but understudied neurological impairments associated with common tropical diseases such as the neglected tropical diseases [7].
Ghana is host to a wide array of medicinal flora and takes pride in the longstanding cultural use of traditional and alternative medicines (TAMs), as exhibited by the several published works on the ethnobotanical use of TAMs in the country [8–11]. Nonetheless, there are concerns about the safety and efficacy claims of some TAMs [12]. In order to address these concerns while enhancing the therapeutic potentials of TAMs and ensuring minimum adverse effects, the Ghanaian Government, academics, and TAM practitioners have institutionalized measures to regulate herbal medicine practice and also integrate TAMs into the mainstream healthcare system. For example, the Ghana Federation of Traditional Medical Practitioners Association (GHAFTRAM) was established in 1999 to help modernize, restructure, and regulate the traditional medical industry in the country [13]. GHAFTRAM has members from all parts of Ghana, working together towards advancing the development of TAMs. In addition, an undergraduate program in herbal medicine which complements university training with hands-on internships at a herbal medicine research centre as well as herbal and allopathic practitioners has been established [14]. On passing their professional qualifying examination, graduates are certified and regulated by Ghana's Traditional Medical Practice Council, and some are employed by the Government to practice as medical herbalists in herbal clinics established within public hospitals to work in partnership with medical and allied health staff to provide curative and preventive medical care [12, 14].
The foregoing measures emphasize that TAMs continue to play a significant role in the treatment of various disorders including those of CNS/PNS origin [15–17]. However, there have been no studies focusing primarily on the documentation of traditional methods of treating mental and neurological disorders in Ghana, and how these may inform healthcare practice, policy, and drug development. Consequently, comprehensive information on plant species, plant parts used, cultural practices, and methods of preparation and use of these TAMs are lacking. Moreover, the therapeutic potential, CNS properties, and the safety profile of most of these products are largely unknown. The present study sought to address this knowledge gap by using a guided survey to document TAMs used in the treatment of mental and neurological disorders in Ghana based on traditional knowledge. Moreover, we aimed to ascertain the scientifically confirmed pharmacological relevance of these medicinal products that may justify their clinical use and further research to isolate compounds of interest to drug discovery and development. Specifically, the study was aimed at (a) identifying commonly used TAMs for CNS/PNS disorders and their modes of preparation and (b) documenting the therapeutic potentials of these products.
2. Materials and Methods
2.1. Selection of Participants, Obtaining prior Informed Consent, Ethical Approval, and Data Collection
An ethnobotanical approach was used to explore the knowledge and treatment practices of mental and nervous system disorders by traditional medical practitioners (TMPs) from various districts and subdistricts of the Greater Accra and Brong-Ahafo regions of Ghana. Study participants were TMPs who were all members of GHAFTRAM attending a meeting in Accra. The study objectives, method, and planned use of information were explained to the TMPs before the interviews. Among the 200 TMPs present at the GHAFTRAM meeting, 66 were included in this study. The excluded delegates were not specialized in the treatment of mental and neurological diseases, as they found such patients quite difficult to manage.
A guided questionnaire interview approach was used: during the interviews conducted in both English and Twi, a local Ghanaian dialect, information on the types and parts of plant materials used, the methods of preparation, the local names of plants, and the mode of administration of herbal products were obtained. To be included in the interview, one had to be a (a) TMP practicing in Ghana, potentially treating mental and neurological diseases directly or having some level of knowledge on products used in treating such patients or (b) registered member of GHAFTRAM willing to participate in the survey. Approval for this study was granted by the Scientific and Technical Committee of the Noguchi Memorial Institute for Medical Research, Accra, Ghana, reference number STC-4 (2) 2013-14. Prior to the study, permission was granted from the leadership of GHAFTRAM and all participants duly signed informed consents.
2.2. Data Management and Analysis
A list of the plants obtained from the survey was subjected to thorough review using Internet search engines (such as google scholar) and journal databases such as Medline, Embase, Scopus, and Pubmed to confirm their therapeutic potential. A search of Ghanaian and West African herbal pharmacopoeias was done using the following search terms: “neurological disorders”, “psychiatric disorders”, “schizophrenia”, “Parkinson's disease”, “Alzheimer's disease”, and “mental disorders” in combination with either “Ghana”, “West Africa”, or “Africa”.
Data obtained from the ethnobotanical study were analyzed using the Statistical Package for Social Sciences version 22.0 for Windows.
3. Results
3.1. Sociodemographic Characteristics of Respondents
In total, 66 TMPs were interviewed: 65 and 1 from the Greater Accra and Brong-Ahafo regions, respectively. About 56.1% were males and 43.9% were females. About 40.9% of the TMPs were either 50-59-year-old or 60 years and above (27.3%). In addition, while 65.2% were married, 22.7% were single, 10.6% were widowed, and 1.5% were divorced. Most TMPs had either primary school (53.1%) or secondary school (28.1%) education (Table 1); only 10.9% had some form of postsecondary education.
Table 1.
Sociodemographic characteristics of traditional medical practitioners who treat mental and neurological disorders in Ghana.
| Variable | Frequency | Percentage (%) |
|---|---|---|
| Sex | ||
| Male | 37 | 56.1 |
| Female | 29 | 43.9 |
|
| ||
| Age (years) | ||
| 20-29 | 1 | 1.5 |
| 30-39 | 8 | 12.1 |
| 40-49 | 12 | 18.2 |
| 50-59 | 27 | 40.9 |
| 60 and above | 18 | 27.3 |
|
| ||
| Marital status | ||
| Single | 15 | 22.7 |
| Married | 43 | 65.2 |
| Divorced | 1 | 1.5 |
| Widowed | 7 | 10.6 |
|
| ||
| Highest educational level | ||
| No Education | 5 | 7.8 |
| Primary | 34 | 53.1 |
| Secondary | 18 | 28.1 |
| Tertiary | 7 | 10.9 |
3.2. Source of the Knowledge of Herbal Medicine Practice and Duration of Practice
The TMPs' knowledge of traditional healing, including knowledge to treat mental and neurological disorders, was mainly acquired from relatives (Figure 1). About 36.5% had practiced for 16-20 years, while 27% had practiced for 1-5 years (Table 2).
Figure 1.
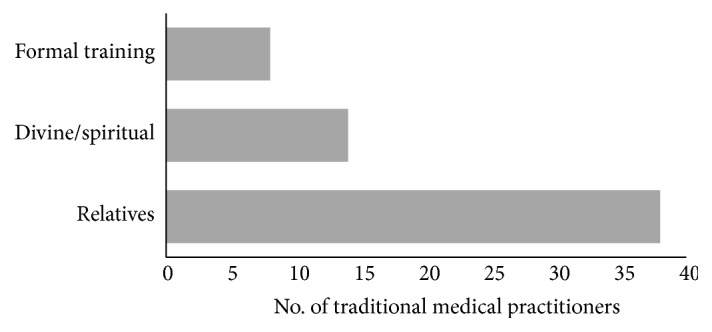
Source of traditional medical practitioners' knowledge for the treatment of mental and nervous system disorders in Ghana.
Table 2.
Sources of knowledge and duration of practice for traditional medicine practitioners who treat mental and neurological disorders in Ghana.
| Variable | Frequency | Percentage (%) |
|---|---|---|
| Source of the knowledge of herbal medical practice | ||
| Inheritance (knowledge passed on from others) | 38 | 63.3 |
| Divine/spiritual | 14 | 23.3 |
| Formal training | 8 | 13.3 |
|
| ||
| Years of herbal medical practice | ||
| 1-5 | 17 | 27.0 |
| 6-10 | 10 | 15.9 |
| 11-15 | 13 | 20.6 |
| 16-20 | 23 | 36.5 |
3.3. Treatment of Mental and Neurological Diseases
Most of the TMPs (60.6%) had specific herbs for treating a variety of mental and neurological disorders. However, only 36.4% had actually treated such patients. Out of these, 19.7% had treated a maximum of 5 patients, with only 1.5% having treated more than 20 patients. Overall, 31.8% of the treated patients had completely recovered (Table 3).
Table 3.
Treatment of mental and neurological disorders by traditional medical practitioners in Ghana.
| Variable | Frequency (n) | Percentage (%) |
|---|---|---|
| Knowledge of herbs for treating mental and neurological disorders | ||
| No | 26 | 39.4 |
| Yes | 40 | 60.6 |
|
| ||
| Total number of patients treated throughout herbal medical practice | ||
| 0 | 42 | 63.6 |
| 1-5 | 13 | 19.7 |
| 6-10 | 5 | 7.6 |
| 11-15 | 3 | 4.5 |
| 16-20 | 2 | 3.0 |
| Above 20 | 1 | 1.5 |
|
| ||
| Treatment options used | ||
| Not applicable | 42 | 63.6 |
| Divine/spiritual only | 1 | 1.5 |
| Herbs only | 16 | 24.3 |
| Herbs and divine/spiritual | 7 | 10.6 |
|
| ||
| Recovery status of patients treated | ||
| Not applicable/no recovery | 42 | 63.6 |
| Partial recovery | 3 | 4.5 |
| Total recovery | 21 | 31.8 |
3.4. Species, Medicinal Uses, and Experimentally Validated Pharmacological Relevance of Plants
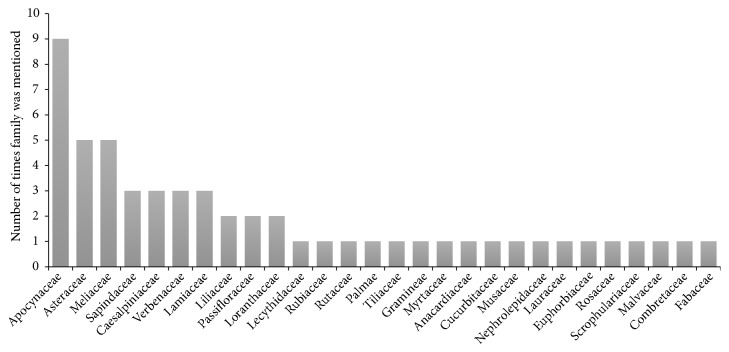
In all, 32 plant species were provided by the TMPs and these came from 28 different plant families (Table 4). The families Apocynaceae, Asteraceae, and Meliaceae were the most mentioned plant families, with Apocynaceae having the highest frequency of mentions and Asteraceae having the highest number of plant species (Figure 2). Rauwolfia vomitoria Afz. was the plant with the highest frequency of mention (mentioned 7 times; Figure 3). About 66% of the plants (21 species) used have been previously reported to have neuropharmacological activities. Half of the identified TAMs had analgesic (50%) properties, with the others having anxiolytic (18.8%), sedative (6.3%), anticonvulsant (15.6%), and antidepressant (9.4%) properties. R. vomitoria Afz., belonging to the family Apocynaceae, however, has been previously reported to have antipsychotic properties [16, 18] (Table 5).
Table 4.
Local names, plant parts, and methods of preparation of traditional African medicines used in treating nervous system and mental disorders in Ghana.
| Species | Family | Growth forms | Local names in different languages | Voucher specimen | Frequency of mention | Plant part used | Method of preparation |
|---|---|---|---|---|---|---|---|
| Ageratum conyzoides | Asteraceae | Herb | Ewe: mima, Nyigbe; Fante: Ahaban Kankan, Efumomoe; Twi: Gu-ekuro, Adwoa-kura, Guakuo, Gu-ekura; Nzema: Ebuakulo; Ga-Dangme: Ntumumu | PA01/UGSOP/GH17 | 3 | Leaves | The fresh leaves are macerated and the liquid obtained is instilled into the nostrils; the fresh leaves can also be boiled, sieved and drank as required. |
| Allium sativum | Liliaceae | Herb | Twi: Gyene Kankan; Ga Adangme: Aya; Hausa: Tafarmuwa | PA02/UGSOP/GH17 | 2 | Whole plant | - |
| Alstonia boonei | Apocynaceae | Tree | Twi: Nyame-dua, Nyamedua, Onyamedua, Osen Nuru; Ewe: Siaketreke Fante: Nyena,Sinuro,Nyamedua; Nzema: Bakunin | PA03/UGSOP/GH17 | 2 | Bark | The leaves are boiled and drank as required |
| Azadiratcha indica | Meliaceae | Tree | Fante: Nim, Aboode,Abodua; Ewe: Liliti; Ga-Dangme: Kintso, Asante: Gyedua; Twi: Nimsi, Dua gyane | PA04/UGSOP/GH17 | 1 | Leaves; Roots | The boiled leaves/roots are drank as required |
| Bertholletia excels | Lecythidaceae | Brazil nut | PA05/UGSOP/GH17 | 1 | Nut; Leaves | The leaves/nuts are boiled and sieved extract is drank as required | |
| Bidens pilosa. | Asteraceae | Herb | Twi: Dwirentwi,Gyinantwi; Ewe: Dzanikpikpi; | PA06/UGSOP/GH17 | 1 | Leaves | The fresh leaves are macerated and the liquid obtained is instilled into the nose |
| Blighia unijucata | Sapindaceae | Tree | Asante: Akye, Akan, Akyibiri, Twi: Akyebiri, Fante: Etedua | PA07/UGSOP/GH17 | 3 | Bark; Roots | The dried barks/roots are boiled and drank as required, the extract can also be smeared on the body |
| Cassia occidentalis. | Caesalpiniaceae | Nkwadowa bɔdeɛ | PA08/UGSOP/GH17 | 3 | Leaves | The leaves are boiled and drank as required | |
| Cinchona pubescens | Rubiaceae | Shrub | PA09/UGSOP/GH17 | 1 | |||
| Citrus aurantifolia | Rutaceae | Twi: Ankaadwea, Akenkaa Ankaatwaree; Fante: Ankama, Ewe: Mumoe; Asante: Ankaatwaree; Dagbani: Nyamsa, Lim buri; Ga-Adangme: Abonua; Hausa: Olomankilisi; Nzema: Domunli; Mole: Leemu; Ga: Kpete | PA10/UGSOP/GH17 | 1 | Peel; Juice | The peels are squeezed directly on the forehead and into the nose | |
| Cocos nucifera | Palmae | Tree | Twi: kube; Ewe: Agone | PA11/UGSOP/GH17 | 1 | Juice | Drinking the fresh coconut juice at will |
| Corchorus olitorius | Tiliaceae | Herbaceous | Ewe: Ademe,Singui; Fante: Oturo; Twi: Otoro | PA12/UGSOP/GH17 | 1 | Jute mallow, Leaves | Hot infusion is made from the leaves and drank as required |
| Cymbopogon citratus | Graminae | Herb | Ewe: Tigbe; Fante: Ti ahaban, Ga-Dangme:Ti-ba | PA13/UGSOP/GH17 | 1 | Leaves; Oil | A decoction is made from the either the fresh/dried leaves and the oily content applied as a massage |
| Eucalyptus globulus | Myrtaceae | Eucalyptus | PA14/UGSOP/GH17 | 1 | Oil | Cold infusion is made and the oily content obtained is rubbed on the body | |
| Khaya senegalensis | Meliaceae | Tree | Hausa: Madwachi, Madachi; Ewe: Logo; Fante: Okum; GaAdangme: Kuga; Twi: Kuntunkuri; Mole: Kuka; Brong: Korobaa; Nzema: Anane | PA15/UGSOP/GH17 | 4 | Bark | The leaves are boiled and drank as required |
| Lantana camara | Verbenaceae | Shrub | Akan,Ananse dokono | PA16/UGSOP/GH17 | 3 | Leaves; Stem | The leaves/stem are boiled and the liquid obtained, drank as required |
| Mangifera indica | Anacardiaceae | Tree | Ewe/Asante/Twi/ Fante Mango, Amango,Ga: Mango | PA17/UGSOP/GH17 | 1 | Bark | A decoction is made from the dried bark and drank as required |
| Momordica charantia | Cucurbitaceae | Herbaceous | Twi: Nyannya, Nyina, Nyinya; Ewe: Kakle; Dangme: Nyanyla, Nyanyra; Ga: Nyanyra; Nzema: Nyanya | PA18/UGSOP/GH17 | 1 | ||
| Musa paradisiaca | Musaceae | Herbaceous | Twi: Brode; Nzema: Banna Ga: Amadaa | PA19/UGSOP/GH17 | 1 | Leaves | The leaves are boiled and drank as required |
| Nephrolepis cordifolia | Nephrolepidaceae | Twi: Mmɛn | PA20/UGSOP/GH17 | 1 | Leaves | The leaves are macerated and the liquid instilled nasally or inhaled. The leaves can be boiled and the extracted liquid used as a bathing liquid. | |
| Occimum gratissimum | Lamiaceae | Shrub | Ewe: Babusui, Dzeveti; Ga: Sulu; Twi: Onunum, Nunum; Asante: Nunum; Ga-Dangme: Sulu; Hausa: Dardoyatagidi; Nzema: Amaloko; Wassa: Aprim; Fante: Onunum | PA21/UGSOP/GH17 | 3 | Leaves | The leaves are boiled and drank as required |
| Passiflora edulis | Passifloraceae | Passion fruit tree | PA22/UGSOP/GH17 | 2 | Leaves; Flowers; Fruit; Leaves | Boiling; grinding | |
| Persea americana | Lauraceae | Tree | Dangme: Paya; Twi: Pee; Akan: Paya, Pae; | PA23/UGSOP/GH17 | 1 | Fresh and dried leaves | A decoction is made from the either the fresh/dried leaves and drank as required |
| Fante: Pae; | |||||||
| Phyllantus nuriri | Euphobiaceae | Herbaceous | Twi: Awommaguwakyi; Ewe: Lane; Krobo: Ofobiokpai, Ofobi; Ga: Omatsoatsi; | PA24/UGSOP/GH17 | 1 | ||
| Rauwolfia vomitoria. | Apocynaceae | Shrub | Twi: Kakapenpen; Ewe: Dodemakpowoe; Fante: Kakapenpen; Ga-Dangme: Apototso; Hausa: Wada, Nzema: Bakapembene; Wassa: Aneene | PA25/UGSOP/GH17 | 7 | Roots | The roots are boiled and the extract obtained are instilled into the nose |
| Rubus fruticosus | Rosaceae | Bramble | PA26/UGSOP/GH17 | 1 | Berries, leaves and flowers | Blend dry leaves and mix with honey | |
| Scoparia dulcis | Scrophulariaceae | PA27/UGSOP/GH17 | 1 | - | - | ||
| Sida acuta | Malvaceae | Branchlets | Ewe: Afideme; Ga: Shwoboto; Twi: Obraneatuto | PA28/UGSOP/GH17 | 1 | Leaves | The leaves are boiled and drank as required |
| Tapinanthus globiferrus | Loranthaceae | Parasitic Tree | Twi: nkranpan Mole: Welebe | PA29/UGSOP/GH17 | 2 | Leaves; stem | The leaves/stem are boiled and the liquid obtained, drank as required |
| Terminalia catapa | Combretaceae | abrɔfo nkateɛ | PA30/UGSOP/GH17 | 1 | Yellowed leaves | The leaves are boiled and the liquid drank as required | |
| Tetrapleura tetraptera | Fabaceae | Tree | Twi: Prɛkesɛ, Zate: Zamturi; Anyi: Aprekese, Kyeke, Fante: Esem, Ewe: Prekese | PA31/UGSOP/GH17 | 1 | Seed | The seeds are ground and the liquid extract drank as required |
| Vernonia amygdalina | Asteraceae | Shrub | Ga:Tatso, Akpa, Dagbani: Biebingira, Ewe: Gbo, Gboti, Asante: Mbonasere, Mponasere; Nzema: Ayeanwole, Ga-Dangme: Tatsho | PA32/UGSOP/GH17 | 1 | Leaves | A decoction is made from the either the fresh/dried leaves and drank as required |
Figure 2.
Plant families commonly used in the treatment of mental and nervous system disorders in Ghana.
Figure 3.
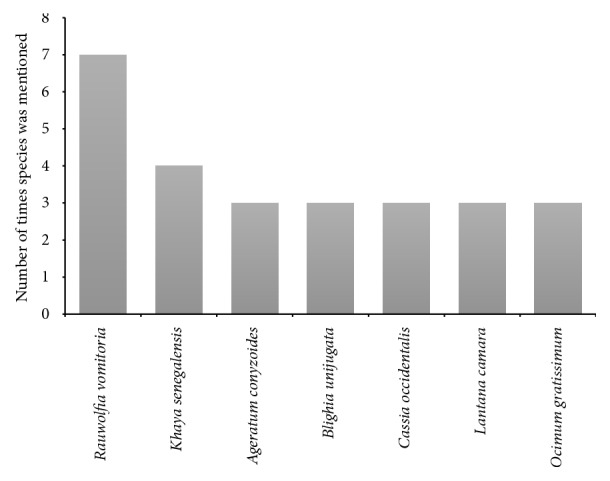
Most frequently used plant species in the treatment of mental and nervous system disorders in Ghana (only species with three or more mentions are shown).
Table 5.
Plant species used for the treatment of mental and nervous system disorders in Ghana and scientific validation of their ethnopharmacological activities.
| Botanical name | Family | CNS uses |
|---|---|---|
| Ageratum conyzoides Linn., | Asteraceae | Analgesia [15, 42, 43] |
| Allium sativum Linn. | Liliaceae | Motor coordination[44]; Analgesia [45] |
| Alstonia boonei De Wild | Apocynaceae | Analgesia [24] |
|
| ||
| Azadirachta indica A. Juss | Meliaceae | Analgesia [25, 46]; Anxiolytic [47]; Alzheimer's disease [26] |
|
| ||
| Bertholletia excelsa H&B | Lecythidaceae | None |
|
| ||
| Bidens pilosa Linn. | Asteraceae | Analgesia [48] |
| Blighia unijugata Bak | Sapindaceae | None |
|
| ||
| Cassia occidentalis Linn. | Caesalpiniaceae | None |
| Cinchona pubescens Vahl. | Rubiaceae | None |
| Citrus aurantifolia Swingle | Rutaceae | None |
|
| ||
| Cocos nucifera Linn. | Palmae | Analgesia [49–51] |
|
| ||
| Corchorus olitorius Linn. | Tiliaceae | Anticonvulsant [52] |
|
| ||
| Cymbopogon citratus DC. | Graminae | Anxiolytic [53–56]; Sedative [53]; Anticonvulsant [53, 54, 57]; Analgesia [58] |
|
| ||
| Eucalyptus globulus Labill. | Myrtaceae | None |
| Khaya senegalensis (Desr.) A. Juss. | Meliaceae | None |
| Lantana camara Linn. | Verbenaceae | Anxiolytic [59, 60] |
| Mangifera indica Linn. F.T.A | Anacardiaceae | Analgesia [61, 62]; |
| Cognitive performance [63] | ||
| [64]; Neuroprotection, anticonvulsant [65] | ||
|
| ||
| Momordica charantia Linn. | Cucurbitaceae | Analgesia [66–68]; Antidepressant Anxiolytic [69] |
|
| ||
| Musa paradisiaca Walker et Sillans | Musaceae | None |
|
| ||
| Nephrolepis cordifolia Linn Presl | Nephrolepidaceae | None |
|
| ||
| Ocimum gratissimum Linn. | Lamiaceae | Analgesia, antidepressant [70–74]; and anxiolytic [75] |
|
| ||
| Passiflora edulis Sims | Passifloraceae | Anxiolytic [76–81] and sedative [78, 81, 82] |
|
| ||
| Persea Americana Mill F.W.T.A | Lauraceae | Analgesia and anticonvulsant [83, 84] |
|
| ||
| Phyllanthus niruri Schum.et Thnn. | Euphobiaceae | Analgesia [85, 86] |
| Rauwolfia vomitoria Afz. | Apocynaceae | Antipsychotic [16, 18, 29] |
| Rubus fruticosus Linn. | Rosaceae | None |
| Scoparia dulcis Linn. | Scrophulariaceae | Analgesia [87, 88] |
| Sida acuta Burn F. | Malvaceae | Analgesia and antidepressant [89, 90] |
|
| ||
| Tapinanthus globiferus A. Rich. | Loranthaceae | None |
| Terminalia catappa Linn. | Combretaceae | None |
| Tetrapleura tetraptera Schum Taub. | Fabaceae | Anticonvulsant [91, 92], Analgesia [92] |
| Vernonia amygdalina Del. Cent. Pl. Afr. | Asteraceae | Analgesia [22] |
3.5. Preparation and Administration of Herbal Products
The TAMs were prepared mostly as mixtures of two or more species. In some cases, however, the products were administered as monopreparations (prepared using a single plant species). The mode of preparation employed included decoction, infusion, and maceration, with decoction being the commonest (Table 4). While roots, fruits, flowers, stems, stem barks, whole plant of shrubs, etc. were all used in the preparation of these products (all together 42.4%), leaves (57.6%) were the commonest plant part used. The Ghanaian vernacular names of the plant species are listed in Table 5.
Given that most TMPs do not preserve these TAMs, they generally prepared the products only when required. The products were administered orally, nasally, or applied on the forehead for periods ranging from one week to several years or until the patient recovers. The TMPs mostly used patient feedback and disappearance of symptoms to assess treatment outcomes. Where there is only a partial recovery or treatment failure, the patients are often referred to the nearest hospital.
4. Discussion
Mental and neurological disorders remain a major public health concern [2]. The disease burden is even more prominent in the developing world, including Ghana [3, 5, 6]. Recent discoveries and clinical usage of the anticancer agent taxol and the antimalarial artemisinin derived from plants have boosted interest in natural products as templates for the development of novel drug scaffolds [19, 20]. TAMs are widely accepted in African communities and there appears to be an increasing reliance on these products [13]. In Ghana, TAMs are used as the main treatment paradigm for a variety of diseases, but they are also used as complements to other medicines or as dietary supplements [21]. However, thorough examination and documentation of the medicinal properties of these products against mental and neurological disorders is lacking.
In the present study, several plant species (32 species) used by local TMPs to treat mental and neurological disorders were reported, with most species belonging to the families Asteraceae, Apocynaceae, and Meliaceae. These are large and widespread plant families with several species. In particular, the Asteraceae family is of great importance due to its high numbers of medicinal species used in the treatment of a wide array of diseases including tuberculosis, malaria, and inflammatory disorders [11, 15, 22]. Members of the Asteraceae family are also known for their wide range of economically important products including cooking oils and phytochemicals such as sesquiterpene lactones, alkaloids, and tannins [23]. The family Apocynaceae also has a wide range of species that are of pharmacological importance, with some members synthesizing alkaloids useful against high blood pressure and inflammation and others synthesizing cardiac glycosides that affect heart function [24]. The family Meliaceae, on the other hand, is known for its species that are processed into important products including vegetable oil, as well as phytochemicals with anti-inflammatory, antioxidant, hepatoprotective, and cognitive-enhancing properties [25, 26].
While the plants used in treating CNS/PNS disorders in Ghana varied greatly, R. vomitoria Afz. was frequently mentioned (17.5%) by the TMPs who had knowledge of natural products for treating these disorders. Herbal preparations of this plant are also used by TMPs in other African countries for the treatment of mental disorders [27] and have been shown to be relatively safe with LD50 of 17.5 g/kg [28]. Remarkably, R. vomitoria Afz. has been found to have activity on the nervous system, especially on locomotor behavior, anxiety, and psychosis [16, 18, 29]. Reserpine, which is one of the numerous alkaloids of this species, has been used in the management of schizophrenia, hypertension, and psychiatric disorders [30]. Beyond its CNS effect, extracts from the plant are reported to have anticancer (due to the alstonine and β-carboline alkaloid) [31], antipyretic, anti-inflammatory [32], and antidiabetic activities [33].
The natural products used by the TMPs in treating mental and neurological disorders fall into the following broad categories: analgesics, anxiolytics, antidepressants, antipsychotics, and anticonvulsants. Of these, those with analgesic (pain relieving), anxiolytic (anti-anxiety), and anticonvulsant (anti-epileptic) effects were the most commonly used, and this possibly reflects the common disorders treated by the TMPs. In particular, half of the identified TAMs were analgesics, possibly suggesting that the TMPs were most often presented with patients suffering from headache, migraine, or other associated conditions. Headache or cephalalgia is used to describe pain in the head and could be a symptom of a number of different conditions associated with the head and neck [1]. Although limited studies have been conducted to assess the epidemiology of headache and migraine in Ghana and Africa, headache is quite common among Africans and is often exacerbated by the hot climate in most African countries [34–36]. In assessing the profile of neurological disorders in an adult neurology clinic in Ghana, clinicians recorded a number of headache and migraine cases, although the frequency was found to be relatively low [37]. This low frequency was suggested to be due to the fact that primary headaches among Ghanaians are commonly reported to and managed by community pharmacists and primary healthcare physicians [37], although there are increasing reports indicating that several individuals with headache or migraine opt for traditional and herbal therapies [34, 35]. The analgesic species frequently used by the TMPs were A. conyzoides L. and O. gratissimum L. Also important are the anxiolytic (antianxiety) and anticonvulsant (antiepileptic) products that were often used by the TMPs, suggesting a potentially high prevalence of anxiety disorders and epilepsy and seizure disorders. Epilepsy, seizures, and anxiety disorders feature prominently among the mental and neurological conditions prevalent in Ghana [5, 37]. On the other hand, L. camara L. was the most frequently mentioned anxiolytic product, while C. citratus DC., M. indica L., T. tetraptera Schum Taub., and Persea Americana Mill were among the most frequently studied anticonvulsants used by the TMPs.
Given that drugs for managing mental disorders are often in shortage in Ghanaian psychiatric hospitals [6, 38], it may be important that TAMs whose therapeutic relevance has been confirmed experimentally are considered for clinical usage. The long history of TAMs usage in African societies with seemingly minimal adverse effects [21, 39] support this perspective. While clinical integration of TAMs may be beneficial, at present, this should be approached with caution due to the inadequacy of studies exploring their efficacy and safety. Therefore, increasing TAMs-based research in Ghana would be a crucial step towards rigorous establishment of their safety, therapeutic, and adverse reaction profiles.
The natural products identified in this study are a valuable collection of resources that may provide leads for drug discovery and development. However, a potential criticism of the traditional approach being employed by the TMPs in relation to the pharmaceutical industry approach to drug discovery is that whole plant extracts may contain several bioactive components, making it difficult to attribute therapeutic benefits and mechanism(s) of action to particular compounds (Rasoanaivo et al., 2011). Moreover, some plant extract components may be negative modulators of active drug ingredients, with adverse implications for drug potential. A feasible means to refine, extend, and enhance the beneficial effects of the plant products identified in this study is to isolate, screen, and characterize bioactive compounds responsible for the positive disease-modifying effects reported. On the other hand, it is possible that components of the different plant extracts used in combination may produce positive interactions leading to complementarity in observed therapeutic effects that are more effective than single components administered at equal doses. In such a case, plant extracts whose benefits are observed when used as combinations by the traditional healers should be explored further to identify their possible synergistic activities. For example, the antimalarial drugs Quinimax® (a combination of quinone, cinchonine, and quinidine) and Malarone® (proguanil and avoquone) are produced and marketed as synergistic complementary drugs (Bunnag et al., 1989; Fivelman et al., 2004). Further drug discovery and development research should be conducted on the reported plant products to identify lead compounds whose in vivo therapeutic capacities would be revealed in preclinical and clinical studies. This would enable the industrial-scale production and marketing of successful drug candidates following drug authority approval. The high cost of the drug discovery and development process would, however, require strengthening academia-industry collaborative research and better provision of research funding and infrastructure [5, 40, 41].
5. Conclusion
The identified natural products used in Ghanaian communities are a potential source of a novel class of drugs for the management of mental and neurological disorders. Many of the plant species used have been investigated for their CNS-specific pharmacologic effects, with the majority having analgesic, anxiolytic, or anticonvulsant properties. However, the most prominent and often used plant, R. vomitoria Afz., has potent antipsychotic properties. The increased reliance and the claimed therapeutic value of the identified TAMs indicate that there is an urgent need for the preservation and extensive investigation of these products to establish their clinical effectiveness. Such studies may help in the isolation and purification of the bioactive compounds, confirm the safety and tolerability of these products, and enable the clinical integration of TAMs.
Acknowledgments
We are grateful to the leadership of GHAFRAM for facilitating the study and the herbal practitioners who spent time participating in the study. This study was funded by the Office of Research, Innovation and Development (ORID), University of Ghana, Accra, Ghana, grant awarded to Dr. Patrick Amoateng (reference number: URF/6/ILG-002/2012-2013). TKK is a member of the Midlands Integrative Biosciences Training Partnership, which is funded by the UK's Biotechnology and Biological Sciences Research Council (BBSRC) Grant no. BB/J014532/1.
Abbreviations
- CNS:
Central nervous system
- GHAFTRAM:
Ghana Federation of Traditional Medical Practitioners' Association
- GPs:
General practitioners
- TAMs:
Traditional African medicines
- TMPs:
Traditional medicine practitioners.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there are no conflicts of interest in the publication of this manuscript.
References
- 1.Calvo M. I., Cavero R. Y. Medicinal plants used for neurological and mental disorders in Navarra and their validation from official sources. Journal of Ethnopharmacology. 2015;169:263–268. doi: 10.1016/j.jep.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 2.WHO. What Are Neurological Disorders. World Health Organization; 2016. http://www.who.int/features/qa/55/en/ [Google Scholar]
- 3.Fekadu A., Hanlon C., Gebre-Eyesus E., et al. Burden of mental disorders and unmet needs among street homeless people in Addis Ababa, Ethiopia. BMC Medicine. 2014;12(1) doi: 10.1186/s12916-014-0138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mamah D., Owoso A., Mbwayo A. W., et al. Classes of psychotic experiences in kenyan children and adolescents. Child Psychiatry & Human Development. 2013;44(3):452–459. doi: 10.1007/s10578-012-0339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quansah E., Karikari T. K. Neuroscience-related research in ghana: A systematic evaluation of direction and capacity. Metabolic Brain Disease. 2016;31(1):11–24. doi: 10.1007/s11011-015-9724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts M., Mogan C., Asare J. B. An overview of Ghana’s mental health system: results from an assessment using the World Health Organization’s Assessment Instrument for Mental Health Systems (WHO-AIMS) International Journal of Mental Health Systems. 2014;8(1):p. 16. doi: 10.1186/1752-4458-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quansah E., Sarpong E., Karikari T. K. Disregard of neurological impairments associated with neglected tropical diseases in Africa. eNeurologicalSci. 2016;3:11–14. doi: 10.1016/j.ensci.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duah F., Owusu P., Knapp J., Slatkin D., Schiff P. Constituents of West African Medicinal Plants. Planta Medica. 1981;42:275–278. doi: 10.1055/s-2007-971640. [DOI] [PubMed] [Google Scholar]
- 9.Dwuma-Badu D., Ayim J. S. K., Dabra T. T., et al. Constituents of West African medicinal plants. XIV. Constituents of Piper guineense Schum. and Thonn. Lloydia. 1976;39(1):60–64. [PubMed] [Google Scholar]
- 10.Kwofie K. D., Tung N. H., Suzuki-Ohashi M., et al. Antitrypanosomal activities and mechanisms of action of novel tetracyclic iridoids from Morinda lucida Benth. Antimicrobial Agents and Chemotherapy. 2016;60(6):3283–3290. doi: 10.1128/AAC.01916-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguta J. M., Appiah-Opong R., Nyarko A. K., et al. In vitro antimycobacterial and cytotoxic data on medicinal plants used to treat tuberculosis. Data in Brief. 2016;7:1124–1130. doi: 10.1016/j.dib.2016.03.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boateng M. A., Danso-Appiah A., Turkson B. K., Tersbøl B. P. Integrating biomedical and herbal medicine in Ghana - experiences from the Kumasi South Hospital: A qualitative study. BMC Complementary and Alternative Medicine. 2016;16(1) doi: 10.1186/s12906-016-1163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. WHO Global Atlas of Traditional, Complementary and Alternative Medicine. World Health Organization; 2005. [Google Scholar]
- 14.Adusi-Poku Y., Okine L. K.-N., Hlortsi-Akakpo F. K., et al. Assesssing herbal medical practitioners in professional qualifying examination in Ghana, a model. African Journal of Traditional, Complementary and Alternative Medicines. 2010;7(1):85–87. [PMC free article] [PubMed] [Google Scholar]
- 15.Abena A. A., Kintsangoula-Mbaya G. S., Diantama J., Bioka D. Analgesic effects of Ageratum conyzoides extract in the rat. L'Encéphale. 1993;19(4):329–332. [PubMed] [Google Scholar]
- 16.Bisong S. A., Brown R., Osim E. E. Comparative effects of Rauwolfia vomitoria and chlorpromazine on locomotor behaviour and anxiety in mice. Journal of Ethnopharmacology. 2010;132(1):334–339. doi: 10.1016/j.jep.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 17.Tabuti J. R. S., Kukunda C. B., Waako P. J. Medicinal plants used by traditional medicine practitioners in the treatment of tuberculosis and related ailments in Uganda. Journal of Ethnopharmacology. 2010;127(1):130–136. doi: 10.1016/j.jep.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 18.Bisong S., Brown R., Osim E. Comparative effects of Rauwolfia vomitoria and chlorpromazine on social behaviour and pain. North American Journal of Medical Sciences. 2011;3(1):48–54. doi: 10.4297/najms.2011.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khanna C., Rosenberg M., Vail D. M. A review of paclitaxel and novel formulations including those suitable for use in dogs. Journal of Veterinary Internal Medicine. 2015;29(4):1006–1012. doi: 10.1111/jvim.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller L. H., Su X. Artemisinin: discovery from the Chinese herbal garden. Cell. 2011;146(6):855–858. doi: 10.1016/j.cell.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguta J. M., Appiah-Opong R., Nyarko A. K., Yeboah-Manu D., Addo P. G. A. Medicinal plants used to treat TB in Ghana. International Journal of Mycobacteriology. 2015;4(2):116–123. doi: 10.1016/j.ijmyco.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Adedapo A. A., Aremu O. J., Oyagbemi A. A. Anti-Oxidant, anti-inflammatory and antinociceptive properties of the acetone leaf extract of Vernonia Amygdalina in some laboratory animals. Advanced Pharmaceutical Bulletin. 2014;4:591–598. doi: 10.5681/apb.2014.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guenne S., Ouattara N., Hilou A., Millogo J. F., Nacoulma O. G. Antioxidant, enzyme inhibition activities and polyphenol contents of three Asteraceae species used in Burkina Faso traditionally medicine. International Journal of Pharmacy and Pharmaceutical Sciences. 2011;3(5):524–528. [Google Scholar]
- 24.Olajide O. A., Awe S. O., Makinde J. M., et al. Studies on the anti-inflammatory, antipyretic and analgesic properties of Alstonia boonei stem bark. Journal of Ethnopharmacology. 2000;71(1-2):179–186. doi: 10.1016/S0378-8741(99)00200-7. [DOI] [PubMed] [Google Scholar]
- 25.Ilango K., Maharajan G., Narasimhan S. Anti-nociceptive and anti-inflammatory activities of Azadirachta indica fruit skin extract and its isolated constituent azadiradione. Natural Product Research. 2013;27(16):1463–1467. doi: 10.1080/14786419.2012.717288. [DOI] [PubMed] [Google Scholar]
- 26.Maiti R., Kumar S., Acharya S., Raghavendra M. Role of aqueous extract of Azadirachta indica leaves in an experimental model of Alzheimer′s disease in rats. International Journal of Applied and Basic Medical Research. 2013;3(1):p. 37. doi: 10.4103/2229-516X.112239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akpanabiatu M. I., Umoh I. B., Eyong E. U., Edet E. E., Uboh F. E. Influence of Rauwolfia vomitoria root bark on cardiac enzymes o normal Wistar albino rats. Biopharmaceuticals. 2006;14:273–278. [Google Scholar]
- 28.Olatokunboh A. O., Kayode Y. O., Adeola O. K. Anticonvulsant activity of Rauvolfia Vomitoria (Afzel) African Journal of Pharmacy and Pharmacology. 2009;3(6):319–322. [Google Scholar]
- 29.Bisong S. A., Brown R. E., Osim E. E. Comparative extrapyramidal effects of Rauwolfia vomitoria, chlorpromazine and reserpine in mice. Journal of Natural Medicines. 2013;67(1):107–112. doi: 10.1007/s11418-012-0657-8. [DOI] [PubMed] [Google Scholar]
- 30.López-Muñoz F., Bhatara V. S., Álamo C., Cuenca E. Historical approach to reserpine discovery and its introduction in psychiatry. Actas Españolas de Psiquiatría. 2004;32(6):387–395. [PubMed] [Google Scholar]
- 31.Bemis D. L., Capodice J. L., Gorroochurn P., Katz A. E., Buttyan R. Anti-prostate cancer activity of a β-carboline alkaloid enriched extract from Rauwolfia vomitoria. International Journal of Oncology. 2006;29(5):1065–1073. [PubMed] [Google Scholar]
- 32.Kweifio-Okai G., Bird D., Field B., et al. Antiinflammatory activity of a Ghanaian antiarthritic herbal preparation: III. Journal of Ethnopharmacology. 1995;46(1):7–15. doi: 10.1016/0378-8741(95)01222-Y. [DOI] [PubMed] [Google Scholar]
- 33.Campbell J. I. A., Mortensen A., Mølgaard P. Tissue lipid lowering-effect of a traditional Nigerian anti-diabetic infusion of Rauwolfia vomitoria foilage and Citrus aurantium fruit. Journal of Ethnopharmacology. 2006;104(3):379–386. doi: 10.1016/j.jep.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 34.Haimanot R. T. Headache in the Tropics: Sub-Saharan Africa. In: Martelletti P., Steiner T. J., editors. Handbook of Headache. Milan: Springer; 2011. pp. 533–540. [Google Scholar]
- 35.Haimanot T., Seraw B., Forsgren L., Ekstedt J., Ekbom K. Migraine, chronic tension-type headache, and cluster headache in an ethiopian rural community. Cephalalgia. 1995;15(6):482–488. doi: 10.1046/j.1468-2982.1995.1506482.x. doi: 10.1046/j.1468-2982.1995.1506482.x. [DOI] [PubMed] [Google Scholar]
- 36.Osuntokun B. O., Schoenberg B. S., Nottidge V., et al. Migraine headache in a rural community in nigeria: Results of a pilot study. Neuroepidemiology. 1982;1(1):31–39. doi: 10.1159/000110687. [DOI] [Google Scholar]
- 37.Sarfo F. S., Akassi J., Badu E., Okorozo A., Ovbiagele B., Akpalu A. Profile of neurological disorders in an adult neurology clinic in Kumasi, Ghana. eNeurologicalSci. 2016;3:69–74. doi: 10.1016/j.ensci.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silberberg D., Katabira E. Neurological Disorders. In: Jamison D. T., Feachem R. G., Makgoba M. W., Bos E. R., Baingana F. K., Hofman K. J., et al., editors. Disease and Mortality in Sub-Saharan Africa. 2nd. Washington, DC, USA: World Bank; 2006. http://www.ncbi.nlm.nih.gov/books/NBK2295/ [PubMed] [Google Scholar]
- 39.McGaw L. J., Lall N., Meyer J. J. M., Eloff J. N. The potential of South African plants against Mycobacterium infections. Journal of Ethnopharmacology. 2008;119(3):482–500. doi: 10.1016/j.jep.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 40.Karikari T. K., Cobham A. E., Ndams I. S. Building sustainable neuroscience capacity in Africa: the role of non-profit organisations. Metabolic Brain Disease. 2015 doi: 10.1007/s11011-015-9687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quansah E., Karikari T. K. Motor neuron diseases in sub-saharan africa: the need for more population-based studies. BioMed Research International. 2015;2015:9. doi: 10.1155/2015/298409.298409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hossain H., Karmakar U. K., Biswas S. K., et al. Antinociceptive and antioxidant potential of the crude ethanol extract of the leaves of Ageratum conyzoides grown in Bangladesh. Pharmaceutical Biology. 2013;51(7):893–898. doi: 10.3109/13880209.2013.770535. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto L. A., Soldera J. C., Emim J. A., Godinho R. O., Souccar C., Lapa A. J. Pharmacological screening of Ageratum conyzoides L. (mentrasto) Memórias do Instituto Oswaldo Cruz. 1991;86(2):145–147. doi: 10.1590/S0074-02761991000600033. [DOI] [PubMed] [Google Scholar]
- 44.Aminuddin M., Partadiredja G., Sari D. C. R. The effects of black garlic (Allium sativum L.) ethanol extract on the estimated total number of Purkinje cells and motor coordination of male adolescent Wistar rats treated with monosodium glutamate. Anatomical Science International. 2014;90(2):75–81. doi: 10.1007/s12565-014-0233-2. [DOI] [PubMed] [Google Scholar]
- 45.Kumar G. R., Reddy K. P. Reduced nociceptive responses in mice with alloxan induced hyperglycemia after garlic (Allium sativum Linn.) treatment. Indian Journal of Experimental Biology (IJEB) 1999;37(7):662–666. [PubMed] [Google Scholar]
- 46.Khanna N., Goswami M., Sen P., Ray A. Antinociceptive action of Azadirachta indica (Neem) in mice: Possible mechanisms involved. Indian Journal of Experimental Biology (IJEB) 1995;33(11):848–850. [PubMed] [Google Scholar]
- 47.Jaiswal A. K., Bhattacharya S. K., Acharya S. B. Anxiolytic activity of Azadirachta indica leaf extract in rats. Indian Journal of Experimental Biology. 1994;32(7):489–491. [PubMed] [Google Scholar]
- 48.Fotso A. F., Longo F., Djomeni P. D. D., et al. Analgesic and antiinflammatory activities of the ethyl acetate fraction of Bidens pilosa (Asteraceae) Inflammopharmacology. 2014;22(2):105–114. doi: 10.1007/s10787-013-0196-2. [DOI] [PubMed] [Google Scholar]
- 49.Alviano D. S., Rodrigues K. F., Leitão S. G., et al. Antinociceptive and free radical scavenging activities of Cocos nucifera L. (Palmae) husk fiber aqueous extract. Journal of Ethnopharmacology. 2004;92(2-3):269–273. doi: 10.1016/j.jep.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 50.Naskar S., Mazumder U. K., Pramanik G., Saha P., Haldar P. K., Gupta M. Evaluation of antinociceptive and anti-inflammatory activity of hydromethanol extract of cocos nucifera l. Inflammopharmacology. 2013;21(1):31–35. doi: 10.1007/s10787-012-0135-7. [DOI] [PubMed] [Google Scholar]
- 51.Rinaldi S., Silva D. O., Bello F., et al. Characterization of the antinociceptive and anti-inflammatory activities from Cocos nucifera L. (Palmae) Journal of Ethnopharmacology. 2009;122(3):541–546. doi: 10.1016/j.jep.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 52.Gupta M., Mazumder U. K., Pal D., Bhattacharya S., Chakrabarty S. Studies on brain biogenic amines in methanolic extract of Cuscuta reflexa Roxb. and Corchorus olitorius linn. sees treated mice. Acta Poloniae Pharmaceutica. Drug Research. 2003;60(3):207–210. [PubMed] [Google Scholar]
- 53.Blanco M. M., Costa C. A. R. A., Freire A. O., Santos J. G., Jr., Costa M. Neurobehavioral effect of essential oil of Cymbopogon citratus in mice. Phytomedicine. 2009;16(2-3):265–270. doi: 10.1016/j.phymed.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 54.Carlini E. A., De D.P. Contar J., Silva-Filho A. R., Da Silveira-Filho N. G., Frochtengarten M. L., Bueno O. F. A. Pharmacology of lemongrass (Cymbopogon citratus Stapf). I. Effects of teas prepared from the leaves on laboratory animals. Journal of Ethnopharmacology. 1986;17(1):37–64. doi: 10.1016/0378-8741(86)90072-3. [DOI] [PubMed] [Google Scholar]
- 55.Costa C. A. R. D. A., Kohn D. O., De Lima V. M., Gargano A. C., Flório J. C., Costa M. The GABAergic system contributes to the anxiolytic-like effect of essential oil from Cymbopogon citratus (lemongrass) Journal of Ethnopharmacology. 2011;137(1):828–836. doi: 10.1016/j.jep.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Leite J., De Lourdes V. Seabra M., Maluf E., et al. Pharmacology of lemongrass (Cymbopogon citratus Stapf). III. Assessment of eventual toxic, hypnotic and anxiolytic effects on humans. Journal of Ethnopharmacology. 1986;17(1):75–83. doi: 10.1016/0378-8741(86)90074-7. [DOI] [PubMed] [Google Scholar]
- 57.Silva M. R., Ximenes R. M., da Costa J. G. M., Leal L. K. A. M., de Lopes A. A., de Barros Viana G. S. Comparative anticonvulsant activities of the essential oils (EOs) from Cymbopogon winterianus Jowitt and Cymbopogon citratus (DC) Stapf. in mice. Naunyn-Schmiedeberg's Archives of Pharmacology. 2010;381(5):415–426. doi: 10.1007/s00210-010-0494-9. [DOI] [PubMed] [Google Scholar]
- 58.Viana G. S. B., Vale T. G., Pinho R. S. N., Matos F. J. A. Antinociceptive effect of the essential oil from Cymbopogon citratus in mice. Journal of Ethnopharmacology. 2000;70(3):323–327. doi: 10.1016/S0378-8741(99)00168-3. [DOI] [PubMed] [Google Scholar]
- 59.Kazmi I., Afzal M., Ali B., Damanhouri Z. A., Ahmaol A., Anwar F. Anxiolytic potential of ursolic acid derivative-a stearoyl glucoside isolated from Lantana camara L. (verbanaceae) Asian Pacific Journal of Tropical Medicine. 2013;6(6):433–437. doi: 10.1016/S1995-7645(13)60069-3. [DOI] [PubMed] [Google Scholar]
- 60.Kazmi I., Gupta G., Afzal M., Rahman M., Anwar F. Pharmacological evaluation of anxiolytic activity of ursolic acid stearoyl glucoside isolated from lantana camara. CNS Neuroscience & Therapeutics. 2012;18(8):707–708. doi: 10.1111/j.1755-5949.2012.00351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garrido G., Gonzalez D., Delporte C., et al. Analgesic and anti-inflammatory effects of Mangifera indica L. extract (Vimang) Phytotherapy Research. 2001;15(1):18–21. doi: 10.1002/1099-1573(200102)15:1<18::AID-PTR676>3.0.CO;2-R. doi: 10.1002/1099-1573(200102)15:1<18::AID-PTR676>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 62.Khan M. A. A., Islam M. T. Analgesic and cytotoxic activity of Acorus calamus L., Kigelia pinnata L., Mangifera indica L. and Tabernaemontana divaricata L. Journal of Pharmacy and Bioallied Sciences. 2012;4(2):149–154. doi: 10.4103/0975-7406.94820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar S., Maheshwari K. K., Singh V. Effects of Mangifera indica fruit extract on cognitive deficits in mice. Journal of Environmental Biology. 2009;30:563–566. [PubMed] [Google Scholar]
- 64.Lemus-Molina Y., Sánchez-Gómez M. V., Delgado-Hernández R., Matute C. Mangifera indica L. extract attenuates glutamate-induced neurotoxicity on rat cortical neurons. NeuroToxicology. 2009;30(6):1053–1058. doi: 10.1016/j.neuro.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 65.Viswanatha G. L., Mohan C. G., Shylaja H., Yuvaraj H. C., Sunil V. Anticonvulsant activity of 1,2,3,4,6-penta-O-galloyl-β-d-glucopyranose isolated from leaves of Mangifera indica. Naunyn-Schmiedeberg's Archives of Pharmacology. 2013;386(7):599–604. doi: 10.1007/s00210-013-0858-z. [DOI] [PubMed] [Google Scholar]
- 66.Biswas A. R., Ramaswamy S., Bapna J. S. Analgesic effect of Momordica charantia seed extract in mice and rats. Journal of Ethnopharmacology. 1991;31(1):115–118. doi: 10.1016/0378-8741(91)90150-C. [DOI] [PubMed] [Google Scholar]
- 67.Jain V., Pareek A., Paliwal N., Ratan Y., Jaggi A. S., Singh N. Antinociceptive and antiallodynic effects of momordica charantia L. in tibial and sural nerve transection-induced neuropathic pain in rats. Nutritional Neuroscience. 2014;17(2):88–96. doi: 10.1179/1476830513Y.0000000069. [DOI] [PubMed] [Google Scholar]
- 68.Patel R., Mahobia N., Upwar N., Waseem N., Talaviya H., Patel Z. Analgesic and antipyretic activities of Momordica charantia linn. fruits. Journal of Advanced Pharmaceutical Technology & Research. 2010;1(4):415–418. doi: 10.4103/0110-5558.76441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ishola I. O., Akinyede A. A., Sholarin A. M. Antidepressant and anxiolytic properties of the methanolic extract of Momordica charantia Linn (Cucurbitaceae) and its mechanism of action. Drug Research. 2014;64(7):368–376. doi: 10.1055/s-0033-1358712. [DOI] [PubMed] [Google Scholar]
- 70.Aziba P. I., Bass D., Elegbe Y. Pharmacological investigation of Ocimum gratissimum in rodents. Phytotherapy Research. 1999;13(5):427–429. doi: 10.1002/(SICI)1099-1573(199908/09)13:5<427::AID-PTR467>3.0.CO;2-T. doi: 10.1002/(SICI)1099-1573(199908/09)13:5<427::AID-PTR467>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 71.Paula-Freire L. I. G., Andersen M. L., Molska G. R., Köhn D. O., Carlini E. L. A. Evaluation of the antinociceptive activity of ocimum gratissimum L. (Lamiaceae) essential oil and its isolated active principles in mice. Phytotherapy Research. 2013;27(8):1220–1224. doi: 10.1002/ptr.4845. [DOI] [PubMed] [Google Scholar]
- 72.Rabelo M., Souza E. P., Soares P. M. G., Miranda A. V., Matos F. J. A., Criddle D. N. Antinociceptive properties of the essential oil of Ocimum gratissimum L. (Labiatae) in mice. Brazilian Journal of Medical and Biological Research. 2003;36(4):521–524. doi: 10.1590/S0100-879X2003000400016. [DOI] [PubMed] [Google Scholar]
- 73.Tanko Y., Magaji G. M., Yerima M., Magaji R. A., Mohammed A. Anti-nociceptive and anti-inflammatory activities of aqueous leaves extract of Ocimum Gratissimum (Labiate) in Rodents. African Journal of Traditional, Complementary and Alternative Medicines. 2008;5(2):141–146. doi: 10.4314/ajtcam.v5i2.31265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zamin M. An analgesic and hepatoprotective plant: Ocimum gratissimum. Pakistan Journal of Biological Sciences. 2011;14(20):954–955. doi: 10.3923/pjbs.2011.954.955. [DOI] [PubMed] [Google Scholar]
- 75.Okoli C. O., Ezike A. C., Agwagah O. C., Akah P. A. Anticonvulsant and anxiolytic evaluation of leaf extracts of Ocimum gratissimum, a culinary herb. Pharmacognosy Research. 2010;2(1):36–40. doi: 10.4103/0974-8490.60580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barbosa P. R., Valvassori S. S., Bordignon C. L., Jr., et al. The aqueous extracts of Passiflora alata and Passiflora edulis reduce anxiety-related behaviors without affecting memory process in rats. Journal of Medicinal Food. 2008;11(2):282–288. doi: 10.1089/jmf.2007.722. [DOI] [PubMed] [Google Scholar]
- 77.Coleta M., Batista M. T., Campos M. G., et al. Neuropharmacological evaluation of the putative anxiolytic effects of Passiflora edulis Sims, its sub-fractions and flavonoid constituents. Phytotherapy Research. 2006;20(12):1067–1073. doi: 10.1002/ptr.1997. [DOI] [PubMed] [Google Scholar]
- 78.Deng J., Zhou Y., Bai M., Li H., Li L. Anxiolytic and sedative activities of Passiflora edulis f. flavicarpa. Journal of Ethnopharmacology. 2010;128(1):148–153. doi: 10.1016/j.jep.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 79.Otify A., George C., Elsayed A., Farag M. A. Mechanistic evidence of Passiflora edulis (Passifloraceae) anxiolytic activity in relation to its metabolite fingerprint as revealed via LC-MS and chemometrics. Food & Function. 2015;6(12):3807–3817. doi: 10.1039/c5fo00875a. [DOI] [PubMed] [Google Scholar]
- 80.Petry R. D., Reginatto F., de-Paris F. Comparative pharmacological study of hydroethanol extracts of Passiflora alata and Passiflora edulis leaves. Phytotherapy Research. 2001;15(2):162–164. doi: 10.1002/ptr.694. [DOI] [PubMed] [Google Scholar]
- 81.Sena L. M., Zucolotto S. M., Reginatto F. H., Schenkel E. P., De Lima T. C. M. Neuropharmacological activity of the pericarp of Passiflora edulis flavicarpa Degener: Putative involvement of C-glycosylflavonoids. Experimental Biology and Medicine. 2009;234(8):967–975. doi: 10.3181/0902-RM-84. [DOI] [PubMed] [Google Scholar]
- 82.Klein N., Gazola A. C., De Lima T. C. M., Schenkel E., Nieber K., Butterweck V. Assessment of sedative effects of passiflora edulis f. flavicarpa and passiflora alata extracts in mice, measured by telemetry. Phytotherapy Research. 2014;28(5):706–713. doi: 10.1002/ptr.5043. [DOI] [PubMed] [Google Scholar]
- 83.Adeyemi O. O., Okpo S. O., Ogunti O. O. Analgesic and anti-inflammatory effects of the aqueous extract of leaves of Persea americana Mill (Lauraceae) Fitoterapia. 2002;73(5):375–380. doi: 10.1016/s0367-326x(02)00118-1. [DOI] [PubMed] [Google Scholar]
- 84.Ojewole J. A. O., Amabeoku G. J. Anticonvulsant effect of Persea americana mill (Lauraceae) (avocado) leaf aqueous extract in mice. Phytotherapy Research. 2006;20(8):696–700. doi: 10.1002/ptr.1940. [DOI] [PubMed] [Google Scholar]
- 85.Moreira J., Klein-Júnior L. C., Cechinel Filho V., de Campos Buzzi F. Anti-hyperalgesic activity of corilagin, a tannin isolated from Phyllanthus niruri L. (Euphorbiaceae) Journal of Ethnopharmacology. 2013;146(1):318–323. doi: 10.1016/j.jep.2012.12.052. [DOI] [PubMed] [Google Scholar]
- 86.Obidike I. C., Salawu O. A., Ndukuba M., Okoli C. O., Osunkwo U. A. The anti-inflammatory and antinociceptive properties of the chloroform fraction from Phyllanthus niruri plant is mediated via the peripheral nervous system. Journal of Dietary Supplements. 2010;7(4):341–350. doi: 10.3109/19390211.2010.522553. [DOI] [PubMed] [Google Scholar]
- 87.Ahmed M., Shikha H. A., Sadhu S. K., Rahman M. T., Datta B. K. Analgesic, diuretic, and anti-inflammatory principle from Scoparia dulcis. Die Pharmazie. 2001;56:657–660. [PubMed] [Google Scholar]
- 88.Freire S. M., Torres L. M., Roque N. F., Souccar C., Lapa A. J. Analgesic activity of a triterpene isolated from Scoparia dulcis L. (vassourinha) Memórias do Instituto Oswaldo Cruz. 1991;86(2):149–151. doi: 10.1590/S0074-02761991000600034. [DOI] [PubMed] [Google Scholar]
- 89.Ibironke G. F., Umukoro A. S., Ajonijebu D. C. Central nervous system activity of the ethanol leaf extract of Sida acuta in rats. African Journal of Medicine and Medical Sciences. 2014;43:11–16. [PubMed] [Google Scholar]
- 90.Konaté K., Bassolé I. H. N., Hilou A., et al. Toxicity assessment and analgesic activity investigation of aqueous acetone extracts of Sida acuta Burn f . and Sida cordifolia L. (Malvaceae), medicinal plants of Burkina Faso. BMC Complementary and Alternative Medicine. 2012;12 doi: 10.1186/1472-6882-12-120.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nwaiwu J. I., Akah P. A. Anticonvulsant activity of the volatile oil from the fruit of tetrapleura tetraptera. Journal of Ethnopharmacology. 1986;18(2):103–107. doi: 10.1016/0378-8741(86)90023-1. [DOI] [PubMed] [Google Scholar]
- 92.Ojewole J. A. O. Analgesic and anticonvulsant properties of Tetrapleura tetraptera (Taub) (Fabaceae) fruit aqueous extract in mice. Phytotherapy Research. 2005;19(12):1023–1029. doi: 10.1002/ptr.1779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.