Abstract
Sirt6 is a member of the sirtuin family involved in physiological and pathological processes including aging, cancer, obesity, diabetes, and energy metabolism. This study is aimed at evaluating the relationship between liver SIRT6 gene expression and the oxidative stress network depending on adiposity levels in Zucker rats, an animal model of metabolic syndrome. We observed that liver-specific SIRT6 expression is reduced in an in vivo model of spontaneous obesity and metabolic syndrome. We also observed that SIRT6 expression in the liver is positively associated with SIRT1 and GST-M2 expressions, two proteins involved in antioxidant protection pathways and inversely related to body weight and plasmatic oxidative status. Interestingly, the SIRT6 expression is upregulated after energy restriction-induced weight loss concomitantly with an improvement in oxidative stress markers. These results suggest that SIRT6 may be a potential therapeutic target for the treatment of obesity and associated metabolic disorders, such as liver disease.
1. Introduction
During the last years, numerous evidences suggested the oxidative stress as a key factor involved in the development of obesity and its comorbidities [1]. The oxidative stress in obesity is induced by an excessive generation and accumulation of reactive oxygen species (ROS) in different cellular structures due to the expansion of the adipose tissue and inefficiency in the energy metabolism leading to cellular damage [2, 3]. The metabolic syndrome associated with obesity identifies subjects who have an increase in morbidity and mortality and is correlated with the development of several pathologies that affect different organs such as the liver and the progression from steatosis to nonalcoholic steatohepatitis and hepatocarcinogenesis in which oxidative stress appears to be involved [4].
Sirtuins are a family of NAD+-dependent protein deacetylases and ADP-ribosyltransferases with an important role in regulating the life span, aging, and cancer as well as energy metabolism and obesity and its metabolic associated disorders [5] and have therefore been proposed as a possible target for future therapies against these diseases.
Seven highly conserved family members of sirtuins have been identified (Sirt1-Sirt7) in mammals [6]. A number of studies revealed that Sirt1 has several beneficial effects on metabolic cell control and enhances the ability of cells to cope with oxidative stress [7, 8]. However, relatively little is known about the other sirtuins Sirt2 to Sirt7 being suggested that seven sirtuins may have redundant or similar cellular functions with Sirt1 [9]. In this regard, the nucleus-specific Sirt6 level is involved in obesity and diabetes [10, 11]. Aging and overnutrition lead to decreased Sirt6 level resulting in alterations of glucose and lipid metabolism [10]. Deletion of Sirt6 in mice resulted in lethal hypoglycemia [9, 12, 13]. On the other hand, overexpression of Sirt6 improves blood lipid profiles in animals fed with high-fat diets [12]. Liver expression of Sirt6 is induced by caloric restriction and suppressed in diseases associated with lipid accumulation in the liver [12]. Hepatic-specific deletion of sirt6 resulted to triglyceride accumulation and liver steatosis [14]. In addition, adipose tissue-specific ablation of Sirt6 resulted in increased blood glucose, hepatic steatosis, and diet-induced obesity [10, 13]. Sirt6 levels are reduced in the adipose tissue of murine models of obesity and increased in the adipose tissue of humans with weight loss [15, 16].
Therefore, the aim of this study was to evaluate the hepatic gene expression of SIRT6 and its relationship with the oxidative stress network depending on adiposity levels in Zucker rats, an animal model of metabolic syndrome.
2. Experimental Procedures
2.1. Animals
Male lean (Fa/fa; n = 10) and obese (fa/fa; n = 10) rats of the Zucker strain, 8 weeks old purchased from Charles River Laboratories (Barcelona, Spain), were maintained in controlled conditions of temperature, humidity, and illumination (12 h controlled photoperiod). They were allowed to acclimatize for 1 week on arrival. All rats had free access to water and standard laboratory diet (SAFE; Panlab, Spain), with 5.5% lipid, 23% protein, and 70% carbohydrate content. Body weight and food and water intake were measured during the experimental period. Finally (22 weeks), animals were euthanized and decapitated, and the livers and blood were obtained, immediately frozen on dry ice, and kept at −80°C until analysis. All animal experiments and procedures involved in this study were approved by the Ethical Committee at the University of Santiago de Compostela, in accordance with the European Union Normative for the use of experimental animals.
In the experimental weight loss protocol, fatty rats (n = 30) were randomly divided into three subgroups: an energy-restriction group (ER; n = 10), an exercise group (EX; n = 10), and an energy restriction plus exercise group (EREX; n = 10). These fatty rats were individually housed for 1 week, and their individual food intake was weighed and recorded. Then, the rats in the ER and the EREX groups were fed a diet 30% less in quantity than their individual food intake during 4 weeks (based on the weight of food).
Animals from the EX and the EREX groups were placed on a monitored rodent treadmill (Treadmill system 303401-R-04/C, TSE-Systems, Inc., Chesterfield, MO, USA) for 10 min/day and increased progressively in intensity from 10 m/min to 20 m/min during 1 week for familiarization. After that, animals were placed on the treadmill for 30 min/day at 20 m/min, 7 days per week for 4 weeks.
2.2. Body Composition
Body composition studies were performed every 2 weeks using a nuclear magnetic resonance imaging (MRI) system (Whole Body Composition Analyser, EchoMRI, Echo Medical Systems, USA).
2.3. RNA Extraction and Quantitative RT-PCR
Total RNA extraction from the liver was performed using Trizol (Invitrogen) according to the manufacturer's recommendations. The RNA (500 ng) was retrotranscribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA). The expression of the genes of interest was studied using TaqMan real-time PCR in Step One Plus system (Applied Biosystems, USA) using specific primers and probes obtained from inventoried TaqMan Gene Expression Assays (Applied Biosystems, USA) for SIRT6, SIRT1, and GST-M2 genes. All reactions were performed using the following cycling parameters: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 60°C for 1 minute. For data analysis, the RNA level of the gene of interest was normalized using the β-actin values, according to the 2-ΔΔCt method.
2.4. Oxidative Stress Blood Analysis
Plasmatic malondialdehyde (MDA) and total antioxidant capacity (TAC) were evaluated using commercially available colorimetric assay kits (OXIS International, Portland, OR, USA).
2.5. Statistical Analysis
The normal distribution was explored with the Kolmogorov-Smirnov test and the Shapiro-Wilk test. Because oxidative stress markers and gene expression levels were not normally distributed, the Mann–Whitney U test was applied to study the differences between obese and lean rats. The fold change in gene expression was calculated using the 2-ΔΔCt relative quantitation method according to the manufacturer's guidelines (Applied Biosystems, USA) and reporting the data as the geometric mean (standard error of the mean, SEM). A p value < 0.05 was considered to be statistically significant, and a p value ≤ 0.1 was considered to be a trend for significance. The potential association between oxidative stress biomarkers and gene expression levels was evaluated using the Spearman rank correlation coefficient. Statistical analysis was performed by SPSS 15.0 software (SPSS Inc., USA) for Windows XP (Microsoft, USA) and GraphPad Prism 6.01 software (GraphPad Software Inc., USA).
3. Results
3.1. Characteristics of the Experimental Animal at 22 Weeks Old
We fed rats a standard diet while monitoring body weight gain and body composition. At the end of the experiment, the obese rats (fa/fa) showed higher body weight and consequently higher fat mass (9×) as well as lower free fat mass than their lean littermates (Fa/fa) (Table 1). In addition, plasma levels of oxidative stress biomarkers as MDA and TAC at the end of the experimental period were lower in lean than in obese phenotype (Table 1).
Table 1.
Characteristics of the experimental animal at 22 weeks old.
| Obese (n = 10) | Lean (n = 10) | p value | |
|---|---|---|---|
| Body weight (g) | 559 ± 28 | 410 ± 30 | <0.001 |
| Fat mass (g) | 213 ± 11 | 24 ± 6 | <0.001 |
| Free fat mass (g) | 261 ± 49 | 303 ± 19 | 0.021 |
| MDA (μM) | 1.75 ± 0.64 | 0.61 ± 0.19 | 0.001 |
| TAC (mM Trolox) | 436 ± 191 | 265 ± 40 | 0.020 |
Data are represented as the mean ± standard error of the mean (SEM). P value shows statistically significant differences compared with the control-lean group. MDA: malondialdehyde; TAC: total antioxidant capacity.
3.2. Hepatic Gene Expression of SIRT6, SIRT1, and GST-M2
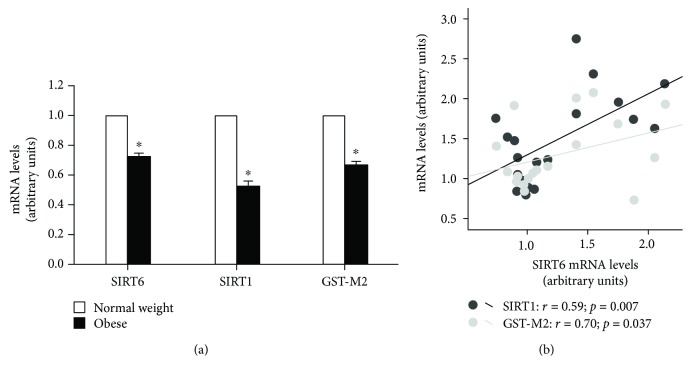
Obese rats showed a marked decrease in the hepatic gene expression of SIRT6 (30%) and SIRT1 (50%). These results in SIRT6 and SIRT1 gene expressions were also observed in an animal model of diet-induced obesity (DIO; Supplementary Figure 1). In addition, the hepatic gene expression of GST-M2, the antioxidant enzyme glutathione-S-transferase Mu2, was also reduced (30%) (Figure 1(a)).
Figure 1.
Liver expression of SIRT6, SIRT1, and GST-M2 genes in lean or obese rats (a). Data are represented as the mean ± standard error of the mean (SEM). Statistically significant differences compared with control-lean counterparts ∗ p < 0.05 vs. normal weight group. Association between SIRT6 mRNA levels with SIRT1 or GST-M2 genes in all animal taking together (b). SIRT1 (r = 0.59; p = 0.007), GST-M2 (r = 0.70; p = 0.037).
Because SIRT1 and GST-M2 are two proteins with proven involvement in the antioxidant protection pathway, we performed an association study between SIRT6 expression and SIRT1 and GST-M2 expression in livers from all rats taken together. Interestingly, the hepatic SIRT6 mRNA levels were positively associated with the gene expression of SIRT 1 (r = 0.59; p = 0.007) and GST-M2 (r = 0.70; p = 0.037) (Figure 1(b)).
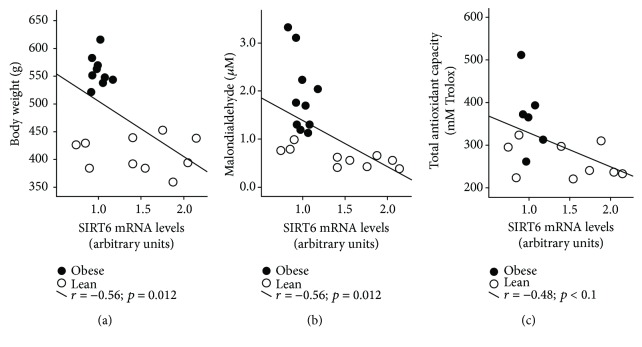
3.3. Association of SIRT6 Gene Expression with Body Weight, MDA, and TAC
In accordance with the involvement of SIRT6 in the regulation of oxidative stress process [10] and its association with SIRT1 and GST-M2 expression levels, we reasoned that the gene expression of SIRT6 at the hepatic level should be correlated with body weight as well as with systemic markers of oxidative stress. In fact, increased hepatic SIRT6 expression was associated with lower body weight (Figure 2(a)), lower plasma MDA levels (Figure 2(b)), and lower plasma TAC (Figure 2(c)).
Figure 2.
Association of SIRT6 gene expression with body weight (a), MDA (b), and TAC (c).
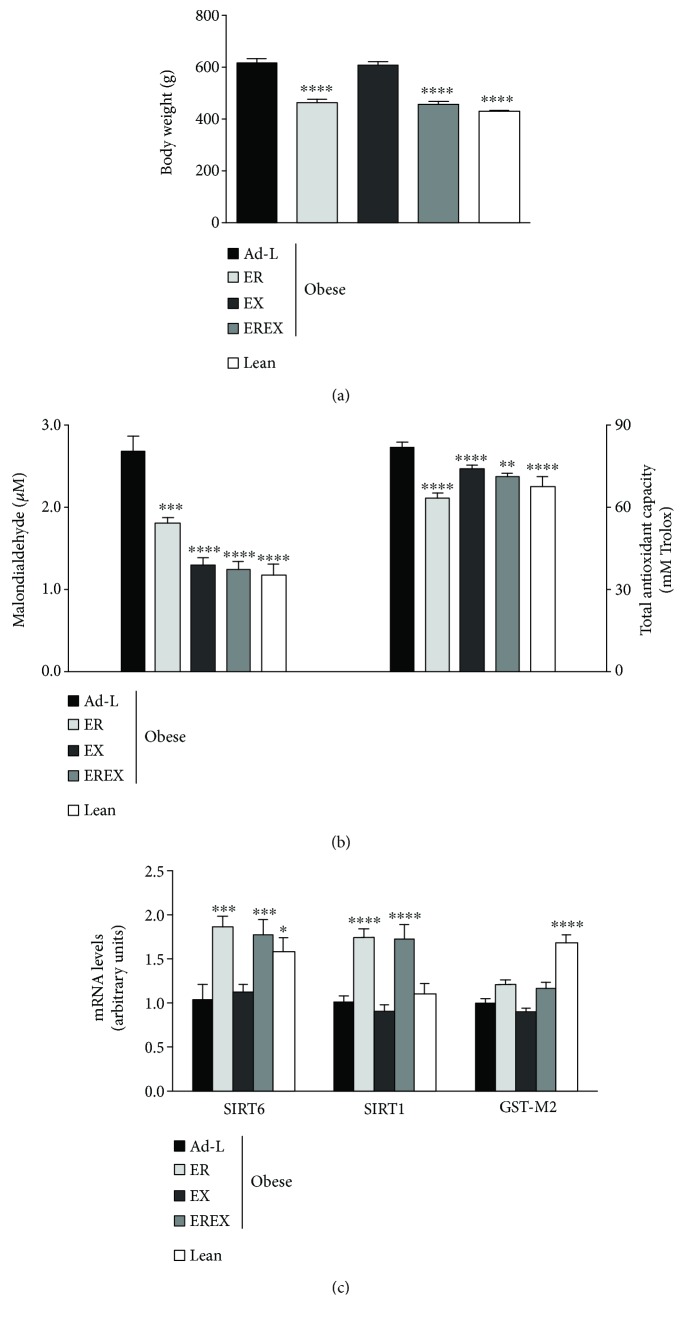
3.4. Weight Loss, Systemic Oxidative Stress, and Hepatic Gene Expression
After the 4 weeks of weight loss treatments, the ER and EREX groups exhibited 26% less body weight (Figure 3(a)) than the Ad-L control group and similar to the lean control animals. No differences were observed between both groups or between the EX and the Ad-L group in body weight. According to the body weight loss data, the ER and EREX groups showed a significant reduction in the circulating levels of MDA and TAC (Figure 3(b)). Interestingly, in the EX group, despite not producing a reduction in body weight, it showed a reduction in the circulating levels of MAD and TAC similar to the effects observed for the EREX group (Figure 3(b)). Then, we investigated the effect of the weight loss interventions on hepatic gene expression of sirtuins and GST-M2. According to the body weight loss data, the ER and EREX groups but not the EX group showed a significant increase in SIRT6 and SIRT1 gene expressions (Figure 3(c)). However, the expression of GST-M2 showed no significant variations after the interventions (Figure 3(c)).
Figure 3.
Effect of 4 weeks of weight loss interventions on body weight (a), serum oxidative stress biomarkers (b), and hepatic gene expression (c). Data are represented as mean and SEM, n = 6 − 10 animals/group. ∗ p < 0.05 vs. the Ad-L group, ∗∗ p < 0.01 vs. the Ad-L group, ∗∗∗ p < 0.001 vs. the Ad-L group, ∗∗∗∗ p < 0.0001 vs. the Ad-L group. Ad-L: ad libitum group; ER: energy-restriction group; EX: exercise group; EREX: energy restriction plus exercise group.
4. Discussion
This work shows that the oxidative stress induced by excess of adiposity is related to a downregulation of hepatic SIRT6 expression in obese individuals. After weight loss induced by energy restriction, the hepatic SIRT6 expression increases, concomitantly with an improvement in oxidative stress markers. Therefore, these results suggest that the potential role of SIRT6 in the protection against oxidative stress damage could be a therapeutic target to treat the damage caused by the association between obesity and oxidative stress.
Sirtuins play an important regulatory role in energy metabolism and they may be a potential therapeutic target for obesity and associated pathologies [5]. Among the sirtuin family members, sirt1 is the most well-studied sirtuin and it has been implicated in the protection against cellular oxidative stress, and it plays an important role in metabolic pathway regulation, specifically acting in adipocytes as an inhibitor of adipogenesis. Additionally, the expression of SIRT1 is modulated by energy restriction in association with improvements in oxidative stress [7]. In this line, SIRT6 was recently discovered as a relevant player in the predisposition to age-associated diseases [17]. The activity of SIRT6 is reduced in obesity and diabetes and its hepatic-specific ablation increases liver steatosis onset [10]. However, the study of SIRT6 is still very fresh [10]. In this work, we showed a downregulation of SIRT6 in the liver of obese rats compared with their lean littermates.
The liver is a key metabolic organ controlling the overall lipid metabolism in response to hormonal and nutritional stimuli received and one of the organs most affected by excessive intake of carbohydrates or fat leading to metabolic pathologies associated with obesity. Several studies highlighted that obesity strongly contributes to the transition of nonalcoholic fatty liver disease (NAFLD) to nonalcoholic steatohepatitis (NASH) and hepatocellular carcinoma (HCC) [18, 19]. The absence of SIRT6 increases the expression of genes responsible for hepatic long-chain fatty acid uptake and reduced expression of genes for β-oxidation leading to accumulation of triglycerides and fatty liver disease and hepatic steatosis [10]. Additionally, the participation of ROS in liver disease has been suggested [20]. Moreover, it was observed that genes related to oxidative stress regulation are overexpressed in early stages of HCC [21]. In this regard, obesity produces various metabolic alterations that contribute very actively to the general oxidative balance, creating the basis for the development of diseases such as diabetes, hypertension, cardiovascular disease, and cancer, among others. According to the literature, the major contributors to systemic oxidative stress in obesity are hyperglycemia, increased muscle activity to support weight gain, high lipid levels in different tissues, chronic inflammation, low antioxidant defenses, endothelial ROS production, and hyperleptinemia [1]. Oxidative stress in obesity is a systematic problem that can be reduced by improving antioxidant defenses through fat reduction, physical activity or exercise, dietary restriction, surgical intervention, or antioxidant therapies which, based on the results showed in this work, may include SIRT6.
In accordance with a potential association between the expression of SIRT6 with oxidative stress, we observed a correlation between SIRT1 and GST-M2, both genes that codify proteins involved in the protection against oxidative stress [7, 22], which were also downregulated in the liver of obese fa/fa rats. These results suggest a dysregulation in the antioxidant defenses that promote the oxidative stress characteristic of obesity [23] which was confirmed by the high circulating levels of MDA and TAC.
The connection between oxidative stress, energy restriction, and sirtuin activity has been shown in the literature. The energy restriction reduces the cellular levels of NADH by increasing the NAD+/NADH ratio and causing an increase in Sirt2 activity [24]. As in the case of Sirt2, Sirt6 activity is also influenced by energy restriction. Prolonged restriction results in increased activity of Sirt6 at the brain, muscle, white adipose tissue, and liver levels [12, 13]. In addition, Sirt6 is also a mediator of the effects induced by energy restriction. SIRT6 suppression decreases life extension activated by energy restriction, and SIRT6 overexpression shows reduced body weight, increased metabolism, and reduced serum levels of insulin, glucose, cholesterol, and several adipokines [13, 25].
In this sense, the data obtained in this study show that body weight loss is associated with an increase in hepatic SIRT6 expression and a reduction in systematic oxidative stress biomarkers in a similar way to the well-studied SIRT1. According to the important role of Sirt6 in the liver related to lipidic and glucose metabolism [10], these effects are observed in models of caloric restriction; however, physical exercise does not seem to have any influence on hepatic SIRT6 expression, although exercise has a potent reducing effect of oxidative stress at the systemic level. This suggests that the exercise model produces a decrease in systemic oxidative stress similar to the energy restriction model but probably through a different mechanism in which the skeletal muscle may be involved. All these data suggest that SIRT6 acts similarly to SIRT1 and may play a key role in regulating energy metabolism and defense against oxidative stress.
In conclusion, the results of the current work evidenced that SIRT6 gene expression shows similar pattern of SIRT1 gene expression, the most-studied sirtuin member, in the context of relationship with excess body weight and the regulation of oxidative stress. It supports the idea of a prominent role for SIRT6 as a potential therapeutic target for the treatment of obesity and associated disease, particularly liver disease.
Acknowledgments
Authors thank Maribel Rendo from the Department of Molecular and Cellular Endocrinology of Instituto de Investigacion Sanitaria de Santiago (IDIS) for her support of research data management. This study was supported by Centro de Investigacion Biomedica en Red de Fisiopatologia de la Obesidad y Nutricion (CIBERobn) and grants from the Instituto de Salud Carlos III (PI17/01287) cofinanced by the European Regional Development Fund (FEDER). Andrea G. Izquierdo is funded by CIBERobn and Ana B. Crujeiras is funded by a research contract “Miguel Servet” (CP17/00088) from the Instituto de Salud Carlos III, cofinanced by the European Regional Development Fund (FEDER).
Abbreviations
- ANOVA:
Analysis of variance
- HCC:
Hepatocellular carcinoma
- HFD:
High-fat diet
- GST-M2:
Glutathione-S-transferase Mu2
- MDA:
Plasmatic malondialdehyde
- MRI:
Magnetic resonance imaging
- NAFLD:
Nonalcoholic fatty liver disease
- NASH:
Nonalcoholic steatohepatitis
- ROS:
Reactive oxygen species
- SIRT:
Sirtuin
- TAC:
Total antioxidant capacity.
Contributor Information
Felipe F. Casanueva, Email: endocrine@usc.es.
Ana B. Crujeiras, Email: anabelencrujeiras@hotmail.com.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
MCC and AGI designed and performed experiments and wrote the manuscript; MA helped with experiments and contributed to the discussion; ABC and FFC obtained funding, designed experiments, and wrote the manuscript. MCC, AGI, ABC, and FFC are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. All authors have reviewed and approved the article and have read the journal's authorship agreement. Marcos C Carreira and Andrea G Izquierdo contributed equally to this work and should be considered co-first authors. Felipe F Casanueva and Ana B. Crujeiras contributed equally to this work and should be considered co-main authors.
Supplementary Materials
Supplementary Figure 1: liver expression of SIRT6 and SIRT1 in lean or diet-induced obesity (DIO) Sprague Dawley rats (A). Data are represented as the mean ± standard error of the mean (SEM). Statistically significant differences compared with control-lean counterparts ∗ p < 0.05 vs. lean group.
References
- 1.Vincent H. K., Innes K. E., Vincent K. R. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes, Obesity & Metabolism. 2007;9(6):813–839. doi: 10.1111/j.1463-1326.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 2.Crujeiras A. B., Parra D., Goyenechea E., Abete I., González-Muniesa P., Martínez J. A. Energy restriction in obese subjects impact differently two mitochondrial function markers. Journal of Physiology and Biochemistry. 2008;64(3):211–219. doi: 10.1007/BF03178844. [DOI] [PubMed] [Google Scholar]
- 3.Dizdar O., Alyamac E. Obesity: an endocrine tumor? Medical Hypotheses. 2004;63(5):790–792. doi: 10.1016/j.mehy.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 4.Streba L. A., Vere C. C., Rogoveanu I., Streba C. T. Nonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma: an open question. World Journal of Gastroenterology. 2015;21(14):4103–4110. doi: 10.3748/wjg.v21.i14.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michan S., Sinclair D. Sirtuins in mammals: insights into their biological function. The Biochemical Journal. 2007;404(1):1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frye R. A. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochemical and Biophysical Research Communications. 1999;260(1):273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- 7.Crujeiras A. B., Parra D., Goyenechea E., Martínez J. A. Sirtuin gene expression in human mononuclear cells is modulated by caloric restriction. European Journal of Clinical Investigation. 2008;38(9):672–678. doi: 10.1111/j.1365-2362.2008.01998.x. [DOI] [PubMed] [Google Scholar]
- 8.Lim C. S. Is SIRT6 a new biomarker for oxidative stress and longevity assurance gene? Medical Hypotheses. 2007;69(1):p. 231. doi: 10.1016/j.mehy.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 9.Kanfi Y., Peshti V., Gil R., et al. SIRT6 protects against pathological damage caused by diet-induced obesity. Aging Cell. 2010;9(2):162–173. doi: 10.1111/j.1474-9726.2009.00544.x. [DOI] [PubMed] [Google Scholar]
- 10.Kuang J., Chen L., Tang Q., Zhang J., Li Y., He J. The role of Sirt6 in obesity and diabetes. Frontiers in Physiology. 2018;9:p. 135. doi: 10.3389/fphys.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michishita E., Park J. Y., Burneskis J. M., Barrett J. C., Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Molecular Biology of the Cell. 2005;16(10):4623–4635. doi: 10.1091/mbc.e05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanfi Y., Shalman R., Peshti V., et al. Regulation of SIRT6 protein levels by nutrient availability. FEBS Letters. 2008;582(5):543–548. doi: 10.1016/j.febslet.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuang J., Zhang Y., Liu Q., et al. Fat-specific Sirt6 ablation sensitizes mice to high-fat diet-induced obesity and insulin resistance by inhibiting lipolysis. Diabetes. 2017;66(5):1159–1171. doi: 10.2337/db16-1225. [DOI] [PubMed] [Google Scholar]
- 14.Kim H. S., Xiao C., Wang R. H., et al. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metabolism. 2010;12(3):224–236. doi: 10.1016/j.cmet.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dominy J. E., Jr., Lee Y., Jedrychowski M. P., et al. The deacetylase Sirt6 activates the acetyltransferase GCN5 and suppresses hepatic gluconeogenesis. Molecular Cell. 2012;48(6):900–913. doi: 10.1016/j.molcel.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moschen A. R., Wieser V., Gerner R. R., et al. Adipose tissue and liver expression of SIRT1, 3, and 6 increase after extensive weight loss in morbid obesity. Journal of hepatology. 2013;59(6):1315–1322. doi: 10.1016/j.jhep.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 17.Zhang P., Tu B., Wang H., et al. Tumor suppressor p53 cooperates with SIRT6 to regulate gluconeogenesis by promoting FoxO1 nuclear exclusion. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(29):10684–10689. doi: 10.1073/pnas.1411026111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marengo A., Rosso C., Bugianesi E. Liver cancer: connections with obesity, fatty liver, and cirrhosis. Annual Review of Medicine. 2016;67(1):103–117. doi: 10.1146/annurev-med-090514-013832. [DOI] [PubMed] [Google Scholar]
- 19.Marrero J. A., Fontana R. J., Su G. L., Conjeevaram H. S., Emick D. M., Lok A. S. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36(6):1349–1354. doi: 10.1002/hep.1840360609. [DOI] [PubMed] [Google Scholar]
- 20.Sánchez-Pérez Y., Carrasco-Legleu C., García-Cuellar C., et al. Oxidative stress in carcinogenesis. Correlation between lipid peroxidation and induction of preneoplastic lesions in rat hepatocarcinogenesis. Cancer Letters. 2005;217(1):25–32. doi: 10.1016/j.canlet.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 21.Beltrán-Ramírez O., Sokol S., le-Berre V., François J. M., Villa-Treviño S. An approach to the study of gene expression in hepatocarcinogenesis initiation. Translational Oncology. 2010;3(2):142–148. doi: 10.1593/tlo.09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crujeiras A. B., Parra D., Goyenechea E., Abete I., Martínez J. A. Tachyphylaxis effects on postprandial oxidative stress and mitochondrial-related gene expression in overweight subjects after a period of energy restriction. European Journal of Nutrition. 2009;48(6):341–347. doi: 10.1007/s00394-009-0019-9. [DOI] [PubMed] [Google Scholar]
- 23.Vincent H. K., Taylor A. G. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. International Journal of Obesity. 2006;30(3):400–418. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 24.Tasselli L., Xi Y., Zheng W., et al. SIRT6 deacetylates H3K18ac at pericentric chromatin to prevent mitotic errors and cellular senescence. Nature Structural & Molecular Biology. 2016;23(5):434–440. doi: 10.1038/nsmb.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanfi Y., Naiman S., Amir G., et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483(7388):218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: liver expression of SIRT6 and SIRT1 in lean or diet-induced obesity (DIO) Sprague Dawley rats (A). Data are represented as the mean ± standard error of the mean (SEM). Statistically significant differences compared with control-lean counterparts ∗ p < 0.05 vs. lean group.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.