Abstract
Background:
The prognosis of advanced pancreatic cancer (APC) is poor and differs considerably among patients. Therefore, it is clinically relevant to identify patients with APC who are more likely to benefit from palliative chemotherapy with reduced risk of toxicity. To date, there is no prognostic score universally recommended to help clinicians in planning the therapeutic management.
Methods:
Using individual patient data from 319 cases of APC treated with gemcitabine-based chemotherapy and enrolled in the SAKK 44/00-CECOG/PAN.1.3.001 randomized trial, several baseline variables, including inflammatory markers, were analysed post hoc as predictors of mortality and/or grade 3 or 4 chemotherapy-related toxicity and separate risk scores were developed.
Results:
Median survival of the study patients was 7.9 months (interquartile range 3.7–13.3 months). Independent predictors of mortality included increased Aspartate transaminase (ASAT), low performance status, increased derived neutrophil to lymphocyte ratio, increased Carbohydrate Antigen 19-9 (CA 19-9), low haemoglobin, presence of pain, presence of metastasis and increased alkaline phosphatase (ALP). During the study, 117 patients experienced at least one grade 3 or 4 adverse event. Independent predictors of toxicity included white blood cells, ALP, renal function and bilirubin levels at baseline. Both models displayed moderate levels of discrimination (C-statistic 0.68 and 0.64 for mortality and toxicity, respectively) and adequate calibration.
Conclusions:
We developed simple-to-use prognostic scores for mortality and severe toxicity for patients with APC. These scores can be useful in daily practice to identify patients with increased risk of death or toxicity and to plan the most appropriate therapeutic strategy to improve survival and quality of life. Further prospective studies to validate such scores are needed.
Keywords: advanced pancreatic cancer, chemotherapy, inflammatory markers, mortality, prediction score, toxicity
Introduction
Pancreatic cancer (PC) is one of the most lethal malignancies worldwide, and most patients are diagnosed too late for curative resection.1 Systemic gemcitabine (Gem)-based chemotherapy has long been used as a standard therapy for patients with advanced pancreatic cancer (APC), however the prognosis differs considerably among patients. Therefore, it is clinically relevant to identify patients with APC who are more likely to benefit from palliative chemotherapy with reduced risk of toxicity.
Some clinical and laboratory parameters have been identified as being associated with negative prognosis in patients with APC,2,3 however, there is great variation in the reporting of potentially confounding or prognostic variables in studies investigating systemic treatment for APC.4 Recently, some studies have shown that different inflammatory markers might have a significant prognostic role in many tumours, but few data exist for APC. In particular, derived neutrophil to lymphocyte ratio (dNLR) and neutrophil–platelet score (NPS), easily obtained from routine blood analysis, were found to be associated with cancer survival, but poorly explored in patients with APC.5–7 Thus, it is necessary to evaluate and subsequently, integrate the available pretreatment factors in order to provide a prognostic model for predicting survival in patients receiving palliative chemotherapy. Furthermore, there is little evidence regarding chemotherapy-related toxicity predictors in patients with APC.
For patients with advanced or metastatic PC, the Swiss Group for Clinical Cancer Research (SAKK) and the Central European Cooperative Oncology Group (CECOG) compared the efficacy and safety of Gem plus capecitabine (Cap) with single-agent Gem in a randomized clinical trial (SAKK 44/00-CECOG/PAN.1.3.001) showing similar safety and efficacy between the two regimens, but the GemCap treatment improved survival in patients with good baseline Karnofsky Performance Score (KPS).8 Given the overall poor prognosis of APC and the symptom burden, symptom palliation and balancing the trade-offs between treatment side effects and benefits on tumour-related symptoms and survival are of paramount importance. Therefore, the aim of this study was to analyse predictors of mortality and chemotherapy-related toxicity in patients with APC treated with gem-based chemotherapy in the controlled setting of this randomized clinical trial. To our knowledge, this is the first study to provide a dedicated analysis and a score of toxicity predictors in this specific setting of patients.
Methods
Study design and patient population
The design and results of the trial have been previously reported.8–10 Briefly, this was a randomized, stratified, multicentre, phase III trial conducted at 30 centres in eight countries comparing the efficacy and safety of GemCap versus single-agent Gem in advanced/metastatic pancreatic cancer (clinicaltrials.gov identifier NCT00030732). A total of 319 patients were enrolled between June 2001 and June 2004 and randomly assigned to receive GemCap (oral capecitabine 650 mg/m2 twice daily on days 1–14 plus Gem 1000 mg/m2 by 30-min infusion on days 1 and 8 every 3 weeks) or Gem (1000 mg/m2 by 30-min infusion weekly for 7 weeks, followed by a 1-week break, and then weekly for 3 weeks every 4 weeks). Treatment was continued until disease progression or for a maximum of 24 weeks except in the case of unacceptable toxicity.
Treatment visits occurred weekly for the first 7 weeks, and subsequently for the administration of Gem. Laboratory tests were performed at regular intervals during treatment, usually coinciding with the administration of Gem. Adverse events were monitored continuously during treatment and for 4 weeks after the last drug administration. All adverse reactions were assessed according to National Cancer Institute Common Toxicity Criteria (version 2.0). Response evaluation was performed at the start of weeks 7, 17 and 25 and every 9 weeks thereafter until disease progression using contrast computed tomography scan; response was defined according to the Response Evaluation Criteria in Solid Tumours. Objective responses (complete or partial) were supposed to be confirmed after a minimum of 4 weeks.
Informed consent was obtained from all patients, and ethical committee approval was received by all participating centres. The trial was conducted in accordance with the Declaration of Helsinki and its subsequent amendments and according to good clinical practice guidelines.
The present study was a post-hoc analysis aimed at exploring predictors of mortality and chemotherapy-related toxicity in patients enrolled in this trial.
Statistical analysis
Patient characteristics assessed for prognostic significance were as follows: age, sex, KPS (60–80 versus 90–100), body mass index, disease status (locally advanced versus metastatic disease), presence of pain, Carbohydrate Antigen 19-9 (CA 19-9) level, liver and renal function (i.e. Aspartate transaminase (ASAT), Alanine transaminase (ALAT), bilirubin, creatinine clearance, protein levels), alkaline phosphatase (ALP), haematological parameters (i.e. haemoglobin, white blood cells [WBC]; neutrophils, platelets). The dNLR was calculated from peripheral blood counts as neutrophils divided by the difference of leucocytes and neutrophils.6 The NPS was calculated as follows: patients with a neutrophil count ⩽ 7.5 × 109/L and platelets ⩽ 400 × 109/L scored 0, patients with neutrophils > 7.5 × 109/L or platelets > 400 × 109/L scored 1, and patients with both neutrophils > 7.5 × 109/L and platelets > 400 × 109/L scored 2.7 All these parameters, including randomized treatment, were tested in univariate analyses and then multivariate analysis to identify the independent predictors of mortality and toxicity (grade 3 or 4 adverse events).
Overall survival (OS) was defined as the period from the date of randomization to the date of death from any cause or censored at the last follow-up visit. OS and 1 year survival rates were analysed using Kaplan–Meier curves, and p values were calculated using the logrank test. A p value < 0.05 was considered statistically significant. To predict mortality, the Cox proportional hazards model was used to estimate the hazard ratio (HR) and 95% confidence interval (CI). The regression coefficient (β) for each independent prognostic factor was derived from the Cox regression equation (HR = eβ). Assignment of points to risk factors to obtain a score was based on a linear transformation of the corresponding β coefficient. The lowest β coefficient was used as reference and the coefficient of each variable was divided by this reference value, then multiplied by a constant (i.e. 2) and finally rounded to the nearest integer as a score. To predict toxicity, the multiple logistic regression model was used to estimate the odds ratio and 95% CI. Similar to mortality, a score was created attributing points to risk and protective factors. The performance of both models was assessed with Harrell’s C-statistic for discrimination and with Hosmer–Lemeshow goodness-of-fit statistic for calibration. To facilitate the use of the scores in daily practice, we also stratified the score values into three risk subgroups (low, intermediate, high risk) based on the best cutoffs optimizing the area under the curve performance.
Results
Patient population
A total of 319 patients (GemCap, n = 160; Gem, n = 159) were analysed. Baseline patient and disease characteristics are summarized in Table 1. Median age was 62 years (61% of patients < 65-years-old) and 54% of patients were men. A majority of patients had normal serum protein, normal body weight, preserved renal function, metastatic disease and pain requiring analgesic medication. The NPS and dNLR scores were increased in 33% and 66% of patients, respectively.
Table 1.
Baseline characteristics of the population.
| Characteristics | Patients n = 319 |
|---|---|
| Age | 62.2 (55.1–68.9) |
| < 65 years | 194 (60.8%) |
| ⩾ 65 years | 125 (39.2%) |
| Patient sex | |
| Male | 171 (53.6%) |
| Female | 148 (46.4%) |
| Body mass index | 23.3 (20.9–25.8) |
| < 18.5 | 19 (6.0%) |
| 18.5–< 25 | 194 (60.8%) |
| 25–< 30 | 84 (26.3%) |
| ⩾ 30 | 22 (6.9%) |
| Treatment arm | |
| Capecitabine + Gemcitabine | 160 (50.2%) |
| Gemcitabine | 159 (49.8%) |
| Karnofsky Performance Score | |
| ⩽ 80 | 151 (47.3%) |
| ⩾ 90 | 168 (52.7%) |
| Distant metastases | |
| Absent | 66 (20.7%) |
| Present | 253 (79.3%) |
| Presence of pain | |
| No | 104 (32.6%) |
| Yes | 215 (67.4%) |
| Haemoglobin (mmol/L) | 7.76 (7.07–8.5) |
| < 8.07 | 197 (61.8%) |
| ⩾ 8.07 | 122 (38.2%) |
| White blood cell count (109/L) | 8.3 (6.4–10.4) |
| > 10.8 | 251 (78.7%) |
| ⩾ 10.8 | 68 (21.3%) |
| Platelet count (109/L) | 262 (211–333) |
| < 400 | 274 (85.9%) |
| ⩾ 400 | 45 (14.1%) |
| Neutrophil count (109/L) | 5.5 (4.1–7.3) (n = 301) |
| < 7.5 | 234 (77.7%) |
| ⩾ 7.5 | 67 (22.3%) |
| Aspartate transaminase (IU/L) | 26.0 (17.0–42.6) (n = 318) |
| < 31 | 188 (59.1%) |
| ⩾ 31 | 130 (40.9%) |
| Alanine transaminase (IU/L) | 31.0 (18.0–52.0) |
| < 33 | 170 (53.3%) |
| ⩾ 33 | 149 (46.7%) |
| Bilirubin (µmol/L) | 11.62 (8.03–18.81) |
| < 22.23 | 248 (77.7%) |
| ⩾ 22.23 | 71 (22.3%) |
| Alkaline phosphatase (IU/L) | 212.0 (124.0–406.0) |
| < 123 | 78 (24.4%) |
| ⩾ 123 | 241 (75.6%) |
| Clearance creatinine (ml/min/ 1.73 m2) | 74.0 (60.0–92.3) |
| < 60 | 74 (23.2%) |
| ⩾ 60 | 245 (76.8%) |
| Serum creatinine (µmol/L) | 76.90 (67.18–88.4) |
| <132.6 | 316 (99.1%) |
| ⩾ 132.6 | 3 (0.9%) |
| Serum protein (g/L) | 7.1 (6.7–7.6) (n = 206) |
| < 6.9 | 72 (35.0%) |
| ⩾ 6.9 | 134 (65.0%) |
| Carbohydrate antigen 19-9 level (IU/L) | 1626.5 (126.7–13399) (n = 296) |
| < 1000 | 138 (46.6%) |
| ⩾ 1000 | 158 (53.4%) |
| Derived neutrophil to lymphocyte ratio | |
| < 2 | 129 (42.9%) |
| ⩾ 2 | 172 (57.1%) |
| Neutrophil to platelet score | |
| 0 | 216 (71.8%) |
| 1 | 66 (21.9%) |
| 2 | 19 (6.3%) |
Predictors of mortality
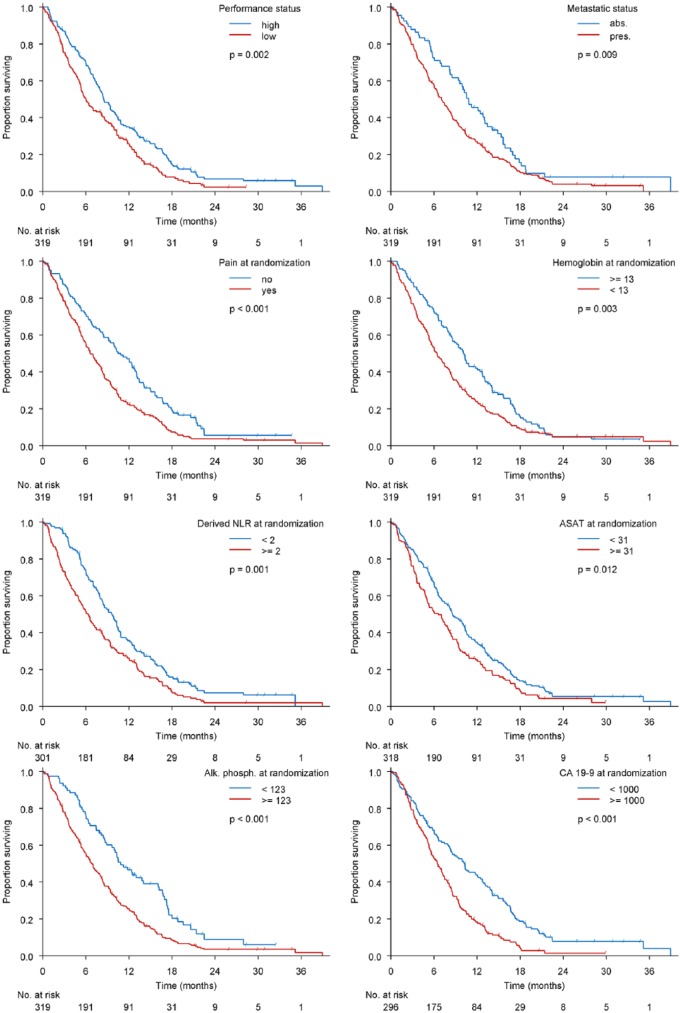
Median survival of the population was 7.9 months (interquartile range 3.7–13.3 months). Point estimates and corresponding 95% CIs for each covariate in the final prediction model of mortality are shown in Table 2. Main predictors of mortality were: increased ASAT, low performance status, increased dNLR, increased CA 19-9, low haemoglobin, presence of pain, presence of metastasis and increased ALP (Figure 1 and Table 2).
Table 2.
Multivariate prediction model of mortality.
| Parameter | Comparison | Hazard ratio | 95% confidence interval | p value | β coefficient | Score |
|---|---|---|---|---|---|---|
| Aspartate transaminase ⩾ 31 IU/L |
⩾ versus < | 1.325 | 1.017–1.727 | 0.0374 | 0.281 | 2 |
| Karnofsky Performance Score | ⩽ 80 versus ⩾ 90 | 1.395 | 1.078–1.806 | 0.0115 | 0.333 | 2 |
| Derived neutrophil to lymphocyte ratio 2 | ⩾ versus < | 1.475 | 1.142–1.905 | 0.0029 | 0.388 | 3 |
| Carbohydrate antigen 19-9 level (1000 U/L) | ⩾ versus < | 1.483 | 1.132–1.943 | 0.0042 | 0.394 | 3 |
| Haemoglobin level (8.07 mmol/L) | < versus ⩾ | 1.514 | 1.163–1.973 | 0.0021 | 0.415 | 3 |
| Presence of pain | Yes versus No | 1.564 | 1.185–2.064 | 0.0016 | 0.447 | 3 |
| Distant metastases | Pre versus Abs | 1.604 | 1.164–2.211 | 0.0039 | 0.473 | 3 |
| Alkaline phosphatase ⩾ 123 IU/L |
⩾ versus < | 1.653 | 1.220–2.241 | 0.0012 | 0.503 | 4 |
| TOTAL SCORE | – | – | – | – | – | 0–23 |
Figure 1.
Predictors of mortality.
ASAT, aspartate transaminase; CA 19-9, carbohydrate antigen 19-9; dNLR, derived neutrophil to lymphocyte ratio.
The score ranged from 0 to 23 and showed a good discrimination (C-statistic = 0.68) with adequate calibration (goodness-of-fit p = 0.94). Based on the distribution of the score values, we stratified the population into three groups (< 12: 27%, 12–16: 37.4% and > 16: 35.6%) and mortality was significantly different among them, progressively increasing with higher scores (p < 0.001) (Figure 2).
Figure 2.
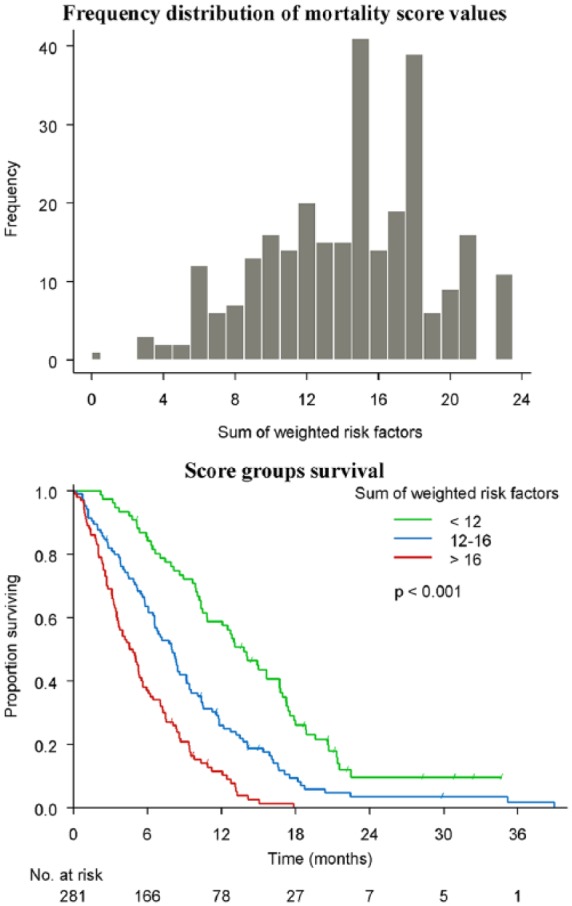
Score for mortality.
Predictors of toxicity
During the treatment, the majority of adverse events were grade 1 or 2 in severity. There were 101 patients who experienced grade 3 or 4 haematological adverse events (including neutropenia, anaemia, thrombocytopenia, febrile neutropenia and leukopenia), and 29 with grade 3 or 4 nonhaematological adverse events (including diarrhoea, nausea, vomiting, stomatitis and hand-foot syndrome). Overall, 117 patients had at least 1 grade 3 or 4 event and 44 patients with at least 2 grade 3 or 4 events.
Point estimates and corresponding 95% CIs for each covariate in the final prediction model of grade 3 or 4 adverse events are shown in Table 3. Patients with increased levels of WBC and ALP had roughly a 50% lower rate of grade 3 or 4 toxicity, while the risk was increased roughly 1.5 times in those with renal dysfunction and doubled in those with increased bilirubin levels. Based on these variables, the score was created and ranged from −6 to 5 with a good discrimination (C-statistic = 0.64) with adequate calibration (goodness-of-fit p = 0.94). Overall, the rate of toxicity was significantly higher in patients with a score ⩾ 0 compared with those with a score value lower than 0 (61 events of 205 patients versus 56 events of 110 patients corresponding to event rates of 50.1% versus 29.8%, respectively; p < 0.001) (Figure 3). The risk progressively increased with higher score values, and the higher rates of grade 3 or 4 toxicity were observed in those with scores 2–5 (18 events of 30 patients corresponding to an event rate of 60%) (Figure 3).
Table 3.
Multivariate prediction model of grade 3 or 4 toxicity.
| Adverse event | Comparison | Odds ratio | 95% confidence interval | p value | β coefficient | Score§ |
|---|---|---|---|---|---|---|
| At least one event* | ||||||
| White blood cell count (⩾ 10.8) | ⩾ versus < | 0.459 | 0.241–0.873 | 0.0176 | −0.779 | −3 |
| Alkaline phosphatase ⩾ 123 IU/L |
⩾ versus < | 0.506 | 0.293–0.871 | 0.0141 | −0.682 | −3 |
| Creatinine clearance < 60$ |
< versus ⩾ | 1.647 | 0.955–2.844 | 0.0729 | 0.499 | 2 |
| Bilirubin (⩾ 1.3) | ⩾ versus < | 2.035 | 1.148–3.607 | 0.0150 | 0.710 | 3 |
| TOTAL SCORE | –6 to 5 | |||||
| Nonhaematological | ||||||
| Alkaline phosphatase ⩾ 123 IU/L | ⩾ versus < | 0.276 | 0.105–0.722 | 0.0087 | – | – |
| Presence of pain | Yes versus No | 2.936 | 1.005–8.578 | 0.0490 | – | – |
| Aspartate transaminase ⩾ 31 IU/L | ⩾ versus < | 4.260 | 1.557–11.657 | 0.0048 | – | – |
| Distant metastases | Pre versus Abs | 8.975 | 1.149–70.125 | 0.0364 | – | – |
| Haematological ‡ | ||||||
| Platelet count (⩾ 400) | ⩾ versus < | 0.448 | 0.202–0.994 | 0.0482 | – | – |
| Karnofsky Performance Score | ⩽ 80 versus ⩾ 90 | 0.559 | 0.337–0.927 | 0.0243 | – | – |
| Aspartate transaminase ⩾ 31 IU/L | ⩾ versus < | 0.562 | 0.330–0.956 | 0.0333 | – | – |
| Bilirubin (⩾ 1.3) | ⩾ versus < | 2.554 | 1.406–4.639 | 0.0021 | – | – |
| At least two events | ||||||
| Alanine transaminase ⩾ 33 IU/L | ⩾ versus < | 0.433 | 0.211–0.888 | 0.0223 | – | – |
| Advanced age (⩾ 65 years) |
⩾ versus < | 2.377 | 1.218–4.640 | 0.0112 | – | – |
| Bilirubin (⩾ 1.3) | ⩾ versus < | 2.416 | 1.094–5.336 | 0.0291 | – | – |
White blood cell count was a stronger predictor and finally included in the model instead of platelet count, neutrophil count and neutrophil–platelet score.
Creatinine clearance < 60 ml/min/1.73 m2 has a borderline p value but was maintained in the final model and score because this model was characterized by a better prediction ability (higher C-statistic).
Platelet count was a stronger predictor and finally included in the model instead of neutrophil–platelet score. A normal value of serum protein level was protective at univariate analysis (odds ratio 0.541, 95% confidence interval 0.297–0.986; p = 0.044) but not included in the final model due to missing values reducing the power of the model.
The score of toxicity was generated only for predicting overall grade 3 or 4 adverse events (at least one event) because this includes the largest number of events and power of the prediction model.
Figure 3.
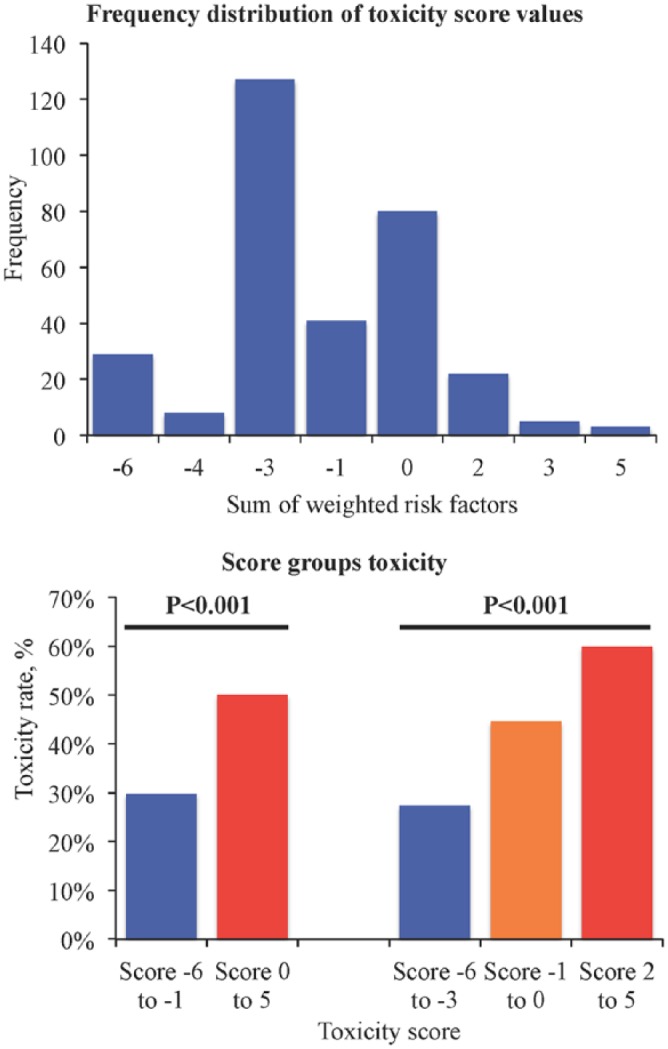
Score for toxicity.
Advanced age and increased bilirubin level were the main predictors of increased risk of experiencing multiple grade 3 or 4 adverse events (Table 3).
Discussion
The present study investigated the association of pretreatment variables, including inflammatory markers, with mortality and toxicity rates among patients with APC enrolled in a multicentre randomized clinical trial of gem-based chemotherapy. The main findings can be summarized as follows.
Eight variables emerged as independent predictors of mortality (i.e. increased ASAT, low performance status, increased dNLR, increased CA 19-9, low haemoglobin, presence of pain, presence of metastasis and increased ALP) and finally used to generate the APC-SAKK mortality score. The score ranges from 0 to 23 and is categorized into three risk groups (low risk: < 12, intermediate risk: 12–16 and high-risk: > 16).
Increased bilirubin level was the main independent predictor of grade 3 or 4 adverse events, including multiple events, and renal dysfunction also was associated with higher rates of grade 3 or 4 toxicity, while increased levels of WBC and ALP were protective factors. These four variables were finally used to generate the APC-SAKK toxicity score, which ranges from −6 to 5 with values ⩾–1 indicating a significantly higher risk of toxicity. Among the other variables, the presence of pain, distant metastasis and increase of ASAT were predictors of nonhaematological toxicity, while advanced age was associated with increased risk of multiple adverse events.
A total of 319 patients were enrolled in the trial and GemCap failed to improve OS at a statistically significant level compared with standard Gem treatment and the safety, clinical benefit response and quality of life were similar, but median OS was improved significantly in patients with good performance status.8,9 Thus, GemCap was proposed as a practical regimen to be considered as an alternative to single-agent Gem for the treatment of patients with APC with a good performance status. Additionally, CA 19-9 concentration was a prognostic factor for survival, but a decrease in concentration during chemotherapy was not significantly associated with lengthened survival compared with those who did not have a corresponding decrease.10
PC is a highly lethal disease associated with poor prognosis, highlighted by the close parallel between disease incidence and mortality.1 Five-year survival in patients with PC remains as low as 6% in the USA.1 The great variability of prognosis of patients treated with palliative chemotherapy underlines the complex interplay between patient, tumour and treatment-related factors. The low survival rate is attributed to numerous factors, but the most important ones are probably the late stage at which the majority of patients are diagnosed and the lack of effective systemic treatment options. Most patients with PC are asymptomatic until the disease develops to an advanced stage.1 Thus, the large majority of patients with APC will not undergo surgical resection, and chemotherapy represents the mainstay of treatment for such cases, and gem-based therapy has been the standard for many years. However, current systemic treatments offer only modest benefits in tumour-related symptoms and survival and, when present, these benefits, are usually limited to nondebilitated patients.
Balancing survival benefits with toxicity complications is fundamental in all patients with cancer undergoing treatment, particularly in those undergoing palliative chemotherapy as patients with APC. Therefore, the evaluation of both mortality and severe toxicity may offer a more appropriate and comprehensive evaluation of the benefit/risk ratio to guide clinical practice and decision-making.
Previous studies on patients with APC have poorly focused on the prognostic role of inflammatory markers,11–15 or have mainly focused on predicting mortality but not toxicity.2,3,16–18 In addition, there is great heterogeneity across APC studies in the reporting of clinical and laboratory variables and their prognostic impact.4 In a recent expert consensus document, the main variables considered to be significantly related to prognosis in randomized controlled trials investigating first-line systemic therapy for unresectable pancreatic cancer were albumin, bilirubin, CA 19-9, disease status, performance status, pain at baseline, ALP, lactate dehydrogenase and liver metastasis, while other variables, even if not with unanimous prognostic significance, were suggested to be mandatory and recommended prognostic variables to be reported (i.e. age, sex, C-reactive protein, NLR, number of metastatic sites, primary tumour location, pulmonary metastasis, previous deep venous thrombosis or embolus, synchronous or metachronous metastasis).4
In our study, we found that main predictors of mortality did not overlap with those predicting severe adverse events, thus, translating into a practical support in daily practice to help identifying patients with most palliative chemotherapy benefits over risks. Notably, an increased level of ALP was the most relevant predictor of mortality but was associated with reduced risk of adverse events, while an increased level of bilirubin was the strongest predictor of toxicity, including multiple adverse events, but not associated with increased mortality. On the other hand, presence of pain and metastasis were associated with reduced survival as well as higher nonhaematological toxicity. As shown in a previous analysis from this study,8 a good performance status identified patients who might benefit most from chemotherapy being associated with better survival in the absence of toxicity risk. Here, we also confirmed previous data suggesting that CA 19-9, one of the most useful tumour markers because of its high sensitivity and specificity, is a predictor of mortality, probably because it reflects overall tumour burden, which influences survival of patients with APC.10,11,14,15
Importantly, we explored the role of some inflammatory markers in this specific setting of patients. There is growing evidence that different inflammatory markers have a significant prognostic role in many tumours. Especially the dNLR and NPS were found to be associated with cancer survival.5,7 The main advantage of these markers is that they can be easily determined from routine blood analysis, however, little is known about their prognostic role in APC, and no studies have investigated their association with chemotherapy-related adverse events in these patients. Notably, we found that the NPS did not emerge as a relevant predictor of either mortality or toxicity, whereas the dNLR was associated with reduced survival but not with increased severe adverse events. We used the dNLR, calculated by the absolute neutrophil count divided by the difference between WBC and neutrophil counts, because lymphocytes were not available, however, the dNLR well approximates the NLR since peripheral blood contains few WBC other than neutrophils or lymphocytes and has demonstrated similar prognostic value to the NLR.6 The exact mechanism behind the negative prognosis is unknown, however, it may relate to increased neutrophil-dependent inflammation, and reduced lymphocyte-mediated tumour response. Whether the systemic inflammation is associated with malignancy or is related to comorbidities that cancer patients may suffer still remains uncertain, however, our finding adds to previous evidence supporting a relevant predicting role of dNLR in APC.2,3,16,18 There is however heterogeneity about the optimal cutoff to be used for this marker. While previous studies showed that cutoff values of 3 or 5 were associated with lower survival, we observed that even a lower increase (dNLR of 2 or more) was able to predict mortality.
The present study includes a large cohort of patients with APC enrolled in a multicentre randomized trial and simplicity is the strength of our models. All clinical and laboratory variables we tested can be easily collected in a routine daily pretreatment assessment, making it feasible for clinicians to estimate risks of mortality and toxicity. Overall, we developed two easy-to-use risk scores that may be valuable in daily decision-making: patients with high risk of mortality and toxicity at baseline would not reach significant benefit from treatment, while those with low risk of both would be the ideal candidates for palliative chemotherapy for APC.
Limitations
This was a retrospective post-hoc analysis, however, it was performed in the controlled setting of patients enrolled in a prospective randomized trial ensuring homogeneous treatments and rigorous collection of data and adverse event definition/adjudication.
The scores were developed in a limited number of patients and need to be validated in larger prospective studies and more recent combined chemotherapies.
The potential prediction role of other variables (i.e. C-reactive protein, albumin, carcinoembryonic antigen, lymphocytes and related ratios) cannot be excluded.
Conclusion
We explored predictors, including inflammatory markers, of mortality and toxicity in a large cohort of patients with APC treated with gem-based chemotherapy and analysed high-quality data from a multicentre randomized trial. We developed simple-to-use prognostic scores for both mortality and severe toxicity that can be useful in daily practice to identify patients with increased risk of death or toxicity and to plan the most appropriate therapeutic strategy in individual patients with the aim of improving both survival and quality of life.
Footnotes
Funding: This work was supported by an European Society for Medical Oncology (ESMO) Clinical Research Fellowship to PG with the aid of a grant from Novartis. Any views, opinions, findings, conclusions or recommendations expressed in this article are those solely of the authors and do not necessarily reflect those of ESMO or Novartis. The SAKK 44/00-CECOG/PAN.1.3.001 trial was sponsored by SAKK, and CECOG played a supportive role in Austria.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
ORCID iD: Piera Gargiulo  https://orcid.org/0000-0002-8828-4209
https://orcid.org/0000-0002-8828-4209
Contributor Information
Piera Gargiulo, Swiss Group for Clinical Cancer Research (SAKK) Coordinating Center, Effingerstrasse 33, CH-3008 Bern, Switzerland.
Daniel Dietrich, Swiss Group for Clinical Cancer Research (SAKK) Coordinating Center, Bern, Switzerland.
Richard Herrmann, University Hospital, Basel, Switzerland.
György Bodoky, Szt. László Teaching Hospital, Budapest, Hungary.
Thomas Ruhstaller, Kantonsspital, St Gallen, Switzerland.
Werner Scheithauer, University of Vienna Medical School, Vienna, Austria.
Bengt Glimelius, Department of Immunology, Genetics and Pathology, University of Uppsala, Uppsala, Sweden.
Simona Berardi, Swiss Group for Clinical Cancer Research (SAKK) Coordinating Center, Bern, Switzerland.
Sandro Pignata, Department of Urology and Gynecology, Istituto Nazionale Tumori “Fondazione G. Pascale”, Naples, Italy.
Peter Brauchli, Swiss Group for Clinical Cancer Research (SAKK) Coordinating Center, Bern, Switzerland.
References
- 1. Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet 2016; 388: 73–85. [DOI] [PubMed] [Google Scholar]
- 2. Park HS, Lee HS, Park JS, et al. Prognostic scoring index for patients with metastatic pancreatic adenocarcinoma. Cancer Res Treat 2016; 48: 1253–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kou T, Kanai M, Yamamoto M, et al. Prognostic model for survival based on readily available pretreatment factors in patients with advanced pancreatic cancer receiving palliative chemotherapy. Int J Clin Oncol 2016; 21: 118–125. [DOI] [PubMed] [Google Scholar]
- 4. Ter Veer E, van Rijssen LB, Besselink MG, et al. Consensus statement on mandatory measurements in pancreatic cancer trials (COMM-PACT) for systemic treatment of unresectable disease. Lancet Oncol 2018; 19: e151–e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mei Z, Shi L, Wang B, et al. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: a systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev 2017; 58: 1–13. [DOI] [PubMed] [Google Scholar]
- 6. van Soest RJ, Templeton AJ, Vera-Badillo FE, et al. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for men with metastatic castration-resistant prostate cancer receiving first-line chemotherapy: data from two randomized phase III trials. Ann Oncol 2015; 26: 743–749. [DOI] [PubMed] [Google Scholar]
- 7. Watt DG, Proctor MJ, Park JH, et al. The Neutrophil-Platelet Score (NPS) predicts survival in primary operable colorectal cancer and a variety of common cancers. PLoS One 2015; 10: e0142159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol 2007; 25: 2212–2217. [DOI] [PubMed] [Google Scholar]
- 9. Bernhard J, Dietrich D, Scheithauer W, et al. Clinical benefit and quality of life in patients with advanced pancreatic cancer receiving gemcitabine plus capecitabine versus gemcitabine alone: a randomized multicenter phase III clinical trial–SAKK 44/00-CECOG/PAN.1.3.001. J Clin Oncol 2008; 26: 3695–3701. [DOI] [PubMed] [Google Scholar]
- 10. Hess V, Glimelius B, Grawe P, et al. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol 2008; 9: 132–138. [DOI] [PubMed] [Google Scholar]
- 11. Ueno H, Okada S, Okusaka T, et al. Prognostic factors in patients with metastatic pancreatic adenocarcinoma receiving systemic chemotherapy. Oncology 2000; 59: 296–301. [DOI] [PubMed] [Google Scholar]
- 12. Tabernero J, Chiorean EG, Infante JR, et al. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist 2015; 20: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geng M, Xu H, Ren R, et al. Prognostic value of clinicopathological characteristics in patients with pancreatic cancer. Chin J Cancer Res 2015; 27: 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Papadoniou N, Kosmas C, Gennatas K, et al. Prognostic factors in patients with locally advanced (unresectable) or metastatic pancreatic adenocarcinoma: a retrospective analysis. Anticancer Res 2008; 28: 543–549. [PubMed] [Google Scholar]
- 15. Vernerey D, Huguet F, Vienot A, et al. Prognostic nomogram and score to predict overall survival in locally advanced untreated pancreatic cancer (PROLAP). Br J Cancer 2016; 115: 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xue P, Kanai M, Mori Y, et al. Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. Cancer Med 2014; 3: 406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kasuga A, Okano N, Naruge D, et al. Retrospective analysis of fixed dose rate infusion of gemcitabine and S-1 combination therapy (FGS) as salvage chemotherapy in patients with gemcitabine-refractory advanced pancreatic cancer: inflammation-based prognostic score predicts survival. Cancer Chemother Pharmacol 2015; 75: 457–464. [DOI] [PubMed] [Google Scholar]
- 18. Luo G, Guo M, Liu Z, et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol 2015; 22: 670–676. [DOI] [PubMed] [Google Scholar]